Abstract
Background: Child obesity is a major problem in the United States. Identifying early-life risk factors is necessary for prevention. Maternal diet during pregnancy is a primary source of fetal energy and might influence risk of child obesity.
Objective: We prospectively investigated the influence of maternal dietary patterns during pregnancy on child growth in the first 3 y of life in 389 mother–child pairs from the Pregnancy, Infection, and Nutrition study.
Methods: Dietary patterns were derived with the use of latent class analysis (LCA) based on maternal diet, collected with the use of a food-frequency questionnaire at 26–29 wk gestation. Associations between maternal dietary patterns and child body mass index (BMI)–for-age z score and overweight or obesity were assessed with the use of linear regression and log-binomial regression, respectively. We used linear mixed models to estimate childhood growth patterns in relation to maternal dietary patterns.
Results: Three patterns were identified from LCA: 1) fruits, vegetables, refined grains, red and processed meats, pizza, french fries, sweets, salty snacks, and soft drinks (latent class 1); 2) fruits, vegetables, baked chicken, whole-wheat bread, low-fat dairy, and water (latent class 2); and 3) white bread, red and processed meats, fried chicken, french fries, and vitamin C–rich drinks (latent class 3). In crude analyses, the latent class 3 diet was associated with a higher BMI-for-age z score at 1 and 3 y of age and a higher risk of overweight or obesity at 3 y of age than was the latent class 2 diet. These associations were not detectable after adjustment for confounding factors. We observed an inverse association between the latent class 3 diet and BMI-for-age z score at birth after adjustment for confounding factors that was not evident in the crude analysis (latent class 3 compared with latent class 2—β: −0.41; 95% CI: −0.79, −0.03).
Conclusion: In this prospective study, a less-healthy maternal dietary pattern was associated with early childhood weight patterns.
Keywords: maternal diet, pregnancy, dietary patterns, child overweight/obesity, latent class analysis
Introduction
Childhood overweight and obesity continues to be a major public health concern in the United States. According to national data, the prevalence of overweight and obesity is nearly 23% in children 2–5 y of age (1). Overweight and obesity during childhood increases the likelihood of adult obesity and the risk of obesity-related chronic conditions such as type 2 diabetes, metabolic syndrome, and cardiovascular disease (2). Moreover, childhood obesity has psychosocial correlates, including negative self-confidence and self-esteem, poor socialization, and depression during childhood and adolescence (2, 3). Growing evidence suggests that development of adipocytes—the cells that regulate fat mass—begins before birth (4), and concentrations of inflammatory biomarkers are higher for children who are overweight starting as young as age 3 y (5). Therefore, identifying risk factors during fetal development that influence childhood growth and obesity could have substantial public health and clinical implications.
Maternal diet during pregnancy is the primary source of energy for the fetus (6), and it has been suggested that it influences the risk of obesity in childhood (7). Studying diet is complex because individuals do not consume foods made up of single nutrients, but instead a diet composed of many foods and combinations of nutrients. As a result, analysis of dietary patterns as a measure of the overall diet is commonly used in nutritional epidemiology (8). However, to our knowledge, few studies have used this method to examine the association of maternal diet with childhood growth and obesity, and the results from previous studies are inconsistent. Maternal dietary patterns during pregnancy were related to birth weight in 2 early studies in Danish and Japanese pregnant women (9, 10); however, this association was not supported in a follow-up study in a diverse pregnancy cohort in the southern region of the United States (11). Similarly, a recent study that used data from the Generation R study found no association between maternal dietary patterns and child body composition at 6 y of age (12).
To our knowledge, previous research involving the association between maternal dietary patterns during pregnancy and childhood weight status has been limited to studies of a single measure at one point in time. Such an approach neglects the dynamic changes that occur in growth during early childhood, which could lead to incorrect interpretations of study results (13). To our knowledge, only one study has examined maternal dietary patterns during pregnancy and early childhood growth patterns. In this study, Poon et al. (14) assessed the association between adherence to 2 a priori dietary patterns, the Alternative Healthy Eating Index and the Mediterranean diet, in relation to change in child weight-for-length z score from 4 to 6 mo of age and found no association. It is possible that the follow-up period was insufficient to observe an effect and that a longer study period is needed. Hence, the purpose of this study was to investigate the influence of maternal dietary patterns during pregnancy on longitudinal childhood growth and obesity through the first 3 y of life.
Methods
Study design and population.
The present study used data from the Pregnancy, Infection, and Nutrition (PIN) study, a prospective cohort study that recruited mothers aged 16–47 y to explore the impact of early-life exposures on child health. Details of study protocols are provided elsewhere (15–17). Briefly, English-speaking pregnant women who were at <20 wk gestation, ≥16 y of age, and currently receiving and planning to continue prenatal care from the University of North Carolina clinics were recruited into the PIN prenatal study and were followed until delivery. Eligible mother–child pairs from the PIN prenatal study (n = 1169) were recruited in 2003 for follow-up at 3 mo (n = 689) and 1 y (n = 550) postpartum. In-home follow-up of the child at 3 y of age began in 2004 (n = 409). All PIN study protocols and procedures were reviewed and approved by the Institutional Review Board of the University of North Carolina School of Medicine.
