Abstract
Methyl tertiary butyl ether (MTBE)—A well known gasoline additive substituting for lead alkyls—causes lipid disorders and liver dysfunctions in animal models. However, whether MTBE exposure is a risk factor for non-alcoholic fatty liver disease (NAFLD) remains uncertain. We evaluate the possible relationship between MTBE exposure and the prevalence of NAFLD among 71 petrol station attendants in southern China. The personal exposure concentrations of MTBE were analyzed by Head Space Solid Phase Microextraction GC/MS. NAFLD was diagnosed by using abdominal ultrasonography according to the guidelines for the diagnosis and treatment of NAFLD suggested by the Chinese Hepatology Association. Demographic and clinical characteristics potentially associated with NAFLD were investigated. Mutivariate logistic regression analysis was applied to measure odds ratios and 95% confidence intervals (CI). The result showed that the total prevalence of NAFLD was 15.49% (11/71) among the study subjects. The average exposure concentrations of MTBE were 292.98 ± 154.90 μg/m3 and 286.64 ± 122.28 μg/m3 in NAFLD and non-NAFLD groups, respectively, and there was no statistically significant difference between them (p > 0.05). After adjusting for age, gender, physical exercise, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), alanine aminotransferase (ALT), white blood cell (WBC), total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL), the odds ratios were 1.31 (95% CI: 0.85–1.54; p > 0.05), 1.14 (95% CI: 0.81–1.32; p > 0.05), 1.52 (95% CI: 0.93–1.61; p > 0.05) in the groups (including men and women) with exposure concentrations of MTBE of 100–200 μg/m3, 200–300 μg/m3, and ≥300 μg/m3, respectively, as compared to the group (including men and women) ≤100 μg/m3. Our investigation indicates that exposure to MTBE does not seem to be a significant risk factor for the prevalence of NAFLD among petrol station attendants in southern China.
Keywords: methyl tertiary butyl ether, environmental pollution, non-alcoholic fatty liver disease, epidemiology
1. Introduction
Methyl tertiary butyl ether (MTBE)—A well known gasoline additive substituting for lead alkyls—is widely used in China and some other countries to increase the octane value of gasoline and reduce harmful emissions [1,2]. In recent years, with more and more people driving gasoline-fueled cars, the consumption of MTBE has dramatically increased, and due to its great volatility, it has become a ubiquitous environmental contaminant which has posed potential health risks to the population, especially the occupational workers with exposure to relatively high concentrations of MTBE [3]. For example, in petrol stations and their surrounding environments, the concentrations of MTBE may be thousands of times higher than in the general environment [3]. MTBE can be rapidly absorbed through respiratory and digestive systems, and rapidly distributed to all major tissues via the bloodstream [4]. Epidemiological investigation revealed that there was a significant positive correlation between ambient air concentrations and blood levels of MTBE [2,5]. The acute, subchronic, and chronic toxicities of MTBE have been explored, and the toxic effects of oxidative stress [6,7,8], DNA damage [5,6,7,8], DNA adducts [9,10], lipid disorder [11], abnormal liver functions [11], vascular lesions [12], and malignant tumors [13] induced by MTBE were found in animal tests. However, there is apparent uncertainty in the extrapolation of animal data to effects on humans [1,14,15]. The complaints of acute adverse health effects from populations exposed to MTBE include headache, cough, nausea, mucosal irritation, dizziness, etc. and these prompted scientific research on the toxicity of MTBE in many countries [3,16]. However, there was not sufficient evidence showing the reliable links between MTBE exposure and these complaints [17,18,19,20]. Because MTBE can cause the adverse effects of oxidative stress [6,7,8], lipid disorder [11], and abnormal liver functions [11] in animal models, we are interested in whether MTBE exposure is a risk factor for non-alcoholic fatty liver disease (NAFLD) in humans, which has not been reported.
NAFLD is the most typical chronic liver disease worldwide [21,22,23,24,25,26]. The spectrum of NAFLD ranges from simple hepatic steatosis to steatohepatitis, which may develop into cirrhosis and ultimately into liver carcinoma [22,23,24,25]. Identification of risk factors for the onset and development of NAFLD is very important for its prevention and control. Although metabolic disorder, body mass index (BMI), and waist circumference have been confirmed as major risk factors for NAFLD, the basic mechanism of hepatic steatosis remains unclear [22,23,25,27]. In recent decades, along with the acceleration of the process of urbanization and industrialization, the emissions of all kinds of environmental pollutants are dramatically increased. More and more evidence suggests that environmental pollutants such as diesel exhaust particles (DEP) [28], particulate matter (PM) suspended in the air [28,29], metals, and polychlorinated compounds [30,31,32], etc. are important risk factors for the progression of NAFLD. In the present study, we focus on the possible association between MTBE exposure and the prevalence of NAFLD in southern China.
