Abstract
The purpose of the current study was to apply a high throughput assay to investigate the structure-activity relationships of fatty acid amides for activating and desensitizing G protein-coupled receptor 119, a promising therapeutic target for both type 2 diabetes and obesity. A cell-based, homogenous time resolved fluorescence (HTRF) method for measuring G protein-coupled receptor 119-mediated increase of cyclic adenosine monophosphate (cAMP) levels was validated and applied in this study. Using novel fatty acid amides and detailed potency and efficacy analyses, we have demonstrated that degree of saturation in acyl chain and charged head groups of fatty acid amides have profound effects on the ability of these compounds to activate G protein-coupled receptor 119. In addition, we have demonstrated for the first time that pretreatments with G protein-coupled receptor 119 agonists desensitize the receptor and the degrees of desensitization caused by fatty acid amides correlate well with their structure-activity relationships in activating the receptor.
Keywords: G protein-coupled receptor 119, Fatty acid amides, Structure-activity relationship
1. Introduction
Type 2 diabetes (T2D) and associated obesity are growing public health concerns. As a result, many pharmaceutical companies have focused their efforts to discover novel, orally effective agents that can modulate glucose homeostasis and concurrently reduce body weight. G protein-coupled receptor 119 is a member of the rhodopsin family of G protein-coupled receptors (GPCRs). Recently G protein-coupled receptor 119 has emerged as a promising therapeutic target for both T2D and obesity (Dhayal and Morgan, 2010; Jones et al., 2009; Overton et al., 2008; Shah and Kowalski, 2010).
G protein-coupled receptor 119 is predominantly expressed in the beta cells of the pancreas and enteroendocrine cells of the gastrointestinal tract (Chu et al., 2007b; Lauffer et al., 2009). G protein-coupled receptor 119 is coupled to Gs, so upon its activation, there is an enhancement of cAMP levels within the cell (Chu et al., 2007b). It has been shown previously that G protein-coupled receptor 119 agonists stimulate insulin release by at least two mechanisms (Flock et al., 2011; Lauffer et al., 2008). The first mechanism is that the increase in cAMP signaling directly leads to an enhanced glucose-dependant insulin secretion. The second mechanism is that the increase in cAMP signaling results in an increased glucagon-like peptide 1 (GLP-1) level. GLP-1 is an anti-diabetic hormone which stimulates glucose-dependant insulin secretion and also inhibits glucagon secretion, appetite, and delays gastric emptying (Baggio and Drucker, 2007; Lauffer et al., 2008). It has been shown that administration of G protein-coupled receptor 119 agonists improves glucose tolerance in rodents (Chu et al., 2007a; Chu et al., 2010; Semple et al., 2008). In addition, it has been demonstrated that G protein-coupled receptor 119 agonists decrease feeding, body weight gain and adiposity in rats (Overton et al., 2006). Thus, G protein-coupled receptor 119 is a highly attractive potential therapeutic target for both diabetes and obesity.
Previously, several studies have demonstrated through phylogenetic analysis that the closest relatives of G protein-coupled receptor 119 are the cannabinoid receptors and placed G protein-coupled receptor 119 to the MECA (melanocortin; endothelial differentiation gene; cannabinoid; adenosine) receptor cluster (Fredriksson et al., 2003; Godlewski et al., 2009; Oh et al., 2006). Since homology clustering analysis revealed that the closest relatives of G protein-coupled receptor 119 are the cannabinoid receptors, it has been hypothesized that fatty acid amides related to the endocannabinoid anandamide, also named arachidonoyl ethanolamide (AEA), may be potential ligands for G protein-coupled receptor 119 (Overton et al., 2006).
A number of cannabinoid ligands and fatty-acid amides have been tested as potential agonists for G protein-coupled receptor 119 (Chu et al., 2010; Overton et al., 2006). However, the data from different research groups have not always been consistent. For example, Overton and coworkers identified oleoyl ethanolamide (OEA) as an endogenous G protein-coupled receptor 119 ligand (Overton et al., 2006). However, not all groups have observed OEA agonism on G protein-coupled receptor 119 (Brown, 2007). Also, detailed pharmacological analyses comparing the potency and efficacy of various fatty acid amides have not been reported. Therefore, the aim of this study is to examine and compare the potency and efficacy of a variety of fatty acid amides, including several novel compounds that have never been tested, towards G protein-coupled receptor 119 and to investigate the structure-activity relationships of the acyl side chains as well as the charged head groups in fatty acid amides for activating G protein-coupled receptor 119.
