Abstract
Background: Increased dietary whole-grain intake may protect against cardiovascular disease (CVD).
Objective: The objective was to evaluate the efficacy of whole grains compared with refined grains on body composition, hypertension, and related mediators of CVD in overweight and obese adults.
Methods: We conducted a double-blind, randomized, controlled crossover trial in 40 overweight or obese men and women aged <50 y with no known history of CVD. Complete whole-grain and refined-grain diets were provided for two 8-wk periods, with a 10-wk washout between diets. Macronutrient composition was matched, except for the inclusion of either whole grains or refined grains (50 g/1000 kcal in each diet). Measurements included blood pressure, body composition, blood lipids and adiponectin, and markers of inflammation and glycemia.
Results: Thirty-three participants (6 men and 27 women) completed the trial [mean ± SD age: 39 ± 7 y; mean ± SD body mass index (in kg/m2): 33.1 ± 4.3]. Decreases in diastolic blood pressure were −5.8 mm Hg (95% CI: −7.7, −4.0 mm Hg) after the whole-grain diet and −1.6 mm Hg (95% CI: −4.4, 1.3 mm Hg) after the control diet (between effect, P = 0.01). Decreases in plasma adiponectin were −0.1 (95% CI: −0.9, 0.7) after the whole-grain diet and −1.4 (95% CI: −2.6, −0.3) after the control diet (between effect, P = 0.05). Decreases in diastolic blood pressure correlated with the circulating adiponectin concentration (r = 0.35, P = 0.04). Substantial reductions in body weight, fat loss, systolic blood pressure, total cholesterol, and LDL cholesterol were observed during both diet periods, with no relevant difference between them.
Conclusions: The improvement in diastolic blood pressure was >3-fold greater in overweight and obese adults when they consumed a whole-grain compared with a refined-grain diet. Because diastolic blood pressure predicts mortality in adults aged <50 y, increased whole-grain intake may provide a functional approach to control hypertension. This may benefit patients at risk of vascular-related morbidity and mortality. This trial was registered at clinicaltrials.gov as NCT01411540.
Keywords: blood pressure, diet, cardiovascular disease, obesity, whole grain
Introduction
Overweight and obesity are global health problems that continue to expand in scope and severity (1) and place ever-increasing demands on health care systems worldwide. Hypertension is a common obesity comorbidity, which affects ∼78 million US adults, is a major risk factor for cardiovascular disease (CVD)9 and is particularly insidious because it often has no symptoms. It is notable that hypertension may have age-dependent links to CVD risk: before age 50 y elevated diastolic blood pressure (DBP) is associated with increased disease risk; after age 50 y elevated systolic blood pressure (SBP) appears to be the more important risk factor (2). In the United States, CVD accounts for 32% of all deaths, at an annual cost of $313 billion (3); globally, the burden of CVD is enormous, contributing to nearly 20 million deaths/y in men and women >40 y of age (4). Although the prevalence and impact of CVD have declined in most economically advanced countries, increases in obesity threaten this progress. Strategies targeting obesity and preventable CVD risk factors including hypertension are urgently needed.
Nutrition and the management of overweight and obesity are central features in all clinical practice guidelines for reducing CVD risk (5). Diets that are rich in whole grains are associated with a decreased risk of many diseases and conditions, including CVD, obesity, type 2 diabetes, cancer, and mortality (6–10). Studies of whole-grain diet interventions have shown reductions in LDL cholesterol (11, 12) and have linked whole-grain intake with reduced inflammation (13). Although studies suggest that whole grains may lower blood pressure, fasting glucose, cholesterol, and inflammation (11, 14–17), some intervention studies have not confirmed these results, even when they have examined high doses of whole grains (up to 120 g/d) (18, 19). Furthermore, a lack of adequate control over intervention diets may explain why some trials with whole grains have not reported improvements in markers of disease risk. To address this issue, we provided the entire diet for each intervention arm and evaluated the effect of a comprehensively controlled whole-grain diet on key clinical outcomes and biomarkers related to CVD in overweight and obese adults. To ensure that subjects complied with the diet, we measured plasma alkylresorcinols. Alkylresorcinols are phenolic lipids present in high amounts in whole-grain wheat and rye. Because they are absorbed well by humans and can be measured in plasma, they are commonly used as biomarkers of whole-grain wheat and rye intake (20). To explore recent hypotheses that glycine betaine (betaine) from whole-grain wheat may make a mechanistic contribution to the health effects of whole grains via the effects on one-carbon metabolism (21, 22), we also measured betaine and related one-carbon metabolites (23–25).
Here, we provide the empirical data from a randomized crossover study of overweight and obese adults that compared the effects of a diet containing whole grains with an energy-matched diet using refined grains on body composition and metabolism. We hypothesized that a diet supplemented with whole grains would result in a greater change in body composition and obesity-related CVD risk factors.
Methods
Design
We conducted a double-blind, randomized, controlled crossover study of 2 fully provisioned diets: a whole-grain (intervention) diet and a refined-grain (control) diet. The study design facilitated examining the within-diet effect as well as the between-diet effects. The study was approved by the institutional review board of the Cleveland Clinic and was registered at clinicaltrials.gov as NCT01411540.
Population
Overweight and obese adults from the Cleveland, Ohio, area were recruited via advertisements. Our inclusion criteria were nonsmoking, <50 y of age, stable weight (<2-kg weight gain or loss in prior 6 mo), physically inactive (<1 h/wk), and BMI (in kg/m2) 28–40. Participants were excluded if they were diagnosed as having chronic disease (e.g., renal, hepatic, CVD, type 2 diabetes) or were taking lipid-lowering medications, including statins. Participants taking antihypertensive medications could be enrolled. Women were to be premenopausal and studied during the midfollicular phase (i.e., 5–10 d postmenses). All of the participants underwent medical examinations, including resting electrocardiogram, urinalysis, and blood biochemistry. Participants were briefed verbally about the study and signed informed consent documents approved by the Cleveland Clinic institutional review board.
Randomization and blinding
Patients were randomly assigned to diet groups before testing. Blinding was achieved by covering whole-grain foods with sauce and by packaging meals into identical containers so that entrees appeared similar for both diets. Entrees were assembled at the Nestlé Product Technology Center in Solon, Ohio.
Intervention
Participants were provided all of their food and fluids during both diet periods. The macronutrient composition of each diet was matched, except for the inclusion of either whole grains or refined grains (50 g/1000 kcal in each diet; Table 1 and Supplemental Table 1). All recipes were identical for the 2 diets, except that entrees, breakfast cereals, and cereal bars differed in whole-grain or refined-grain content. The main cereals used were wheat (57%), rice (21%), and oats (16%). The target whole-grain amount was designed to be easily achievable on a controlled diet and at the higher range of whole-grain intake recommended by the USDA. Cereal foods were selected for the study on the basis that they would be familiar and palatable to the study population.