2. Experimental Section
2.1. Study Population and Ethical Permission
This study was carried out among petrol station attendants recruited from southern China during April to September 2014, all of whom had been working at the petrol stations for more than 3 years and had no history of chronic diseases and had not been taking any medicines for at least one year before this investigation. Some of the petrol station attendants (alcohol intake >0 g/day, hepatitis B antigen or hepatitis C virus antibody positive, autoimmune hepatitis, primary biliary cirrhosis, and other chronic liver disease with clear causes) were strictly excluded. A face-to-face investigation was carried out among the participants by trained interviewers with self-made questionnaires. The individual characteristic data, including age, gender, education, smoking habit, physical exercise, working types, and length of service, etc., were collected. A total of 71 eligible petrol station attendants were included in the final analysis. The procedures for the recruitment and data collection were approved by the local ethical research committee (No. 201158), and the purpose of the study was described in detail to each participant prior to obtaining written informed consent.
2.2. Diagnosis of NAFLD
Abdominal ultrasonography is the most commonly used method for the diagnosis of NAFLD in epidemiological investigation in China [33,34,35]. According to the definition of NAFLD in the guidelines for the diagnosis and treatment of NAFLD suggested by the Chinese Hepatology Association, NAFLD was also diagnosed by using abdominal ultrasonography [36], and some populations (alcohol intake > 20 g/day, hepatitis B antigen or hepatitis C virus antibody positive, autoimmune hepatitis, primary biliary cirrhosis, and other chronic liver disease with clear causes) were strictly excluded. In order to control bias, diagnoses of NAFLD were performed by the same medical doctor with the same instrument in the Baoan Center for Disease Control and Prevention in the period between April to September 2014.
2.3. Basic Examinations and Laboratory Tests
The basic examination (including body mass index, systolic blood pressure, and diastolic blood pressure, etc.) for each subject was conducted with standard operating procedure in Baoan Center for Disease Control and Prevention from April to September, 2014. Eight milliliters of peripheral venous blood was collected from each selected subject at the end of work time by using a single-use syringe for laboratory tests (including alanine aminotransferase (ALT), white blood cell (WBC), platelet (PLT), blood urea nitrogen (BUN), serum creatinine (CREA), total glucose (GLU), serum globulins (GLO), serum albumin (ALB), hemoglobin (Hb), total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL), etc. which potentially associated with NAFLD). Each sample was labeled with serial number, date, and time of collection to avoid possible confusion.
2.4. Analysis of Personal Exposure Level of MTBE
Personal exposure sampling of each participant was performed by using a charcoal-based organic vapor monitor (OVM; 3M 3520). MTBE was analyzed by using Head Space Solid Phase Microextraction GC/MS (model QP2010plus, Shimadzu, Japan), equipped with an AOC-20i+s automatic sampler (Shimadzu, Japan) and a Rtx-Wax column (30 m × 0.32 mm × 0.25 μm). The details were described previously [37].
2.5. Statistical Analysis
SPSS16.0 (SPSS Inc., IBM Company, Chicago, IL, USA) for Windows was used for statistical analysis. Descriptive analyses were conducted to characterize the demographics of the participants, including mean ± SD for continuous data and numbers and percentages for categorical data. The independent-sample Student’s t-test was adopted to assess between-group differences of quantitative materials. Chi-square test was conducted to compare categorical variables. Multivariate logistic regression analysis was conducted to estimate the odds ratio (OR) and 95% confidence interval (CI), in which the study population was classified into four groups according to the personal exposure concentrations of MTBE: ≤100 μg/m3, 100–200 μg/m3, 200–300 μg/m3, and ≥300 μg/m3, respectively. p-values were two-tailed, and the cutoff for statistical significance was set up at α = 0.05.
