Abstract
Background: Many factors have been associated with serum 25-hydroxyvitamin D [25(OH)D] concentrations in observational studies, with variable consistency. However, less information is available on factors affecting the magnitude of changes in serum 25(OH)D resulting from vitamin D supplementation.
Objective: This study aimed to identify factors associated with the serum 25(OH)D response to supplementation with 1000 IU cholecalciferol/d during the first year of a large, multicenter, randomized, placebo-controlled colorectal adenoma chemoprevention trial.
Methods: Eligible older adults who were not vitamin D–deficient [serum 25(OH)D ≥12 ng/mL] were randomly assigned in a modified 2 × 2 factorial design to 1 of 4 groups: daily 1000 IU cholecalciferol, 1200 mg Ca as carbonate, both, or placebo. Women could elect 2-group (calcium ± cholecalciferol) random assignment. In secondary analyses, we used multivariable models to assess factors associated with serum 25(OH)D concentrations in all enrollees (n = 2753) and with relative changes in serum 25(OH)D after 1 y cholecalciferol supplementation among those randomly assigned (n = 2187).
Results: In multivariable models, 8 factors accounted for 50% of the variability of proportional change in serum 25(OH)D after cholecalciferol supplementation. Larger increases were associated with being female (34.5% compared with 20.5%; P < 0.001) and with lower baseline serum 25(OH)D (P < 0.0001), optimal adherence to study pill intake (P = 0.0002), wearing long pants and sleeves during sun exposure (P = 0.0002), moderate activity level (P = 0.01), use of extra vitamin D–containing supplements during the trial (P = 0.03), and seasons of blood draw (P ≤ 0.002). Several genetic polymorphisms were associated with baseline serum 25(OH)D and/or serum response, but these did not substantially increase the models’ R2 values. Other factors, including body mass index, were associated with serum 25(OH)D at baseline but not with its response to supplemental cholecalciferol.
Conclusions: The factors that most affected changes in serum 25(OH)D concentrations in response to cholecalciferol supplementation included sex, baseline serum 25(OH)D, supplement intake adherence, skin-covering clothes, physical activity, and season. Genetic factors did not play a major role. This trial was registered at www.clinicaltrials.gov as NCT00153816.
Keywords: randomized controlled trial, cholecalciferol, vitamin D3, calcium, 25-hydroxyvitamin D, dose response
Introduction
Observational data suggest that inadequate vitamin D status is associated with an increased risk of fracture, cancer, and other chronic diseases (1–4). Although the definition of an adequate serum concentration remains controversial (12 ng/mL) (5, 6), it has been argued that one-quarter of Americans have inadequate serum concentrations of 25-hydroxyvitamin D [25(OH)D]15 (12–20 ng/mL), and 8% are deficient (<12 ng/mL) (7, 8). However, the National Academy of Medicine (NAM) made relatively minor changes to DRIs in 2010, stating that “a majority of the population is meeting its needs for vitamin D” (7).
Many factors are known to be associated with serum 25(OH)D concentrations, including sun exposure, BMI, skin pigmentation, medications, and comorbidities (5, 7–26), but, to our knowledge, there is limited evidence on a few factors that may affect the magnitude of serum response to cholecalciferol supplementation, e.g., baseline serum 25(OH)D, BMI, age, calcium intake, and season (27, 28). The NAM provides intake recommendations by age and sex, but it is unclear whether supplementation should be varied according to vitamin D status, weight, or other factors (7). In addition, although common genetic variants have been associated with circulating serum 25(OH)D concentrations in genome-wide association studies (29, 30), their role in affecting the magnitude of serum response to supplementation is also less clear (31–33).
In this study, we report the results of analyses of the potential impact of a large number of personal, medical, environmental, lifestyle, and genetic factors on the serum 25(OH)D concentration response to 1 y of oral supplementation with 1000 IU cholecalciferol/d during a multicenter, randomized, placebo-controlled colorectal adenoma chemoprevention trial.
Methods
As described previously (34), we conducted a randomized, double-blind, placebo-controlled trial (NCT00153816) to test the effects of daily supplementation with 1000 IU cholecalciferol and/or 1200 mg elemental calcium (as calcium carbonate) on large-bowel adenoma recurrence. From May 2004 through July 2008, we recruited participants from 11 centers in the continental United States and Puerto Rico. Participants aged 45–75 y were eligible for enrollment if they had ≥1 large-bowel adenoma removed and no adenomas remaining after a complete colonoscopic surveillance examination within 4 mo of enrollment. Participants had no familial colorectal cancer syndromes, no history of serious gastrointestinal disease, no serious health concerns that might jeopardize several years of participation in the trial, and no contraindications to, or medical indications for, vitamin D or calcium supplementation.
Participants provided detailed information at enrollment on health, medications, and behavioral factors, such as sun exposure and exercise [International Physical Activity Questionnaire, Short Form (35)], and completed the Block Brief 2000 FFQ (Nutritionquest). They were counseled by study coordinators to help them avoid consuming >1200 mg Ca and 400 IU vitamin D/d in their food during the study. Participants also agreed to avoid taking vitamin D or calcium supplements other than those provided for the trial, but because vitamin D use in the community gained popularity, the protocol was amended to promote participant retention. From April 2008 onward, if personal supplements ≤1000 IU vitamin D and/or 400 mg elemental calcium were reported as being taken, these were permitted in addition to the study pills. For those who wished to take a multivitamin during the study, we provided one that lacked calcium and vitamin D. Blood drawn at enrollment and 1 y after random assignment (mean of 14.5 mo after the baseline blood draw) was tested for 25(OH)D with the use of a Gamma-B 25-Hydroxy Vitamin D Liquid-Phase RIA kit (IDS) in laboratory processes monitored through the international Vitamin D External Quality Assessment Scheme. In addition, we genotyped single-nucleotide polymorphisms (SNPs) in selected genes related to vitamin D metabolism, as described previously (31): genomic DNA was isolated from buffy coat samples by BioServe Biotechnologies with the use of DNAQuiK (36). Genotyping was conducted by KBioscience with the use of KASP technology (37); or by Genome Quebec Innovation Center with the use of Sequenom iPLEX Gold (38) or predesigned TaqMan assays (rs228570 and rs10766197) (39).
After a placebo run-in phase of 56–84 d, eligible participants who reported taking ≥80% of run-in study pills were randomly assigned, provided that their baseline serum 25(OH)D concentration was ≥12 ng/mL. Participants were randomly assigned in a modified 2 × 2 factorial design stratified by study center, sex, and surveillance interval to 1 of 4 study arms: daily 1000 IU cholecalciferol, 1200 mg Ca as carbonate, both, or placebo. Women with concerns about osteoporosis who declined to forego calcium supplementation were randomly assigned to calcium alone or calcium with vitamin D. Tablets for the study were prepared and tested according to the FDA’s guidelines for Good Manufacturing Practices. Trained study coordinators interviewed randomly assigned participants by telephone biannually to document changes in health and medications and ascertain self-reported adherence to study-pill intake. Medications were classified according to their constituent drug groups (e.g., loop or thiazide diuretic), but for logistical reasons, drug doses and adherence were not considered in this analysis. Participation in the trial continued through the next surveillance colonoscopy after 3 or 5 y, a surveillance interval determined by each participant’s medical provider. All candidate variables that were examined are shown in Supplemental Table 1.
All participants provided written informed consent; the research was approved by the Committee for the Protection of Human Subjects at Dartmouth College and by institutional review boards at each clinical center.
In secondary analyses, we analyzed data from all participants for whom we had a baseline serum 25(OH)D concentration to identify factors associated with serum concentrations at enrollment. We identified potential predictors of serum 25(OH)D concentrations from published research, and used this information to inform our selection of variables for analysis. To explore associations of potential exposures with baseline serum 25(OH)D concentrations, we used linear regression models. Variables with a univariate P < 0.05 were carried forward to the multiple linear regression analysis. The general strategy for modeling included 2 stages: 1) Potential predictors were considered in groups of similar factors, including demographic, sun-related, behavioral, health, medication-related, and nutritional. The best model was obtained within each group by including all variables and then removing the one with the largest P value > 0.05. The model was then refitted and the process repeated until all variables remaining had P < 0.05, giving the best model. 2) All variables from the best group models were entered simultaneously into a further model, and the same procedure was used to reduce the variables to a set of variables that were all significant with P < 0.05, giving the final best model. The 25(OH)D data were positively skewed and were log transformed for analysis to fulfill the distributional assumptions.
With the use of the same modeling strategy, we next examined factors influencing the serum response to vitamin D supplementation in an intention-to-treat analysis of randomly assigned participants for whom we knew both baseline and year 1 serum concentrations. The outcome variable was the logarithm of year 1 serum 25(OH)D concentration; the potential predictors included baseline serum 25(OH)D concentration and randomly assigned treatment group (vitamin D or placebo). The rationale for this analysis approach was to adjust the year 1 serum concentration for baseline serum 25(OH)D concentration and test the effect of each factor on year 1 serum 25(OH)D concentration while removing the effect of regression to the mean. Effects of each factor on response to vitamin D supplementation were tested by fitting the interaction between that factor and the treatment variable. Estimates represent the percentage increase in serum 25(OH)D from baseline associated with supplementation; these are presented separately for individuals in each category of the variable (e.g., in men and women). Statistical significance was assessed with likelihood ratio tests. Because serum 25(OH)D concentration was log transformed, estimated effects are ratios of geometric means, which are expressed as the percentage differences in final serum 25(OH)D concentration between those receiving vitamin D and those receiving placebo. To assist in interpreting the proportional effects, we also calculated the equivalent changes in absolute serum 25(OH)D concentration for participants with median baseline serum 25(OH)D who had increases of 20%, 25%, 30%, 35%, and 40%. In the final model, we included terms for the main effects of the stratification factors: study center, colonoscopy surveillance interval, study arm, and sex.
