Abstract
In this review, reports were retrieved in which vitamin D status, as assessed by serum 25-hydroxyvitamin D [25(OH)D] levels, was measured in South African population groups with varied skin colours and ethnicities. Healthy children and adults were generally vitamin D-sufficient [25(OH)D level >50 nmol/L] but the majority of those aged above 65 years were deficient. A major role for exposure to solar ultraviolet radiation (UVR) in determining 25(OH)D levels was apparent, with the dietary contribution being minor. Limited data exist regarding the impact of recent changes in lifestyles on vitamin D status, such as urbanisation. With regard to disease susceptibility, 11 of 22 relevant publications indicated association between low 25(OH)D levels and disease, with deficiency most notably found in individuals with tuberculosis and HIV-1. Information on the relationship between vitamin D receptor variants and ethnicity, disease or treatment response in the South African population groups demonstrated complex interactions between genetics, epigenetics and the environment. Whether vitamin D plays an important role in protection against the range of diseases that currently constitute a large burden on the health services in South Africa requires further investigation. Only then can accurate advice be given about personal sun exposure or dietary vitamin D supplementation.
Keywords: 25(OH)D levels, HIV-1, tuberculosis, vitamin D receptor, sun exposure
1. Introduction
Vitamin D deficiency is a risk factor for rickets in children and osteomalacia in adults [1]. Recently, with the recognition that the active form of vitamin D is immunoregulatory, vitamin D deficiency has been proposed to increase the likelihood of a wide range of common human disorders [2,3], including various autoimmune, infectious and cardiovascular diseases, and internal cancers, several of which occur in South Africa at rates exceeding those reported globally. While there has been considerable research on vitamin D in developed countries [4,5], this has not been matched in developing countries where the impact of vitamin D deficiency on health may be greater in terms of disease burden. Therefore in this review, we gather together all the published information regarding vitamin D in the context of South Africa. Our main aims were to establish whether there was widespread vitamin D deficiency in any population group and to investigate whether there was robust evidence to associate low vitamin D status with any of the diseases, prevalent currently in South Africa.
In most people, exposure of the skin to solar ultraviolet radiation (UVR) in the UV-B range (290–315 nm) is the main route for synthesis of vitamin D. Absorption of UVR converts 7-dehydrocholesterol in the lower epidermis to previtamin D3 which then undergoes thermal isomerisation to vitamin D3. This is followed by hydroxylation, first to 25-hydroxyvitamin D [25(OH)D] mainly in the liver, and then to 1,25-dihydroxyvitamin D [1,25(OH)2D] mainly in the kidney [6]. Both 25(OH)D and 1,25(OH)2D are transported in the blood linked to the vitamin D binding protein (DBP, also known as group-specific component [Gc]). 1,25(OH)2D binds to the vitamin D receptor (VDR) in the membrane and/or the nucleus of a wide variety of cell types. In addition many non-renal cells can convert 25(OH)D to 1,25(OH)2D, thus providing a source of 1,25(OH)2D for tissue-specific intracellular action [7]. Binding of 1,25(OH)2D facilitates the VDR to form a heterodimer with the retinoid X receptor (RXR), leading to the activation of the VDR-RXR transcription complex. This regulates the expression of more than 900 genes, thus affecting a myriad of signalling cascades in the body [8].
Vitamin D is present in some foods, as vitamin D3 (cholecalciferol) from animal sources and as vitamins D3 and D2 (ergocalciferol) from plant sources. Both forms are absorbed from the small intestine together with dietary fat, and incorporated into chylomicrons which are rapidly transported via the lymphatics and then via the venous blood, bound to the DBP, to the liver. A limited number of staple foodstuffs, such as cereals and dairy products, are fortified with vitamin D in many developed countries. Finally dietary supplements are widely available, containing either vitamin D2 or D3 in a wide range of dosages.
Vitamin D status is commonly measured as the total plasma or serum concentration of the sum of 25(OH)D2 and 25(OH)D3, both bound to DBP and albumin, as well as unbound (“free”) [9]. The cut-off points for sufficiency, insufficiency and deficiency are not agreed at present. In their 2011 report, the US Institute of Medicine focussed on bone health and defined <30 nmol/L 25(OH)D as deficient, 30–50 nmol/L as insufficient and >50 nmol/L as sufficient [10]. Others such as Bischoff-Ferrari et al. [11] and the US Endocrine Society [12] have defined <50 nmol/L as deficient, 50–75 nmol/L as insufficient and >75 nmol/L as sufficient, based on the skeletal and non-skeletal health benefits of vitamin D. In the following section, a 25(OH)D concentration of >50 nmol/L in people without disease was taken as being sufficient as the levels relating to bone are not generally disputed, while those relating to the other possible in vivo benefits of vitamin D remain uncertain. It should be noted that the accuracy and reproducibility of the assays that measure 25(OH)D are variable [13,14]. At present quality assurance-validated liquid chromatography-tandem mass spectroscopy (LC-MS/MS) is considered by many as the “gold standard” method [13], but the lower-cost competitive immunoassay, which varies by supplier, is often used and may give different results from LC-MS/MS, thus confounding comparison between studies.
South Africa consists of nine provinces, spanning latitudes 22.34° S to 34.28° S (Figure 1). The country has a narrow coastal plain at sea level, rising to mountains over 3000 m in height, with a large plateau, the Highveld, at an altitude of 1200 m. High atmospheric pressure over this Northern plateau frequently results in cloudless skies and, in combination with the altitude, leads to high levels of ambient solar UVR. As an illustration of the climatic variation between the Northern and Southern regions of South Africa, the temperature, UV Index, and number of hours of sunshine per day in the winter and summer are shown in Table 1 for Cape Town representing the South, and Pretoria representing the North Highveld. These conditions have the potential to result in variable seasonal personal exposure to solar UVR, with implications for the production of vitamin D [15].
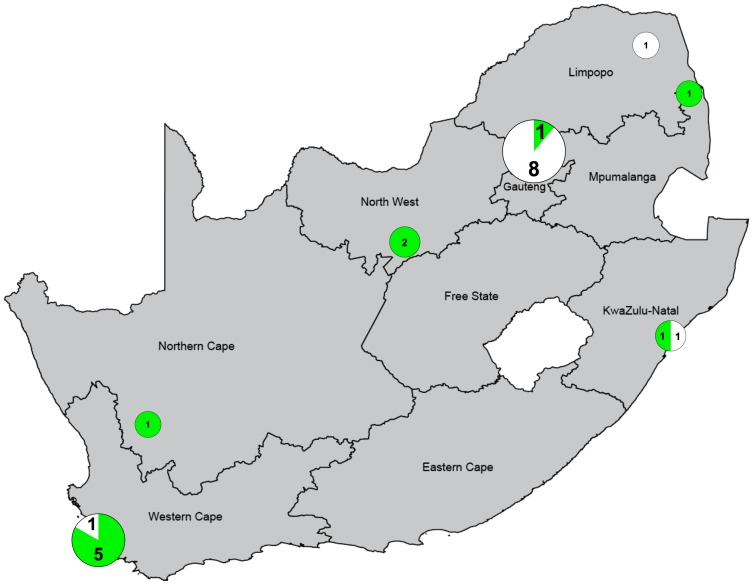
Figure 1.
The number of publications investigating the association between vitamin D status and disease in the Provinces of South Africa. Green indicates a significant association, and white indicates no significant association. The size of the Venn diagrams is proportional to the number of studies. Map adapted from Global Security [19] and the Venn diagrams were plotted using GraphPad PRISM 6.
Table 1.
| Parameter | Cape Town Latitude 35° S, Altitude 0–300 m | Pretoria Latitude 26° S, Altitude 1339 m | ||
|---|---|---|---|---|
| Summer | Winter | Summer | Winter | |
| Average hours of sunshine per day | 11 | 5 | 10 | 8 |
| Ultraviolet index | 9–10 | 2–3 | 11+ | 4–6 |
| Average temperature in °C, minimum/maximum | 16/26 | 7/18 | 18/28 | 5/20 |
The population of South Africa is multi-ethnic and complex, with phototypes ranging from deeply pigmented to fair [20]. The South African government collects demographic data on four ethnic groups: in 2014, the National Census estimated the population as 54 million of whom 80.2% were Black African, 8.8% Coloured (mixed ancestry between White and Black or Black and Asian, with skin colour ranging from pale to dark brown), 2.5% Asian/Indian (frequently termed Indian in South Africa) and 8.4% White [21]. Approximately 10% of the population is infected with HIV-1 currently, one of the highest prevalences in the world, and the incidence of tuberculosis (TB) is the third highest in the world. Epidemiological transition in South Africa, occurring particularly since the end of apartheid in 1994, means that many social factors relating to personal sun exposure and hence to vitamin D status may have changed in recent years.
In this article, published studies measuring the vitamin D status of various healthy South African populations are reviewed first. These provide information to indicate whether any population group, defined by age or ethnicity or location of residence, is vitamin D-deficient. Such a status may lead to deleterious consequences for health. It is important to try to construct simple messages for the public regarding “safe” sun exposure that ensures sufficient vitamin D status while not increasing the risk of the adverse effects of intense UVR. This topic is considered in the context of the large range of South African phototypes. Next, as urbanisation may affect vitamin D status, either positively through improvements in lifestyle and diet, or negatively through less time spent outdoors, this aspect is reviewed. Information regarding disease and vitamin D deficiency is then evaluated in the context of South Africa. This country has a high burden of diseases such as TB and HIV, so the possibility of a low vitamin D status increasing the risk of the disorder or its severity is of particular interest. Finally recent information is reviewed regarding the effect of the VDR polymorphisms, found in the diverse populations of South Africa, on susceptibility to disease, particularly TB.
