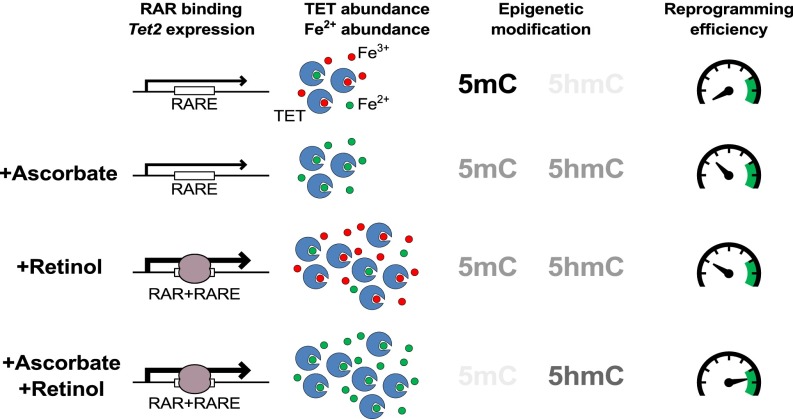
Significance
Naïve embryonic stem cells are characterized by genome-wide low levels of cytosine methylation, a property that may be intrinsic to their function. We found that retinol/retinoic acid (vitamin A) and ascorbate (vitamin C) synergistically diminish DNA methylation levels and in doing so enhance the generation of naïve pluripotent stem cells. This is achieved by two complementary mechanisms. Retinol increases cellular levels of TET proteins (which oxidize DNA methylation), whereas ascorbate affords them greater activity by reducing cellular Fe3+ to Fe2+. This mechanistic insight is relevant for the production of induced pluripotent stem cells used in regenerative medicine, and contributes to our understanding of how the genome is connected to extrinsic and intrinsic signals.
Keywords: epigenetic memory, naive pluripotency, DNA methylation, vitamin A/C, TET
Abstract
Epigenetic memory, in particular DNA methylation, is established during development in differentiating cells and must be erased to create naïve (induced) pluripotent stem cells. The ten-eleven translocation (TET) enzymes can catalyze the oxidation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) and further oxidized derivatives, thereby actively removing this memory. Nevertheless, the mechanism by which the TET enzymes are regulated, and the extent to which they can be manipulated, are poorly understood. Here we report that retinoic acid (RA) or retinol (vitamin A) and ascorbate (vitamin C) act as modulators of TET levels and activity. RA or retinol enhances 5hmC production in naïve embryonic stem cells by activation of TET2 and TET3 transcription, whereas ascorbate potentiates TET activity and 5hmC production through enhanced Fe2+ recycling, and not as a cofactor as reported previously. We find that both ascorbate and RA or retinol promote the derivation of induced pluripotent stem cells synergistically and enhance the erasure of epigenetic memory. This mechanistic insight has significance for the development of cell treatments for regenenerative medicine, and enhances our understanding of how intrinsic and extrinsic signals shape the epigenome.
Epigenetic modification is a mechanism used to stably enforce and maintain gene expression patterns between different cell types. Cytosine methylation is perhaps the most intensively studied of these modifications. A low level of cytosine methylation (<30% of CpG dinucleotides) is one of the few features that distinguish the most basal stem cells of the body—naïve embryonic stem cells (nESCs)—from stem cells primed for differentiation (1–5) or committed to somatic lineages (70–85% CpG methylation) (6, 7). Importantly, inhibitors of the DNA methylation maintenance machinery can accelerate the reprogramming of differentiated cells into induced pluripotent stem cells (iPSCs) (8, 9). These features suggest that reduced DNA methylation, either globally or at specific genomic regions, is a fundamental property of naïve pluripotency (Fig. 1A).
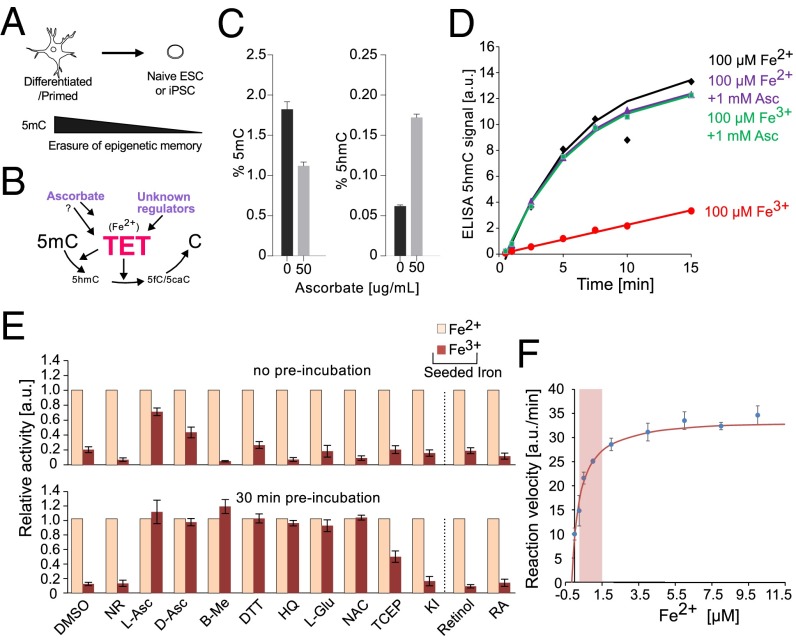
Fig. 1.
TET protein catalytic activity in vitro is rescued by the addition of Fe2+, ascorbate, or other antioxidants. (A) Major loss of DNA methylation, globally and at gene promoters, is associated with naïve pluripotent cells. (B) Demethylation during reprogramming is affected by the activity of the Fe2+-dependent TET hydroxylases, which create 5hmC and other oxidized derivatives (5fC and 5caC). However, what regulates TET levels is largely unknown, and the mechanism by which factors such as ascorbate affect TET-mediated oxidation are unclear. (C) Global levels of 5mC and 5hmC (%) in nESCs following 72 h of supplementation with 50 μg/mL ascorbate (± SD; n = 3). (D) Kinetics of TET1-CD–mediated 5hmC production when supplemented with 100 μM iron (Fe2+ or Fe3+) and 1 mM ascorbate (corresponds to 172.12 μg/mL). (E, Upper) Relative activity of TET1-CD when supplemented with 100 μM iron (Fe2+ or Fe3+) and various antioxidants. (E, Lower) The same experiment repeated, with the antioxidant and iron mix preincubated for 30 min before the addition of TET1-CD (± SD; n = 4). NR represents reaction conditions without added reducing agents. (F) Relative activity of TET1-CD at various Fe2+ concentrations encompassing those seen in cellular contexts (0.2–1.5 μM, indicated by a red box). The mean apparent dissociation constant (Kd) for this reaction was determined to be 0.41 ± 0.05 µM (n = 2).
A key pathway of active DNA demethylation involves the ten-eleven translocation (TET) protein family. These Fe2+- and oxoglutarate-dependent enzymes remove methylated cytosine (5mC) by converting it to 5-hydroxymethylcytosine (5hmC) and further oxidized derivatives (10–13) (Fig. 1B). TET proteins can contribute to locus- specific demethylation in nESCs (1, 14), and their depletion reduces the expression of pluripotency genes and increases methylation at their promoters (12, 15). Furthermore, forced expression of TET1 and TET2 dramatically enhances iPSC reprogramming in a catalytically dependent manner (16–18). Nevertheless, the molecular signals that control TET activity in nESCs, and how they can be manipulated during reprogramming, are poorly characterized. For example, although ascorbate (vitamin C) is known to enhance 5hmC production in a TET-dependent manner (19–23), the mechanism by which this occurs is unclear (Fig. 1B).
Here we report that ascorbate enhances 5hmC production and potentiates TET catalysis, not as a cofactor as reported previously, but rather by reduction of Fe3+ to Fe2+, making it available for participation in the TET enzyme catalytic center. Retinol, the most common form of vitamin A in the body, is chemically unrelated to ascorbate but similarly enhances the production of iPSCs (24). We discovered that it also increases 5hmC production and DNA demethylation in a TET-dependent manner. This is achieved not by an effect on enzymatic activity, but rather through increased TET2 and TET3 expression. We show that increased TET2 mRNA is dependent on an evolutionary conserved retinoic acid (RA) receptor element (RARE) in the first intron of its underlying gene. Finally, given the overlapping effects of retinol and ascorbate on 5hmC production and DNA demethylation, we tested their effects on the reprogramming of primed cells to naïve pluripotency. We found synergistic effects between these two vitamins in a manner predicted by their interdependent effects on epigenetic memory erasure.
Results
Ascorbate Enhances 5hmC Production Not as a Cofactor of TET, but by Reduction of Fe3+ to Fe2+.
Ascorbate has been shown to drive DNA demethylation in cultured cells in a TET-dependent manner (19, 21, 22, 25); however, this effect was not measured in a genome-wide or fully quantitative fashion. We determined cytosine modification levels in nESCs using mass spectrometry and found that 50 µg/mL ascorbate supplementation over 72 h decreased 5mC by almost one half (1.7-fold reduction) and increased 5hmC by 2.8-fold (Fig. 1C). The absolute loss of 5mC (0.71 pp) was 6.5-fold greater than the increase in 5hmC (0.11 pp), implying that ascorbate drives a genuine loss of cytosine modification along with increasing 5hmC equilibrium levels.
