Abstract
Costus speciosus is native to South East Asia, especially found in India, Srilanka, Indonesia and Malaysia. C. speciosus have numerous therapeutic potentials against a wide variety of complains. The therapeutic properties of C. speciosus are attributed to the presence of various ingredients such as alkaloids, flavonoids, glycosides, phenols, saponins, sterols and sesquiterpenes. This review presented the past, present, and the future status of C. speciosus active ingredients to propose a future use as a potential anticancer agent. All possible up-regulation of cellular apoptotic molecules as p53, p21, p27, caspases, reactive oxygen species (ROS) generation and others attribute to the anticancer activity of C. speciosus along the down-regulation of anti-apoptotic agents such as Akt, Bcl2, NFκB, STAT3, JAK, MMPs, actin, surviving and vimentin. Eventually, we recommend further investigation of different C. speciosus extracts, using some active ingredients and evaluate the anticancer effect of these chemicals against different cancers.
Keywords: Costus speciosus, anticancer mechanisms, future vision
Introduction
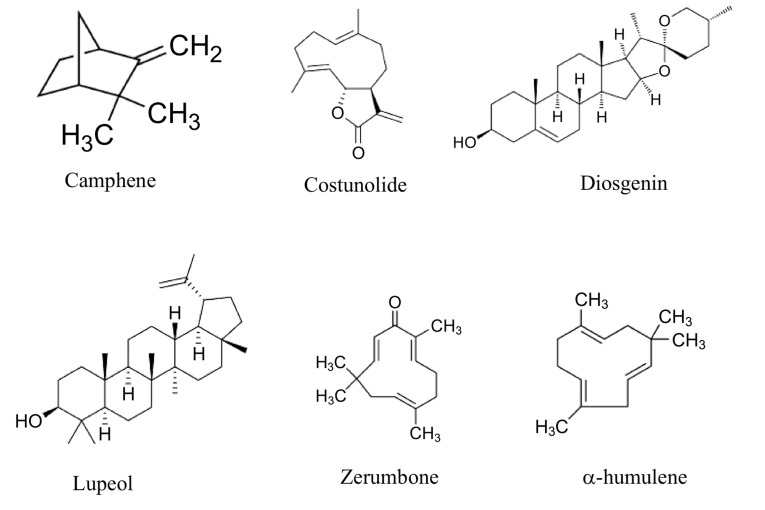
Plant active principles are important task for developing therapeutic agents. Herbal products are greatly safe in comparison to the synthetics. Herbal natural products are a components of different parts of medicinal herb [1]. From the important medicinal plant families is the Zingiberaceae that distributed throughout tropical Africa, Asia, Americas and Indo-Malayan region, Sri Lanka and in India. It is commonly grown along road sides and streams [2]. The rhizomes and roots are ascribed to have an anthelmintic, expectorant, tonic, aphrodisiac, flatulence, anti-inflammatory, antidiabetic, hepatoprotective, antihyperlipidemic, antispasmodic and antimicrobial activities [3]. Indeed, leaf extract of C. speciosus shows potential in vitro anticancer activity toward liver cancer [4]. C. speciosus serves as an important source of numerous compounds owning many pharmacological benefits as diosgenin, tigogenin, saponins and β-sitosterol; diosgenin, 5α-stigmast-9 (11)-en-3β-ol, β-sitosterol-β-D-glucoside, dioscin, prosapogenins A and B of dioscin, gracillin, α-tocopherol; diosgenone, cycloartanol, 25-en-cycloartenol and octacosanoic acid [5]. The major compounds C. speciosus oils such as α-humulene, zerumbone, camphene, α-amyrin stearate, β-amyrin, costunolide and lupeol have been isolated from its rhizomes and their structural formula are illustrated in Fig. 1 [6,7].
Fig. (1).
Shows the structural formulae of some active ingredients present in C. speciosus.
Anticancer Activity Evaluation and Mechanism of Costus speciosus Active Ingredients
In Vitro Anticancer Activity
Up-Regulation of p53, p21 and p27
The tumor suppressor p53 is a transcription factor that responds to diverse cases of cellular stress. It is recognized as the guardian of the genome [8]. P53 promotes growth arrest genes as p21. The p21 is a tumor suppressor able to suppress cancer cell proliferation [9]. The pro-apoptotic gene products such as the PUMA, Noxa, BAX and p53AIP1 localize to the mitochondria and promote the loss of mitochondrial membrane potential and cytochrome c release. Moreover, Fas or DR5/KILLER, the components of the extrinsic pathway of apoptosis were regulated by p53. Finally, p53 induces in reactive oxygen species (ROS) production that damage the mitochondria, leading to apoptosis [10].
Cyclin-dependent kinase inhibitor 1B (p27Kip1) is an enzyme inhibitor that binds to and inhibits the activation of cyclin E-CDK2 and cyclin D-CDK4 complexes, and thus induces G1 phase arrest that may be stop or slow down the cancer cell growth [11]. The up-regulation of p53, p21 and p27 by C. speciosus active ingredients in different cancer cells is illustrated in Table 1.
Table 1.
Up-regulation of p53, p21 and p27 by C. speciosus active ingredients.
| Mechanism | Ingredients | Cell | References |
|---|---|---|---|
| Up-regulation of p53 | Diosgenin | Human osteosarcoma (1547) | [21] |
| Cervix carcinoma (HEp-2) | |||
| Human melanoma (M4Beu) | |||
| Human osteosarcoma (1547) | [22] | ||
| [23] | |||
| Zerumbone | Human lung cancer (NSCLC) | [24] | |
| Human pancreatic carcinoma (PANC-1) | [25] | ||
| Up-regulation of p21 | Costunolide | Human prostate cancer (PC-3) | [26] |
| Breast cancer (MDA-MB-231) | [27] | ||
| Diosgenin | Human hepatoma (Bel-7402) | [28] | |
| Human hepatocellular carcinoma (HepG2) | |||
| Human hepatoma cells (SMMC-7721) | |||
| Human osteosarcoma (1547) | [23] | ||
| Human erythromyeloblastoid leukemia (K562) | [29] | ||
| Human colon carcinoma (HCT-116) | [30] | ||
| Lupeol | Human osteosarcoma cells (MNNG/HOS) | [31] | |
| Human osteosarcoma cells (MG-63) | |||
| Human pancreatic cancer (PCNA-1) | [32] | ||
| Melanoma (451Lu) | [33] | ||
| Zerumbone | Human pancreatic carcinoma (PANC-1) | [25] | |
| Up-regulation of p27 | Diosgenin | Human hepatoma (Bel-7402) | [28] |
| Human hepatocellular carcinoma (HepG2) | |||
| Human hepatoma cells (SMMC-7721) | |||
| Lupeol | Human osteosarcoma cells (MNNG/HOS) | [31] | |
| Human osteosarcoma cells (MG-63) | |||
| Human pancreatic cancer (PCNA-1) | [32] |
Up-Regulation of Caspases
Caspases are endo-proteases that accomplish their activity by hydrolysing cell protein peptide bonds. The apoptotic caspases have been sub-classified by their mechanism of action into initiator caspases (caspase-8 and -9) or executioner caspases (caspase-3, -6, and -7) [12]. They are activated in both main apoptotic pathways: extrinsic, mediated by death receptors, and intrinsic, where mitochondria play a central role. The mitochondrial pathway activates caspase-9, which, when activated, forms an apoptosome in the cytosol, together with cytochrome c, Apaf-1 and deoxyadenosine triphosphate (dATP). The apoptosome activates caspase-3 [13]. Whereas, the extrinsic death receptor Fas pathway is activated by Fas ligand interaction with Fas complexes those activate caspase 3 and induce apoptosis [14]. The up-regulation of apoptotic initiators and executioner caspases by C. speciosus active ingredients in numerous cancer cells are illustrated in Table 2.
Table 2.
Up-regulation of caspases by C. speciosus active ingredients.