2. Vitamin D Status in South Africa
Several recent reviews have provided information on global vitamin D status [4,5,22], but all of these lack data for most African countries, including South Africa. In this section, all the publications were retrieved in which 25(OH)D was assessed with the aim of determining whether any South African population group, defined by age or skin colour or place of residence, was vitamin D-deficient. A systematic search was conducted using PubMed, covering the period 1978 to mid-2015, with the terms “vitamin D” AND “South Africa” AND “(status or prevalence or deficiency)”. By title, 220 papers were found; this number was reduced to 85 after reading the abstracts, and further reduced to 19 after considering the full reports: these are listed in Table 2 with a summary of each set of findings. Publications involving case reports, clinical management and reviews were excluded. Reports assessing 25(OH)D levels in people with various diseases and the control groups are described in a later section.
Table 2.
Studies assessing vitamin D status, as 25-hydroxyvitamin D [25(OH)D] levels, in various populations of children, adults and the elderly in South Africa (listed chronologically by year in each section).
| Study Population, Sampling Frame | Age (Years) | Location (Latitude), Season of Sampling when Specified | Assay | Serum or Plasma 25(OH)D Concentration Means (Unless Specified), nmol/L | Reference (Year) |
|---|---|---|---|---|---|
| Children | |||||
| 285 Coloured (mixed race) children; community based | 1–17 | Western Township, Johannesburg (26° S), winter | Competitive protein binding | 78.0 in age 1–12 y, 58.5 in age 13–14 y, 56.8 in age 15–16 y; none <25; no difference between boys and girls | [23] (1978) |
| 60 Black African children; school sample | 7–12 | Rural, small urban and large urban communities near Johannesburg (26° S) | Competitive protein binding | 72.3 in rural, 77.3 in small urban, 82.8 in large urban; none <25 | [24] (1979) |
| 114 hospitalised Black African infants; random hospital admissions | 0–2 | Witwatersrand (26° S), throughout year | Competitive protein binding | 37.3 aged 1–24 months, no correlation with age or season; <12.5 in 7%. 19.8 aged 0–1 month, probably reflecting vitamin D status of mother | [25] (1985) |
| 20 Black African pre-school children; cluster sample of villages | 3–5 | Villages in Northern Transvaal, now Limpopo (24° S), end of summer | Competitive protein binding | 85.5, no difference between underweight and normal weight children | [26] (1986) |
| 82 Black Africans with OCA, 58 Black Africans; school sample | 6–18 | Pietersburg, Northern Province (24° S) | Radioimmunoassay | 125 in OCA Blacks, 103 in Blacks, 6–9 y; 116 in OCA Blacks, 86.3 in Blacks, 10–13 y; 90.3 in OCA Blacks, 90.8 in Blacks,14–18 y | [27] (2000) |
| 295 Black African children, 90 White children; bone health subcohort of Birth-to-Twenty longitudinal cohort | 10 | Johannesburg (26° S), all seasons | Chemiluminescence (DiaSorin Liaison) | 100 in Black boys, 129 in White boys, 86 in Black girls, 112 in White girls; <50 in 8% Blacks and 1% Whites; higher values in White children in summer/autumn than in winter/spring, no seasonal variation in Blacks | [28] (2011) |
| Adults | |||||
| 43 healthy Black African women and cord blood of their babies, shortly after delivery; hospital based | 16–40 | Transkei (31° S) | Competitive protein binding | 81.8 in mothers; 171 in cord blood | [29] (1987) |
| 105 healthy White and 74 Black African premenopausal nurses; 50 healthy White and 65 Black African postmenopausal nurses; hospital based | 20–64 | Witwatersrand (26° S) | Competitive protein binding | Medians–65.8 in premenopausal White, 48.3 Black; 64.5 in postmenopausal White, 67.5 Black | [30] (1997) |
| 216 requests for vitamin D testing (39% with suspected osteoporosis); hospital based | All ages, peaks at 2 and 64 | Western Cape (32° S), all seasons | Competitive protein binding | Medians–48.3 (range 5.5–106); <45 in 41%; no seasonal effect on level | [31] (2009) |
| 658 rural healthy Black African women, 603 urban healthy Black African women; random selection from Prospective Urban and Rural Epidemiology Study or community based | >35 | North West Province (27° S), rural and urban seasonally matched | Roche Eledsys 2010 COBAS system | Levels decreased with age in both rural and urban women from about 78 at <50 y to about 65 at >70 y; levels lower in urban than in rural women, aged 50–70 y | [32] (2011) |
| 373 Black Africans, 344 Asian/Indians; cohort from Birth-to-Twenty longitudinal study | Mean 42 | Johannesburg-Soweto (26° S), all seasons | HPLC | 70.9 Blacks, 41.8 Asian/Indian; <30 3% Blacks and 13% Asian/Indians; females lower than males in both groups; highest level in autumn | [33] (2013) |
| 291 healthy urban Black African women; random selection from Prospective Urban and Rural Epidemiology Study or community based | >47 (mean 57.6) | North West Province (27° S) | Roche Elecsys 2010 COBAS system | 65; those with levels <75 two-times more likely to have higher systolic blood pressure than those with >75 (151 vs. 146 mmHg) | [34] (2013) |
| 368 healthy Black Africans and 347 healthy Asian/Indians; random selection from Birth-to-Twenty longitudinal study | 18–65 | Johannesburg (26° S) | HPLC | 58.3 in Black females, 72.7 in Black males, 35.7 in Asian/Indian females, 45.4 in Asian/Indian males | [35] (2014) |
| 371 healthy Black Africans and 343 healthy Asian/Indians; random selection from Birth-to-Twenty longitudinal study | 18–65 | Johannesburg (26° S) | HPLC | 56.8 in Black females, 72.4 in Black males, 32.4 in Asian/Indian females, 43.9 in Asian/Indian males; <30 in 5% Blacks and 28.6% Asian/Indians; levels 40%–60% higher in autumn than in winter/spring; little 25(OH)D2 | [36] (2014) |
| 502 Black Africans; population sample from Modelling of the Epidemiologic Transition Study | 25–45 (mean 33.4) | Cape Town (34° S), winter and summer months | LC-MS/MS | 59.3; <30 in 6.6%, >50 in 65.9%; negative correlation of 25(OH)D level with distance from the equator (by comparing levels in Blacks living at latitudes 41° N, 17° N, 6° N, 4° S and Cape Town) | [37] (2014) |
| 50 healthy Black Africans, 50 healthy Coloured (Cape mixed); community based longitudinal study | 18–24 | Cape Town (34° S), winter and summer months | Chemiluminescence (DiaSorin, Liaison) | Medians: 72.6 Black, 65.5 Coloured in summer; 45.5 Black, 43.8 Coloured in winter | [38] (2015) |
| Elderly | |||||
| 232 patients with femoral neck fractures, ethnicity not specified; hospital admissions | Mean 72.7 | Johannesburg (26° S), throughout year | Competitive protein binding | 44.3 throughout year; 51 in summer/autumn, 38.1 in winter and spring; <25 in 17% subjects in winter/spring | [39] (1978) |
| 60 females living in old-age homes, ethnicity not specified | Mean 80 | Pretoria (26° S), winter | Not specified | 32 | [40] (1991) |
| 173 non-institutionalised Coloured (mixed race), 52% women; population sample | 65–92 (mean 73.7) | Cape Town (34° S), late winter | Not specified | 37; <25 in 17% | [41] (1996) |
HPLC, high-performance liquid chromatography; LC-MS/MS, liquid chromatography-tandem mass spectroscopy; OCA, oculocutaneous albinism; y, years.
The results were compared for variation in the methods assessing 25(OH)D level, sample size, the age and population groups of the participants, the latitude of the study location and the time of year of sampling. Several different assays were used to determine the concentration of 25(OH)D in plasma or serum, ranging from competitive protein binding to LC-MS/MS more recently. Results from these different methods may not be comparable. Sample sizes tended to be smaller in older reports compared with more recent studies. The findings of each investigation varied, and included people at only one location. The sampling frames are indicated in Table 2, and show that they were limited to particular groups and did not reflect the demographics of the population as a whole. Thus none of the results in the 19 publications could be considered as providing an accurate reflection of the entire South African population. The participants varied in age from infants to the elderly, and were stated as belonging to the Black African, Coloured, Asian/Indian or White population groups in all but three cases [31,39,40]. Residence ranged from latitude 24–35° S, within both rural and urban communities, but it should be noted that only five of the studies were based in the South of the country. Samples were collected throughout the year in seven investigations and, of these, 25(OH)D levels were assessed in each of the four seasons in five [25,28,31,36,39], with a mean value for the year given in the other two [33,37]. The season of sampling was stated as being solely in the winter in three instances [23,39,41] and at the end of the summer in another one [26]. There was one longitudinal study in which 25(OH)D levels were assessed in the summer and the following winter in the same participants [38]. It is particularly important to obtain this information because 25(OH)D levels may decrease in the winter months, especially in the southern part of South Africa, as the quantity of UV-B in solar UVR reduces. In the remaining eight investigations, the time of year when the samples were collected was not specified.
