Significance
Chloramphenicol and linezolid interfere with translation by targeting the ribosomal catalytic center and are viewed as universal inhibitors of peptide bond formation. We show that, contrary to this view, the activity of these antibiotics critically depends on the nature of specific amino acids of the nascent chain carried by the ribosome and by the identity of the residue entering the A site. These findings indicate that the nascent protein modulates the properties of the ribosomal catalytic center and affects binding of its ligands. Understanding the principles of context specificity of ribosomal drugs may help develop better antibiotics.
Keywords: ribosome, antibiotics, protein synthesis, nascent peptide, oxazolidinones
Abstract
The first broad-spectrum antibiotic chloramphenicol and one of the newest clinically important antibacterials, linezolid, inhibit protein synthesis by targeting the peptidyl transferase center of the bacterial ribosome. Because antibiotic binding should prevent the placement of aminoacyl-tRNA in the catalytic site, it is commonly assumed that these drugs are universal inhibitors of peptidyl transfer and should readily block the formation of every peptide bond. However, our in vitro experiments showed that chloramphenicol and linezolid stall ribosomes at specific mRNA locations. Treatment of bacterial cells with high concentrations of these antibiotics leads to preferential arrest of translation at defined sites, resulting in redistribution of the ribosomes on mRNA. Antibiotic-mediated inhibition of protein synthesis is most efficient when the nascent peptide in the ribosome carries an alanine residue and, to a lesser extent, serine or threonine in its penultimate position. In contrast, the inhibitory action of the drugs is counteracted by glycine when it is either at the nascent-chain C terminus or at the incoming aminoacyl-tRNA. The context-specific action of chloramphenicol illuminates the operation of the mechanism of inducible resistance that relies on programmed drug-induced translation arrest. In addition, our findings expose the functional interplay between the nascent chain and the peptidyl transferase center.
The key chemical reaction catalyzed by the ribosome is peptide bond formation. This reaction occurs in the peptidyl transferase center (PTC) where the transfer of the nascent peptide from the P-site–bound peptidyl-tRNA (pept-tRNA) to the A-site–bound aminoacyl tRNA (aa-tRNA) leads to elongation of the growing protein chain by one amino acid at each round of translation (reviewed in ref. 1). Although the peptidyl transfer reaction is potentially assisted by the chemical environment of the PTC (2–4), the main contribution of the ribosome to peptide bond formation is entropic, as the major acceleration of the reaction stems from the proper orientation of the donor and acceptor substrates within the catalytic center (5–7).
The PTC is a primary target for antibiotics that inhibit protein synthesis (see ref. 8 for review), such as chloramphenicol (CHL), one of the first broad-spectrum antibacterials in clinical use (9). CHL binds to the PTC A site, preventing proper placement of the aminoacyl moiety of aa-tRNA (Fig. 1) (10–12). Because the key interactions with the ribosome in the A site are shared by all aa-tRNAs, CHL is viewed as a universal inhibitor of peptide bond formation (reviewed in ref. 13). However, this commonly accepted model of CHL action fails to explain several experimental observations such as the differential inhibition of translation of specific mRNA templates (14, 15) or only partial inhibition of puromycin-mediated release of nascent chains in polysomes even at saturating CHL concentrations (16). The perceived ability of CHL to indiscriminately inhibit formation of any peptide bond also conflicts with its role as an inducer of resistance genes. Activation of a number of CHL resistance genes relies on the antibiotic-promoted arrest of translation at a specific codon within the regulatory leader ORF (reviewed in ref. 17). Thus, to activate the expression of the resistance locus in response to the antibiotic assault, the ribosome should be able to progress through several leader ORF codons to reach the site of the programmed translation arrest. How the ribosome can polymerize a segment of the leader peptide, if CHL indiscriminately inhibits formation of any peptide bond, remained unclear.
Fig. 1.
The binding site of CHL and LZD in the peptidyl transferase center of the 50S ribosomal subunit. (Top) The chemical structures of CHL and LZD. (Bottom) When bound to the large ribosomal subunit in the PTC A site, the molecules of CHL (orange) and LZD (blue) would sterically clash with the aminoacyl moiety of the aminoacyl-tRNA (yellow). P-site–bound peptidyl-tRNA esterified with the nascent peptide chain is purple. The images were prepared based on the structures with the Protein Data Bank ID codes 4V7T, 3DLL, and 3J5L (10, 20, 46).
Although CHL is one of the oldest known antibiotics, linezolid (LZD) belongs to one of the newest classes of clinically important PTC-targeting protein synthesis inhibitors, the oxazolidinones (Fig. 1). Genetic, biochemical, and crystallographic evidence showed that LZD binds in the PTC A site, at a location overlapping with the CHL binding pocket. Similar to CHL, LZD directly clashes with the placement of the aminoacyl moiety of the aa-tRNA, suggesting that the drug should indiscriminately inhibit peptidyl transfer reaction (Fig. 1) (18–21). It remained unexplained, however, why LZD, which readily interfered with in vivo protein synthesis or cell-free translation (22), failed to inhibit peptide bond formation between fMet-tRNA and puromycin (18, 23).
Because of the accumulating biochemical evidence that contradicted the conventional view of the mode of action of CHL and LZD, we questioned whether these antibiotics universally block the formation of every peptide bond during protein synthesis. Here, we show that CHL and LZD do not act as global inhibitors of peptide bond formation, but rather block translation at specific locations within the mRNA in a context-specific manner. Furthermore, we present evidence that the specificity of action of these drugs is defined by the nature of the penultimate residue of the nascent peptide as well as by the amino acid residues directly participating in peptide bond formation. Our findings unveiled an important functional influence of the nascent peptide on the properties of the PTC and expand the concept of the context-specific action of ribosomal antibiotics.
Results
Toeprinting Analysis Reveals Site Specificity of CHL and LZD Action.
To assess the mode of action of CHL and LZD in vitro, we used toeprinting analysis, which determines the position of the antibiotic-stalled ribosome on an mRNA (24, 25). If, as alleged, CHL and LZD act as global inhibitors of the PTC catalytic function, preincubation of the ribosome with these antibiotics should prevent the formation of the very first peptide bond. However, consistent with our previous observations (25), even at very high concentrations of CHL or LZD, ribosomes were not arrested at the initiator codon of any of the tested mRNA templates (Fig. S1A). Instead, translation progressed through few codons until the ribosome was stalled, with varying efficiency, at specific downstream sites. In an attempt to understand the requirement for the preferential action of CHL and LZD at specific mRNA locations, we aligned the encoded protein sequences leading up to the experimentally identified sites of CHL- or LZD-induced arrest and examined the nature of the P- or A-site amino acids directly participating in peptide bond formation (Fig. S1B). Nonetheless, no clear conservation of the donor or acceptor residues was immediately obvious. We realized that, to search for the motifs conducive to the action of CHL and LZD, we needed to operate with a broader array of sites of the preferred action of these drugs. Therefore, we used ribosome profiling, which allows analyzing the mode of action of protein synthesis inhibitors at the genome-wide level (26–29).
Fig. S1.
Ribosome stalling induced by CHL or LZD during in vitro translation. (A) Translation arrest within the E. coli genes osmC, cspA, and mipA was analyzed by toeprinting. The bands representing CHL-induced arrest are marked by orange dots; LZD-induced arrest sites are indicated by blue dots. The control antibiotic clindamycin (Cld) arrests translation at the initiation codon (purple dots). The nucleotide sequences of the relevant genes segments and the encoded amino acids are shown. A- and G-specific sequencing lanes are indicated. CHL and LZD were present in the reactions at the final concentrations of 200 µM and 1 mM, respectively. (B) A list of the CHL- or LZD-induced arrest sites identified by toeprinting analysis in a number of bacterial genes. The sites are aligned according to the codon located in the A site of the arrested ribosome. The amino acids in the P (0) and A (+1) sites, and the penultimate position (−1) of the nascent chain are boxed. The three amino acids preceding the penultimate position (nascent-chain positions −2 to −4) are also shown. Arrowheads indicate those sites of drug-induced arrest where Ala, Ser, or Thr are present in the penultimate position of the nascent peptide (see Fig. 2 in the main text and Fig. S4).
Identity of the Penultimate Residue of the Nascent Peptide Is Critical for the Action of CHL and LZD.
The ribosome-profiling experiments were carried out with the Escherichia coli strain BWDK, a descendant of the WT E. coli K-12 strain, where the absence of the tolC gene (a key component of the multidrug efflux pump) renders the cells hypersusceptible to antibiotics. Exponentially growing cells were exposed to a 100-fold excess over the minimal inhibitory concentration of CHL or LZD for 2.5 min, a time period sufficient to reach maximum inhibition of translation (Fig. S2). The ribosome-protected mRNA fragments were then prepared, sequenced, and mapped to the genome using established procedures (30, 31). Treatment with any of the two inhibitors caused a modest redistribution of ribosome density along the genes relative to the untreated control (Fig. S3). Thus, it became evident that exposure to the antibiotic does not immediately “freeze” translation. Instead, ribosomes can still polymerize a few peptide bonds before pausing at particular codons. This observation is consistent with our in vitro toeprinting results, which showed that CHL and LZD stall translation at a number of specific locations within the protein-coding sequences (Fig. S1).
