Abstract
Background: Cocoa flavanols may improve cardiometabolic health. Evidence from small short-term randomized clinical trials (RCTs) remains inconsistent, and large long-term RCTs testing the efficacy of cocoa flavanols are still lacking.
Objective: We performed a systematic review and meta-analysis of RCTs to quantify the effect of cocoa flavanol intake on cardiometabolic biomarkers.
Methods: We searched PubMed, Web of Science, and the Cochrane Library for RCTs that evaluated the effects of cocoa flavanols on biomarkers relevant to vascular disease pathways among adults. Data were extracted following a standardized protocol. We used DerSimonian and Laird random-effect models to compute the weighted mean differences (WMDs) and 95% CIs. We also examined potential modification by intervention duration, design, age, sex, comorbidities, and the form and amount of cocoa flavanol intake.
Results: We included 19 RCTs that comprised 1131 participants, and the number of studies for a specific biomarker varied. The amount of cocoa flavanols ranged from 166 to 2110 mg/d, and intervention duration ranged from 2 to 52 wk. Cocoa flavanol intake significantly improved insulin sensitivity and lipid profile. The WMDs between treatment and placebo were −0.10 mmol/L (95% CI: −0.16, −0.04 mmol/L) for total triglycerides, 0.06 mmol/L (95% CI: 0.02, 0.09 mmol/L) for HDL cholesterol, −2.33 μIU/mL (95% CI: −3.47, −1.19 μIU/mL) for fasting insulin, −0.93 (95% CI: −1.31, −0.55) for the homeostatic model assessment of insulin resistance, 0.03 (95% CI: 0.01, 0.05) for the quantitative insulin sensitivity check index, 2.54 (95% CI: 0.63, 4.44) for the insulin sensitivity index, −0.83 mg/dL (95% CI: −0.88, −0.77 mg/dL) for C-reactive protein, and 85.6 ng/mL (95% CI: 16.0, 155 ng/mL) for vascular cell adhesion molecule 1. No significant associations were found for other biomarkers. None of the modifiers seemed to qualitatively modify the effects of cocoa flavanol intake.
Conclusions: Our study suggests that cocoa flavanol intake has favorable effects on select cardiometabolic biomarkers among adults. These findings support the need for large long-term RCTs to assess whether cocoa flavanol intake reduces the risk of diabetes and cardiovascular events.
Keywords: cocoa flavanols, cardiometabolic health, randomized controlled trials, meta-analysis, biomarkers
Introduction
Cardiometabolic diseases are among the leading causes of morbidity and mortality worldwide (1, 2). In observational studies, dietary intake of flavanol-rich cocoa products, such as dark chocolate, has been associated with a reduced risk of cardiometabolic diseases, including cardiovascular disease (3), hypertension (4), metabolic syndrome (5), and diabetes (6). Given these possible protective effects on cardiometabolic health, cocoa products may add to the armamentarium of bioactives.
Cocoa products are generally considered a source of dietary flavanols that may underlie their purported health benefits (5–8), although their flavanol profile and content vary by cultivars and fermentation procedures. Evidence from previous meta-analyses of randomized clinical trials (RCTs)1010 suggest that chocolate, cocoa, or cocoa flavanols may lower blood pressure (9) and improve cardiometabolic health (10, 11). Unfortunately, the evidence from RCTs for cocoa flavanols remains limited (10). Synthesized evidence from observational studies has also shown that chocolate consumption may reduce the risk of ischemic heart disease (12) and stroke (13). Hypothesized mechanisms that underlie potential associations between cocoa flavanols and a reduced risk of chronic diseases include improvements in the lipid profile (14), insulin sensitivity (15, 16), and endothelial function (17) and the alleviation of systemic inflammation (18), thrombosis (19, 20), and oxidation (14, 21).
We therefore conducted a meta-analysis of RCTs to assess the effects of cocoa flavanol intake on a variety of circulating cardiometabolic biomarkers. We also examined whether the effects of cocoa flavanols differ by study design, participant age, sex, intervention duration, existing comorbidities, and the form and amount of cocoa flavanol intake.
Methods
Data sources and searches.
We followed a standardized protocol in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines to conduct this meta-analysis (22). Two investigators independently conducted literature searches of PubMed, Web of Science, and the Cochrane Central Register of Controlled Trials published from January 1965 (index date) to December 2015 with the use of terms from Medical Subject Headings, including cacao, cocoa, chocolate, clinical trial, controlled clinical trial, and RCT. The search was limited to trials on human participants and articles in English. All relevant studies and review articles (including meta-analysis) and the reference lists of the identified articles were checked manually. Any disagreement between the 2 investigators was resolved by consensus. An institutional review board review was not applicable because we conducted a systematic review and meta-analysis, which do not directly involve human subjects.
Study selection.
Articles were included if the study was 1) an RCT that assigned ≥1 group of participants to cocoa products, chocolate, or cocoa flavanol supplements and 1 group to placebo and 2) circulating cardiometabolic biomarkers in blood samples, including plasma, serum, and whole blood, were measured at baseline and at the end of each intervention. All abstracts that reported the effects of cocoa flavanols on cardiometabolic biomarkers were included for screening. Studies were excluded if 1) the study design was not an RCT or there was no placebo group; 2) the intervention was not cocoa products, chocolate, or cocoa flavanol supplements; 3) the biomarker concentrations were monitored ≤1 wk after the acute intervention; 4) the amount of cocoa flavanols in the active intervention was <100 mg/d; 5) the participants were pregnant women, children, or adolescents; or 6) values of outcome measures at the end of the trial or changes from baseline were not reported.