Despite these difficulties, an overview of the studies in Table 2 indicates that 25(OH)D levels were generally sufficient in most healthy children (birth to 18 years old) with black, coloured and white skin (mean value approximately 84 nmol/L) [23,24,26,28], but were about 24% lower in Black African children compared with age-matched White children [28]. It is of note that all of these studies were located in the Northern provinces of South Africa and that four of them were conduced prior to 1986 [27,28,29,30] so their relevance to the current vitamin D status of children throughout South Africa who are undergoing rapid urbanisation, and particularly to those living in the Southern provinces is uncertain. In addition the results using assays for 25(OH)D at that time may not correlate with the assays used currently. The levels of 25(OH)D were sufficient in most healthy Black African (mean approximately 67 nmol/L) and White (mean approximately 65 nmol/L) adults (aged 18–54 years) [29,30,32,37], but insufficient in many Asian/Indians (mean approximately 42 nmol/L) [33,35,36], hypothesised to be due to the cultural preference to clothe most of the body. Three studies undertaken prior to 1997 in elderly people (>65 years) showed a high prevalence of vitamin D deficiency (mean approximately 38 nmol/L) [39,40,41]. However in the only study since then which included data on the elderly, a mean level of 65 nmol/L in healthy Black African women over the age of 70 years was found [32]. Males tended to have higher 25(OH)D levels than age-matched females in two recent reports [28,33], and the levels decreased with age, both during adolescence [27], and in adults [32]. Levels were higher in the summer/autumn (peak in autumn) compared with the winter/spring [33,36,39], and, in the only longitudinal study, decreased from sufficiency in the summer to less than 50 nmol/L in the winter in both the Black African and Coloured groups in Cape Town [38]. They also tended to decrease with increasing distance from the Equator [37].
3. Sun Exposure and Photoprotection in Relation to Vitamin D Production in South Africa
The Cancer Association of South Africa produced a Vitamin D Sun Exposure Statement in 2014 [42]. While this document details dietary sources of vitamin D and mentions links between vitamin D deficiency and certain diseases, it does not provide guidance on how much time to spend in the sun to produce sufficient vitamin D in South Africa, and does not distinguish between people with fair and darkly pigmented skin. In fact, there is no scientifically validated and agreed safe threshold level of sun exposure that allows for optimal vitamin D synthesis without increasing the risk of harm. Chronic unprotected personal exposure to solar UVR and excess exposure leading to sunburn are associated with several adverse health effects, including skin cancer and cataract [43]. Personal photoprotection, such as broad-brimmed hats, clothing, shade and sunscreen, is recommended by the World Health Organization during peak solar UVR hours around midday, and at any time when the UV Index is ≥3 [44]. A combination of these methods reduces over-exposure effectively. Studies carried out predominantly in people with fair skin living in Australia, USA and Europe indicate that sunscreen usage does not affect 25(OH)D levels significantly [45], although a small decrease occurs if they are applied at frequent intervals and at the correct thickness, neither of which are common practice [46].
A systematic search using PubMed with the terms “sun exposure/sun protection” AND “South Africa” AND “vitamin D” identified 18 articles by title. After excluding case and clinical management reports and reviews, and consideration of the full papers, the number was reduced to nine. One study in Durban (latitude 30° S) showed that children, aged 6 years, received about 5% of the total daily ambient solar UVR [47], similar to that received by children in Australia [48] and England [49]. A nationwide study among primary schoolchildren in South Africa suggested that about half of the children ever wore a hat and/or used sunscreen [50]. Even amongst those at high risk of sunburn and skin cancer, as is the case for individuals with oculocutaneous albinism, personal photoprotection is frequently minimal [51,52].
As already indicated, several reports based in South Africa demonstrate a major role of solar UVR in determining vitamin D status [28,33,36,38,39]. Daily sunshine hours by season were noted in a study of the vitamin D status of children in Johannesburg but were not analysed in relation to 25(OH)D level [28]. One in vitro study showed that exposure of vials containing 7-dehydrocholesterol to sunlight for one hour at different times on one day in each month of the year resulted in the same quantity of vitamin D being produced in Johannesburg throughout the year, but the quantity was reduced to about one-third during the winter months in Cape Town compared with the summer months at this location [15]. It was suggested that the decrease in the vitamin D status of people living in Johannesburg in the winter months may be due to the drop in temperature, and consequently increased clothing and decreased time spent outdoors, rather than to the reduction in ambient solar UVR. Two studies, one in Johannesburg and the other in Cape Town, have quantified the participants’ recollection of daily sun exposure during the past week using a questionnaire, and then related this to their 25(OH)D level [36,38]. Daily sun exposure scores, based on duration of time spent outdoors and amount of skin exposed, were positive predictors of 25(OH)D concentration among Black Africans in Johannesburg, and season and sun exposure explained 17% of the variance in 25(OH)D concentration [36]. Daily sun exposure scores did not predict 25(OH)D concentration among Asian/Indian participants who were mainly veiled females. In the Cape Town populations, personal sun exposure was the strongest determinant of 25(OH)D level, driven by the area of skin exposed and duration of exposure [38]. Black African participants spent about 4 h longer each week in the sun in both winter and summer than Coloured participants, and they exposed larger areas of their bodies in the summer, while both groups reduced their body exposure to similar levels in the winter [38].
4. Effect of Urbanisation and Diet on Vitamin D Status in South Africa
African countries are amongst the fastest urbanising in the world [53]. In South Africa, official government projections estimate that, between 2011 and 2016, the provinces of Gauteng and Western Cape will experience an inflow of 1.5 million migrants, many from the Eastern Cape and Limpopo [21]. Generally these people feel driven to move because of population growth and associated economic and social pressures. The situation has worsened over the past 25 years due to large-scale intensive farming at the cost of traditional small-scale subsistence-based production. With urbanisation, changes in lifestyle occur which may lead to reduced personal solar UVR exposure, with the potential to decrease vitamin D status. For example, office work is less conducive to time spent in the sun, and urban environments, such as tall buildings and narrow streets, may limit direct sun exposure. As urbanization frequently leads to changes in the diet which may, in turn, affect vitamin D status, these two aspects are considered in this section.
A systematic search was conducted using PubMed for papers published in English, with no limit on years and with the terms “vitamin D” AND “South Africa” AND “rural” OR “urbanisation” OR “semi-rural”. By title 165 articles were found. After excluding duplicates and studies that were not about South Africa or vitamin D, 50 papers remained which were reduced to 15 after reading the abstracts. These provided relevant information on the diet of South African populations, defined as living in urban, semi-rural or rural settings. Papers were excluded if they focused on vitamin D in relation to a disease outcome, if they did not present original data, or if they did not specifically present results for vitamin D intakes or serum 25(OH)D levels.
Only two investigations compared 25(OH)D levels in rural and urban populations in South Africa. In a study of Black African women in the North West Province in 2011, Kruger et al. [32] found that serum 25(OH)D levels were lower in urban women aged between 50 and 70 years (urban: 63 nmol/L; rural: 71 and 75 nmol/L, depending on age decade), but similar between urban- and rural-living women <50 years and >70 years. More than 30 years earlier, Pettifor et al. [24] analysed 25(OH)D levels in 60 black children living in rural, small urban and large urban communities near Johannesburg. The mean values were 72.3, 77.3 and 82.8 nmol/L respectively, thus indicating a small increase in vitamin D status with urbanisation. However it should be noted that the sample size was small, the assay may have given different results from the assays currently employed, the standard deviations were large and, as the children were of primary school age, they might spend considerable time outdoors, even in an urban environment.
Dietary intake of vitamin D is low in African countries in both urban and rural environments, and the contribution of 25(OH)D2 to the overall 25(OH)D status is minor [36,37]. In South Africa, many margarines are fortified with vitamin D (one brand contains 9200 IU/Kg) but other foods are not, and few foods available to the majority of the population contain significant amounts of vitamin D. In rural KwaZulu-Natal, the mean dietary vitamin D intake was considerably less than the dietary recommended intake of 15 μg/day (600 IU/day) [54,55]. Although the diets of urban Black African women in the north-west Provinces of South Africa were healthier than those of rural women, all of the participants had low vitamin D intakes (means from 1.79 to 3.28 μg/day), and urban women were more likely to be obese [32]. Elderly Black African people in a peri-urban environment near Cape Town also had low average vitamin D intake [56], as did healthy Black African and Coloured 18–24 year olds in the same region [38]. Low vitamin D intake was consistently found in both Black African (median 2.96 µg/day) and Asian-Indian (median 1.17 µg/day) adults in the Johannesburg area, with a marked difference in the prevalence of vitamin D supplementation use: 1.9% of Black African adults and 14.9% of Asian-Indian adults [36].
Low intake of vitamin D and calcium have been hypothesised as a cause of the high prevalence of stunting in children aged 2–5 years (36.9% had stunted growth) in a semi-urban impoverished setting in the Hantam district of the Northern Cape Province [57]. Similar low vitamin D intake was reported amongst 5-year old children living in the urban Johannesburg/Soweto area [58].
With such limited information, it is not possible currently to determine whether urbanisation with or without associated changes in the diet leads to an overall increase or decrease in the vitamin D status of an individual in South Africa. In addition the definition of what constitutes urban and rural may differ between the studies described above.
5. Association of Vitamin D Status with Diseases in South Africa
One of the major research interests in vitamin D at the present time lies in investigating the possible association of low 25(OH)D levels with various diseases. This aspect is considered in detail below in the context of South Africa, and is of particular relevance to the infectious diseases commonly found in this country. A systematic search was conducted using PubMed, covering 1973 to mid-2015, with the terms “vitamin D” AND “South Africa” AND “(disease or health or infection)”. By title, 117 papers were found; this was reduced to 25 after reading the abstracts, and to 22 after reading the full reports. Papers were excluded for six reasons: a review or commentary; non-South African population; no in vivo data; no disease association; not human; 25(OH)D not measured. Details of each study are shown in Table 3 and their geographic location in Figure 1. It is notable that 15 of these papers have been published since 2011, reflecting the recent rise of interest in vitamin D and health outcomes. The most common diseases studied were bone pathologies and infectious disease (ID), each representing 9 of the 22 reports, with one investigating bone growth and vitamin D status in HIV-1-infected women [62]. The threshold for vitamin D sufficiency and deficiency as defined in each of these articles was used as it was not possible to re-calculate statistical significance using one standard value from the data presented.
Table 3.