Current biochemical studies report that ascorbate acts as a bound cofactor of the TET proteins (19, 21–23). This hypothesis is based largely on the observation that antioxidants other than ascorbate have no effect on TET activity in cultured cells (19, 21–23). Moreover, several other Fe2+- and oxoglutarate-dependent enzymes are reliant on ascorbate as a bound cofactor (26). The classic example of this is collagen prolyl 4-hydroxylase (C-P4H), which in the absence of substrate undergoes a partial reaction involving decarboxylation of oxoglutarate and oxidation of the iron center to the inactive Fe3+ ion. Without ascorbate, this “uncoupled” reaction essentially destroys C-P4H activity in <1 min (27, 28) and is thought to be responsible for many of the symptoms associated with vitamin C deficiency.
To test whether ascorbate is required for TET function, we measured the in vitro activity of the recombinant murine TET1 catalytic domain (TET1-CD) using an ELISA plate assay. Under neutral conditions (pH 6.8) and 10 or 100 µM Fe2+ supplementation, no stimulation of hydroxymethylation activity was observed (Fig. 1D and Fig. S1B). This observation contrasts previous findings, including those of Yin et al. (23), who reported that 50–500 μM ascorbate was able to enhance TET function. One possible explanation for these discordant results could be related to the more basic reaction conditions used by Yin et al. (pH 8.0), in which Fe2+ is >100 times more likely to spontaneously oxidize to Fe3+ (29). We tested this hypothesis and found that at pH 8.0, nearly all Fe2+ ions became oxidized within 3 min (Fig. S1A), and ascorbate was able to rescue TET activity (Fig. S1 B and C). In contrast, at pH 6.8, only negligible oxidation of Fe2+ occurred after 15 min, and, accordingly, ascorbate was not able to enhance TET catalytic activity (Fig. S1 A–C). When TET1-CD was preincubated in the absence of methylated DNA substrate for 30 min (conditions that promote uncoupled reactions with other Fe2+- and oxoglutarate-dependent enzymes), no loss of TET activity was seen (Fig. S1D). This demonstrates that unlike C-P4H (27, 28), there is no obligate requirement of ascorbate for TET activity.
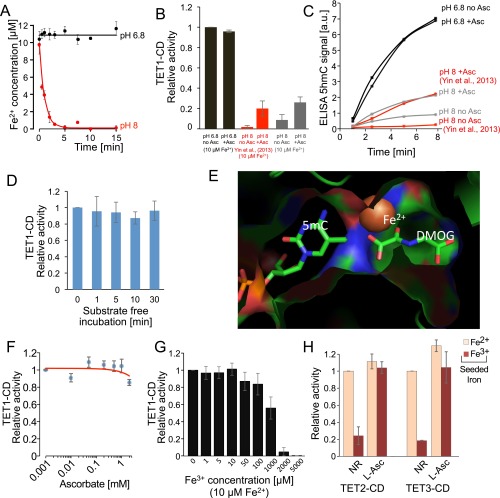
Fig. S1.
Ascorbate rescues TET activity by Fe3+ reduction, not as a cofactor. (A) Fe2+ concentration within reaction mixes used to test TET activity. Although Fe2+ is stable for 15 min at pH 6.8 (black bar), it is rapidly lost at pH 8 (red bar) (± SD; n = 3). (B) Comparison of relative activity of TET1-CD (at 10 μM Fe2+) in the presence or absence of ascorbate at different reaction conditions. Black bars show enzyme activity (± SD; n = 3) under optimized conditions (at pH 6.8) as in Fig. 3, or using the conditions described by Yin et al. (23) at pH 8 (red) and our conditions, but at pH 8.0 (gray). (C) Reaction progression curves of 5hmC production from B. (D) Probing uncoupled partial reaction of the TET1-CD enzyme. TET1-CD was preincubated in the reaction mixture in the presence of oxoglutarate but the absence of substrate DNA. After the indicated time (on the x-axis), the reactions were started by the addition of DNA substrate, and the reaction kinetics were followed as before. Subsequently, the initial reaction rates were extracted and compared with the reaction rate without preincubation. (E) Catalytic center of human TET2 (PDB ID code 4NM6) (53) focused on the direct neighborhood of the bound catalytic iron. The Fe2+ ion is tightly contacted by oxoglutarate (a noncatalyzed analog, DMOG, is shown here) and 5mC, leaving no space for binding of an ascorbate molecule. (F) Relative activity of recombinant TET1-CD in the presence of increasing concentrations of ascorbate (± SD; n = 3). No stimulation of TET1 oxidation activity is seen; however, at high concentrations (2 mM ascorbate), weak suppression of TET activity is observed. (G) Competition between Fe2+ and Fe3+ for occupancy at the catalytic center. TET1-CD reactions containing 10 μM Fe2+ were supplemented with increasing amounts of Fe3+ ions. The initial rates of these reactions were compared with the oxidation reactions without the addition of Fe3+ (± SD; n = 4). (H) Relative activity of TET2-CD and TET3-CD when supplemented with 100 μM iron (Fe2+ or Fe3+) and either no reducing agent (NR) or l-ascorbate (l-Asc) (± SD; n = 3).
To further test the hypothesis that ascorbate functions as a bound cofactor, we inspected the structure of the TET2 catalytic domain. In the presence of oxoglutarate and the target base, there is insufficient space for ascorbate to also bind in the catalytic pocket (Fig. S1E). Consequently, if ascorbate acts to reduce TET-bound iron, then at active concentrations it should compete with substrate for a position in the catalytic pocket. However, even with 2 mM l-ascorbate, TET1-CD activity was not stimulated and was only slightly inhibited (Fig. S1F). As such, we conclude that ascorbate does not act within the catalytic pocket to reduce bound iron.
An alternative hypothesis is that ascorbate supports TET function by converting Fe3+ (the most common form of iron in the cell) into Fe2+, the catalytically relevant state for this class of enzymes. To test this hypothesis, we performed in vitro 5mC oxidation reactions of TET1-CD in the presence of Fe3+ (Fig. 1E). Consistent with the dependency of TET enzymes on Fe2+, we observed only weak enzymatic function (∼5–12% of previous activity); however, on addition of 1 mM ascorbate to the reaction mixture, the oxidative capacity was reinstated (Fig. 1E). To verify that this effect is not specific to TET1, we repeated our experiments using recombinant TET2 and TET3 catalytic domain proteins (TET2-CD and TET3-CD, respectively), and found similar results (Fig. S1H). Along with demonstrating stereoisotopic insensitivity (both d and l forms were effective), our results suggest that Fe3+ is weakly bound by TET and can be readily replaced by Fe2+. A subsequent competition assay confirmed this point. A small amount of Fe2+ relative to Fe3+ (up to 100-fold less) was sufficient to retain full TET1-CD activity in vitro (Fig. S1G).
Prompted by the foregoing observations, we analyzed whether other common reducing agents can cause a similar effect as ascorbate in vitro. Following supplementation with DTT, tris(2-carboxyethyl)phosphine and reduced glutathione, 5mC oxidation activity under Fe3+ conditions recovered mildly (Fig. 1E). In contrast, hydroquinone and N-acetyl-l-cysteine showed little or no effect. Nevertheless, different reducing agents are known to show variable efficiencies for Fe3+ reduction (30). As such, we preincubated the reaction mixture containing Fe3+ ions for 30 min in the presence of a reducing agent and subsequently started the reactions by the addition of TET1-CD. This time, we observed robust recovery of TET1-CD activity for almost all reducing agents used (Fig. 1E). These results further emphasize the critical role of appropriate iron levels and valency for TET activity, as opposed to the presence of ascorbate per se.
To support this proposition, we examined the rates of reactions performed at different Fe2+ concentrations (Fig. 1F), and were able to infer an apparent dissociation constant (0.41 ± 0.05 µM). Significantly, this value falls within the reported range of labile Fe2+ concentrations in resting erythroid and myeloid cultured cells (0.2–1.5 µM) (31), suggesting that cellular labile iron concentration can directly modulate TET function in cells.
Retinol Enhances 5hmC by Activating TET Expression.
We and others have previously reported that reprogramming of serum-grown ESCs to naïve pluripotency causes a profound decrease in DNA methylation levels (1–4), an effect precipitated by the “2i” signaling inhibitors PD0325091 and CHIR99021. We also have shown that at 72 h of reprogramming, there is a simultaneous peak of 5hmC in the genome (1). To identify the molecular mechanism underlying this increase in 5hmC, we investigated the composition of the base medium used for growing the cells. There are two different formulations of the defined base medium used with 2i inhibition, the standard N2-B27 mixture originally designed for supporting the growth of neural stem cells (32) and another mixture in which the B27 supplement is without vitamin A, referred to herein as VitA+ and VitA−, respectively. When these two media types were used to convert serum-grown ESCs to naïve conditions, VitA+ treated cells were found to have greater loss of 5mC and increased 5hmC after 72 h (Fig. 2A).