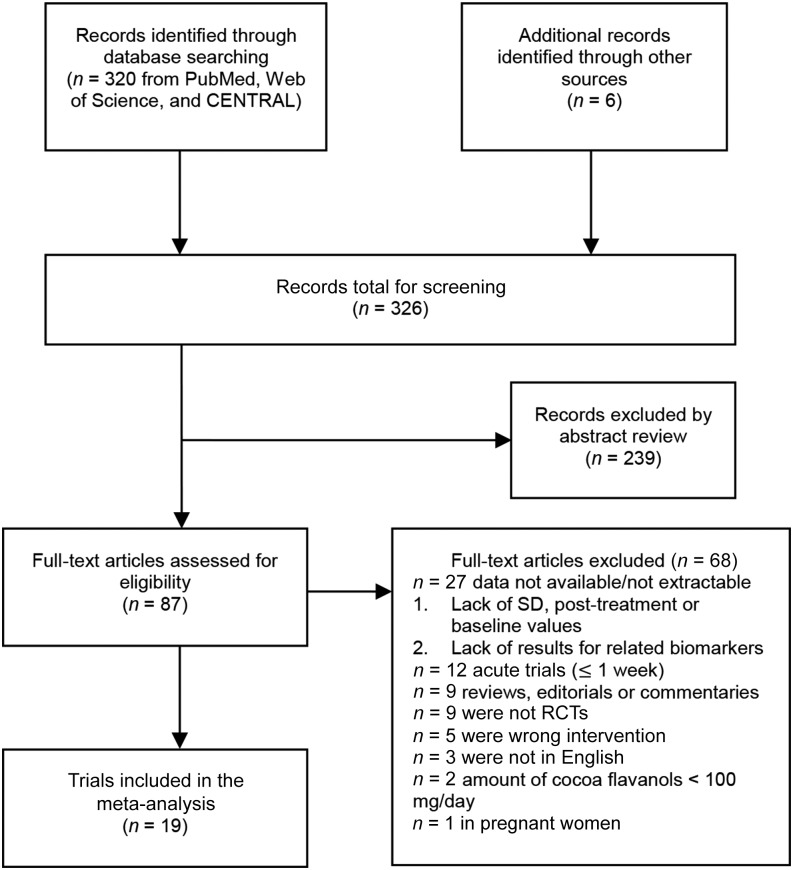
In total, 320 articles were retrieved from the literature search, and 6 additional articles were retrieved from cross-reference and expert sources (Figure 1). We excluded 239 articles after reviewing the titles and abstracts and 68 more after examining the full text. The final set of articles for our systematic review and meta-analysis included 1131 participants from 19 unique RCTs.
FIGURE 1.
Flowchart of the study selection of 19 RCTs eligible for the meta-analysis. In total, 326 articles were identified that evaluated the effect of cocoa flavanols on cardiometabolic biomarkers. We excluded 239 articles after abstract review and 68 after full-text examination. After exclusion, 19 RCTs (n = 1131) were included in the meta-analysis. RCT, randomized control trial.
Data extraction and quality assessment.
Data were extracted according to a pre-established protocol. The following information was extracted from the included RCTs: general information (first author’s name, year of publication, title); study characteristics (study design, eligibility criteria, trial quality, intervention duration, and the form and amount of cocoa flavanol intake); participant characteristics (age, proportion of men, race/ethnicity, and comorbidities); and outcome measures (definition of outcomes, statistical methods, pre- and postintervention means and SDs, sample size of each arm, and adverse events). Methodologic quality was assessed with the use of the Cochrane Collaboration’s tool for assessing the risk of bias (23) and included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. For each trial, the risk of bias was reported as low, unclear, or high. The criteria used for quality assessment have been described in detail elsewhere (23).
Data synthesis and analysis.
Mean changes and SDs of cardiometabolic biomarkers from baseline in the treatment and placebo groups were used to calculate the weighted mean differences (WMDs) and 95% CIs with the use of the DerSimonian and Laird random-effects models (24). Between-study heterogeneity was examined with the use of I2 statistics (25), with an I2 of 25%, 50%, and 75% denoting low, medium, and high heterogeneity, respectively. Begg’s and Egger’s tests formally tested for publication bias (26, 27). If there were any evidence of publication bias, the trim and fill method evaluated its impact (28).
Meta-regressions evaluated the overall impact of the predetermined potential modifiers, including study design, the form of cocoa flavanol intake, the amount of cocoa flavanol intake, age, sex, intervention duration, and existing comorbidity. Cutoffs of 200 and 600 mg/d for the categorical cocoa flavanol amount tested were selected based on prior knowledge and what has been used in previous studies. The categorical variable for intervention duration was created based on the median duration of all included RCTs.
P ≤ 0.05 was considered significant except for the tests of publication bias (P = 0.10) (29). All statistical analyses were performed with Stata version 13 (StataCorp LP).
Results
Figure 1 shows how the participants from the 19 RCTs were selected for the meta-analysis. The actual number of studies synthesized varied across biomarkers. Characteristics of eligible trials are summarized in Table 1. Among all participants, the proportion of men ranged from 0% to 100%, with a mean age of 30–71 y. The amount of cocoa flavanols tested for the RCTs ranged from 166 to 2110 mg/d, and intervention duration ranged from 2 to 52 wk. Among the included RCTs, 11 trials used a crossover design, 8 trials used a placebo-controlled parallel design, and 13 trials were conducted among participants with existing comorbidities, including hypertension, coronary artery disease, diabetes, overweight, hypercholesterolemia, metabolic syndrome, or mild cognitive impairment.
TABLE 1.
Characteristics of trials included in the meta-analysis1
| Author (reference) | Year | Country | nI/nC | Age, y | Men, % | Comorbidity | Cocoa flavanols, mg/d | Cocoa flavanol form | Duration, wk | Source of blood measurements | Fasting status |
| Grassi et al. (30) | 2005 | Italy | 15/15 | 34 | 47 | None | 500 | Chocolate bars | 2 | Serum: lipids; plasma: insulin and glucose | Yes |
| Grassi et al. (31) | 2005 | Italy | 20/20 | 44 | 50 | Hypertensive | 500 | Chocolate bars | 2 | Serum: lipids; plasma: insulin and glucose | Yes |
| Farouque et al. (32) | 2006 | Australia | 20/20 | 61 | 75 | Coronary artery disease | 444 | Chocolate bars and beverage | 6 | Serum: lipids, lipoproteins, and glucose; plasma: insulin and biomarkers of endothelial function | Yes |
| Baba et al. (33) | 2007 | Japan | 13/12 | 38 | 43 | None | 199 | Beverage | 12 | Plasma | Yes |
| Shiina et al. (34) | 2009 | Japan | 20/19 | 30 | 100 | None | 550 | Chocolate bars | 2 | Serum | NR |
| Balzer et al. (35) | 2008 | Germany | 21/20 | 64 | 29 | Diabetic | 963 | Beverage | 4 | Plasma | Yes |
| Davison et al. (36) | 2008 | Australia | 12/12 | 45 | 33 | Overweight | 902 | Beverage | 12 | Plasma | Yes |
| 12/13 | 45 | 31 | Overweight | 902 | Beverage | 12 | Plasma | ||||
| Grassi et al. (4) | 2008 | Italy | 19/19 | 45 | 58 | Hypertensive | 1080 | Chocolate bars | 2 | Serum: lipids, CRP, and insulin; plasma: glucose | Yes |
| Muniyappa et al. (37) | 2008 | United States | 20/20 | 51 | 40 | Hypertensive | 902 | Beverage | 2 | Serum: lipids, biomarkers of endothelial function, and adipocytokines; plasma: glucose and insulin | Yes |
| Monagas et al. (38) | 2009 | Spain | 42/42 | 70 | 45 | High CV risk | 495 | Beverage | 4 | Serum: glucose, lipids, inflammatory biomarkers, biomarkers of endothelial function | Yes |
| Njike et al. (39) | 2011 | United States | 38/39 | 52 | 15 | Overweight | 805 | Beverage | 6 | Serum | Yes |
| Mellor et al. (40) | 2010 | United Kingdom | 12/12 | 68 | 58 | Diabetic | 166 | Chocolate bars | 8 | Serum: insulin and lipids; plasma: glucose | Yes |
| Almoosawi et al. (41) | 2012 | United Kingdom | 21/21 | NR | 0 | None | 200 | Beverage | 4 | NR | Yes |
| 21/21 | NR | 0 | Overweight | 200 | Beverage | 4 | NR | Yes | |||
| Curtis et al. (42) | 2012 | United Kingdom | 50/59 | 62 | 0 | Diabetic | 850 | Chocolate bars | 52 | Plasma | Yes |
| Desideri et al. (43) | 2012 | Italy | 30/30 | 71 | 48 | Cognitively impaired | 990 | Beverage | 8 | Plasma | Yes |
| Neufingerl et al. (44) | 2013 | France | 37/37 | 55 | 50 | None | 325 | Beverage | 4 | Serum | Yes |
| 32/37 | 55 | 50 | None | 325 | Beverage | 4 | Serum | Yes | |||
| Sarria et al. (45) | 2014 | Spain | 24/24 | 27 | 46 | None | 400 | Beverage | 2 | Serum: lipids, lipoprotein, glucose, urea, uric acid, and creatinine | Yes |
| 20/20 | 30 | 45 | High cholesterol | 400 | Beverage | 2 | Plasma: inflammatory biomarkers | Yes | |||
| West et al. (46) | 2014 | United States | 30/30 | 53 | 0 | None | 814 | Chocolate bars and beverage | 4 | Serum: lipids, lipoproteins, CRP, and insulin; plasma: inflammatory biomarkers and glucose | Yes |
| D’Anna et al. (47) | 2014 | Italy | 30/30 | 56 | 0 | Metabolic syndrome | 2110 | Beverage | 24 | Serum | Yes |
CRP, C-reactive protein; nI, sample size of the intervention group; nC, sample size of the placebo group; NR, not reported.