Fig. S2.
Time dependence of translation inhibition by CHL or LZD. Antibiotic hypersusceptible E. coli cells growing in defined medium lacking methionine were exposed to a 100-fold excess over the minimal inhibitory concentration of the drugs for varying time periods before addition of l-[35S]methionine. After 1 min of addition of methionine, the incorporation of radioactivity into the trichloracetic acid-precipitable material was determined by scintillation counting.
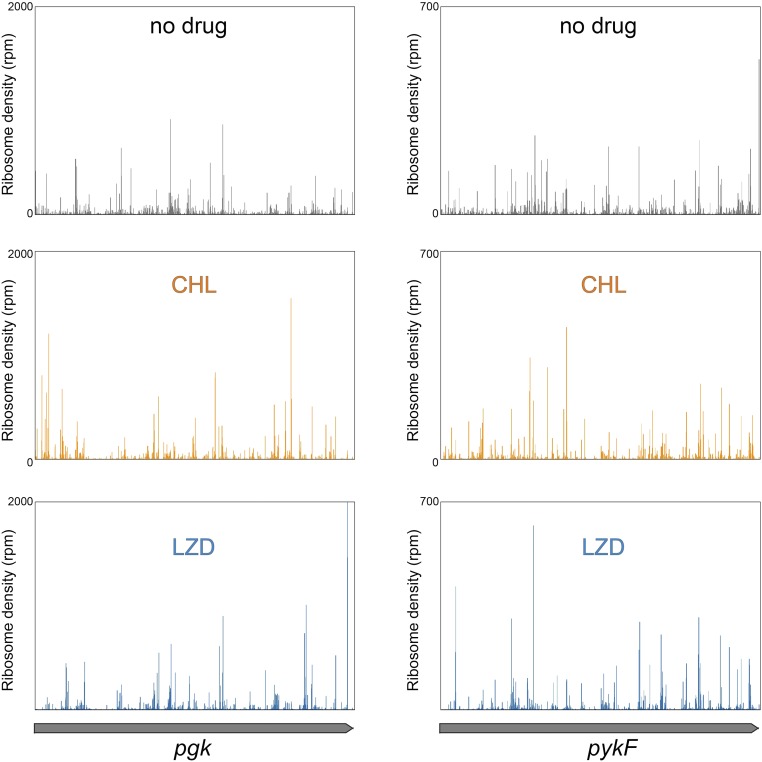
Fig. S3.
CHL and LZD cause redistribution of ribosomes during translation of E. coli genes. Distribution of ribosomes along the two sample genes (pgk on the panels on the left side and pykF genes on the panels on the right side) in the absence (“no drug”) or presence of CHL or LZD. Exponentially growing E. coli cells were exposed for 2.5 min to 100-fold MIC of CHL (100 µg/mL) or LZD (800 µg/mL), and distribution of ribosomes along actively translated genes was analyzed by ribosome profiling. See Materials and Methods for experimental details.
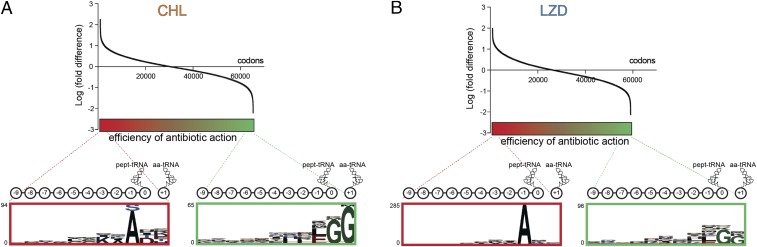
We identified the preferential sites of antibiotic action by computing changes in ribosome occupancy at ∼60,000 individual codons between the antibiotic-treated and untreated cells and ranking all of the analyzed codons by the magnitude of the change (Fig. 2) (see SI Materials and Methods for detail). For each antibiotic, we then selected the top 1,000 codons, where the strongest drug-induced translation arrest was observed. Within these sites, we searched for a specific sequence signature among amino acids encoded within the nine codons preceding the arrest site (positions −1 to −9), the arrest codon (position 0), which occupies the P site of the stalled ribosome, and the following codon (position +1), corresponding to the A-site codon (Fig. 2). Remarkably, the preferential CHL arrest sites showed significant enrichment in Ala (38.1%) and, to a lesser extent, of Ser (14.8%) or Thr (6.3%) codons, in the −1 position compared with the expected random occurrence of these residues (15.2%, 7.8%, and 5.5%, respectively) (Fig. 2A and Fig. S4). The sites of LZD-induced arrest exhibited an even stronger preference for Ala codons (69.9%) in the same position (Fig. 2B and Fig. S4). Although Ala and Thr can be defined by four codons each and Ser is defined by six codons, no preference for any specific Ala, Ser, or Thr codon at the sites of arrest was apparent. This lack of codon bias argues that the specificity of antibiotic action is defined by the nature of the encoded amino acids rather than the mRNA sequence or tRNA structure. The occurrence of Ala, Ser, or Thr in the penultimate peptide position strongly correlated with the drug-induced translation stalling throughout the entire range of the analyzed locations, and their presence progressively decreased toward the end of the spectrum where codons with the least pronounced ribosome stalling were grouped (Fig. S5A). Furthermore, when all of the ∼60,000 analyzed sites were grouped into 20 bins, according to the nature of the nascent-chain penultimate residue, the sites with Ala, Ser, or Thr in position (−1) showed a significant increase of the cumulative ribosome occupancy across all of the analyzed codons (Fig. S5B). All of these data strongly argue that presence of Ala (and, to some degree, Thr or Ser) as the penultimate amino acid in the nascent chain stimulates the inhibitory action of CHL and LZD.
Fig. 2.
Context specificity of the action of CHL and LZD in vivo. (A and B) Ranking of genomic sites according to the relative fold difference in the ribosome occupancy in cells treated with CHL (A) or LZD (B) vs. the untreated control. The higher relative fold difference values (left side of the spectrum, colored in red) correspond to the sites of more pronounced inhibition of translation by the antibiotics. The low fold difference values (right side of the spectrum, shown in green) represent the sites where antibiotics were least efficient. The magnified panels at the Bottom show the pLogo analysis of amino acid bias within the top 1,000 (red frame) and bottom 1,000 (green frame) sites of action of CHL or LZD. The 10 C-terminal residues of the nascent chains (marked as 0 to −9 of pept-tRNA) and the incoming amino acids (marked as +1, aa-tRNA) in these sites are indicated in the cartoons. The y axes of the pLogo panels correspond to the odds of the binomial probability (in logarithmic scale) of occurrence of specific amino acids at the defined positions (47).
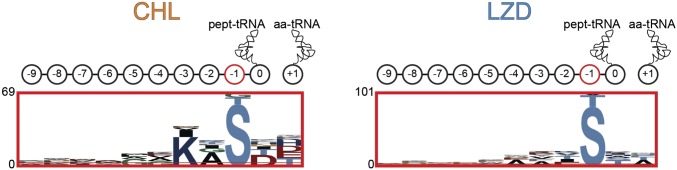
Fig. S4.
Presence of Ser or Thr residues at the penultimate position of the nascent chain contributes to CHL- and LZD-translation arrest. pLogo analysis of amino acid bias at the top 1,000 sites of CHL and LZD action after elimination of sites containing an Ala codon in the −1 position. The cartoons above the logos represent the 10 C-terminal nascent-chain residues (0, which is the P site, to −9) and the amino acid at the +1 position (aa-tRNA).
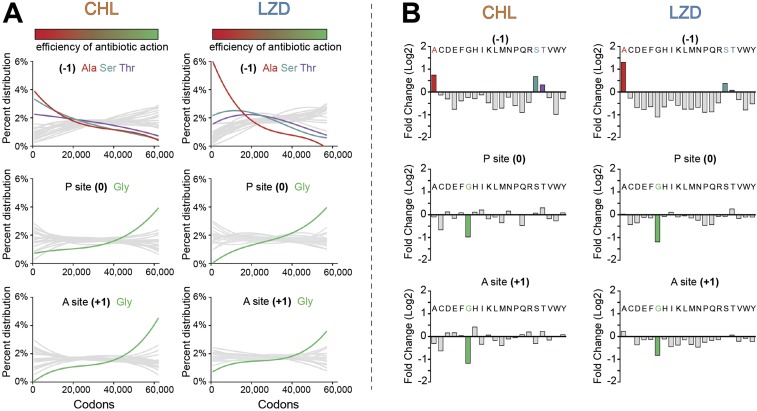
Fig. S5.