Vitamin D deficiency and disease associations in South African populations, by disease, ethnicity, location, age and gender.
| Category | Disease/Study Groups | Assoc 1 | Study Type | Location | Population | Sample Size | Age Group | Gender | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Bone | Fractures | Yes | longitudinal | Gauteng | NA | 20, 20 | 6–29 y | M & F | [59] |
| Bone | Rickets | Yes | cross-sectional | KwaZulu-Natal | Black African | 37 | 1–12 y | M & F | [60] |
| Bone | Bone mineral density | Yes | cross-sectional | North-West | Black African | 658 | >45 y | F | [32] |
| Bone | Growth stunting | Yes | cross-sectional | Northern Cape | NA | 150 | 2–5 y | M & F | [57] |
| Bone | Rickets | No | cross-sectional | Gauteng | Black African | 114 | <2 y | M & F | [25] |
| Bone | Metabolic bone disease | No | cross-sectional | Gauteng | Black African | 26 | 16–19 y | M | [61] |
| Bone | Bone mineral density | No | cross-sectional | Gauteng | Black & Asian-Indian | 371, 343 | 18–65 y | M & F | [35] |
| Bone | Under weight vs. normal weight | No | case-control | Limpopo | Black African | 145 | 3–5 y | M & F | [22] |
| Bone/ID | BMD in HIV uninfected vs. HIV high CD4 vs. HIV low CD4 count | No | case-control | Gauteng | Black African | 98, 74, 75 | ≥18 y | F | [62] |
| ID | Schistosomiasis (PZQ vs. PZQ + vitamin D vs. vitamin D vs. placebo) | Yes 2 | RCT | Mozambique border | NA | 14, 16, 14, 15 | 14–18 y | M | [63] |
| ID | TB-Meningitis | Yes 3 | case-control | Western Cape | Black & Coloured | 42, 147 | 0–13 y | M & F | [64] |
| ID | HIV replication (Summer vs. winter vs. winter + vitamin D) | Yes | longitudinal | Western Cape | Black African | 30 | 18–24 y | M & F | [38] |
| ID | TB HIV (TB vs. HIV vs. TB-HIV vs. OD) | Yes | case-control | Western Cape | Black African | 93, 75, 99, 103 | ≥18 y | M & F | [65] |
| ID | HIV-Cryptococcal Meningitis vs. HIV | No 4 | case-control | Western Cape | NA | 150, 150 | ≥21 y | M & F | [66] |
| ID | HIV ART initiation | NA 5 | cross-sectional | Gauteng/KWN | NA | 270 | ≥18 y | M & F | [67] |
| ID | HIV ART initiation | NA 5 | cross-sectional | Gauteng/KWN | NA | 270 | ≥18 y | M & F | [68] |
| ID | Paradoxical TB-HIV IRIS vs TB-HIV no IRIS | No | case-control | KwaZulu-Natal | Black African | 11, 11 | 24–50 y | M & F | [69] |
| ID | ART-associated TB vs HIV+TB- | No | case-control | KwaZulu-Natal | Black African | 18, 38 | 23–57 y | M & F | [69] |
| NCD | Cardiovascular disease (blood pressure and pulse) | Yes | cross-sectional | North-West | Black African | 291 | >47 y | F | [34] |
| NCD | Metabolic syndrome | No | cross-sectional | Gauteng | Black & Asian-Indian | 374, 350 | 18–65 y | M & F | [33] |
| NCD | Obesity (total body fat, fat distribution) | No | cross-sectional | Gauteng | Black & Asian-Indian | 371, 343 | 18–65 y | M & F | [36] |
| Nutrition | Alzheimer’s zinc deficiency (Zn vs. Zn ± vitamin A ± vitamin D) | Yes 6 | RCT | Western Cape | NA | 70/group | 55 y | M | [70] |
| Nutrition | Alcohol use disorders vs. matched controls | Yes | cross-sectional | Western Cape | Mixed ancestry | 81, 81 | 12–16 y | M & F | [71] |
Assoc., association; ART, antiretroviral therapy; BMD, bone mineral density; C:C, case vs. control; CVD, cardiovascular disease; F, female; HIV, human immunodeficiency virus; ID, infectious disease; IRIS, immune reconstitution inflammatory disease; M, male; NA, not available; NCD, Non communicable disease; OD, other diseases; PZQ, praziquantel; RCT, randomised controlled trial; TB, tuberculosis. Bold indicates significant association. y, year; 1 Disease prevalence or disease-associated functional response negatively correlated with serum 25(OH)D levels or positively with vitamin D deficiency. 2 Vitamin D supplementation improved lymphocyte and eosinophil function in schistosomiasis-infected individuals, disease association not investigated. 3 Winter sunlight hours associated with TB-meningitis. 4 74% deficiency in HIV-meningitis patients, but no difference to HIV-only. 5 Multinational cohort (n = 30 of 270 from South Africa).HIV disease association not assessed (40%–50% deficient); initiation of Efavirenz containing ART regimes significantly decreased 25(OH)D levels. 6 Improved plasma zinc levels in combination Zn + vitamin A + vitamin D.
Of the 22 studies, 11 found a significant inverse association between 25(OH)D level and disease presentation, severity or pathogenesis (Table 3). It should be noted that, in four of the other 11 studies that did not find association, the comparison was between HIV-1-associated disease states, such as HIV-1 vs. HIV-1-cryptococcal meningitis [63], rather than between disease vs. healthy control, and vitamin D deficiency was present in 42%–74% of the HIV-1 infected persons. The significant inverse correlation between disease presentation and 25(OH)D levels was more prevalent in studies conducted in southern and western provinces where, due to the higher latitude in the south and lower altitude in the south and west compared with the central/northern Highveld, there is less solar UV-B radiation in the winter months, and thus the likelihood of seasonal deficiency is greater (Figure 1). The association between disease and seasonal sunlight hours was highlighted in three studies, two indicating lower incidences of TB and TB meningitis following the summer increase in solar UVB radiation [64,65]. The third showed a decrease in HIV-1 replication in peripheral blood mononuclear cells (PBMCs), collected in the summer and infected ex vivo in autologous serum, compared with HIV-1 replication in PBMCs from the same individual collected in the winter [38].
Aside from the accepted link between vitamin D deficiency and bone mineralisation, the recent focus of vitamin D research in South Africa on HIV-1 and TB reflects the burden of these chronic diseases in this country. Seasonal vitamin D deficiency was significantly associated with disease or HIV-1 replication in three studies from the Western Cape [38,64,65], with vitamin D deficiency more severe in patients co-infected with HIV-1 and Mycobacterium tuberculosis [64]. Three multinational cohort studies which included participants from Gauteng and/or KwaZulu-Natal also found vitamin D deficiency in approximately 40%–50% of HIV-1-infected patients, but there was no control comparison group so that disease association could not be assessed [67,68,69]. Participants from the PEARLS (Prospective Evaluation of Antiretrovirals in Resource Limited Settings) trial were used in two of these studies. At 24 weeks after initiation of antiretroviral therapy (ART), patients receiving efavirenz-containing regimes had significantly decreased serum 25(OH)D concentration, with the greatest decrease occurring in those with higher baseline 25(OH)D levels. Notably ART did not improve micronutrient deficiency in HIV-1-infected persons [67,68].
Vitamin D deficiency may be the result of both HIV-1 infection and loss of HIV-1 killing mechanisms with a further decrease in CD4+ T cells [72]. The HIV-1 viral envelope protein gp120 induces CYP27B1 expression [73], the enzyme which metabolises 25(OH)D, indicating that infection may contribute to decreased circulating 25(OH)D and vitamin D deficiency. There is also a variety of immune pathways regulated by vitamin D, leading to inhibition of HIV replication [74,75,76]. As well as this antimicrobial activity, vitamin D can also reduce HIV replication by affecting the production of factors, such as NF-kB, which are required for transcription of the long terminal repeat of HIV-1 [77,78,79].
The nine studies on bone health included six investigating rickets, stunting or metabolic bone disease in children and adolescents in the Northern and Eastern Provinces [22,25,57,59,61]. Three found significant association between low vitamin D intake and disease, or clinical improvement following three months of vitamin D supplementation (5000 IU/day) [25,57,59]. In the three that did not detect association, there was a high prevalence of vitamin D deficiency in the children investigated, particularly in those hospitalised. It is possible that the duration of deficiency could be an important determinant of disease development [25].
Bone mineral density (BMD) and vitamin D status were also examined in two studies of older adults in Gauteng and North-West Province [32,35]. No association between 25(OH)D levels and BMD was demonstrated in either report. However the concentration of parathyroid hormone (PTH), which upregulates 1,25(OH)2D production from 25(OH)D, thus increasing intestinal calcium absorption, was inversely correlated with BMD in Asian-Indian participants in Gauteng [35]. In Black African women in the North-West Province, 25(OH)D levels were inversely correlated with the concentrations of PTH and C-terminal telopeptide of Type 1 colleagen (CTX, a measure of bone resorption) in rural participants; urban participants had lower CTX levels which correlated with dietary calcium and PTH, the PTH in turn correlating with 25(OH)D. This suggests that urban women might be at risk of high bone resorption in response to calcium insufficiency and low vitamin D status [32].
Three of the 22 reports considered association between cardiovascular disease or obesity/metabolic syndrome and 25(OH)D levels. The incidences of cerebrovascular disease, heart disease, and type 2 diabetes mellitus (T2DM), representing the third, fourth and fifth highest cause of mortality in South Africa, after TB and influenza/pneumonia, are all increasing currently, indicating their importance to the health of the country [80]. Only one study investigated vitamin D status and indicators of cardiovascular disease, finding that women with low 25(OH)D levels had significantly higher systolic blood pressure [34]. Using the same cohort of adults in greater Johannesburg, elevated PTH, not low 25(OH)D, predicted metabolic syndrome, although 25(OH)D inversely correlated with PTH [33,34]. It should be noted that it is not possible to separate cause and effect from such cross-sectional studies, and there many be confounding by factors such as physical activity which may relate to both time in the sun and disease risk. Any link between vitamin D status and T2DM in South African populations has not been investigated thus far. In addition, although cancer causes 8.3% of all deaths in South Africa, no association with vitamin D status has been sought. This is despite some evidence supporting a role for vitamin D in the regulation of cell differentiation, angiogenesis and matrix remodelling, all key components of metastasis [81].