TABLE 1.
Composition of whole-grain and refined-grain diets1
| Diet constituents | Whole grain | Refined grain |
| Energy intake, kcal/d | ||
| Prescribed | 2168 ± 330 | 2175 ± 372 |
| Actual | 2065 ± 351 | 2044 ± 352 |
| Carbohydrate, g/d | 287 ± 50 | 281 ± 50 |
| Carbohydrate, % energy | 56 ± 2 | 55 ± 1 |
| Fiber, g/d | 29 ± 5 | 21 ± 4 |
| Sugar, g/d | 130 ± 28 | 129 ± 28 |
| Whole grain, g/d | 93 ± 19 | 0 ± 0 |
| Fat, g/d | 66 ± 12 | 67 ± 12 |
| Fat, % energy | 29 ± 1 | 29 ± 1 |
| Saturated fat, g/d | 21 ± 4 | 23 ± 4 |
| Trans fat, g/d | 1 ± 1 | 1 ± 1 |
| Cholesterol, mg/d | 275 ± 66 | 277 ± 62 |
| Protein, g/d | 92 ± 15 | 91 ± 15 |
| Protein, % energy | 18 ± 1 | 18 ± 1 |
| Sodium, mg/d | 3310 ± 546 | 3390 ± 541 |
| Potassium, mg/d | 1320 ± 371 | 1300 ± 412 |
Values are means ± SDs. n = 33.
Each diet period lasted 8 wk with a 10-wk washout period between diets. During washout, participants were instructed to resume their usual eating patterns. Diets were energy matched and isocaloric to each individual’s daily requirements (measured resting metabolic rate × 1.3 activity factor). The resting metabolic rate was determined before the start of each diet by using a ventilated hood and indirect calorimetry (Vmax Encore, Viasys) (26). Dietary compliance was estimated by weekly weigh-backs of food containers and calculated as the percentage of difference between prescribed and actual caloric intake.
At the beginning and end of each diet period, participants spent 3 d in our clinical research unit for metabolic testing. Plasma alkylresorcinols, which are objective biomarkers of whole-grain intake (20), were measured to confirm compliance with the diet. Diet analysis was performed using the Food Processor Nutritional Analysis Pro version 10.80 (ESHA Research). During their inpatient stay, participants were provided all meals and instructed to avoid strenuous activity.
Outcome measures
Body composition.
Height and weight were obtained with participants wearing a standard hospital gown and by use of a wall-mounted stadiometer and a calibrated scale. BMI was calculated as body mass (kilograms) divided by the square of height (meters). Total body fat (percentage), fat mass, and fat-free mass were assessed using dual-energy x-ray absorptiometry (iDXA, Lunar Prodigy; GE Healthcare). Waist circumference was measured up to 3 times with the use of a plastic tape measure ∼2 cm above the umbilicus. Measurements within 0.5 cm were averaged and used for analysis.
Blood pressure.
Participants slept overnight in the clinical research unit. On the third morning, research nurses used an automated platform (DINAMAPProcare 400; GE Medical Systems) to obtain morning brachial SBP and DBP measurements, which were performed on the left arm in a lowly lighted room while participants lay semisupine after 10 min of awake rest. Reported data were based on the mean of 3 measurements, with 1 min between each measurement. Mean arterial pressure was calculated as 2/3(DBP) + 1/3(SBP). Pulse pressure was estimated by subtracting DBP from SBP.
Cardiovascular risk assessment.
A polyethylene catheter was inserted into an antecubital vein and fasting blood samples were obtained for the determination of glycated hemoglobin (HbA1c); insulin; liver enzymes; high-sensitivity C-reactive protein (hs-CRP); total adiponectin; plasma glucose; TGs; and total cholesterol (TC), LDL cholesterol, VLDL cholesterol, and HDL cholesterol. Homocysteine, betaine, choline, and N-N-dimethylglycine (DMG) were also determined to characterize intermediate metabolites of cholesterol metabolism. Sex-specific z scores were calculated to determine the efficacy of each diet on decreasing the severity of the metabolic syndrome (27). The National Cholesterol Education Program Adult Treatment Panel (ATP) III criteria for the metabolic syndrome also were calculated based on the sum of risk factors for the syndrome. Insulin resistance was estimated by use of the HOMA-IR, as described elsewhere (27).
Biochemical analyses.
Blood samples were obtained at baseline and after completing each diet period. We measured plasma glucose immediately after collection using the glucose-oxidase method (YSI 2300 STAT Plus). HbA1c was measured in whole blood by turbidimetric inhibition immunoassay (Cleveland Clinic Laboratories). Alanine transaminase and aspartate transaminase (AST) also were assessed to characterize liver inflammation or injury (28). The remaining blood was centrifuged at 4°C for 10 min and frozen at −70°C until subsequent analysis.
To minimize interassay variability, all of the frozen samples from preintervention and postintervention collections were analyzed in the same assay. Homocysteine was analyzed by high-performance liquid chromatography (Dionex) with the use of a commercial kit. Betaine, choline, DMG, and carnitine were analyzed by isotope dilution with high-performance liquid chromatography combined with mass spectrometry (22). Plasma alkylresorcinols were analyzed by gas chromatography coupled to a mass spectrometer (Agilent Technologies) (29). Plasma total adiponectin concentration was analyzed by enzyme-linked immunosorbent assay (Biovendor Laboratory Medicine Inc.). Plasma TGs and cholesterol were analyzed with the use of enzymatic methods with an automated platform (Roche Modular Diagnostics). Plasma hs-CRP was measured with an automated analyzer (Dimension EXL 2000; Siemens Healthcare Diagnostic Inc.). Plasma insulin was assayed by radioimmunoassay (EMD Millipore).
Data analysis
We assessed the data using R software, Mavericks build 6913, version 3.1.3 (R Foundation). ANOVA with linear mixed effects was used to compare baseline characteristics and detect between-diet differences (i.e., Δ of postintervention to preintervention) with order and period effects added to the model. Although there were no pretest differences between diets, suggesting that there was no carryover effect, we analyzed the treatment × period interaction to confirm that there was no carryover effect (data not shown), and results are reported as treatment effect for ease of interpretation. Results between diets were further adjusted for age, sex, change in body fat, dietary fiber, and baseline concentrations. Within-diet effects were assessed using paired t tests. Non-normally distributed data were log transformed for analysis. Pearson’s product-moment correlation was used to examine the associations between physiologic characteristics using Δ-Δ (i.e., whole-group) condition values. Significance was set at P < 0.05, and data were reported as means, SDs, and CIs.