The efficiency of CHL- or LZD-induced translation arrest correlates with the presence of Ala, Ser, or Thr in the penultimate position of the nascent chain and countercorrelates with the presence of Gly in the P or A site. (A, graphs at Top) Occurrence of Ala, Ser, or Thr in the penultimate position (−1) of the nascent chain along the spectrum of the analyzed sites arranged according to the efficiency of antibiotic action (see SI Materials and Methods for detail). (Middle and Bottom) Occurrence of Gly in the P site (position 0) (Middle) or A site (position +1) (Bottom) of the drug-stalled ribosome. The colored bars at the Top mark all of the analyzed sites arranged as in Fig. 2 in the main text according to the efficiency of antibiotic action: from the sites of the strongest drug-induced arrest on the Left (red) to the sites of the least pronounced arrest on the Right (green). (B) Analysis of the cumulative drug-induced changes in the ribosome density at all of the analyzed sites according to the nature of amino acid in the penultimate position (−1) of the nascent chain (Top), P site (0) (Middle), or A site (+1) (Bottom).
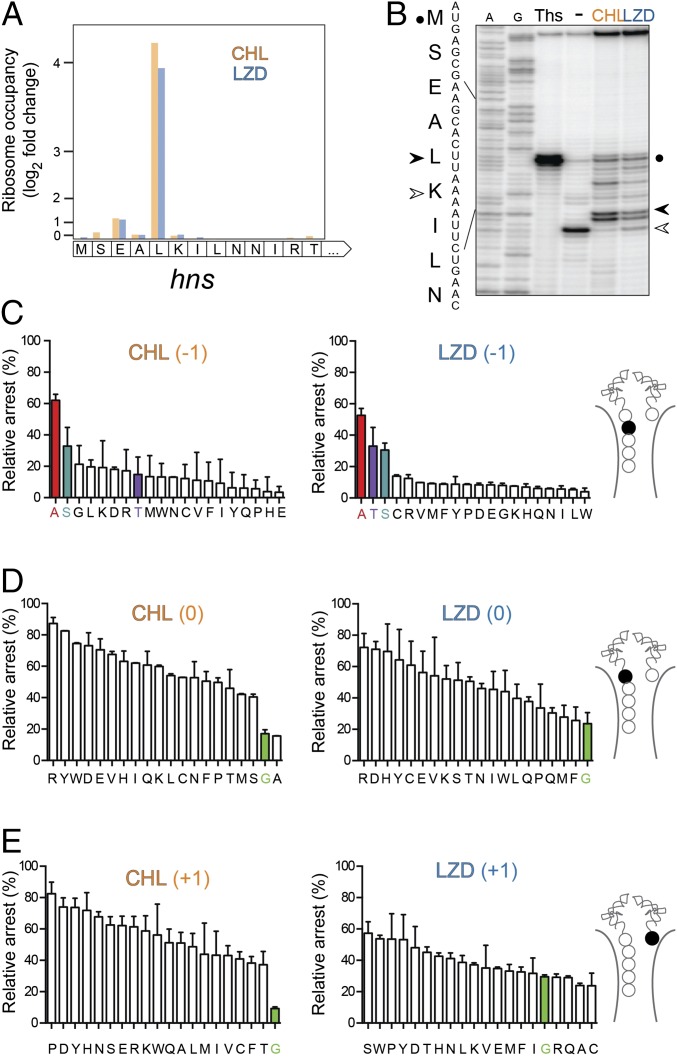
To further examine the influence of the sequence context on drug-induced arrest, we selected a well-defined model system appealingly suited for cell-free translation experiments (32). According to the ribosome-profiling data, the drug-induced ribosome stalling at the Leu5 codon of the hns gene represents one of the 10 strongest arrest sites common for both CHL and LZD (Fig. 3A). Drug-induced ribosome stalling at the hns Leu5 codon was readily reproduced in vitro in the toeprinting assay (Fig. 3B). By comparing the fraction of the ribosomes arrested at the Leu5 codon with those that bypassed the site of drug-induced translation stalling, we compared the efficiency of translation inhibition for the H-NS mutants containing every possible amino acid substitutions of Ala4 (Fig. 3 C and D). The results of the in vitro experiments confirmed that the presence of Ala in the penultimate position of the H-NS N-terminal pentapeptide significantly enhanced the action of CHL and LZD. Replacing Ala4 with any other amino acid greatly reduced the efficiency of drug-induced ribosome stalling. Furthermore, in agreement with the profiling data (Fig. S4), Ser and, in the case of LZD, also Thr at position −1 were more conducive to the antibiotic action than any other substitution of Ala4 (Fig. 3 C and D).
Fig. 3.
Amino acid residues of the PTC donor and acceptor substrates influence antibiotic action. (A) Changes in the ribosome occupancy of the first codons of the hns gene in cells treated with CHL or LZD compared with that in the untreated cell culture. (B) In vitro toeprinting analysis of CHL- or LZD-induced ribosome stalling close to the 5′-end of the hns gene. The control antibiotic thiostrepton (Ths) was used to arrest translation at the start codon (black circle). The prominent CHL- and LZD-induced arrest site at the Leu5 codon of the gene is indicated by a black arrowhead. Due to the presence of the Ile-tRNA synthetase inhibitor mupirocin in all of the samples, the ribosomes that were not arrested by CHL or LZD at the hns Leu5 codon, were “caught” at the following Lys6 codon (open arrowhead). A- and G-specific sequencing lanes are indicated. The sequence of the first nine codons of the hns gene and the encoded amino acids are indicated on the side of the gel. (C–E) The effect of mutagenizing (C) the Ala4 (position −1), (D) Leu5 (position 0), or (E) Lys6 (position +1) codons of hns on CHL- or LZD-induced translation arrest. The cartoons showing the PTC region of the CHL- or LZD-stalled ribosomal complexes highlight the mutagenized amino acid residue (filled spheres) in each set. The efficiency of antibiotic-mediated arrest (“relative arrest”) was calculated by comparing the fraction of arrested ribosomes at codon 5 (estimated from the intensity of the CHL- or LZD-specific toeprint bands) with the fraction of ribosomes trapped at codon 6 (calculated from the intensity of the mupirocin-specific toeprint bands) during the translation of the WT or mutant hns templates (see B for reference). For the templates where the specified codon was replaced with an Ile codon, the Ile7 codon was mutated to Thr and borrelidin (and inhibitor of Thr-RS) was used instead of mupirocin. The bars representing H-NS mutants with Ala, Ser, or Thr in the penultimate peptide position are highlighted in red; those corresponding to the mutants with Gly in the P or A sites are highlighted in green. The error bars show deviation from the mean in two independent experiments.
Having verified the importance of the penultimate peptide residue in the mechanism of LZD and CHL action, we reexamined the collection of the arrest sites identified by toeprinting in our initial experiments (Fig. S1B). Consistent with the trend observed in vivo, more than 80% of the drug-induced arrests detected in those experiments occurred when the penultimate amino acid of the nascent peptide was Ala, Ser, or Thr (marked by arrowheads in Fig. S1B).
Collectively, the results of the genome-wide analysis and of the in vitro testing converge on the key stimulatory role of Ala, Ser, or Thr in the penultimate position of the nascent peptide in the mechanism of action of the PTC-targeting antibiotics CHL and LZD.
CHL and LZD Poorly Inhibit Peptide Bond Formation When Glycine Residues Are Involved.
The codons with the maximally increased ribosomal occupancy in drug-treated cells represent the sites where antibiotic action is most pronounced (red frames in Fig. 2). Conversely, the codons whose occupancy significantly decreases in the presence of the drug are those where the antibiotic fails to hinder peptide bond formation. Despite the presence of the inhibitor, the ribosomes proceed with translation, traversing these codons until they encounter the nearest “strong” arrest location. In contrast to our finding for the top arrest sites, the analysis of the 1,000 codons characterized by the most dramatic decrease in ribosome occupancy in the drug-treated cells (green frames in Fig. 2) did not show a dramatic bias in favor of any particular amino acid in the position (−1) of the peptide. Instead, it revealed a strong prevalence for the presence of glycine residues in either the P site (position 0) or the A site (position +1) of the translating ribosome. These data suggest that the peptidyl transfer reaction involving a Gly residue either as a donor or as an acceptor is poorly inhibited by CHL or LZD.
The occurrence of Gly codons in either the P or A sites negatively correlated with the efficiency of drug-inflicted stalling throughout the entire range of analyzed sites (Fig. S5A). Consistently, the presence of glycine codons in the P or A sites correlated with the most pronounced decrease of the cumulative ribosomal density at the corresponding codons in antibiotic-treated cells (Fig. S5B). These findings derived from the ribosome-profiling analysis were in keeping with the results of the subsequent in vitro testing with the hns gene: presence of a Gly residue in the P or the A site made the action of CHL or LZD inefficient (Fig. 3 D and E).
Thus, we concluded that the amino acid sequence context is capable of either stimulating or counteracting the inhibition of peptide bond formation by CHL and LZD.
Induction of CHL Resistance Genes Relies on the Stimulatory Effect of the Penultimate Residue of the Nascent Chain on Drug Action.