The population for the current study was derived from mothers who participated in follow-up at 1 y postpartum (n = 550) and their children. A flow diagram summarizing the study population is provided in Figure 1. Mothers with incomplete dietary information (n = 55) were excluded, as were children with physician-diagnosed illnesses related to growth (n = 3), children missing weight and height measurements (n = 33), and preterm infants (gestational age <37 wk; n = 58). In addition, 12 mother–child pairs with missing covariate information [poverty level (n = 1), smoking during pregnancy (n = 7), birth weight category (n = 2), prepregnancy BMI (n = 1), and pre-existing diabetes (n = 1)] were excluded. The remaining mother–child pairs (n = 389) that had ≥1 weight and height measurement were included in the study population, resulting in n = 202, n = 254, n = 282, and n = 244 mother–child pairs at birth and 6-mo, 1-y, and 3-y follow-up, respectively. Distributions of selected baseline characteristics were compared for mother–child pairs included (n = 389) and excluded (n = 161) from the study population. In comparison with mothers included, those who were excluded were more likely to be younger, black, and smokers, and have a lower education level, lower income, and higher BMI.
FIGURE 1.
Flow diagram of the study population for this analysis (n = 389) from the PIN study, 1996–2006. PIN, Pregnancy, Infection, and Nutrition.
Dietary pattern assessment.
Maternal diet during pregnancy was collected at 26–29 wk gestation with the use of a self-administered, semiquantitative 119-item Block FFQ to assess dietary intake over the previous 3 mo. Detailed information on the validity of the FFQ has been described elsewhere (18). Dietsys+Plus version 5.6, with an updated food composition table based on nutrient values from NHANES III, and USDA 1998 nutrient databases were used to calculate daily energy intake in kilocalories and food intake in grams per day from the FFQ data.
The number of FFQ food items was reduced from 119 to 105 because some food items were rarely consumed (<10% of women) and were thus excluded, and because low-fat milks (skim, 1%, and 2%) were combined into one group because of small cell counts. Many of the food items had zero-inflated distributions because of the high prevalence of nonconsumers; therefore, items were grouped into categories of intake. Food items with a high prevalence of consumption (nonconsumption <10%; n = 17 items), such as green beans and pizza, were dichotomized at the median. Food items with a low prevalence of consumption (nonconsumption >70%; n = 8), such as grapefruit and whole milk, were dichotomized as consumed or nonconsumed. The remaining food items (n = 80) were categorized into 3 levels: 1) nonconsumers, 2) below the median of consumption among consumers, and 3) above the median of consumption among consumers. Latent class analysis (LCA) for categorical outcomes was used to derive dietary patterns from the FFQ data (19, 20). Briefly, LCA models were energy-adjusted with the use of Mplus 7.3, and the number of classes were determined based on a Lo–Mendell–Rubin likelihood ratio test (21). Women were classified into mutually exclusive classes according to their highest predicted probability of class membership.
Child anthropometric assessment.
Child birth weight and sex were abstracted from delivery records. Subsequently, during regular pediatrician visits, infant weights and heights were measured by medical staff and provided to mothers on standardized forms supplied by the PIN study. Child age at each measurement was calculated with the use of the difference between the documented date of pediatrician visit and date of birth. Children’s weight and height were measured during the 3-y home visit by trained PIN research staff, at a mean ± SD of 3.0 ± 0.2 y of age by trained PIN research staff who used stadiometers and scales according to the NHANES protocols (22). Age- and sex-specific BMI-for-age z scores were calculated with the use of the 2006 WHO growth charts for children <24 mo of age and the 2000 CDC growth charts for children ≥24 mo (23, 24). Children were further classified as overweight or obese based on a BMI (in kg/m2) ≥85th percentile for age and sex. The prevalence of biologically implausible heights and weights according to the growth charts was low (<1%). Regression models were estimated with and without children with implausible anthropometric measures included, and results did not differ; therefore, all children were included in the analysis.
Covariates.
Information regarding maternal age, race, marital status, parity, household income, educational level, prepregnancy weight, and smoking status were collected from telephone interviews at study enrollment. Maternal prepregnancy BMI was calculated from self-reported prepregnancy weight (kilograms) and measured height (meters squared) at the first clinic visit. Missing or implausible prepregnancy weights were imputed with the use of weight at the first prenatal care visit. BMI classifications followed the 2009 Institute of Medicine recommendations: underweight, <18.5; normal weight, 18.5–24.9; overweight, 25.0–29.9; and obese, ≥30.0. Gestational weight gain was defined as the difference between prepregnancy weight and weight measured near the time of delivery. Adequacy of gestational weight gain was categorized as inadequate, adequate, or excessive on the basis of the Institute of Medicine 2009 recommendations (25). Information on gestational age at birth was available from delivery records.
Statistical analysis.
Descriptive statistics for mother and child characteristics are presented as frequencies and percentages for categorical variables. Differences in maternal dietary intake by latent dietary class were assessed with the use of ANOVA. Visual examination of BMI-for-age z score distributions was conducted by latent dietary class with the use of box-plots. Associations of maternal latent dietary patterns with continuous measures of child weight status (e.g., BMI-for-age z score at birth and follow-up) were estimated with the use of linear regression models, and associations with dichotomous overweight or obesity (BMI ≥85th percentile) across the study period were estimated with the use of log-binomial regression with robust SEs.