3. Results and Discussion
3.1. Demographic and Clinical Characteristics of Study Population
As indicated in Table 1, a total of 71 eligible subjects were eventually recruited from different petrol stations in southern China, which consisted of 41 males (57.75%) and 30 females (42.25%). Among the study population, 11 were diagnosed with NAFLD. The prevalence of NAFLD was 24.39% (10/41) in males, 3.33% (1/30) in females, there was a statistically significant difference between them (p = 0.039), and the total prevalence of NAFLD was 15.49% (11/71). It was reported that NAFLD has been found worldwide, and it is recognized as the most common chronic liver disease in China and Western countries, with a prevalence ranging from 3%–46% in the general population [38,39,40,41,42]. However, for different countries or different studies, there existed some apparent difference in the prevalence of NAFLD—the reason for which has not yet been fully clarified. In our study, demographic and clinical characteristics potentially associated with NAFLD (e.g., age, gender, education, smoking habit, physical exercise, working types, and length of service, etc.) were also investigated during April to September, 2014. The result showed that there was a statistically significant difference in age (p = 0.000), gender (p = 0.037), physical exercise (p = 0.046), body mass index (BMI, p = 0.000), systolic blood pressure (SBP, p = 0.000 ), diastolic blood pressure (DBP, p = 0.000 ), ALT (p = 0.000), WBC (p = 0.000), TC (p = 0.000), TG (p = 0.002), LDL (p = 0.012), and HDL (p = 0.023) between the NAFLD and non-NAFLD groups, and there were no significant differences in education (p = 0.785) , smoking habit (p = 0.680), working types (p = 0.917), length of service (p = 0.365), PLT (p = 0.071), BUN (p = 0.067), CREA (p = 0.190), GLU (p = 0.076), GLO (p = 0.097), ALB (p = 0.061), and Hb (p = 0.053) between the two groups. Our study is consistent with the reported data [34,38,39,40,41,42]: for example, our study indicates that there was a higher prevalence of NAFLD in men (10/41 = 24.39%) than in women (1/30 = 3.33%) (p = 0.037), the reason for which may be the effect of estrogen on the amount and distribution of fat in men and women, and different blood lipid levels in men and women may directly affect the prevalence of NAFLD [34].
Table 1.
Analysis of factors potentially associated with non-alcoholic fatty liver disease (NAFLD).
| Factors | NAFLD | Non–NAFLD | X2/t Value | p Value |
|---|---|---|---|---|
| Age (mean ± SD) (years) | 39.75 ± 8.61 | 27.11 ± 6.97 | 8.760 | 0.000 |
| Gender | ||||
| Male | 10 (90.91%) | 31 (51.67%) | 4.369 (continuity correction) |
0.037 |
| Female | 1 (9.09%) | 29 (48.33%) | ||
| Education | ||||
| High school | 8 (72.72%) | 49 (81.67%) | 0.074 (continuity correction) |
0.785 |
| College | 3 (27.28) | 11 (18.33%) | ||
| Smoking habit | ||||
| Yes | 3 (27.27%) | 10 (16.67%) | 0.170 (continuity correction) |
0.680 |
| No | 8 (72.73%) | 50 (83.33%) | ||
| Physical exercise | ||||
| Yes | 1 (9.09%) | 28 (46.67%) | 3.988 (continuity correction) |
0.046 |
| No | 10 (90.91%) | 32 (53.33%) | ||
| Working types | ||||
| Oil suppliers | 9 (81.82%) | 53 (88.33%) | 0.011 (continuity correction) |
0.917 |
| Office workers | 2 (18.18%) | 7 (11.67%) | ||
| Length of service (years) | 5.60 ± 2.51 | 4.68 ± 2.03 | 0.936 | 0.365 |
| BMI (kg/m2) | 25.63 ± 1.46 | 21.29 ± 1.98 | 17.637 | 0.000 |
| SBP (mmHg) | 126.64 ± 10.32 | 109.45 ± 9.67 | 14.971 | 0.000 |
| DBP (mmHg) | 80.03 ± 7.34 | 71.25 ± 6.87 | 11.670 | 0.000 |
| ALT (U/L) | 36.54 ± 6.75 | 22.17 ± 5.71 | 9.584 | 0.000 |
| WBC (×109/L) | 7.16 ± 2.64 | 5.69 ± 1.67 | 8.483 | 0.000 |
| PLT (×109/L) | 220 ± 30.38 | 203 ± 23.77 | 21.371 | 0.071 |
| BUN (mmol/L) | 5.61 ± 1.92 | 4.63 ± 1.37 | 7.692 | 0.067 |
| CREA (μmol/L) | 87.61 ± 23.15 | 81.59 ± 21.64 | 13.593 | 0.190 |
| GLU (mmol/L) | 5.37 ± 1.62 | 5.13 ± 1.35 | 8.691 | 0.076 |
| GLO (g/L) | 28.34 ± 7.65 | 27.97 ± 6.8 | 16.452 | 0.097 |
| ALB (g/L) | 46.74 ± 8.15 | 44.69 ± 7.98 | 18.723 | 0.061 |
| Hb (g/L) | 150.79 ± 12.67 | 145.93 ± 11.77 | 17.871 | 0.053 |
| TC (mmol/L) | 5.26 ± 0.87 | 4.07 ± 0.73 | 7.572 | 0.000 |
| TG (mmol/L) | 2.17 ± 0.81 | 1.41 ± 0.30 | 5.033 | 0.002 |
| LDL (mmol/L) | 2.51 ± 0.71 | 2.11 ± 0.52 | 6.971 | 0.012 |
| HDL (mmol/L) | 1.21 ± 0.12 | 1.51 ± 0.22 | −2.722 | 0.023 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; ALT: alanine aminotransferase; WBC: white blood cell; PLT: platelet; BUN: blood urea nitrogen; CREA: serum creatinine; GLU: total glucose; GLO: serum globulins; ALB: serum albumin; Hb: hemoglobin; TC: Total cholesterol; TG: triglycerides; LDL: low-density lipoprotein; HDL: high-density lipoprotein.