| Mechanism | Ingredients | Cell | References |
|---|---|---|---|
| Up-regulation of caspase-3 | Camphene | Murine melanoma cell (B16F10-Nex2) | [34] |
| Human pancreatic carcinoma (MIA PaCa-2) | [35] | ||
| Human hepatocellular carcinoma (HepG2) | |||
| Human colon adenocarcinoma (SW-480) | |||
| Costunolide | Human promyelocytic leukemia (HL-60) | [36] | |
| Breast cancer (MDA-MB-231) | [27] | ||
| Human bladder carcinoma (T24) | [6] | ||
| Ovarian cancer (MPSC1) | [37] | ||
| Human ovarian carcinoma (A2780) | |||
| Human ovarian carcinoma (SKOV3) | |||
| Human breast adenocarcinoma (MCF-7) | [38] | ||
| Breast cancer (MDA-MB-231) | |||
| Diosgenin | Human erythromyeloblastoid leukemia (K562) | [39] | |
| Human promyelocytic leukemia (HL-60) | |||
| Human osteosarcoma (1547) | [21] | ||
| Cervix carcinoma (HEp-2) | |||
| Human melanoma (M4Beu) | |||
| Human lung carcinoma (A549) | [40] | ||
| Human erythroleukemia (HEL) | [41] | ||
| Human hepatocellular carcinoma (HepG2) | [42] | ||
| Human breast adenocarcinoma (MCF-7) | |||
| Human hepatoma (Bel-7402) | [28] | ||
| Human hepatocellular carcinoma (HepG2) | |||
| Human hepatoma cells (SMMC-7721) | |||
| Human epidermoid carcinoma (A431) | [43] | ||
| Human hepatocellular carcinoma (Hep2) | |||
| Human erythroleukemia (HEL) | [44] | ||
| Human erythromyeloblastoid leukemia (K562) | [29] | ||
| Human colon adenocarcinoma (HT-29) | [45] | ||
| Up-regulation of caspase-3 | Lupeol | Melanoma (451Lu) | [33] |
| Head and neck squamous cell carcinoma (HNSCC) | [46] | ||
| Human hepatoma cells (SMMC-7721) | [47] | ||
| Human hepatocellular carcinoma (HepG2) | |||
| Zerumbone | Acute promyelocytic leukemia (NB4) | [48] | |
| Chronic myeloid leukemia (CML) | [49] | ||
| Human erythromyeloblastoid leukemia (K562) | |||
| Human T-cell (Jurkat) | [50] | ||
| Human lung cancer (NSCLC) | [24] | ||
| Human renal carcinoma (786-0) | [51] | ||
| Human renal carcinoma (769-P) | |||
| Human brain malignant glioma (GBM8401) | [52] | ||
| Human pancreatic carcinoma (PANC-1) | [25] | ||
| Human epithelioid cervical carcinoma (HeLa) | [53] | ||
| leaves methanol extract | Human hepatocellular carcinoma (HepG2) | [4] | |
| Up-regulation of caspase-7 | Camphene | Human pancreatic carcinoma (MIA PaCa-2) | [35] |
| Human hepatocellular carcinoma (HepG2) | |||
| Human colon adenocarcinoma (SW-480) | |||
| Costunolide | Human promyelocytic leukemia (HL-60) | [36] | |
| Human neuroblastoma (IMR-32) | [54] | ||
| Human neuroblastoma (NB-39) | |||
| Human neuroblastoma (SK-N-SH) | |||
| Human neuroblastoma (LA-N-1) | |||
| Up-regulation of caspase-6 | Costunolide | Human promyelocytic leukemia (HL-60) | [36] |
| Up-regulation of caspase-8 | Costunolide | Breast cancer (MDA-MB-231) | [27] |
| Ovarian cancer (MPSC1) | [37] | ||
| Human ovarian carcinoma (A2780) | |||
| Human ovarian carcinoma (SKOV3) | |||
| Diosgenin | Human lung carcinoma (A549) | [40] | |
| Human erythroleukemia (HEL) | [41] | ||
| Human hepatoma (Bel-7402) | [28] | ||
| Human hepatocellular carcinoma (HepG2) | |||
| Human hepatoma cells (SMMC-7721) | |||
| Lupeol | Pancreatic cancer (PaCa) | [55] | |
| Zerumbone | Acute promyelocytic leukemia (NB4) | [48] | |
| Up-regulation of caspase-9 | Costunolide | Ovarian cancer (MPSC1) | [37] |
| Human ovarian carcinoma (A2780) | |||
| Human ovarian carcinoma (SKOV3) | |||
| Human breast adenocarcinoma (MCF-7) | [38] | ||
| Breast cancer (MDA-MB-231) | |||
| Diosgenin | Human lung carcinoma (A549) | [40] | |
| Human erythroleukemia (HEL) | [41] | ||
| Human erythromyeloblastoid leukemia (K562) | [39] | ||
| Human promyelocytic leukemia (HL-60) | |||
| Human hepatoma (Bel-7402) | [28] | ||
| Human hepatocellular carcinoma (HepG2) | |||
| Human hepatoma cells (SMMC-7721) | |||
| Lupeol | Human hepatoma cells (SMMC-7721) | [56] | |
| Zerumbone | Chronic myeloid leukemia (CML) | [49] | |
| Human erythromyeloblastoid leukemia (K562) | |||
| Human T-cell (Jurkat) | [50] | ||
| Human lung cancer (NSCLC) | [24] | ||
| Human renal carcinoma (786-0) | [51] | ||
| Human renal carcinoma (769-P) | |||
| Acute promyelocytic leukemia (NB4) | [48] |
Calcium Overload Induce Apoptosis
Variation in cytosolic calcium concentration promotes numerous cellular functions as contraction of myofilaments, secretion of hormonal secretion and metabolic regulation [15]. However, it has become clear that cellular Ca2+ overload can cause cytotoxicity and trigger apoptosis [16]. The up-regulation of intracellular Ca2+ by C. speciosus active ingredients presented in Table 3.
Table 3.
Up-regulation of Bax by C. speciosus active ingredients.
| Mechanism | Ingredients | Cell | References |
|---|---|---|---|
| Up-regulation of Bax | Costunolide | Human bladder carcinoma (T24) | [6] |
| Diosgenin | Human erythromyeloblastoid leukemia (K562) | [29] | |
| Human erythroleukemia (HEL) | [57] | ||
| Human lung carcinoma (A549) | [40] | ||
| Human epidermoid carcinoma (A431) | [43] | ||
| Human hepatocellular carcinoma (Hep2) | |||
| Human erythroleukemia (HEL) | [41] | ||
| Lupeol | Human epidermoid carcinoma (A431) | [58] | |
| Melanoma (451Lu) | [33] | ||
| Head and neck squamous cell carcinoma (HNSCC) | [46] | ||
| Zerumbone | Human lung cancer (NSCLC) | [24] | |
| Human hepatocellular carcinoma (HepG2) | [59] | ||
| Intracellular Ca2+ increase | Camphene | Murine melanoma cell (B16F10-Nex2) | [34] |
| Zerumbone | Human prostate cancer (PC-3) | [60] | |
| Human prostate cancer (DU-145) | |||
| Chronic myeloid leukemia (CML) | [49] | ||
| Human erythromyeloblastoid leukemia (K562) | |||
| Overload of nuclear Ca2+ | Costunolide | Human prostate cancer (PC-3) | [26] |
| Human prostate cancer (DU-145) | |||
| Human prostate adenocarcinoma (LNCaP) |
Up-regulation of ROS Generation
Nitric oxide synthase (nNOS) is a Ca2+-dependent cytosolic enzyme that forms nitric oxide (NO) from l-arginine, and NO reacts with the free superoxide radical (O2−) to form the toxic free peroxynitrite radical (ONOO−). These free radicals predispose the damage of cellular membranes and intracellular proteins, enzymes and DNA. COX-2-dependent reactions generate ROS during the conversion of arachidonic acid to prostaglandin G2, causing direct oxidative damage to DNA and favour apoptosis [17]. The ROS generation in cancer cells and the antioxidant status augmentation of cancer bearing animal by C. speciosus active ingredients are tabulated in Table 4.
Table 4.
Up-regulation of antioxidant status by C. speciosus active ingredients.
| Mechanism | Ingredients | Cell | References |
|---|---|---|---|
| Intracellular thiols depletion | Costunolide | Human prostate cancer (PC-3) | [26] |
| Human prostate cancer (DU-145) | |||
| Human prostate adenocarcinoma (LNCaP) | |||
| Up-regulation of 5-LOX | Diosgenin | Human colon carcinoma (HCT-116) | [61] |
| Human colon adenocarcinoma (HT-29) | |||
| Up-regulation of COX-2 | Diosgenin | Human colon adenocarcinoma (HT-29) | [61] |
| Human colon carcinoma (HCT-116) | |||
| Human colon carcinoma (HCT-116) | [62] | ||
| Human colon adenocarcinoma (HT-29) | |||
| ROS generation | Costunolide | Breast cancer (MDA-MB-231) | [27] |
| Human promyelocytic leukemia (HL-60) | [36] | ||
| Human bladder carcinoma (T24) | [6] | ||
| Ovarian cancer (MPSC1) | [37] | ||
| Human ovarian carcinoma (A2780) | |||
| Human ovarian carcinoma (SKOV3) | |||
| Diosgenin | Human erythromyeloblastoid leukemia (K562) | [29] | |
| Lupeol | Human prostate adenocarcinoma (LNCaP) | [63] | |
| Human epidermoid carcinoma (A431) | [58] | ||
| Zerumbone | Human lung cancer (NSCLC) | [24] | |
| Chronic myeloid leukemia (CML) | [49] | ||
| Human erythromyeloblastoid leukemia (K562) | |||
| Human colon carcinoma (HCT116) | [64] | ||
| Human pancreatic carcinoma (PANC-1) | [25] | ||
| α-Humulene | Human colon carcinoma (CaCo-2) | [65] | |
| β-amyrin | Human bladder carcinoma (NTUB1) | [66] |
Induction of Apoptosis and Oppose Metastasis
The up-regulation of the following mentioned apoptotic molecules by C. speciosus active ingredients is illustrated in Table 5. In which, the apoptosis inducing factor (AIF) is a mitochondrial intermembrane flavoprotein that induce chromatin condensation and DNA cleavage. AIF can also participate in the regulation of apoptosis by means of mitochondrial membrane permeabilization [18].
Table 5.
Up-regulation of some apoptotic molecules by C. speciosus active ingredients.
| Mechanism | Ingredients | Cell | References |
|---|---|---|---|
| Up-regulation of Apaf1 | Lupeol | Human epidermoid carcinoma (A431) | [58] |
| Up-regulation of AIF | Diosgenin | Human osteosarcoma (1547) | [21] |
| Cervix carcinoma (HEp-2) | |||
| Human melanoma (M4Beu) | |||
| Up-regulation of ATF3 | Zerumbone | Human colon carcinoma (HCT116) | [67] |
| Human colon adenocarcinoma (SW-480) | |||
| Up-regulation of E-cadherin | Diosgenin | Human gastric cancer (BGC-823) | [68] |
| Up-regulation of FADD | Lupeol | Human hepatoma cells (SMMC-7721) | [69] |
| Up-regulation of Fas | Costunolide | Breast cancer (MDA-MB-231) | [27] |
| Zerumbone | Acute promyelocytic leukemia (NB4) | [48] | |
| Up-regulation of integrin α5 | Diosgenin | Human gastric cancer (BGC-823) | [68] |
| Up-regulation of integrin β6. | Diosgenin | Human gastric cancer (BGC-823) | [68] |
| Up-regulation of Notch2 | Zerumbone | Human breast adenocarcinoma (MCF-7) | [70] |
| Breast cancer (MDA-MB-231) | [70] | ||
| Up-regulation of PTEN | Lupeol | Hepatocellular carcinoma (MHCC-LM3 HCC) |
[71] |
| Up-regulation of Rab27a | Lupeol | Mouse melanoma (B16 2F2) | [72] |
| Up-regulation of thromboxane synthase | Diosgenin | Human erythroleukemia (HEL) | [41] |
| Up-regulation of DR4 | Zerumbone | Human colon carcinoma (HCT116) | [64] |
E-cadherin plays important roles in cell-cell adhesion. Cancer cell metastasis include loss of cell-cell adhesion that leads to increased invasiveness, entry into the circulation, dispersion to distant anatomic sites, extravasation and colonization. Therefore, down-regulation of E-cadherin facilitates metastasis. The combination of diosgenin and HIF-1α silencing RNAs can enhance the expression of E-cadherin [19]. Phosphatase and tensin homolog (PTEN) inhibits p-Akt and mouse double minute 2 homolog (MDM2), and then increases the level of p53, thereby inducing G1 phase arrest and apoptosis. PTEN functions by dephosphorylation of
phosphatidyl inositol 3-phosphate (PIP3) and negatively regulating survival signalling mediated by protein kinase B/Akt (PKB/Akt) [20].
Down-Regulation of Akt
Akt is a serine-threonine kinase which regulates cell growth, survival and proliferation. The phosphatidylinositol 3-kinase/Akt pathway plays a key role in cancer cell survival [73]. Foxo inhibits tumor growth in breast cancer, and cytoplasmic localization of Foxo interrelated with poorer cancer cell survival. Phosphorylation of Foxos by Akt inhibits transcriptional functions of Foxos and contributes to cell survival, growth and proliferation [73]. The cell survival encouraged by Akt was diminished by C. speciosus active ingredients (Table 6).