We conducted exploratory analyses of the effects of medications by adding each separately to the final model, whether or not it was significant in univariate analyses. This was done to provide information systematically on each medication, even though, because of power issues, most had been excluded from the final model. We also defined optimal adherence as the self-reported intake of ≥80% of study pills, on average, over the entire study period, no personal vitamin D supplementation, and no gaps in pill intake of >7 d. To assess the potential impact of adherence on these results, we repeated the analyses on the subgroup of optimally adherent participants.
In a simple exercise, we classified participants at baseline and year 1 into categories of deficiency (<12 ng/mL), inadequacy (12 to <20 ng/mL), and adequacy (≥20 ng/mL) in order to describe changes in the categories of vitamin D sufficiency in those treated with vitamin D or placebo (7). We restricted this analysis to participants who had not been taking vitamin D supplements at baseline, because only in those participants would the change in serum 25(OH)D be unaffected by the study requirement to stop those supplements at enrollment.
Finally, we genotyped 41 SNPs in selected genes involved in the vitamin D and/or calcium metabolism pathways [selected previously (31)] to assess their effects on the final model. Population stratification can lead to spurious associations between genetic markers and disease phenotypes because of subpopulation heterogeneity in allele frequencies and disease rates (40); to address this concern, a common statistical approach is to restrict genetic analyses to a single ethnic or racial group. We restricted these analyses to our largest subgroup, non-Hispanic whites, and then developed new multivariable models for baseline serum 25(OH)D concentrations and for serum response to 1000 IU cholecalciferol supplementation with the use of the approach described previously. All 41 SNPs were analyzed univariately with the use of an additive model. For SNPs that were associated with serum 25(OH)D baseline concentrations or follow-up changes at P < 0.05, correlations among them were explored, and for those SNPs in high linkage disequilibrium, defined as r2 > 0.95, only 1 SNP was included in the group SNP model. We then added to the final model all SNPs that remained significant in the group model after backward elimination. For serum response, we also assessed genetic variant associations within the optimally adherent subgroup. Analyses were performed with the use of SAS software version 9.3 and Stata software version 12.
Results
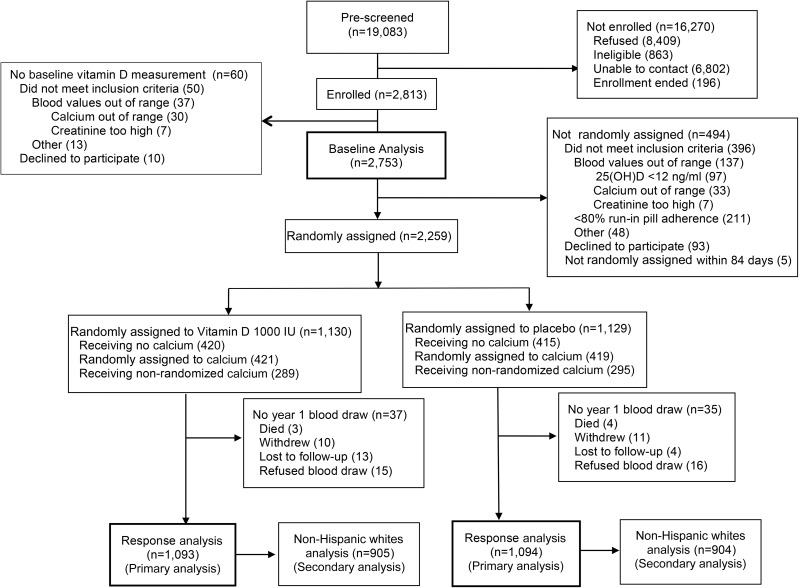
Of 19,083 individuals who were potentially eligible for the trial, 2813 were enrolled, 2753 had baseline serum 25(OH)D measured, 2259 were randomly assigned, and 2187 were included in the response analysis (Figure 1). The mean baseline serum 25(OH)D concentration for all 2753 enrollees was (mean ± SD) 23.9 ± 8.6 ng/mL. At enrollment, 54% of participants had been taking vitamin D supplements and 56% had been taking calcium supplements; they were asked (and agreed) to stop doing so. Subsequently, 107 of 2186 (4.9%) reported taking any personal vitamin D supplements up to 1000 IU/d between random assignment and year 1. Adequate serum 25(OH)D concentrations (i.e., ≥20 ng/mL) (7) were seen at baseline in 69% of those taking supplements before enrollment and 54% of those not taking supplements (Table 1). Mean concentrations ranged from 24.8 ng/mL in whites to 18.1 ng/mL in blacks, with the highest concentrations during the summer and fall, and a downward trend with increasing BMI (Table 2).
FIGURE 1.
CONSORT flow diagram. CONSORT, Consolidated Standards of Reporting Trials; 25(OH)D, 25-hydroxyvitamin D.
TABLE 1.
Baseline characteristics of participants1
| Random assignment of participants (n = 2187) |
|||||
| All participants (n = 2187) |
Optimally adherent participants (n = 1767) |
||||
| Enrolled participants (n = 2753) | Randomly assigned to cholecalciferol (n = 1093) | Randomly assigned to placebo (n = 1094) | Randomly assigned to cholecalciferol (n = 875) | Randomly assigned to placebo (n = 892) | |
| Age, y | 58.0 ± 6.9 | 58.2 ± 6.9 | 58.0 ± 6.8 | 58.3 ± 6.8 | 58.1 ± 6.8 |
| Male | 1705 [62] | 699 [64] | 692 [63] | 589 [67] | 580 [65] |
| Race | |||||
| White | 2248 [82] | 923 [84] | 922 [84] | 751 [86] | 761 [85] |
| Black | 276 [10] | 89 [8.1] | 83 [7.6] | 63 [7.2] | 57 [6.4] |
| Asian or Pacific Islander | 62 [2.3] | 25 [2.3] | 27 [2.5] | 22 [2.5] | 24 [2.7] |
| Other/unknown/refused | 167 [6.1] | 56 [5.1] | 62 [5.7] | 39 [4.5] | 50 [5.6] |
| Hispanic ethnicity | 201 [7.3] | 71 [6.5] | 67 [6.1] | 47 [5.4] | 55 [6.2] |
| Latitude of study center2 | |||||
| Northern (43–45°) | 759 [28] | 291 [27] | 300 [27] | 236 [27] | 247 [28] |
| Middle (35–42°) | 974 [35] | 424 [39] | 421 [38] | 352 [40] | 357 [40] |
| Southern (<35°) | 1020 [37] | 378 [35] | 373 [34] | 287 [33] | 288 [32] |
| Smoking status | |||||
| Never | 1435 [52] | 575 [53] | 589 [54] | 457 [52] | 467 [52] |
| Former | 1031 [37] | 406 [37] | 413 [38] | 328 [37] | 350 [39] |
| Current | 287 [10] | 112 [10] | 92 [8.4] | 90 [10] | 75 [8.4] |
| BMI, kg/m2 | |||||
| <25 | 598 [22] | 266 [24] | 241 [22] | 208 [24] | 200 [22] |
| 25–29.9 | 1108 [40] | 443 [41] | 447 [41] | 358 [41] | 368 [41] |
| 30–34.9 | 695 [25] | 253 [23] | 277 [25] | 204 [23] | 218 [24] |
| ≥35 | 347 [13] | 130 [12] | 127 [12] | 104 [12] | 105 [12] |
| Baseline 25(OH)D, ng/mL | |||||
| Arithmetic mean | 23.9 ± 8.6 | 24.7 ± 8.2 | 24.6 ± 8.6 | 24.8 ± 8.1 | 24.8 ± 8.6 |
| Geometric mean | 22.4 (22.2, 22.7) | 23.4 (23.0, 23.9) | 23.3 (22.9, 23.8) | 23.6 (23.1, 24.1) | 23.5 (23.0, 24.0) |
| Baseline 25(OH)D3 | |||||
| All | 2,753 | 1093 | 1094 | 875 | 892 |
| Deficient | 97 [3.5] | — | — | — | — |
| Inadequate | 950 [35] | 366 [33] | 384 [35] | 282 [32] | 304 [34] |
| Adequate | 1706 [62] | 727 [67] | 710 [65] | 593 [68] | 588 [66] |
| Taking vitamin D at baseline | 1493 | 620 | 619 | 497 | 502 |
| Deficient | 30 [2.0] | — | — | — | — |
| Inadequate | 440 [29] | 180 [29] | 183 [30] | 137 [28] | 145 [29] |
| Adequate | 1023 [69] | 440 [71] | 436 [70] | 360 [72] | 357 [71] |
| Not taking vitamin D at baseline | 1260 | 473 | 475 | 378 | 390 |
| Deficient | 67 [5.3] | — | — | — | — |
| Inadequate | 510 [40] | 186 [39] | 201 [42] | 145 [38] | 159 [41] |
| Adequate | 683 [54] | 287 [61] | 274 [58] | 233 [62] | 231 [59] |
| Vitamin D intake, IU/d | |||||
| Dietary | 132 ± 96 | 132 ± 98 | 137 ± 96 | 130 ± 91 | 138 ± 95 |
| Supplements or multivitamins | |||||
| None | 1260 [46] | 473 [43] | 475 [43] | 378 [43] | 390 [44] |
| 1–399 | 66 [2.4] | 26 [2.4] | 29 [2.7] | 17 [1.9] | 21 [2.4] |
| ≥400 | 1289 [47] | 544 [50] | 532 [49] | 440 [50] | 440 [49] |
| Unknown amount | 138 [5.0] | 50 [4.6] | 58 [5.3] | 40 [4.6] | 41 [4.6] |
| Calcium allocation | |||||
| No calcium | |||||
| Run-in failure (not randomly assigned) | 494 [18] | — | — | — | — |
| Randomly assigned to take no calcium | 835 [30] | 409 [37] | 405 [37] | 339 [39] | 332 [37] |
| Calcium (1200 mg/d) | |||||
| Randomly assigned to take calcium | 840 [31] | 410 [38] | 403 [37] | 334 [39] | 335 [38] |
| Calcium not randomly assigned4 | 584 [21] | 274 [25] | 286 [26] | 192 [22] | 225 [25] |
Values are means ± SDs, means (95% CIs), or n [%], unless otherwise indicated. When numbers do not sum to column totals, this is due to missing data. 25(OH)D, 25-hydroxyvitamin D.
