The 2016 Rio Olympics remind us that the amazing feats of athletes are made possible by intense training, often from an early age, and then a cerebral focus in the face of competition and enthusiastic audiences. Underpinning these impressive feats of athleticism is something crucial that is rarely highlighted by the media: adequate nutrition. Vertebrates other than humans provide us with insights into how nutrition affects animal performance within the context of a variety of essential life history events such as growth, reproduction, and migration. Especially interesting and relevant to the health and performance of any animal is the acquisition of “essential” nutrients, those that must be acquired in the diet because they cannot be manufactured by the animals from precursors. Olympic athletes have available special diets formulated to develop and fuel their high performance, but how do wild vertebrates acquire essential nutrients in an environment that often varies in food availability and quality, and how does this acquisition affect their performance? In PNAS, Twining et al. (1) report on an experiment in which they answered this question for one of the supreme aerial acrobats: tree swallows (Tachycineta albilinea).
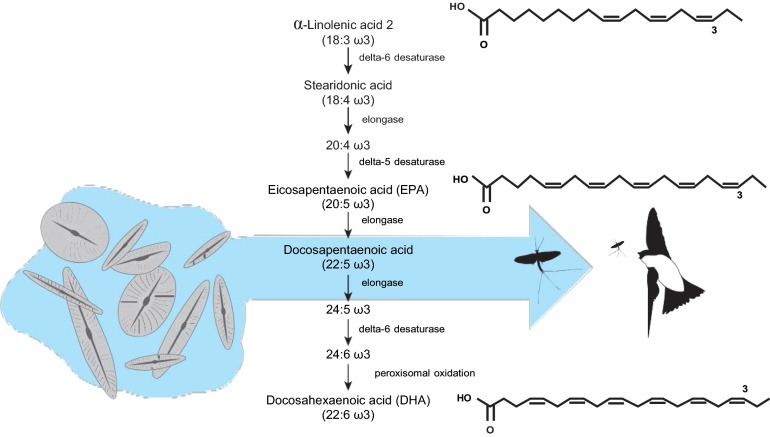
They supplemented the diets of growing chicks with certain long-chain omega-3 poyunsaturated fatty acids (LCPUFA). In the wild, these are primarily found in aquatic, but not terrestrial, insect prey. They found that supplemented chicks grew faster, were in better body condition, and had lower metabolic rates and higher immune competence than unsupplemented ones. The effect of supplementation was so strong that supplemented chicks fed lower quantities of food did better than those fed higher amounts of unsupplemented diets. The diets of unsupplemented chicks contained relatively high amounts of the essential/indispensable precursor of LCPUFA, alpha-linolenic acid (ALA). Therefore, Twining et al. (1) provide evidence of the limiting step in the metabolic availability of LCPUFA: Like many other animals, tree swallow chicks have limited ability to elongate and desaturate ALA to make enough LCPUFA to optimize physiological needs (Fig. 1). Twining et al. (1) argue that this physiological limitation has important implications for understanding the ecology of tree swallows and other aerial insectivores as well as for their conservation.
Fig. 1.
Some aquatic producers such as diatoms synthesize LCPUFA. These fatty acids play an important role in the development of aerial insectivores that feed on emerging aquatic invertebrates such as T. albilinea. LCPUFA are limiting for a large number of terrestrial consumers that rely on aquatic sources for them. They are a pivotal link between aquatic and terrestrial ecosystems.
The LCPUFA eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) participate in critical physiological functions including supporting the function of the central nervous system, cardiovascular health, growth, reproduction, and the immune system (2). Tree swallow chicks join a long list of organisms ranging from water fleas (Daphnia magna) (2) to humans that require dietary LCPUFA for adequate growth and reproduction (3). Aquaculturists have long recognized the importance of LCPUFA supplementation for the production of farmed fish and crustaceans and there is growing recognition of their physiological importance for other animals (4). In humans, the beneficial effects on LCPUFA for growth and health have led to increasing demand and pressure on the marine resources that provide them (5). Fat quality, in general, and certain LCPUFAs, in particular, also affect general health and performance of wild animals in a variety of ways including key attributes of hibernation in small mammals (6), swimming efficiency of salmon (7), energy costs of flying in migratory birds (8), and longevity and aging in a variety of vertebrates (9). Thanks to Twining et al. (1) we now know that dietary LCPUFA affect growth rate, immunocompetence, and energy expenditure of young tree swallows, and that this effect on chick performance may explain at least in part the population decline of this aerial insectivore and thus the need to conserve aquatic habitats that provide LCPUFA-rich aquatic insects.