Table 6.
Down-regulation of PI3-kinase/Akt by C. speciosus active ingredients.
| Mechanism | Ingredients | Cell | References |
|---|---|---|---|
| Down-regulation of PI3-kinase/Akt | Lupeol | Human hepatocellular carcinoma (HepG2) | [86] |
| Human hepatoma cells (SMMC-7721) | |||
| Diosgenin | Human prostate cancer (PC-3) | [87] | |
| Down-regulation of Akt | Diosgenin | Human erythroleukemia (HEL) | [88] |
| Mouse melanoma (B16) | [89] | ||
| Human prostate cancer (DU145) | [90] | ||
| Human epidermoid carcinoma (A431) | [43] | ||
| Human hepatocellular carcinoma (Hep2) | |||
| Breast cancer (HER2) | [91] | ||
| Human breast carcinoma (BCa) | [92] | ||
| Human breast carcinoma (BCa) | |||
| Lupeol | Human epidermoid carcinoma (A431) | [58] | |
| Zerumbone | Human colon carcinoma (HCT116) | [93] | |
| Human brain malignant glioma (GBM8401) | [52] | ||
| Non-Small Cell Lung Cancer (A549) | [94] | ||
| Down-regulation of (p-PI3K) | Lupeol | Human osteosarcoma cells (MNNG/HOS) | [31] |
| Human osteosarcoma cells (MG-63) | |||
| Human pancreatic cancer (PCNA-1) | [32] | ||
| Down-regulation of p-AKT | Lupeol | Gallbladder carcinoma (GBC-SD) | [95] |
| Human osteosarcoma cells (MNNG/HOS) | [31] | ||
| Human osteosarcoma cells (MG-63) | |||
| Human pancreatic cancer (PCNA-1) | [32] |
Cell Cycle Arrest
The cell cycle starts by G1 phase, during which cytoplasmic organelles are replicated. Afterward, the cell enters into the S phase where the DNA is replicated. After which cell reaches the second phase, G2 where proteins and other cellular elements are synthesized. Eventually, the cell enters M phase where it splits into two daughter cells [74]. Cell cycle progression is forcefully regulated by interaction between cyclin-dependent kinases (Cdk1, 2, 4, or 6) and regulatory cyclin subunits (cyclin A, B, Ds, or E). The cell arrest is accompanied by micro-nucleation resulting from chromosome fragments [75]. This cycle arrest was accomplished by C. speciosus active ingredients in different cell cycle phases as presented in Table 8.
Table 8.
Down-regulation of Bcl-2 and Bcl-xL by C. speciosus active ingredients.
| Mechanism | Ingredients | Cell | References |
|---|---|---|---|
| Down-regulation of Bcl-2 | Costunolide | Human bladder carcinoma (T24) | [6] |
| Human ovarian carcinoma (SKOV3) | [37] | ||
| Human ovarian carcinoma (A2780) | |||
| Human ovarian carcinoma (SKOV3) | |||
| Diosgenin | Human lung carcinoma (A549) | [40] | |
| Human epidermoid carcinoma (A431) | [43] | ||
| Human hepatocellular carcinoma (Hep2) | |||
| Human erythroleukemia (HEL) | [41] | ||
| Human breast carcinoma (BCa) | [92] | ||
| Human erythromyeloblastoid leukemia (K562) | [29] | ||
| Human colon adenocarcinoma (HT-29) | [45] | ||
| Human erythroleukemia (HEL) | [57] | ||
| Human erythromyeloblastoid leukemia (K562) | [39] | ||
| Human promyelocytic leukemia (HL-60) | |||
| Lupeol | Human epidermoid carcinoma (A431) | [58] | |
| Human breast adenocarcinoma (MCF-7) | [106] | ||
| Melanoma (451Lu) | [33] | ||
| Head and neck squamous cell carcinoma (HNSCC) | [46] | ||
| Zerumbone | Human renal carcinoma (786-0) | [51] | |
| Human renal carcinoma (769-P) | |||
| Human hepatocellular carcinoma (HepG2) | [59] | ||
| Down-regulation of Bcl-xL | Diosgenin | Human erythroleukemia (HEL) | [44] |
| Human erythromyeloblastoid leukemia (K562) | [29] | ||
| Lupeol | Human breast adenocarcinoma (MCF-7) | [106] | |
| Zerumbone | Human prostate cancer (PC-3) | [60] | |
| Human prostate cancer (DU-145) | |||
| Human colon carcinoma (HCT116) | [93] |
Down-Regulation of BCL2
B cell lymphoma-2 (BCL2) family proteins are key regulators of the apoptotic process and classified into three subgroups anti-apoptotic (BCL2, BCL-XL, and BCL2L10), pro-apoptotic (e.g. BAX, BAK, and BOK) and BH3-only pro-apoptotic members (e.g. BID, BAD, and BIM) [76]. BCL2 and the BCL2-associated X protein gene (BAX) are an oncogene and a cancer suppressor gene, respectively. Overexpression of BCL2 promotes cell survival in vitro and in vivo. When Bax is overexpressed, cell apoptosis will be hastened. Hence, the ratio BCL2/Bax governs the cell survival or death [77]. Moreover, NF-κB p65/p52 signalling mediated the effects of Glial-cell-line-derived neurotrophic factor (GDNF) on BCL2 and BCL2-w expressions [78]. The up-regulation of Bax by C. speciosus active ingredients in different cancer cells is illustrated in Table 3. Whereas BCL2 down-regulations is presented in Table 8.
Down-Regulation of NFκB
Nuclear factor κB (NFκB) is a transcription factor that activates its own inhibitor (IκB) as well as groups of pro-apoptotic and anti-apoptotic genes [79]. NFκB activates the inhibitor of apoptosis protein (IAP) gene transcription and down-regulate the activity of the caspase cascade.
Following stimulation of the cell by a variety of agents, IκB is degraded, allowing NF-κB to translocate to the nucleus and bind to the promoter regions of its multiple target genes to promote cell survival [80,81]. The cell survival induced by NF-κB was down-regulated by C. speciosus active ingredients (Table 9).
Table 9.
Down-regulation of NF-κB, JAKs and JNK by C. speciosus active ingredients.
| Mechanism | Ingredients | Cell | References |
|---|---|---|---|
| Down-regulation of NF-κB | Costunolide | Breast cancer (MDA-MB-231) | [107] |
| Diosgenin | Human prostate cancer (PC-3) | [87] | |
| Human erythroleukemia (HEL) | [44] | ||
| Human breast carcinoma (BCa) | [92] | ||
| Lupeol | Head and neck squamous cell carcinoma (HNSCC) | [46] | |
| human pancreatic adenocarcinoma cells (AsPC-1) | [108] | ||
| Zerumbone | Pancreatic cancer (PaCa) | [109] | |
| Breast cancer (HER2) | [110] | ||
| Breast cancer (MDA-MB-231) | [111] | ||
| Human gastric carcinoma (AGS) | [112] | ||
| Down-regulation of JAK1 | Diosgenin | hepatocellular carcinoma (HCC) | [113] |
| Down-regulation of JAK2 | Diosgenin | hepatocellular carcinoma (HCC) | [113] |
| Zerumbone | Human prostate cancer (DU145) | [101] | |
| Human prostate cancer (PC-3) | |||
| Down-regulation of JAKs | Lupeol | Human hepatocellular carcinoma (HepG2) | [56] |
| Human liver hepatoma (PLC/PRF5) | |||
| Human hepatoma-derived (C3A) | |||
| Hepatocarcinoma (HUH-7) | |||
| Human hepatoma (Hep3B) | |||
| Down-regulation of JNK | Diosgenin | Breast cancer (HER2) | [91] |
| Human prostate cancer (PC-3) | [87] | ||
| Human epidermoid carcinoma (A431) | [43] | ||
| Human hepatocellular carcinoma (Hep2) |
PARP Cleavage
DNA damage activates nuclear poly (ADP-ribose) polymerase-1 (PARP-1) to repair DNA. The activated PARP-1 uses NAD+ to form polymers of ADP-ribose that amend PARP-1 and DNA repair proteins [82]. PARP-1 was cleaved by C. speciosus active ingredients that inhibit DNA repair of cancer cells apoptosis (Table 10).
Table 10.
Down-regulation of PARP, STAT3 and MMPs by C. speciosus active ingredients.
| Mechanism | Ingredients | Cell | References |
|---|---|---|---|
| PARP cleavage | Costunolide | Breast cancer (MDA-MB-231) | [27] |
| Human bladder carcinoma (T24) | [6] | ||
| Human neuroblastoma (IMR-32) | [54] | ||
| Diosgenin | Human erythroleukemia (HEL) | [44] | |
| Human lung carcinoma (A549) | [40] | ||
| Lupeol | Human hepatoma cells (SMMC-7721) | [47] | |
| Human hepatocellular carcinoma (HepG2) | |||
| Pancreatic cancer (PaCa) | [55] | ||
| Human epidermoid carcinoma (A431) | [58] | ||
| Human prostate cancer (CWR22Rnu1) | [108] | ||
| Melanoma (451Lu) | [33] | ||
| Zerumbone | Chronic myeloid leukemia (CML) | [49] | |
| Human erythromyeloblastoid leukemia (K562) | |||
| Human renal carcinoma (786-0) | [51] | ||
| Human renal carcinoma (769-P) | |||
| Acute promyelocytic leukemia (NB4) | [48] | ||
| Down-regulation of STAT3 | Diosgenin | hepatocellular carcinoma (HCC) | [113] |
| Lupeol | Human hepatocellular carcinoma (HepG2) | [56] | |
| Human liver hepatoma (PLC/PRF5) | |||
| Human hepatoma-derived (C3A) | |||
| Hepatocarcinoma (HUH-7) | |||
| Human hepatoma (Hep3B) | |||
| Zerumbone | Human prostate cancer (DU145) | [101] | |
| Breast cancer cells | [114] | ||
| Down-regulation of MMP-2 | Diosgenin | Human prostate cancer (PC-3) | [87] |
| Lupeol | Prostate cancer (CaP) | [115] | |
| Down-regulation of MMP-3 | Zerumbone | Breast cancer (Hs578T) | [116] |
| Breast cancer (MDA-MB-231) | |||
| Down-regulation of MMP-9 | Costunolide | Breast cancer (MDA-MB-231) | [27] |
| Diosgenin | Human prostate cancer (PC-3) | [87] | |
| Lupeol | Gallbladder carcinoma (GBC-SD) | [95] |
Down-Regulation of STAT3, JAK and MMPs
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor which in humans is encoded by the STAT3 gene. The Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway regulate signals for development and homeostasis. JAK activation stimulates cell proliferation, differentiation, cell migration and apoptosis [83]. The down-regulation of STAT3 and JAKs by C. speciosus active ingredients was presented in Table 10.