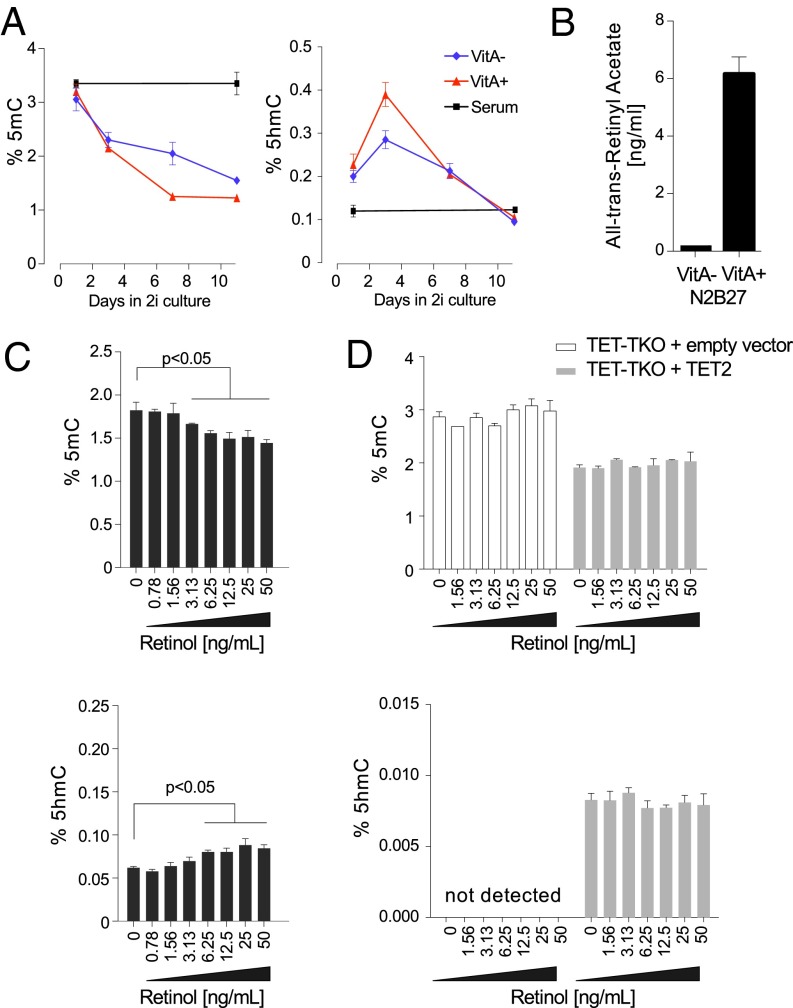
Fig. 2.
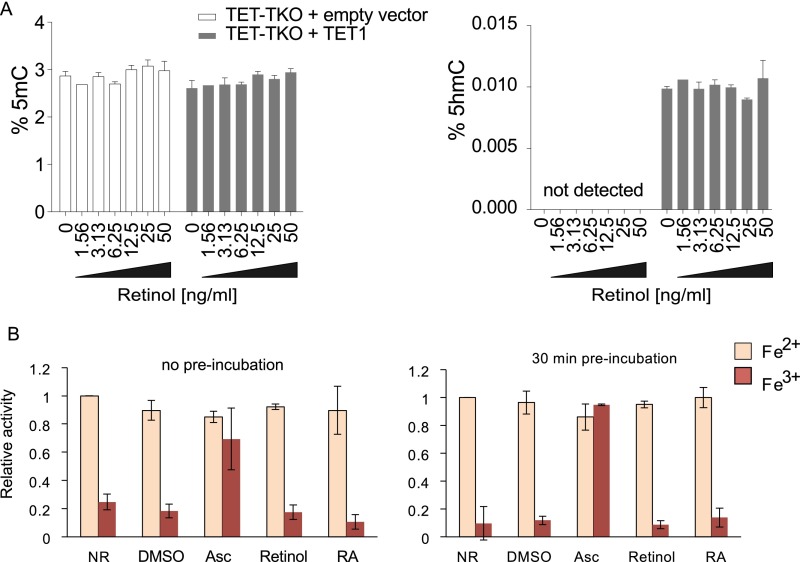
Retinol increases 5hmC and reduces 5mC in nESCs. (A) Global levels of 5mC and 5hmC (%) in serum-grown ESCs following 11 d of reprogramming in 2i/LIF medium either with vitamin A (VitA+) or without vitamin A (VitA−) (± SD; n = 3). (B) Retinyl acetate levels in VitA+/− 2i/LIF media formulations (± SD; n = 3). (C) Global levels of 5mC (Upper) and 5hmC (Lower) in nESCs supplemented with increasing levels of retinol for 72 h (black bars) (± SD; n = 3). (D) Global levels of 5mC (Upper) and 5hmC (Lower) in 2i/LIF-conditioned TET-TKO ESCs that were partially rescued by forced expression of TET2 (dark gray) and were exposed to retinol for 72 h (± SD; n = 3).
Mass spectrometry analysis of these different formulations at working concentrations revealed that the VitA+ medium contains ∼6 ng/mL of all-trans retinyl acetate (Fig. 2B), a common form of vitamin A used in cell culture media. Retinyl acetate and retinol show similar biological effects at equal concentrations; however, retinol is the most common form of vitamin A in human blood. As such, we supplemented nESCs grown in VitA− media with increasing levels of retinol up to 50 ng/mL, and found that it resulted in a 27% increase in 5hmC and a 26% decrease in 5mC (Fig. 2C, black bars). Cosupplementation of ascorbate with increasing retinol concentrations additively decreased 5mC and increased 5hmC, except at high doses, where 5hmC levels became saturated (Fig. S2A, gray bars).
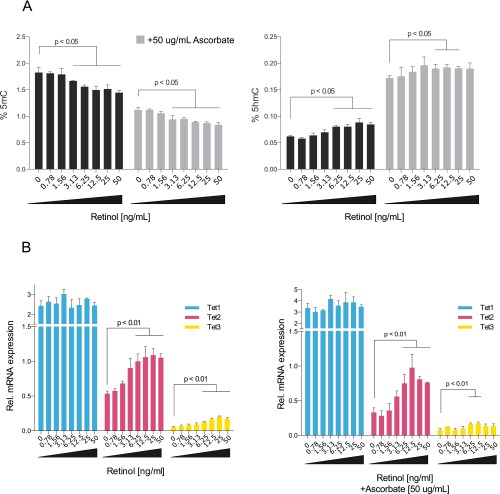
Fig. S2.
Effect of combined ascorbate and retinol treatment on nESCs. (A) Global levels of 5mC (Left) and 5hmC (Right) in nESCs supplemented with increasing levels of retinol for 72 h (black bars) (± SD; n = 3) and ascorbate plus increasing levels of retinol (gray bars). (B) Relative mRNA levels from TET1-3 in nESCs supplemented with retinol for 72 h (Left) and with retinol plus ascorbate (Right) (± SD; n = 3).
To understand how 5hmC is increased and 5mC is decreased by retinol treatment, we tested TET triple-knockout (TET-TKO) ESCs under the same conditions (Fig. 2D). No retinol-dependent decrease in 5mC was observed in these cells, indicating a requirement of the original retinol effect on TET proteins and 5hmC. When we partially rescued 5hmC production in the TET-TKO cells by forced expression of either TET1 or TET2 (the two most abundant TETs in ESCs), we found no increase in 5hmC upon retinol supplementation, and also no loss of 5mC (Fig. 2D and Fig. S3A). Thus, we reasoned that retinol does not affect TET protein stability or the efficiency of catalysis. Supporting this proposition, even with 30 min of preincubation, neither retinol nor RA could rescue TET1-CD function in the presence of Fe3+ (Fig. 1D), nor could it enhance TET1-CD in the presence of optimal Fe2+ (Fig. S3B). As such, we reasoned that retinol might instead affect TET transcription.
Fig. S3.
Retinol does not affect TET function posttranslation. (A) Global levels of 5mC (Left) and 5hmC (Right) in 2i/LIF-grown TET-TKO ESCs that were partially rescued by forced expression of TET1 (dark gray) and exposed to retinol for 72 h. Similar data were observed for TET2 (Fig. 2D). (± SD; n = 3). (B) Relative activity of TET1-CD when supplemented with 100 μM iron (Fe2+ or Fe3+) and retinol or RA, compared with ascorbate, and controls such as DMSO (carrier) or no reducing agent (NR). (± SD; n = 3).