Results
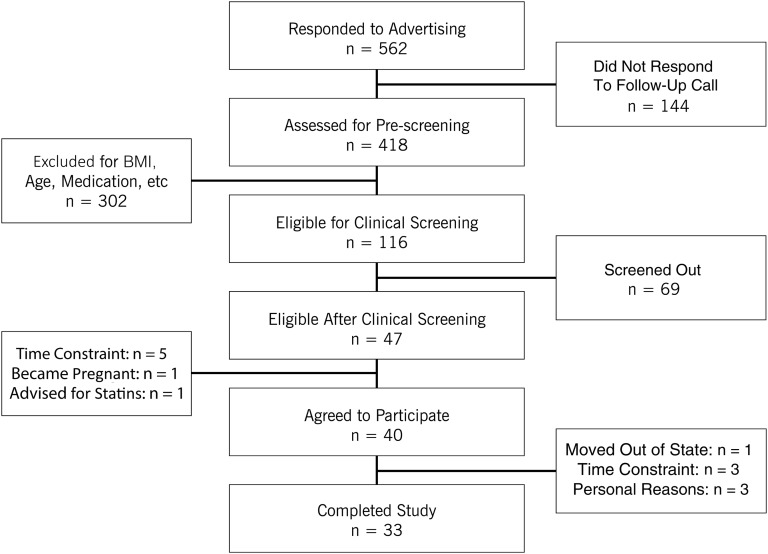
Forty overweight or obese adults with a mean age of 40 ± 7 y were enrolled. Seven participants dropped out (Figure 1), so 33 subjects completed the trial. Participants taking antihypertensive medication (n = 5) were instructed to maintain medication usage throughout the study.
FIGURE 1.
Participant flow diagram.
Diet.
Diet compliance based on weigh-backs was comparable in both arms [94.6% ± 6.4% (whole grain) compared with 92.9% ± 5.7% (refined grain); P = 0.18]. As expected, whole-grain (between effect, P < 0.001) and total fiber consumption (between effect, P < 0.001; Table 1) were higher during the whole-grain arm than during the control, refined-grain arm. There was a slightly smaller intake of saturated fat with the whole-grain diet (between effect, P = 0.03; Table 1). Also, plasma alkylresorcinols were not different at baseline for either diet arm, but they measured significantly higher after whole-grain intake (between effect, P < 0.001; Table 2).
TABLE 2.
Baseline values and changes in body composition, CVD risk factors, and plasma metabolites in overweight and obese adults before and after the 8-wk whole-grain and refined-grain interventions1
| Whole grain |
Refined grain |
ANOVA, P |
||||||
| Characteristic | Pretest | Δ2 | t Test, P | Pretest | Δ2 | t Test, P | Pretest | Δ |
| Body composition | ||||||||
| Weight, kg | 93.2 ± 16.1 | −2.4 (−3.4, −1.4) | <0.001 | 93.7 ± 16.2 | −2.5 (−3.5, −1.5) | <0.001 | 0.63 | 0.94 |
| BMI, kg/m2 | 32.9 ± 4.5 | −0.9 (−1.2, −0.5) | <0.001 | 33.1 ± 4.3 | −0.9 (−1.2, −0.5) | <0.001 | 0.48 | 0.81 |
| Fat mass, kg | 38.1 ± 9.4 | −1.8 (−2.6, −1.0) | <0.001 | 38.6 ± 9.6 | −2.2 (−3.0, −1.4) | <0.001 | 0.58 | 0.71 |
| Total body fat, % | 42.0 ± 6.2 | −1.1 (−1.8, −0.5) | 0.001 | 42.3 ± 6.4 | −1.1 (−1.6, −0.6) | <0.001 | 0.59 | 0.71 |
| FFM, kg | 55.0 ± 10.6 | −0.6 (−1.1, −0.2) | 0.009 | 55.1 ± 10.9 | −0.5 (−0.9, −0.1) | 0.02 | 0.63 | 0.64 |
| Waist circumference, cm | 96.4 ± 14.7 | −2.1 (−3.9, −0.3) | 0.02 | 95.5 ± 12.1 | −2.4 (−4.1, −0.7) | 0.009 | 0.45 | 0.73 |
| Cardiovascular | ||||||||
| MAP, mm Hg | 90 ± 9.1 | −5.0 (−7.2, −2.9) | <0.001 | 89 ± 9.3 | −2.4 (−5.3, 0.5) | 0.11 | 0.32 | 0.10 |
| Pulse pressure, mm Hg | 42 ± 9.2 | 2.3 (−2.1, 6.7) | 0.31 | 45 ± 7.0 | −2.6 (−5.8, 0.6) | 0.12 | 0.10 | 0.03 |
| hs-CRP, mg/L | 3.7 ± 3.3 | 0.8 (−1.1, 2.6) | 0.75 | 5.9 ± 7.1 | −2.3 (−4.8, 0.1) | 0.007 | 0.04 | 0.06 |
| Metabolic syndrome | ||||||||
| z Score | −2.0 ± 3.7 | −0.7 (−1.3, −0.1) | 0.04 | −2.2 ± 3.1 | −0.2 (−0.8, 0.3) | 0.36 | 0.48 | 0.26 |
| Glucose metabolism | ||||||||
| HbA1c, % | 5.9 ± 0.5 | −0.13 (−0.01, −0.26) | 0.04 | 5.8 ± 0.5 | −0.07 (−0.001, −0.14) | 0.06 | 0.18 | 0.36 |
| FPG, mg/dL | 88.6 ± 18.3 | −3.0 (−7.2, 1.1) | 0.16 | 89.2 ± 15.5 | 1.3 (-2.1, 4.8) | 0.45 | 0.95 | 0.14 |
| FPI, μU/mL | 19.6 ± 10.5 | −2.9 (−5.3, −0.5) | 0.02 | 22.6 ± 14.9 | −3.7 (-8.4, 1.0) | 0.13 | 0.06 | 0.76 |