Because California is long and the study center was based in Los Angeles, we used the latitude for Los Angeles (34.1°) (41). Northern centers (43–45°) included New Hampshire and Minnesota; middle centers (35–42°) included Ohio, Colorado, Iowa, and North Carolina; and Southern centers (18–34°) included Puerto Rico, Texas, Georgia, South Carolina, and California.
Deficiency (<12 ng/mL) was an exclusion criterion. Inadequate 25(OH)D was defined as 12 to <20 ng/mL; adequate was defined as ≥20 ng/mL (7).
In the 2-arm randomization protocol, all women received 1200 mg Ca and were randomly assigned to either 1000 IU cholecalciferol/d or placebo.
TABLE 2.
Factors associated with serum 25(OH)D at baseline in older adults who were not vitamin D–deficient who received daily supplements containing cholecalciferol, calcium, both, or placebo1
| Univariate model |
Final multivariable model—R2 = 0.32 (n = 2465) |
|||||
| n | Baseline serum 25(OH)D,2 ng/mL | Estimate3 | P | Estimate3 | P | |
| Demographic factors | ||||||
| Sex | 0.001 | <0.0001 | ||||
| M | 1705 | 24.2 ± 8.5 | Reference | Reference | ||
| F | 1048 | 23.3 ± 8.7 | −4.5 (−7.1, −1.9) | −7.8 (−10.8, −4.8) | ||
| Race | <0.0001 | <0.0001 | ||||
| White | 2248 | 24.8 ± 8.6 | Reference | Reference | ||
| Black | 276 | 18.1 ± 6.8 | −26.9 (−30.0, −23.8) | −18.4 (−22.0, −14.7) | ||
| Asian or Pacific Islander | 62 | 19.9 ± 6.0 | −18.7 (−25.3, −11.4) | −19.1 (−25.5, −12.2) | ||
| Other/unknown/refused | 167 | 22.4 ± 7.3 | −9.0 (−13.7, −4.0) | −5.8 (−10.6, −0.8) | ||
| Year of blood draw | <0.0001 | <0.0001 | ||||
| 2004 | 237 | 26.0 ± 8.7 | Reference | Reference | ||
| 2005 | 704 | 25.9 ± 8.9 | −0.9 (−5.8, 4.4) | 6.0 (1.1, 11.1) | ||
| 2006 | 836 | 22.7 ± 7.9 | −12.8 (−17.0, −8.3) | −5.3 (−9.6, −0.8) | ||
| 2007 | 688 | 22.7 ± 8.6 | −13.5 (−17.8, −8.9) | −5.0 (−9.4, −0.3) | ||
| 2008 | 288 | 23.4 ± 8.5 | −10.6 (−15.8, −5.1) | 3.2 (−2.6, 9.3) | ||
| Sun-related factors | ||||||
| Season of blood draw | <0.0001 | <0.0001 | ||||
| December–February | 624 | 22.5 ± 8.1 | Reference | Reference | ||
| March–May | 690 | 21.7 ± 8.0 | −3.7 (−7.2, 0.0) | −4.6 (−7.7, −1.4) | ||
| June–August | 758 | 25.8 ± 8.7 | 15.4 (11.2, 19.6) | 14.8 (11.0, 18.6) | ||
| September–November | 681 | 25.3 ± 8.8 | 12.8 (8.7, 17.1) | 11.4 (7.7, 15.3) | ||
| Latitude of study center | <0.0001 | <0.0001 | ||||
| Northern (43–45°) | 759 | 23.5 ± 8.6 | Reference | Reference | ||
| Middle (35–42°) | 974 | 25.0 ± 8.8 | 6.5 (3.0, 10.2) | 7.0 (3.8, 10.2) | ||
| Southern (<35°) | 1020 | 23.1 ± 8.3 | −1.4 (−4.6, 1.9) | 8.1 (4.7, 11.6) | ||
| Artificial tanning | <0.0001 | <0.0001 | ||||
| No | 2664 | 23.6 ± 8.4 | Reference | Reference | ||
| Yes | 83 | 32.8 ± 10.4 | 40.1 (29.8, 51.2) | 29.1 (20.7, 38.1) | ||
| Sunscreen use | <0.0001 | 0.03 | ||||
| Never or rarely (0–10%) | 1486 | 22.8 ± 8.5 | Reference | Reference | ||
| Sometimes (11–85%) | 1044 | 25.1 ± 8.5 | 10.7 (7.7, 13.8) | 3.0 (0.4, 5.7) | ||
| Almost always (86–100%) | 217 | 25.4 ± 9.1 | 11.3 (5.9, 17.0) | 4.4 (−0.3, 9.3) | ||
| Wears pants and long sleeves in 3-mo period when outside most | <0.0001 | <0.0001 | ||||
| Never or rarely (0–10%) | 1378 | 25.3 ± 8.9 | Reference | Reference | ||
| Sometimes (11–50%) | 497 | 24.0 ± 8.4 | −5.0 (−8.3, −1.5) | −4.3 (−7.3, −1.2) | ||
| Usually (51–85%) | 269 | 22.4 ± 8.0 | −11.3 (−15.2, −7.2) | −7.2 (−11.0, −3.3) | ||
| Almost always (86–100%) | 602 | 21.1 ± 7.6 | −16.5 (−19.2, −13.6) | −13.1 (−15.7, −10.4) | ||
| Vacation days in warm climate, n | <0.0001 | |||||
| None | 1221 | 22.9 ± 8.3 | Reference | — | ||
| 1–7 | 670 | 24.3 ± 8.8 | 6.4 (3.0, 10.0) | — | ||
| 8–14 | 430 | 25.0 ± 8.8 | 9.8 (5.7, 14.1) | — | ||
| 15–120 | 430 | 24.9 ± 8.6 | 9.1 (5.0, 13.4) | — | ||
| Behavioral factors | ||||||
| BMI, kg/m2 | <0.0001 | <0.0001 | ||||
| <25 | 598 | 26.0 ± 9.1 | Reference | Reference | ||
| 25–29 | 1108 | 24.3 ± 8.4 | −6.0 (−9.2, −2.7) | −6.9 (−9.7, −3.9) | ||
| 30–34 | 695 | 23.4 ± 8.4 | −10.0 (−13.4, −6.6) | −9.9 (−12.9, −6.7) | ||
| ≥35 | 347 | 19.9 ± 7.1 | −23.2 (−26.6, −19.6) | −19.8 (−23.0, −16.3) | ||
| Smoking status | 0.0003 | 0.002 | ||||
| Never | 1435 | 23.8 ± 8.4 | Reference | Reference | ||
| Former | 1031 | 24.4 ± 8.8 | 2.6 (−0.2, 5.6) | 1.7 (−0.9, 4.3) | ||
| Current | 287 | 22.5 ± 8.9 | −6.5 (−10.6, −2.3) | −5.8 (−9.5, −1.9) | ||
| Alcohol intake, g/d | <0.0001 | 0.01 | ||||
| ≤1 | 875 | 22.4 ± 7.9 | Reference | Reference | ||
| 1.1–13.5 | 903 | 24.5 ± 8.6 | 9.2 (5.8, 12.8) | 2.9 (0.0, 5.8) | ||
| 13.6–30 | 414 | 25.2 ± 8.7 | 12.6 (8.1,17.2) | 3.3 (−0.4, 7.1) | ||
| >30 | 326 | 25.9 ± 9.5 | 14.9 (10.0, 20.1) | 6.9 (2.7, 11.2) | ||
| Activity level4 | <0.0001 | <0.0001 | ||||
| Low | 657 | 22.4 ± 8.2 | Reference | Reference | ||
| Moderate | 872 | 23.4 ± 8.2 | 4.2 (0.6, 8.0) | 1.6 (−1.6, 4.8) | ||
| High | 1185 | 25.0 ± 8.9 | 11.3 (7.7, 15.1) | 6.4 (3.2, 9.6) | ||
| Health-related factors | ||||||
| Ever had hypertension | <0.0001 | |||||
| No | 1696 | 24.4 ± 8.8 | Reference | — | ||
| Yes | 1056 | 23.0 ± 8.3 | −5.5 (−8.1, −3.0) | — | ||
| Ever had high cholesterol | 0.04 | |||||
| No | 1415 | 24.3 ± 8.9 | Reference | — | ||
| Yes | 1331 | 23.5 ± 8.2 | −2.7 (−5.2, −0.1) | — | ||
| Diabetes | <0.0001 | |||||
| No history | 2471 | 24.2 ± 8.7 | Reference | — | ||
| Diet controlled | 60 | 22.6 ± 8.0 | −6.7 (−14.7, 2.1) | — | ||
| Not insulin dependent | 182 | 21.1 ± 7.2 | −12.5 (−17.0, −7.7) | — | ||
| Insulin dependent | 37 | 19.5 ± 6.2 | −18.7 (−27.5, −8.9) | — | ||
| Hormone replacement therapy | 0.001 | 0.005 | ||||
| Never | 548 | 22.6 ± 8.3 | Reference | Reference | ||
| Former | 338 | 23.6 ± 8.7 | 4.3 (−0.8, 9.6) | 0.8 (−3.3, 5.2) | ||
| Current | 148 | 26.0 ± 10.1 | 13.8 (6.5, 21.6) | 9.8 (3.7, 16.2) | ||
| Taking oral corticosteroid | 0.002 | 0.02 | ||||