Tree swallows are members of a guild of birds that is declining in North America (10). They are aerial insectivores that twist and turn above marshes and rivers, catching emerging aquatic insects on the wing. By feeding on aquatic insects, and depending on them to feed their chicks (11), tree swallows link the aquatic and terrestrial realms (12). A growing ecological literature highlights the importance of the reciprocal interdependence of terrestrial and aquatic ecosystems (13). Twining et al.’s (1) experimental results suggest that an important, but still underappreciated, aspect of this interdependence is the supply of LCPUFA from aquatic ecosystems to terrestrial consumers (5).
Almost 20 y ago Brett and Müller-Navarra (14) found a strong correlation between the taxonomic identity of autotrophs at the base of aquatic food webs and the magnitude of energy transfer to consumers, their biomass, and their production rate. Autotrophs with high LCPUFA contents such as diatoms, dinophytes, and cryptophytes sustain highly productive food webs, whereas green algae and cyanobacteria sustain less-productive ones. Although the vascular plants at the base of terrestrial ecosystems can be tremendously productive, they lack the ability to synthesize LCPUFA. Therefore, terrestrial animals that require them must obtain them from aquatic ecosystems. The importance of the reciprocal flow of materials across aquatic and terrestrial ecosystems has emphasized the flow of energy and elements (15). The potentially limiting role that LCPUFAs can play for consumers and their higher concentration in aquatic relative to terrestrial sources suggests a crucial ecological role for these fatty acids. It is perhaps not surprising that so many terrestrial consumers take advantage of and shape their life histories to the timing of higher availability of high-quality aquatic resources (16, 17).
Together with many other aerial insectivores, the populations of tree swallows have declined over the last decades (10). There are many potential reasons for the decline of aerial insectivores, such as decreases in aerial insect populations, direct effects of environmental contaminants, and climate change accompanied by phenological mismatches (17). Twining et al. (1) add the possibility of decreases in LCPUFA-providing aquatic invertebrates to the list. These decreases are likely (albeit undocumented), given historical reductions in the extent of wetlands and streams (18) and increased pesticide use (19). Twining et al.’s (1) experimental research suggests that LCPUFA limitation might be widespread among terrestrial animals, and might have profound ecological consequences. How widespread is the LCPUFA physiological limitation among animals, and hence how widespread are their ecological effects?
The biochemistry of the synthesis of unsaturated fatty acids has been known for quite a while and we have glimpses of patterns that begin, but only begin, to answer these questions. Animals differ in their ability to synthesize LCPUFAs: They are essential for some animals, conditionally essential for others, and might not be required by other animals provided that they have an adequate source of precursors. EPA and DHA seem to be indispensable for many marine fish species because they seem to have lost the expression of either elongases or desaturases needed to synthesize them from ALA, but they are conditionally essential or nonessential for freshwater fish species (20). The expression of desaturases and elongases in anadromous Atlantic salmon (Salmo salar) depends on both life history stage and diet. Atlantic salmon spend 1–3 y in fresh water and then move to salt water.
Synthesis of EPA and DHA is higher in the freshwater phase, increases to a peak during the transition from fresh- to saltwater,
Twining et al.'s experimental research suggests that LCPUFA limitation might be widespread among terrestrial animals, and might have profound ecological consequences.
and declines thereafter. Salmon fed on ALA-containing (but LCPUFA-deficient) plant oils have higher levels of synthesis of LCPUFA and more expression of elongases and desaturases than those fed on LCPUFA-rich fish oils (21). The provisioning of EPA and DHA in maternal milk is necessary for the adequate growth of mammalian young (22). In some mammals, the mammary glands have increased expression of elongases and desaturases during late pregnancy and lactation (23), and this expression seems to be higher in animals fed diets low in LCPUFA than in animals fed diets containing adequate amounts (24). Clearly, there is a large range of variation in the degree of dependence of different consumers on dietary LCPUFAs and at least some animals are capable of adjusting to their deficiency in diet.