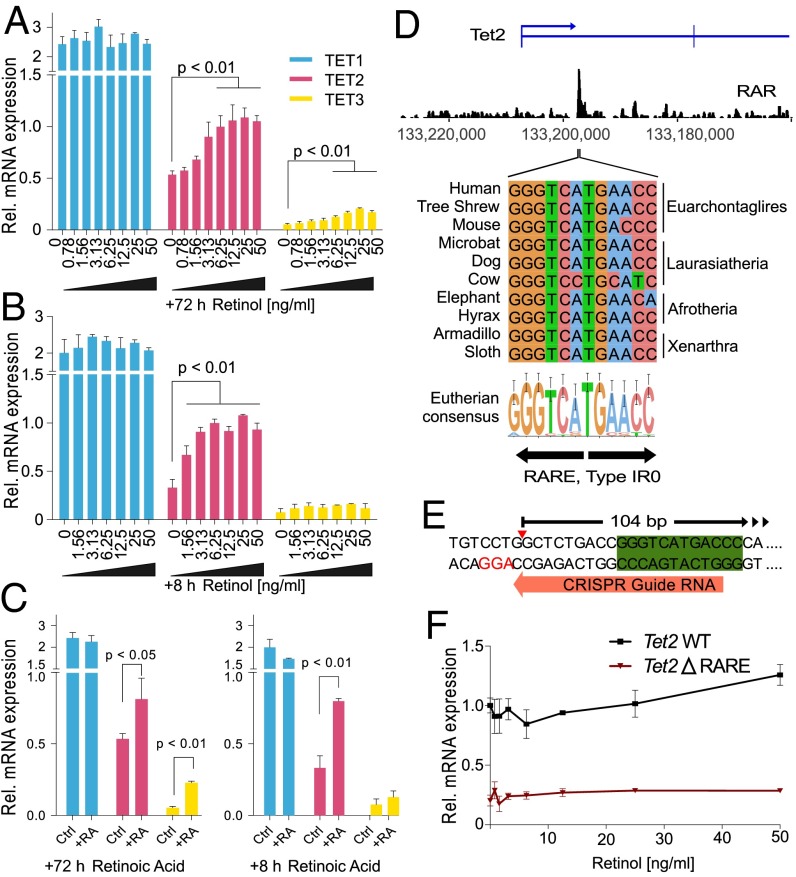
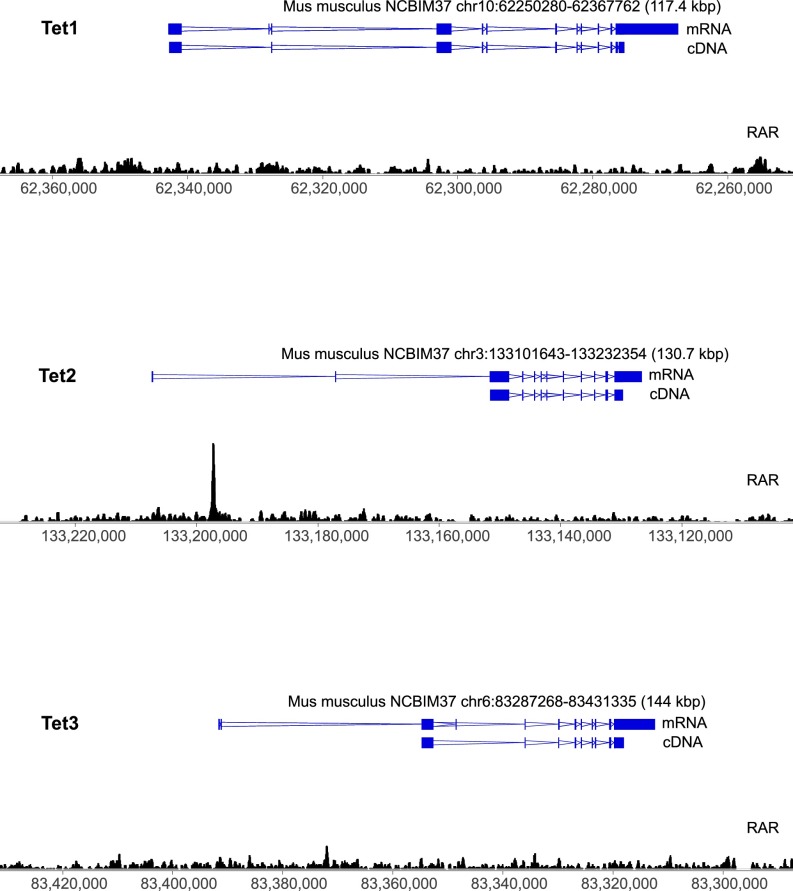
To explore this idea further, we examined TET mRNA levels in nESCs over the same range of retinol concentrations as shown in Fig. 2. After 72 h, there was a clear dose-dependent increase in both TET2 and TET3 mRNA in response to retinol supplementation (up to a 1.5- and 4.3-fold increase, respectively) (Fig. 3A and Fig. S2B), indicating a direct transcriptional effect. Shorter stimulation of the cells with retinol (8 h) activated only TET2 (Fig. 3B). Retinol is a metabolic precursor of RA, which is a potent signaling molecule and morphogen that promotes the anteriorization of developing vertebrate embryos (33). Direct supplementation of cells with RA (1 mg/mL) for 72 h also increased TET2 and TET3 expression, with only TET2 activated after 8 h (Fig. 3C). Analysis of published chromatin immunoprecipitation and sequencing (ChIP-seq) data using a pan-RA receptor (RAR) antibody (34) revealed a strong peak of enrichment in ESCs 10.1 kb downstream of the Tet2 promoter (Fig. 3D and Fig. S4). Underneath the apex of this peak, we discovered an inverted repeat consistent with IR0-type RAR elements (RAREs) (34, 35). This IR0 element is highly conserved throughout eutherian mammals, including all four major superorders (Fig. 3D and Table S1). In contrast, we found no RAR enrichment at Tet1, and although there was a small enrichment peak at Tet3, we could not detect any RAREs associated with this sequence (Fig. S4). To test whether the RARE is responsible for the effect of retinol on TET2 expression, we deleted it using CRISPR/Cas9 (Tet2ΔRARE; Fig. 3E). Stimulation of Tet2ΔRARE cells with retinol did not increase TET2 expression (Fig. 3F), indicating a direct role of the RARE in retinol-dependent regulation of TET2.
Fig. 3.
Retinol enhances 5hmC in a TET2 and RA signaling-dependent manner. (A) Relative mRNA levels from TET1-3 in nESCs supplemented with retinol for 72 h (± SD; n = 3). (B) Relative mRNA levels from TET1-3 in nESCs supplemented with retinol for 8 h (± SD; n = 3). (C) Relative mRNA levels from TET1-3 in nESCs supplemented with RA (± SD; n = 3). (D) ChIP-seq of a pan-RAR antibody in ESCs (data analyzed from ref. 34). Underneath the major enrichment peak is an IR0-type RARE that is conserved throughout all mammalian superorders. (E) A 104-bp deletion was created encompassing the Tet2 RARE (green box) using a CRISPR guide RNA (orange arrow) downstream of a protospacer adjacent motif (red text). The full deletion coordinates are NCBI37, chr3:133197151–133197253. (F) Relative TET2 mRNA levels in retinol-supplemented nESCs with the Tet2 RARE deleted (Tet2 ΔRARE). Wild-type (WT) control cells are included for comparison (± SD; n = 3).
Fig. S4.
RAR binding at Tet1-3. ChIP-seq profiles using a pan-RAR antibody (black wiggle plot) in serum-grown ESCs following 8 h of treatment with RA (+8 h RA). Regions overlapping the Tet1, Tet2, and Tet3 genes are displayed. Exons are blue blocks and introns are double blue lines, with the apex of these lines representing the direction of transcription (data analyzed from ref. 34).
Table S1.
Tet2 IR0-type RARE sequences from eutherian mammals
| Name | Sequence |
| Alpaca | AGGTCATGAACC |
| Armadillo | GGGTCATGAACC |
| Bushbaby | AGGTCATGAACC |
| Cat | GGGTCATGAACC |
| Chimpanzee | GGGTCATGAACC |
| Cow | GGGTCCTGCATC |
| Dog | GGGTCATGAACC |
| Dolphin | GGGTCATGAATC |
| Elephant | GGGTCATGAACA |
| Ferret | GGGTCATGAACC |
| Gibbon | GGGTCATGAACC |
| Gorilla | GGGTCATGAACC |
| Hedgehog | GGGTCGTGAACC |
| Human | GGGTCATGAACC |
| Hyrax | GGGTCATGAACC |
| Lesser hedgehog tenrec | AGGTCGTGAACC |
| Macaque | GGGTCATGAACC |
| Marmoset | GGGTCATGAACC |
| Megabat | GGGTTATGAACC |
| Microbat | GGGTCATGAACC |
| Mouse | GGGTCATGACCC |
| Mouse lemur | GGGCCATGCACC |
| Olive baboon | GGGCCATGAACC |
| Orangutan | GGGTCATGAACC |
| Panda | GGGTCATGAACC |
| Pig | GGGTCATGAGCC |
| Pika | GGGTCATGAACC |
| Rabbit | GGGTCATGAACC |
| Rat | GGGTCATGACCC |
| Sheep | GGGTCCTGCATC |
| Sloth | GGGTCATGAACC |
| Squirrel | GGGTCATGAACT |
| Tarsier | GGGTCATGAACC |
| Tree shrew | GGGTCATGAACC |
| Vervet | GGGTCATGAACC |
Retinol and Ascorbate Enhance Reprogramming of Epiblast Stem Cells to Naïve Pluripotency.
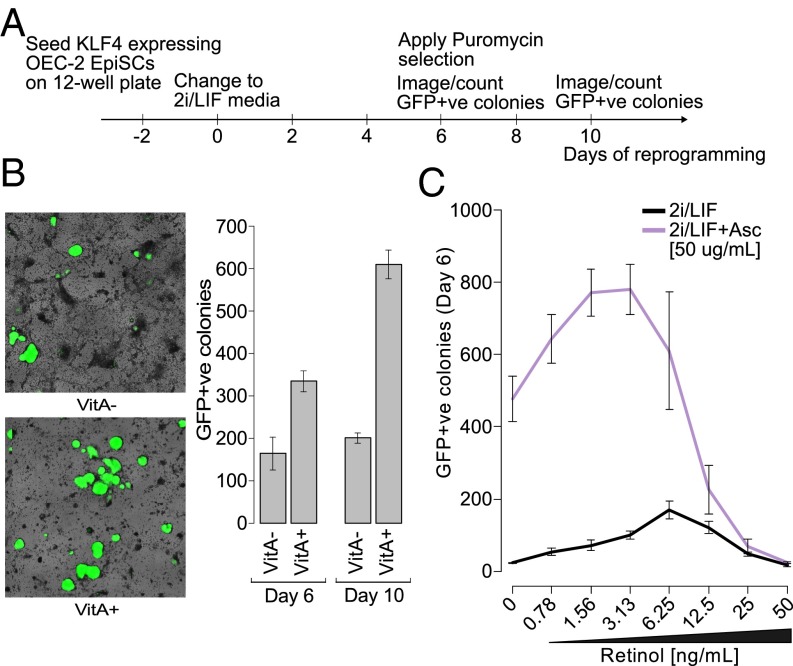
To understand the relevance of our results from the perspective of DNA demethylation in a biological context, we asked how retinol and ascorbate affect iPSC production. Epiblast stem cells (EpiSCs) are derived from the embryo postimplantation (E6.5), have a highly methylated genome, and are unable to create chimeric mice on injection into a host blastocyst (36–38). They can be reprogrammed to naïve iPSCs in 2i/LIF media (albeit relatively inefficiently) by overexpression of at least one regulator of naïve pluripotency (36) and, in the case of the OEC-2 EpiSC line (39), show bright and homogenous expression of an Oct4:GFP reporter gene after 6–10 d of reprogramming (Fig. 4A).