3.2. Personal Exposure Concentrations of MTBE among Petrol Station Attendants
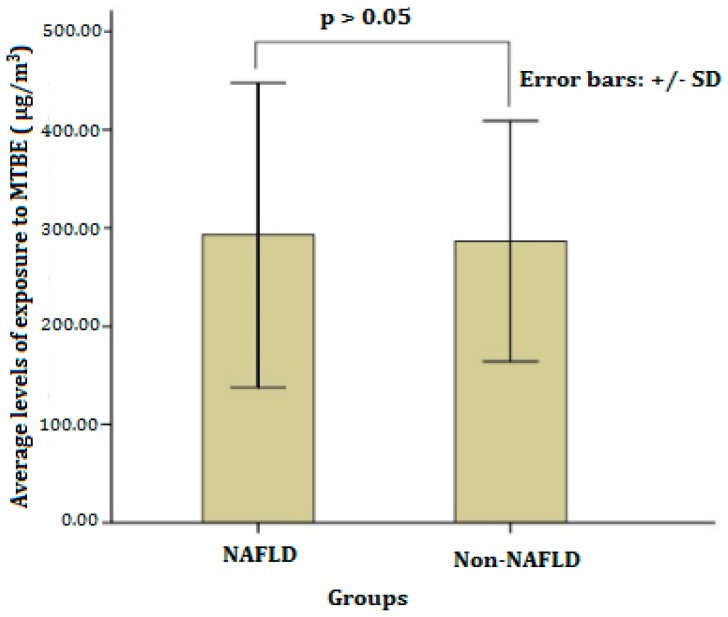
Personal exposure concentrations of MTBE among petrol station attendants were analyzed by using Head Space Solid Phase Microextraction (HSSPME) GC–MS. The results showed that the average exposure concentrations of MTBE were 292.98 ± 154.90 μg/m3 and 286.64 ± 122.28 μg/m3 in NAFLD and non-NAFLD groups, respectively, and there was no statistically significant difference between the the them (p > 0.05, as shown in Figure 1). These occupational exposure concentrations of MTBE among petrol station attendants were much higher than that of general residents in the same area of the Pearl River Delta in Southern China, which ranged from 0 to 1.25 μg/m3 [43]. Fortunately, all of the observed values in each group did not exceed the ACGIH (American Conference of Governmental and Industrial Hygienists) Threshold Limit Value (TLV®) of MTBE (50 ppm; 1 ppm MTBE = 3.61 mg/m3; 50 ppm = 180.5 mg/m3 = 180,500 µg/m3). This situation may mainly be attributed to air purification equipment, which has been enforced to be established in each petrol station in southern China.
Figure 1.
Average levels of exposure to methyl tertiary butyl ether (MTBE) in the NAFLD and Non-NAFLD groups.