The expression of matrix metalloproteinases (MMPs) correlates with the extracellular matrix degradation and tumor metastasis. The expression of MMP-2 and MMP-9 is associated with metastasis of numerous human cancers because they play an important role in the degradation of type IV collagen, which is a major component of the basement membrane [84]. Therefore, MMP-2, -3 and -9 may be involved in the process of metastasis of breast cancer to the brain. MMPs were down-regulated by C. speciosus active ingredients that inhibit DNA repair of cancer cells apoptosis (Table 10).
Down-Regulation of p38 MAPK
The p38 is a member of Ser/Thr kinases family called the mitogen-activated protein kinase (MAPKs) (Table 12). The p38 MAPK signalling coordinates cellular responses during erythropoiesis in which the proliferation and differentiation of erythroid progenitors are controlled by erythropoietin through the p38 (Table 11) and Jun N-terminal kinase (JNK) (Table 9) signalling cascades [85].
Table 12.
Down-regulation of some anti-apoptotic molecules by C. speciosus active ingredients.
| Mechanism | Ingredients | Cell | References |
|---|---|---|---|
| Down-regulation of actin | Diosgenin | Breast cancer (MDA-MB-231) | [98] |
| Down-regulation of β-catenin | Diosgenin | Human colon carcinoma (HCT-116) | [30] |
| Lupeol | Prostate cancer (CaP) | [115] | |
| Down-regulation of FADD-like IL-1β-converting enzyme)-inhibitory protein (c-FLIP) | Lupeol | Pancreatic cancer (PaCa) | [55] |
| Down-regulation of c-Src | Diosgenin | Hepatocellular carcinoma (HCC) | [113] |
| Down-regulation of epidermal growth factor receptor (EGFR) | Lupeol | Gallbladder carcinoma (GBC-SD) | [95] |
| Down-regulation of extracellular signal-regulated kinase (ERK) | Diosgenin | Human prostate cancer (PC-3) | [87] |
| Human erythroleukemia (HEL) | [118] | ||
| Down-regulation of GLI | Diosgenin | Human erythroleukemia (HEL) | [118] |
| Down-regulation of Gli-1 | Zerumbone | Human renal carcinoma (786-0) | [51] |
| Human renal carcinoma (769-P) | |||
| Down-regulation of glycogen synthase kinase-3 (GSK3beta) | Diosgenin | Mouse melanoma (B16) | [89] |
| Down-regulation of Hepatocyte growth factor (HGF) | Diosgenin | Human prostate cancer (DU145) | [90] |
| Down-regulation of hypoxia-inducible factor 1 (HIF-1α) | Diosgenin | Human gastric cancer (BGC-823) | [68] |
| Gastric carcinoma (NCI-N87) | |||
| Human gastric adenocarcinoma (MGC80-3) | |||
| Human gastric cancer (SGC-7901) | |||
| Down-regulation of human telomerase reverse transcriptase (hTERT) | Diosgenin | Human lung carcinoma (A549) | [119] |
| Human lung carcinoma (A549) | [120] | ||
| Down-regulation of myeloid leukemia cell differentiation protein (Mcl-1) | Zerumbone | Human prostate cancer (PC-3) | [60] |
| Human prostate cancer (DU-145) | |||
| Down-regulation of mouse double minute 2 homolog (Mdm2) | Diosgenin | Human prostate cancer (DU145) | [90] |
| Down-regulation of MAPKs | Zerumbone | Human colon carcinoma (CaCo-2) | [121] |
| Human colon carcinoma (Colo320DM) | |||
| Human colon adenocarcinoma (HT-29) | |||
| Down-regulation of mammalian target of rapamycin (mTOR) | Diosgenin | Human prostate cancer (DU145) | [90] |
| Breast cancer (HER2) | [91] | ||
| Down-regulation of Polo-like kinase 1 (PLK-1) | Lupeol | Human prostate cancer (PC-3) | [96] |
| Down-regulation of Smoothened (SMO) | Diosgenin | Human erythroleukemia (HEL) | [118] |
| Down-regulation of survivin | Costunolide | Human bladder carcinoma (T24) | [6] |
| Diosgenin | Human breast carcinoma (BCa) | [92] | |
| Lupeol | Prostate cancer (CaP) | [99] | |
| Zerumbone | Human colon carcinoma (HCT116) | [93] | |
| Down-regulation of tumor necrosis factor-alpha (TNF-α) | Costunolide | Breast cancer (MDA-MB-231) | [122] |
| Down-regulation of vascular endothelial growth factor (VEGF) | Zerumbone | Human gastric carcinoma (AGS) | [112] |
| Down-regulation of Vav2 | Diosgenin | Breast cancer (MDA-MB-231) | [98] |
| Down-regulation of vimentin | Diosgenin | Human prostate cancer (DU145) | [90] |
| Down-regulation of Wnt | Lupeol | Human Melanoma (Mel 928) | [123] |
| Down-regulation of X-linked inhibitor of apoptosis | Diosgenin | Human breast carcinoma (BCa) | [92] |
| Zerumbone | Human colon carcinoma (HCT116) | [93] |
Table 11.
Down-regulation of CXCR4, CXCL12, p52, p65, p70S6K and p100 by C. speciosus active ingredients.
| Mechanism | Ingredients | Cell | References |
|---|---|---|---|
| Down-regulation of C-X-C chemokine receptor type 4 (CXCR-4) | Zerumbone | Breast cancer (HER2) | [110] |
| Chronic Myelogenous Leukemia (KBM-5) | |||
| Human myeloma (U266) | |||
| Human squamous carcinoma (SCC4) | |||
| Human embryonic kidney (A293) | |||
| Human non-small cell lung carcinoma (H1299) | |||
| Human pancreatic carcinoma (PANC-1) | |||
| Pancreatic carcinoma (PANC-28) | |||
| Human pancreatic carcinoma (MIA PaCa-2) | |||
| Down-regulation of C-X-C motif chemokine 12 (CXCL12) | Zerumbone | Breast cancer (HER2) | [110] |
| Human pancreatic carcinoma (PANC-1) | |||
| Pancreatic carcinoma (PANC-28) | |||
| Human pancreatic carcinoma (MIA PaCa-2) | |||
| Down-regulation of p38 | Diosgenin | Human esophageal cancer (Eca109) | [117] |
| Human erythroleukemia (HEL) | [44] | ||
| Down-regulation of p52 | Costunolide | Breast cancer (MDA-MB-231) | [107] |
| Down-regulation of p65 | Costunolide | Breast cancer (MDA-MB-231) | |
| Down-regulation of p70S6K | Lupeol | human osteosarcoma cells (MG-63) | [31] |
| human pancreatic cancer (PCNA-1) | [32] | ||
| Down-regulation of p100 | Costunolide | Breast cancer (MDA-MB-231) | [107] |
Down-Regulation of Cell Survival and Angiogenesis Molecules
The down-regulation of numerous anti-apoptotic molecules such as actin, survivin, vimentin and others by C. speciosus active ingredients is illustrated in Tables (12).
In Vivo Anticancer Activity
The in vivo anticancer activity of diosgenin, lupeol and zerumbone was conducted by up-
regulation of caspase, Bax, antioxidant potential and PTEN. In contrary, they induce down-regulation of cyclin B, G2/M phase, Bcl-2, NF-κB and surviving as presented in Table 13.
Table 13.
In vivo anticancer activity of C. speciosus active ingredients.
| Mechanism | Ingredients | Animal | References |
|---|---|---|---|
| Up-regulation of caspase-3 | Lupeol | Hamster buccal pouch carcinogenesis | [124] |
| Skin of Swiss albino mice | [100] | ||
| Up-regulation of caspase-9 | Lupeol | Hamster buccal pouch carcinogenesis | [124] |
| Up-regulation of Bax | Lupeol | Hamster buccal pouch carcinogenesis | [124] |
| Skin of Swiss albino mice | [100] | ||
| Zerumbone | Hepatocarcinogenesis in rat | [125] | |
| Antioxidant activity | Diosgenin | Breast carcinoma in female rats | [126] |
| Mouse colon carcinogenesis | [127] | ||
| Hamster buccal pouch carcinogenesis | [128] | ||
| Lupeol | Oral carcinogenesis | [128] | |
| Zerumbone | Hepatocarcinogenesis in rat | [125] | |
| Up-regulation of PTEN | Lupeol | Bladder carcinogenesis in rats | [129] |
| Down-regulation of cyclin B | Lupeol | Skin of Swiss albino mice | [100] |
| G2/M phase arrest | Lupeol | Skin of Swiss albino mice | [100] |
| Down-regulation of Bcl-2 | Lupeol | Hamster buccal pouch carcinogenesis | [124] |
| Skin of Swiss albino mice | [100] | ||
| Zerumbone | Hepatocarcinogenesis in rat | [125] | |
| Down-regulation of NF-κB | Lupeol | Skin cancer in CD-1 mice | [130] |
| Zerumbone | Colonic adenocarcinomas in mice | [131] | |
| Lung adenomas in mice | [131] | ||
| Down-regulation of survivin | Lupeol | Skin of Swiss albino mice | [100] |
Conclusion and Recomendations
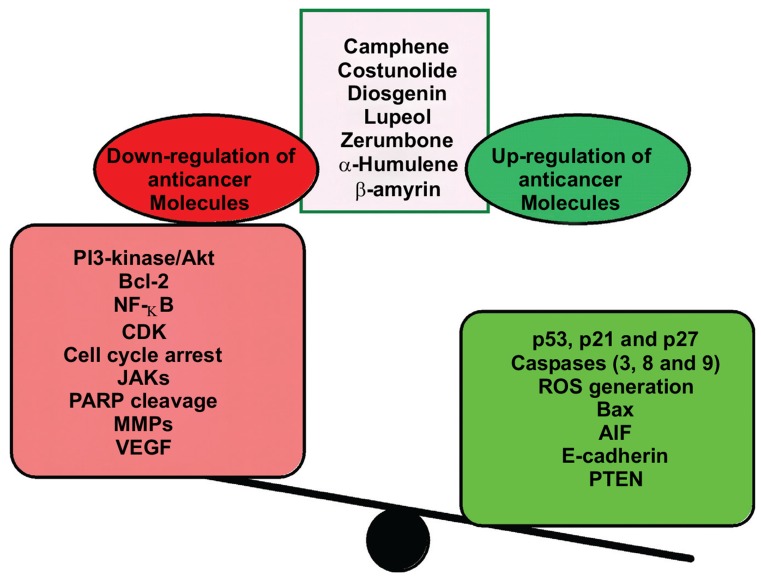
Chemical ingredients of C. speciosus have been described as potent anticancer therapy through induction of cancer cell apoptosis and weaken the cell survival through various mechanisms as illustrated in Fig. 2. From the data of this review we can propose research suggestions as a future plan outline that include:
Fig. (2).