2. Materials and Methods
2.1. Materials
Dulbecco’s Modified Eagles’s Medium (DMEM), penicillin/streptomycin, L-glutamine, trypsin, and geneticin were purchased from Mediatech (Manassas, VA). Fetal bovine serum was obtained from Atlanta Biologicals (Lawrenceville, GA). Glass tubes used for cAMP accumulation assays were obtained from Kimble Chase (Vineland, NJ). These tubes were silanized by exposure to dichlorodimethylsilane (Sigma-Aldrich, St. Louis, MO) vapor for 3 h under vacuum. 384-well, round bottom, low volume white plates were purchased from Grenier Bio One (Monroe, NC). The cell-based HTRF cAMP HiRange assay kits were purchased from CisBio International (Bedford, MA).
Forskolin was obtained from Sigma (St. Louis, MO). AR231453, Ro 20-1724 and palmitoyl ethanolamide were purchased from Enzo Life Sciences (Farmingdale, NY). PSN632408, oleoyl ethanolamide, linoleoyl ethanolamide, dihomo-gamma-linolenoyl ethanolamide, docosatetra-7Z,10Z,13Z,16Z-enoyl ethanolamide, eicosapentaenoyl ethanolamide, docosahexaenoyl ethanolamide, anandamide, N-oleoyl glycine, and N-oleoyl dopamine were purchased from Cayman Chemical Company (Ann Arbor, Michigan). Oleamide and N-oleoyl GABA were purchased from Tocris Bioscience (Ellisville, MO).
2.2. Cell Transfection and Culture
Human Embryonic Kidney 293 (HEK293) cells (purchased from ATCC, Manassas, VA) were maintained in DMEM containing 10% fetal bovine serum, 2 mM glutamine, 100 units/ml penicillin, and 100 µg/ml streptomycin in a humidified atmosphere consisting of 5% CO2 at 37°C. Expression plasmid containing the human GPR119 receptor was stably transfected into HEK293 cells using lipofectamine, according to manufacturer’s instructions. Stably transfected cells were selected in culture medium containing 800 µg/ml geneticin and maintained in growth medium containing 400 µg/ml of geneticin (G418) until needed for experiments.
2.3.Cell-based HTRF cAMP assay
Cellular cAMP levels were measured as described previously (Kumar and Song, 2013) using reagents supplied by Cisbio International (HTRF HiRange cAMP kit). Compounds were diluted in drug buffer (DMEM plus 2.5 % fatty acid free bovine serum albumin) and added to the assay plate at 5 µl per well. Following incubation of cells with the drugs or vehicle for 30 min at room temperature, d2-conjugated cAMP and Europium cryptate-conjugated anti-cAMP antibody were added to the assay plate at 5 µl per well. After 2 hour incubation at room temperature, the plate was read on a TECAN GENious Pro microplate reader with excitation at 337 nm and emissions at 665 nm and 620 nm. To assess receptor desensitization, HEK293 cells stably expressing G protein-coupled receptor 119 were pre-incubated for 20 min with vehicle or drugs at a concentration of 10 µM before subject to stimulation with OEA.
2.4. Data Analysis
Data analyses were performed based on the ratio of fluorescence intensity of each well at 620 nm and 665 nm. Data are expressed as delta F%, which is defined as [(standard or sample ratio – ratio of the negative control) / ratio of the negative control] × 100. The standard curves were generated by plotting delta F% versus cAMP concentrations using non-linear least squares fit (Prism software, GraphPad, San Diego, CA). Unknowns are determined from the standard curve as nanomolar concentrations of cAMP. After the unknowns are determined, the sigmoidal concentration-response equations were used (via GraphPad Prism) to determine EC50 and Emax values of the tested compounds.
3. Results
3.1. Z’ Factor Determination
To determine the Z’ value, experiments were performed in 384-well plates using many replicates of the cell-based HTRF cAMP assay with positive and negative controls (Fig. 1A). For positive controls, the HEK293 cells stably expressing G protein-coupled receptor 119 were treated with the potent G protein-coupled receptor 119 agonist AR231453 at a concentration of 10 µM for 30 min at room temperature. For negative controls, the cells were treated with vehicle for 30 min. The Z’ value was calculated using the formula: Z’ = 1–3[(standard deviation of negative control) + standard deviation of positive control)]/ [(mean of negative control) − (mean of positive control)] (Zhang et al., 1999). In the current study, the Z factor was determined to be 0.71.
Fig. 1. Validation of the cell-based, HTRF cAMP assay for G protein-coupled receptor 119.