Other non-communicable outcomes investigated included the management of zinc deficiency in Alzheimer’s [59], and the association between alcohol use disorders and vitamin D status in adolescents [60]. The former study found that adjunct vitamin D supplementation together with zinc and vitamin A was more effective at increasing zinc levels, than zinc alone [70], suggesting an interaction between micronutrients. Chronic alcohol use in Western Cape adolescent 12–16 year olds was significantly associated with lower serum 25(OH)D and calcium levels and all the participants had inadequate dietary intakes of vitamin D [71].
6. Vitamin D Receptor Gene (VDR) Polymorphisms and Ethnicity in South Africa
The VDR, the gene encoding the vitamin D receptor protein (VDR), spans more than 100 kb on chromosome 12. Besides eight protein-coding exons (2–9), almost half of the VDR sequence serves regulation, including six, largely noncoding, exons (1a–1f). Four promoters and four enhancers regulate transcription from the VDR and alternative splicing delivers at least nine splice variants encoding different proteins. Having nine bona fide CpG islands (CGIs) and three miRNA recognition elements in its 3’ UTR, VDR is regulated in a complex fashion through genetics, epigenetics and environment [82]. VDR polymorphisms most commonly studied include FokI, BsmI, ApaI and TaqI, named after restriction enzyme cleaving sites. Their alleles are referred to as either letters or nucleotides, such as FokI (rs2228570, previously rs10735810, C/T or F/f [lower case indicating restriction site presence]), BsmI (rs1544410, A/G or A/a), and TaqI (rs731236, T/C or T/t).
Different ethnicities in South Africa have evolved at different geographical locations under diverse conditions. Selection factors established unique combinations of genetic and epigenetic variants for optimal function of genes and pathways, including that of vitamin D [83,84]. Recent lifestyles changes which lead to less exposure to solar UVR, together with DBP and VDR genetic variants with low affinity for 25(OH)D, the frequency of which differs between population groups, may contribute to disease susceptibility.
In the following section, information on the VDR variants found in the populations of South Africa was collated, with the aim of determining the function of such polymorphisms in relation to the risk of disease or an association with ethnicity. Reports on the association of VDR variants with ethnicity, disease or treatment response, VDR DNA methylation and VDR function in South Africans were identified using the PubMed search terms: VDR polymorphism AND South Africa AND (disease OR health OR infection OR infectious OR seasonal OR ethnicity OR pigmentation) OR (seasonal variation AND infectious disease AND polymorphism AND pigmentation AND nutrition). Of the eleven papers identified, seven were relevant experimental reports investigating VDR polymorphisms in South African populations (Table 4). Five related to disease [38,86,87,89,90], and two to ethnicity alone [85,88]. The first study on VDR single nucleotide polymorphisms (SNPs) in South African Whites, Blacks and Asian-Indians showed a significantly higher frequency of TaqI “TT” and ApaI “AA” genotypes in Blacks compared to Whites and Asian-Indians [85]. A case-control study in the Black Venda people living in Limpopo Province found no independent VDR SNP association with pulmonary TB, but protection by FokI-BsmI-ApaI-TaqI haplotype, “F-b-A-T”, proposed to encode a VDR variant with more effective transactivation of target genes [86]. In a South African Coloured population in the Western Cape, VDR polymorphisms were associated with time to conversion of sputum to smear negativity during chemotherapy of patients with pulmonary TB, but there was no association with susceptibility to TB [87]. A second study of the Venda revealed a complex association of TaqI with the methylation status of the 3’ region CGI 1060 of the VDR, in which site-specific methylation distinguished ethnicity and TB status [88]. In South African Black children, the minor “T” (f) allele of FokI correlated with susceptibility to respiratory syncytial virus infection [89], as similarly reported in Dutch children [91]. A recent study of Black African and Coloured adults in Cape Town found FokI “AG” associated with lower serum 25(OH)D levels, as well as a lower 25(OH)D status in winter and following supplementation with vitamin D [38]. The impact of FokI on VDR function was examined using monocytes from healthy South African Blacks and Whites [90]. Despite having higher levels of more active VDR, monocytes from Blacks showed reduced transactivation of the gene encoding the cathelicidin antimicrobial protein (CAMP), compared to Whites, unless supplemented in vitro with 1,25(OH)2D3. Thus the association of VDR polymorphisms with disease in the population groups of South Africa appears to be ethnicity-dependent, involving complex interactions between genetics, epigenetics and the environment.
Table 4.
Vitamin D receptor gene (VDR) variants in South Africans, associated with ethnicity, disease, treatment response, DNA methylation, VDR expression, vitamin D receptor protein (VDR) level or VDR transactivation of target genes.
| SNP (rs) | Study Population; Gender when Specified | Factor Investigated | Study Design | Median/Mean Age, Years (Range) | Association | Reference |
|---|---|---|---|---|---|---|
|
FokI (rs2544037) (rs10783219) (rs10735810) TaqI (rs731236) |
22 male, 28 female Cape mixed; 26 male, 24 female Black Africans (Zhosa) | Determinants of 25(OH)D status, before and after vitamin D supplementation | Longitudinal | (18–24) | FokI (rs10735810) “AG” contributed to lower 25(OH)D, and lower 25(OH)D in winter and post-supplementation, while “AA” contributed to higher 25(OH)D, in a linear model incorporating UVB exposure, skin pigmentation, ethnicity, age and sex | [38] |
|
ApaI (rs7975232) TaqI (rs731236) |
264 Black Africans, 247 Whites, 194 Asian-Indians | Ethnicity | Retrospective cross-sectional cohort of healthy male and female blood donors | NA | Higher ApaI “AA” and TaqI “TT” in Blacks than Whites or Asian-Indians; no difference between Whites and Asian-Indians. Higher frequency of TaqI “T” may contribute to lower incidence of osteoporosis in Blacks | [85] |
|
FokI (rs2228570) BsmI (rs1544410) ApaI (rs7975232) TaqI (rs731236) |
95 Black Africans (Venda) with TB, 117 ethnicity matched controls | TB | Case-control | NA | No independent SNP association; FokI-BsmI-ApaI-TaqI haplotype “F-b-A-T” (C-G-T-T) protects | [86] |
|
FokI (rs2228570) ApaI (rs7975232) TaqI (rs731236) |
All Colored: 249 TB cases, 352 ethnicity matched controls, 220 TB cases converting from positive to negative |
TB and chemotherapy for TB | Case-control for TB; longitudinal for TB conversion | (18–65) | No association of VDR allele, genotype, haplotype or diplotype with TB. Quicker response to TB chemotherapy in ApaI “AA” (TT) vs. “aa” (GG), in TaqI “Tt” (TC) vs. “tt” (CC) and in TaqI “TT” (TT) vs. “tt” (CC) | [87] |
|
ApaI (rs7975232) TaqI (rs731236) |
15 male, 15 female CAU (North America); 15 male, 15 female YRI (Nigeria); 16 male, 16 female Black Africans (Venda) with TB together with 12 male and 17 female healthy Black African controls | Ethnicity, TB, and DNA methylation of the VDR 3’ region CGI 1060 | Ethnicity; case-control for TB | CAU 32 (22–44), YRI unknown, TB cases 38 (18–62) TB controls 34 (21–62) |
Methylation variable positions in 3’ end of VDR distinguish ethnicity and TB status. Higher regional methylation in TaqI “TC/CC” than “TT” in YRI and Venda, but not in CAU | [88] |
| FokI (rs10735810 merged into rs2228570) | 296 Black Africans with RSV, 113 Black African controls | RSV-related disease | Case-control | Cases 3.0 months, controls 3.5 months |
FokI “C” frequency higher in Black South Africans than European, Asian and Japanese populations. “CT” and “T” predispose to RSV disease, while “CC” protects | [89] |
| FokI (rs2228570) | 40 healthy Black Africans, 20 healthy Whites | VDR expression and VDR function, considering ethnicity and 25(OH)D levels | Cross-sectional cohort of male and female blood donors | 35 (17–65) | Higher frequency FokI “CC” genotype and VDR protein in Blacks than Whites, but less VDR mRNA and CAMP mRNA. CAMP mRNA higher in FokI “CT/TT” genotypes than “CC”. Circulating 25(OH)D levels ≥50 nmol/L, and comparable between Blacks and Whites. Different effects on CAMP and CYP24A1 expression in Whites and Blacks on supplementation with 1,25(OH)2D | [90] |
1,25(OH)2D: 1,25-Dihydroxyvitamin D; 25(OH)D: 25-Hydroxyvitamin D; CAMP: Gene coding cathelicidin antimicrobial protein; CAU: Caucasian; CGI: CpG island; CYP24A1: Gene coding cytochrome P450 family 24 subfamily A member 1; NA: Not available; RSV: Respiratory syncytial virus; SNP (rs): Single nucleotide polymorphism (reference SNP cluster ID to access a specific SNP in the dbSNP database); TB: Tuberculosis; UVB: Ultraviolet B radiation; YRI: Yoruba (African ancestry, Ibadan, Nigeria).