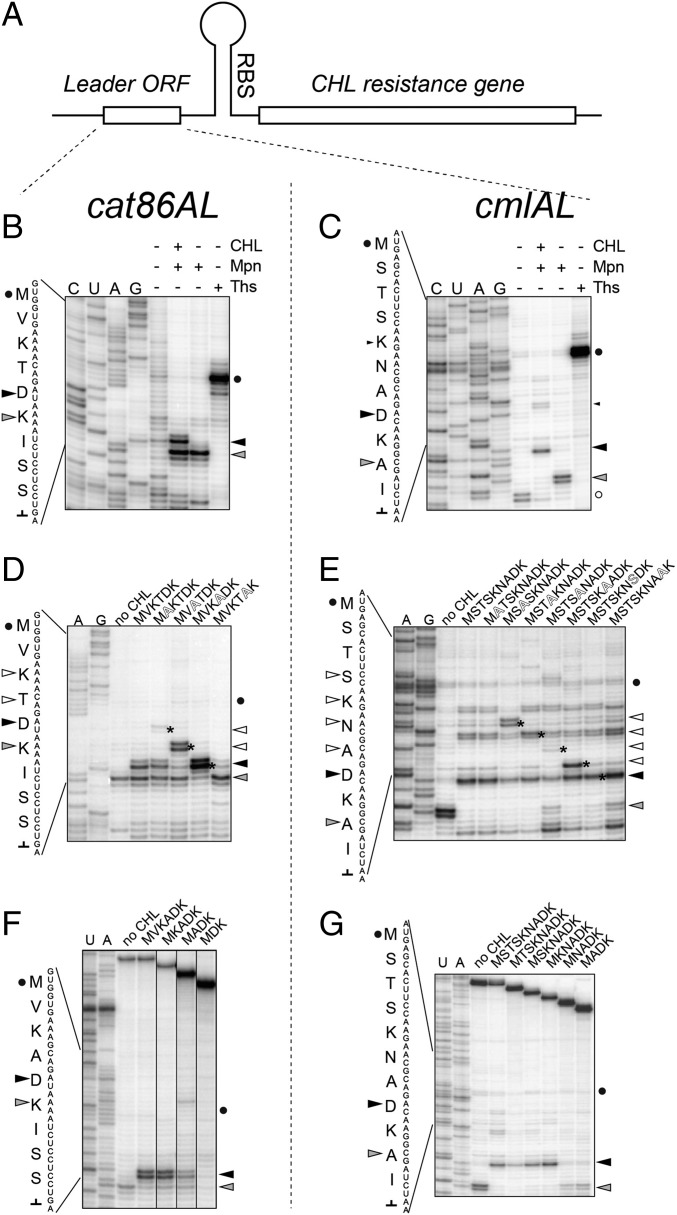
Our newly gained understanding of the site specificity of CHL action prompted us to reevaluate the mechanism of inducible CHL resistance. We analyzed CHL-dependent programmed translation arrest at the leader ORFs of two resistance genes, catA86 (originated in Gram-positive bacteria) and cmlA (common to Gram-negative species) (33) (Fig. 4A). Toeprinting analysis showed that, in the presence of the antibiotic, translation driven by E. coli ribosomes stalls when the fifth codon of catA86L or the eighth codon of the cmlAL ORF enter the ribosomal P site (Fig. 4 B and C). The same stalling site was observed when translation of cat86AL was catalyzed by ribosomes isolated from Gram-positive Bacillus subtilis (Fig. S6A). These results are in line with the conclusions of the previous genetic testing, which suggested that the N-terminal pentapeptide MVKTD of Cat86AL or the octapeptide MSTSKNAD of CmlAL are synthesized in the presence of CHL (33, 34). Remarkably, translation arrest at the leader ORFs occurs when the nascent chain carries in the penultimate positions amino acid residues conducive to CHL action: Ala (in the case of CmlAL) or Thr (Cat86AL) (Fig. 4 B and C). Thus, the mechanism of induction of CHL resistance simply exploits the site specificity rules that have emerged from the profiling and toeprinting analyses. In agreement with this assertion, in the alanine-scanning mutagenesis experiment carried out with cat86AL and cmlAL, we observed the appearance of new ribosome stalling sites consistently occurring at the codon subsequent to the engineered Ala mutation (asterisks in Fig. 4 D and E). In addition, replacement of Thr4 of the wt Cat86AL with Ala significantly enhanced CHL-mediated stalling at the “native site” (compare lanes MVKTDK and MVKADK in Fig. 4D), corroborating our conclusion that an Ala residue in the penultimate peptide position is the most stimulatory for drug activity.
Fig. 4.
CHL-induced arrest at the leader ORFs of inducible resistance genes. (A) The general organization of inducible CHL resistance genes. In the absence of antibiotic, the leader ORF is constitutively translated, but the expression of the resistance cistron is attenuated because its ribosome-binding site (RBS) is sequestered in the mRNA secondary structure. In the presence of CHL, translation of the leader ORF is arrested at a specific internal codon. Ribosome stalling mediates rearrangement of the mRNA structure resulting in activation of expression of the resistance gene. (B and C) CHL-induced translation arrest at the fifth codon of the cat86A leader ORF (B) or the eighth codon of the cmlA leader ORF (C). The control antibiotic thiostrepton (Ths) stalls translation at the start codon (black circle). A black triangle indicates the toeprint band representing CHL-induced programmed translation arrest. The gray triangle points to the band produced by ribosomes stalled before the “hungry” Ile codon [because of the presence in the reaction of mupirocin (Mpn), an Ile-tRNA synthetase inhibitor]. The band produced by ribosomes paused during termination of the cmlAL translation is marked with an open circle. (D and E) Alanine-scanning mutagenesis of the cat86AL (D) or cmlAL (E) alters the location of CHL-mediated translation arrest. As in B and C, the translation initiation site is marked by a circle, the site of the programmed CHL-induced arrest is shown by a black triangle, and the “Mpn band” is indicated by a gray triangle. The new sites of arrest, which appear due to the presence of new Ala residues in the mutant ORFs, are marked by the asterisks on the gel and are indicated by the open triangles. (F and G) N-terminal truncations of the cat86AL (F) or cmlAL (G) and their impact on the efficiency of CHL-induced translation arrest. The start codon, CHL band, and mupirocin band are indicated by a circle, black triangle, and gray triangle, respectively.
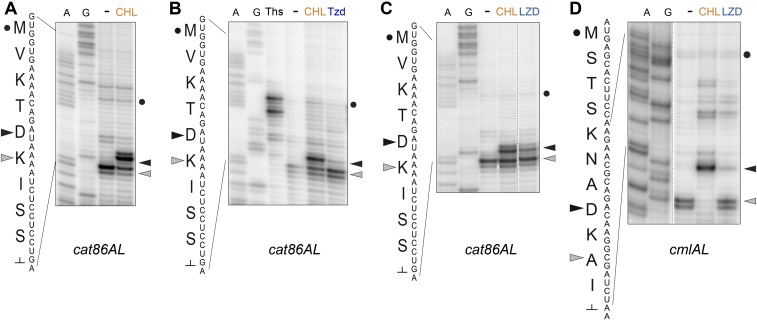
Fig. S6.
Drug-induced translation arrest within the leader ORFs of CHL resistance genes. Toeprinting analysis of drug-dependent ribosome stalling within the leader ORFs of (A–C) cat86AL and (D) cmlAL CHL resistance genes. All of the reactions loaded onto gels shown in A–D contained mupirocin, an inhibitor of Ile-tRNA synthetase. In all of the panels, the site of CHL-induced arrest is indicated by a black triangle, the “mupirocin band” is indicated by a gray triangle, and the start codon is marked by a black dot. (A) CHL-dependent arrest of translation of the cat86AL ORF driven by Bacillus subtilis ribosomes. (B and C) Oxazolidinone antibiotics (B) tedizolid (Tzd) and (C) LZD stall the E. coli ribosome within the cat86AL gene at the site of programmed CHL-induced arrest. (D) LZD arrests the E. coli ribosome at the site of CHL-induced arrest within the cmlAL leader ORF.
Codons 2–4 of the cmlAL ORF specify Ser and Thr, which are expected to moderately stimulate CHL action (Fig. 3 and Fig. S4). Curiously, however, translation of the ORF in the presence of the antibiotic proceeds fairly efficiently until the eighth codon (large black triangle in Fig. 4C), with only a moderate arrest at the codon 5 (small black triangle in Fig. 4C). One possible explanation is that efficient CHL-induced stalling requires the nascent chain to reach a certain length. Indeed, sequential 5′-terminal truncations of the cmlAL or cat86AL ORFs diminished the efficiency of drug-induced arrest at the native site when the nascent chain became shorter than 4 (Cat86AL) or 5 (CmlAL) aa long (Fig. 4 F and G).
Although the regulatory ORFs of the CHL resistance genes have been evolutionarily selected to respond specifically to the presence of natural CHL, the similarity in the context specificity of action of CHL and LZD suggested that the latter, a synthetic antibiotic, could also act as an inducer. Indeed, LZD and even the newest oxazolidinone antibiotic, tedizolid, were able to stimulate ribosome stalling at the fifth codon of cat86AL or the eighth codon of cmlAL, although less efficiently than CHL (Fig. S6). Thus, synthetic oxazolidinones likely would be able to induce the natural CHL resistance genes.