To examine the longitudinal growth trajectory of child BMI-for-age z score from birth through 3 y of age in relation to maternal latent dietary class, linear mixed models with the use of restricted maximum likelihood with unstructured correlation were estimated. Linear mixed models are useful to model continuous repeated outcome measures over time, and can account for unbalanced data (varying number of repeated outcomes across children), unequal spacing of measurements across time, and the correlations between measurements within each child (26). A graphic examination of BMI-for-age z score between birth and 3 y of age suggested a linear relation. Age (in years) represented the unit of time. Children with ≥1 measurement were included in the analysis. Random intercept for each child and random slope for the linear age term were included to allow both the intercept and rates of overall growth to vary across children. To determine whether associations between child growth and maternal latent dietary class differed from birth through 3 y of age, an interaction term for child age and latent dietary class was tested with the use of Wald tests with an a priori significance P value of <0.20. Potential confounding factors for all models were determined a priori from the research literature and directed acyclic graph analysis (27). Variables were included in the final models as potential confounding factors if they resulted in at least a ±10% change in the effect estimates when included in the crude regression models individually (28). Prepregnancy BMI was assessed as both a potential confounding factor and effect measure modifier. All analyses were performed with the use of SAS version 9.3.
Results
Maternal baseline characteristics.
Three latent dietary classes were identified from the LCA models. The first class was characterized by a higher probability of consuming greater amounts of peaches, strawberries, canned fruits, broccoli, green beans or peas, corn, cabbage or coleslaw, white potatoes, sweet potatoes, refined grains, red and processed meats, pizza, french fries, sweets, salty snacks, and soft drinks (latent class 1; n = 139). The second class was characterized by a higher probability of consuming apples, bananas, oranges, tomatoes, broccoli, spinach, carrots, green salads, whole-wheat bread, low-fat dairy, baked chicken, and water (latent class 2; n = 174). The third class was characterized by a higher probability of consuming white bread, red and processed meats, fried chicken, french fries, and vitamin C–rich drinks (latent class 3; n = 76).
The overall distribution of selected maternal characteristics by latent dietary class is shown in Table 1. A higher proportion of mothers consuming a latent class 3 diet were younger, black, unmarried, and obese before pregnancy. Mothers classified into latent class 3 also were less likely to have a family income >350% of the federal poverty level or higher than a college education. Mothers consuming diets consistent with latent class 3 had a lower consumption of vegetables, nuts and legumes, whole grains, and low-fat dairy, but a higher consumption of red meat and sweetened beverages than did mothers grouped into latent class 1 and latent class 2 (Table 2). Mothers with a diet consistent with latent class 1 had a lower percentage of energy from carbohydrates, but a higher percentage of energy from fat and a higher intake of saturated fat than those consuming diets consistent with latent class 2 and latent class 3.
TABLE 1.
Selected maternal and child characteristics according to latent dietary class at 24–29 wk gestation in the Pregnancy, Infection and Nutrition study, 1996–20051
| Overall (n = 389) | Latent class 12 (n = 139) | Latent class 23 (n = 174) | Latent class 34 (n = 76) | |
| Maternal characteristics | ||||
| Age, y | ||||
| 16–24 | 53 (13.6) | 22 (15.8) | 8 (4.6) | 23 (30.3) |
| 25–29 | 111 (28.5) | 44 (31.7) | 45 (25.9) | 22 (29.0) |
| 30–34 | 150 (38.6) | 49 (35.3) | 79 (45.4) | 22 (29.0) |
| 35–47 | 75 (19.3) | 24 (17.3) | 42 (24.1) | 9 (11.8) |
| Race | ||||
| Nonblack | 356 (91.5) | 125 (89.9) | 173 (99.4) | 58 (76.3) |
| Black | 33 (8.5) | 14 (10.1) | 1 (0.6) | 18 (23.7) |
| Marital status | ||||
| Married | 340 (87.4) | 121 (87.1) | 165 (94.8) | 54 (71.1) |
| Unmarried | 49 (12.6) | 18 (13.0) | 9 (5.2) | 22 (29.0) |
| Federal poverty level, % | ||||
| <185 | 51 (13.1) | 28 (20.1) | 8 (4.6) | 15 (19.7) |
| 185–350 | 72 (18.5) | 25 (18.0) | 22 (12.6) | 25 (32.9) |
| >350 | 266 (68.4) | 86 (61.9) | 144 (82.8) | 36 (47.4) |
| Education level | ||||
| High school education or lower | 38 (9.8) | 16 (11.5) | 3 (1.7) | 19 (25.0) |