(A) Z’ factor determination. Open symbols represent positive controls (cells stimulated with 10 µM AR231453), while solid symbols represent negative controls (cells stimulated with vehicle). The Z’ factor was calculated to be 0.71 using 57 positive and 57 negative control points. (B) DMSO tolerance. HEK293 cells stably expressing G protein-coupled receptor 119 was treated with different concentrations of DMSO. Delta F % was calculated using the following formula: Delta F % = [(standard or sample ratio – ratio of the negative control) / ratio of the negative control] × 100. Values represent the mean ± S.E.M. of three independent experiments, each performed in duplicate. (C) The effects of known agonists to activate G protein-coupled receptor 119. HEK293 stably expressing G protein-coupled receptor 119 were treated with G protein-coupled receptor 119 agonists AR231453, oleoyl ethanolamide (OEA), and PSN632408 for 30 min. Results are expressed as percent of maximum OEA-induced cAMP accumulation. Values represent the mean ± S.E.M. of five independent experiments.
3.2.Tolerance to Dimethyl Sulfoxide (DMSO)
One important condition to define is the concentration of dimethyl sulfoxide (DMSO) that the HTRF cAMP assay is able to tolerate without any loss in signal. For this purpose, we tested the effect of DMSO at concentrations ranging from 0.001% to 100%. As shown in Fig. 1B, the cell-based HTRF cAMP assay for G protein-coupled receptor 119 can tolerate DMSO up to 1% without any loss of signal.
3.3. Pharmacological Testing of Known G protein-coupled receptor 119 Agonists
The ability of known agonists to activate G protein-coupled receptor 119 was tested using the HTRF cAMP assay in HEK293 cells stably expressing G protein-coupled receptor 119. As shown in Fig. 1C and Table 1, all three previously reported G protein-coupled receptor 119 ligands, AR231453 (Semple et al., 2008), OEA (Overton et al., 2006), and PSN632408 (Overton et al., 2006), increased the cellular cAMP levels in a concentration-dependent manner, with a rank order of potency of AR231453 > OEA = PSN632408, and a rank order of efficacy of AR231453 = OEA > PSN632408. In addition, these three compounds failed to elicit any response in HEK293 cells transfected with an empty vector (data not shown).
Table 1.
The effects of known G protein-coupled receptor 119 agonists on increasing cAMP in HEK293 cells stably expressing G protein-coupled receptor 119.
| Drug | Structure | EC50 (95% CI) (µM) |
Emax (95% CI) (% OEA response) |
|---|---|---|---|
| AR231453 |  |
0.011 (0.0090 – 0.0131)a |
98.23 (95.71 – 100.80) |
| Oleoyl ethanolamide (OEA) |
7.65 (7.56 – 7.74) |
100.00 (99.70 – 100.30) |
|
| PSN632408 |  |
7.61 (7.01 – 8.26) |
88.72 (87.04 – 90.40)a |
Significantly different (P < 0.05) from OEA.
3.4. The Effects of Acyl Chain Degree of Saturation on the Ability of Fatty Acid Ethanolamides to Activate G protein-coupled receptor 119
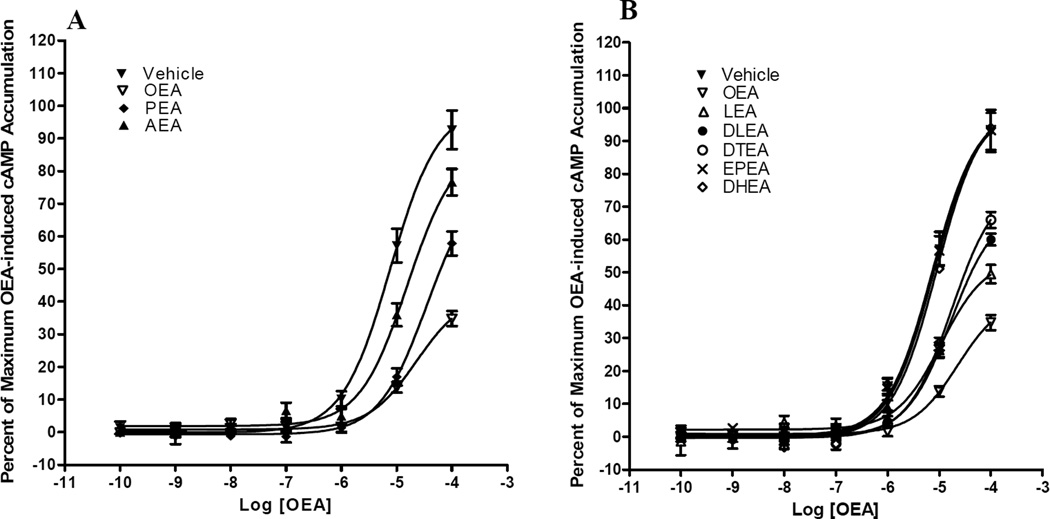
Three endogenous fatty acid, oleoyl ethanolamide (OEA), palmitoyl ethanolamide (PEA) and arachidonoyl ethanolamide (AEA) were tested for their activity on G protein-coupled receptor 119 (Fig. 2A and Table 2). All three compounds significantly increased cAMP levels in a concentration-dependent manner, with rank orders of both potency and efficacy of OEA > PEA > AEA.