Description of study quality.
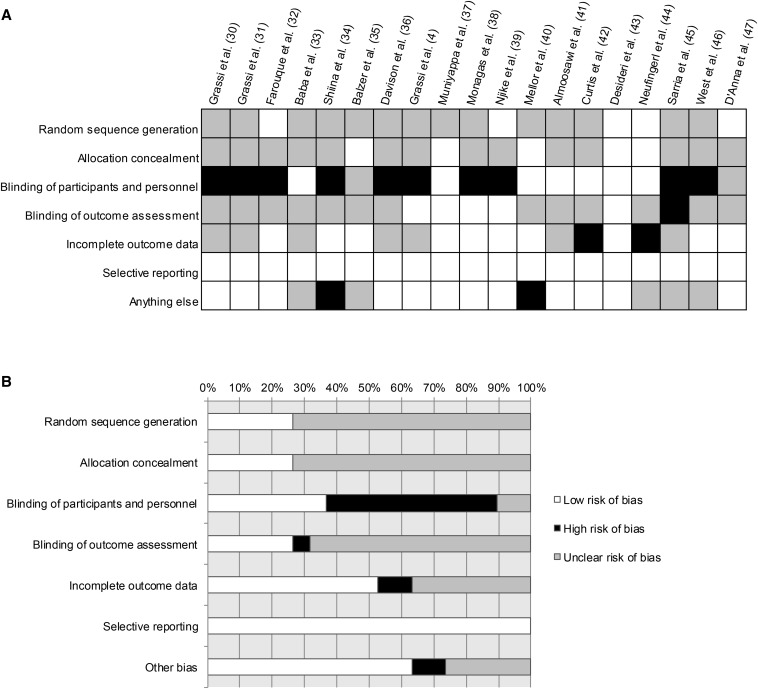
The quality of studies included was heterogeneous (Figure 2). Random sequence generation and allocation concealment were reported in 6 trials. Allocation concealment was also reported in 6 trials. The risk of potential performance bias was low in 7 trials. Among 6 trials with available information on whether outcome assessment was blinded, the risk of detection bias was high in only 1 trial. The outcome data were incomplete in 2 trials. The risk of another bias was high in 2 trials because of poor compliance or other study-specific limitations.
FIGURE 2.
Assessment of the risk of bias for 19 selected randomized control trials: summary for items of bias. (A) Risk of bias for all trials included in the meta-analysis presented for individual trials (represented by author name and year) as low, high, or unclear risk of bias in each assessment item; (B) risk of bias for all trials included in the meta-analysis presented as the percentages of trials with low, high, or unclear risk of bias in each assessment item.
Lipid and lipoprotein biomarkers.
The number of trials included for each lipid and lipoprotein biomarker is shown in Table 2. Cocoa flavanol intake significantly lowered TGs (P < 0.001) and increased HDL cholesterol concentrations (P < 0.001) compared with placebo (Table 2). The WMDs were −0.10 mmol/L (95% CI: −0.16, −0.04 mmol/L; I2 = 28%) for TGs (Table 2, Supplemental Figure 1) and 0.06 mmol/L (95% CI: 0.02, 0.09 mmol/L; I2 = 71%) for HDL cholesterol (Table 2, Supplemental Figure 2). The P values of the Egger’s or Begg’s tests for all lipid biomarkers were >0.10, suggesting that the small study effects were not significant. After correcting for multiple comparisons, the associations observed for TGs and HDL cholesterol remained significant (q = 0.002 and 0.003).
TABLE 2.