| College education | 191 (49.1) | 71 (51.1) | 78 (44.8) | 42 (55.3) |
| Higher than college education | 160 (41.1) | 52 (37.4) | 93 (53.5) | 15 (19.7) |
| Prepregnancy BMI category, kg/m2 | ||||
| <18.5 | 20 (5.1) | 8 (5.8) | 11 (6.3) | 1 (1.3) |
| 18.5–24.9 | 257 (66.1) | 87 (62.6) | 129 (74.1) | 41 (54.0) |
| 25.0–29.9 | 64 (16.5) | 25 (18.0) | 22 (12.6) | 17 (22.4) |
| ≥30.0 | 48 (12.3) | 19 (13.7) | 12 (6.9) | 17 (22.4) |
| Parity | ||||
| Nulliparous | 190 (48.8) | 60 (43.2) | 105 (60.3) | 25 (32.9) |
| Parous | 199 (51.2) | 79 (56.8) | 69 (39.7) | 51 (67.1) |
| Smoking status during pregnancy | ||||
| No | 361 (92.8) | 126 (90.7) | 170 (97.7) | 65 (85.5) |
| Yes | 28 (7.2) | 13 (9.4) | 4 (2.3) | 11 (14.5) |
| Gestational weight gain | ||||
| Inadequate | 45 (11.6) | 12 (8.6) | 21 (12.1) | 12 (15.8) |
| Adequate | 116 (29.9) | 40 (28.8) | 56 (32.4) | 20 (26.3) |
| Excessive | 227 (58.5) | 87 (62.6) | 96 (55.5) | 44 (57.9) |
| Gestational diabetes | ||||
| No | 380 (97.7) | 137 (98.6) | 173 (99.4) | 70 (92.1) |
| Yes | 9 (2.3) | 2 (1.4) | 1 (0.6) | 6 (7.9) |
| Pregnancy-induced hypertension | ||||
| No | 366 (94.1) | 130 (93.5) | 166 (95.4) | 70 (92.1) |
| Yes | 23 (5.9) | 9 (6.5) | 8 (4.6) | 6 (7.9) |
| Chronic hypertension | ||||
| No | 364 (93.6) | 130 (93.5) | 164 (94.3) | 70 (92.1) |
| Yes | 17 (4.4) | 7 (5.0) | 6 (5.0) | 4 (5.3) |
| Pregestational diabetes | ||||
| No | 373 (95.9) | 133 (95.7) | 167 (96.0) | 73 (96.1) |
| Yes | 16 (4.1) | 6 (4.3) | 7 (4.0) | 3 (3.9) |
| Child characteristics | ||||
| Birth weight, g | 3430 ± 427 | 3450 ± 382 | 3450 ± 466 | 3360 ± 406 |
| Sex | ||||
| M | 197 (50.6) | 80 (57.6) | 85 (48.9) | 32 (42.1) |
| F | 192 (49.4) | 59 (42.6) | 89 (51.2) | 44 (57.9) |
| Gestational age, wk | 39 [2] | 39 [2] | 39 [1] | 39 [2] |
| Child overweight or obese | ||||
| at 6 mo of age5 | 37 (14.6) | 11 (12.0) | 17 (14.9) | 9 (18.7) |
| at 1 y of age6 | 68 (24.1) | 23 (22.3) | 28 (22.2) | 17 (32.1) |
| at 3 y of age7 | 48 (19.7) | 16 (19.3) | 18 (15.8) | 14 (29.8) |
Values are means ± SDs, medians [IQRs], or n (%).
Represents a high probability of consuming peaches, strawberries, canned fruits, broccoli, green beans or peas, corn, cabbage or coleslaw, white potatoes, sweet potatoes, refined grains, red and processed meats, pizza, french fries, sweets, salty snacks, and soft drinks.
Represents a high probability of consuming apples, bananas, oranges, tomatoes, broccoli, spinach, carrots, green salads, whole-wheat bread, low-fat dairy, baked chicken, and water.
Represents a high probability of consuming white bread, red and processed meats, fried chicken, french fries, and vitamin C–rich drinks.
Overall n = 254; Latent class 1 n = 92; Latent class 2 n = 114; Latent class 3 n = 48.
Overall n = 282; Latent class 1 n = 103; Latent class 2 n = 126, Latent class 3 n = 53.
Overall n = 244; Latent class 1 n = 83; Latent class 2 n = 114; Latent class 3 n = 47.
TABLE 2.
Food group and energy intake according to latent dietary class at 24–29 wk gestation in the Pregnancy, Nutrition, and Infection study, 1996–20051
| Latent class 12 (n = 139) | Latent class 23 (n = 174) | Latent class 34 (n = 76) | |
| Food group, g/d | |||
| Fruits | 541 ± 333 | 478 ± 273 | 497 ± 400 |
| Vegetables | 148 ± 90 | 160 ± 110 | 78 ± 71 |
| Nuts and legumes | 45 ± 32 | 35 ± 25 | 31 ± 34 |
| Whole grains | 49 ± 39 | 61 ± 49 | 22 ± 31 |
| Low-fat dairy | 297 ± 246 | 454 ± 293 | 200 ± 253 |
| Red meat | 57 ± 33 | 27 ± 26 | 65 ± 126 |
| Sweetened beverages | 376 ± 337 | 172 ± 205 | 524 ± 593 |
| Energy intake variable | |||
| Energy intake, kcal | 2480 ± 732 | 1920 ± 504 | 2000 ± 1000 |
| Carbohydrates, % energy | 53.0 ± 6.6 | 55.0 ± 6.3 | 55.0 ± 7.4 |
| Protein, % energy | 14.3 ± 2.1 | 15.5 ± 2.6 | 13.4 ± 2.6 |
| Fat, % energy | 34.6 ± 5.5 | 32.1 ± 5.3 | 33.3 ± 6.0 |
Values are means ± SDs, n = 389. Differences were observed in the means for each food group and energy intake variable, with the exception of fruits, in the 3 latent dietary classes (ANOVA; P < 0.05).
Represents a high probability of consuming peaches, strawberries, canned fruits, broccoli, green beans or peas, corn, cabbage or coleslaw, white potatoes, sweet potatoes, refined grains, red and processed meats, pizza, french fries, sweets, salty snacks, and soft drinks.
Represents a high probability of consuming apples, bananas, oranges, tomatoes, broccoli, spinach, carrots, green salads, whole-wheat bread, low-fat dairy, baked chicken, and water.
Represents a high probability of consuming white bread, red and processed meats, fried chicken, french fries, and vitamin C–rich drinks.
Child adiposity at birth through 3 y of age.