SI Materials and Methods
Kinetics of Translation Inhibition by CHL and LZD.
Kinetics of inhibition of protein synthesis in Escherichia coli was followed as previously described (32) by monitoring incorporation of l-[35S]methionine into the trichloracetic acid-precipitable material after exposure of cells to 100-fold minimum inhibitory concentration (MIC) of CHL (100 µg/mL) or 50-fold MIC of LZD (400 µg/mL).
pLogo Analysis of the Amino Acid Biases at the Strongest and Weakest Arrest Sites.
The total number of reads assigned to the three nucleotides of a codon was normalized to the total reads within the gene, generating the relative codon density (RCD). For analysis of the changes in the relative codon occupancy, only genes that had a gene score greater than 100 in drug-treated and control samples were used, and for those, only the codons with at least five assigned reads were included. The gene score was calculated according to the following equation:
The relative fold difference values (RFDVs) for individual codons were calculated as the ratio of RCD in the drug-treated sample relative to the control. The sites located at a distance of less than 10 codons from the beginning of the gene and those followed immediately by the stop codon were excluded.
The RFDVs (61,250 for CHL vs. control or 56,251 for LZD vs. control) were then arranged in a descending order. For each of the sites, the encoded peptide sequence was extracted; this included the 10-aa C-terminal sequence of the nascent chain ending with the codon specifying the residue at the P site to which the ribosome density was assigned, and the following A-site amino acid. pLogo analysis (47) was carried out using this 11-aa sequence. The pLogo foreground was the amino acid sequences of either the 1,000 strongest or weakest arrest sites. The background was amino acid sequences of all analyzed sites (61,250 for CHL or 56,251 for LZD).
Computing the Changes of Occurrence of Individual Amino Acids at Specific Sites as a Function of Efficiency of CHL or LZD Action.
A 1,000-site sliding window was used to compute the occurrence of each of the 20 aa in positions −1 (the penultimate peptide residue), 0 (P site), or +1 (A site). The occurrence was calculated as the total number of sites within a window with a specific amino acid in the corresponding position relative to the total count of that amino acid in that position in all sites. For example, the first 1,000-site window for the CHL sample contains 298 sites with Ala in the position (−1) relative to a total of 9,370 sites with Ala in position (−1) among all of the analyzed 61,250 sites. After plotting the obtained values the curves were smoothed by using the best-fit quadratic slope algorithm of Prism 7 (GraphPad).
Estimation of the Cumulative Differences in Relative Codon Occupancy.
For computing cumulative change in ribosomal occupancy, all of the RFDVs were split into 20 bins according to the chemical nature of the amino acid at the penultimate (−1) position in the nascent chain, the P site or the A site, and the average fold difference was computed for every bin. Those sites within nine codons of the start site or having a stop codon in the A site were ignored. In a similar fashion, the average RFDVs for all possible 2-aa combinations (400 bins) found the in the pairs −1 and P, −1 and A, or P and A sites were calculated.
Generation of Templates for in Vitro Translation and Toeprinting Analysis.
All DNA templates for toeprinting were produced using AccuPrime DNA Polymerase (Thermo Fisher). The names and sequences of all of the primers are listed in Table S1. The osmC, cspA, hns, and mipA templates were generated by PCR amplification of E. coli genomic DNA. All of the templates contained T7 RNA polymerase promoter and initiation region of the ermCL gene at their 5′-end and the sequence complementary to the toeprinting primer NV1 at the 3′-end (43). The cat86L, cmlAL, the WT, and −1 and P-site position mutant hns templates, as well as the alanine scanning mutagenesis or N-terminal truncations of H-NS, were generated by four-primer PCRs (44), using the pair of template-specific “long” primers at 10 µM and the pair of “short” primers (T7 and NV1, or T7 and a short template-specific reverse primer) at 100 µM. The hns templates with mutant A sites were generated by a five-primer PCR. For this, each reaction contained 10 µM of one of 19 primers designed to introduce the mutant A-site codon, in combination with 10 µM of hns_trunc_rev primer corresponding to the 3′-end of the template. After the first three PCR cycles, 10 µM of primer T7_hns_fwd, corresponding to the 5′-end of the template, and 100 µM of primers T7 and NV1 were added, and the PCR continued for 27 more cycles.
Table S1.
DNA primers used in the study
| Primer name | Sequence |
| Ala -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTGCAAC TCTGAACAAC ATCCGTAC |
| Arg -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTCGAAC TCTGAACAAC ATCCGTAC |
| Asn -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTAACAC TCTGAACAAC ATCCGTAC |
| Asp -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTGATAC TCTGAACAAC ATCCGTAC |
| Cys -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTTGCAC TCTGAACAAC ATCCGTAC |
| Gln -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTCAGAC TCTGAACAAC ATCCGTAC |
| Glu -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTGAAAC TCTGAACAAC ATCCGTAC |
| Gly -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTGGCAC TCTGAACAAC ATCCGTAC |
| His -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTCATAC TCTGAACAAC ATCCGTAC |
| Ile -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTATTAC TCTGAACAAC ATCCGTAC |
| Leu -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTCTTAC TCTGAACAAC ATCCGTAC |
| Lys -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTAAAAC TCTGAACAAC ATCCGTAC |
| Met -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTATGAC TCTGAACAAC ATCCGTAC |
| Phe -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTTTTAC TCTGAACAAC ATCCGTAC |
| Pro -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTCCGAC TCTGAACAAC ATCCGTAC |
| Ser -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTAGCAC TCTGAACAAC ATCCGTAC |
| Thr -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTACCAT TCTGAACAAC ATCCGTAC |
| Trp -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTTGGAC TCTGAACAAC ATCCGTAC |
| Tyr -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTTATAC TCTGAACAAC ATCCGTAC |
| Val -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTGTGAC TCTGAACAAC ATCCGTAC |
| hns-FL-fwd | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CACTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| hns-S-fwd | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGGAAG CACTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| hns-SE-fwd | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGG CACTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| hns-SEA-fwd | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| hns-SEAL-fwd | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAAAAC TCTGAACAAC ATCCGTACTC TTC |
| hns-SEALK-fwd | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAC TCTGAACAAC ATCCGTACTC TTC |
| hns-rev-trunc | GGT TAT AAT GAA TTT TGC TTA TTA ACC TTG CCT GCG CAC GAA GAG TAC GGA TGT TGT TCA GAG T |
| T7 | ATTAATACGACTCACTATAGGG |
| NV1 | GGTTATAATGAATTTTGCTTATTAAC |
| Arg -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAACGACTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Asn -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAAACCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Asp -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAGATCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Cys -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAATGCCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Gln -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAACAGCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Glu -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAGAACTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Gly -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAGGCCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| His -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAACATCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Ile -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAATTCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Leu -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAACTTCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Lys -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAAAACTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Met -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAATGCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Phe -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAATTTCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Pro -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAACCGCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Ser -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAAGCCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Thr -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAACCCTTAAAAT TCTGAACAAC ATCCGTACTC TTC |