| HOMA-IR | 4.5 ± 3.0 | −0.8 (−1.4, −0.2) | 0.02 | 5.2 ± 4.0 | −0.6 (−1.7, 0.4) | 0.27 | 0.07 | 0.78 |
| Lipids, mg/dL | ||||||||
| TG | 99.8 ± 64.1 | −10.7 (−23.9, 2.4) | 0.11 | 94.6 ± 52.8 | −10.0 (−19.8, −0.2) | 0.05 | 0.56 | 0.70 |
| TC | 176.2 ± 52.6 | −20.0 (−35.3, −4.7) | 0.01 | 175.2 ± 38.0 | −11.8 (−20.2, −3.4) | 0.01 | 0.97 | 0.27 |
| HDL-C | 48.8 ± 11.9 | −3.5 (−6.0, −1.1) | 0.007 | 48.9 ± 10.4 | −2.3 (−4.4, −0.3) | 0.03 | 0.94 | 0.38 |
| VLDL-C | 19.9 ± 12.8 | −2.1 (−4.7, 0.6) | 0.13 | 18.9 ± 10.6 | −2.0 (−4.0, −0.1) | 0.05 | 0.61 | 0.77 |
| LDL-C | 107.5 ± 50.1 | −14.4 (−28.5, −0.3) | 0.05 | 107.4 ± 32.8 | −7.4 (−14.5, −0.3) | 0.04 | 0.99 | 0.34 |
| Phenolic lipids, nM | ||||||||
| Alkylresorcinols | 40.7 ± 20.6 | 85.2 (38.2, 132.2) | <0.001 | 42.2 ± 22.9 | −36.8 (−51.1, −22.5) | 0.07 | 0.30 | <0.001 |
| One-carbon metabolites, μM | ||||||||
| Choline | 6.3 ± 2.0 | −0.8 (−1.4, −0.2) | 0.009 | 6.6 ± 2.1 | −1.0 (−1.5, −0.6) | <0.001 | 0.27 | 0.54 |
| DMG | 1.3 ± 1.3 | 0.0 (−0.1, 0.1) | 0.35 | 1.4 ± 1.4 | 0.1 (0.0, 0.2) | 0.03 | 0.13 | 0.06 |
| Homocysteine | 6.6 ± 2.4 | 0.2 (−0.4, 0.7) | 0.53 | 6.6 ± 1.9 | 0.1 (−0.2, 0.5) | 0.34 | 0.94 | 0.94 |
| Betaine | 17.7 ± 7.0 | −0.3 (−1.6, 1.1) | 0.72 | 18.0 ± 7.2 | −1.8 (−2.9, −0.6) | 0.006 | 0.61 | 0.07 |
| Carnitine | 32.7 ± 11.1 | 0.8 (−1.6, 3.2) | 0.53 | 32.9 ± 11.0 | −1.5 (−3.3, 0.2) | 0.09 | 0.81 | 0.12 |
| Liver enzymes, U/L | ||||||||
| ALT | 18.8 ± 12.2 | −0.6 (−3.5, 2.3) | 0.68 | 18.4 ± 14.1 | −1.5 (−3.5, 0.4) | 0.12 | 0.74 | 0.58 |
| AST | 19.6 ± 6.4 | 0.9 (−1.7, 3.6) | <0.001 | 22.0 ± 11.5 | −2.3 (−6.0, 1.4) | <0.001 | 0.14 | 0.19 |
Values are means ± SDs. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CVD, cardiovascular disease; DMG, N-N-dimethylglycine; FFM, fat-free mass; FPG, fasting plasma glucose; FPI, fasting plasma insulin; HbA1c, glycated hemoglobin; HDL-C, HDL cholesterol; hsCRP, high-sensitivity C-reactive protein; LDL-C, LDL cholesterol; MAP, mean arterial pressure; TC, total cholesterol; VLDL-C, VLDL cholesterol; Δ, absolute change postintervention.
Data are reported as means (95% CIs), n = 33.
Body weight and composition.
Both diets induced 3% and 6% weight loss and fat loss, respectively (within effect, P < 0.001; Table 2), with no significant difference between diets. Both diets also promoted similar reductions in waist circumference and fat-free mass (∼2 cm and ∼0.6 kg, respectively; Table 2).
Blood pressure.
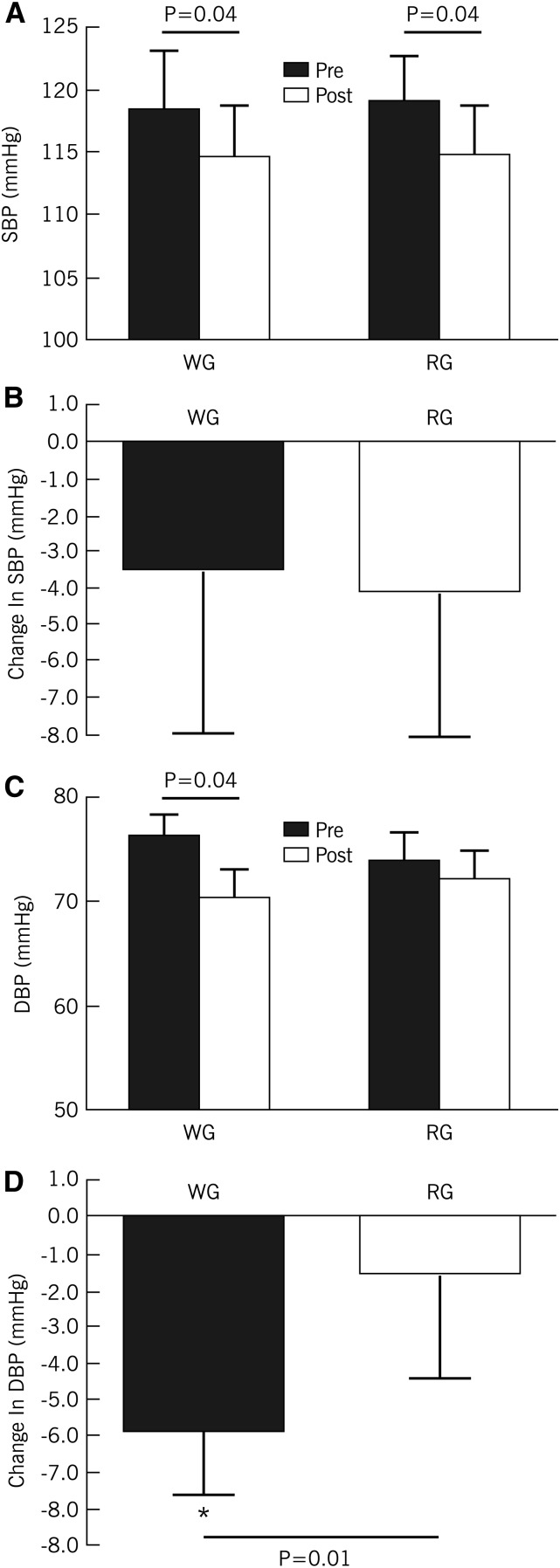
Although both diets lowered SBP, there were no significant differences in the magnitude of reduction between interventions (between effect, P = 0.80, Figure 2). Whole grains lowered DBP by ∼8% (compared with 1% for refined grains; P = 0.01; Figure 2), however. This change in DBP translated to a 10% improvement in pulse pressure (Table 2). After adjustment for changes in body fat, age, sex, dietary fiber, and baseline variables, improvements in both DBP and pulse pressure remained significant.
FIGURE 2.