| No | 2718 | 23.9 ± 8.6 | Reference | Reference | ||
| Yes | 35 | 19.5 ± 5.8 | −16.8 (−26.0, −6.4) | −11.6 (−20.3, −2.1) | ||
| Taking any diuretic | <0.0001 | |||||
| No | 2243 | 24.2 ± 8.7 | Reference | — | ||
| Yes | 510 | 22.3 ± 8.0 | −7.8 (−10.9, −4.6) | — | ||
| Taking protein pump inhibitor | 0.001 | |||||
| No | 2375 | 24.1 ± 8.7 | Reference | — | ||
| Yes | 378 | 22.7 ± 8.2 | −6.1 (−9.7, −2.5) | — | ||
| Nutritional factors5 | ||||||
| Vitamin D intake via personal supplements, IU/d | <0.0001 | 0.04 | ||||
| None | 1260 | 23.3 ± 8.2 | Reference | Reference | ||
| 1–399 | 66 | 25.8 ± 8.5 | 17.4 (7.8, 28.0) | 13.3 (2.2, 25.5) | ||
| ≥400 | 1289 | 25.2 ± 8.6 | 14.1 (11.1, 17.3) | 10.3 (2.4, 18.8) | ||
| Unknown amount | 138 | 25.4 ± 9.6 | 13.5 (6.8, 20.7) | 12.6 (2.8, 23.5) | ||
| Calcium intake via personal supplements, mg/d | <0.0001 | <0.0001 | ||||
| None | 1211 | 22.3 ± 8.3 | Reference | Reference | ||
| 1–200 | 774 | 24.7 ± 8.5 | 11.5 (8.1, 15.1) | −3.4 (−10.6, 4.3) | ||
| >200 | 582 | 25.9 ± 8.8 | 17.3 (13.4, 21.4) | 6.6 (−1.3, 15.1) | ||
| Unknown amount | 186 | 24.5 ± 8.8 | 10.4 (4.7, 16.5) | −1.3 (−9.3, 7.4) | ||
| Dairy servings6 | <0.0001 | <0.0001 | ||||
| Quartile 1 | 629 | 22.2 ± 8.1 | Reference | Reference | ||
| Quartile 2 | 636 | 23.6 ± 8.7 | 5.7 (1.8, 9.8) | 2.6 (−0.7, 6.0) | ||
| Quartile 3 | 613 | 24.2 ± 8.3 | 9.5 (5.4, 13.7) | 4.8 (1.4, 8.4) | ||
| Quartile 4 | 641 | 26.3 ± 8.7 | 19.4 (15.0, 24.0) | 12.6 (8.8, 16.5) | ||
| Dietary vitamin D intake7 | <0.0001 | |||||
| Quartile 1 | 626 | 23.3 ± 9.1 | Reference | — | ||
| Quartile 2 | 634 | 23.6 ± 8.4 | 2.2 (−1.6, 6.2) | — | ||
| Quartile 3 | 631 | 23.7 ± 8.2 | 3.1 (−0.8, 7.1) | — | ||
| Quartile 4 | 628 | 25.7 ± 8.6 | 12.4 (8.1, 16.8) | — | ||
| Dietary calcium intake8 | <0.0001 | |||||
| Quartile 1 | 632 | 22.6 ± 8.4 | Reference | — | ||
| Quartile 2 | 622 | 23.9 ± 8.7 | 5.7 (1.8, 9.9) | — | ||
| Quartile 3 | 635 | 23.9 ± 8.2 | 6.2 (2.2, 10.3) | — | ||
| Quartile 4 | 630 | 25.9 ± 8.7 | 15.5 (11.2, 20.0) | — | ||
| Dietary magnesium intake9 | 0.01 | |||||
| Quartile 1 | 630 | 23.3 ± 8.7 | Reference | — | ||
| Quartile 2 | 630 | 24.3 ± 8.5 | 4.8 (0.8, 8.9) | — | ||
| Quartile 3 | 631 | 23.9 ± 8.2 | 3.5 (−0.4, 7.5) | — | ||
| Quartile 4 | 628 | 24.9 ± 9.0 | 7.1 (3.1, 11.3) | — | ||
| Multivitamin use | <0.0001 | |||||
| No | 1265 | 22.5 ± 8.4 | Reference | — | ||
| Yes | 1478 | 25.1 ± 8.6 | 12.7 (9.8, 15.7) | — | ||
All variables shown had a univariate association with 25(OH)D after all variables in Supplemental Table 1 were tested. When numbers do not sum to 2573, this is due to missing data. MET, metabolic task equivalent; 25(OH)D, 25-hydroxyvitamin D.
Values are means ± SDs.
Percentage change (95% CI) in serum 25(OH)D concentration from baseline to year 1 relative to controls, adjusted for study center, colonoscopy surveillance follow-up interval (3 or 5 y), sex, and randomization protocol (2- or 4-group).
High activity: ≥3 d/wk of vigorous activity achieving ≥1500 MET-min/wk, or 7 d of any combination of activities, achieving ≥3000 MET-min/wk. Moderate activity: ≥3 d/wk of vigorous activity of ≥20 min/d, or ≥5 d/wk of moderate activity and/or walking of ≥30 min/d, or ≥5 d/wk of any activity achieving ≥600 MET-min/wk. Low: less than moderate activity level.
Sex-specific quartiles were used in nutrition analyses.
Cutoffs for quartiles—men: 0.6, 1.0, and 1.7; women: 0.5, 1.0, and 1.5.
Cutoffs for quartiles—men: 67.31, 109.12, and 176.10 IU/d; women: 57.37, 99.80, and 157.35 IU/d.
Cutoffs for quartiles—men: 444.6, 612.1, and 840.8 mg/d; women: 397.5, 559.4, and 784.5 mg/d.
Cutoffs for quartiles—men: 188.0, 242.6, and 310.7 mg/d; women: 159.8, 206.0, and 266.9 mg/d.
At baseline, the final multivariable model incorporated 17 factors that were significantly associated with serum 25(OH)D concentration, including demographic, sun-related, health-related, behavioral, and nutritional factors; those factors explained 32% of the variability in baseline serum 25(OH)D in 2753 individuals (Table 2). Several medications were associated with baseline serum 25(OH)D concentrations in univariate analyses; in multivariable models, current or former users of hormone replacement therapy had higher serum 25(OH)D concentrations (P = 0.005), and oral corticosteroid users had lower concentrations (P = 0.02). When we tested all medications (even if not significant in univariate analyses) by adding each to the final adjusted model, calcium channel blockers also were statistically significantly associated with higher baseline serum 25(OH)D concentrations (P = 0.02) (Supplemental Table 2).
Of 2753 participants included in the baseline analysis, 566 were excluded from the response analysis: 494 failed run-in [including 97 whose baseline serum 25(OH)D measurement was <12 ng/mL], and a further 72 randomly assigned participants did not have a 1-y blood draw (Figure 1). Optimal adherence was achieved by 1767 (81%). Of the 420 with suboptimal adherence, 228 (10% of the total) reported taking <80% of the pills, on average; 223 (10%) reported periods of ≥1 wk without taking pills; 73 (3%) had taken personal vitamin D supplements <400 IU/d; 30 (1%) had taken between 400 and 1000 IU/d; 4 had taken large doses (up to 50,000 IU/wk for a limited period); and 1 failed to report pill intake during the year 1.
After 1 y of supplementation, the unadjusted mean serum 25(OH)D concentration for those randomly assigned to 1000 IU cholecalciferol/d increased by 6.3 ng/mL, from 24.6 to 30.9 ng/mL. For those randomly assigned to calcium only or placebo, the mean serum 25(OH)D concentration fell by 1.1 ng/mL, from 24.5 to 23.4 ng/mL (data not shown), yielding a relative increase of 7.4 ng/mL, just over 1 SD of baseline concentrations, attributable to cholecalciferol supplementation.