Fig. 4.
Retinol and ascorbate enhance reprogramming of epiblast stem cells to naïve pluripotency. (A) Experimental setup for EpiSC reprogramming experiments. (B) Oct4:GFP+ colonies following 6–10 d of reprogramming in VitA+/− 2i/LIF media. (Left) Representative microscopic field views at day 10 (4× magnification; the GFP channel is colored green and superimposed over a brightfield image of the same view). (Right) Frequency of reprogrammed colonies (± SD; n = 3). (C) Frequency of Oct4:GFP+ colonies after 6 d of reprogramming in VitA− 2i/LIF media with supplemented retinol and ascorbate (± SD; n = 3).
We first compared VitA+ and VitA− reprogramming media (i.e., with and without retinyl acetate, respectively) on KLF4-overexpressing OEC-2 EpiSCs, and found that the latter resulted in less than one-half the number of Oct4:GFP+ colonies after 6 d of reprogramming and almost one-third fewer after 10 d (Fig. 4B). When we tested this effect over the same range of retinol from Fig. 2, we found that the number of Oct4:GFP+ colonies increased proportionally up to a concentration of 6.25 ng/mL (Fig. 4C, black line) but that beyond this point, further increases in retinol actually decreased the number of Oct4:GFP+ colonies, such that at 50 ng/mL of retinol, almost no reprogrammed colonies were found. Combining ascorbate treatment with retinol enhanced reprogramming considerably, as previously reported in other systems (40, 41), but exhibited an interesting additional effect (Fig. 4C, purple line). The dose–response curve was clearly shifted to the left, meaning that the maximum number of Oct4:GFP+ colonies produced resulted from a lower retinol concentration when cosupplemented with ascorbate (3.13 ng/mL). These results are consistent with the hypothesis that increased 5hmC and decreased 5mC due to retinol and ascorbate enhance the reprogramming of EpiSCs, and that their costimulation results in increased reprogramming synergistically.
Discussion
DNA methylation erasure in the germ line and during experimental reprogramming are closely linked to the acquistition of pluripotency. TET-mediated production of 5hmC can enhance DNA demethylation (reviewed in ref. 6); however, the mechanistic details involved with this process and what can be done to manipulate it are much less understood. Here we show that DNA demethylation and steady-state 5hmC levels can be enhanced in nESCs by various retinoid forms and ascorbate through distinct mechanisms (Fig. 5).
Fig. 5.
Retinol and ascorbate enhance DNA demethylation, 5hmC production, and pluripotent stem cell reprogramming by synergistic mechanisms. The RARE in the first intron of Tet2 allows increased expression of TET2 mRNA on stimulation of RA signaling (by retinol, retinyl acetate, or RA itself) and enhanced binding of the RAR (brown enzyme). In contrast, ascorbate increases the active iron (Fe2+, green circles) required for the TET catalytic center by reduction from Fe3+ (red circles). Together, retinol and ascorbate additively enhance 5hmC production, resulting in greater removal of methylation from DNA. The enhancing effect of ascobate and retinol on naïve pluripotent stem cell reprogramming is greater than the sum of their individual effects.
We find that ascorbate supports TET activity not as an essential cofactor, but rather by reduction of nonenzyme-bound Fe3+ to Fe2+. Unlike C-P4H, which is reliant on ascorbate for its activity, TET does not undergo uncoupled reactions that destroy its activity in the absence of substrate (Fig. S1G). Moreover, under conditions of sufficient Fe2+ and neutral pH, ascorbate does not enhance TET function (Fig. 1D). When faced with insufficient Fe2+ (and excess Fe3+), ascorbate dramatically rescues TET activity, but other reducing agents can do this as well, provided that sufficient incubation time is provided (Fig. 1E). Although these results challenge the findings reported in the literature (19, 21–23), they are in complete agreement with the recent finding that redox-active quinones stimulate TET activity in cell culture through reduction of enzyme-free Fe3+ to Fe2+ (42). Taken together, these results imply that TET proteins are inherently sensitive to labile iron concentrations in the cell, an idea supported by the fact that the dissociation constant of Fe2+ and TET1-CD overlaps with the physiological range of labile iron in the cell (Fig. 1F).
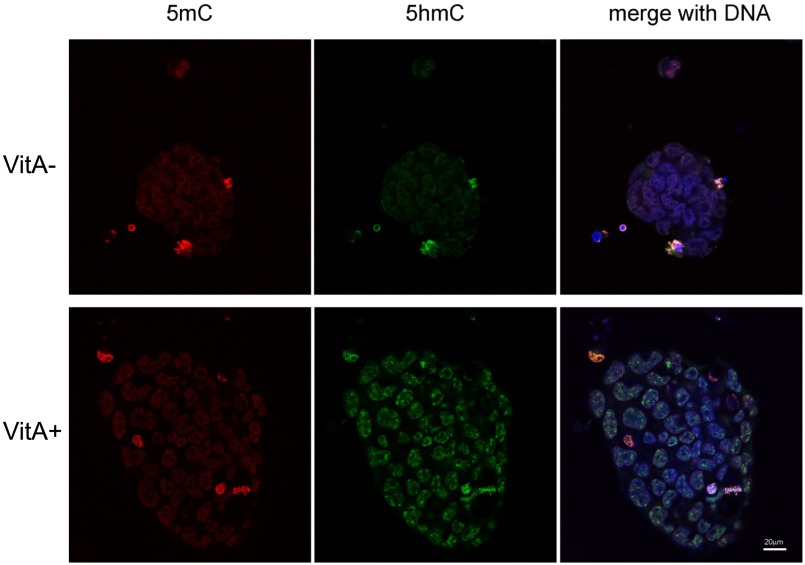
In contrast to ascorbate, we found that retinol and RA do not have any effect on TET enzyme efficiency in cell culture (Fig. 2D) or in vitro (Fig. 1E), but instead enhance DNA demethylation and increase 5hmC by activating TET2 and TET3 expression (Fig. 3). We also found that human nESCs grown in media with vitamin A supplementation (i.e., retinyl acetate; Fig. 2B) have more genomic 5hmC than those grown without it (Fig. S5), supporting our original observations in mouse nESCs (Fig. 2C) and implying that this is a conserved regulatory effect. TET2 activation has been previously associated with RA treatment in embryonal carcinoma cells (43); however, the nature of this association is unknown. Given that TET2 responds to retinol and RA stimulation within 8 h in a manner dependent on a eutherian-conserved IR0-type RARE within its first intron (Fig. 3), we conclude that Tet2 is a direct target of RA signaling.
Fig. S5.
Retinol enhances 5hmC in human naïve ESCs. Immunofluorescence using antibodies specific to 5mC (red) and 5hmC (green) on human naïve ESCs grown with retinol (VitA+) and without retinol (VitA−).
When ascorbate and retinol are supplemented in combination, RA signaling will increase TET protein levels and ascorbate will potentiate its activity (Fig. 5). A prediction arising from this scenario is that the combined effect of retinol and ascorbate should be greater than the sum of their individual effects, owing to complementary effects on shared cellular components. Indeed, ascorbate treatment essentially “sensitizes” EpiSCs to lower levels of retinol (Fig. 4C), which suggests that the improvement in reprogramming by both small molecules is affecting the same pathway, the nexus of which is 5hmC.
Our results demonstrate that intermediate levels of RA signaling enhance the reprogramming of EpiSCs to naïve pluripotency (Fig. 4). Supplemented retinol above 6.25 ng/mL suppresses reprogramming in a dose-dependent manner, an effect that we speculate is due to the well-described ability of RA to stimulate differentiation. Interestingly, retinol levels in the serum of mice (44) and humans (45) is higher than the range that we tested; thus, adult levels of RA signaling may form one potential barrier by which somatic cells are protected from spontaneous de-differentation. A previous study showed that optimum levels of retinoid stimulation are critical for the reprogramming of EpiSCs to iPSCs, and that RA signaling strongly affects the reprogramming of mouse embryonic fibroblasts (24). That study further showed that small-molecule antagonists of RA signaling attenuate β-catenin and activate Wnt signaling in EpiSCs, thus providing a likely mechanism for how RA enhances reprogramming. We suggest that in addition to this effect, RA signaling enhances reprogramming by directly activating TET2 expression, a known reprogramming factor (16, 18).