| Trp -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAATGGCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Tyr -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAATATCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Val -FL-1 | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAGTGCTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Ala -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CAGCAAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Arg -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CACGAAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Asn -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CAAACAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Asp -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CAGATAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Cys -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CATGCAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Gln -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CACAGAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Glu -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CAGAAAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Gly -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CAGGCAAAAC TCTGAACAAC ATCCGTACTC TTC |
| His -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CACATAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Ile -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CAATTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Lys -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CAAAAAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Met -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CAATGAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Phe -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CATTTAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Pro -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CACCGAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Ser -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CAAGCAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Thr -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CAACCAAAAT TCTGAACAAC ATCCGTACTC TTC |
| Trp -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CATGGAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Tyr -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CATATAAAAC TCTGAACAAC ATCCGTACTC TTC |
| Val -FL-P | ATTAATACGACTCACTATA GGGATATA AGGAGGA AAACAT ATGAGCGAAG CAGTGAAAAC TCTGAACAAC ATCCGTACTC TTC |
| T7-cmlaL | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGAGCACTTCCAAGAACGCAGACAAGGTCTGCGTTC |
| T7-cmlaL-2A | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGGCCACTTCCAAGAACGCAGACAAGGTCTGCGTTC |
| T7-cmlaL-3A | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGAGCGCTTCCAAGAACGCAGACAAGGTCTGCGTTC |
| T7-cmlaL-4A | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGAGCACTGCCAAGAACGCAGACAAGGTCTGCGTTC |
| T7-cmlaL-5A | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGAGCACTTCCGCGAACGCAGACAAGGTCTGCGTTC |
| T7-cmlaL-6A | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGAGCACTTCCAAGGCCGCAGACAAGGTCTGCGTTC |
| T7-cmlaL-7A | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGAGCACTTCCAAGAACTCAGACAAGGTCTGCGTTC |
| T7-cmlaL-8A | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGAGCACTTCCAAGAACGCAGCCAAGGTCTGCGTTC |
| T7-cmlaL-9A | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGAGCACTTCCAAGAACGCAGACGCGGTCTGCGTTC |
| cmaL-toe2 | CGTAGAGCAAAAGGATTCATGAGAACGCCGCAACAACCGAAAAATGAAGGTTGCTGCGGCTTACTTGTCTGCGTTCTTG |
| cmaL-toe2-6A | CGTAGAGCAAAAGGATTCATGAGAACGCCGCAACAACCGAAAAATGAAGGTTGCTGCGGCTTACTTGTCTGCGGCC |
| cmaL-toe2-7A | CGTAGAGCAAAAGGATTCATGAGAACGCCGCAACAACCGAAAAATGAAGGTTGCTGCGGCTTACTTGTCTGAGTTC |
| cmaL-toe2-8A | CGTAGAGCAAAAGGATTCATGAGAACGCCGCAACAACCGAAAAATGAAGGTTGCTGCGGCTTACTTGGCTGCGTTC |
| cmaL-toe2-9A | CGTAGAGCAAAAGGATTCATGAGAACGCCGCAACAACCGAAAAATGAAGGTTGCTGCGGCTTACGCGTCTGCGTTC |
| rev-cmlaL-toe2 | CGTAGAGCAAAAGGATTCATG |
| T7-catL | TAATACGACTCACTATAGGGGGACAGAAACATGACATATCTCTTGAAAGGATGATTGTGGTGGTGAAAACAGATAAAATCTCCTCC |
| T7-catL-2A | TAATACGACTCACTATAGGGGGACAGAAACATGACATATCTCTTGAAAGGATGATTGTGGTGGCGAAAACAGATAAAATCTCCTCC |
| T7-catL-3A | TAATACGACTCACTATAGGGGGACAGAAACATGACATATCTCTTGAAAGGATGATTGTGGTGGTGGCAACAGATAAAATCTCCTCC |
| T7-catL-4A | TAATACGACTCACTATAGGGGGACAGAAACATGACATATCTCTTGAAAGGATGATTGTGGTGGTGAAAGCAGATAAAATCTCCTCC |
| T7-catL-5A | TAATACGACTCACTATAGGGGGACAGAAACATGACATATCTCTTGAAAGGATGATTGTGGTGGTGAAAACAGCTAAAATCTCCTCC |
| T7-catL-6A | TAATACGACTCACTATAGGGGGACAGAAACATGACATATCTCTTGAAAGGATGATTGTGGTGGTGAAAACAGATGCAATCTCCTCC |
| catl-toe1 | CAGATAATTTTCGTCTATTTGTTTAAACAATTTTATCTCCTCCTGAATGTGATTTACTGTATTCAGGAGGAGATTTTATCTGTTTTCACC |
| catl-toe1-2A | CAGATAATTTTCGTCTATTTGTTTAAACAATTTTATCTCCTCCTGAATGTGATTTACTGTATTCAGGAGGAGATTTTATCTGTTTTCGC |
| catl-toe1-3A | CAGATAATTTTCGTCTATTTGTTTAAACAATTTTATCTCCTCCTGAATGTGATTTACTGTATTCAGGAGGAGATTTTATCTGTTGCCAC |
| catl-toe1-4A | CAGATAATTTTCGTCTATTTGTTTAAACAATTTTATCTCCTCCTGAATGTGATTTACTGTATTCAGGAGGAGATTTTATCTGCTTTCAC |
| catl-toe1-5A | CAGATAATTTTCGTCTATTTGTTTAAACAATTTTATCTCCTCCTGAATGTGATTTACTGTATTCAGGAGGAGATTTTAGCTGTTTTCAC |
| catl-toe1-6A | CAGATAATTTTCGTCTATTTGTTTAAACAATTTTATCTCCTCCTGAATGTGATTTACTGTATTCAGGAGGAGATTGCATCTGTTTTCAC |
| rev-cat-toe1 | CAGATAATTTTCGTCTATTTGTTTAAAC |
| T7-cat-2Al-V | TAATACGACTCACTATAGGGGGACAGAAACATGACATATCTCTTGAAAGGATGATTGTGGTGAAAGCAGATAAAATCTCCTCC |
| T7-catl-2A-VK | TAATACGACTCACTATAGGGGGACAGAAACATGACATATCTCTTGAAAGGATGATTGTGGTGGCAGATAAAATCTCCTCC |
| T7-catl-2A-VKA | TAATACGACTCACTATAGGGGGACAGAAACATGACATATCTCTTGAAAGGATGATTGTGGTGGATAAAATCTCCTCC |
| catl-toe1-2A-V | CAGATAATTTTCGTCTATTTGTTTAAACAATTTTATCTCCTCCTGAATGTGATTTACTGTATTCAGGAGGAGATTTTATCTGCTTTCAC |
| catl-toe1-2A-VK | CAGATAATTTTCGTCTATTTGTTTAAACAATTTTATCTCCTCCTGAATGTGATTTACTGTATTCAGGAGGAGATTTTATCTGCCACCAC |
| catl-toe1-2A-VKA | CAGATAATTTTCGTCTATTTGTTTAAACAATTTTATCTCCTCCTGAATGTGATTTACTGTATTCAGGAGGAGATTTTATCCACCACAAT |
| T7-cmlal-S | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGACTTCCAAGAACGCAGACAAGGCGATCTAAGC |
| T7-cmlal-ST | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGTCCAAGAACGCAGACAAGGCGATCTAAGC |
| T7-cmlal-STS | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGAAGAACGCAGACAAGGCGATCTAAGC |
| T7-cmlal-STSK | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGAACGCAGACAAGGCGATCTAAGC |
| T7-cmlal-STSKN | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGGCAGACAAGGCGATCTAAGC |
| T7-cmlal-STSKNA | TAATACGACTCACTATAGGGCGATTCAAATTCAATCATGAGATAGTCAGCAGATGGACAAGGCGATCTAAGC |
| cmlaL-STSKN-toe2 | CGTAGAGCAAAAGGATTCATGAGAACGCCGCAACAACCGAAAAATGAAGGTTGCTGCGGCTTAGATCGCCTTGTCTGCC |
| cmlaL-STSKNA-toe2 | CGTAGAGCAAAAGGATTCATGAGAACGCCGCAACAACCGAAAAATGAAGGTTGCTGCGGCTTAGATCGCCTTGTCCATC |
| Ala -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTGCAAC TCTGAACAAC ATCCGTAC |
| Arg -FL-A | GATATA AGGAGGA AAACAT ATGAGCGAAGCACTTCGAAC TCTGAACAAC ATCCGTAC |
Discussion
We have presented evidence that antibiotics CHL and LZD, belonging to two different classes of ribosomal PTC inhibitors, do not actively block formation of every peptide bond, but instead interfere with translation in a context-specific manner. The nature of the two C-terminal nascent peptide residues as well as of the A-site acceptor strongly influence the ability of these drugs to inhibit peptidyl transfer. The presence of Ala, and to a lesser extent of Ser and Thr, in the penultimate position of the peptide stimulates the action of the drugs. In contrast, Gly in the P or A sites of the PTC strongly counteracts the antibiotics’ inhibitory effect.
Our findings reveal the most extreme manifestation of the dependency of CHL or LZD action on the nature of the PTC donor and acceptor substrates. It is very likely that the action of these antibiotics is affected by a more extended context. Thus, despite a strong preference for the presence of Ala at position (−1) in the sites of the strongest arrest, this amino acid can be found in the penultimate peptide position throughout the entire spectrum of ∼60,000 codons that were analyzed in the CHL and LZD samples (Fig. S5A). Of note, the positive effect of Ala (−1) on CHL-dependent stalling at the fifth codon of the hns gene seems to be alleviated by the Leu5 to Ala mutation (Fig. 3D), an effect that was not observed at other examined sites. Therefore, the context in which Ala (−1) appears affects the strength of the drug-dependent translation arrest. For example, the stimulatory effect of Ala (−1) upon CHL or LZD action can be completely negated when Gly is present at the peptide’s C terminus (Fig. S7A), whereas C-terminal Asp seems to additionally boost the effect of penultimate Ala, Ser, or Thr on CHL-dependent translation arrest (Fig. S7E). A more comprehensive analysis of the cumulative changes in codon-specific ribosome occupancy showed that both the stimulatory effect of Ala (−1) and the counteracting influence of Gly (0) and Gly (+1) may be modulated by the identity of the other amino acid residues in these three positions (Fig. S7 A–D).
Fig. S7.
Extended context for CHL and LZD action. (A–D) The cumulative drug-induced changes in the ribosome density at all of the analyzed sites with (A and C) Ala (−1) and various P- and A-site codons, (C) Gly in the P site and various −1 codons or (D) Gly in the A-site and various −1 codons. The bars representing sites with Ala in the penultimate position of the nascent peptide and Gly in the P site are indicated by arrows. (E) The presence of C-terminal Asp and acceptor Lys stimulates CHL action at the sites with Ala or Thr in position −1. pLogo analysis of amino acids of the top 500 sites of CHL-induced translation arrest that contain exclusively Ala or Thr in position −1. Note the preferential presence of Asp in position 0 (P site).