Summarizes the anticancer effect of Costus speciosus active ingredients.
Study the preclinical novel angiogenesis inhibitor of C. speciosus
Study the critical component of multiple signalling pathways that regulate proliferation, survival, metastasis, and angiogenesis especially Myeloid leukemia.
Study the possible use of some C. speciosus ingredients as adjuvant therapy in chemoresistant cancer; especially hepatocellular carcinoma, breast cancer, and colorectal cancer.
Study the possible use of some C. speciosus ingredients as anti-RAGE, the receptor for advanced glycation end products therapy.
Study the change of the common regimen for treatment of cancer on a basis to improve the efficacy and reduce the common serious side effects.
Studies the dose-dependent antiproliferative activity in the human breast cancer MCF-7 cells as a microtubule-interacting agent. These studies demonstrated that costunolide can be related to an interaction with microtubules and inhibits the proliferation of breast cancer cells.
Table 7.
Down-regulation of cell cycle components by C. speciosus active ingredients.
| Mechanism | Ingredients | Cell | References |
|---|---|---|---|
| Down-regulation of cdc25B | Lupeol | Human prostate cancer (PC-3) | [96] |
| Zerumbone | Human prostate cancer (PC-3) | [60] | |
| Human prostate cancer (DU-145) | |||
| Human breast adenocarcinoma (MCF-7) | [97] | ||
| Breast cancer (MDA-MB-231) | |||
| Human breast adenocarcinoma (MCF-7) | |||
| Breast cancer (MDA-MB-231) | |||
| Down-regulation of cdc42 | Diosgenin | Breast cancer (MDA-MB-231) | [98] |
| Down-regulation of cdk 1 | Zerumbone | Human breast adenocarcinoma (MCF-7) | [97] |
| Breast cancer (MDA-MB-231) | |||
| Down-regulation of cdk 2 | Diosgenin | Human breast carcinoma (BCa) | [92] |
| Lupeol | Melanoma (451Lu) | [33] | |
| Human prostate adenocarcinoma (LNCaP) | [99] | ||
| Human prostate cancer (DU145) | |||
| Down-regulation of cdk 4 | Diosgenin | Human breast carcinoma (BCa) | [92] |
| Down-regulation of cyclin A | Lupeol | Human prostate adenocarcinoma (LNCaP) | [99] |
| Human prostate cancer (DU145) | |||
| Down-regulation of cyclin B | Lupeol | Swiss albino mice | [100] |
| Human prostate cancer (PC-3) | [96] | ||
| Down-regulation of cyclin B1 | Diosgenin | Human erythromyeloblastoid leukemia (K562) | [29] |
| Lupeol | Human prostate adenocarcinoma (LNCaP) | [99] | |
| Human prostate cancer (DU145) | |||
| Zerumbone | Human breast adenocarcinoma (MCF-7) | [97] | |
| Breast cancer (MDA-MB-231) | |||
| Acute promyelocytic leukemia (NB4) | [48] | ||
| Down-regulation of cyclin D1 | Diosgenin | Human breast carcinoma (BCa) | [92] |
| Lupeol | Melanoma (451Lu) | [33] | |
| Human prostate adenocarcinoma (LNCaP) | [99] | ||
| Human prostate cancer (DU145) | |||
| Human osteosarcoma cells (MNNG/HOS) | [31] | ||
| Human osteosarcoma cells (MG-63) | |||
| Human pancreatic cancer (PCNA-1) | [32] | ||
| Down-regulation of cyclin D2 | Lupeol | Melanoma (451Lu) | [33] |
| Human prostate adenocarcinoma (LNCaP) | [99] | ||
| Human prostate cancer (DU145) | |||
| Human prostate adenocarcinoma (LNCaP) | |||
| Human prostate cancer (DU145) | |||
| G0/G1 phase arrest | Lupeol | Human osteosarcoma cells (MG-63) | [31] |
| Human osteosarcoma cells (MNNG/HOS) | |||
| Human pancreatic cancer (PCNA-1) | [32] | ||
| Zerumbone | Human prostate cancer (DU145) | [101] | |
| Human prostate cancer (PC-3) | |||
| Human colon adenocarcinoma (HT-29) | [102] | ||
| G1 phase arrest | Costunolide | Human prostate cancer (PC-3) | [26] |
| Human prostate cancer (DU-145) | |||
| Human prostate adenocarcinoma (LNCaP) | |||
| Diosgenin | Human erythroleukemia (HEL) | [103] | |
| Human osteosarcoma (1547) | [23] | ||
| Human breast carcinoma (BCa) | [92] | ||
| G1/S phase arrest | Lupeol | Melanoma (451Lu) | [33] |
| G2/M phase arrest | Costunolide | Breast cancer (MDA-MB-231) | [27] |
| Human bladder carcinoma (T24) | [6] | ||
| Human breast adenocarcinoma (MCF-7) | [38] | ||
| Breast cancer (MDA-MB-231) | |||
| Diosgenin | Human erythroleukemia (HEL) | [57] | |
| Human hepatoma (Bel-7402) | [28] | ||
| Human hepatocellular carcinoma (HepG2) | |||
| Human hepatoma cells (SMMC-7721) | |||
| Human erythromyeloblastoid leukemia (K562) | [39] | ||
| Human promyelocytic leukemia (HL-60) | |||
| Human erythromyeloblastoid leukemia (K562) | [29] | ||
| Lupeol | Human prostate cancer (PC-3) | [96] | |
| Zerumbone | Human ovarian cancer (Caov-3) | [104] | |
| Human epithelioid cervical carcinoma (HeLa) | [104] | ||
| Human colorectal cancer (CRC) | [105] | ||
| Human colon adenocarcinoma (HT-29) | [102] | ||
| Human breast adenocarcinoma (MCF-7) | [97] | ||
| Breast cancer (MDA-MB-231) | |||
| Acute promyelocytic leukemia (NB4) | [48] |
ACKNOWLEDGeMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Rajashree R., Gangolli D., Patil S., Ingawale K. Amla, Ashwagandha and Shatavari Formulations as Herbal Medicines and Nutraceuticals. Res J Pharm Sci. 2012;1:10–15. [Google Scholar]
- 2.Gupta A.K., Tondon N., Sharma M. Publ by Indian Counc Med Res; 2008. Quality Standards of Indian Medicinal Plants, Medicinal Plants Unit. . [Google Scholar]
- 3.Saraf A. Phytochemical and Antimicrobial Studies of Medicinal Plant Costus Speciosus (Koen.). E-Journal Chem Hindawi Publishing Corporation. 2010;7:S405–S413. [Google Scholar]
- 4.Nair SVG, Hettihewa M, Rupasinghe HPV, Nair SVG, Hettihewa M, Rupasinghe HPV. Apoptotic and Inhibitory Effects on Cell Proliferation of Hepatocellular Carcinoma HepG2 Cells by Methanol Leaf Extract of Costus speciosus. 2014 doi: 10.1155/2014/637098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiao C., Li Q., Dong H., Xu L., Wang Z. Zhongguo Zhong Yao Za Zhi. 2002;27:123–125. [Studies on chemical constituents of two plants from Costus]. [PubMed] [Google Scholar]
- 6.Rasul A., Bao R., Malhi M., et al. Induction of apoptosis by costunolide in bladder cancer cells is mediated through ROS generation and mitochondrial dysfunction. Molecules. 2013;18:1418–1433. doi: 10.3390/molecules18021418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos F., Frota J., Arruda B., et al. Antihyperglycemic and hypolipidemic effects of α, β-amyrin, a triterpenoid mixture from Protium heptaphyllum in mice. Lipids Health Dis. 2012;11:98. doi: 10.1186/1476-511X-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyle S., Hoover K., Colpan C., Zhu Z., Matijasevic Z., Jones S.N. Dicer cooperates with p53 to suppress DNA damage and skin carcinogenesis in mice. PLoS One. 2014;9:e100920. doi: 10.1371/journal.pone.0100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masgras I., Carrera S., de Verdier P.J., et al. Reactive oxygen species and mitochondrial sensitivity to oxidative stress determine induction of cancer cell death by p21. J. Biol. Chem. 2012;287:9845–9854. doi: 10.1074/jbc.M111.250357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polyak K., Xia Y., Zweier J.L., Kinzler K.W., Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 11.Møller M.B. P27 in cell cycle control and cancer. Leuk. Lymphoma. 2000;39:19–27. doi: 10.3109/10428190009053535. [DOI] [PubMed] [Google Scholar]
- 12.McIlwain D.R., Berger T., Mak T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou H., Henzel W.J., Liu X., Lutschg A., Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 14.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 15.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 16.Mattson M.P., Chan S.L. Calcium orchestrates apoptosis. Nat Cell Biol Nature. 2003;5:1041–1043. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- 17.Nikolic D., van Breemen R.B. DNA Oxidation Induced by Cyclooxygenase-2. Chem. Res. Toxicol. 2001;14:351–354. doi: 10.1021/tx010004x. [DOI] [PubMed] [Google Scholar]
- 18.Candé C., Cohen I., Daugas E., et al. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84:215–222. doi: 10.1016/s0300-9084(02)01374-3. [DOI] [PubMed] [Google Scholar]
- 19.Berx G., Cleton-Jansen A.M., Nollet F., et al. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 1995;14:6107–6115. doi: 10.1002/j.1460-2075.1995.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas-Kogan D., Shalev N., Wong M., Mills G., Yount G., Stokoe D. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr. Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 21.Corbiere C., Liagre B., Terro F., Beneytout J.L. Induction of antiproliferative effect by diosgenin through activation of p53, release of apoptosis-inducing factor (AIF) and modulation of caspase-3 activity in different human cancer cells. Cell Res. 2004;14:188–196. doi: 10.1038/sj.cr.7290219. [DOI] [PubMed] [Google Scholar]
- 22.Corbiere C., Liagre B., Bianchi A., et al. Different contribution of apoptosis to the antiproliferative effects of diosgenin and other plant steroids, hecogenin and tigogenin, on human 1547 osteosarcoma cells. Int. J. Oncol. 2003;22:899–905. [PubMed] [Google Scholar]
- 23.Moalic S., Liagre B., Corbière C., et al. A plant steroid, diosgenin, induces apoptosis, cell cycle arrest and COX activity in osteosarcoma cells. FEBS Lett. 2001;506:225–230. doi: 10.1016/s0014-5793(01)02924-6. [DOI] [PubMed] [Google Scholar]
- 24.Hu Z, Zeng Q, Zhang B, Liu H, Wang W. Promotion of p53 expression and reactive oxidative stress production is involved in zerumbone-induced cisplatin sensitization of non-small cell lung cancer cells. 2014. [DOI] [PubMed]