3.3. Multivariate-Adjusted Odds Ratios for the Prevalence of NAFLD in Groups with Different Exposure Concentrations of MTBE
Multivariate logistic analysis showed that the crude odds ratios were 1.87 (95% CI: 0.91–2.11; p > 0.05), 1.51 (95% CI: 0.87–1.86; p > 0.05), and 1.91 (95% CI: 1.07–2.34; p < 0.05) in the groups (including men and women) with personal exposure concentrations of MTBE of 100–200 μg/m 3, 200–300 μg/m3, and ≥ 300 μg/m3, respectively, as compared to the group (including men and women) with the personal exposure concentration of MTBE ≤ 100 μg/m3. After adjusting for the confounding factors potentially associated with NAFLD (p < 0.05, as indicated in Table 1), including age, gender, physical exercise, BMI, SBP, DBP, ALT, WBC, TC, TG, LDL, and HDL, the odds ratios were 1.31 (95% CI: 0.85–1.54; p > 0.05), 1.14 (95% CI: 0.81–1.32; p > 0.05) and 1.52 (95% CI: 0.93–1.61; p > 0.05), respectively. At the same time, there was no significant association between MTBE exposure and the prevalence of NAFLD separately in men and women (p > 0.05, as shown in Table 2). Therefore, although the acute, subchronic, and chronic toxic effects of MTBE, such as lipid disorder [11], abnormal liver function [11], and oxidative stress [6,7,8], etc., were found in animal tests, there is no significant evidence showing an association between MTBE exposure and the prevalence of NAFLD in humans in our study. Of course, there were some limitations to this study, the first being that the exposure concentrations of MTBE among the subjects were relatively low (did not exceed the ACGIH TLV® of MTBE, 50 ppm); thus, it can hardly shed light on the association between relatively high exposure concentrations of MTBE and the prevalence of NAFLD. The second point is that our study is an epidemiological cross-sectional survey, which can not reveal the toxic effects related to long-term exposure to MTBE. The third point is that the sample size of our study is relatively small, which may limit the representativeness.
Table 2.
Odds ratios for the prevalence of NAFLD among groups with different MTBE exposure concentrations.
| MTBE (μg/m3) |
Gender | n | Crude | Adjusted c | ||||
|---|---|---|---|---|---|---|---|---|
| OR a | 95% CI b | p Value | OR | 95% CI | p Value | |||
| ≤100 | M | 4 | 1.00 | 1.00–1.00 | - | 1.00 | 1.00–1.00 | - |
| W | 7 | 1.00 | 1.00–1.00 | - | 1.00 | 1.00–1.00 | - | |
| T | 11 | 1.00 | 1.00–1.00 | - | 1.00 | 1.00–1.00 | - | |
| 100–200 | M | 4 | 1.93 | 0.78–2.31 | >0.05 | 1.64 | 0.84–1.83 | >0.05 |
| W | 8 | 0.97 | 0.85–1.41 | >0.05 | 1.17 | 0.79–1.32 | >0.05 | |
| T | 12 | 1.87 | 0.91–2.11 | >0.05 | 1.31 | 0.85–1.54 | >0.05 | |
| 200–300 | M | 25 | 1.68 | 0.78–1.89 | >0.05 | 1.32 | 0.80–1.63 | >0.05 |
| W | 9 | 0.93 | 0.81–1.33 | >0.05 | 1.02 | 0.79–1.26 | >0.05 | |
| T | 34 | 1.51 | 0.87–1.86 | >0.05 | 1.14 | 0.81–1.32 | >0.05 | |
| ≥300 | M | 7 | 1.35 | 0.77–2.41 | >0.05 | 1.21 | 0.77–1.73 | >0.05 |
| W | 7 | 1.27 | 0.83–1.67 | >0.05 | 1.11 | 0.75–1.41 | >0.05 | |
| T | 14 | 1.91 | 1.07–2.34 | <0.05 | 1.52 | 0.93–1.61 | >0.05 | |
M = Man; W = Woman; T = M + W; a: OR = Odds ratio; b: CI = Confidence interval; c: Adjusted for age, gender, physical exercise, BMI, SBP, DBP, ALT, WBC, TC, TG, LDL, and HDL, which potentially associated with NAFLD (p < 0.05, as indicated in Table 1).
4. Conclusions
Personal exposure concentrations of MTBE among petrol station attendants were relatively low, and do not seem to be a significant risk factor for the prevalence of NAFLD among petrol station attendants in southern China.
Acknowledgments
We gratefully acknowledge the cooperation of South China Institute of Environmental Sciences, Ministry of Environment Protection, P.R.C; Shenzhen Centre for Disease Control and Prevention; Shenzhen Prevention and Treatment Centre for Occupational Diseases; The Disease Prevention and Control Center of Shenzhen City, Baoan District. This study was supported by a research grant (201009008) from the Ministry of Environment Protection, P.R.C, a grant from the Department of Science and Technology of Guangdong Province (2014A020212206), and a Grant from Shenzhen Municipal Science and Technology Innovation Committee (JCYJ20150331102453903).