In the multivariable model of serum response to supplementation, a greater proportional increase in serum 25(OH)D concentration in the vitamin D group relative to placebo was associated with lower baseline serum 25(OH)D, being female, season of each blood draw, wearing long pants and sleeves during sun exposure, moderate activity level, optimal adherence to study-pill intake, and use of personal vitamin D–containing supplements during the trial; these factors explained 50% of the variability in the serum response in 2157 individuals (R2, Table 3). To illustrate how the model for all participants might be interpreted, consider an example in which an individual with a median baseline serum 25(OH)D concentration (23.2 ng/mL) could expect a 33% (7.6 ng/mL) relative increase after supplementation, to an adjusted year 1 value of 30.8 ng/mL. In post hoc analysis, we explored the unexpected finding that sex was associated with serum response to vitamin D supplementation by adding body weight to the final model. Weight did not contribute significantly to the model, nor did it materially alter the coefficients for sex in the model.
TABLE 3.
Factors associated with serum 25(OH)D response to supplementation with 1000 IU cholecalciferol/d in older adults who were not vitamin D–deficient1
| Univariate model |
Multivariable model—R2 = 0.50 (n = 2157) |
|||||
| Randomly assigned to cholecalciferol (n = 1093) | Randomly assigned to placebo (n = 1094) | Estimate,2% | P | Estimate,2% | P | |
| Baseline serum 25(OH)D,3 ng/mL | <0.0001 | <0.0001 | ||||
| 25th percentile (18.2) | 41.0 (37.1, 45.1) | 32.6 (23.2, 42.7) | ||||
| 50th percentile (23.2) | 34.4 (31.4, 37.4) | 27.4 (18.5, 36.9) | ||||
| 75th percentile (30.0) | 27.8 (24.3, 31.5) | 22.2 (13.5, 31.7) | ||||
| Optimal adherence4 | <0.0001 | 0.0002 | ||||
| No | 218 [20] | 201 [18] | 21.4 (15.3, 27.8) | 20.0 (11.6, 29.1) | ||
| Yes | 875 [80] | 892 [82] | 37.7 (34.3, 41.1) | 35.0 (24.3, 46.8) | ||
| Demographic factors | ||||||
| Sex | <0.0001 | <0.0001 | ||||
| M | 699 [64] | 692 [63] | 29.5 (25.9, 33.2) | 20.5 (11.8, 29.9) | ||
| F | 394 [36] | 402 [37] | 42.8 (37.6, 48.3) | 34.5 (24.7, 45.1) | ||
| Race | 0.002 | |||||
| White | 923 [84] | 922 [84] | 32.2 (29.0, 35.4) | — | ||
| Black | 89 [8.1] | 83 [7.6] | 52.5 (40.7, 65.1) | — | ||
| Asian or Pacific Islander | 25 [2.3] | 27 [2.5] | 53.9 (33.1, 78.0) | — | ||
| Other/unknown/refused | 56 [5.1] | 62 [5.7] | 34.1 (21.7, 47.7) | — | ||
| Sun-related factors | ||||||
| Season of baseline blood draw | <0.0001 | 0.002 | ||||
| December–February | 249 [23] | 247 [23] | 48.6 (41.9, 55.6) | 30.8 (19.8, 42.7) | ||
| March–May | 257 [24] | 281 [26] | 24.4 (19.0, 30.0) | 15.8 (6.4, 26.0) | ||
| June–August | 308 [28] | 288 [26] | 28.2 (22.9, 33.7) | 30.6 (19.7, 42.4) | ||
| September–November | 279 [26] | 278 [25] | 39.8 (33.8, 46.0) | 32.9 (21.9, 44.9) | ||
| Season of year 1 blood draw | <0.0001 | 0.001 | ||||
| December–February | 272 [25] | 265 [24] | 42.1 (36.0, 48.4) | 31.1 (20.3, 42.8) | ||
| March–May | 269 [25] | 267 [24] | 47.6 (41.3, 54.2) | 38.4 (27.0, 50.9) | ||
| June–August | 276 [25] | 274 [25] | 24.8 (19.5, 30.4) | 23.6 (13.4, 34.8) | ||
| September–November | 276 [25] | 288 [26] | 24.8 (19.6, 30.3) | 17.1 (7.4, 27.7) | ||
| Wears pants and long sleeves in 3-mo period when outside most | <0.0001 | 0.0002 | ||||
| Never or rarely (0–10%) | 560 [51] | 565 [52] | 28.4 (24.4, 32.5) | 19.7 (11.3, 28.8) | ||
| Sometimes (11–50%) | 175 [16] | 205 [19] | 33.0 (26.0, 40.4) | 22.0 (12.0, 32.9) | ||
| Usually (51–85%) | 111 [10] | 96 [8.8] | 46.0 (35.6, 57.0) | 35.4 (22.9, 49.2) | ||
| Almost always (86–100%) | 245 [22] | 227 [21] | 45.6 (38.7, 52.8) | 32.8 (22.5, 43.9) | ||
| Behavioral factors | ||||||
| Alcohol intake, g/d | 0.04 | |||||
| ≤1 | 319 [32] | 342 [33] | 41.1 (35.5, 47.0) | — | ||
| 1.1–13.5 | 371 [37] | 394 [38] | 30.9 (26.0, 35.9) | — | ||
| 13.6–30 | 179 [18] | 155 [15] | 32.6 (25.2, 40.4) | — | ||
| >30 | 136 [14] | 134 [13] | 31.1 (23.0, 39.7) | — | ||
| Activity level5 | 0.0001 | 0.01 | ||||
| Low | 267 [25] | 247 [23] | 40.2 (33.9, 46.9) | 27.6 (17.5, 38.4) | ||
| Moderate | 344 [32] | 350 [32] | 41.4 (35.9, 47.2) | 31.9 (22.1, 42.6) | ||
| High | 467 [43] | 485 [45] | 27.1 (22.9, 31.5) | 22.6 (13.7, 32.2) | ||
| Health-related factors | ||||||
| Taking proton-pump inhibitor | 0.02 | |||||
| No | 942 [86] | 922 [84] | 32.7 (29.5, 36.0) | — | ||
| Yes | 151 [14] | 171 [16] | 43.3 (35.1, 52.0) | — | ||
| Nutritional factors6 | ||||||
| Vitamin D intake via personal supplements during year 1, IU/d | 0.0003 | 0.03 | ||||
| None | 1037 [95] | 1042 [95] | 35.2 (32.2, 38.4) | 36.6 (32.1, 41.4) | ||
| 1–399 | 39 [3.6] | 34 [3.1] | 29.1 (14.1, 46.1) | 40.1 (24.2, 58.1) | ||
| ≥400 | 17 [1.6] | 17 [1.6] | −6.4 (−21.8, 12.1) | 7.8 (−9.4, 28.2) | ||
| Calcium intake via supplements during year 1, mg/d | 0.046 | |||||
| None | 1032 [94] | 1041 [95] | 35.2 (32.2, 38.4) | — | ||
| 1–200 | 49 [4.5] | 30 [2.7] | 23.3 (9.1, 39.4) | — | ||
| >200 | 12 [1.1] | 22 [2.0] | 10.8 (−8.3, 34.0) | — | ||
| Dietary vitamin D intake during year 17 | 0.04 | |||||
| Quartile 1 | 282 [26] | 264 [24] | 36.1 (30.1, 42.3) | — | ||
| Quartile 2 | 267 [24] | 280 [26] | 39.7 (33.5, 46.1) | — | ||
| Quartile 3 | 279 [26] | 268 [25] | 34.4 (28.5, 40.6) | — | ||
| Quartile 4 | 265 [24] | 281 [26] | 27.5 (21.9, 33.4) | — | ||
| Dietary calcium intake during year 18 | 0.04 | |||||
| Quartile 1 | 274 [25] | 271 [25] | 40.5 (34.3, 47.0) | — | ||
| Quartile 2 | 289 [26] | 259 [24] | 35.3 (29.4, 41.6) | — | ||
| Quartile 3 | 267 [24] | 280 [26] | 33.8 (27.9, 39.9) | — | ||
| Quartile 4 | 263 [24] | 283 [26] | 28.0 (22.4, 33.9) | — | ||
Values are means (95% CIs) or n [%]. All variables shown had a univariate association with 25(OH)D response after all variables in Supplemental Table 1 were tested. When numbers do not sum to column totals, this is due to missing data. 25(OH)D, 25-hydroxyvitamin D.
Percentage change in serum 25(OH)D concentration from baseline to year 1 relative to controls, adjusted for study center, colonoscopy surveillance follow-up interval (3 or 5 y), sex, and random assignment group (2- or 4-group).
Percentiles of baseline serum 25(OH)D are derived from all participants with measured year 1 serum 25(OH)D. To assist with interpretation, the equivalent year 1 serum 25(OH)D concentration for participants with a median baseline concentration (23.2 ng/mL) and an increase of 20% would be 27.8 ng/mL. Concentrations for other increases are as follows: 25%, 29.0 ng/mL; 30%, 30.2 ng/mL; 35%, 31.3 ng/mL; and 40%, 32.5 ng/mL.
Self-reported intake of ≥80% of study pills, no personal vitamin D supplementation, and no gaps in pill intake of ≥7 d.