Changes in SBP (A and B) and DBP (C and D) in overweight and obese adults who consumed WG and RG diets, each for 8 wk. Values are means ± SEMs, n = 33. DBP, diastolic blood pressure; RG, refined grain; SBP, systolic blood pressure; WG, whole grain.
Blood lipids, glycemic control, and insulin resistance.
There were no significant differences in lipid or glycemic risk factors between the diets. Both diets significantly reduced TC and LDL cholesterol by ∼9–20% (Table 2). Each diet comparably reduced HOMA-IR, but only whole grains significantly lowered HbA1c and fasting plasma insulin concentrations (Table 2). Moreover, although participants presented with few ATP III criteria (i.e., number of risk factors) for the metabolic syndrome before each diet intervention (1.6 ± 1.2 compared with 1.4 ± 1.1, P = 0.29) and neither diet greatly reduced the number of ATP III criteria (−0.2 ± 1.0 compared with 0.6 ± 1.0, P = 0.18), only the whole-grain intervention reduced metabolic syndrome severity (i.e., z score calculation; P = 0.04; Table 2).
Adiponectin, hs-CRP, liver enzymes, and one-carbon metabolites.
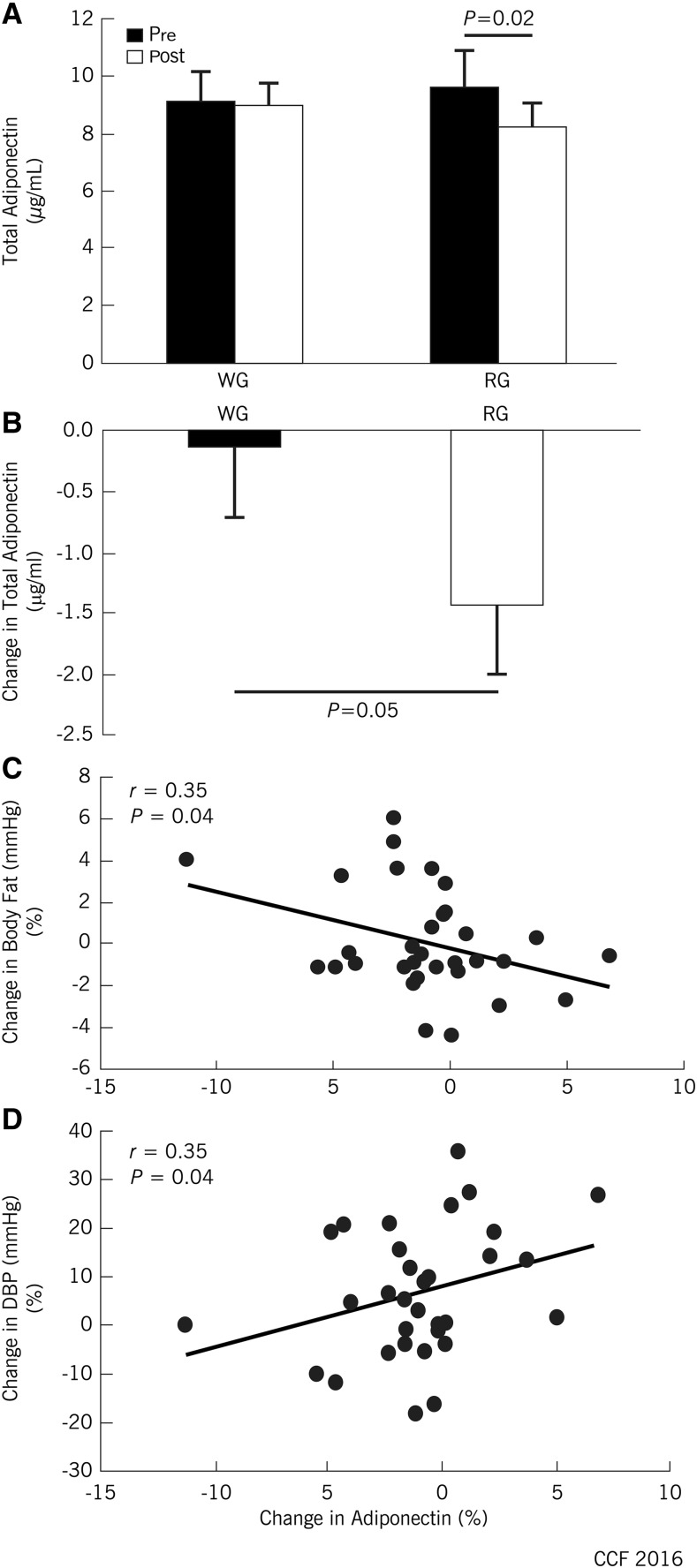
Whole-grain intake preserved circulating total adiponectin concentrations compared with an ∼10% decline after refined-grain intake, independent of changes in body fat, baseline age, sex, and dietary fiber (P = 0.02; Figure 3A, B). There was no between-trial effect on hs-CRP, alanine transaminase, AST, or one-carbon metabolites (Table 2).
FIGURE 3.
Changes (%) in plasma adiponectin (A and B) and correlations between plasma adiponectin and body fat (C) or blood pressure (D) in overweight and obese adults who consumed whole- and refined-grain diets, each for 8 wk. Values are means ± SEMs, n = 33. DBP, diastolic blood pressure; RG, refined grain; WG, whole grain.
Correlation analyses.
Postintervention, adiponectin correlated with reductions in body fat (Figure 3C) and DBP (Figure 3D). Further exploratory analyses revealed that changes in adiponectin also significantly correlated with changes in AST (r = 0.33, P = 0.05) and betaine (r = 0.44, P = 0.009). Changes in betaine significantly correlated with changes in HOMA-IR (r = 0.54, P = 0.001) and DMG (r = 0.51, P = 0.002). DMG significantly correlated with TC (r = 0.35, P = 0.04), LDL cholesterol (r = 0.37, P = 0.03), and carnitine (r = 0.39, P = 0.02). Moreover, lower fasting insulin concentration significantly correlated with reductions in both body weight and body fat (both r = −0.46, P = 0.008).
Discussion
We evaluated the effects of a whole-grain diet on CVD and metabolic syndrome risk factors in overweight and obese adults <50 y of age. Compared with a control, refined-grain diet, the whole-grain diet reduced DBP by 5.8 mm Hg, or an additional 4.2 mm Hg beyond any change attributable to weight loss. In population studies this improvement approximates to a 40% lower risk of dying from stroke and a 30% lower risk of dying from ischemic heart disease or other vascular causes (30). Because increased DBP was the primary risk factor for CVD in the study population—adults aged <50 y—these findings have important implications for both patient care and public health.