In summary, our work provides mechanistic insight into how TET proteins remove epigenetic information during reprogramming to naïve pluripotency, and how this process can be manipulated through the use of retinol and ascorbate. Nevertheless, the significance of our work is not limited to the elucidation of these fundamental processes and their application to iPSC reprogramming. For example, our finding that TET activity is sensitive to physiological changes in iron suggests that TET may represent a conduit through which alterations in this ion are signaled to the genome. Moreover, the observation that RA signaling enhances TET2 expression could be relevant for the treatment of certain cancers. TET2 is a well-described tumor suppressor that is regularly mutated in a number of hematopoietic malignancies (46). Acute promyelocytic leukemia (APL) is a form of myeloid malignancy characterized by PML-RARα translocation and sensitivity to RA treatment, such that RA used in combination with arsenic trioxide can provide a 5-year event-free survival rate of >90% (47, 48), a dramatic improvement for what was once considered the deadliest form of acute leukemia. Nevertheless, a significant number of patients are resistant to RA treatment. A recent analysis found that 4.5% of patients with APL have mutations in TET2, and that a mutation in this and other epigenetic modifiers is a significant indicator of poor disease outcome (49). Our work provides a potential mechanistic explanation for RA insensitivity in patients with APL with TET2 mutations, and if proven in further experimentation, could affect the management of this disease.
Methods
Our methodology is described in detail in SI Methods. In brief, stem cell culture was performed according to standard techniques as reported previously (1, 16), with modifications as described in Tables S2 and S3. Mass spectrometry analysis of nucleosides was performed as described previously (50). An ELISA-based plate assay was used to quantify the 5hmC produced by a TET1 catalytic domain protein (TET1-CD) in vitro. EpiSC reprogramming was performed as described previously (39), with modifications as outlined in SI Methods.
Table S2.
Cell lines used in this study
Table S3.
Media and additives used in this study
| Medium/additive | Composition (final concentration) | Notes |
| Base media | ||
| Serum | DMEM (Gibco, 11995–065); 15% FBS (Gibco, 10270–106); 1× pen/strep solution (Gibco, 15140–122); 1× nonessential amino acids (Gibco, 11140–035); 50 nM β-mercaptoethanol (Gibco, 31350–010) | |
| N2B27 | 1:1 DMEM/F12 (Gibco 21331):Neurobasal (Gibco 21103); 1× N2 (Gibco, 17502–048); 1× B27* (Gibco 17504–044 or 12587–010); 0.4 mM l-glutamine (Gibco 25030–024); 1× pen/strep solution (Gibco, 15140–122); 50 nM β-mercaptoethanol (Gibco, 31350–010) | *B27 with retinyl acetate (VitA+) is 17504–044; B27 without retinyl acetate (VitA−) is 12587–010 |
| Additives | ||
| 2i | 3 µM, CHIR99021 (Stemgent, 04–0004); 1 µM PD0325901 (Stemgent, 04–0006) | |
| LIF | 10 μg/mL LIF (Wellcome Trust Centre for Stem Cell Research) | |
| FGF2 | 12.5 ng/mL FGF2 (Wellcome Trust Centre for Stem Cell Research) | |
| Activin A | 20 ng/mL Activin A (Wellcome Trust Centre for Stem Cell Research) | |
| Blasticidin | 20 μg/mL Blasticidine S hydrochloride (Sigma-Aldrich, 15205) | |
| Retinol | Variable concentrations (Sigma-Aldrich, R7632) | |
| Retinoic acid | 1 mg/mL (Sigma-Aldrich, R2625) | |
| Ascorbate | 50 μg/mL (Sigma-Aldrich, A7631) |
SI Methods
Cell Lines and Culturing Conditions.
All ESC and EpiSC lines used in this study are listed in Table S2, and respective culture conditions and media compositions are listed in Table S3. All cells were grown on gelatin-coated plates without feeders except for EpiSCs, which were grown on fibronectin coated plates.
Nucleic Acid Extraction, Manipulation, and qRT-PCR.
Total nucleic acid was extracted from cell pellets using the Agencourt RNAdvance Cell v2 Kit (Beckman Coulter, catalog no. A47942) according to the manufacturer’s instructions. Total nucleic acid was DNase-treated using the Ambion TURBO DNA-free Kit (Thermo Fisher Scientific, catalog no. AM1907), from which 500–2000 ng of RNA was converted into cDNA using the RevertAid First-Strand cDNA Synthesis Kit (Thermo Fisher Scientific, catalog no. K1622). qRT-PCR was performed on a Bio-Rad 384-well machine using the primers and reaction conditions listed in Table S4. Cycle number differences related to input RNA level were normalized using a standard curve and two reference genes (Atp5b and Hsp90ab1).
Table S4.
Primers used in this study
| Primer name | Sequence | Target |
| Hspcb_RT_F | GCTGGCTGAGGACAAGGAGA | Hsp90ab1 (reference gene) |
| Hspcb_RT_R | CGTCGGTTAGTGGAATCTTCA | |
| Atp5b_RT_F | GGCCAAGATGTCCTGCTGTT | Atp5b (reference gene) |
| Atp5b_RT_R | GCTGGTAGCCTACAGCAGAAGG | |
| Tet1_FW_GF | CCATTCTCACAAGGACATTCACA | Tet1 (catalytic domain) |
| Tet1_RV_GF | GCAGGACGTGGAGTTGTTCA | |
| Tet2_FW_GF | GCCATTCTCAGGAGTCACTGC | Tet2 (catalytic domain) |
| Tet2_RV_GF | ACTTCTCGATTGTCTTCTCTATTGAGG | |
| Tet3_FW_GF | TCCGGATTGAGAAGGTCATC | Tet3 (catalytic domain) |
| Tet3_RV_GF | CCAGGCCAGGATCAAGATAA |
Cycling conditions: 98 °C, 2 min; 40× {98 °C, 20 s; 72 °C, 20 s}; 72 °C, 5 min. Reaction conditions: SYBR Green qRT-PCR Master Mix (Stratagene, catalog no. 600886) according to the manufacturer’s instructions.
Reprogramming and TET-TKO Rescue Experiments.
EpiSCs were transfected using FuGENE reagent (E2311; Promega) with 1 μg of PB-flox-Klf4-Pgk-Hygro, 1 μg PB-flox-Empty-IRES-Blast, and 2 μg of PBase expression vector pCAGPBase54 (16). Dual hygromycin and blasticidin selection was applied to transfectants for a minimum of 10 d. Stable transgene expression was confirmed by qRT-PCR. Here 20,000 stable transfectant cells were seeded per well in a 12-well plate in standard EpiSC conditions (N2B27 + FGF2 + Activin A). After 24 h, the medium was switched to N2B27 + 2i/LIF. Puromycin selection for an Oct4-GFP-IRES-Puro reporter transgene was applied from day 6 of 2i/LIF treatment. Image tiles covering entire wells were recorded using both the transmitted and GFP channels (±VitA), or only the GFP channel (retinol series) using a BD Pathway 855 microscope (BD Biosciences). Mosaic images were created using on-board CALIPER software. GFP-positive colonies were counted manually from GFP images, taking into consideration both signal morphology and intensity.
2i/LIF conditioned TET-TKO cells were transfected with 1 μg of PB-flox-HsaTET1-IRES-Blast, PB-flox-HsaTET2-IRES-Blast (16), or an empty vector control according to the foregoing protocol. Single colonies of stable transfectants were selected for 10 d and then subjected to retinol treatment for 72 h.
TET2 RARE Deletion.
The Tet2 RARE element was deleted in E14 ESCs using CRISPR/Cas9. ESCs were transfected with 1 µg of pSpCas9(BB)-2A-GFP (PX458) (Addgene plasmid 48138) (51) expressing Cas9 and GFP and a plasmid expressing the guide RNA (GTCATGACCCGGTCAGAGCCAGG), targeting the Tet2 RARE (position 210–232; minus strand). Single cells expressing high levels of GFP after 48 h were sorted into 96-well plates, expanded, screened for deletions of the Tet2 RARE, and the expression of Tet2 after retinol treatment for 72 h was assayed.
Mass Spectrometry.
Here 500 ng of genomic DNA was incubated with DNA Degradase Plus (Zymo Research) at 37 °C for 4 h. Analysis of global 5mC and 5hmC levels was carried out with an AB Sciex Triple Quad 6500 mass spectrometer as described previously (48). Results are expressed as percentage of the total cytosines.
For retinoid mass spectrometry, all manipulations of samples were carried out below 10 °C with exposure to light kept to a minimum. Media samples were diluted with acetonitrile (3 volumes), incubated at 4 °C for 30 min, and then centrifuged at 14,000 × g for 5 min. The supernatants were diluted with water (2 volumes), then further diluted with 50% aqueous acetonitrile as required immediately before analysis.
Samples were analyzed by LC-MS on a Q-Exactive Plus high-resolution mass spectrometer (Thermo Fisher Scientific) coupled, via a Proxeon nanoelectrospray ion source, to a Dionex UltiMate 3000 Nano LC System. LC separation was achieved isocratically with a solvent of 0.1% formic acid in 90% aqueous acetonitrile, at a flow rate of 800 nL/min, on a reversed-phase column packed with ReproSil-Pur C18AQ (250 × 0.1 mm; 3-µm particles).
Because both retinol (Sigma-Aldrich; R2625) and retinyl acetate (Sigma-Aldrich; R7882) fragment spontaneously during electrospray ionization to generate the C20H29+ ion (monoisotopic m/z, 269.2264), both compounds were quantified from extracted ion chromatograms of this ion in high-resolution (140,000 nominal) SIM scans, recorded over the m/z range of 264.2–274.2. For confirmation of identity, MS2 spectra were also recorded, with an isolation window of 1 Thomson unit and a normalized collision energy of 30 V.
Cloning, Expression, and Purification of Recombinant mTET1-CD.