WMDs in biomarkers in cocoa flavonol intervention groups compared with placebo groups1
| Biomarkers | n | WMD (95% CI) | P | I2, % |
| Glucose, mmol/L | 18 | −0.19 (−0.40, 0.03) | 0.09 | 82 |
| Insulin, μIU/mL | 10 | −2.33 (−3.47, −1.19) | <0.001 | 66 |
| HOMA-IR | 11 | −0.93 (−1.31, −0.55) | <0.001 | 85 |
| HbA1c, % | 3 | 0.00 (−0.23, 0.24) | 0.99 | 0 |
| QUICKI | 7 | 0.03 (0.01, 0.05) | 0.01 | 97 |
| ISI | 4 | 2.54 (0.63, 4.44) | 0.01 | 86 |
| TC, mmol/L | 19 | −0.07 (−0.15, 0.01) | 0.10 | 27 |
| TG, mmol/L | 20 | −0.10 (−0.16, −0.04) | 0.001 | 28 |
| HDL cholesterol, mmol/L | 20 | 0.06 (0.02, 0.09) | 0.001 | 71 |
| LDL cholesterol, mmol/L | 19 | −0.26 (−0.56, 0.04) | 0.09 | 95 |
| ApoAI, g/L | 2 | 0.05 (−0.04, 0.14) | 0.29 | 0 |
| ApoB, g/L | 2 | −0.02 (−0.08, 0.05) | 0.65 | 0 |
| CRP, mg/dL | 5 | −0.83 (−0.88, −0.77) | <0.001 | 0 |
| IL-6, pg/mL | 3 | 0.04 (−0.34, 0.43) | 0.82 | 0 |
| TNF-α, pg/mL | 2 | −0.09 (−0.45, 0.27) | 0.62 | 0 |
| ICAM-1, ng/mL | 4 | −0.52 (−4.30, 3.27) | 0.79 | 0 |
| VCAM-1, ng/mL | 3 | 85.6 (16.0, 155) | 0.02 | 0 |
| MCP-1, pg/mL | 2 | −8.49 (−67.1, 50.1) | 0.78 | 0 |
| Creatinine, mg/L | 4 | −0.22 (−0.82, 0.38) | 0.47 | 77 |
| Uric acid, mg/L | 3 | −0.64 (−2.79, 1.50) | 0.56 | 0 |
| Urea, mg/dL | 3 | −13.7 (−62.7, 35.2) | 0.58 | 71 |
| E-selectin, ng/mL | 3 | −2.48 (−10.5, 5.52) | 0.54 | 0 |
| P-selectin, ng/mL | 2 | 5.96 (−28.6, 40.5) | 0.74 | 29 |
| Adiponectin, μg/mL | 2 | 0.78 (−1.73, 3.28) | 0.54 | 0 |
| Oxidized LDL, unit/L | 2 | 2.88 (−3.49, 9.24) | 0.38 | 0 |
CRP, C-reactive protein; HbA1c, glycated hemoglobin; ICAM-1, intercellular adhesion molecule 1; ISI, insulin sensitivity index; MCP-1, monocyte chemoattractant protein 1; QUICKI, quantitative insulin sensitivity check index; TC, total cholesterol; VCAM-1, vascular cell adhesion molecule 1; WMD, weighted mean difference.
Biomarkers of insulin resistance.
Table 2 also shows the effects of cocoa flavanol intake on insulin resistance. Fasting insulin concentrations and HOMA-IR were each significantly lower in the cocoa flavanol groups than the placebo group (each P < 0.001), whereas the quantitative insulin sensitivity check index (QUICKI) and insulin sensitivity index (ISI) were significantly improved among the cocoa flavanol groups compared with placebo (each P = 0.01) (Table 2). The WMDs between the cocoa flavanol and placebo groups were −2.33 μIU/mL (95% CI: −3.47, −1.19 μIU/mL; I2 = 66%) for fasting insulin (Table 2, Supplemental Figure 3) and −0.93 (95% CI: −1.31, −0.55; I2 = 85%) for HOMA-IR (Table 2, Supplemental Figure 4). The WMD for QUICKI was 0.03 (95% CI: 0.01, 0.05; I2 = 97%), and the WMD for ISI based on postintervention values was 2.54 (95% CI: 0.63, 4.44; I2 = 86%) (Table 2). There was no evidence of publication bias from the Egger’s or Begg’s tests (all P > 0.10). After correcting for multiple comparisons, the associations observed for insulin (q < 0.001), HOMA-IR (q < 0.001), QUICKI (q = 0.02), and ISI (q = 0.02) remained significant.
Other biomarkers.
The WMDs were −0.22 mg/L (95% CI: −0.82, 0.38 mg/L; I2 = 77%) for creatinine and −0.64 mg/L (95% CI: −2.79, 1.50 mg/L; I2 = 0%) for uric acid. Because of the limited information reported by individual trials, we were unable to compute WMDs of change scores for other biomarkers. Therefore, postintervention values and change scores reported in the original articles were used for those biomarkers. A significant difference between the cocoa flavanol intervention and placebo groups was found for C-reactive protein (WMD = −0.83 mg/dL; 95% CI: −0.88, −0.77 mg/dL; P < 0.001; I2 = 0%) and vascular cell adhesion molecule 1 (VCAM-1) (WMD = 85.6 mg/L; 95% CI: 16.0, 155 mg/L; P = 0.02; I2 = 0%) (Table 2). After correcting for multiple comparisons, the associations observed for C-reactive protein (q < 0.001) and VCAM-1 (q = 0.02) remained significant. With a relatively limited number of RCTs available, our analyses did not show any statistically significant overall effects of cocoa flavanols on biomarkers of oxidative stress.
Modifying effects of age, sex, duration, design, comorbidities, and form and amount of cocoa flavanols.
Meta-regressions and subgroup analyses were conducted for biomarkers with ≥10 trials included in the primary analyses. Age and sex did not modify the effects of cocoa flavanol intake on lipid metabolism and insulin resistance (all Pmeta-regression ≥ 0.05). Cocoa flavanols improved the profiles of lipid metabolism and insulin resistance regardless of intervention duration, design, or form of cocoa flavanols (Tables 3–7). Cocoa flavanol intake <200 mg/d was significantly associated with elevated circulating HDL cholesterol concentrations (WMD = 0.19 mmol/L; 95% CI: 0.12, 0.26 mmol/L; P < 0.001); cocoa flavanol intake between ≥200 and <600 mg/d showed significant beneficial effects on fasting glucose (WMD = −0.26 mmol/L; 95% CI: −0.40, −0.13 mmol/L; P < 0.001), fasting insulin (WMD = −2.43 μIU/mL; 95% CI: −4.81, −0.05 μIU/mL; P = 0.05), HOMA-IR (WMD = −0.72; 95% CI: −1.15, −0.29; P = 0.001), and HDL cholesterol (WMD = 0.06 mmol/L; 95% CI: 0.02, 0.09 mmol/L; P = 0.001); and cocoa flavanol intake ≥600 mg/d significantly reduced fasting insulin (WMD = −2.19 μIU/mL; 95% CI: −3.69, −0.69 μIU/mL; P = 0.004), HOMA-IR (WMD = −1.05; 95% CI: −1.69, −0.41; P = 0.001), and TGs (WMD = −0.09 mmol/L; 95% CI: −0.16, −0.02 mmol/L; P = 0.01) (Table 6).
TABLE 3.