Consuming a diet characterized by a high intake of white bread, red and processed meat, french fries, fried chicken, and vitamin C–rich drinks (latent class 3) was associated with a decrease of −0.41 SD (95% CI: −0.79, −0.03 SD) in child BMI-for-age z score at birth compared with a diet consisting of a high intake of fruits, vegetables, whole-wheat bread, low-fat dairy, baked chicken, and water (latent class 2) after adjustment for maternal age, race, education, income, marital status, parity, smoking status, and prepregnancy BMI (Table 3). In crude models during the follow-up period, latent class 3 was associated with an increase of 0.45 SD (95% CI: 0.12, 0.78 SD) and 0.42 SD (95% CI: 0.09, 0.75 SD) in child BMI-for-age z score at 1 and 3 y of age, respectively, compared with latent class 2. Adjustment for potential confounding factors attenuated the effect estimates, and associations were no longer statistically significant (1-y—β: 0.32; 95% CI: −0.04, 0.69; 3-y—β: 0.24; 95% CI: −0.12, 0.60). Compared with children of women characterized by a latent class 2 diet, those of women characterized by a latent class 3 diet had an increased risk of overweight or obesity at 3 y of age (RR: 1.83; 95% CI: 1.02, 3.28) (Table 4); however, this association was no longer statistically significant after adjusting for potential confounding factors (RR: 1.10; 95% CI: 0.69, 1.75).
TABLE 3.
Associations between latent dietary pattern at 24–29 wk gestation and BMI-for-age z score at birth and 6 mo, 1 y, and 3 y of age in the Pregnancy, Infection, and Nutrition study, 1996–20051
| Latent class 12 | Latent class 23 | Latent class 34 | |
| BMI-for-age z score at birth (n = 202) | |||
| Crude | −0.05 (−0.32, 0.22) | 0.0 (Ref) | −0.24 (−0.57, 0.10) |
| Adjusted5 | −0.12 (−0.39, 0.14) | 0.0 (Ref) | −0.41 (−0.79, −0.03)* |
| BMI-for-age z score at 6 mo of age (n = 254) | |||
| Crude | 0.03 (−0.25, 0.31) | 0.0 (Ref) | 0.23 (−0.11, 0.57) |
| Adjusted5 | 0.05 (−0.23, 0.34) | 0.0 (Ref) | 0.13 (−0.25, 0.50) |
| BMI-for-age z score at 1 y of age (n = 282) | |||
| Crude | 0.08 (−0.19, 0.34) | 0.0 (Ref) | 0.45 (0.12, 0.78)* |
| Adjusted5 | 0.03 (−0.24, 0.30) | 0.0 (Ref) | 0.32 (−0.04, 0.69) |
| BMI-for-age z score at 3 y of age (n = 244) | |||
| Crude | 0.19 (−0.09, 0.46) | 0.0 (Ref) | 0.42 (0.09, 0.75)* |
| Adjusted5 | 0.09 (−0.19, 0.38) | 0.0 (Ref) | 0.24 (−0.12, 0.60) |
Values are β coefficients (95% CIs). *Statistically significant; 95% CI does not cross the null value 0.0. Ref, reference.
Represents a high probability of consuming peaches, strawberries, canned fruits, broccoli, green beans or peas, corn, cabbage or coleslaw, white potatoes, sweet potatoes, refined grains, red and processed meats, pizza, french fries, sweets, salty snacks, and soft drinks.
Represents a high probability of consuming apples, bananas, oranges, tomatoes, broccoli, spinach, carrots, green salads, whole-wheat bread, low-fat dairy, baked chicken, and water.
Represents a high probability of consuming white bread, red and processed meats, fried chicken, french fries, and vitamin C–rich drinks.
Adjusted for maternal age categories, maternal race, education at enrollment, income level, marital status, parity, smoking status, and prepregnancy BMI.
TABLE 4.
Associations between latent dietary pattern at 24–29 wk gestation and child overweight or obesity (BMI ≥85th percentile) at 6 mo, 1 y, and 3 y of age in the Pregnancy, Infection, and Nutrition study, 1996–20051
| Latent class 12 | Latent class 23 | Latent class 34 | |
| 6 mo of age (n = 254) | |||
| Crude | 0.81 (0.42, 1.57) | 1.0 (Ref) | 1.24 (0.62, 2.48) |
| Adjusted5 | 0.83 (0.43, 1.60) | 1.0 (Ref) | 1.11 (0.52, 2.35) |
| 1 y of age (n = 282) | |||
| Crude | 1.00 (0.63, 1.60) | 1.0 (Ref) | 1.42 (0.87, 2.33) |
| Adjusted5 | 0.85 (0.53, 1.35) | 1.0 (Ref) | 1.15 (0.69, 1.91) |
| 3 y of age (n = 244) | |||
| Crude | 1.21 (0.68, 2.15) | 1.0 (Ref) | 1.83 (1.02, 3.28)* |
| Adjusted5 | 0.88 (0.49, 1.57) | 1.0 (Ref) | 1.10 (0.69, 1.75) |
Values are RRs (95% CIs). *Statistically significant; 95% CI does not cross the null value 1.0. Ref, reference.
Represents a high probability of consuming peaches, strawberries, canned fruits, broccoli, green beans or peas, corn, cabbage or coleslaw, white potatoes, sweet potatoes, refined grains, red and processed meats, pizza, french fries, sweets, salty snacks, and soft drinks.
Represents a high probability of consuming apples, bananas, oranges, tomatoes, broccoli, spinach, carrots, green salads, whole-wheat bread, low-fat dairy, baked chicken, and water.
Represents a high probability of consuming white bread, red and processed meats, fried chicken, french fries, and vitamin C–rich drinks.
Adjusted for maternal age categories, maternal race, education at enrollment, income level, marital status, parity, smoking status, and prepregnancy BMI.