Although data are still scanty, elongases and desaturases seem to conform to the predictions of Karasov’s adaptive modulation hypothesis: The pathways for the uptake and synthesis of a potentially limiting nutrient should be modulated downward by its availability and up-modulated in deficiency (25). This hypothesis also predicts that among species the expression of these pathways should be higher in habitats where LCPUFA availability is low than in those where it is high. This pattern has been confirmed by the aquaculture literature but remains to be tested in terrestrial ecosystems. A large number of terrestrial animals inhabit areas that are far from sources of LCPUFAs and/or have diets that are deficient in them. Because terrestrial plants do not synthesize LCPUFA (26), it is unlikely that terrestrial herbivore animals have the severe limitations in the synthesis of LCPUFA from ALA that marine animals and animals that evolve with access to aquatic sources have.
Because we do not yet have enough data to know the ecological correlates of LCPUFA limitation among terrestrial consumers, it is difficult to assess the extent of the limiting role that they play on land. LCPUFA seem to be pivotal for the structure and function of aquatic ecosystems (27) and seem to be critical for consumers that rely on resources at the boundaries between aquatic and terrestrial systems (1).
The exchange of materials from aquatic to terrestrial ecosystems is a major water–land linkage in the biosphere. Twining et al.’s (1) results suggest that LCPUFA may play a pivotal role in this linkage.
Acknowledgments
We thank Hannah L. Sease for drafting Fig. 1. This work was supported by National Science Foundation Grant IOS-0748349 (to S.R.M.) and US Department of Agriculture Grant RIAES-538748 (to S.R.M.).
Footnotes
The authors declare no conflict of interest.
See companion article on page 10920 in issue 39 of volume 113.
References
- 1.Twining CW, et al. Omega-3 long-chain polyunsaturated fatty acids support aerial insectivore performance more than food quantity. Proc Natl Acad Sci USA. 2016;113(39):10920–10925. doi: 10.1073/pnas.1603998113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenna JT, Carlson SE. Docosahexaenoic acid and human brain development: Evidence that a dietary supply is needed for optimal development. J Hum Evol. 2014;77:99–106. doi: 10.1016/j.jhevol.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Twining CW, Brenna JT, Hairston NG, Flecker AS. Highly unsaturated fatty acids in nature: What we know and what we need to learn. Oikos. 2016;125:749–760. [Google Scholar]
- 4.Tocher DR. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in prespective. Aquaculture. 2015;449:94–107. [Google Scholar]
- 5.Gladyshev MI, Sushchik NN, Makhutova ON. Production of EPA and DHA in aquatic ecosystems and their transfer to the land. Prostaglandins Other Lipid Mediat. 2013;107:117–126. doi: 10.1016/j.prostaglandins.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Geiser F, Kenagy GJ. Polyunsaturated lipid diet lengthens torpor and reduces body temperature in a hibernator. Am J Physiol. 1987;252(5 Pt 2):R897–R901. doi: 10.1152/ajpregu.1987.252.5.R897. [DOI] [PubMed] [Google Scholar]
- 7.Wagner GN, Balfry SK, Higgs DA, Lall SP, Farrell AP. Dietary fatty acid composition affects the repeat swimming performance of Atlantic salmon in seawater. Comp Biochem Physiol A Mol Integr Physiol. 2004;137(3):567–576. doi: 10.1016/j.cbpb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Pierce BJ, McWilliams SR. The fat of the matter: How dietary fatty acids can affect exercise performance. Integr Comp Biol. 2014;54(5):903–912. doi: 10.1093/icb/icu098. [DOI] [PubMed] [Google Scholar]
- 9.Valencak TG, Azzu V. Making heads or tails of mitochondrial membranes in longevity and aging: A role for comparative studies. Longev Healthspan. 2014;3(1):3. doi: 10.1186/2046-2395-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith AC, Hudson MR, Downes CM, Francis CM. Change points in the population trends of aerial-insectivores in North America: Synchronized in time and across species and regions. PLoS One. 2015;10(7):e0130768. doi: 10.1371/journal.pone.0130768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler DW, et al. 2011 Tree Swallow (Tachycineta bicolor). The Birds of North America Online, ed Poole A (Cornell Lab of Ornithology, Cornell University, Ithaca, NY). Available at https://birdsna.org/Species-Account/bna/species/treswa.