WMDs in lipids and insulin resistance-related biomarkers in cocoa flavonol intervention groups compared with placebo groups by health status1
| With existing comorbidities |
Without existing comorbidities |
||||||
| Biomarker | n | WMD (95% CI) | P | n | WMD (95% CI) | P | P-meta-regression |
| Glucose, mmol/L | 13 | −0.22 (−0.48, 0.04) | 0.10 | 2 | 0.16 (−0.25, 0.57) | 0.44 | 0.31 |
| Insulin, μIU/mL | 7 | −2.63 (−4.20, −1.01) | 0.001 | 0 | NA | NA | NA |
| HOMA-IR | 7 | −1.15 (−1.71, −0.60) | <0.001 | 1 | −0.71 (−1.24, −0.18) | 0.01 | 0.62 |
| TC, mmol/L | 12 | −0.12 (−0.25, 0.02) | 0.10 | 6 | −0.03 (-0.14, 0.09) | 0.67 | 0.40 |
| TG, mmol/L | 13 | −0.09 (−0.14, −0.03) | 0.002 | 6 | −0.17 (−0.34, −0.00) | 0.05 | 0.22 |
| HDL cholesterol, mmol/L | 13 | 0.05 (0.00, 0.09) | 0.04 | 6 | 0.08 (0.02, 0.15) | 0.02 | 0.38 |
| LDL cholesterol, mmol/L | 12 | −0.35 (−0.77, 0.08) | 0.11 | 6 | −0.06 (−0.18, 0.07) | 0.37 | 0.35 |
TC, total cholesterol; WMD, weighted mean difference.
TABLE 7.
WMDs in lipids and insulin resistance-related biomarkers in cocoa flavonol intervention groups compared with placebo groups by form of cocoa flavanols1
| Chocolate bars |
Powder or powder-based beverage |
Combined |
||||||||
| Biomarker | n | WMD (95% CI) | P | n | WMD (95% CI) | P | n | WMD (95% CI) | P | P-meta-regression |
| Glucose, mmol/L | 5 | −0.36 (−0.52, −0.19) | <0.001 | 11 | −0.10 (−0.42, 0.21) | 0.52 | 2 | 0.05 (−0.19, 0.29) | 0.70 | 0.40 |
| Insulin, μIU/mL | 5 | −2.21 (−3.70, −0.73) | 0.003 | 4 | −2.71 (−5.86, 0.44) | 0.09 | 1 | −2.30 (−3.99, −0.61) | 0.01 | 0.98 |
| HOMA-IR | 7 | −1.06 (−1.61, −0.51) | <0.001 | 3 | −0.82 (−1.60, −0.04) | 0.04 | 1 | −0.47 (−0.85, −0.09) | 0.01 | 0.72 |
| TC, mmol/L | 6 | −0.08 (−0.24, 0.08) | 0.31 | 12 | −0.04 (−0.14, 0.06) | 0.42 | 1 | −0.30 (−0.64, 0.04) | 0.08 | 0.49 |
| TG, mmol/L | 5 | −0.21 (−0.37, −0.05) | 0.01 | 13 | −0.09 (−0.14, −0.05) | <0.001 | 2 | 0.14 (−0.20, 0.48) | 0.42 | 0.12 |
| HDL cholesterol, mmol/L | 6 | 0.06 (0.004, 0.12) | 0.04 | 13 | 0.06 (0.01, 0.10) | 0.02 | 1 | 0.03 (−0.05, 0.11) | 0.48 | 0.94 |
| LDL cholesterol, mmol/L | 5 | −0.17 (−0.32, −0.02) | 0.03 | 12 | −0.28 (−0.76, 0.20) | 0.25 | 2 | −0.43 (−0.70, −0.16) | 0.002 | 0.89 |
TC, total cholesterol; WMD, weighted mean difference.
TABLE 6.
WMDs in lipids and insulin resistance-related biomarkers in cocoa flavonol intervention groups compared with placebo groups by amount of cocoa flavanols1
| <200 mg/d |
≥200 to <600 mg/d |
≥600 mg/d |
||||||||
| n | WMD (95% CI) | P | n | WMD (95% CI) | P | n | WMD (95% CI) | P | P-meta-regression | |
| Glucose, mmol/L | 2 | 0.31 (−0.06, 0.68) | 0.10 | 7 | −0.26 (−0.40, −0.13) | <0.001 | 9 | −0.20 (−0.56, 0.16) | 0.29 | 0.02 |
| Insulin, μIU/mL | 1 | −3.80 (−8.24, 0.64) | 0.09 | 3 | −2.43 (−4.81, −0.05) | 0.05 | 6 | −2.19 (−3.69, −0.69) | 0.004 | 0.86 |
| HOMA-IR | 1 | −1.20 (−2.57, 0.17) | 0.09 | 4 | −0.72 (−1.15, −0.29) | 0.001 | 6 | −1.05 (−1.69, −0.41) | 0.001 | 0.28 |
| TC, mmol/L | 2 | −0.07 (−0.39, 0.26) | 0.68 | 9 | −0.09 (−0.20, 0.02) | 0.10 | 8 | −0.06 (−0.22, 0.10) | 0.46 | 0.90 |
| TG, mmol/L | 2 | −0.11 (−0.36, 0.14) | 0.40 | 9 | −0.13 (−0.26, 0.01) | 0.06 | 9 | −0.09 (−0.16, −0.02) | 0.01 | 0.44 |
| HDL cholesterol, mmol/L | 2 | 0.19 (0.12, 0.26) | <0.001 | 9 | 0.06 (0.02, 0.09) | 0.001 | 9 | 0.02 (−0.04, 0.08) | 0.54 | 0.48 |
| LDL cholesterol, mmol/L | 2 | −0.25 (−0.58, −0.09) | 0.15 | 9 | −0.13 (−0.28, 0.01) | 0.07 | 8 | −0.40 (−1.02, 0.21) | 0.20 | 0.31 |
TC, total cholesterol; WMD, weighted mean difference.
TABLE 4.
WMDs in lipids and insulin resistance-related biomarkers in cocoa flavonol intervention groups compared with placebo groups by trial design1
| Crossover |
Parallel |
||||||
| Biomarker | n | WMD (95% CI) | P | n | WMD (95% CI) | P | P-meta-regression |
| Glucose, mmol/L | 10 | −0.09 (−0.27, 0.10) | 0.36 | 8 | −0.31 (−0.69, 0.06) | 0.10 | 0.23 |
| Insulin, μIU/mL | 6 | −2.02 (−3.49,-0.56) | 0.01 | 4 | −3.08 (−5.37, −0.79) | 0.01 | 0.47 |
| HOMA-IR | 7 | −1.00 (−1.51,-0.49) | <0.001 | 4 | −0.82 (−1.40, −0.25) | 0.01 | 0.68 |
| TC, mmol/L | 9 | −0.03 (−0.15, 0.10) | 0.69 | 10 | −0.13 (−0.23, −0.02) | 0.02 | 0.26 |
| TG, mmol/L | 9 | −0.07 (−0.13, −0.01) | 0.02 | 11 | −0.11 (−0.21, −0.01) | 0.03 | 0.58 |
| HDL cholesterol, mmol/L | 9 | 0.06 (0.02, 0.10) | 0.003 | 11 | 0.05 (−0.00, 0.11) | 0.06 | 0.88 |
| LDL cholesterol, mmol/L | 9 | −0.04 −0.21, 0.13) | 0.62 | 10 | −0.39 (−0.86, 0.08) | 0.10 | 0.21 |
TC, total cholesterol; WMD, weighted mean difference.