High activity: ≥3 d/wk of vigorous activity achieving ≥1500 MET-min/wk, or 7 d of any combination of activities, achieving ≥3000 MET-min/wk. Moderate activity: ≥3 d/wk of vigorous activity of ≥20 min/d, or ≥5 d/wk of moderate activity and/or walking of ≥30 min/d, or ≥5 d/wk of any activity achieving ≥600 MET-min/wk. Low: less than moderate activity level.
Sex-specific quartiles are calculated for the whole study population.
Cutoffs for quartiles—men: 26.6, 59.65, and 114.15 IU/d; women: 18.05, 42.6, and 101.8 IU/d.
Cutoffs for quartiles—men: 251.63, 384.35, and 589.65 mg/d; women: 206.80, 317.50, and 497.90 mg/d.
A model developed for the subsample of optimally adherent participants was similar to the overall model (Supplemental Table 3), except that race was included in the model, whereas physical activity did not contribute significantly; use of personal vitamin D supplements could not be included because of the optimal adherence restriction. Significant univariate SNP analyses in baseline and response models restricted to non-Hispanic whites are shown in Supplemental Tables 4 and 5. The serum 25(OH)D concentration was significantly associated with rs10741657 in cytochrome P450 family 2 subfamily R member 1 (CYP2R1; P = 0.0003), and rs4588 in the vitamin D binding protein (GC; P < 0.0001). However, inclusion of these variables in the final multivariable model barely changed the R2 from 0.28 to 0.29 and had a substantial impact only on the coefficients for smoking status (Supplemental Table 4).
When we examined serum response to vitamin D supplementation (Supplemental Table 5), only the rs10766197 polymorphism of CYP2R1 contributed significantly (P = 0.001), but, again, this did not affect the R2 value of 0.48 (Supplemental Table 5) or the coefficients of the other variables. When we restricted to optimally adherent participants, 3 polymorphisms contributed significantly to the final model: rs10766197 in CYP2R1 (P = 0.01), rs1801725 in the calcium-sensing receptor (CASR; P = 0.02), and rs4516035 in the vitamin D receptor (VDR; P = 0.04) (Supplemental Table 6). Inclusion of these variables in the model marginally increased R2 from 0.49 to 0.51 (Supplemental Table 6).
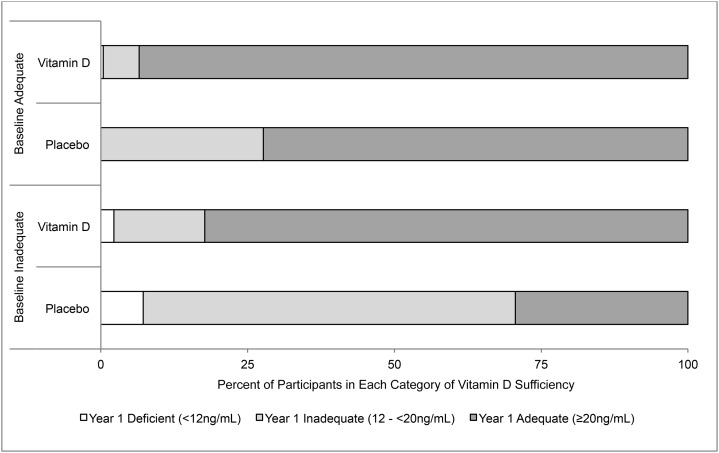
In crude analyses of individuals who had not been taking vitamin D supplements before the study or during year 1, we also assessed changes in proportions of individuals in the categories of inadequate (12 to <20 ng/mL) and adequate (≥20 ng/mL) serum 25(OH)D concentrations (7) (Figure 2). After 1 y, adequate concentrations were seen in 89% of participants who had received 1000 IU cholecalciferol/d and in 54% of controls. In stratified analyses, 83% of those who began with inadequate concentrations reached adequacy after 1 y of supplementation compared with 30% of controls. Among those who began the study with adequate concentrations, 94% of those randomly assigned to vitamin D were still adequate at 1 y compared with 72% of controls. These figures were similar in participants with optimal adherence (data not shown).
FIGURE 2.
Changes in serum 25-hydroxyvitamin D concentrations in older adults who received daily supplements containing cholecalciferol, calcium, both, or placebo. Values shown are the proportions achieving deficient, inadequate, or adequate status after 1 y in each of 4 baseline categories: 1) inadequate vitamin D status randomly assigned to placebo (n = 194), 2) inadequate vitamin D status randomly assigned to cholecalciferol (n = 181), 3) adequate vitamin D status randomly assigned to placebo (n = 267), and 4) adequate vitamin D status randomly assigned to cholecalciferol (n = 279). Individuals were excluded from analysis if they were vitamin D–deficient at baseline, had been taking vitamin D supplements before enrollment, or took personal vitamin D supplements during year 1. All measures are unadjusted.
Discussion
We modeled the determinants of serum 25(OH)D response to supplementation with 1000 IU cholecalciferol/d after 1 y of treatment in a large randomized trial in 2187 men and women aged 45–75 y, from 11 geographically diverse regions of the United States. At baseline, 17 factors were independently associated with baseline serum 25(OH)D concentrations, but we found that 50% of the variability in an individual’s response to supplementation could be explained by baseline serum 25(OH)D concentration and just a few other factors. A greater proportional serum response was seen with a lower baseline serum 25(OH)D concentration, being female, optimal adherence, season of baseline and year 1 blood draws, wearing pants and sleeves when out in the sun, physical activity, and personal vitamin D supplement intake beyond that prescribed. The rs10766197 polymorphism of CYP2R1 was significantly associated with serum response in non-Hispanic whites, but it did not improve the explanatory value (R2) of the multivariable model. Although our model confirmed the findings of others that response to supplementation varies with season of blood collection and baseline serum 25(OH)D concentration (27, 42), other aspects of the analysis were unexpected. For example, we found a significantly greater proportional serum 25(OH)D concentration increase from supplementation in women than in men, a finding that, to our knowledge, has not been reported in other trials (5, 21, 27, 43, 44). We may have seen this result because our sample size was very large and we could adjust for many potential confounders. Our results also contrast with studies that reported a significant association between response to supplementation and BMI (27), including a systematic review suggesting that the dose per kilogram of body weight explains 34.5% of the serum response (28). Although BMI affected baseline serum 25(OH)D concentrations, it did not independently affect the serum response to supplementation in our study. Other studies provide mixed or limited evidence that response to supplementation is associated with calcium intake, estrogen use, and dietary fat (27), but our results supported none of these associations. The inconsistencies seen across studies may reflect small sample sizes, as well as differences in the covariates collected and how they were measured. Age and race were not associated with response to supplementation in our study or in several others (21, 27, 45–51). Several variables (being female, greater physical activity, wearing long pants and sleeves while outdoors, and personal vitamin D supplement use) contributed significantly to both baseline and response models; this suggests either that these factors are independent determinants of both baseline serum 25(OH)D concentration and response to supplementation, or that they have residual effects in the response model because they are imperfectly represented by baseline serum 25(OH)D concentration, e.g., because of measurement error. Similarly, some other predictors in the response model are correlated—e.g., seasons of baseline and year 1 blood draws and baseline serum 25(OH)D concentration and physical activity—yet they contributed independently to the final model. It is possible that some of the variability between studies in the predictors of response to supplementation may be due to the intercorrelation of such variables and the impact of collinearity in model building.
Overall, our model for baseline concentrations contained 17 factors that explained 32% of the variability in baseline serum 25(OH)D. We confirmed the observations of others that baseline concentrations reflect a variety of personal characteristics; nutritional, behavioral and lifestyle factors; and measures of sun exposure (5, 7–26, 42). Our finding of an association between glucocorticoid use and lower serum 25(OH)D concentrations adds to mixed evidence from previous studies. Furthermore, it is unclear whether there is a biological basis for the association, or if confounding by comorbid illness accounts for it; the latter possibility is supported by a small meta-analysis that showed significantly lower serum 25(OH)D in patients treated with glucocorticoids than in healthy controls, but not in diseased controls (52, 53). Our finding that hormone replacement therapy was associated with significantly higher serum 25(OH)D baseline concentrations is supported by Cheng et al. (54) in univariate but not multivariable models. Alcohol intake was independently associated with higher baseline serum 25(OH)D concentrations in our study, whereas the evidence from other studies is mixed (15, 55–59). Surprisingly, we found that sunscreen use was associated with slightly higher serum 25(OH)D concentrations; this may be because individuals who regularly use sunscreen have greater sun exposure, but may use sunscreen ineffectively (7, 60). In contrast, individuals who regularly wore long pants and long sleeves outdoors had lower concentrations of serum 25(OH)D, a finding that may relate to more effective sun protection, or may reflect confounding by other factors.
Incorporation of selected genetic variants in the full baseline and response models extended our previous work in minimally adjusted models (31). Although the association between rs10766197 and serum response is consistent with our previous results (31), 2 polymorphisms (rs6013897 and rs7968585) that contributed significantly to the minimally adjusted model did not do so in the full model. Furthermore, the inclusion of genetic information in the full model in non-Hispanic whites did not substantially improve the R2 value of the model (R2 = 48%). The finding that genetic factors may have only a small impact on serum response is supported by some research (43), but not all (42). However, even if the impact of the genetic variants appears to be relatively small overall, there may be cumulative effects in individuals with multiple risk alleles (61). In the baseline model, our findings implicating rs10741657 in CYP2R1 and rs4588 (a missense mutation) in GC are largely consistent with other reports (30, 42, 43, 61, 62). In optimally adherent participants, our findings implicating rs4516035 in VDR and rs1801725 (a missense mutation) in CASR appear to be novel and merit further investigation. Recent evidence also suggests that DNA methylation in CYP2R1 and cytochrome P450 family 24 subfamily A member 1 (CYP24A1) may be associated with poorer response to vitamin D supplementation (63); we could not address this question in our study. Ideally, a more comprehensive examination of rare and common variations in the genome will be necessary to further our understanding.