Child growth trajectory from birth through 3 y of age.
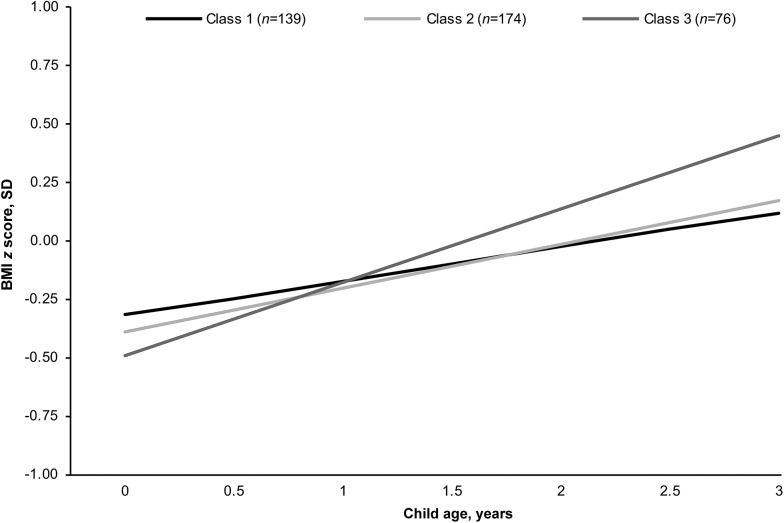
Mean BMI-for-age z scores across latent dietary classes are shown in Supplemental Figure 1. Children of mothers consuming a latent class 3 diet had a slightly lower BMI-for-age z score at birth, but a higher BMI-for-age z score at 6 mo, 1 y, and 3 y of age than did children of mothers consuming latent class 1 or latent class 2 diets. The association between maternal latent dietary class and child growth trajectory from birth through 3 y of age was further examined. Child BMI-for-age z scores consistently increased from birth through 3 y of age for children of mothers in each latent dietary class (Figure 2). At birth, the BMI-for-age z score was lower for children born to women consuming a latent class 3 diet during pregnancy than for children of women consuming a latent class 1 or latent class 2 diet. Children of mothers consuming a latent class 3 diet also experienced a faster rate of change in BMI-for-age z score across the study period (interaction of latent class 3 and child’s age, P = 0.10), resulting in a significantly higher BMI-for-age z score from 6 mo to 3 y of age than in children of mothers consuming a latent class 1 or latent class 2 diet.
FIGURE 2.
Predicted child BMI-for-age z score from birth to 3 y of age according to each latent dietary class at 24–29 wk gestation, based on results from our linear mixed model (n = 389) in the Pregnancy, Infection, and Nutrition study, 1996–2005. Child BMI-for-age z score trajectories were predicted for a specific maternal profile [30–34 y of age, nonblack maternal race, higher than college education, >350% of federal poverty level, married, parous, nonsmoker, prepregnancy BMI (in kg/m2) 18.5–24.9]. Latent class 1 represents a high probability of consuming peaches, strawberries, canned fruits, broccoli, green beans or peas, corn, cabbage or coleslaw, white potatoes, sweet potatoes, refined grains, red and processed meats, pizza, french fries, sweets, salty snacks, and soft drinks. Latent class 2 represents a high probability of consuming apples, bananas, oranges, tomatoes, broccoli, spinach, carrots, green salads, whole-wheat bread, low-fat dairy, baked chicken, and water. Latent class 3 represents a high probability of consuming white bread, red and processed meats, fried chicken, french fries, and vitamin C–rich drinks.
Discussion
In this prospective study, we found that maternal dietary patterns during pregnancy were associated with growth patterns from birth through 3 y of age. Specifically, adherence to a dietary pattern characterized by the consumption of white bread, red and processed meats, fried chicken, french fries, and vitamin C–rich drinks (latent class 3) during pregnancy was associated with lower child birth size and a faster rate of growth during the study period. Our results also suggested an increased risk of child overweight or obesity at age 3 y in children of mothers who consumed a latent class 3 diet compared with those of mothers who had a dietary pattern consisting of a high consumption of fruits, vegetables, whole-wheat bread, low-fat dairy, baked chicken, and water (latent class 2 diet).
To our knowledge, studies on maternal dietary patterns during pregnancy and child anthropometric measurements and growth are limited, and the findings are mixed. Generally, our findings are consistent with previous studies examining the association with child birth weight outcomes. A prospective study in pregnant women in Japan showed that children of mothers who consumed a diet consisting of meat and eggs (beef, pork, processed meat, chicken, eggs, butter, and dairy products) or one consisting of wheat products (bread, confectioneries, fruit and vegetable juice, and soft drinks) had a lower birth weight, on average, than children of mothers consuming a diet consisting of rice, fish, and vegetables (e.g., rice, potatoes, nuts, legumes, fruits, vegetables, tea, and fish) (10). Similarly, a maternal Western dietary pattern (e.g., high-fat dairy, refined grains, processed and red meat, and sweets) was associated with a lower birth weight (9). In contrast, a recent study in a largely African-American, low-income population did not find an association between maternal dietary patterns and child weight variables at birth (11). The discrepant findings could be attributed to limited variation between the 7 identified dietary patterns in the latter study, which might have masked any possible associations between maternal dietary patterns and birth weight outcomes.