TABLE 5.
WMDs in lipids and insulin resistance-related biomarkers in cocoa flavonol intervention groups compared with placebo groups by intervention duration1
| >4 wk |
≤4 wk |
||||||
| Biomarker | n | WMD (95% CI) | P | n | WMD (95% CI) | P | P-meta-regression |
| Glucose, mmol/L | 9 | −0.26 (−0.66, 0.15) | 0.21 | 9 | −0.14 (−0.29, 0.02) | 0.09 | 0.08 |
| Insulin, μIU/mL | 5 | −3.12 (−5.06, −1.19) | 0.002 | 5 | −1.88 (−3.43, −0.33) | 0.02 | 0.70 |
| HOMA-IR | 5 | −0.87 (−1.38, −0.35) | 0.001 | 6 | −0.98 (−1.52, −0.45) | <0.001 | 0.66 |
| TC, mmol/L | 8 | −0.07 (−0.22, 0.09) | 0.38 | 11 | −0.08 (−0.18, 0.02) | 0.11 | 0.88 |
| TG, mmol/L | 9 | −0.08 (−0.15, −0.01) | 0.03 | 11 | −0.15 (−0.26, −0.04) | 0.01 | 0.89 |
| HDL cholesterol, mmol/L | 9 | 0.09 (0.04, 0.15) | 0.001 | 11 | 0.03 (−0.01, 0.07) | 0.11 | 0.52 |
| LDL cholesterol, mmol/L | 8 | −0.44 (−1.00, 0.13) | 0.13 | 11 | −0.10 (−0.21, 0.01) | 0.06 | 0.72 |
TC, total cholesterol; WMD, weighted mean difference.
Discussion
In this systematic review and meta-analysis of 1131 participants from 19 RCTs, we found that cocoa flavanol intake from cocoa products, chocolate, or cocoa flavanol supplements significantly improved biomarkers of lipid metabolism and insulin resistance. Our meta-analysis of RCTs is among the first to our knowledge to characterize how cocoa flavanols affect cardiometabolic biomarkers. We found that cocoa flavanol intake may reduce dyslipidemia, insulin resistance, and systemic inflammation, which are all major subclinical risk factors for cardiometabolic diseases.
The favorable associations between cocoa flavanols and cardiometabolic health have been reported in 3 major US prospective cohort studies: Nurses’ Health Study I, Nurses’ Health Study II, and the Health Professionals Follow-Up Study. Wedick et al. (48) followed >130,000 women and men, identified 12,611 incident type 2 diabetes cases, and reported a modestly reduced risk in type 2 diabetes of similar magnitude across quintiles 2–5 of flavanol intake compared with the lowest quintile (pooled HRs: 0.92, 0.91, 0.94, and 0.91, respectively). Similar findings were also reported in the European Prospective Investigation into Cancer and Nutrition study (49, 50). However, these findings were not confirmed in a study of 35,816 postmenopausal women (51).
Flavanols have been shown to inhibit glucosidase and glucose absorption from the intestine, protect pancreatic β cells, increase insulin secretion, activate insulin receptors and glucose uptake in insulin-sensitive tissues, and modulate intracellular signaling pathways and genes involved in gluconeogenesis and glycogenesis (15, 52). Flavanols may also improve insulin sensitivity by increasing NO bioavailability and inhibiting production of reactive oxygen species and nitrogen species (5, 6). The increased bioavailability of NO may also mediate the beneficial effects of cocoa flavanols on endothelial function (53). Experimental studies have shown that cocoa supplementation slowed body weight gain, increased plasma concentrations of adiponectin, and attenuated insulin resistance, as indicated by improved HOMA-IR (54, 55). In addition, a growing body of evidence derived from both in vitro studies and animal studies also demonstrates the antidyslipidemia and anti-inflammation effects of cocoa and cocoa flavanols (14, 56, 57) (58–60). However, evidence from humans is restricted to observational studies and smaller short-term trials. Only a few studies to our knowledge have synthesized the evidence for the effects of cocoa flavanol intake on specific biological parameters beyond those characterized by excess oxidative stress.
Previous reviews and meta-analyses have supported the notion that chocolate, cocoa products, and cocoa flavanol supplements may improve cardiometabolic health (10–13, 61, 62). A recent systematic review suggests beneficial effects of food sources of flavan-3-ols (green tea and cocoa) on cardiovascular health (57). Ding et al. (12) reported in a systematic review of observational studies that cocoa consumption may reduce ischemic heart disease mortality. In a meta-analysis of prospective cohorts of men, Larsson et al. (62) found that chocolate consumption was associated with a lower risk of stroke (RR = 0.83 comparing the highest and the lowest quartile of chocolate consumption). For intermediate cardiometabolic biomarkers, a meta-analysis of 20 RCTs reported that cocoa product consumption had a small but statistically significant effect on lowering blood pressure (−2.8 mm Hg systolic and −2.2 mm Hg diastolic) (63). Another meta-analysis of RCTs conducted by Hooper et al. (10) showed that both flow-mediated dilation and HOMA-IR were also improved after chocolate consumption, and Shrime et al. (64) showed beneficial effects of cocoa products of lowering blood pressure and improving insulin sensitivity, lipid profiles, and flow-mediated dilation in a meta-analysis of short-term studies. Our updated meta-analysis of RCTs has not only updated previous findings (10) but also added cardiometabolic biomarkers involved in lipid metabolism, insulin resistance, systemic inflammation, renal function, and oxidative stress.
Strengths of this meta-analysis include the synthesis of evidence from RCTs that examined both conventional and novel cardiometabolic biomarkers, detailed subgroup analyses for potential effect modifiers, and a comprehensive evaluation of potential bias. Our meta-analysis is among the first to our knowledge to synthesize evidence for the novel, less-studied cardiometabolic biomarkers. In addition, our subgroup analysis found that cocoa flavanol interventions may have consistent effects on the biomarkers of lipid metabolism and insulin resistance regardless of age, sex, existing comorbidities, intervention duration, RCT design, and the form and amount of cocoa flavanols. Although the meta-regression analysis showed that the difference between people with and without comorbidities did not reach statistically significant cutoffs, possibly because of the limited number of trials available, the benefits for people with existing comorbidities seemed to be more substantial than those without comorbidities, which warrant further investigations.