Author Contributions
Dalin Hu is the corresponding author, who responsible for design, organizing implementation and paper writing of this project; Jianping Yang and Jianhui Yuan performed epidemiological investigation; Qinzhi Wei, Xiaochun Peng, Xiaowu Peng provided important suggestions and revised the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bogen K.T., Heilman J.M. Reassessment of MTBE cancer potency considering modes of action for MTBE and its metabolites. Crit. Rev. Toxicol. 2015;45(Suppl 1):1–56. doi: 10.3109/10408444.2015.1052367. [DOI] [PubMed] [Google Scholar]
- 2.Phillips S., Palmer R.B., Brody A. Epidemiology, toxicokinetics, and health effects of methyl tert-butyl ether (MTBE) J. Med. Toxicol. 2008;4:115–126. doi: 10.1007/BF03160966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown S.L. Atmospheric and potable water exposures to methyl tert-butyl ether (MTBE) Regul. Toxicol. Pharmacol. 1997;25:256–276. doi: 10.1006/rtph.1997.1104. [DOI] [PubMed] [Google Scholar]
- 4.Moolenaar R.L., Hefflin B.J., Ashley D.L., Middaugh J.P., Etzel R.A. Methyl tertiary butyl ether in human blood after exposure to oxygenated fuel in Fairbanks, Alaska. Arch. Environ. Health. 1994;49:402–409. doi: 10.1080/00039896.1994.9954993. [DOI] [PubMed] [Google Scholar]
- 5.White M.C., Johnson C.A., Ashley D.L., Buchta T.M., Pelletier D.J. Exposure to methyl tertiary-butyl ether from oxygenated gasoline in Stamford, Connecticut. Arch. Environ. Health. 1995;50:183–189. doi: 10.1080/00039896.1995.9940385. [DOI] [PubMed] [Google Scholar]
- 6.Li D., Yin D., Han X. Methyl tert-butyl ether (MTBE)-induced cytotoxicity and oxidative stress in isolated rat spermatogenic cells. J. Appl. Toxicol. 2007;27:10–17. doi: 10.1002/jat.1178. [DOI] [PubMed] [Google Scholar]
- 7.Sgambato A., Iavicoli I., De Paola B., Bianchino G., Boninsegna A., Bergamaschi A., Pietroiusti A., Cittadini A. Differential toxic effects of methyl tertiary butyl ether and tert-butanol on rat fibroblasts in vitro. Toxicol. Ind. Health. 2009;25:141–151. doi: 10.1177/0748233709104867. [DOI] [PubMed] [Google Scholar]
- 8.Williams-Hill D., Spears C.P., Prakash S., Olah G.A., Shamma T., Moin T., Kim L.Y., Hill C.K. Mutagenicity studies of methyl-tert-butylether using the Ames tester strain TA102. Mutat. Res. 1999;446:15–21. doi: 10.1016/S1383-5718(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 9.Yuan Y., Wang H.F., Sun H.F., Du H.F., Xu L.H., Liu Y.F., Ding X.F., Fu D.P., Liu K.X. Adduction of DNA with MTBE and TBA in mice studied by accelerator mass spectrometry. Environ. Toxicol. 2007;22:630–635. doi: 10.1002/tox.20295. [DOI] [PubMed] [Google Scholar]
- 10.Du H.F., Xu L.H., Wang H.F., Liu Y.F., Tang X.Y., Liu K.X., Peng S.X. Formation of MTBE-DNA adducts in mice measured with accelerator mass spectrometry. Environ. Toxicol. 2005;20:397–401. doi: 10.1002/tox.20124. [DOI] [PubMed] [Google Scholar]
- 11.Dong-mei L., Yi G., Chun-Tao Y., Yu-feng H., Xiao-dong H. Effects of subchronic methyl tert-butyl ether ether exposure on male Sprague-Dawley rats. Toxicol. Ind. Health. 2009;25:15–23. doi: 10.1177/0748233708101594. [DOI] [PubMed] [Google Scholar]
- 12.Bonventre J.A., Kung T.S., White L.A., Cooper K.R. Manipulation of the HIF-Vegf pathway rescues methyl tert-butyl ether (MTBE)-induced vascular lesions. Toxicol. Appl. Pharmacol. 2013;273:623–634. doi: 10.1016/j.taap.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belpoggi F., Soffritti M., Maltoni C. Methyl-tertiary-butyl ether (MTBE)—A gasoline additive-causes testicular and lymphohaematopoietic cancers in rats. Toxicol. Ind. Health. 1995;11:119–149. doi: 10.1177/074823379501100202. [DOI] [PubMed] [Google Scholar]
- 14.Froines J.R., Collins M., Fanning E., McConnell R., Robbins W., Silver K., Kun H., Mutialu R., Okoji R., Taber R., et al. An evaluation of the scientific peer-reviewed research and literature on the human health effects of mtbe, its metabolites, combustion products and substitute compounds. In: Davis C.A., editor. Health and Environmental Assessment of MTBE: Report to the Governor and Legislature of the State of California as Sponsored by SB 521. Volume II: Human Health Effects. University of California Toxic Substances Research & Teaching Program; Sacramento, CA, USA: 1998. p. 267. [Google Scholar]
- 15.California Environmental Protection Agency Public Health Goal for Methyl Tertiary Butyl Ether (MTBE) in Drinking Water: Sacramento, CA, California Environmental Protection Agency, Pesticide and Environmental Toxicology Section, Office of Environmental Health Hazard Assessment. [(accessed on 4 February 2016)];:124. Avaialble online: http://www.oehha.ca.gov/water/phg/pdf/mtbe_f.pdf.