Only one other study, to our knowledge, has investigated the association between maternal dietary patterns during pregnancy and child growth trajectory (14). When data from the Infant Feeding Practices Study II were used, no association was demonstrated between maternal diet during pregnancy and change in weight for length from 4 to 6 mo of age (14). Infant weight and length were collected from the mother’s report from the last pediatric visit, which may have introduced some measurement error. In addition, it is possible that the association was examined at an age too early to observe an effect, as evidenced by the lack of association found with child BMI-for-age z score results at 6 mo of age in our study.
Our findings were suggestive of an association between maternal dietary pattern during pregnancy and child overweight or obesity at 3 y of age. Children of mothers who consumed a latent class 3 diet had an increased risk of being overweight or obese at 3 y of age; however, associations were attenuated after adjustment for important prenatal factors. Similarly, data from the Generation R study, a prospective cohort study in Dutch mother–child pairs, did not observe an association between maternal dietary patterns and child body composition at 6 y of age after adjustment for confounding factors, which included both maternal and childhood factors (12). Our study did not adjust for childhood factors because of our interest in the total effect of maternal dietary patterns on child growth variables, and childhood factors are potentially mediators of this association.
Although it is unknown whether our findings are attributable to fetal programming, the postnatal environment, genetics, or a combination of several factors, our current observations are supported by animal research, which suggests a link between maternal diet composition during gestation and child weight status. Pregnant rats fed a junk-food diet during gestation and lactation produced offspring with higher adiposity (29, 30). One potential biological mechanism that may explain the associations found in the present study is leptin action, synthesis, and secretion (31). An intrauterine environment exposed to poor maternal nutrition could cause low fetal glucose, insulin, and leptin concentrations, leading to reduced birth weight and rapid postnatal growth because of increased appetite in early postnatal life (31). Unfortunately, testing this hypothesis directly is impractical because of the difficulty of measuring fetal glucose, insulin, and leptin concentrations directly.
Our findings must be interpreted within the context of study limitations. First, because both pediatric records and standardized study measurements were used for child anthropometric measures, not all measurements were standardized, and measurement error might have influenced the observed associations. Second, although the prospective cohort design allowed for adjustment of important confounding factors, the effect of unmeasured confounding on study results cannot be ignored. Third, our FFQ collected dietary information at 26–29 wk gestation to reflect intake over the previous 3 mo. It is possible that women with pre-existing conditions (e.g., hypertensive disorders and type 2 diabetes) and pregnancy-related complications (e.g., pregnancy-induced hypertension and gestational diabetes) might change their dietary patterns after diagnosis or take medications to treat their conditions. We performed sensitivity analyses to examine the robustness of our results after excluding women with pregestational diabetes, chronic hypertension, gestational diabetes, and pregnancy-induced hypertension. We observed slight changes in our associations. The associations between latent class 1 and child BMI-for-age z score and risk of overweight or obesity at 1 y of age became inversely associated; however, it remained nonsignificant. In addition, the association between latent class 3 and risk of overweight or obesity at 6 mo and 1 y of age was attenuated after exclusions. Consequently, the effect of residual confounding on our results cannot be ruled out.
Fourth, it is possible that our findings were affected by loss to follow-up experienced over the study period, which resulted in a lower proportion of women from high-risk groups (e.g., black women, smokers, and those with lower education and income levels and higher BMIs). We also observed a significantly disproportionate loss of mothers who consumed the least healthy dietary pattern (latent class 3). As a result, our findings are likely an underestimation of the true associations. Finally, the generalizability of our study population is limited because the women were mainly well-educated, white subjects with high income levels; this led to a lower prevalence of pregnant women with unhealthy dietary patterns.
Despite these limitations, our study had several strengths. Data were collected from a longitudinal, prospective pregnancy cohort with mother–child follow-up data through 3 y of age, which allowed for adjustment of important confounding factors. Dietary intake was collected with the use of an FFQ that was validated in several populations, including the PIN study population (18), and, to our knowledge, a novel method was used to examine overall maternal diet during pregnancy. We also incorporated repeated measures of height and weight per child with follow-up of children at 3 y of age, which provided an opportunity to examine growth patterns and weight outcomes throughout early childhood.
In summary, our findings suggest an association between maternal diet during pregnancy and risk of child overweight or obesity, which is a potentially modifiable maternal behavior. Specifically, a diet composed of a high intake of white bread, red and processed meats, fried chicken, french fries, and vitamin C–rich drinks was associated with a lower birth weight and a faster rate of growth in early childhood, and it was suggestive of an increased risk of overweight or obesity. Our study highlights the need for further investigations in larger, more diverse longitudinal studies to better understand the implications of maternal diet on infant and childhood growth and obesity. Maternal diet is an important modifiable risk factor that could be incorporated into intervention strategies to reduce the burden of childhood obesity.