There are several potential limitations to our study. First, although 19 trials were included in the meta-analysis, the number of studies for a specific biomarker varied. The number of available RCTs is especially limited for lipoprotein(a), oxidized LDL, VCAM-1, and leptin. In addition, the effect sizes were small to moderate for most biomarkers. Therefore, findings for those biomarkers need to be confirmed by further investigations and interpreted with caution. In addition, the precision of the estimates from individual RCTs was subject to small samples and variable intervention durations. In addition to providing comprehensive evidence for biomarkers of lipid metabolism and insulin resistance, our study highlights the urgent need for large long-term RCTs that improve our understanding of how the short-term benefits of cocoa flavanol intake on cardiometabolic biomarkers may be translated into clinical outcomes. Second, the subgroup analyses were restricted to biomarkers with ≥10 studies, and cutoffs used for categorizing modifiers were selected on an ad hoc basis. Third, because of the heterogeneity of cocoa flavanol interventions and limited evidence reported from RCTs, we were not able to distinguish the effects of different active compounds of cocoa flavanol-rich foods. However, we sought to maximize those data available from RCTs and found that any favorable effects of cocoa flavanols on cardiometabolic risk factors did not seem to be modified by the form or amount of cocoa flavanols. In addition, because of the limited number of trials available, studies that used different sources of blood for biomarker measurements were synthesized together, which may have introduced additional heterogeneity. Fourth, as shown in Figure 2, our results may be prone to the inherent weaknesses of individual RCTs.
In summary, our meta-analysis of RCTs indicates that cocoa flavanol intake from cocoa products, chocolate, or cocoa flavanol supplements may have modest but significant benefits in lipid metabolism, insulin resistance, and systemic inflammation. Further investigations, particularly large long-term RCTs, are urgently needed to confirm or refute whether cocoa flavanols represent a promising bioactive in the prevention of cardiometabolic diseases.
Acknowledgments
XL, LW, and SL designed the study; XL, IZ, and AL collected the data; XL performed the statistical analysis and drafted the manuscript; XL, JEM, HDS, LW, and SL critically revised the manuscript for important intellectual content; and SL supervised all the work and had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations: ISI, insulin sensitivity index; QUICKI, quantitative insulin sensitivity check index; RCT, randomized clinical trial; VCAM-1, vascular cell adhesion molecule 1; WMD, weighted mean difference.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014;129:e28–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones DS, Greene JA. The decline and rise of coronary heart disease: understanding public health catastrophism. Am J Public Health 2013;103:1207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z, Xu G, Liu X. Chocolate intake reduces risk of cardiovascular disease: evidence from 10 observational studies. Int J Cardiol 2013;168:5448–50. [DOI] [PubMed] [Google Scholar]
- 4.Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, Blumberg JB, Ferri C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr 2008;138:1671–6. [DOI] [PubMed] [Google Scholar]
- 5.Gu Y, Lambert JD. Modulation of metabolic syndrome-related inflammation by cocoa. Mol Nutr Food Res 2013;57:948–61. [DOI] [PubMed] [Google Scholar]
- 6.Grassi D, Desideri G, Ferri C. Protective effects of dark chocolate on endothelial function and diabetes. Curr Opin Clin Nutr Metab Care 2013;16:662–8. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Murga L, Tarin JJ, Garcia-Perez MA, Cano A. The impact of chocolate on cardiovascular health. Maturitas 2011;69:312–21. [DOI] [PubMed] [Google Scholar]
- 8.Grassi D, Desideri G, Ferri C. Blood pressure and cardiovascular risk: what about cocoa and chocolate? Arch Biochem Biophys 2010;501:112–5. [DOI] [PubMed] [Google Scholar]
- 9.Desch S, Schmidt J, Kobler D, Sonnabend M, Eitel I, Sareban M, Rahimi K, Schuler G, Thiele H. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens 2010;23:97–103. [DOI] [PubMed] [Google Scholar]
- 10.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 2012;95:740–51. [DOI] [PubMed] [Google Scholar]
- 11.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2008;88:38–50. [DOI] [PubMed] [Google Scholar]
- 12.Ding EL, Hutfless SM, Ding X, Girotra S. Chocolate and prevention of cardiovascular disease: a systematic review. Nutr Metab (Lond) 2006;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walters MR, Williamson C, Lunn K, Munteanu A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology 2013;80:1173–4. [DOI] [PubMed] [Google Scholar]
- 14.Kondo K, Hirano R, Matsumoto A, Igarashi O, Itakura H. Inhibition of LDL oxidation by cocoa. Lancet 1996;348:1514. [DOI] [PubMed] [Google Scholar]
- 15.Wolfram S. Effects of green tea and EGCG on cardiovascular and metabolic health. J Am Coll Nutr 2007;26:373S–88S. [DOI] [PubMed] [Google Scholar]
- 16.Liu K, Zhou R, Wang B, Chen K, Shi LY, Zhu JD, Mi MT. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr 2013;98:340–8. [DOI] [PubMed] [Google Scholar]
- 17.Heiss C, Dejam A, Kleinbongard P, Schewe T, Sies H, Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA 2003;290:1030–1. [DOI] [PubMed] [Google Scholar]
- 18.Ramiro E, Franch A, Castellote C, Perez-Cano F, Permanyer J, Izquierdo-Pulido M, Castell M. Flavonoids from Theobroma cacao down-regulate inflammatory mediators. J Agric Food Chem 2005;53:8506–11. [DOI] [PubMed] [Google Scholar]
- 19.Rein D, Paglieroni TG, Pearson DA, Wun T, Schmitz HH, Gosselin R, Keen CL. Cocoa and wine polyphenols modulate platelet activation and function. J Nutr 2000;130:2120S–6S. [DOI] [PubMed] [Google Scholar]
- 20.Rein D, Paglieroni TG, Wun T, Pearson DA, Schmitz HH, Gosselin R, Keen CL. Cocoa inhibits platelet activation and function. Am J Clin Nutr 2000;72:30–5. [DOI] [PubMed] [Google Scholar]
- 21.Waterhouse AL, Shirley JR, Donovan JL. Antioxidants in chocolate. Lancet 1996;348:834. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 29.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119–29. [DOI] [PubMed] [Google Scholar]
- 30.Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr 2005;81:611–4. [DOI] [PubMed] [Google Scholar]
- 31.Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, Desideri G, Blumberg JB, Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005;46:398–405. [DOI] [PubMed] [Google Scholar]
- 32.Farouque HM, Leung M, Hope SA, Baldi M, Schechter C, Cameron JD, Meredith IT. Acute and chronic effects of flavanol-rich cocoa on vascular function in subjects with coronary artery disease: a randomized double-blind placebo-controlled study. Clin Sci (Lond) 2006;111:71–80. [DOI] [PubMed] [Google Scholar]