- 25.Zhang S, Liu Q, Liu Y, Qiao H, Liu Y. Zerumbone, a Southeast Asian Ginger Sesquiterpene, Induced Apoptosis of Pancreatic Carcinoma Cells through p53 Signaling Pathway. Evid Based Complement Alternat Med. 2012 doi: 10.1155/2012/936030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu J.L., Pan S.L., Ho Y.F., Hwang T.L., Kung F.L., Guh J.H. Costunolide induces apoptosis through nuclear calcium2+ overload and DNA damage response in human prostate cancer. J. Urol. 2011;185:1967–1974. doi: 10.1016/j.juro.2010.12.091. [DOI] [PubMed] [Google Scholar]
- 27.Choi Y.K., Seo H.S., Choi H.S., et al. Induction of Fas-mediated extrinsic apoptosis, p21WAF1-related G2/M cell cycle arrest and ROS generation by costunolide in estrogen receptor-negative breast cancer cells, MDA-MB-231. Mol. Cell. Biochem. 2012;363:119–128. doi: 10.1007/s11010-011-1164-z. [DOI] [PubMed] [Google Scholar]
- 28.Li Y., Wang X., Cheng S., et al. Diosgenin induces G2/M cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Oncol. Rep. 2015;33:693–698. doi: 10.3892/or.2014.3629. [DOI] [PubMed] [Google Scholar]
- 29.Liu M.J., Wang Z., Ju Y., Wong R.N., Wu Q.Y. Diosgenin induces cell cycle arrest and apoptosis in human leukemia K562 cells with the disruption of Ca2+ homeostasis. Cancer Chemother. Pharmacol. 2005;55:79–90. doi: 10.1007/s00280-004-0849-3. [DOI] [PubMed] [Google Scholar]
- 30.Raju J., Bird R.P. Diosgenin, a naturally occurring steroid [corrected] saponin suppresses 3-hydroxy-3-methylglutaryl CoA reductase expression and induces apoptosis in HCT-116 human colon carcinoma cells. Cancer Lett. 2007;255:194–204. doi: 10.1016/j.canlet.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y., Bi T., Dai W., et al. Lupeol Induces Apoptosis and Cell Cycle Arrest of Human Osteosarcoma Cells Through PI3K/AKT/mTOR Pathway. Technol. Cancer Res. Treat. 2015;•••:1533034615609014. doi: 10.1177/1533034615609014. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y., Bi T., Wang G., et al. Lupeol inhibits proliferation and induces apoptosis of human pancreatic cancer PCNA-1 cells through AKT/ERK pathways. Naunyn Schmiedebergs Arch. Pharmacol. 2015;388:295–304. doi: 10.1007/s00210-014-1071-4. [DOI] [PubMed] [Google Scholar]
- 33.Saleem M., Maddodi N., Abu Zaid M., et al. Lupeol inhibits growth of highly aggressive human metastatic melanoma cells in vitro and in vivo by inducing apoptosis. Clin. Cancer Res. 2008;14:2119–2127. doi: 10.1158/1078-0432.CCR-07-4413. [DOI] [PubMed] [Google Scholar]
- 34.Girola N., Figueiredo C.R., Farias C.F., et al. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem. Biophys. Res. Commun. 2015;467:928–934. doi: 10.1016/j.bbrc.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 35.Mulyaningsih S., Youns M., El-Readi M.Z., et al. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components. J. Pharm. Pharmacol. 2010;62:1037–1044. doi: 10.1111/j.2042-7158.2010.01119.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee M.G., Lee K.T., Chi S.G., Park J.H. Costunolide induces apoptosis by ROS-mediated mitochondrial permeability transition and cytochrome C release. Biol. Pharm. Bull. 2001;24:303–306. doi: 10.1248/bpb.24.303. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y.I., Kim J.H., Lee K.T., Choi J.H. Costunolide induces apoptosis in platinum-resistant human ovarian cancer cells by generating reactive oxygen species. Gynecol. Oncol. 2011;123:588–596. doi: 10.1016/j.ygyno.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 38.Roy A., Manikkam R. Cytotoxic Impact of Costunolide Isolated from Costus speciosus on Breast Cancer via Differential Regulation of Cell Cycle-An In-vitro and In-silico Approach. Phytother. Res. 2015;29:1532–1539. doi: 10.1002/ptr.5408. [DOI] [PubMed] [Google Scholar]
- 39.Liu M.J., Wang Z., Ju Y., Zhou J., Wang Y., Wong R.N. The mitotic-arresting and apoptosis-inducing effects of diosgenyl saponins on human leukemia cell lines. Biol. Pharm. Bull. 2004;27:1059–1065. doi: 10.1248/bpb.27.1059. [DOI] [PubMed] [Google Scholar]
- 40.He Y., Wang J.S., Zhang P., Zhang W.J., Huang Q.L., Hua Z.C. Yao Xue Xue Bao. 2013;48:45–51. [Synergistic apoptotic effect of the combination of diosgenin and TRAIL on non-small-cell lung cancer cell line A549 evaluated with the Chou-Talalay method]. [PubMed] [Google Scholar]
- 41.Cailleteau C., Liagre B., Beneytout J.L. A proteomic approach to the identification of molecular targets in subsequent apoptosis of HEL cells after diosgenin-induced megakaryocytic differentiation. J. Cell. Biochem. 2009;107:785–796. doi: 10.1002/jcb.22176. [DOI] [PubMed] [Google Scholar]
- 42.Selim S., Al Jaouni S. Anticancer and apoptotic effects on cell proliferation of diosgenin isolated from Costus speciosus (Koen.). Sm BMC Complement Altern Med. 2015;15:301. doi: 10.1186/s12906-015-0836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das S., Dey K.K., Dey G., et al. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgenin in squamous cell carcinoma. PLoS One. 2012;7:e46641. doi: 10.1371/journal.pone.0046641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leger D.Y., Liagre B., Beneytout J.L. Role of MAPKs and NF-kappaB in diosgenin-induced megakaryocytic differentiation and subsequent apoptosis in HEL cells. Int. J. Oncol. 2006;28:201–207. [PubMed] [Google Scholar]
- 45.Raju J., Patlolla J.M., Swamy M.V., Rao C.V. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol. Biomarkers Prev. 2004;13:1392–1398. [PubMed] [Google Scholar]
- 46.Prasad S., Kalra N., Shukla Y. Hepatoprotective effects of lupeol and mango pulp extract of carcinogen induced alteration in Swiss albino mice. Mol. Nutr. Food Res. 2007;51:352–359. doi: 10.1002/mnfr.200600113. [DOI] [PubMed] [Google Scholar]
- 47.He Y., Liu F., Zhang L., et al. Growth inhibition and apoptosis induced by lupeol, a dietary triterpene, in human hepatocellular carcinoma cells. Biol. Pharm. Bull. 2011;34:517–522. doi: 10.1248/bpb.34.517. [DOI] [PubMed] [Google Scholar]
- 48.Xian M., Ito K., Nakazato T., et al. Zerumbone, a bioactive sesquiterpene, induces G2/M cell cycle arrest and apoptosis in leukemia cells via a Fas- and mitochondria-mediated pathway. Cancer Sci. 2007;98:118–126. doi: 10.1111/j.1349-7006.2006.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rajan I., Jayasree P.R., Kumar P.R. Zerumbone induces mitochondria-mediated apoptosis via increased calcium, generation of reactive oxygen species and upregulation of soluble histone H2AX in K562 chronic myelogenous leukemia cells. Tumour Biol. 2015;36:8479–8489. doi: 10.1007/s13277-015-3583-z. [DOI] [PubMed] [Google Scholar]
- 50.Rahman H.S., Rasedee A., Abdul A.B., et al. Zerumbone-loaded nanostructured lipid carrier induces G2/M cell cycle arrest and apoptosis via mitochondrial pathway in a human lymphoblastic leukemia cell line. Int. J. Nanomedicine. 2014;9:527–538. doi: 10.2147/IJN.S54346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Y., Sheng Q., Cheng Y., et al. Zerumbone induces apoptosis in human renal cell carcinoma via Gli-1/Bcl-2 pathway. Pharmazie. 2013;68:141–145. [PubMed] [Google Scholar]
- 52.Weng H.Y., Hsu M.J., Wang C.C., et al. Zerumbone suppresses IKKα, Akt, and FOXO1 activation, resulting in apoptosis of GBM 8401 cells. J. Biomed. Sci. 2012;19:86. doi: 10.1186/1423-0127-19-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdel Wahab SI, Abdul AB, Alzubairi AS, Mohamed Elhassan M, Mohan S. 2009. [DOI] [PMC free article] [PubMed]
- 54.Tabata K., Nishimura Y., Takeda T., Kurita M., Uchiyama T., Suzuki T. Sesquiterpene lactones derived from Saussurea lappa induce apoptosis and inhibit invasion and migration in neuroblastoma cells. J. Pharmacol. Sci. 2015;127:397–403. doi: 10.1016/j.jphs.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Murtaza I., Saleem M., Adhami V.M., Hafeez B.B., Mukhtar H. Suppression of cFLIP by lupeol, a dietary triterpene, is sufficient to overcome resistance to TRAIL-mediated apoptosis in chemoresistant human pancreatic cancer cells. Cancer Res. 2009;69:1156–1165. doi: 10.1158/0008-5472.CAN-08-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siveen K.S., Nguyen A.H., Lee J.H., et al. Negative regulation of signal transducer and activator of transcription-3 signalling cascade by lupeol inhibits growth and induces apoptosis in hepatocellular carcinoma cells. Br. J. Cancer. 2014;111:1327–1337. doi: 10.1038/bjc.2014.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Léger D.Y., Liagre B., Cardot P.J., Beneytout J.L., Battu S. Diosgenin dose-dependent apoptosis and differentiation induction in human erythroleukemia cell line and sedimentation field-flow fractionation monitoring. Anal. Biochem. 2004;335:267–278. doi: 10.1016/j.ab.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Prasad S., Madan E., Nigam N., Roy P., George J., Shukla Y. Induction of apoptosis by lupeol in human epidermoid carcinoma A431 cells through regulation of mitochondrial, Akt/PKB and NFkappaB signaling pathways. Cancer Biol. Ther. 2009;8:1632–1639. doi: 10.4161/cbt.8.17.9204. [DOI] [PubMed] [Google Scholar]
- 59.Sakinah S.A., Handayani S.T., Hawariah L.P. Zerumbone induced apoptosis in liver cancer cells via modulation of Bax/Bcl-2 ratio. Cancer Cell Int. 2007;7:4. doi: 10.1186/1475-2867-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan M.L., Liang J.W., Hsu L.C., Chang W.L., Lee S.S., Guh J.H. Zerumbone, a ginger sesquiterpene, induces apoptosis and autophagy in human hormone-refractory prostate cancers through tubulin binding and crosstalk between endoplasmic reticulum stress and mitochondrial insult. Naunyn Schmiedebergs Arch. Pharmacol. 2015;388:1223–1236. doi: 10.1007/s00210-015-1152-z. [DOI] [PubMed] [Google Scholar]