- 12.Baxter CV, Fausch KD, Saunders WC. Tangled webs: Reciprocal flows of invertebrate prey link streams and riparian zones. Freshw Biol. 2005;50(2):201–220. [Google Scholar]
- 13.Soininen J, Bartels P, Jani Heino J, Luoto M, Hillebrand H. Toward more integrated ecosystem research in aquatic and terrestrial environments. Bioscience. 2015;65(2):174–182. [Google Scholar]
- 14.Brett MT, Müller-Navarra DC. The role of highly unsaturated fatty acids in aquatic foodweb processes. Freshw Biol. 1997;38(3):483–499. [Google Scholar]
- 15.Nakano S, Murakami M. Reciprocal subsidies: Dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci USA. 2001;98(1):166–170. doi: 10.1073/pnas.98.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gende SM, Quinn TP, Willson MF, Heintz R, Scott TM. Magnitude and fate of salmon- derived nutrients and energy in a coastal stream ecosystem. J Freshwat Ecol. 2004;19:149–160. [Google Scholar]
- 17.Nebel S, Mills A, McCracken JD, Taylor PD. 2010 Declines of aerial insectivores in North America follow a geographic gradient. Avian Conservation and Ecology - Écologie et conservation des oiseaux 5(2):1. Available at www.ace-eco.org/vol5/iss2/art1/
- 18.Dahl TE, Steadman AM. 2011. Status and trends of wetlands in the conterminous United States 2004 to 2009 (US Department of the Interior, Fish and Wildlife Service, Washington, DC)
- 19.Van Dijk TC, Van Staalduinen MA, Van der Sluijs JP. Macro-invertebrate decline in surface water polluted with imidacloprid. PLoS One. 2013;8(5):e62374. doi: 10.1371/journal.pone.0062374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glencross BD. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev Aquacult. 2009;1(2):71–124. [Google Scholar]
- 21.Zheng X, et al. Environmental and dietary influences on highly unsaturated fatty acid biosynthesis and expression of fatty acyl desaturase and elongase genes in liver of Atlantic salmon (Salmo salar) Biochim Biophys Acta. 2005;1734(1):13–24. doi: 10.1016/j.bbalip.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Crawford MA. The role of essential fatty acids in neural development: Implications for perinatal nutrition. Am J Clin Nutr. 1993;57(5) Suppl:703S–709S, discussion 709S–710S. doi: 10.1093/ajcn/57.5.703S. [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez-Cruz M, et al. Participation of mammary gland in long-chain polyunsaturated fatty acid synthesis during pregnancy and lactation in rats. Biochim Biophys Acta. 2011;1811(4):284–293. doi: 10.1016/j.bbalip.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 24.González RS, Rodriguez-Cruz M, Maldonado J, Saavedra FJ. Role of maternal tissue in the synthesis of polyunsaturated fatty acids in response to a lipid-deficient diet during pregnancy and lactation in rats. Gene. 2014;549(1):7–23. doi: 10.1016/j.gene.2014.06.057. [DOI] [PubMed] [Google Scholar]
- 25.Karasov WH. Tests of the adaptive modulation hypothesis for dietary control of intestinal nutrient transport. Am J Physiol. 1992;263(3 Pt 2):R496–R502. doi: 10.1152/ajpregu.1992.263.3.R496. [DOI] [PubMed] [Google Scholar]
- 26.Qi B, et al. Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat Biotechnol. 2004;22(6):739–745. doi: 10.1038/nbt972. [DOI] [PubMed] [Google Scholar]
- 27.Müller-Navarra DC, Brett MT, Liston AM, Goldman CR. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature. 2000;403(6765):74–77. doi: 10.1038/47469. [DOI] [PubMed] [Google Scholar]