The worldwide prevalence of hypertension in adults and children has increased in parallel with the pandemic of obesity. Many individuals with hypertension remain undiagnosed, making treatment and remission challenging. Lifestyle changes including diet and exercise are recommended first-line treatments for prehypertension or stage 1 hypertension, and even modest weight loss benefits individuals (31–33). Although the evidence is limited, the consumption of more whole-grain foods has been associated with reduced CVD risk, including reductions in SBP in older adults (34–38). Participants in our study represent a relatively young group at high risk for developing debilitating, complex, and long-term complications associated with obesity, such as hypertension and CVD. The ability of the whole-grain diet to attenuate blood pressure—particularly DBP—opens an important clinically relevant therapeutic direction for improving health over and above improvements resulting from weight loss.
There are few detailed mechanistic studies using randomized prospective study designs. A comprehensive meta-analysis by Ye and colleagues (39) concluded that although the evidence to support a link between whole grain intake and reduced CVD risk, including lower hypertension and blood lipids, is strong, a major gap exists in our knowledge of the mechanisms that underlie the benefits of whole-grain intake. A recent study by Jenkins et al. (40) suggests that one potential mechanism linking whole-grain intake to hypertension may work through increased vascular reactivity. Data also support the suggestion that whole grain–induced lowering of DBP may be mediated through enhanced endothelial function. Intriguing data from the Coronary Artery Risk Development in Young Adults (commonly known as CARDIA) study (41) showed that a diet that includes high intake of whole-grain foods has a strong inverse association with specific cellular-adhesion molecules that indicate endothelial dysfunction and subclinical CVD. Consistent with these studies, we noted a significant correlation between plasma adiponectin and DBP in our sample. Adiponectin, a 244–amino acid peptide secreted from adipocytes, protects against metabolic diseases and CVD (42, 43). Low plasma adiponectin concentrations are common in obesity and coronary artery disease and are associated with increased hypertension and impaired vasoreactivity (44, 45). In our study, systemic adiponectin concentrations were maintained when the diet was enriched with whole grains, but they declined when whole grains were absent, which was all the more remarkable because weight loss and fat loss were similar in both diets. Dietary whole-grain intake and adiponectin have been associated before (46), and together support the view that diets enriched with whole grains may be linked to hypertension and CVD through an anti-inflammatory–related mechanism. The active compound or compounds that contribute to blood-pressure regulation within the whole-grain diet remains to be discovered, but it does not appear to be any of the one-carbon metabolites that we measured in this study.
In epidemiological studies, dietary whole grains were associated with a reduced risk of stroke, and the effect appears to be independent of fiber intake (47). In the case of CVD, data suggest a lowering of circulating lipids, particularly cholesterol (12, 14, 39, 48), although a recent meta-analysis (49) found that a reduction in blood lipids was consistently associated only with whole-grain oats, not other cereals. The main grains we used in this study were wheat and rice. Because TC, VLDL cholesterol, and LDL cholesterol were significantly reduced in both diets, we cannot conclude that the improved lipid profile in our participants was the exclusive domain of whole grains. We suspect that the improvements stemmed from the controlled nature of the dietary intervention and the resultant weight loss.
Modest weight loss during controlled feeding studies is not unusual. In our experience, this is because snacking and consuming the extra calories that are common in food-rich environments is minimized; this observation is supported by published reports (50, 51). Most studies that have examined whole-grain foods allow participants to remain in “free-living” environments, where whole-grain foods are provided in their usual diets. This approach can lead to participants adding—instead of substituting—the study foods to their diet, leading to higher overall energy intake, which mitigates any benefit gained from the added whole-grain foods (13). This factor may have been at play in studies in which no effect of eating more whole-grain foods was observed (18, 19). In contrast, studies reporting a lowering of risk factors related to CVD have been tightly controlled crossover studies with all foods provided (11, 14, 15, 35).
Because of the length of the study, we had concerns about compliance. As a nonsubjective measure of compliance, we measured plasma alkylresorcinols, which correlate with whole-grain intake (20), and have been used as compliance biomarkers in several whole-grain intervention studies (12, 52, 53). Alkylresorcinols are found almost exclusively in the outer layers of wheat, rye, and barley (54); are absorbed; and can be measured in plasma (55). Plasma alkylresorcinols substantially increased during the whole-grain intervention, indicating excellent overall compliance.
The USDA recommends that Americans eat ≥48 g whole grains/d, as part of their six 1-ounce servings of cereal-based foods. No upper limit for whole-grain intake is given, although the 100 g/2000 kcal provided during this study is substantially higher than average intakes reported in the United States (<16 g/d) (56). Because we observed no differences in compliance or preference for either diet (although participants were blinded), 100 g whole grains/2000 kcal was easily achievable in this population. Our experience is consistent with several studies that have substantially increased whole-grain intake in populations with habitually low whole-grain intake (12, 57). More important, the foods we used in this study were based on commercially available ingredients and recipes and were well accepted by the participants.
The limitations of our study include sample size, which although large for a dietary study of this kind, was moderate in the context of clinical intervention trials. The design is similar to a crossover study design, which allowed tight individual control and minimized intersubject variance. This design is similar but superior to 66 subjects in a between-group design. In addition, our study population included mainly women, which may limit generalization of the results to the population as a whole. Moreover, we recognize that adjustments were not made for multiple comparisons. There was no particular subset of analyses for which we desired to control an overall type I error rate; therefore, the results of all of the statistical tests were judged individually for significance at the 0.05 level. The probability of a type I error is 5% in each case that a null hypothesis is true, and individual significant results should be understood in that context. Lastly, despite instructions to consume dietary foods provided during the study, it remains possible that subjects consumed foods of which we were not aware and our estimation of caloric intake underestimates true nutrition.
The strengths of our study include the rigorous control of diet in an outpatient setting, and the pivotal observation that whole grains attenuate a key CVD risk factor in a high-risk segment of the population. These data build on strong observational evidence that whole grains are an important dietary adjunct for the prevention and treatment of hypertension, even in people already at risk of CVD-related complications.
In summary, a diet based on whole grains reduced multiple risk factors for CVD and selectively decreased DBP in overweight and obese adults <50 y of age. The improvement in DBP was independent of weight loss or fat loss. Our results suggest that whole grains may be a key nutrient regulator of hypertension and could provide an effective nutritional strategy to lower CVD-related morbidity and mortality.
Acknowledgments
We thank Kay Stelmach for CRU support, Marianne Fischer for dietetic support, Velma Stephens and Brenda Foley-Murray for excellent organization of food distribution and biospecimen collection, Teresa Markle for outstanding biospecimen organization, Jeff Hammel for statistical advice, Ciarán Fealy for technical support, and Isabelle Breton, Anne-France Kapp, Corinne Ammon-Zufferey, Laurence Guignard, and Alicia Zangger for biochemical analyses. JPK and ABR designed the study; JPK and SKM produced the initial draft of the manuscript; JPK had responsibility for the final content; and all authors contributed to data collection, data organization, or sample/statistical analysis; provided edits; and approved the final version of the manuscript.