7. Conclusions
A systematic search of the published literature provided limited but important information relating to the vitamin D status of the South African population. The study protocols were varied, involving males and females of different ages, ethnicities, skin colours and places of residence, together with a range of methods to measure 25(OH)D levels. Despite these considerable shortcomings, 25(OH)D levels were generally found to be sufficient (>50 nmol/L) in most healthy people, although they were frequently insufficient in Asian/Indians and in the elderly (>65 years) and it should be noted that little data are available for those living at the higher southern latitudes of the country, especially during the winter months. The few studies investigating whether urbanisation might lead to an increase or decrease in vitamin D status have yielded contradictory results. A combination of factors is involved here: for example, less exposure to the sun might be predicted in urban compared with rural environments with a consequent reduction in 25(OH)D levels, but on the other hand, urbanisation might lead to a higher standard of living with the consumption of more foods containing vitamin D and its metabolites. A low dietary intake of vitamin D occurs throughout South Africa, emphasising the importance of exposure to the sun in determining vitamin D status. The use of vitamin D supplements amongst South Africans is not common.
Reports investigating vitamin D status in relation to disease, have yielded inconsistent results, with half finding a relationship between 25(OH)D deficiency and disease or its severity, and half finding no such association. The majority of the studies falling into the latter category were in the North of the country where the mean 25(OH)D levels tended to be higher compared with the South (Figure 1, Table 3). Most studies were cross-sectional or case-control so that establishing the temporal relationships and thus revealing whether low 25(OH)D levels are a cause, consequence or proxy for a “true” risk factor is not possible. Similarly, the sample sizes tended to be small, especially in the earlier reports, and were underpowered to detect an effect on disease risk. Furthermore the population groups and ages varied, and the disorders covered a very wide range, with bone pathologies and IDs being most common. It is of interest that 1,25(OH)2D interacts with cells of the immune system including monocytes/macrophages, dendritic cells, B and T cells, leading to modulation of many inflammatory responses through various signalling pathways [91]. Inflammation and infection can associate with low 25(OH)D levels, and linkage studies may identify consequence rather than cause. Whether vitamin D deficiency is causal to disease or not, the significant burden of deficiency in individuals with a variety of disorders in South Africa suggests that vitamin D supplements should be part of the therapy, at least to maintain bone health but also possibly to enhance recovery and disease resolution. In addition it is becoming clear that not all the immunomodulatory effects of exposure to solar UVR are due to vitamin D [92]. Further work in this area is merited, particularly concentrating on the disorders that present major health burdens in South Africa currently, such as HIV, TB, cardiovascular diseases, T2DM and various cancers.
It is not possible at the present time to give accurate advice to the general public, based on scientific findings, regarding how much sun exposure is optimal for health. There are many environmental variables to take into consideration, such as the latitude of residence, season, time of day, cloud cover and surface reflectivity. In addition variations in personal factors, including skin pigmentation and the extent of clothing, require that the health messages are adapted to make them relevant for each ethnic group. Given the climate of South Africa, there should be ample opportunity to attain vitamin D sufficiency throughout the year for those living in the northern Provinces and in all but the winter months for those living in the southern Provinces when there is limited UVB in sunlight. Our review shows that some populations are at increased risk of vitamin D deficiency. These include the elderly, those in residential care, Asian-Indian women who tend to be fully clothed, those with deeply pigmented skin, and people infected with HIV and M. tuberculosis. It is possible that the obese and workers employed underground or on night shifts may also be at risk of deficiency. All, or at least some, of these groups may benefit from vitamin D supplementation.
Acknowledgments
Anna K. Coussens is supported by the Academy of Science of South Africa (Sydney Brenner Fellowship), Medical Research Council of South Africa-SHIP-02-2013; Robert J. Wilkinson by the Wellcome Trust Grants 084323 and 104893, Francis Crick Institute 10218, Medical Research Council of South Africa, National Research Foundation of South Africa 96841 and US National Institutes of Health U19AI111276; Liza Bornman by the Cancer Association of South Africa and the National Research Foundation of South Africa, Robyn M. Lucas by an Australian National Health and Medical Research Council Senior Research Fellowship 1107343; and Caradee Y. Wright by the Medical Research Council of South Africa and the National Research Foundation of South Africa.
Author Contributions
The initial drafts of the sections on vitamin D status, diet/urbanisation, disease association, VDR polymorphisms, and sun exposure/photoprotection were written by Mary Norval, Robyn M. Lucas, Anna K. Coussens and Robert J. Wilkinson, Liza Bornman, and Caradee Y. Wright respectively. All the authors contributed comments on all sections and their revisions. Mary Norval had primary responsibility for the final content.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Holick M.F. Sunlight, UV-radiation, vitamin D and skin cancer. Adv. Exp. Med. Biol. 2008;624:1–15. doi: 10.1007/978-0-387-77574-6_1. [DOI] [PubMed] [Google Scholar]
- 2.Wimalawansa S.J. Non-musculoskeletal benefits of vitamin D. J. Steroid Biochem. Mol. Biol. 2016 doi: 10.1016/j.jsbmb.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Hossein-nezhad A., Holick M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013;88:720–755. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahl D.A., Cooper C., Ebeling P.R., Eggersdorfer M., Hilger J., Hoffmann K., Josse R., Kasis J.A., Mithal A., Pierroz D.D., et al. A global representation of vitamin D status in healthy populations. Arch. Osteoporos. 2012;7:155–172. doi: 10.1007/s11657-012-0093-0. [DOI] [PubMed] [Google Scholar]
- 5.Hilger J., Friedel A., Herr R., Rausch T., Roos F., Wahl D.A., Pierros D.D., Weber P., Hoffmann K. A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 2014;111:23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 6.Holick M.F. Sunlight “D”ilemma: Risk of skin cancer or bone disease and muscle weakness. Lancet. 2001;357:4–6. doi: 10.1016/S0140-6736(00)03560-1. [DOI] [PubMed] [Google Scholar]
- 7.Hewison M., Zehnder D., Chakraverty R., Adams J.S. Vitamin D and barrier function: A novel role for extra-renal 1 alpha-hydroxylase. Mol. Cell. Endocrinol. 2004;215:31–38. doi: 10.1016/j.mce.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Wang T.T., Tavera-Mendoza L.C., Laperriere D., Libby E., MacLeod N.B., Nagal Y., Bourdeau V., Konstorum A., Lallemant B., Zhang R., et al. Large-scale in silico and microarrany-based indentification of direct 1,25-dihydroxyvitamin D3 target genes. Mol. Endocrinol. 2005;19:2685–2695. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz J.B., Lai J., Lizaola B., Kane L., Markova S., Weyland P., Terrault N.A., Stotland N., Bikle D. A comparison of measured and calculated free 25(OH)D levels in clinical populations. J. Clin. Endocrinol. Metab. 2014;99:1631–1637. doi: 10.1210/jc.2013-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium . Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; Washington, DC, USA: 2011. [PubMed] [Google Scholar]
- 11.Bischoff-Ferrari H.A., Giovannucci E., Willett W.C., Dietrich T., Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 12.Holick M.F., Binkley N.C., Bischoff-Ferrari A., Girdin C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 13.Carter G.D., Carter R., Jones J., Berry J. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin. Chem. 2004;50:2195–2197. doi: 10.1373/clinchem.2004.040683. [DOI] [PubMed] [Google Scholar]
- 14.Sempos C.T., Vesper H.W., Phinney K.W., Thienpont L.M., Coates P.M. Vitamin D status as an international issue: National surveys and the problem of standardization. Scand. J. Lab. Investig. Suppl. 2012;243:32–40. doi: 10.3109/00365513.2012.681935. [DOI] [PubMed] [Google Scholar]
- 15.Pettifor J.M., Moodley G.P., Hough F.S., Koch H., Chen T., Lu Z., Holick M.F. The effect of season and latitude on in vitro vitamin D formation by sunlight in South Africa. S. Afr. Med. J. 1996;86:1270–1272. [PubMed] [Google Scholar]
- 16.Weather 2 Travel. [(accessed on 14 October 2016)]. Available online: www.weather2travel.com.
- 17.World Weather Online. [(accessed on 14 October 2016)]. Available online: www.worldweatheronline.com.
- 18.Weather South African. [(accessed on 14 October 2016)]. Available online: www.weathersa.co.za.
- 19.Global Security. [(accessed on 5 August 2016)]. Available online: www.globalsecurity.org.
- 20.Wilkes M., Wright C.Y., du Plessis A., Reeder A. Fitzpatrick skin type, individual topology angle, and melanin index in an African population: Steps towards universally applicable skin photosensitivity assessments. JAMA Dermatol. 2015;151:902–903. doi: 10.1001/jamadermatol.2015.0351. [DOI] [PubMed] [Google Scholar]
- 21.Statistics South Africa. [(accessed on 14 October 2016)]; Available online: http://www.statssa.gov.za/?page_id=1854&PPN=P0302&SCH=6334.