- 16.Mehlman M.A. Dangerous and cancer-causing properties of products and chemicals in the oil-refining and petrochemical industry—Part XXII: Health hazards from exposure to gasoline containing methyl tertiary butyl ether: Study of New Jersey residents. Toxicol. Ind. Health. 1996;12:613–627. doi: 10.1177/074823379601200502. [DOI] [PubMed] [Google Scholar]
- 17.Mohr S.N., Fiedler N., Weisel C., Kelly-McNeil K. Health effects of MTBE among New Jersey garage workers. Inhalation Tox. 1994;6:553–562. doi: 10.3109/08958379409003040. [DOI] [Google Scholar]
- 18.Cain W., Leaderer B., Ginsberg G., Andrews L., Cometto-Muniz J., Gent J., Buck M., Berglund L., Mohseinin V., Monahan E., Kjaergaard S. Acute exposure to low-level methyl tertiary butyl ether (MTBE): Human reactions and pharmacokinetic response. Inhal. Toxicol. 1996;8:21–48. doi: 10.3109/08958379609005425. [DOI] [Google Scholar]
- 19.Prah J.D., Goldstein G.M., Devlin R., Otto D., Ashley D., House D., Willingham F., Cohen K.L., Gerrity T. Sensory, symptomatic, inflammatory, and ocular responses to and the metabolism of methyl tertiary butyl ether in a controlled human exposure experiment. Inhal. Toxicol. 1994;6:521–538. doi: 10.3109/08958379409003038. [DOI] [Google Scholar]
- 20.Nihlen A., Lof A., Johanson G. Experimental exposure to methyl tertiary-butyl ether. Toxicol. Appl. Pharm. 1998;148:274–280. doi: 10.1006/taap.1997.8333. [DOI] [PubMed] [Google Scholar]
- 21.Mehta K., Van Thiel D.H., Shah N., Mobarhan S. Nonalcoholic fatty liver disease: Pathogenesis and the role of antioxidants. Nutr. Rev. 2002;60:289–293. doi: 10.1301/002966402320387224. [DOI] [PubMed] [Google Scholar]
- 22.Arciello M., Gori M., Maggio R., Barbaro B., Tarocchi M., Galli A., Balsano C. Environmental pollution: A tangible risk for NAFLD pathogenesis. Int. J. Mol. Sci. 2013;14:22052–22066. doi: 10.3390/ijms141122052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angulo P., Lindor K.D. Non-alcoholic fatty liver disease. J. Gastroenterol Hepatol. 2002;17:S186–S190. doi: 10.1046/j.1440-1746.17.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 24.Marrero J.A., Fontana R.J., Su G.L., Conjeevaram H.S., Emick D.M., Lok A.S. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1002/hep.1840360609. [DOI] [PubMed] [Google Scholar]
- 25.Starley B.Q., Calcagno C.J., Harrison S.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 26.Luyckx F.H., Lefebvre P.J., Scheen A.J. Non-alcoholic steatohepatitis: Association with obesity and insulin resistance, and influence of weight loss. Diabetes Metab. 2000;26:98–106. doi: 10.1016/S0140-6736(05)76069-4. [DOI] [PubMed] [Google Scholar]
- 27.Fan J.G., Saibara T., Chitturi S., Kim B.I., Sung J.J., Chutaputti A. Asia-Pacific Working Party for NAFLD. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J. Gastroenterol Hepatol. 2007;22:794–800. doi: 10.1111/j.1440-1746.2007.04952.x. [DOI] [PubMed] [Google Scholar]
- 28.Tomaru M., Takano H., Inoue K., Yanagisawa R., Osakabe N., Yasuda A., Shimada A., Kato Y., Uematsu H. Pulmonary exposure to diesel exhaust particles enhances fatty change of the liver in obese diabetic mice. Int. J. Mol. Med. 2007;19:17–22. doi: 10.3892/ijmm.19.1.17. [DOI] [PubMed] [Google Scholar]