Because CHL has been traditionally viewed as a universal inhibitor of peptide bond formation, it has been used to “freeze” the ribosomes on mRNA in some profiling experiments (30, 35), an approach challenged by several studies where trends similar to the ones we presented here were noted (29, 31, 36, 37). We show that exposure of the cells to high concentrations of CHL (or LZD) does not in fact freeze the ribosomes on mRNA, but allows them to redistribute from the sites less favorable for the drug-dependent arrest to the downstream strong-arrest codons. This effect, for example, can be readily observed near the sites of translation initiation. A peak of ribosome density is prominent at the start codons of many genes in the untreated cells (30) and can be visualized by the metagene analysis (Fig. S8, gray plot). In the CHL- or LZD-treated cells, the relative overall start codon occupancy is dramatically decreased, indicative of poor ability of LZD or CHL to inhibit the first peptide bond formation and the resulting redistribution of the ribosomes from the translation initiation site to the downstream codons (Fig. S8, blue and orange plots). This observation is in line with the results of toeprinting experiments where both antibiotics failed to arrest translation at the start codon (Fig. 3 and Fig. S1). Altogether, our results strongly argue against the use of CHL in the ribosome-profiling experiments if precise position of the ribosomes on mRNA is being analyzed.
Fig. S8.
CHL and LZD fail to inhibit formation of the first peptide bond and therefore cause redistribution of ribosomes from the start codons of the genes. Metagene analysis of the relative ribosome occupancy of the first 30 codons of the actively translated genes in the untreated E. coli cells (gray line) or cells treated with CHL (orange line) or LZD (blue line). The relative ribosome occupancy is calculated as the fraction (percentage) of cumulative ribosome density at a specific codon relative to the total ribosome density at the 105-nt-long region surrounding the initiation codon (positions −15 to +90 relative to the first nucleotide of the start codon). Note the decrease of the start codon occupancy in the CHL- and LZD-treated samples.
Our finding that CHL and LZD act at the defined locations within the gene extends the concept of context-specific action of ribosome-targeting inhibitors (32). Macrolides, which bind to the nascent peptide exit tunnel, arrest translation at a limited number of codons within the ORF also depending on the nature of the nascent peptide and of the incoming amino acid (27, 28, 32, 38). The context requirements for macrolides, however, are principally different from those for the PTC-targeting antibiotics. The locations where LZD or CHL would arrest translation are defined primarily by individual amino acids, but the sites of macrolide action are delineated by short amino acid sequences (27, 28). As a result, PTC inhibitors halt translation at multiple locations along the gene, whereas macrolides arrest ribosome only at a limited number of codons within the ORF, and synthesis of the proteins lacking the “problematic” sequences may not be inhibited at all (32, 39). For this reason, cells treated with excess of macrolide antibiotics continue to synthesize a limited subset of polypeptides (32), whereas protein translation in cells exposed to CHL or LZD is essentially abolished (Fig. S2). Nevertheless, our data suggest that CHL and LZD should be viewed as “poor” inhibitors of peptide bond formation even within the preferred context. At high drugs concentration, translation is only transiently paused at specific codons,, leading to the appearance of a number of “arrest bands” in toeprinting gels, rather than at a unique stalling site (Fig. S1A and Fig. 3B).
Similar to the inducible macrolide resistance genes, induction of CHL resistance requires programmed ribosome stalling within the leader ORFs (40). Our findings revealed the principles of antibiotic-induced site-specific arrest of translation of the regulatory ORFs of two model CHL resistance genes. Translation of the cmlAL ORF is stalled by CHL after Ala, the amino acid most conducive to antibiotic action, appears in the penultimate position of the nascent peptide (Fig. 4C). Furthermore, synthesis of the CmlAL peptide is halted within the sequence ADK, one of the best motifs for CHL-dependent arrest (Fig. S7E). Arrest of translation of the Cat86AL leader peptide occurs within a similar context, except that Ala is replaced with Thr, another amino acid conducive to CHL action (Fig. 4C and Fig. S7E). Thus, programmed ribosome stalling responsible for induction of CHL resistance simply exploits the sequence context most favorable for the action of this antibiotic. Although the cmlA and catA86 resistance genes come from evolutionarily distant bacterial species, the sites of CHL-induced ribosome stalling within the leader ORFs of these genes conform to the contexts that we identified when exploring the CHL and LZD action in E. coli. Therefore, we believe that the uncovered trends in the specificity of LZD and CHL action are universal, rather than strain or species specific.
We still lack the understanding of the mechanistic principles of context specificity of CHL and LZD action. Crystallographic reconstructions have uncovered how these drugs bind to the PTC of the vacant bacterial ribosome (10–12, 19, 20). However, the gained structural and functional implications are likely incomplete or possibly even misleading because our data clearly show that the key aspects of the drug binding and action must critically depend on the properties of the peptidyl nascent chain and the aminoacyl-acceptor. The effects of the PTC substrates could be direct, involving immediate interactions between the key amino acid residues and the drug, or indirect, when the properties of the drug-binding pocket are allosterically affected by the ribosomal ligands.
The context-specific action of the PTC inhibitors reflects the functional interplay between the nascent chain and the PTC, which has been demonstrated in a range of ribosomal functions: from programmed translation arrest, to termination and recoding (reviewed in ref. 41). Our previous studies (32) and the findings presented here strongly suggest that many antibiotics that directly or indirectly influence peptide bond formation, translocation of the substrates through the PTC, or egress of the nascent protein chain will be influenced by the sequence context. Improving our understanding of these principles will pave the way for the knowledge-based discovery of better protein synthesis inhibitors.
Materials and Methods
Ribosome Profiling.
Ribosome profiling was carried out using the antibiotic hypersusceptible E. coli strain BWDK, a derivative of the strain BW25113 with inactivated tolC gene [F−, DE(araD-araB)567, lacZ4787(del)::rrnB-3, λ−, rph-1, DE(rhaD-rhaB)568, hsdR514, tolC] (32). The overnight culture grown in LB medium was diluted 100-fold into three conical baffled 1-L flasks with 100 mL of LB supplemented with 0.2% glucose and grown with vigorous shaking at 37 °C. When the culture density reached OD600 of ∼0.5, CHL or LZD were added to a final concentration of 100 or 800 µg/mL (100-fold minimum inhibitory concentration) and incubation continued for 2.5 min. The control culture did not receive a drug treatment. Cells were harvested by rapid filtration, frozen in liquid nitrogen, and processed for ribosome profiling as described (30). The library for Illumina sequencing was prepared using RNA fragments ranging in size between 28 and 42 nt. Analysis of ribosome profiling was carried out using the GALAXY pipeline (28, 42). The first position of the P-site codon was assigned at 15 nt away from the 3′-end of the read (37). Details of analysis of the ribosome-profiling data are presented in SI Materials and Methods.
Generation of Templates for in Vitro Translation and Toeprinting Analysis.
Preparation of DNA templates for toeprinting is described in SI Materials and Methods, and the primers used are listed in Table S1. Toeprinting reactions were carried out in 5 µL of PURExpress transcription–translation system (New England Biolabs) as previously described (43, 44). The final concentrations of LZD, CHL, mupirocin, borrelidin, or thiostrepton in the reactions were 50 µM, unless otherwise indicated. Gels were quantified using ImageJ (45), and relative stalling efficiency was calculated using the following equation:
where Ab is the intensity of the band representing CHL- or LZD-dependent stalling, Cc is the intensity of the band representing ribosome stalling at the downstream “catch” codon, and Bd is the background.
Acknowledgments
We thank Joseph Dang for carrying out early experiments, Jiyoung Lee for the invaluable assistance with bioinformatics analysis, Yury Polikanov for fruitful discussions and help with preparation of Fig. 1, and Alejandro Mankin for proofreading the manuscript. This work was supported by National Institutes of Health Grant AI125518.
Footnotes
Conflict of interest statement: The research in the laboratory was supported by, among other sources, grants from the pharmaceutical companies Melinta Therapeutics and Cempra Pharmaceuticals.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE86536).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613055113/-/DCSupplemental.