Footnotes
Abbreviations used: AST, aspartate transaminase; ATP, Adult Treatment Panel (National Cholesterol Education Program); CVD, cardiovascular disease; DBP, diastolic blood pressure; DMG, N-N-dimethylglycine; HbA1c, glycated hemoglobin; hs-CRP, high-sensitivity C-reactive protein; SBP, systolic blood pressure; TC, total cholesterol.
References
- 1.Ogden C, Carroll M, Fryar C, Flegal K. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS data brief, no. 219, Hyattsville (MD): National Center for Health Statistics; 2015. [Google Scholar]
- 2.Li Y, Wei FF, Thijs L, Boggia J, Asayama K, Hansen TW, Kikuya M, Bjorklund-Bodegard K, Ohkubo T, Jeppesen J, et al. Ambulatory hypertension subtypes and 24-hour systolic and diastolic blood pressure as distinct outcome predictors in 8341 untreated people recruited from 12 populations. Circulation 2014;130:466–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013;127:e6–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128. Erratum in: Lancet 2013;381:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol 2014;63(25 Pt B):2985–3023. [DOI] [PubMed] [Google Scholar]
- 6.Esmaillzadeh A, Azadbakht L. Whole-grain intake, metabolic syndrome, and mortality in older adults. Am J Clin Nutr 2006;83:1439–40. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs DR Jr, Gallaher DD. Whole grain intake and cardiovascular disease: a review. Curr Atheroscler Rep 2004;6:415–23. [DOI] [PubMed] [Google Scholar]
- 8.Qi L, Hu FB. Dietary glycemic load, whole grains, and systemic inflammation in diabetes: the epidemiological evidence. Curr Opin Lipidol 2007;18:3–8. [DOI] [PubMed] [Google Scholar]
- 9.Williams PG, Grafenauer SJ, O’Shea JE. Cereal grains, legumes, and weight management: a comprehensive review of the scientific evidence. Nutr Rev 2008;66:171–82. [DOI] [PubMed] [Google Scholar]
- 10.Wu H, Flint AJ, Qi Q, van Dam RM, Sampson LA, Rimm EB, Holmes MD, Willett WC, Hu FB, Sun Q. Association between dietary whole grain intake and risk of mortality: two large prospective studies in US men and women. JAMA Intern Med 2015;175:373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giacco R, Clemente G, Cipriano D, Luongo D, Viscovo D, Patti L, Di Marino L, Giacco A, Naviglio D, Bianchi MA, et al. Effects of the regular consumption of wholemeal wheat foods on cardiovascular risk factors in healthy people. Nutr Metab Cardiovasc Dis 2010;20:186–94. [DOI] [PubMed] [Google Scholar]
- 12.Ross AB, Bruce SJ, Blondel-Lubrano A, Oguey-Araymon S, Beaumont M, Bourgeois A, Nielsen-Moennoz C, Vigo M, Fay LB, Kochhar S, et al. A whole-grain cereal-rich diet increases plasma betaine, and tends to decrease total and LDL-cholesterol compared with a refined-grain diet in healthy subjects. Br J Nutr 2011;105:1492–502. [DOI] [PubMed] [Google Scholar]
- 13.Katcher HI, Legro RS, Kunselman AR, Gillies PJ, Demers LM, Bagshaw DM, Kris-Etherton PM. The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr 2008;87:79–90. [DOI] [PubMed] [Google Scholar]
- 14.Behall KM, Scholfield DJ, Hallfrisch J. Diets containing barley significantly reduce lipids in mildly hypercholesterolemic men and women. Am J Clin Nutr 2004;80:1185–93. [DOI] [PubMed] [Google Scholar]
- 15.Behall KM, Scholfield DJ, Hallfrisch J. Whole-grain diets reduce blood pressure in mildly hypercholesterolemic men and women. J Am Diet Assoc 2006;106:1445–9. [DOI] [PubMed] [Google Scholar]
- 16.Hallfrisch J, Behall KM. Mechanisms of the effects of grains on insulin and glucose responses. J Am Coll Nutr 2000;19:320S–5S. [DOI] [PubMed] [Google Scholar]
- 17.Vitaglione P, Mennella I, Ferracane R, Rivellese AA, Giacco R, Ercolini D, Gibbons SM, La Storia A, Gilbert JA, Jonnalagadda S, et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. Am J Clin Nutr 2015;101:251–61. [DOI] [PubMed] [Google Scholar]
- 18.Andersson A, Tengblad S, Karlstrom B, Kamal-Eldin A, Landberg R, Basu S, Aman P, Vessby B. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J Nutr 2007;137:1401–7. [DOI] [PubMed] [Google Scholar]
- 19.Brownlee IA, Moore C, Chatfield M, Richardson DP, Ashby P, Kuznesof SA, Jebb SA, Seal CJ. Markers of cardiovascular risk are not changed by increased whole-grain intake: the WHOLEheart study, a randomised, controlled dietary intervention. Br J Nutr 2010;104:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross AB. Present status and perspectives on the use of alkylresorcinols as biomarkers of wholegrain wheat and rye intake. J Nutr Metab 2012;2012:462967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross AB, Godin JP, Minehira K, Kirwan JP. Increasing whole grain intake as part of prevention and treatment of nonalcoholic fatty liver disease. Int J Endocrinol 2013;2013:585876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross AB, Zangger A, Guiraud SP. Cereal foods are the major source of betaine in the Western diet—analysis of betaine and free choline in cereal foods and updated assessments of betaine intake. Food Chem 2014;145:859–65. [DOI] [PubMed] [Google Scholar]
- 23.Konstantinova SV, Tell GS, Vollset SE, Nygard O, Bleie O, Ueland PM. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr 2008;138:914–20. [DOI] [PubMed] [Google Scholar]
- 24.Wald NJ, Watt HC, Law MR, Weir DG, McPartlin J, Scott JM. Homocysteine and ischemic heart disease: results of a prospective study with implications regarding prevention. Arch Intern Med 1998;158:862–7. [DOI] [PubMed] [Google Scholar]
- 25.Pang H, Han B, Fu Q, Zong Z. Association of high homocysteine levels with the risk stratification in hypertensive patients at risk of stroke. Clin Ther 2016;38:1184–92. [DOI] [PubMed] [Google Scholar]
- 26.Solomon TP, Marchetti CM, Krishnan RK, Gonzalez F, Kirwan JP. Effects of aging on basal fat oxidation in obese humans. Metabolism 2008;57:1141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malin SK, Finnegan S, Fealy CE, Filion J, Rocco MB, Kirwan JP. Beta-cell dysfunction is associated with metabolic syndrome severity in adults. Metab Syndr Relat Disord 2014;12:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fealy CE, Haus JM, Solomon TP, Pagadala M, Flask CA, McCullough AJ, Kirwan JP. Short-term exercise reduces markers of hepatocyte apoptosis in nonalcoholic fatty liver disease. J Appl Physiol 2012;113:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landberg R, Man P, Kamal-Eldin A. A rapid gas chromatography-mass spectrometry method for quantification of alkylresorcinols in human plasma. Anal Biochem 2009;385:7–12. [DOI] [PubMed] [Google Scholar]