Acknowledgments
CLM conceptualized the study, analyzed and interpreted the data, and wrote the paper; AMS-R was a coinvestigator of the Pregnancy, Infection, and Nutrition study, was responsible for the acquisition of the data, and assisted with the design, analysis, and interpretation of the results for this manuscript; DS-A assisted with the data analysis and interpretation of the results; JLD was a coinvestigator of the Pregnancy, Infection, and Nutrition Kids study and assisted with acquisition of the data and interpretation of the results; and WRR, EMP, and AMS provided assistance with interpretation of the results and provided instrumental substantive knowledge. All authors provided intellectual input into the paper, and read and approved the final manuscript.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daniels SR. The consequences of childhood overweight and obesity. Future Child 2006;16:47–67. [DOI] [PubMed] [Google Scholar]
- 3.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics 1998;101:518–25. [PubMed] [Google Scholar]
- 4.Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T. Endocrine and nutritional regulation of fetal adipose tissue development. J Endocrinol 2003;179:293–9. [DOI] [PubMed] [Google Scholar]
- 5.King JC. Physiology of pregnancy and nutrient metabolism. Am J Clin Nutr 2000;71:1218S–25S. [DOI] [PubMed] [Google Scholar]
- 6.Skinner AC, Steiner MJ, Henderson FW, Perrin EM. Multiple markers of inflammation and weight status: cross-sectional analyses throughout childhood. Pediatrics 2010;125:e801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murrin C, Shrivastava A, Kelleher CC, Lifeways Cross-Generation Cohort Study Steering Committee. Maternal macronutrient intake during pregnancy and 5 years postpartum and associations with child weight status aged five. Eur J Clin Nutr 2013;67:670–9. [DOI] [PubMed] [Google Scholar]
- 8.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 9.Knudsen VK, Orozova-Bekkevold IM, Mikkelsen TB, Wolff S, Olsen SF. Major dietary patterns in pregnancy and fetal growth. Eur J Clin Nutr 2008;62:463–70. [DOI] [PubMed] [Google Scholar]
- 10.Okubo H, Miyake Y, Sasaki S, Tanaka K, Murakami K, Hirota Y; Oksaka Maternal and Child Health Study Group, Kanzaki H, Kitada M, et al. Maternal dietary patterns in pregnancy and fetal growth in Japan: the Osaka Maternal and Child Health Study. Br J Nutr 2012;107:1526–33. [DOI] [PubMed] [Google Scholar]
- 11.Colón-Ramos U, Racette SB, Ganiban J, Nguyen TG, Kocak M, Carroll KN, Volgyi E, Tylavsky FA. Association between dietary patterns during pregnancy and birth size measures in a diverse population in Southern US. Nutrients 2015;7:1318–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Broek M, Leermakers E, Jaddoe V, Steegers E, Rivadeneira F, Raat H, Hofman A, Franco OH, Kiefte-de Jong JC. Maternal dietary patterns during pregnancy and body composition of the child at 6 y: the Generation R study. Am J Clin Nutr 2015;102:873–80. [DOI] [PubMed] [Google Scholar]
- 13.Ziyab AH, Karmaus W, Kurukulaaratchy RJ, Zhang H, Arshad SH. Developmental trajectories of body mass index from infancy to 18 years of age: prenatal determinants and health consequences. J Epidemiol Community Health 2014;68:934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poon AK, Yeung E, Boghossian N, Albert PS, Zhang C. Maternal dietary patterns during third trimester in association with birthweight characteristics and early infant growth. Scientifica 2013;2013:786409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta UJ, Siega-Riz AM, Herring AH. Effect of body image on pregnancy weight gain. Matern Child Health J 2011;15:324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siega-Riz AM, Herring AH, Carrier K, Evenson KR, Dole N, Deierlein A. Sociodemographic, perinatal, behavioral, and psychosocial predictors of weight retention at 3 and 12 months postpartum. Obesity (Silver Spring) 2010;18:1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deierlein AL, Siega-Riz AM, Herring AH, Adair LS, Daniels JL. Gestational weight gain and predicted changes in offspring anthropometrics between early infancy and 3 years. Pediatr Obes 2012;7:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saldana TM, Siega-Riz AM, Adair LS. Effect of macronutrient intake on the development of glucose intolerance during pregnancy. Am J Clin Nutr 2004;79:479–86. [DOI] [PubMed] [Google Scholar]
- 19.Sotres-Alvarez D, Herring AH, Siega-Riz AM. Latent class analysis is useful to classify pregnant women into dietary patterns. J Nutr 2010;140:2253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padmadas SS, Dias JG, Willekens FJ. Disentangling women’s responses on complex dietary intake patterns from an Indian cross-sectional survey: a latent class analysis. Public Health Nutr 2006;9:204–11. [DOI] [PubMed] [Google Scholar]
- 21.Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika 2001;88:767–78. [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS). National health and human examination protocol 1999–2000 [cited 2015 Mar 15]. Available from: http://www.cdc.gov.libproxy.lib.unc.edu/nchs/data/nhanes/meccomp.pdf.
- 23.de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicentre Growth Reference Study: planning, study design, and methodology. Food Nutr Bull 2004;25:S15–26. [DOI] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegel KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11 2002;(246):1–190. [PubMed] [Google Scholar]
- 25.Institute of Medicine. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): The National Academies Press; 2009. [PubMed] [Google Scholar]
- 26.Cheng J, Edwards LJ, Maldonado-Molina MM, Komro KA, Muller KE. Real longitudinal data analysis for real people: building a good enough mixed model. Stat Med 2010;29:504–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology 1999;10:37–48. [PubMed] [Google Scholar]
- 28.Maldonado G, Greenland S. Simulation study of confounder–selection strategies. Am J Epidemiol 1993;138:923–36. [DOI] [PubMed] [Google Scholar]
- 29.Bayol SA, Farrington SJ, Stickland NC. A maternal ‘junk food’ diet in pregnancy and lactation promotes an exacerbated taste for ‘junk food’ and a greater propensity for obesity in rat offspring. Br J Nutr 2007;98:843–51. [DOI] [PubMed] [Google Scholar]
- 30.Bayol SA, Simbi BH, Stickland NC. A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol 2005;567:951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMillen IC, Edwards LJ, Duffield J, Muhlhausler BS. Regulation of leptin synthesis and secretion before birth: implications for the early programming of adult obesity. Reproduction 2006;131:415–27. [DOI] [PubMed] [Google Scholar]