References
- 1.Beringer M, Rodnina MV. The ribosomal peptidyl transferase. Mol Cell. 2007;26(3):311–321. doi: 10.1016/j.molcel.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Weinger JS, Parnell KM, Dorner S, Green R, Strobel SA. Substrate-assisted catalysis of peptide bond formation by the ribosome. Nat Struct Mol Biol. 2004;11(11):1101–1106. doi: 10.1038/nsmb841. [DOI] [PubMed] [Google Scholar]
- 3.Erlacher MD, et al. Chemical engineering of the peptidyl transferase center reveals an important role of the 2′-hydroxyl group of A2451. Nucleic Acids Res. 2005;33(5):1618–1627. doi: 10.1093/nar/gki308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polikanov YS, Steitz TA, Innis CA. A proton wire to couple aminoacyl-tRNA accommodation and peptide-bond formation on the ribosome. Nat Struct Mol Biol. 2014;21(9):787–793. doi: 10.1038/nsmb.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polacek N, Gaynor M, Yassin A, Mankin AS. Ribosomal peptidyl transferase can withstand mutations at the putative catalytic nucleotide. Nature. 2001;411(6836):498–501. doi: 10.1038/35078113. [DOI] [PubMed] [Google Scholar]
- 6.Youngman EM, Brunelle JL, Kochaniak AB, Green R. The active site of the ribosome is composed of two layers of conserved nucleotides with distinct roles in peptide bond formation and peptide release. Cell. 2004;117(5):589–599. doi: 10.1016/s0092-8674(04)00411-8. [DOI] [PubMed] [Google Scholar]
- 7.Sievers A, Beringer M, Rodnina MV, Wolfenden R. The ribosome as an entropy trap. Proc Natl Acad Sci USA. 2004;101(21):7897–7901. doi: 10.1073/pnas.0402488101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson DN. The A-Z of bacterial translation inhibitors. Crit Rev Biochem Mol Biol. 2009;44(6):393–433. doi: 10.3109/10409230903307311. [DOI] [PubMed] [Google Scholar]
- 9.Pestka S. Chloramphenicol. In: Corcoran JW, Hahn FE, editors. Antibiotics: Mechanism of Action of Antimicrobial and Antitumor Agents. Vol III. Springer; Berlin: 1975. pp. 370–395. [Google Scholar]
- 10.Dunkle JA, Xiong L, Mankin AS, Cate JH. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc Natl Acad Sci USA. 2010;107(40):17152–17157. doi: 10.1073/pnas.1007988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulkley D, Innis CA, Blaha G, Steitz TA. Revisiting the structures of several antibiotics bound to the bacterial ribosome. Proc Natl Acad Sci USA. 2010;107(40):17158–17163. doi: 10.1073/pnas.1008685107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlünzen F, et al. Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria. Nature. 2001;413(6858):814–821. doi: 10.1038/35101544. [DOI] [PubMed] [Google Scholar]
- 13.Wilson DN. On the specificity of antibiotics targeting the large ribosomal subunit. Ann N Y Acad Sci. 2011;1241:1–16. doi: 10.1111/j.1749-6632.2011.06192.x. [DOI] [PubMed] [Google Scholar]
- 14.Kucan Z, Lipmann F. Differences in chloramphenicol sensitivity of cell-free amino acid polymerization systems. J Biol Chem. 1964;239(2):516–520. [PubMed] [Google Scholar]
- 15.Vazquez D. Antibiotics affecting chloramphenicol uptake by bacteria. Their effect on amino acid incorporation in a cell-free system. Biochim Biophys Acta. 1966;114(2):289–295. doi: 10.1016/0005-2787(66)90310-8. [DOI] [PubMed] [Google Scholar]
- 16.Cannon M. The puromycin reaction and its inhibition by chloramphenicol. Eur J Biochem. 1968;7(1):137–145. doi: 10.1111/j.1432-1033.1968.tb19584.x. [DOI] [PubMed] [Google Scholar]
- 17.Lovett PS. Translational attenuation as the regulator of inducible cat genes. J Bacteriol. 1990;172(1):1–6. doi: 10.1128/jb.172.1.1-6.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kloss P, Xiong L, Shinabarger DL, Mankin AS. Resistance mutations in 23 S rRNA identify the site of action of the protein synthesis inhibitor linezolid in the ribosomal peptidyl transferase center. J Mol Biol. 1999;294(1):93–101. doi: 10.1006/jmbi.1999.3247. [DOI] [PubMed] [Google Scholar]
- 19.Leach KL, et al. The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Mol Cell. 2007;26(3):393–402. doi: 10.1016/j.molcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Wilson DN, et al. The oxazolidinone antibiotics perturb the ribosomal peptidyl-transferase center and effect tRNA positioning. Proc Natl Acad Sci USA. 2008;105(36):13339–13344. doi: 10.1073/pnas.0804276105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ippolito JA, et al. Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J Med Chem. 2008;51(12):3353–3356. doi: 10.1021/jm800379d. [DOI] [PubMed] [Google Scholar]
- 22.Shinabarger DL, et al. Mechanism of action of oxazolidinones: Effects of linezolid and eperezolid on translation reactions. Antimicrob Agents Chemother. 1997;41(10):2132–2136. doi: 10.1128/aac.41.10.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin AH, Murray RW, Vidmar TJ, Marotti KR. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob Agents Chemother. 1997;41(10):2127–2131. doi: 10.1128/aac.41.10.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartz D, McPheeters DS, Traut R, Gold L. Extension inhibition analysis of translation initiation complexes. Methods Enzymol. 1988;164:419–425. doi: 10.1016/s0076-6879(88)64058-4. [DOI] [PubMed] [Google Scholar]
- 25.Orelle C, et al. Tools for characterizing bacterial protein synthesis inhibitors. Antimicrob Agents Chemother. 2013;57(12):5994–6004. doi: 10.1128/AAC.01673-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324(5924):218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis AR, Gohara DW, Yap MN. Sequence selectivity of macrolide-induced translational attenuation. Proc Natl Acad Sci USA. 2014;111(43):15379–15384. doi: 10.1073/pnas.1410356111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kannan K, et al. The general mode of translation inhibition by macrolide antibiotics. Proc Natl Acad Sci USA. 2014;111(45):15958–15963. doi: 10.1073/pnas.1417334111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakahigashi K, et al. Comprehensive identification of translation start sites by tetracycline-inhibited ribosome profiling. DNA Res. 2016;23(3):193–201. doi: 10.1093/dnares/dsw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh E, et al. Selective ribosome profiling reveals the cotranslational chaperone action of trigger factor in vivo. Cell. 2011;147(6):1295–1308. doi: 10.1016/j.cell.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker AH, Oh E, Weissman JS, Kramer G, Bukau B. Selective ribosome profiling as a tool for studying the interaction of chaperones and targeting factors with nascent polypeptide chains and ribosomes. Nat Protoc. 2013;8(11):2212–2239. doi: 10.1038/nprot.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kannan K, Vázquez-Laslop N, Mankin AS. Selective protein synthesis by ribosomes with a drug-obstructed exit tunnel. Cell. 2012;151(3):508–520. doi: 10.1016/j.cell.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Lovett PS. Translation attenuation regulation of chloramphenicol resistance in bacteria—a review. Gene. 1996;179(1):157–162. doi: 10.1016/s0378-1119(96)00420-9. [DOI] [PubMed] [Google Scholar]
- 34.Alexieva Z, Duvall EJ, Ambulos NP, Jr, Kim UJ, Lovett PS. Chloramphenicol induction of cat-86 requires ribosome stalling at a specific site in the leader. Proc Natl Acad Sci USA. 1988;85(9):3057–3061. doi: 10.1073/pnas.85.9.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li GW, Oh E, Weissman JS. The anti-Shine-Dalgarno sequence drives translational pausing and codon choice in bacteria. Nature. 2012;484(7395):538–541. doi: 10.1038/nature10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakahigashi K, et al. Effect of codon adaptation on codon-level and gene-level translation efficiency in vivo. BMC Genomics. 2014;15:1115. doi: 10.1186/1471-2164-15-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohammad F, Woolstenhulme CJ, Green R, Buskirk AR. Clarifying the translational pausing landscape in bacteria by ribosome profiling. Cell Rep. 2016;14(4):686–694. doi: 10.1016/j.celrep.2015.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sothiselvam S, et al. Binding of macrolide antibiotics leads to ribosomal selection against specific substrates based on their charge and size. Cell Rep. 2016;16(7):1789–1799. doi: 10.1016/j.celrep.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Starosta AL, et al. Interplay between the ribosomal tunnel, nascent chain, and macrolides influences drug inhibition. Chem Biol. 2010;17(5):504–514. doi: 10.1016/j.chembiol.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Lovett PS. Nascent peptide regulation of translation. J Bacteriol. 1994;176(21):6415–6417. doi: 10.1128/jb.176.21.6415-6417.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito K, Chiba S. Arrest peptides: Cis-acting modulators of translation. Annu Rev Biochem. 2013;82:171–202. doi: 10.1146/annurev-biochem-080211-105026. [DOI] [PubMed] [Google Scholar]
- 42.Afgan E, et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 2016;44(W1):W3–W10. doi: 10.1093/nar/gkw343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazquez-Laslop N, Thum C, Mankin AS. Molecular mechanism of drug-dependent ribosome stalling. Mol Cell. 2008;30(2):190–202. doi: 10.1016/j.molcel.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 44.Orelle C, et al. Identifying the targets of aminoacyl-tRNA synthetase inhibitors by primer extension inhibition. Nucleic Acids Res. 2013;41(14):e144. doi: 10.1093/nar/gkt526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arenz S, et al. Molecular basis for erythromycin-dependent ribosome stalling during translation of the ErmBL leader peptide. Nat Commun. 2014;5:3501. doi: 10.1038/ncomms4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Shea JP, et al. pLogo: A probabilistic approach to visualizing sequence motifs. Nat Methods. 2013;10(12):1211–1212. doi: 10.1038/nmeth.2646. [DOI] [PubMed] [Google Scholar]