Fig. 2. The effects of acyl chain degree of saturation on the ability of fatty acid ethanolamides to activate G protein-coupled receptor 119.
(A) HEK293 stably expressing G protein-coupled receptor 119 were treated with oleoyl ethanolamide (OEA), palmitoyl ethanolamide (PEA), and AEA for 30 min. (B) HEK293 stably expressing G protein-coupled receptor 119 were treated with oleoyl ethanolamide (OEA), linoleoyl ethanolamide (LEA), dihomo-gamma-linolenoyl ethanolamide (DLEA), docosatetra-7Z,10Z,13Z,16Z-enoyl ethanolamide (DTEA), eicosapentaenoyl ethanolamide (EPEA) and docosahexaenoyl ethanolamide (DHEA) for 30 min. Results are expressed as percent of maximum OEA-induced cAMP accumulation. Values represent the mean ± S.E.M. of five independent experiments.
Table 2.
The effects of acyl chain degree of saturation on the ability of fatty acid ethanolamides to increase cAMP levels in HEK293 cells stably expressing G protein-coupled receptor 119.
| Drug | Structure | EC50 (95% CI) (µM) |
Emax (95% CI) (% OEA response) |
|---|---|---|---|
| Oleoyl ethanolamide (OEA) |
7.65 (7.56 – 7.74) |
100.00 (99.70 – 100.30) |
|
| Palmitoyl ethanolamide (PEA) |
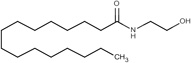 |
10.12 (8.72 – 11.73) a |
45.76 (44.08 – 47.44)a |
| Linoleoyl ethanolamide (LEA) |
 |
8.11 (7.55 – 8.70) |
46.41 (45.69 – 47.13)a |
| Dihomo-γ-linolenoyl ethanolamide (DLEA) |
 |
25.64 (22.93 – 28.67)a |
43.07 (41.63 – 44.51)a |
| Docosatetra- 7Z,10Z,13Z,16Z-enoyl ethanolamide (DTEA) |
 |
40.16 (37.41 – 43.11)a |
43.35 (42.38 – 44.33)a |
| Arachidonoyl ethanolamide (AEA) |
 |
19.67 (16.35 – 23.67)a |
33.79 (31.72 – 35.87)a |
| Eicosapentaenoyl ethanolamide (EPEA) |
 |
65.60 (43.56 – 98.78)a |
33.96 (31.44 – 36.47)a |
| Docosahexxaenoyl ethanolamide (DHEA) |
 |
63.79 (47.50 – 85.68)a |
34.87 (30.97 – 38.77)a |
Significantly different (P < 0.05) from OEA.
Furthermore, we examined the structure-activity relationship on a subset of novel fatty acid ethanolamides, whose potency (EC50 values) and efficacy (Emax values) toward G protein-coupled receptor 119 has not been previously analyzed in detail (Fig. 2B and Table 2). Among fatty acid ethanolamides that we tested, the rank order of potency was OEA = linoleoyl ethanolamide (LEA) > dihomo-γ-linolenoyl ethanolamide (DLEA) > docosatetra-7Z,10Z,13Z,16Z-enoyl ethanolamide (DTEA) > eicosapentaenoyl ethanolamide (EPEA) = docosahexaenoyl ethanolamide (DHEA). The rank of order of efficacy was OEA > LEA > DLEA = DTEA > EPEA = DHEA. In addition, none of the above compounds elicited any response in HEK293 cells transfected with an empty vector (data not shown).
3.5. The Effects of Different Head Groups on the Ability of Oleoyl Amides to Activate G protein-coupled receptor 119
We hypothesized that different head groups on the oleoyl amides may impact the ability of oleoyl amides to activate G protein-coupled receptor 119. To test this hypothesis, N-oleoyldopamine (OLDA), oleamide, OEA, oleoyl alanine, oleoyl glycine, and oleoyl GABA were tested for their ability to increase cAMP levels in HEK293 cells stably expressing G protein-coupled receptor 119. Fig. 3 and Table 3 demonstrate the agonist activity of different oleoyl amides as compared to OEA. In HEK293 cells stably expressing G protein-coupled receptor 119, both OLDA and oleamide increased cAMP levels in a concentration-dependent manner, with a rank order of potency of OEA > OLDA = oleamide, and a rank order of efficacy of OEA > OLDA > oleamide. On the contrary, oleoyl alanine, oleoyl glycine, and oleoyl GABA failed to activate G protein-coupled receptor 119 with concentrations up to 100 µM. In addition, none of the above compounds elicited any response in HEK293 cells transfected with an empty vector (data not shown).