In a crude analysis of participants who had not been taking vitamin D supplements before enrollment or during year 1 of the study, adequate concentrations of serum 25(OH)D (≥20 ng/mL) (7) were found after 1 y in 89% of those randomly assigned to 1000 IU cholecalciferol daily, as well as in 54% of controls. The NAM’s RDA [defined as the intake likely to “meet or exceed the needs of about 97.5% of the population” (7)] is 600 and 800 IU/d for those aged ≤70 and >70 y, respectively. Supplementation with 1000 IU/d did not meet the NAM target in our participants, a finding that is consistent with the assertion that the RDA may not achieve its target in the general population (64, 65). However, this finding was not adjusted for potential confounding (e.g., by season), and our population had special characteristics that may affect generalizability, including a history of colorectal adenoma (but not familial polyposis syndromes), the exclusion of vitamin D–deficient participants (serum 25(OH)D concentration <12 ng/mL), a lower prevalence of minorities (18% compared with 28%), and a higher prevalence of overweight participants (78% compared with 72%) than in the general population (66, 67).
Our models account for 32% and 50% of the variability in baseline serum 25(OH)D and the response to supplementation, respectively. The unexplained variability may relate to differences in absorption, metabolism, and genetic factors, which we could not address in this study. Predictions from our overall model are consistent with those of others. For example, 1000 IU/d is expected to increase a baseline serum 25(OH)D of 30 to 38 ng/mL in Garland’s model (68) and to 39 ng/mL in ours, although in our model, optimal adherence increases the postsupplementation concentration to 42 ng/mL. This difference is not unexpected, but illustrates the importance of adherence as a source of error in dose–response estimation during long-term supplementation. Perhaps not surprisingly, there is considerable variation within (5) and between (69) studies in the slope representing the increase in serum 25(OH)D concentration after vitamin D supplementation. Relative to other studies (69), we observed only a modest mean increase in serum 25(OH)D concentration of 6.3 ng/mL after supplementation with 1000 IU cholecalciferol/d, and a decrease of 1.1 ng/mL in controls. Several study-related factors may have affected these changes: 1) more than one-half of participants were taking vitamin D supplements at baseline, and they agreed to stop doing so; 2) participants were counseled to avoid excessive vitamin D and calcium intake during the study; and 3) three-quarters of our participants were overweight or obese, and so would expect a smaller effect than that seen in other studies (69).
Strengths of our study include the large sample size, inclusion of racial and ethnic minorities of both sexes, availability of detailed personal data, good adherence to pill intake, and detailed information on lapses in pill intake and use of personal vitamin D supplements (which occurred in <5% of participants). We avoided bias that could have resulted from regression to the mean by examining relative proportional increases in our models, comparing those receiving supplements or placebo. Limitations of the study include factors affecting generalizability, such as our enrollment of individuals with colorectal polyps and our counseling of participants to avoid excessive dietary intake of calcium and vitamin D during the study.
In this large, multicenter chemoprevention trial, we found that 50% of the variability in serum response to supplementation with 1000 IU cholecalciferol/d was explained by 8 factors: baseline serum 25(OH)D concentration, sex, optimal adherence, season, use of long pants and long sleeves for protection from the sun, physical activity level, and personal vitamin D supplementation. In contrast to previous smaller studies, we identified a significantly higher response to supplementation in women than in men. Associations were identified with several genetic factors that merit further investigation.
Acknowledgments
JRR, LAM, ELB, JAB, and JLP designed the research; JRR, ELB, JAB, RMB, JCF, RSB, DJR, and JLP conducted the research and/or collected the essential data; LAM analyzed the data; JRR, LAM, ELB, JAB, RMB, JCF, DJR, and JLP wrote the paper; and JRR had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CASR, calcium sensing receptor; CYP2R1, cytochrome P450 family 2 subfamily R member 1; CYP24A1, cytochrome P450 family 24 subfamily A member 1; GC, vitamin D binding protein; NAM, National Academy of Medicine; SNP, single-nucleotide polymorphism; VDR, vitamin D receptor; 25(OH)D, 25-hydroxyvitamin D.
References
- 1.Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol 2008;3:1535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2011;155:827–38. [DOI] [PubMed] [Google Scholar]
- 3.Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, Lichtenstein A, Patel K, Raman G, Tatsioni A, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep) 2009;(183):1–420. [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S. Epidemiology of vitamin D in health and disease. Nutrition research reviews 2009;22:188–203. [DOI] [PubMed] [Google Scholar]
- 5.Aloia JF, Patel M, Dimaano R, Li-Ng M, Talwar SA, Mikhail M, Pollack S, Yeh JK. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr 2008;87:1952–8. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28. Corrected and republished from: Am J Clin Nutr 2007;86(3):809. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington (DC): The National Academies Press, 2010. [PubMed] [Google Scholar]
- 8.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr 2008;88:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006;98:451–9. [DOI] [PubMed] [Google Scholar]
- 10.Brock K, Huang WY, Fraser DR, Ke L, Tseng M, Stolzenberg-Solomon R, Peters U, Ahn J, Purdue M, Mason RS, et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J Steroid Biochem Mol Biol 2010;121:462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brock KE, Graubard BI, Fraser DR, Weinstein SJ, Stolzenberg-Solomon RZ, Lim U, Tangrea JA, Virtamo J, Ke L, Snyder K, et al. Predictors of vitamin D biochemical status in a large sample of middle-aged male smokers in Finland. Eur J Clin Nutr 2010;64:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001–2006. NCHS Data Brief 2011;(59):1–8. [PubMed] [Google Scholar]
- 13.Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr 2008;88:558S–64S. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Dawsey SM, Qiao YL, Mark SD, Dong ZW, Taylor PR, Zhao P, Abnet CC. Prospective study of serum 25(OH)-vitamin D concentration and risk of oesophageal and gastric cancers. Br J Cancer 2007;97:123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egan KM, Signorello LB, Munro HM, Hargreaves MK, Hollis BW, Blot WJ. Vitamin D insufficiency among African-Americans in the southeastern United States: implications for cancer disparities (United States). Cancer Causes Control 2008;19:527–35. [DOI] [PubMed] [Google Scholar]
- 16.Glass D, Lens M, Swaminathan R, Spector TD, Bataille V. Pigmentation and vitamin D metabolism in Caucasians: low vitamin D serum levels in fair skin types in the UK. PLoS One 2009;4:e6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter D, De Lange M, Snieder H, MacGregor AJ, Swaminathan R, Thakker RV, Spector TD. Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Miner Res 2001;16:371–8. [DOI] [PubMed] [Google Scholar]
- 18.Park S, Johnson MA. Living in low-latitude regions in the United States does not prevent poor vitamin D status. Nutr Rev 2005;63:203–9. [DOI] [PubMed] [Google Scholar]
- 19.Rockell JE, Green TJ, Skeaff CM, Whiting SJ, Taylor RW, Williams SM, Parnell WR, Scragg R, Wilson N, Schaaf D, et al. Season and ethnicity are determinants of serum 25-hydroxyvitamin D concentrations in New Zealand children aged 5–14 y. J Nutr 2005;135:2602–8. [DOI] [PubMed] [Google Scholar]
- 20.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008;88:582S–6S. [DOI] [PubMed] [Google Scholar]
- 21.Blum M, Dallal GE, Dawson-Hughes B. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. J Am Coll Nutr 2008;27:274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab 2003;88:157–61. [DOI] [PubMed] [Google Scholar]
- 23.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008;168:1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao G, Ford ES, Li C, Kris-Etherton PM, Etherton TD, Balluz LS. Independent associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with blood pressure among US adults. J Hypertens 2010;28:1821–8. [DOI] [PubMed] [Google Scholar]
- 25.Bogh MK, Schmedes AV, Philipsen PA, Thieden E, Wulf HC. Interdependence between body surface area and ultraviolet B dose in vitamin D production: a randomized controlled trial. Br J Dermatol 2011;164:163–9. [DOI] [PubMed] [Google Scholar]
- 26.Nesby-O’Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr 2002;76:187–92. [DOI] [PubMed] [Google Scholar]
- 27.Mazahery H, von Hurst PR. Factors affecting 25-hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients 2015;7:5111–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zittermann A, Ernst JB, Gummert JF, Borgermann J. Vitamin D supplementation, body weight and human serum 25-hydroxyvitamin D response: a systematic review. Eur J Nutr 2014;53:367–74. [DOI] [PubMed] [Google Scholar]
- 29.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet 2010;19:2739–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barry EL, Rees JR, Peacock JL, Mott LA, Amos CI, Bostick RM, Figueiredo JC, Ahnen DJ, Bresalier RS, Burke CA, et al. Genetic variants in CYP2R1, CYP24A1, and VDR modify the efficacy of vitamin D3 supplementation for increasing serum 25-hydroxyvitamin D levels in a randomized controlled trial. J Clin Endocrinol Metab 2014;99:E2133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Didriksen A, Grimnes G, Hutchinson MS, Kjaergaard M, Svartberg J, Joakimsen RM, Jorde R. The serum 25-hydroxyvitamin D response to vitamin D supplementation is related to genetic factors, BMI, and baseline levels. Eur J Endocrinol 2013;169:559–67. [DOI] [PubMed] [Google Scholar]
- 33.Fu L, Yun F, Oczak M, Wong BY, Vieth R, Cole DE. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem 2009;42:1174–7. [DOI] [PubMed] [Google Scholar]
- 34.Baron JA, Barry EL, Mott LA, Rees JR, Sandler RS, Snover DC, Bostick RM, Ivanova A, Cole BF, Ahnen DJ, et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N Engl J Med 2015;373:1519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International Physical Activity Questionnaire [Internet]. [updated 2016; cited 2016 Aug 9]. Available from: http://www.ipaq.ki.se.