- 22.Palacios C., Gonzalez L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014;144:138–145. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pettifor J.M., Ross F.P., Moodley G., Wang J., Margo G., Skjolde C. Serum calcium, magnesium, phosphorus, alkaline phosphatase and 25-hydroxyvitamin D concentrations in children. S. Afr. Med. J. 1978;53:751–754. [PubMed] [Google Scholar]
- 24.Pettifor J.M., Ross P., Moodley G., Shuenyane E. Calcium deficiency in rural black children in South Africa—A comparison between rural and urban communities. Am. J. Clin. Nutr. 1979;32:2477–2483. doi: 10.1093/ajcn/32.12.2477. [DOI] [PubMed] [Google Scholar]
- 25.Sochett E.B., Pettifor J.M., Moodley G., Pentopoulos M. Vitamin D status in hospitalized black children under 2 years of age. S. Afr. Med. J. 1985;67:1041–1044. [PubMed] [Google Scholar]
- 26.Van der Westhuyzen J. Biochemical evaluation of black preschool children in the northern Transvaal. S. Afr. Med. J. 1986;70:146–148. [PubMed] [Google Scholar]
- 27.Cornish D.A., Maluleke V., Mhlanga T. An investigation into a possible relationship between vitamin D, parathyroid hormone, calcium and magnesium in normally pigmented and an albino rural black population in the Northern Province of South Africa. Biofactors. 2000;11:35–38. doi: 10.1002/biof.5520110110. [DOI] [PubMed] [Google Scholar]
- 28.Poopedi M.A., Norris S.A., Pettifor J.M. Factors influencing the vitamin D status of 10-year-old urban South African children. Public Health Nutr. 2011;14:334–339. doi: 10.1017/S136898001000234X. [DOI] [PubMed] [Google Scholar]
- 29.Fairney A., Sloan M.A., Patel K.V., Coumbe A. Vitamin A and D status of black South African women and their babies. Hum. Nutr. Clin. Nutr. 1987;41:81–87. [PubMed] [Google Scholar]
- 30.Daniels E.D., Pettifor J.M., Schnitzlet C.M., Moodley G.P., Zachen D. Differences in mineral homeostasis, volumetric bone mass and femoral neck axis length in black and white South African women. Osteoporos. Int. 1997;7:105–112. doi: 10.1007/BF01623684. [DOI] [PubMed] [Google Scholar]
- 31.Haarburger D., Hoffman M., Erasmus R.T., Pillay T.S. Relationship between vitamin D, calcium and parathyroid hormone in Cape Town. J. Clin. Pathol. 2009;26:567–569. doi: 10.1136/jcp.2008.062877. [DOI] [PubMed] [Google Scholar]
- 32.Kruger M.C., Kruger I.M., Wentzel-Viljoen E., Kruger A. Urbanization of black South African women may increase risk of low bone mass due to low vitamin D status, low calcium intake, and high bone turnover. Nutr. Res. 2011;31:748–758. doi: 10.1016/j.nutres.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 33.George J.A., Norris S.A., van Deventer H.E., Crowther N.J. The association of 25 hydroxyvitamin D and parathyroid hormone with metabolic syndrome in two ethnic groups in South Africa. PLoS ONE. 2013;8:1019. doi: 10.1371/journal.pone.0061282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruger I.M., Kruger M.C., Doak C.M., Schutte A.E., Huisman H.W., Van Rooyen J.M., Schutte R., Malan L., Malan N.T., Fourie C.M., et al. The association of 25(OH)D with blood pressure, pulse pressure and carotid-radial pulse wave velocity in African women. PLoS ONE. 2013;8:1019. doi: 10.1371/journal.pone.0054554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George J.A., Micklesfield L.K., Norris S.A., Crowther N.J. The association between body composition, 25(OH)D, and PTH and bone mineral density in black African and Asian Indian population groups. J. Clin. Endocrinol. Metab. 2014;99:2146–2154. doi: 10.1210/jc.2013-3968. [DOI] [PubMed] [Google Scholar]
- 36.George J.A., Norris S.A., van Deventer H.E., Pettifor J.M., Crowther N.J. Effect of adiposity, season, diet and calcium or vitamin D supplementation on the vitamin D status of healthy urban African and Asian-Indian adults. Br. J. Nutr. 2014;112:590–599. doi: 10.1017/S0007114514001202. [DOI] [PubMed] [Google Scholar]
- 37.Durazo-Arvizu R.A., Camacho P., Bovet P., Forrester T., Lambert E.V., Plage-Rhule J., Hoofnagle A.N., Aloia J., Tayo B., Dugas L.R., et al. 25-Hydroxyvitamin D in African-origin populations at varying latitudes challenges the construct of a physiologic norm. Am. J. Clin. Nutr. 2014;100:908–914. doi: 10.3945/ajcn.113.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coussens A.K., Naude C.E., Goloath R., Chaplin G., Wilkinson R.J., Jablonski N.G. High-dose vitamin D3 reduces deficiency caused by low UVB exposure and limits HIV-1 replication in urban Southern Africans. Proc. Natl. Acad. Sci. USA. 2015;112:8052–8057. doi: 10.1073/pnas.1500909112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pettifor J.M., Ross F.P., Solomon L. Seasonal variation in serum 25-hydroxycholecalciferol concentrations in elderly South African patients with fractures of femoral neck. Br. Med. J. 1978;1:826–827. doi: 10.1136/bmj.1.6116.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Papendorp D.H. The vitamin D status of South African women living in old-age homes. S. Afr. Med. J. 1990;78:556. [PubMed] [Google Scholar]
- 41.Charlton K.E., Labadarios D., Lombard C.J., Louw M.E. Vitamin D status of older South Africans. S. Afr. Med. J. 1996;86:1406–1410. [PubMed] [Google Scholar]
- 42.Cancer Association of South Africa (CANSA) Fact Sheet on Vitamin D. [(accessed on 14 October 2016)]. Available online: http://www.cansa.org.za/files/2015/10/Fact-Sheet-Vitamin-D-October-2015.pdf.
- 43.Lucas R.M., Norval M., Neale R.E., Young A.R., de Gruijl F.R., Takizawa Y., van der Leun J.C. The consequences for human health of stratospheric ozone depletion in association with other environmental factors. Photochem. Photobiol. Sci. 2015;14:53–87. doi: 10.1039/C4PP90033B. [DOI] [PubMed] [Google Scholar]
- 44.Ultraviolet Radiation and the INTERSUN Programme . Sun Protection. World Health Organization (WHO); Geneva, Switzerland: 2015. [Google Scholar]
- 45.Norval M., Wulf H.C. Does chronic sunscreen use reduce vitamin D production to insufficient levels? Br. J. Dermatol. 2009;161:732–736. doi: 10.1111/j.1365-2133.2009.09332.x. [DOI] [PubMed] [Google Scholar]
- 46.Petersen B., Thieden E., Philipsen P.A., Heydenreich J., Wulf H.C., Young A.R. Determinants of personal ultraviolet-radiation exposure doses on a sun holiday. Br. J. Dermatol. 2013;168:1073–1079. doi: 10.1111/bjd.12211. [DOI] [PubMed] [Google Scholar]
- 47.Guy C.Y., Diab R., Martincigh B. Ultraviolet radiation exposure of children and adolescents in Durban, South Africa. Photochem. Photobiol. 2003;77:265–270. doi: 10.1562/0031-8655(2003)077<0265:UREOCA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 48.Gies P., Roy C., Toomey S., MacLennan R., Watson M. Solar UVR exposures of primary school children at three locations in Queensland. Photochem. Photobiol. 1998;68:78–83. doi: 10.1111/j.1751-1097.1998.tb03255.x. [DOI] [PubMed] [Google Scholar]
- 49.Diffey B.L., Gibson C.J., Haylock R., McKinlay A.F. Outdoor ultraviolet exposure of children and adolescents. Br. J. Dermatol. 1996;124:1030–1034. doi: 10.1111/j.1365-2133.1996.tb07937.x. [DOI] [PubMed] [Google Scholar]
- 50.Wright C.Y., Albers P.N., Oosthuizen M.A., Phala N. Self-reported sun-related knowledge, attitudes and behaviours among schoolchildren attending South African primary schools. Photodermatol. Photoimmunol. Photomed. 2014;30:266–276. doi: 10.1111/phpp.12107. [DOI] [PubMed] [Google Scholar]
- 51.Lund P.M., Taylor J.S. Lack of adequate sun protection for children with oculocutaneous albinism in South Africa. BMC Public Health. 2008;8:1019. doi: 10.1186/1471-2458-8-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lund P.M., Gaigher R. A health intervention programme for children with albinism at a special school in South Africa. Health Educ. Res. 2002;17:365–372. doi: 10.1093/her/17.3.365. [DOI] [PubMed] [Google Scholar]
- 53.United Nations Human Settlements Programme (UN-Habitat) The State of African Cities 2014. UN-Habitat; Nairobi, Kenya: 2014. [Google Scholar]
- 54.Kolahdooz F., Spearing K., Sharma S. Dietary adequacies among South African adults in rural KwaZulu-Natal. PLoS ONE. 2013;8:1019. doi: 10.1371/journal.pone.0067184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spearing K., Kolahdooz F., Lukasewich M., Mathe N., Khamis T., Sharma S. Nutritional composition of commonly consumed composite dishes from rural villages in Empangeni, KwaZulu-Natal, South Africa. J. Hum. Nutr. Diet. 2013;26:222–229. doi: 10.1111/jhn.12001. [DOI] [PubMed] [Google Scholar]
- 56.Bourne L.T., Steyn K., Laubscher J.A. Poor nutritional status in older black South Africans. Asia Pac. J. Clin. Nutr. 2001;10:31–38. doi: 10.1046/j.1440-6047.2001.00195.x. [DOI] [PubMed] [Google Scholar]
- 57.Van Stuijvenberg M.E., Nel J., Schoeman S.E., Lombard C.J., du Plessis L.M., Dhansay M.A. Low intake of calcium and vitamin D, but not zinc, iron or vitamin A, is associated with stunting in 2- to 5-year-old children. Nutrition. 2015;31:841–846. doi: 10.1016/j.nut.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 58.MacKeown J.M., Cleaton-Jones P.E., Edwards A.W., Turgeon-O’Brien H. Energy, macro- and micronutrient intake of 5-year-old urban black South African children in 1984 and 1995. Paediatr. Perinat. Epidemiol. 1998;12:297–312. doi: 10.1046/j.1365-3016.1998.00128.x. [DOI] [PubMed] [Google Scholar]