- 33.Baba S, Osakabe N, Kato Y, Natsume M, Yasuda A, Kido T, Fukuda K, Muto Y, Kondo K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am J Clin Nutr 2007;85:709–17. [DOI] [PubMed] [Google Scholar]
- 34.Shiina Y, Funabashi N, Lee K, Murayama T, Nakamura K, Wakatsuki Y, Daimon M, Komuro I. Acute effect of oral flavonoid-rich dark chocolate intake on coronary circulation, as compared with non-flavonoid white chocolate, by transthoracic Doppler echocardiography in healthy adults. Int J Cardiol 2009;131:424–9. [DOI] [PubMed] [Google Scholar]
- 35.Balzer J, Rassaf T, Heiss C, Kleinbongard P, Lauer T, Merx M, Heussen N, Gross HB, Keen CL, Schroeter H, et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J Am Coll Cardiol 2008;51:2141–9. [DOI] [PubMed] [Google Scholar]
- 36.Davison K, Coates AM, Buckley JD, Howe PR. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes (Lond) 2008;32:1289–96. [DOI] [PubMed] [Google Scholar]
- 37.Muniyappa R, Hall G, Kolodziej TL, Karne RJ, Crandon SK, Quon MJ. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am J Clin Nutr 2008;88:1685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monagas M, Khan N, Andres-Lacueva C, Casas R, Urpi-Sarda M, Llorach R, Lamuela-Raventos RM, Estruch R. Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. Am J Clin Nutr 2009;90:1144–50. [DOI] [PubMed] [Google Scholar]
- 39.Njike VY, Faridi Z, Shuval K, Dutta S, Kay CD, West SG, Kris-Etherton PM, Katz DL. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. Int J Cardiol 2011;149:83–8. [DOI] [PubMed] [Google Scholar]
- 40.Mellor DD, Sathyapalan T, Kilpatrick ES, Beckett S, Atkin SL. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in Type 2 diabetes patients. Diabet Med 2010;27:1318–21. [DOI] [PubMed] [Google Scholar]
- 41.Almoosawi S, Tsang C, Ostertag LM, Fyfe L, Al-Dujaili EA. Differential effect of polyphenol-rich dark chocolate on biomarkers of glucose metabolism and cardiovascular risk factors in healthy, overweight and obese subjects: a randomized clinical trial. Food Funct 2012;3:1035–43. [DOI] [PubMed] [Google Scholar]
- 42.Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012;35:226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desideri G, Kwik-Uribe C, Grassi D, Necozione S, Ghiadoni L, Mastroiacovo D, Raffaele A, Ferri L, Bocale R, Lechiara MC, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) study. Hypertension 2012;60:794–801. [DOI] [PubMed] [Google Scholar]
- 44.Neufingerl N, Zebregs YE, Schuring EA, Trautwein EA. Effect of cocoa and theobromine consumption on serum HDL-cholesterol concentrations: a randomized controlled trial. Am J Clin Nutr 2013;97:1201–9. [DOI] [PubMed] [Google Scholar]
- 45.Sarriá B, Martinez-Lopez S, Sierra-Cinos JL, Garcia-Diz L, Mateos R, Bravo L. Regular consumption of a cocoa product improves the cardiometabolic profile in healthy and moderately hypercholesterolaemic adults. Br J Nutr 2014;111:122–34. [DOI] [PubMed] [Google Scholar]
- 46.West SG, McIntyre MD, Piotrowski MJ, Poupin N, Miller DL, Preston AG, Wagner P, Groves LF, Skulas-Ray AC. Effects of dark chocolate and cocoa consumption on endothelial function and arterial stiffness in overweight adults. Br J Nutr 2014;111:653–61. [DOI] [PubMed] [Google Scholar]
- 47.D’Anna R, Santamaria A, Cannata ML, Interdonato ML, Giorgianni GM, Granese R, Corrado F, Bitto A. Effects of a new flavonoid and Myo-inositol supplement on some biomarkers of cardiovascular risk in postmenopausal women: a randomized trial. Int J Endocrinol 2014;2014:653561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B, Willett W, Hu FB, Sun Q, van Dam RM. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr 2012;95:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zamora-Ros R, Forouhi NG, Sharp SJ, Gonzalez CA, Buijsse B, Guevara M, van der Schouw YT, Amiano P, Boeing H, Bredsdorff L, et al. The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in European populations: the EPIC-InterAct study. Diabetes Care 2013;36:3961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zamora-Ros R, Forouhi NG, Sharp SJ, Gonzalez CA, Buijsse B, Guevara M, van der Schouw YT, Amiano P, Boeing H, Bredsdorff L, et al. Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. J Nutr 2014;144:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nettleton JA, Harnack LJ, Scrafford CG, Mink PJ, Barraj LM, Jacobs DR Jr. Dietary flavonoids and flavonoid-rich foods are not associated with risk of type 2 diabetes in postmenopausal women. J Nutr 2006;136:3039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanhineva K, Torronen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkanen H, Poutanen K. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci 2010;11:1365–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace TC. Anthocyanins in cardiovascular disease. Adv Nutr 2011;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu Y, Yu S, Lambert JD. Dietary cocoa ameliorates obesity-related inflammation in high fat-fed mice. Eur J Nutr 2014;53:149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomaru M, Takano H, Osakabe N, Yasuda A, Inoue K, Yanagisawa R, Ohwatari T, Uematsu H. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition 2007;23:351–5. [DOI] [PubMed] [Google Scholar]
- 56.Friedrich M, Petzke KJ, Raederstorff D, Wolfram S, Klaus S. Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. Int J Obes (Lond) 2012;36:735–43. [DOI] [PubMed] [Google Scholar]
- 57.van Dam RM, Naidoo N, Landberg R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: review of recent findings. Curr Opin Lipidol 2013;24:25–33. [DOI] [PubMed] [Google Scholar]
- 58.Chen YK, Cheung C, Reuhl KR, Liu AB, Lee MJ, Lu YP, Yang CS. Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. J Agric Food Chem 2011;59:11862–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamashita Y, Okabe M, Natsume M, Ashida H. Prevention mechanisms of glucose intolerance and obesity by cacao liquor procyanidin extract in high-fat diet-fed C57BL/6 mice. Arch Biochem Biophys 2012;527:95–104. [DOI] [PubMed] [Google Scholar]
- 60.Ramos-Romero S, Perez-Cano FJ, Ramiro-Puig E, Franch A, Castell M. Cocoa intake attenuates oxidative stress associated with rat adjuvant arthritis. Pharmacol Res 2012;66:207–12. [DOI] [PubMed] [Google Scholar]
- 61.Buitrago-Lopez A, Sanderson J, Johnson L, Warnakula S, Wood A, Di Angelantonio E, Franco OH. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ 2011;343:d4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology 2012;79:1223–9. [DOI] [PubMed] [Google Scholar]
- 63.Ried K, Sullivan TR, Fakler P, Frank OR, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev 2012;8:CD008893. [DOI] [PubMed] [Google Scholar]
- 64.Shrime MG, Bauer SR, McDonald AC, Chowdhury NH, Coltart CE, Ding EL. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J Nutr 2011;141:1982–8. [DOI] [PubMed] [Google Scholar]