- 30.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–13. [DOI] [PubMed] [Google Scholar]
- 31.Look AHEAD Research Group, Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007;30:1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dengo AL, Dennis EA, Orr JS, Marinik EL, Ehrlich E, Davy BM, Davy KP. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension 2010;55:855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sacre JW, Jennings GL, Kingwell BA. Exercise and dietary influences on arterial stiffness in cardiometabolic disease. Hypertension 2014;63:888–93. [DOI] [PubMed] [Google Scholar]
- 34.Pins JJ, Geleva D, Keenan JM, Frazel C, O’Connor PJ, Cherney LM. Do whole-grain oat cereals reduce the need for antihypertensive medications and improve blood pressure control? J Fam Pract 2002;51:353–9. [PubMed] [Google Scholar]
- 35.Hallfrisch J, Schofield DJ, Behall KM. Blood pressure reduced by whole grain diet containing barley or whole wheat and brown rice in moderately hypercholesterolemic men. Nutr Res 2003;23:1631–42. [Google Scholar]
- 36.Tighe P, Duthie G, Vaughan N, Brittenden J, Simpson WG, Duthie S, Mutch W, Wahle K, Horgan G, Thies F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. Am J Clin Nutr 2010;92:733–40. [DOI] [PubMed] [Google Scholar]
- 37.Bodinham CL, Hitchen KL, Youngman PJ, Frost GS, Robertson MD. Short-term effects of whole-grain wheat on appetite and food intake in healthy adults: a pilot study. Br J Nutr 2011;106:327–30. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Gaziano JM, Liu S, Manson JE, Buring JE, Sesso HD. Whole- and refined-grain intakes and the risk of hypertension in women. Am J Clin Nutr 2007;86:472–9. [DOI] [PubMed] [Google Scholar]
- 39.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr 2012;142:1304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenkins DJ, Kendall CW, Vuksan V, Faulkner D, Augustin LS, Mitchell S, Ireland C, Srichaikul K, Mirrahimi A, Chiavaroli L, et al. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: a randomized controlled trial. Diabetes Care 2014;37:1806–14. [DOI] [PubMed] [Google Scholar]
- 41.Sijtsma FP, Meyer KA, Steffen LM, Van Horn L, Shikany JM, Odegaard AO, Gross MD, Kromhout D, Jacobs DR Jr. Diet quality and markers of endothelial function: the CARDIA study. Nutr Metab Cardiovasc Dis 2014;24:632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia 2012;55:2319–26. [DOI] [PubMed] [Google Scholar]
- 43.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004;291:1730–7. [DOI] [PubMed] [Google Scholar]
- 44.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension 2004;43:1318–23. [DOI] [PubMed] [Google Scholar]
- 45.Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 2003;42:231–4. [DOI] [PubMed] [Google Scholar]
- 46.Yannakoulia M, Yiannakouris N, Melistas L, Kontogianni MD, Malagaris I, Mantzoros CS. A dietary pattern characterized by high consumption of whole-grain cereals and low-fat dairy products and low consumption of refined cereals is positively associated with plasma adiponectin levels in healthy women. Metabolism 2008;57:824–30. [DOI] [PubMed] [Google Scholar]
- 47.Liu S, Manson JE, Stampfer MJ, Rexrode KM, Hu FB, Rimm EB, Willett WC. Whole grain consumption and risk of ischemic stroke in women: a prospective study. JAMA 2000;284:1534–40. [DOI] [PubMed] [Google Scholar]
- 48.Maki KC, Beiseigel JM, Jonnalagadda SS, Gugger CK, Reeves MS, Farmer MV, Kaden VN, Rains TM. Whole-grain ready-to-eat oat cereal, as part of a dietary program for weight loss, reduces low-density lipoprotein cholesterol in adults with overweight and obesity more than a dietary program including low-fiber control foods. J Am Diet Assoc 2010;110:205–14. [DOI] [PubMed] [Google Scholar]
- 49.Hollaender PL, Ross AB, Kristensen M. Whole-grain and blood lipid changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr 2015;102:556–72. [DOI] [PubMed] [Google Scholar]
- 50.Solomon TP, Haus JM, Kelly KR, Cook MD, Filion J, Rocco M, Kashyap SR, Watanabe RM, Barkoukis H, Kirwan JP. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr 2010;92:1359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris H, Mallan KM, Nambiar S, Daniels LA. The relationship between controlling feeding practices and boys’ and girls’ eating in the absence of hunger. Eat Behav 2014;15:519–22. [DOI] [PubMed] [Google Scholar]
- 52.Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M, Bugel S, Tetens I, Astrup A. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr 2012;142:710–6. [DOI] [PubMed] [Google Scholar]
- 53.Harris Jackson K, West SG, Vanden Heuvel JP, Jonnalagadda SS, Ross AB, Hill AM, Grieger JA, Lemieux SK, Kris-Etherton PM. Effects of whole and refined grains in a weight-loss diet on markers of metabolic syndrome in individuals with increased waist circumference: a randomized controlled-feeding trial. Am J Clin Nutr 2014;100:577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ross AB. Analysis of alkylresorcinols in cereal grains and products using ultrahigh-pressure liquid chromatography with fluorescence, ultraviolet, and CoulArray electrochemical detection. J Agric Food Chem 2012;60:8954–62. [DOI] [PubMed] [Google Scholar]
- 55.Ross AB, Kamal-Eldin A, Lundin EA, Zhang JX, Hallmans G, Aman P. Cereal alkylresorcinols are absorbed by humans. J Nutr 2003;133:2222–4. [DOI] [PubMed] [Google Scholar]
- 56.Cleveland LE, Moshfegh AJ, Albertson AM, Goldman JD. Dietary intake of whole grains. J Am Coll Nutr 2000;19(3 Suppl):331S–8S. [DOI] [PubMed] [Google Scholar]
- 57.Kuznesof S, Brownlee IA, Moore C, Richardson DP, Jebb SA, Seal CJ. WHOLEheart study participant acceptance of wholegrain foods. Appetite 2012;59:187–93. [DOI] [PubMed] [Google Scholar]