Fig. 3. The effects of different head groups on the ability of oleoyl amides to activate G protein-coupled receptor 119.
HEK293 stably expressing G protein-coupled receptor 119 were treated with oleoyl ethanolamide (OEA), oleoyl dopamine (OLDA), oleamide, oleoyl alanine, oleoyl glycine, and oleoyl GABA for 30 min. Results are expressed as percent of maximum OEA-induced cAMP accumulation. Values represent the mean ± S.E.M. of five independent experiments.
Table 3.
The effects of different head groups on the ability of oleoyl amides to increase cAMP levels in HEK293 cells stably expressing G protein-coupled receptor 119.
| Drug | Structure | EC50(95% CI) (µM) |
Emax (95% CI) (% OEA response) |
|---|---|---|---|
| Oleoyl ethanolamide (OEA) |
7.65 (7.56 – 7.74) |
100.0 (99.7 – 100.3) |
|
| Oleoyl dopamine (OLDA) |
 |
54.79 (37.64 – 79.75)a |
43.15 (37.43 – 48.87)a |
| Oleamide |  |
42.86 (37.06 – 49.58)a |
33.48 (31.56 – 35.39)a |
| Oleoyl alanine |  |
N.D. | N.D. |
| Oleoyl glycine |  |
N.D. | N.D. |
| Oleoyl GABA |  |
N.D. | N.D. |
Significantly different (P < 0.05) from OEA. N.D., not determined.
3.6.Receptor Desensitization Produced by Pretreatment with G protein-coupled receptor 119 Agonists
To study receptor desensitization, HEK293 cells stably expressing G protein-coupled receptor 119 were pretreated for 20 min with 10 µM of various G protein-coupled receptor 119 agonists. Subsequently, OEA-induced enhancement of cAMP was measured as an indicator of receptor activation. As shown in Fig. 4, AR231453 pretreatment completely abolished OEA-induced activation of G protein-coupled receptor 119, whereas OEA and PSN632408 pretreatments significantly desensitized the OEA-induced activation of G protein-coupled receptor 119. As shown in Fig. 5A, pretreatments with fatty acid amides OEA, PEA, and AEA caused a desensitization of OEA-induced G protein-coupled receptor 119-activation, and the degree of desensitization follows the order of OEA > PEA > AEA. Fig. 5B demonstrates that pretreatments with OEA, LEA, DLEA, and DTEA caused a desensitization of OEA-induced activation of G protein-coupled receptor 119 and the degree of desensitization follows the order of OEA > LEA > DLEA > DTEA. In contrast, at a concentration of 10 µM, neither EPEA nor DHEA caused G protein-coupled receptor 119 receptor desensitization. Furthermore, as shown in Fig. 6, pretreatments with OEA, OLDA, and oleamide led to a desensitization of OEA-induced activation of G protein-coupled receptor 119 and the degree of desensitization follows the order of OEA > OLDA > oleamide. On the contrary, pretreatments with oleoyl alanine, oleoyl glycine, and oleoyl GABA did not result in a desensitization of the G protein-coupled receptor 119 receptor.
Fig. 4. The effects of known agonists to desensitize G protein-coupled receptor 119.
.HEK293 cells stably expressing G protein-coupled receptor 119 were pretreated with known G protein-coupled receptor 119 agonists AR231453, oleoyl ethanolamide (OEA), and PSN632408 for 20 min, followed by stimulation with OEA for 30 min. Results are expressed as percent of maximum OEA-induced cAMP accumulation. Values represent the mean ± S.E.M. of five independent experiments.
Fig. 5. The effects of acyl chain degree of saturation on the ability of fatty acid ethanolamides to desensitize G protein-coupled receptor 119.
(A) HEK293 cells stably expressing G protein-coupled receptor 119 were pretreated with oleoyl ethanolamide (OEA), palmitoyl ethanolamide (PEA), and AEA for for 20 min, followed by stimulation with OEA for 30 min. (B) HEK293 cells stably expressing G protein-coupled receptor 119 were pretreated with oleoyl ethanolamide (OEA), linoleoyl ethanolamide (LEA), dihomo-gamma-linolenoyl ethanolamide (DLEA), docosatetra-7Z,10Z,13Z,16Z-enoyl ethanolamide (DTEA), eicosapentaenoyl ethanolamide (EPEA) and docosahexaenoyl ethanolamide (DHEA) for 20 min, followed by stimulation with OEA for 30 min. Results are expressed as percent of maximum OEA-induced cAMP accumulation. Values represent the mean ± S.E.M. of five independent experiments.