- 29.Tan H.H., Fiel M.I., Sun Q., Guo J., Gordon R.E., Chen L.C., Friedman S.L., Odin J.A., Allina J. Kupffer cell activation by ambient air particulate matter exposure may exacerbate non-alcoholic fatty liver disease. J. Immunotoxicol. 2009;6:266–275. doi: 10.3109/15476910903241704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cave M., Appana S., Patel M., Falkner K.C., McClain C.J., Brock G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environ. Health Perspect. 2010;118:1735–1742. doi: 10.1289/ehp.1002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyder O., Chung M., Cosgrove D., Herman J.M., Li Z., Firoozmand A., Gurakar A., Koteish A., Pawlik T.M. Cadmium exposure and liver disease among U.S. adults. J. Gastrointest Surg. 2013;17:1265–1273. doi: 10.1007/s11605-013-2210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Sevillano M.Á., García-Barrera T., Navarro-Roldán F., Montero-Lobato Z., Gómez-Ariza J.L. A combination of metallomics and metabolomics studies to evaluate the effects of metal interactions in mammals: Application to Mus musculus mice under arsenic/cadmium exposure. J. Proteomics. 2014;104:66–79. doi: 10.1016/j.jprot.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Lin H., Zhang C., Wang L., Wu S., Zhang D., Tang F., Xue F., Liu Y. Non-alcoholic fatty liver disease associated with gallstones in females rather than males: A longitudinal cohort study in Chinese urban population. BMC Gastroenterol. 2014;14:946. doi: 10.1186/s12876-014-0213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Z.Y., Shao Z., Li Y.L., Wulasihan M., Chen X.H. Prevalence of and risk factors for non-alcoholic fatty liver disease in a Chinese population: An 8-year follow-up study. World J. Gastroenterol. 2016;22:3663–3669. doi: 10.3748/wjg.v22.i13.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan J.G., Farrell G.C. Epidemiology of non-alcoholic fatty liver disease in China. J. Hepatol. 2009;50:204–210. doi: 10.1016/j.jhep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Zeng M.D., Fan J.G., Lu L.G., Li Y.M., Chen C.W., Wang B.Y., Mao Y.M. Chinese National Consensus Workshop on Nonalcoholic Fatty Liver Disease. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J. Dig. Dis. 2008;9:108–112. doi: 10.1111/j.1751-2980.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- 37.Hu D., Yang J., Liu Y., Zhang W., Peng X., Wei Q., Yuan J., Zhu Z. Health risk assessment for inhalation exposure to methyl tertiary butyl ether at petrol stations in southern China. Int. J. Environ. Res. Public Health. 2016;13:204. doi: 10.3390/ijerph13020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y.J., Li Y.Y., Nie Y.Q., Ma J.X., Lu L.G., Shi S.L., Chen M.H., Hu P.J. Prevalence of fatty liver disease and its risk factors in the population of South China. World J. Gastroenterol. 2007;13:6419–6424. doi: 10.3748/wjg.v13.i47.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Browning J.D., Szczepaniak L.S., Dobbins R., Nuremberg P., Horton J.D., Cohen J.C., Grundy S.M., Hobbs H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 40.Clark J.M. The epidemiology of nonalcoholic fatty liver disease in adults. J. Clin. Gastroenterol. 2006;40(Suppl 1):S5–S10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 41.Weiß J., Rau M., Geier A. Non-alcoholic fatty liver disease: Epidemiology, clinical course, investigation, and treatment. Dtsch. Arztebl. Int. 2014;111:447–452. doi: 10.3238/arztebl.2014.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vernon G., Baranova A., Younossi Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang B.G., Shao M., Zhang Y.H., Lü W.M., Zhou Y. Methyl tert-butyl ether (MTBE) in atmosphere of the Pearl River Delta, China. Huan Jing Ke Xue. 2007;28:1614–1620. [PubMed] [Google Scholar]