- 61.Lepage C., Liagre B., Cook-Moreau J., Pinon A., Beneytout J.L. Cyclooxygenase-2 and 5-lipoxygenase pathways in diosgenin-induced apoptosis in HT-29 and HCT-116 colon cancer cells. Int. J. Oncol. 2010;36:1183–1191. doi: 10.3892/ijo_00000601. [DOI] [PubMed] [Google Scholar]
- 62.Lepage C., Léger D.Y., Bertrand J., Martin F., Beneytout J.L., Liagre B. Diosgenin induces death receptor-5 through activation of p38 pathway and promotes TRAIL-induced apoptosis in colon cancer cells. Cancer Lett. 2011;301:193–202. doi: 10.1016/j.canlet.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 63.Prasad S., Kalra N., Shukla Y. Induction of apoptosis by lupeol and mango extract in mouse prostate and LNCaP cells. Nutr. Cancer. 2008;60:120–130. doi: 10.1080/01635580701613772. [DOI] [PubMed] [Google Scholar]
- 64.Yodkeeree S., Sung B., Limtrakul P., Aggarwal B.B. Zerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: Evidence for an essential role of reactive oxygen species. Cancer Res. 2009;69:6581–6589. doi: 10.1158/0008-5472.CAN-09-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ambrož M., Boušová I., Skarka A., et al. The Influence of Sesquiterpenes from Myrica rubra on the Antiproliferative and Pro-Oxidative Effects of Doxorubicin and Its Accumulation in Cancer Cells. Molecules. 2015;20:15343–15358. doi: 10.3390/molecules200815343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin K.W., Huang A.M., Tu H.Y., et al. Xanthine oxidase inhibitory triterpenoid and phloroglucinol from guttiferaceous plants inhibit growth and induced apoptosis in human NTUB1 cells through a ROS-dependent mechanism. J. Agric. Food Chem. 2011;59:407–414. doi: 10.1021/jf1041382. [DOI] [PubMed] [Google Scholar]
- 67.Edagawa M., Kawauchi J., Hirata M., et al. Role of activating transcription factor 3 (ATF3) in endoplasmic reticulum (ER) stress-induced sensitization of p53-deficient human colon cancer cells to tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through up-re. J. Biol. Chem. 2014;289:21544–21561. doi: 10.1074/jbc.M114.558890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mao Z.J., Tang Q.J., Zhang C.A., et al. Anti-proliferation and anti-invasion effects of diosgenin on gastric cancer BGC-823 cells with HIF-1α shRNAs. Int. J. Mol. Sci. 2012;13:6521–6533. doi: 10.3390/ijms13056521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang L., Zhang Y., Zhang L., Yang X., Lv Z. Lupeol, a dietary triterpene, inhibited growth, and induced apoptosis through down-regulation of DR3 in SMMC7721 cells. Cancer Invest. 2009;27:163–170. doi: 10.1080/07357900802210745. [DOI] [PubMed] [Google Scholar]
- 70.Sehrawat A., Sakao K., Singh S.V. Notch2 activation is protective against anticancer effects of zerumbone in human breast cancer cells. Breast Cancer Res. Treat. 2014;146:543–555. doi: 10.1007/s10549-014-3059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee T.K., Castilho A., Cheung V.C., Tang K.H., Ma S., Ng I.O. Lupeol targets liver tumor-initiating cells through phosphatase and tensin homolog modulation. Hepatology. 2011;53:160–170. doi: 10.1002/hep.24000. [DOI] [PubMed] [Google Scholar]
- 72.Ogiwara K., Hata K. Melanoma cell differentiation induced by lupeol separates into two stages: morphological and functional changes. J. Nat. Med. 2009;63:323–326. doi: 10.1007/s11418-009-0319-7. [DOI] [PubMed] [Google Scholar]
- 73.West K.A., Castillo S.S., Dennis P.A. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist. Updat. 2002;5:234–248. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 74.Pisu M., Concas A., Cao G. A novel quantitative model of cell cycle progression based on cyclin-dependent kinases activity and population balances. Comput. Biol. Chem. 2015;55:1–13. doi: 10.1016/j.compbiolchem.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Simon H.U., Friis R. ATG5: a distinct role in the nucleus. Autophagy. 2014;10:176–177. doi: 10.4161/auto.26916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Youle R.J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 77.Chao D.T., Korsmeyer S.J. BCL-2 family: regulators of cell death. Annu. Rev. Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 78.Cao J.P., Niu H.Y., Wang H.J., Huang X.G., Gao D.S. -κB p65/p52 plays a role in GDNF up-regulating Bcl-2 and Bcl-w expression in 6-OHDA-induced apoptosis of MN9D cell. Int. J. Neurosci. 2013;123:705–710. doi: 10.3109/00207454.2013.795149. [DOI] [PubMed] [Google Scholar]
- 79.Perkins N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 80.Pahl H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 81.Park M., Hong J. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells. 2016;5:15. doi: 10.3390/cells5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krishnakumar R., Kraus W.L. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol. Cell. 2010;39:8–24. doi: 10.1016/j.molcel.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Shea J.J., Gadina M., Schreiber R.D. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl.):S121–S131. doi: 10.1016/s0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 84.Sansone P., Piazzi G., Paterini P., et al. Cyclooxygenase-2/carbonic anhydrase-IX up-regulation promotes invasive potential and hypoxia survival in colorectal cancer cells. J. Cell. Mol. Med. 2009;13:3876–3887. doi: 10.1111/j.1582-4934.2008.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagata Y., Takahashi N., Davis R.J., Todokoro K. Activation of p38 MAP kinase and JNK but not ERK is required for erythropoietin-induced erythroid differentiation. Blood. 1998;92:1859–1869. [PubMed] [Google Scholar]
- 86.Liu F., He Y., Liang Y., et al. PI3-kinase inhibition synergistically promoted the anti-tumor effect of lupeol in hepatocellular carcinoma. Cancer Cell Int. 2013;13:108. doi: 10.1186/1475-2867-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen P.S., Shih Y.W., Huang H.C., Cheng H.W. Diosgenin, a steroidal saponin, inhibits migration and invasion of human prostate cancer PC-3 cells by reducing matrix metalloproteinases expression. PLoS One. 2011;6:e20164. doi: 10.1371/journal.pone.0020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Léger D.Y., Battu S., Liagre B., Beneytout J.L., Cardot P.J. Megakaryocyte cell sorting from diosgenin-differentiated human erythroleukemia cells by sedimentation field-flow fractionation. Anal. Biochem. 2006;355:19–28. doi: 10.1016/j.ab.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 89.Lee J., Jung K., Kim Y.S., Park D. Diosgenin inhibits melanogenesis through the activation of phosphatidylinositol-3-kinase pathway (PI3K) signaling. Life Sci. 2007;81:249–254. doi: 10.1016/j.lfs.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Chang H.Y., Kao M.C., Way T.D., Ho C.T., Fu E. Diosgenin suppresses hepatocyte growth factor (HGF)-induced epithelial-mesenchymal transition by down-regulation of Mdm2 and vimentin. J. Agric. Food Chem. 2011;59:5357–5363. doi: 10.1021/jf200598w. [DOI] [PubMed] [Google Scholar]
- 91.Chiang C.T., Way T.D., Tsai S.J., Lin J.K. Diosgenin, a naturally occurring steroid, suppresses fatty acid synthase expression in HER2-overexpressing breast cancer cells through modulating Akt, mTOR and JNK phosphorylation. FEBS Lett. 2007;581:5735–5742. doi: 10.1016/j.febslet.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 92.Srinivasan S., Koduru S., Kumar R., Venguswamy G., Kyprianou N., Damodaran C. Diosgenin targets Akt-mediated prosurvival signaling in human breast cancer cells. Int. J. Cancer. 2009;125:961–967. doi: 10.1002/ijc.24419. [DOI] [PubMed] [Google Scholar]
- 93.Sobhan P.K., Seervi M., Deb L., et al. Calpain and Reactive Oxygen Species Targets Bax for Mitochondrial Permeabilisation and Caspase Activation in Zerumbone Induced Apoptosis. PLoS One. 2013;8:e59350. doi: 10.1371/journal.pone.0059350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kang C.G., Lee H.J., Kim S.H., Lee E.O. Zerumbone Suppresses Osteopontin-Induced Cell Invasion Through Inhibiting the FAK/AKT/ROCK Pathway in Human Non-Small Cell Lung Cancer A549 Cells. J. Nat. Prod. 2016;79:156–160. doi: 10.1021/acs.jnatprod.5b00796. [DOI] [PubMed] [Google Scholar]
- 95.Liu Y., Bi T., Shen G., et al. Lupeol induces apoptosis and inhibits invasion in gallbladder carcinoma GBC-SD cells by suppression of EGFR/MMP-9 signaling pathway. Cytotechnology. 2016;68:123–133. doi: 10.1007/s10616-014-9763-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Prasad S., Nigam N., Kalra N., Shukla Y. Regulation of signaling pathways involved in lupeol induced inhibition of proliferation and induction of apoptosis in human prostate cancer cells. Mol. Carcinog. 2008;47:916–924. doi: 10.1002/mc.20442. [DOI] [PubMed] [Google Scholar]