Fig. 6. The effects of different head groups on the ability of oleoyl amides to desensitize G protein-coupled receptor 119.
HEK293 cells stably expressing G protein-coupled receptor 119 were pretreated with oleoyl ethanolamide (OEA), oleoyl dopamine (OLDA), oleamide, oleoyl alanine, oleoyl glycine, and oleoyl GABA for 20 min, followed by stimulation with OEA for 30 min. Results are expressed as percent of maximum OEA-induced cAMP accumulation. Values represent the mean ± S.E.M. of five independent experiments.
4. Discussion
Agonist binding to G protein-coupled receptor 119 leads to Gs coupling and activation of adenylate cyclase (Dhayal and Morgan, 2010; Jones et al., 2009; Overton et al., 2008; Shah and Kowalski, 2010). As a result, there is an increase in intracellular cAMP levels which was measured as a decrease in HTRF signal in this study. We have shown that the cell-based HTRF cAMP assay is a suitable technology for assaying ligands that may act on G protein-coupled receptor 119.
The Z’factor is a standard statistical parameter used to evaluate the robustness of a high throughput assay (Zhang et al., 1999). The Z’factor value can range between 0 and 1, with values approaching 1 indicates excellent assay robustness. In this study the calculated Z’factor for the assay was 0.71. Since Z’ factor greater than 0.5 indicates a suitable difference between signal and background values with low variability, our results demonstrate that the cell-based, HTRF cAMP assay is robust and suitable for testing ligands that activate G protein-coupled receptor 119.
Since most chemical compound libraries come pre-dissolved in dimethyl sulfoxide (DMSO), it is critical to determine the maximum concentration that a compound can be assayed before DMSO reaches a concentration that is too high to be tolerated by the assay (Williams, 2004). Therefore, we determined the effect of DMSO on the cell-based HTRF cAMP assay. We tested DMSO at a variety of concentrations and the results showed that the assay can tolerate DMSO up to 1 %. These data indicate that the assay is suitable for testing ligands that may act on G protein-coupled receptor 119 at a DMSO concentration of less than 1 %.
To validate that the cell-based HTRF cAMP assay is suitable for assaying ligands that may activate G protein-coupled receptor 119 we performed concentration-response studies for three previously reported G protein-coupled receptor 119 agonists AR231453, OEA, and PSN632408. Both the rank order of potency and the rank order of efficacy of these three known G protein-coupled receptor 119 agonists in enhancing cAMP levels in G protein-coupled receptor 119-expressing HEK293 cells are consistent with previous reports (Overton et al., 2006; Semple et al., 2008). These results also confirmed the suitability of this cell-based HTRF cAMP assay for testing ligands for G protein-coupled receptor 119.
Recently, the fatty acid ethanolamide OEA has been reported to be a putative endogenous ligand for G protein-coupled receptor 119 (Overton et al., 2006). However, not all groups have observed OEA agonism on G protein-coupled receptor 119 (Brown, 2007). Overton and coworkers have also tested the endogenous cannabinoid agonist AEA and the saturated fatty-acid ethanolamide PEA for G protein-coupled receptor 119 activity in a yeast-based assay. Their results showed that OEA was the most efficacious, followed by PEA and then AEA. Based on the data with OEA, PEA, and AEA, it has been proposed that the degree of saturation in fatty acid aryl chain might be important for these fatty-acid ethanolamides to activate G protein-coupled receptor 119 (Chu et al., 2010; Overton et al., 2006). Our results on OEA, PEA, and AEA with the cAMP assay demonstrated rank orders of both potency and efficacy of OEA > PEA > AEA. Thus, our data on these three fatty acid amides with the human G protein-coupled receptor 119 stably expressed in HEK293 cells are consistent with those reported by Overton et al. with the yeast-based assay (Overton et al., 2006).
In this study, we report for the first time the detailed potency and efficacy analyses of a novel subset of fatty acid ethanolamides, including linoleoyl ethanolamide (LEA), dihomo-gamma-linolenoyl ethanolamide (DLEA), docosatetra-7Z,10Z,13Z,16Z-enoyl ethanolamide (DTEA), eicosapentaenoyl ethanolamide (EPEA), and docosahexaenoyl ethanolamide (DHEA). Overall, our new data in the present study provide direct evidence to support the hypothesis that the degree of saturation in the acyl chain of fatty acid ethanolamides affects the ability of these compounds to activate G protein-coupled receptor 119.