The genes coding for the catalytic domains of murine TET1, TET2, and TET3 proteins were amplified from pFastBac-mTet1, -mTet2, and -mTet3 full-length constructs (generously provided by Yi Zhang, Howard Hughes Medical Institute, Boston) and cloned in pET28a (+) vector. For TET2 and TET3, the variable regions found between two parts of dioxygenase domains were substituted by a 15-aa flexible linker. Recombinant TET-CD proteins were expressed in Escherichia coli strain BL21 (DE3) CodonPlus-RIL at 20 °C overnight (12–13 h) in LB medium after the addition of 1 mM isopropyl β-D-1-thiogalactopyranoside. The cells were harvested and resuspended in sonication/wash buffer containing 50 mM Hepes pH 8.0, 30 mM imidazole, 500 mM NaCl, 10 mM α-ketoglutarate, and 10% glycerol, after which the cells were lysed by sonication. The crude lysate was clarified by centrifugation, and the protein was purified using Ni-NTA affinity chromatography (Genaxxon). The proteins were eluted with wash buffer supplemented with 250 mM imidazole and dialyzed against buffer containing 50 mM Hepes pH 8.0, 300 mM NaCl, 10 mM α-ketoglutarate, and 10% glycerol for 3 h. After dialysis, the proteins were aliquoted and stored at −80 °C for further use.
ELISA-Based Plate Assay for the Detection of 5hmC.
The ELISA plate assay for measuring TET activity was performed in 96-well white high-binding plates (Lumitrac 600; Greiner), precoated overnight with 100 µL containing 1.3 µg of avidin (Sigma-Aldrich) dissolved in 0.1 M sodium bicarbonate (pH 9.6). At the start of the assay, the wells were washed five times with PBST-500 wash buffer (PBS supplemented with 0.01% Tween 20 and 0.5 M NaCl) and then filled with 100 µL of 0.05 M NaOH stop solution.
DNA oxidation reaction mixtures (20 µL) were prepared in microcentrifuge tubes and comprised 0.5 µM methylated substrate DNA (86-mer) and 1.5 µM recombinant murine TET-CD in buffer containing 50 mM Hepes pH 6.8, 1 mM ascorbate, 10 or 100 µM Fe2+ or Fe3+ (FeCl3) and 1 mM α-ketoglutarate. The reactions were started by addition of the enzyme, and 2-µL samples were taken from the reaction mixture at respective time points. The samples were added to the corresponding well of a microtiter plate containing stop solution and incubated on a rotating platform for at least 20 min. Then the wells were washed five times with PBST-500 wash buffer and blocked with 300 µL of 2% BSA dissolved in PBST for 1 h on a rotating platform. Subsequently, the wells were washed five times with 300 µL of PBST-500 per well, and 100 µL of primary antibody [anti-5hmC; 1:10,000 (ActiveMotif) diluted in 2% BSA in PBST solution] was added to each well. The plate was incubated for a1 h on a rotating platform, followed by five washes with 300 µL of PBST-500 wash buffer. Next, 100 µL of secondary antibody [anti-rabbit conjugated with HRP (GE Healthcare); 1:6,000 dilution in 2% BSA/PBST] was added to each well, followed by incubation for 1 h. The wells were washed again five times with 300 µL per well of PBST-500. Finally, 100 µL of the ECL substrate solution (Pierce ECL Plus) was added, and luminescence was measured for 5 s in each well using an EnSpire microtiter plate reader (PerkinElmer).
To check the effect of various reducing agents on Tet activity, the reaction mixtures containing 10 or 100 µM Fe2+ or Fe3+ ions were supplemented with reducing agents, and the reaction was started by adding the enzyme either directly or after a 30-min preincubation. Beta-mercaptoethanol, DTT, tris(2-carboxyethyl)phosphine, l-glutathione, d-iso ascorbate, N-acetyl l-cysteine, and sodium l-ascorbate were used at a concentration of 1 mM in the reaction. Hydroquinone was used at 50 µM, potassium iodide was used at 10 µM, and retinol and RA were used at 0.2 µM and 100 µM.
Preparation of Biotinylated Substrate.
The biotinylated substrate (86-mers) used in the assay was amplified from a pUC19 vector by qRT-PCR using oligos (DpnII_pUC19_f: 5′- GAG TAA ACT TGG TCT GAC AGT TAC CA -3′ and DpnII_pUC19_r: 5′- CAA CTA TGG ATG AAC GAA ATA GAC AGA T -3′) with forward primer carrying biotin molecules. The substrate- containing modified cytosines (5mC) were generated using 5mdCTP (New England Biolabs) instead of dCTP in the amplification reaction.
Fe2+ Binding Assay.
To investigate the Tet1-CD and Fe2+ binding affinity, we assayed the enzyme activity at different Fe2+ concentrations. The kinetic data were plotted in Microsoft Excel, and the initial reaction rates were extracted using linear regression. The reaction rates were fitted using a least squares fitting procedure to a bimolecular binding equilibrium model. To account for prebound Fe2+, which copurifies with the enzyme from bacteria, a variable representing the concentration of prebound Fe2+ was added to the concentration of supplemented Fe2+.
Quantification of Ferrous Iron.
The concentration of ferrous iron present in the reaction mixtures was quantified with the ferrozine method (52). The reaction mixture containing 10 µM ammonium iron (II) sulfate hexahydrate, 50 mM HEPES (at either pH 6.8 or 8.8) was prepared and incubated at 37 °C. At defined time points, 500-µL aliquots were removed from the reaction mixture and added to the tube containing 100 µL of 500 µM ferrozine and 100 µL of 2 M ammonium acetate at pH 6.0 to quench potential further oxidation of ferrous iron during the subsequent incubation step. The samples were kept at room temperature for 20 min to ensure full color development, and their absorbance was measured at 562 nm in 10-mm glass cuvettes using a Hitachi U-2810 spectrophotometer. Samples containing 50 mM HEPES buffer (at either pH 6.8 or 8.8) without ammonium iron (II) sulfate hexahydrate served as blanks. The data were plotted in Microsoft Excel and fitted with a single exponential function. A standard curve prepared with known concentrations of ferrous iron was used to quantify the observed Fe2+ concentrations in the samples.
Bioinformatics.
ChIP datasets (32) (GSM482749 and GSM482749) were analyzed using SeqMonk and custom R scripts. RAREs were discovered within the Tet family sequence by a text search and homology alignment. Regions orthologous to the Tet2 IR0-type RARE were retrieved from 35 eutherian mammals using Ensembl release 77 (www.ensembl.org). The eutherian consensus logo was created using Weblogo3 (weblogo.threeplusone.com).
Immunofluorescence, Microscopy, and Image Analysis.
Staining of methylated and hydroxymethylated DNA was performed as described previously (1) with modifications. Cells were fixed in 2% paraformaldehyde for 30 min. After permeabilization by PBS + 0.5% Triton X-100 for 1 h, fixed cells were incubated in 2 N HCl for 30 min at 37 °C, washed in PBS-Tween, and then blocked overnight. Cells were incubated in 1:500 anti-5mC (Eurogentec, BI-MECY) and 1:1,000 anti-5hmC (Active Motif, 39769). Secondary antibodies (Alexa Fluor) were diluted 1:1,000 and incubated for 1 h. Nuclei were stained with YOYO1 (Molecular Probes). Single optical sections were captured with a Zeiss LSM780 microscope (63× oil-immersion objective), and the images were pseudocolored using Adobe Photoshop.
Acknowledgments
We thank Austin Smith, Guoliang Xu, and Jose Silva for providing human ESCs, TET triple-KO ESCs, and OEC-2 EpiSCs, respectively. We also thank Felix Krueger and Simon Andrews for bioinformatic help and Simon Walker for assistance with imaging. This work was funded by the Wellcome Trust (senior investigators W.R. and S.B.; 095645/Z/11/Z and 099232/z/12/z, respectively), the Biotechnology and Biological Sciences Research Council (BB/K010867/1 to W.R.), the Medical Research Council, the European Union EpiGeneSys Network of Excellence, the European Union BLUEPRINT Consortium, the Human Frontier Science Program (T.H.), the Swiss National Science Foundation/Novartis (F.v.M.), and the German Research Foundation (Grant Deutsche Forschungsgemeinschaft SPP1784, to T.P.J).
Footnotes
Conflict of interest statement: W.R. and S.B. serve as consultants for Cambridge Epigenetix Ltd.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608679113/-/DCSupplemental.