- 36.Bioserve Biotechnologies, Limited[Internet]. [updated 2015; cited 2016 Aug 9]. Available from: http://www.bioserve.com.
- 37.LGC Limited [Internet]. [updated 2015; cited 2016 Aug 9]. Available from: http://www.lgcgenomics.com.
- 38.Sequenom [Internet]. [updated 2016; cited 2016 Aug 9]. Available from: http://www.sequenom.com.
- 39.Thermofisher Scientific[Internet]. [updated 2016; cited 2016 Aug 9]. Available from: http://www.invitrogen.com.
- 40.Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet 2003;361:598–604. [DOI] [PubMed] [Google Scholar]
- 41.Maxmind. Average latitude and longitude for US states [Internet]. [updated 2016; cited 2016 Aug 9]. Available from: http://www.maxmind.com/app/state_latlon.
- 42.Sollid ST, Hutchinson MY, Fuskevag OM, Joakimsen RM, Jorde R. Large individual differences in serum 25-hydroxyvitamin D response to vitamin D supplementation: effects of genetic factors, body mass index, and baseline concentration. Results from a randomized controlled trial. Horm Metab Res 2016;48:27–34. [DOI] [PubMed] [Google Scholar]
- 43.Waterhouse M, Tran B, Armstrong BK, Baxter C, Ebeling PR, English DR, Gebski V, Hill C, Kimlin MG, Lucas RM, et al. Environmental, personal, and genetic determinants of response to vitamin D supplementation in older adults. J Clin Endocrinol Metab 2014;99:E1332–40. [DOI] [PubMed] [Google Scholar]
- 44.Ng K, Scott JB, Drake BF, Chan AT, Hollis BW, Chandler PD, Bennett GG, Giovannucci EL, Gonzalez-Suarez E, Meyerhardt JA, et al. Dose response to vitamin D supplementation in African Americans: results of a 4-arm, randomized, placebo-controlled trial. Am J Clin Nutr 2014;99:587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallagher JC, Sai A, Templin T 2nd, Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med 2012;156:425–37. [DOI] [PubMed] [Google Scholar]
- 46.Zhao LJ, Zhou Y, Bu F, Travers-Gustafson D, Ye A, Xu X, Hamm L, Gorsage DM, Fang X, Deng HW, et al. Factors predicting vitamin D response variation in non-Hispanic white postmenopausal women. J Clin Endocrinol Metab 2012;97:2699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner D, Hanwell HE, Schnabl K, Yazdanpanah M, Kimball S, Fu L, Sidhom G, Rousseau D, Cole DE, Vieth R. The ratio of serum 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) is predictive of 25-hydroxyvitamin D(3) response to vitamin D(3) supplementation. J Steroid Biochem Mol Biol 2011;126:72–7. [DOI] [PubMed]
- 48.Forsythe LK, Livingstone MB, Barnes MS, Horigan G, McSorley EM, Bonham MP, Magee PJ, Hill TR, Lucey AJ, Cashman KD, et al. Effect of adiposity on vitamin D status and the 25-hydroxycholecalciferol response to supplementation in healthy young and older Irish adults. Br J Nutr 2012;107:126–34. [DOI] [PubMed] [Google Scholar]
- 49.Zwart SR, Mehta SK, Ploutz-Snyder R, Bourbeau Y, Locke JP, Pierson DL, Smith SM. Response to vitamin D supplementation during Antarctic winter is related to BMI, and supplementation can mitigate Epstein-Barr Virus Reactivation. J Nutr 2011;141:692–7. [DOI] [PubMed] [Google Scholar]
- 50.Giusti A, Barone A, Pioli G, Girasole G, Razzano M, Pizzonia M, Pedrazzoni M, Palummeri E, Bianchi G. Heterogeneity in serum 25-hydroxy-vitamin D response to cholecalciferol in elderly women with secondary hyperparathyroidism and vitamin D deficiency. J Am Geriatr Soc 2010;58:1489–95. [DOI] [PubMed] [Google Scholar]
- 51.Talwar SA, Aloia JF, Pollack S, Yeh JK. Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr 2007;86:1657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skversky AL, Kumar J, Abramowitz MK, Kaskel FJ, Melamed ML. Association of glucocorticoid use and low 25-hydroxyvitamin D levels: results from the National Health and Nutrition Examination Survey (NHANES): 2001–2006. J Clin Endocrinol Metab 2011;96:3838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davidson ZE, Walker KZ, Truby H. Clinical review: Do glucocorticosteroids alter vitamin D status? A systematic review with meta-analyses of observational studies. J Clin Endocrinol Metab 2012;97:738–44. [DOI] [PubMed] [Google Scholar]
- 54.Cheng TY, Millen AE, Wactawski-Wende J, Beresford SA, LaCroix AZ, Zheng Y, Goodman GE, Thornquist MD, Neuhouser ML. Vitamin D intake determines vitamin d status of postmenopausal women, particularly those with limited sun exposure. J Nutr 2014;144:681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janssen HC, Emmelot-Vonk MH, Verhaar HJ, van der Schouw YT. Determinants of vitamin D status in healthy men and women aged 40–80 years. Maturitas 2013;74:79–83. [DOI] [PubMed] [Google Scholar]
- 56.Jacques PF, Felson DT, Tucker KL, Mahnken B, Wilson PW, Rosenberg IH, Rush D. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am J Clin Nutr 1997;66:929–36. [DOI] [PubMed] [Google Scholar]
- 57.McCullough ML, Weinstein SJ, Freedman DM, Helzlsouer K, Flanders WD, Koenig K, Kolonel L, Laden F, Le Marchand L, Purdue M, et al. Correlates of circulating 25-hydroxyvitamin D: Cohort consortium vitamin D pooling project of rarer cancers. Am J Epidemiol 2010;172:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirazi L, Almquist M, Malm J, Wirfalt E, Manjer J. Determinants of serum levels of vitamin D: a study of life-style, menopausal status, dietary intake, serum calcium, and PTH. BMC Womens Health 2013;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee K. Sex-specific relationships between alcohol consumption and vitamin D levels: the Korea National Health and Nutrition Examination Survey 2009. Nutr Res Pract 2012;6:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diehl JW, Chiu MW. Effects of ambient sunlight and photoprotection on vitamin D status. Dermatol Ther 2010;23:48–60. [DOI] [PubMed] [Google Scholar]
- 61.Nissen J, Vogel U, Ravn-Haren G, Andersen EW, Madsen KH, Nexo BA, Andersen R, Mejborn H, Bjerrum PJ, Rasmussen LB, et al. Common variants in CYP2R1 and GC genes are both determinants of serum 25-hydroxyvitamin D concentrations after UVB irradiation and after consumption of vitamin D(3)-fortified bread and milk during winter in Denmark. Am J Clin Nutr 2015;101:218–27. [DOI] [PubMed] [Google Scholar]
- 62.Engelman CD, Meyers KJ, Iyengar SK, Liu Z, Karki CK, Igo RP Jr, Truitt B, Robinson J, Sarto GE, Wallace R, et al. Vitamin D intake and season modify the effects of the GC and CYP2R1 genes on 25-hydroxyvitamin D concentrations. J Nutr 2013;143:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Y, Zhao LJ, Xu X, Ye A, Travers-Gustafson D, Zhou B, Wang HW, Zhang W, Lee Hamm L, Deng HW, et al. DNA methylation levels of CYP2R1 and CYP24A1 predict vitamin D response variation. J Steroid Biochem Mol Biol 2014;144 Pt A:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veugelers PJ, Ekwaru JP. A statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients 2014;6:4472–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res 2011;26:455–7. [DOI] [PubMed] [Google Scholar]
- 66.Humes KR, Jones NA, Ramirez RR. Overview of Race and Hispanic Origin: 2010. 2010 Census Briefs [Internet]. Washington (DC): US Census Bureau; 2011 [cited 2016 May 26]. Available from: http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf. [Google Scholar]
- 67.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006;295:1549–55. [DOI] [PubMed] [Google Scholar]
- 68.Garland CF, French CB, Baggerly LL, Heaney RP. Vitamin D supplement doses and serum 25-hydroxyvitamin D in the range associated with cancer prevention. Anticancer Res 2011;31:607–11. [PubMed] [Google Scholar]
- 69.Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int 1998;8:222–30. [DOI] [PubMed] [Google Scholar]