OEA, LEA, DLEA, DTEA, EPEA and DHEA contain one, two, three, four, five, and six double bonds in their fatty acid acyl chain, respectively. Our results indicate that increasing the number of double bonds reduces the ability of these ligands to activate G protein-coupled receptor 119; with compounds containing 1–2 double bonds have significantly higher efficacy and potency than those compounds containing 3–6 double bonds.
Chu and coworkers reported that a diverse set of lipid amides, including N-oleoyldopamine (OLDA) and oleamide, activate G protein-coupled receptor 119 (Chu et al., 2010). Thus, they suggested that there might be a broad permissiveness in the amine-derived moieties (the head groups) of lipid amides for being an agonist for G protein-coupled receptor 119 (Chu et al., 2010). In the present study, we demonstrated that both OLDA and oleamide activate G protein-coupled receptor 119, with a rank order of potency of OEA > OLDA = oleamide, and a rank order of efficacy of OEA > OLDA > oleamide. These new data on the potency and efficacy of these fatty acid amides confirm the notion that there is a considerable level of permissiveness in the head group of oleoyl amides. However, our data also demonstrate that to achieve the maximum efficacy in activating G protein-coupled receptor 119, the ethanolamide head group is necessary.
Furthermore, in the current study, we also demonstrated that oleoyl alanine, oleoyl glycine, and oleoyl GABA were unable to activate G protein-coupled receptor 119. These data suggest that although there are certain levels of permissiveness, in order to activate G protein-coupled receptor 119, there are also certain structural requirements for the head groups of oleoyl amides. An interesting observation is that all three compounds (oleoyl alanine, oleoyl glycine, and oleoyl GABA) that failed to activate G protein-coupled receptor 119 have a carboxyl group. This suggests that a plausible explanation that these ligands failed to activate G protein-coupled receptor 119 might be due to either the steric hindrance or the acidic nature of the carboxyl group.
Desensitization is the attenuation of receptor responsiveness to agonist after prior agonist exposure and represents an important feedback mechanism for preventing receptor overstimulation (Kohout and Lefkowitz, 2003). Although it is a well known phenomenon for GPCRs, receptor desensitization has not been reported for G protein-coupled receptor 119. To our knowledge, this is the first characterization of agonist-induced desensitization of the G protein-coupled receptor 119 receptor which appears to be due to a reduction both in potency and in efficacy of OEA to elevate cAMP.
In this study, our data demonstrate that the degree of receptor desensitization produced by a certain agonist correlates well with the potency and efficacy of the agonist. For example, the most potent and efficacious G protein-coupled receptor 119 agonist AR231453 induced the greatest degree of receptor desensitization. Furthermore, among a subset of fatty acid amides, the degree of receptor desensitization follows the order of OEA > LEA > DLEA > DTEA > EPEA=DHEA, which correlates closely with their ability to activate G protein-coupled receptor 119. These results indicate that increasing the number of double bonds reduces the ability of these fatty acid amides to activate, as well as to desensitize G protein-coupled receptor 119.
We have shown that pretreatment with OEA, PEA, LEA, DLEA, DTEA, EPEA, and DHEA is able to inhibit the OEA-induced response to different extents that correlate with their ability to activate G protein-coupled receptor 119. These results suggest that these fatty acid amides share the same binding sites. This suppression of OEA-induced response could be due to (1) desensitization of the receptor, (2) competition between pre- and post-treated ligands, (3) both receptor desensitization and competition between the pre- and post-treated ligands. With our experimental protocol, we believe possibility number 3 is most likely to be the mechanism. To further differentiate and/or exclude these possible mechanisms, and to further confirm that the suppression of OEA response was from ligand binding to the orthosteric rather than allosteric site, one of the critical experiments needed is the radioligand binding experiment with pre-treated and post-treated cells. However, currently there is no commercially available radioligand for G protein-coupled receptor 119 for us to conduct ligand binding experiments. Even though we are unable to differentiate/exclude the possible mechanisms at the present time, our main conclusion regarding the degree of saturation in the acyl chain and the head group of the fatty acid amides are still strongly supported by our structure-activity relationship data.
5. Conclusions
In this study we first validated a cell-based, homogenous time resolved fluorescence (HTRF) method for measuring G protein-coupled receptor 119-mediated increase of cAMP levels in HEK293 cells stably expressing G protein-coupled receptor 119. Using novel fatty acid amide ligands and detailed potency and efficacy analyses, we then demonstrated that degree of saturation in acyl chain and charged head groups of fatty acid amides have profound effects on the ability of fatty acid amides to activate G protein-coupled receptor 119. Finally, we have demonstrated for the first time that pretreatments with G protein-coupled receptor 119 agonists desensitize the receptor, and the degrees of desensitization caused by fatty acid amides correlate well with their structure-activity relationships in activating the receptor.