- 59.Bischof F., Basu D., Pettifor J.M. Pathological long-bone fractures in residents with cerebral palsy in a long-term care facility in South Africa. Dev. Med. Child Neurol. 2002;44:119–122. doi: 10.1017/S0012162201001773. [DOI] [PubMed] [Google Scholar]
- 60.Bhimma R., Pettifor J.M., Coovadia H.M., Moodley M., Adhikari M. Rickets in black children beyond infancy in Natal. S. Afr. Med. J. 1995;85:668–672. [PubMed] [Google Scholar]
- 61.Schnitzler C.M., Pettifor J.M., Patel D., Mesquita J.M., Moodley G.P., Zachen D. Metabolic bone disease in black teenagers with genu valgum or varum without radiologic rickets: A bone histomorphometric study. J. Bone Miner. Res. 1994;9:479–486. doi: 10.1002/jbmr.5650090407. [DOI] [PubMed] [Google Scholar]
- 62.Hamill M.M., Ward K.A., Pettifor J.M., Norris S.A., Prentice A. Bone mass, body composition and vitamin D status of ARV-naive, urban, black South African women with HIV infection, stratified by CD4 count. Osteoporos. Int. 2013;24:2855–2861. doi: 10.1007/s00198-013-2373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snyman J.R., de Sommers K., Steinmann M.A., Lizamore D.J. Effects of calcitriol on eosinophil activity and antibody responses in patients with schistosomiasis. Eur. J. Clin. Pharmacol. 1997;52:277–280. doi: 10.1007/s002280050289. [DOI] [PubMed] [Google Scholar]
- 64.Visser D.H., Schoeman J.F., Van Furth A.M. Seasonal variation in the incidence rate of tuberculous meningitis is associated with sunshine hours. Epidemiol. Infect. 2013;141:459–462. doi: 10.1017/S0950268812001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martineau A.R., Nhamoyebonde S., Oni T., Rangaka M.X., Marais S., Bangani N., Tsekela R., Bash L., de Azevedo V., Caldwell J., et al. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proc. Natl. Acad. Sci. USA. 2011;108:19013–19017. doi: 10.1073/pnas.1111825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jarvis J.N., Bicanic T., Loyse A., Meintjes G., Hogan L., Roberts C.H., Shoham S., Perfect J.R., Govender N.P., Harrison T.S. Very low levels of 25-hydroxyvitamin D are not associated with immunologic changes or clinical outcome in South African patients with HIV-associated cryptococcal meningitis. Clin. Infect. Dis. 2014;59:493–500. doi: 10.1093/cid/ciu349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shivakoti R., Christian P., Yang W.T., Gupte N., Mwelase N., Kanyama C., Pillay S., Samaneka W., Santos B., Poongulali S., et al. Prevalence and risk factors of micronutrient deficiencies pre- and post-antiretroviral therapy (ART) among a diverse multicountry cohort of HIV-infected adults. Clin. Nutr. 2015;61:102–110. doi: 10.1016/j.clnu.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Havers F.P., Detrick B., Cardoso S.W., Bwewbdes S., Lama J.R., Sugandhavesa P., Mwelase N.H., Campbell T.B., Gupta A. Change in vitamin d levels occurs early after antiretroviral therapy initiation and depends on treatment regimen in resource-limited settings. PLoS ONE. 2014;9:1019. doi: 10.1371/journal.pone.0095164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Price P., Haddow L.J., Affandi J., Agerwal U., Easterbrook P.J., Elliott J., French M., Kumar M., Moosa M.Y., Oliver B., et al. Short communication: Plasma levels of vitamin D in HIV patients initiating antiretroviral therapy do not predict immune restoration disease associated with Mycobacterium tuberculosis. AIDS Res. Hum. Retrovir. 2012;28:216–219. doi: 10.1089/aid.2011.0272. [DOI] [PubMed] [Google Scholar]
- 70.Potocnik F.C., van Rensburg S.J., Hon D., Emsley R.A., Moodie I.M., Erasmus R.T. Oral zinc augmentation with vitamins A and D increases plasma zinc concentration: Implications for burden of disease. Metab. Brain Dis. 2006;21:139–147. doi: 10.1007/s11011-006-9023-4. [DOI] [PubMed] [Google Scholar]
- 71.Naude C.E., Carey P.D., Laubscher R., Fein G., Senekal M. Vitamin D and calcium status in South African adolescents with alcohol use disorders. Nutrients. 2012;4:1076–1094. doi: 10.3390/nu4081076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coussens A.K., Martineau A.R., Wilkinson R.J. Anti-inflammatory and antimicrobial actions of vitamin D in combating TB/HIV. Scientifica. 2014 doi: 10.1155/2014/903680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pinzone M.R., di Rosa M., Celesia B.M., Condorelli F., Malaguarnera M., Madeddu G. LPS and HIV gp120 modulate monocyte/macrophage CYP27B1 and CYP24A1 expression leading to vitamin D consumption and hypovitaminosis in HIV-infected individuals. Eur. Rev. Med. Pharmacol. Sci. 2013;17:1938–1950. [PubMed] [Google Scholar]
- 74.Campbell G.R., Spector S.A. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophage in human macrophages that inhibits HIV-1 infection. J. Biol. Chem. 2011;286:18890–18902. doi: 10.1074/jbc.M110.206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quinones-Mateu M.E., Lederman M.M., Feng Z., Chakraborty B., Weber J., Rangel H.R. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:39–48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 76.Feng Z., Dubyak G.R., Lederman M.M., Weinberg A. Cutting edge: Human beta-defensin 3—A novel antagonist of the HIV-coreceptor CXCR4. J. Immunol. 2006;177:782–786. doi: 10.4049/jimmunol.177.2.782. [DOI] [PubMed] [Google Scholar]
- 77.Alvarez-Diaz S., Valle N., Ferrer-Mayorga G., Lombardia L., Herrara M., Dominguez O. MicroRNA-22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Hum. Mol. Genet. 2012;21:2157–2165. doi: 10.1093/hmg/dds031. [DOI] [PubMed] [Google Scholar]
- 78.Ranijbar S., Jasenosky L.D., Chow N., Goldfield A.E. Regulation of Mycobacterium tuberculosis-dependent HIV-1 transcription reveals a new role for NFAT5 in the toll-like receptor pathway. PLoS Pathogens. 2012;8:1019. doi: 10.1371/journal.ppat.1002620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nunnari G., Fagone P., Lazzara F., Longo A., Cambria D., Di Stefano G. Vitamin D inhibitis TNFalpha-induced latent HIV reactivation in J-LAT cells. Mol. Cell. Biochem. 2016;418:49–57. doi: 10.1007/s11010-016-2732-z. [DOI] [PubMed] [Google Scholar]
- 80.Statistics South Africa . Mortality and Causes of Death in South Africa, 2011: Findings from Death Notification. Statistics South Africa; Pretoria, South Africa: 2014. [Google Scholar]
- 81.Feldman D., Krishnan A.V., Swami S., Giovannucci E., Feldman B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 82.Saccone D., Asani F., Bornman L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene. 2015;561:171–180. doi: 10.1016/j.gene.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 83.Creemers P.C., Du Toit E.D., Kriel J. DBP (vitamin D binding protein) and BF (properdin factor B) allele distribution in Namibian San and Khoi and in other South African populations. Gene Geogr. 1995;9:185–189. [PubMed] [Google Scholar]
- 84.Luxwolda M.F., Kuipers R.S., Kema I.P., van der Veer E., Dijck-Brouwer D.A., Muskiet F.A. Vitamin D status indicators in indigenous populations in East Africa. Eur. J. Nutr. 2013;52:1115–1125. doi: 10.1007/s00394-012-0421-6. [DOI] [PubMed] [Google Scholar]
- 85.Ojwang P.J., Pegoraro R.J., Rom L., Lanning P. Collagen Ialpha1 and vitamin D receptor gene polymorphisms in South African whites, blacks and Indians. East Afr. Med. J. 2001;78:604–607. doi: 10.4314/eamj.v78i11.8951. [DOI] [PubMed] [Google Scholar]
- 86.Lombard Z., Dalton D.-L., Venter P.A., Williams R.C., Bornman L. Association of HLA-DR, -DQ, and vitamin D receptor alleles and haplotypes with tuberculosis in the Venda of South Africa. Hum. Immunol. 2006;67:643–654. doi: 10.1016/j.humimm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Babb C., van der Merwe L., Beyers N., Pheiffer C., Walzl G., Duncan K., van Helden P., Hoal E.G. Vitamin D receptor gene polymorphisms and sputum conversion time in pulmonary tuberculosis patients. Tuberculosis. 2007;87:295–302. doi: 10.1016/j.tube.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Andraos C., Koorsen G., Knight J.C., Bornman L. Vitamin D receptor gene methylation is associated with ethnicity, tuberculosis, and TaqI polymorphism. Hum. Immunol. 2011;72:262–268. doi: 10.1016/j.humimm.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kresfelder T.L., Janssen R., Bont L., Pretorius M., Venter M. Confirmation of an association between single nucleotide polymorphisms in the VDR gene with respiratory syncytial virus related disease in South African children. J. Med. Virol. 2011;83:1834–1840. doi: 10.1002/jmv.22179. [DOI] [PubMed] [Google Scholar]
- 90.Neill V., Asani F.F., Jeffery T.J., Saccone D.S., Bornman L. Vitamin D receptor gene expression and function in a South African population: Ethnicity, vitamin D and FokI. PLoS ONE. 2013;8:1019. doi: 10.1371/journal.pone.0067663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janssen R., Bont L., Siezen C.L., Hodemaekers H.M., Ermers M.J., Doornbos G., van’t Slot R., Wijmenga C., Goeman J.J., Kimpen J.L., et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J. Infect. Dis. 2007;196:826–834. doi: 10.1086/520886. [DOI] [PubMed] [Google Scholar]
- 92.Hart P.H., Gorman S. Exposure to UV wavelengths in sunlight suppresses immunity. To what extent is UV-induced vitamin D3 the mediator responsible? Clin. Biochem. Rev. 2013;34:3–13. [PMC free article] [PubMed] [Google Scholar]