References
- 1.Ficz G, et al. FGF signaling inhibition in ESCs drives rapid genome-wide demethylation to the epigenetic ground state of pluripotency. Cell Stem Cell. 2013;13(3):351–359. doi: 10.1016/j.stem.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habibi E, et al. Whole-genome bisulfite sequencing of two distinct interconvertible DNA methylomes of mouse embryonic stem cells. Cell Stem Cell. 2013;13(3):360–369. doi: 10.1016/j.stem.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Leitch HG, et al. Naive pluripotency is associated with global DNA hypomethylation. Nat Struct Mol Biol. 2013;20(3):311–316. doi: 10.1038/nsmb.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaji M, et al. PRDM14 ensures naive pluripotency through dual regulation of signaling and epigenetic pathways in mouse embryonic stem cells. Cell Stem Cell. 2013;12(3):368–382. doi: 10.1016/j.stem.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Theunissen TW, et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell. 2014;15(4):471–487. doi: 10.1016/j.stem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HJ, Hore TA, Reik W. Reprogramming the methylome: Erasing memory and creating diversity. Cell Stem Cell. 2014;14(6):710–719. doi: 10.1016/j.stem.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hon GC, et al. Epigenetic memory at embryonic enhancers identified in DNA methylation maps from adult mouse tissues. Nat Genet. 2013;45(10):1198–1206. doi: 10.1038/ng.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikkelsen TS, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454(7200):49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theunissen TW, et al. Nanog overcomes reprogramming barriers and induces pluripotency in minimal conditions. Curr Biol. 2011;21(1):65–71. doi: 10.1016/j.cub.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurkowski TP, Jeltsch A. Burning off DNA methylation: New evidence for oxygen-dependent DNA demethylation. ChemBioChem. 2011;12(17):2543–2545. doi: 10.1002/cbic.201100549. [DOI] [PubMed] [Google Scholar]
- 11.Tahiliani M, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324(5929):930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ficz G, et al. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature. 2011;473(7347):398–402. doi: 10.1038/nature10008. [DOI] [PubMed] [Google Scholar]
- 13.Ito S, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333(6047):1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Meyenn F, et al. Impairment of DNA methylation maintenance is the main cause of global demethylation in naive embryonic stem cells. Mol Cell. 2016;62(6):848–861. doi: 10.1016/j.molcel.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams K, et al. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473(7347):343–348. doi: 10.1038/nature10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa Y, et al. NANOG-dependent function of TET1 and TET2 in establishment of pluripotency. Nature. 2013;495(7441):370–374. doi: 10.1038/nature11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y, et al. Replacement of Oct4 by Tet1 during iPSC induction reveals an important role of DNA methylation and hydroxymethylation in reprogramming. Cell Stem Cell. 2013;12(4):453–469. doi: 10.1016/j.stem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Doege CA, et al. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488(7413):652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blaschke K, et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500(7461):222–226. doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, et al. Vitamin C modulates TET1 function during somatic cell reprogramming. Nat Genet. 2013;45(12):1504–1509. doi: 10.1038/ng.2807. [DOI] [PubMed] [Google Scholar]
- 21.Minor EA, Court BL, Young JI, Wang G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Biol Chem. 2013;288(19):13669–13674. doi: 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickson KM, Gustafson CB, Young JI, Züchner S, Wang G. Ascorbate-induced generation of 5-hydroxymethylcytosine is unaffected by varying levels of iron and 2-oxoglutarate. Biochem Biophys Res Commun. 2013;439(4):522–527. doi: 10.1016/j.bbrc.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin R, et al. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc. 2013;135(28):10396–10403. doi: 10.1021/ja4028346. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, et al. Signalling through retinoic acid receptors is required for reprogramming of both MEFs and EpiSCs to iPSCs. Stem Cells. 2014;33(5):1390–1404. doi: 10.1002/stem.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung T-L, et al. Vitamin C promotes widespread yet specific DNA demethylation of the epigenome in human embryonic stem cells. Stem Cells. 2010;28(10):1848–1855. doi: 10.1002/stem.493. [DOI] [PubMed] [Google Scholar]
- 26.Kuiper C, Vissers MCM. Ascorbate as a co-factor for Fe- and 2-oxoglutarate dependent dioxygenases: Physiological activity in tumor growth and progression. Front Oncol. 2014;4:359. doi: 10.3389/fonc.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myllylä R, Majamaa K, Günzler V, Hanauske-Abel HM, Kivirikko KI. Ascorbate is consumed stoichiometrically in the uncoupled reactions catalyzed by prolyl 4-hydroxylase and lysyl hydroxylase. J Biol Chem. 1984;259(9):5403–5405. [PubMed] [Google Scholar]
- 28.De Jong L, Albracht SPJ, Kemp A. Prolyl 4-hydroxylase activity in relation to the oxidation state of enzyme-bound iron: The role of ascorbate in peptidyl proline hydroxylation. Biochim Biophys Acta. 1982;704(2):326–332. doi: 10.1016/0167-4838(82)90162-5. [DOI] [PubMed] [Google Scholar]
- 29.Morgan B, Lahav O. The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution: Basic principles and a simple heuristic description. Chemosphere. 2007;68(11):2080–2084. doi: 10.1016/j.chemosphere.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Petrat F, et al. Reduction of Fe(III) ions complexed to physiological ligands by lipoyl dehydrogenase and other flavoenzymes in vitro: Implications for an enzymatic reduction of Fe(III) ions of the labile iron pool. J Biol Chem. 2003;278(47):46403–46413. doi: 10.1074/jbc.M305291200. [DOI] [PubMed] [Google Scholar]
- 31.Epsztejn S, Kakhlon O, Glickstein H, Breuer W, Cabantchik I. Fluorescence analysis of the labile iron pool of mammalian cells. Anal Biochem. 1997;248(1):31–40. doi: 10.1006/abio.1997.2126. [DOI] [PubMed] [Google Scholar]
- 32.Ying Q-L, Smith AG. Defined conditions for neural commitment and differentiation. Methods Enzymol. 2003;365:327–341. doi: 10.1016/s0076-6879(03)65023-8. [DOI] [PubMed] [Google Scholar]
- 33.Rhinn M, Dollé P. Retinoic acid signalling during development. Development. 2012;139(5):843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- 34.Mahony S, et al. Ligand-dependent dynamics of retinoic acid receptor binding during early neurogenesis. Genome Biol. 2011;12(1):R2. doi: 10.1186/gb-2011-12-1-r2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moutier E, et al. Retinoic acid receptors recognize the mouse genome through binding elements with diverse spacing and topology. J Biol Chem. 2012;287(31):26328–26341. doi: 10.1074/jbc.M112.361790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martello G, Smith A. The nature of embryonic stem cells. Annu Rev Cell Dev Biol. 2014;30(1):647–675. doi: 10.1146/annurev-cellbio-100913-013116. [DOI] [PubMed] [Google Scholar]
- 37.Senner CE, Krueger F, Oxley D, Andrews S, Hemberger M. DNA methylation profiles define stem cell identity and reveal a tight embryonic-extraembryonic lineage boundary. Stem Cells. 2012;30(12):2732–2745. doi: 10.1002/stem.1249. [DOI] [PubMed] [Google Scholar]
- 38.Hackett JA, et al. Synergistic mechanisms of DNA demethylation during transition to ground-state pluripotency. Stem Cell Rep. 2013;1(6):518–531. doi: 10.1016/j.stemcr.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo G, et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136(7):1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esteban MA, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6(1):71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz BA, Bar-Nur O, Silva JC, Hochedlinger K. Nanog is dispensable for the generation of induced pluripotent stem cells. Curr Biol. 2014;24(3):347–350. doi: 10.1016/j.cub.2013.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao B, et al. Redox-active quinones induces genome-wide DNA methylation changes by an iron-mediated and Tet-dependent mechanism. Nucleic Acids Res. 2014;42(3):1593–1605. doi: 10.1093/nar/gkt1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bocker MT, et al. Hydroxylation of 5-methylcytosine by TET2 maintains the active state of the mammalian HOXA cluster. Nat Commun. 2012;3(818):818. doi: 10.1038/ncomms1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei S, et al. van Bennekum AM Biochemical basis for depressed serum retinol levels in transthyretin-deficient mice. J Biol Chem. 2001;276(2):1107–1113. doi: 10.1074/jbc.M008091200. [DOI] [PubMed] [Google Scholar]
- 45.Thurnham DI, Mburu ASW, Mwaniki DL, De Wagt A. Micronutrients in childhood and the influence of subclinical inflammation. Proc Nutr Soc. 2005;64(4):502–509. doi: 10.1079/pns2005468. [DOI] [PubMed] [Google Scholar]
- 46.Ko M, et al. TET proteins and 5-methylcytosine oxidation in hematological cancers. Immunol Rev. 2015;263(1):6–21. doi: 10.1111/imr.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu J, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci USA. 2009;106(9):3342–3347. doi: 10.1073/pnas.0813280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lo-Coco F, et al. Gruppo Italiano Malattie Ematologiche dell’Adulto German-Austrian Acute Myeloid Leukemia Study Group Study Alliance Leukemia Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 49.Shen Y, et al. Mutations of epigenetic modifier genes as a poor prognostic factor in acute promyelocytic leukemia under treatment with all-trans retinoic acid and arsenic trioxide. EBioMedicine. 2015;2(6):563–571. doi: 10.1016/j.ebiom.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bachman M, et al. 5-Hydroxymethylcytosine is a predominantly stable DNA modification. Nat Chem. 2014;6(12):1049–1055. doi: 10.1038/nchem.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ran FA, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verschoor MJ, Molot LA. A comparison of three colorimetric methods of ferrous and total reactive iron measurement in freshwaters. Limnol Oceanogr Methods. 2013;11(3):113–125. [Google Scholar]
- 53.Hu L, et al. Crystal structure of TET2-DNA complex: Insight into TET-mediated 5mC oxidation. Cell. 2013;155(7):1545–1555. doi: 10.1016/j.cell.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 54.Smith AG, Hooper ML. Buffalo rat liver cells produce a diffusible activity which inhibits the differentiation of murine embryonal carcinoma and embryonic stem cells. Dev Biol. 1987;121(1):1–9. doi: 10.1016/0012-1606(87)90132-1. [DOI] [PubMed] [Google Scholar]
- 55.Hu X, et al. Tet and TDG mediate DNA demethylation essential for mesenchymal-to-epithelial transition in somatic cell reprogramming. Cell Stem Cell. 2014;14(4):512–522. doi: 10.1016/j.stem.2014.01.001. [DOI] [PubMed] [Google Scholar]