Acknowledgments
This study was supported in part by the National Institutes of Health Grants EY13632 and DA11551.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Brown AJ. Novel cannabinoid receptors. Br J Pharmacol. 2007;152:567–575. doi: 10.1038/sj.bjp.0707481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, Moloney M, Gao H, Mondala H, Bagnol D, Unett D, Liang Y, Demarest K, Semple G, Behan DP, Leonard J. A Role for {beta}-Cell-Expressed GPR119 in Glycemic Control by Enhancing Glucose-Dependent Insulin Release. Endocrinology. 2007a doi: 10.1210/en.2006-1608. [DOI] [PubMed] [Google Scholar]
- Chu ZL, Carroll C, Chen R, Alfonso J, Gutierrez V, He H, Lucman A, Xing C, Sebring K, Zhou J, Wagner B, Unett D, Jones RM, Behan DP, Leonard J. N-oleoyldopamine enhances glucose homeostasis through the activation of GPR119. Mol Endocrinol. 2010;24:161–170. doi: 10.1210/me.2009-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ZL, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, Moloney M, Gao H, Mondala H, Bagnol D, Unett D, Liang Y, Demarest K, Semple G, Behan DP, Leonard J. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology. 2007b;148:2601–2609. doi: 10.1210/en.2006-1608. [DOI] [PubMed] [Google Scholar]
- Dhayal S, Morgan NG. The significance of GPR119 agonists as a future treatment for type 2 diabetes. Drug News Perspect. 2010;23:418–424. doi: 10.1358/dnp.2010.23.7.1468395. [DOI] [PubMed] [Google Scholar]
- Flock G, Holland D, Seino Y, Drucker DJ. GPR119 regulates murine glucose homeostasis through incretin receptor-dependent and independent mechanisms. Endocrinology. 2011;152:374–383. doi: 10.1210/en.2010-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. Seven evolutionarily conserved human rhodopsin G protein-coupled receptors lacking close relatives. FEBS Lett. 2003;554:381–388. doi: 10.1016/s0014-5793(03)01196-7. [DOI] [PubMed] [Google Scholar]
- Godlewski G, Offertaler L, Wagner JA, Kunos G. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 2009;89:105–111. doi: 10.1016/j.prostaglandins.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Leonard JN, Buzard DJ, Lehmann J. GPR119 agonists for the treatment of type 2 diabetes. Expert Opin Ther Pat. 2009;19:1339–1359. doi: 10.1517/13543770903153878. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lefkowitz RJ. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- Kumar P, Song ZH. Identification of raloxifene as a novel CB2 inverse agonist. Biochem Biophys Res Commun. 2013;435:76–81. doi: 10.1016/j.bbrc.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer L, Iakoubov R, Brubaker PL. GPR119:"double-dipping" for better glycemic control. Endocrinology. 2008;149:2035–2037. doi: 10.1210/en.2008-0182. [DOI] [PubMed] [Google Scholar]
- Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58:1058–1066. doi: 10.2337/db08-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Kim K, Kwon HB, Seong JY. Cellular and molecular biology of orphan G protein-coupled receptors. International review of cytology. 2006;252:163–218. doi: 10.1016/S0074-7696(06)52003-0. [DOI] [PubMed] [Google Scholar]
- Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, Widdowson PS, Williams GM, Reynet C. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Overton HA, Fyfe MC, Reynet C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br J Pharmacol. 2008;153(Suppl 1):S76–S81. doi: 10.1038/sj.bjp.0707529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple G, Fioravanti B, Pereira G, Calderon I, Uy J, Choi K, Xiong Y, Ren A, Morgan M, Dave V, Thomsen W, Unett DJ, Xing C, Bossie S, Carroll C, Chu ZL, Grottick AJ, Hauser EK, Leonard J, Jones RM. Discovery of the first potent and orally efficacious agonist of the orphan G-protein coupled receptor 119. J Med Chem. 2008;51:5172–5175. doi: 10.1021/jm8006867. [DOI] [PubMed] [Google Scholar]
- Shah U, Kowalski TJ. GPR119 agonists for the potential treatment of type 2 diabetes and related metabolic disorders. Vitam Horm. 2010;84:415–448. doi: 10.1016/B978-0-12-381517-0.00016-3. [DOI] [PubMed] [Google Scholar]
- Williams C. cAMP detection methods in HTS: selecting the best from the rest. Nat Rev Drug Discov. 2004;3:125–135. doi: 10.1038/nrd1306. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]