- 97.Sehrawat A., Arlotti J.A., Murakami A., Singh S.V. Zerumbone causes Bax- and Bak-mediated apoptosis in human breast cancer cells and inhibits orthotopic xenograft growth in vivo. Breast Cancer Res. Treat. 2012;136:429–441. doi: 10.1007/s10549-012-2280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He Z., Chen H., Li G., et al. Diosgenin inhibits the migration of human breast cancer MDA-MB-231 cells by suppressing Vav2 activity. Phytomedicine. 2014;21:871–876. doi: 10.1016/j.phymed.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 99.Saleem M., Murtaza I., Witkowsky O., Kohl A.M., Maddodi N. Lupeol triterpene, a novel diet-based microtubule targeting agent: disrupts survivin/cFLIP activation in prostate cancer cells. Biochem. Biophys. Res. Commun. 2009;388:576–582. doi: 10.1016/j.bbrc.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nigam N., Prasad S., George J., Shukla Y. Lupeol induces p53 and cyclin-B-mediated G2/M arrest and targets apoptosis through activation of caspase in mouse skin. Biochem. Biophys. Res. Commun. 2009;381:253–258. doi: 10.1016/j.bbrc.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 101.Jorvig J.E., Chakraborty A. Zerumbone inhibits growth of hormone refractory prostate cancer cells by inhibiting JAK2/STAT3 pathway and increases paclitaxel sensitivity. Anticancer Drugs. 2015;26:160–166. doi: 10.1097/CAD.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 102.Kirana C., McIntosh G.H., Record I.R., Jones G.P. Antitumor activity of extract of Zingiber aromaticum and its bioactive sesquiterpenoid zerumbone. Nutr. Cancer. 2003;45:218–225. doi: 10.1207/S15327914NC4502_12. [DOI] [PubMed] [Google Scholar]
- 103.Cailleteau C., Micallef L., Lepage C., et al. Investigating the relationship between cell cycle stage and diosgenin-induced megakaryocytic differentiation of HEL cells using sedimentation field-flow fractionation. Anal. Bioanal. Chem. 2010;398:1273–1283. doi: 10.1007/s00216-010-4062-4. [DOI] [PubMed] [Google Scholar]
- 104.Abdelwahab S.I., Abdul A.B., Zain Z.N., Hadi A.H. Zerumbone inhibits interleukin-6 and induces apoptosis and cell cycle arrest in ovarian and cervical cancer cells. Int. Immunopharmacol. 2012;12:594–602. doi: 10.1016/j.intimp.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 105.Deorukhkar A., Ahuja N., Mercado A.L., et al. Zerumbone increases oxidative stress in a thiol-dependent ROS-independent manner to increase DNA damage and sensitize colorectal cancer cells to radiation. Cancer Med. 2015;4:278–292. doi: 10.1002/cam4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pitchai D., Roy A., Ignatius C. In vitro evaluation of anticancer potentials of lupeol isolated from Elephantopus scaber L. on MCF-7 cell line. J. Adv. Pharm. Technol. Res. 2014;5:179–184. doi: 10.4103/2231-4040.143037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pitchai D., Roy A., Banu S. In vitro and in silico evaluation of NF-κB targeted costunolide action on estrogen receptor-negative breast cancer cells--a comparison with normal breast cells. Phytother. Res. 2014;28:1499–1505. doi: 10.1002/ptr.5155. [DOI] [PubMed] [Google Scholar]
- 108.Saleem M., Kweon M.H., Yun J.M., et al. A novel dietary triterpene Lupeol induces fas-mediated apoptotic death of androgen-sensitive prostate cancer cells and inhibits tumor growth in a xenograft model. Cancer Res. 2005;65:11203–11213. doi: 10.1158/0008-5472.CAN-05-1965. [DOI] [PubMed] [Google Scholar]
- 109.Shamoto T., Matsuo Y., Shibata T., et al. Zerumbone inhibits angiogenesis by blocking NF-κB activity in pancreatic cancer. Pancreas. 2014;43:396–404. doi: 10.1097/MPA.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 110.Sung B., Jhurani S., Ahn K.S., et al. Zerumbone down-regulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res. 2008;68:8938–8944. doi: 10.1158/0008-5472.CAN-08-2155. [DOI] [PubMed] [Google Scholar]
- 111.Sung B., Murakami A., Oyajobi B.O., Aggarwal B.B. Zerumbone abolishes RANKL-induced NF-kappaB activation, inhibits osteoclastogenesis, and suppresses human breast cancer-induced bone loss in athymic nude mice. Cancer Res. 2009;69:1477–1484. doi: 10.1158/0008-5472.CAN-08-3249. [DOI] [PubMed] [Google Scholar]
- 112.Tsuboi K., Matsuo Y., Shamoto T., et al. Zerumbone inhibits tumor angiogenesis via NF-κB in gastric cancer. Oncol. Rep. 2014;31:57–64. doi: 10.3892/or.2013.2842. [DOI] [PubMed] [Google Scholar]
- 113.Li F., Fernandez P.P., Rajendran P., Hui K.M., Sethi G. Diosgenin, a steroidal saponin, inhibits STAT3 signaling pathway leading to suppression of proliferation and chemosensitization of human hepatocellular carcinoma cells. Cancer Lett. 2010;292:197–207. doi: 10.1016/j.canlet.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 114.Kim S., Kil W.H., Lee J., et al. Zerumbone suppresses EGF-induced CD44 expression through the inhibition of STAT3 in breast cancer cells. Oncol. Rep. 2014;32:2666–2672. doi: 10.3892/or.2014.3514. [DOI] [PubMed] [Google Scholar]
- 115.Saleem M., Murtaza I., Tarapore R.S., et al. Lupeol inhibits proliferation of human prostate cancer cells by targeting beta-catenin signaling. Carcinogenesis. 2009;30:808–817. doi: 10.1093/carcin/bgp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Han J., Bae S.Y., Oh S.J., et al. Zerumbone suppresses IL-1β-induced cell migration and invasion by inhibiting IL-8 and MMP-3 expression in human triple-negative breast cancer cells. Phytother. Res. 2014;28:1654–1660. doi: 10.1002/ptr.5178. [DOI] [PubMed] [Google Scholar]
- 117.Han J., Bae S.Y., Oh S.J., et al. Zerumbone Suppresses IL-1β-induced Cell Migration and Invasion by Inhibiting IL-8 and MMP-3 Expression in Human Triple-negative Breast Cancer Cells. Phytother. Res. 2014;28:1654–1660. doi: 10.1002/ptr.5178. [DOI] [PubMed] [Google Scholar]
- 118.Ghezali L., Liagre B., Limami Y., Beneytout J.L., Leger D.Y. Sonic Hedgehog activation is implicated in diosgenin-induced megakaryocytic differentiation of human erythroleukemia cells. PLoS One. 2014;9:e95016. doi: 10.1371/journal.pone.0095016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohammad R.Y., Somayyeh G., Gholamreza H., Majid M., Yousef R. Diosgenin inhibits hTERT gene expression in the A549 lung cancer cell line. Asian Pac. J. Cancer Prev. 2013;14:6945–6948. doi: 10.7314/apjcp.2013.14.11.6945. [DOI] [PubMed] [Google Scholar]
- 120.Rahmati-Yamchi M., Ghareghomi S., Haddadchi G., Milani M., Aghazadeh M., Daroushnejad H. Fenugreek extract diosgenin and pure diosgenin inhibit the hTERT gene expression in A549 lung cancer cell line. Mol. Biol. Rep. 2014;41:6247–6252. doi: 10.1007/s11033-014-3505-y. [DOI] [PubMed] [Google Scholar]
- 121.Murakami A., Miyamoto M., Ohigashi H. Zerumbone, an anti-inflammatory phytochemical, induces expression of proinflammatory cytokine genes in human colon adenocarcinoma cell lines. Biofactors. 2004;21:95–101. doi: 10.1002/biof.552210118. [DOI] [PubMed] [Google Scholar]
- 122.Choi YK, Cho SG, Woo SM, et al. Saussurea lappa Clarke-Derived Costunolide Prevents TNF α -Induced Breast Cancer Cell Migration and Invasion by Inhibiting NF- B Activity. Evid Based Complement Alternat Med 2013. 2013. [DOI] [PMC free article] [PubMed]
- 123.Tarapore R.S., Siddiqui I.A., Saleem M., Adhami V.M., Spiegelman V.S., Mukhtar H. Specific targeting of Wnt/β-catenin signaling in human melanoma cells by a dietary triterpene lupeol. Carcinogenesis. 2010;31:1844–1853. doi: 10.1093/carcin/bgq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Manoharan S., Palanimuthu D., Baskaran N., Silvan S. Modulating effect of lupeol on the expression pattern of apoptotic markers in 7, 12-dimethylbenz(a)anthracene induced oral carcinogenesis. Asian Pac. J. Cancer Prev. 2012;13:5753–5757. doi: 10.7314/apjcp.2012.13.11.5753. [DOI] [PubMed] [Google Scholar]
- 125.Taha M.M., Abdul A.B., Abdullah R., Ibrahim T.A., Abdelwahab S.I., Mohan S. Potential chemoprevention of diethylnitrosamine-initiated and 2-acetylaminofluorene-promoted hepatocarcinogenesis by zerumbone from the rhizomes of the subtropical ginger (Zingiber zerumbet). Chem. Biol. Interact. 2010;186:295–305. doi: 10.1016/j.cbi.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 126.Jagadeesan J., Nandakumar N., Rengarajan T., Balasubramanian M.P. Diosgenin, a steroidal saponin, exhibits anticancer activity by attenuating lipid peroxidation via enhancing antioxidant defense system during NMU-induced breast carcinoma. J. Environ. Pathol. Toxicol. Oncol. 2012;31:121–129. doi: 10.1615/jenvironpatholtoxicoloncol.v31.i2.40. [DOI] [PubMed] [Google Scholar]
- 127.Miyoshi N., Nagasawa T., Mabuchi R., et al. Chemoprevention of azoxymethane/dextran sodium sulfate-induced mouse colon carcinogenesis by freeze-dried yam sanyaku and its constituent diosgenin. Cancer Prev. Res. (Phila.) 2011;4:924–934. doi: 10.1158/1940-6207.CAPR-10-0279. [DOI] [PubMed] [Google Scholar]
- 128.Rajalingam K., Sugunadevi G., Arokia Vijayaanand M., Kalaimathi J., Suresh K. Anti-tumor and anti-oxidative potential of diosgenin against 7, 12-dimethylbenz(a)anthracene induced experimental oral carcinogenesis. Pathol. Oncol. Res. 2012;18:405–412. doi: 10.1007/s12253-011-9460-1. [DOI] [PubMed] [Google Scholar]
- 129.Prabhu B., Balakrishnan D., Sundaresan S. Antiproliferative and anti-inflammatory properties of diindolylmethane and lupeol against N-butyl-N-(4-hydroxybutyl) nitrosamine induced bladder carcinogenesis in experimental rats. Hum. Exp. Toxicol. 2016;35:685–692. doi: 10.1177/0960327115597985. [DOI] [PubMed] [Google Scholar]
- 130.Saleem M., Afaq F., Adhami V.M., Mukhtar H. Lupeol modulates NF-kappaB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene. 2004;23:5203–5214. doi: 10.1038/sj.onc.1207641. [DOI] [PubMed] [Google Scholar]
- 131.Kim M., Miyamoto S., Yasui Y., Oyama T., Murakami A., Tanaka T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int. J. Cancer. 2009;124:264–271. doi: 10.1002/ijc.23923. [DOI] [PubMed] [Google Scholar]