Significance
Although implicated, the role of herpes simplex virus (HSV) infected cell culture polypeptide 27 (ICP27) in cotranscriptional pre-mRNA processing remains poorly understood. We show that ICP27 promotes cotranscriptional cellular pre-mRNA 3′ end formation using cryptic polyadenylation signals in introns, generating hundreds of novel, intronless GC-rich cellular transcripts that resemble HSV genes. ICP27 also causes aberrant pre-mRNA splicing of some genes. ICP27-targeted genes share common features such as high GC content, cytosine-rich sequences, and suboptimal splice sites, providing an explanation for the observed target specificity of ICP27 and suggesting an overlapping mechanism for ICP27-mediated aberrant pre-mRNA splicing and polyadenylation. By specifically modifying pre-mRNA processing of HSV-like GC-rich transcripts that are likely spared by the virion host shutoff protein, ICP27 contributes to virus-induced host shutoff required for efficient viral growth.
Keywords: polyadenylation, alternative splicing, DNA viruses, host–pathogen interactions, RNA 3′ polyadenylation signals
Abstract
The herpes simplex virus (HSV) infected cell culture polypeptide 27 (ICP27) protein is essential for virus infection of cells. Recent studies suggested that ICP27 inhibits splicing in a gene-specific manner via an unknown mechanism. Here, RNA-sequencing revealed that ICP27 not only inhibits splicing of certain introns in <1% of cellular genes, but also can promote use of alternative 5′ splice sites. In addition, ICP27 induced expression of pre-mRNAs prematurely cleaved and polyadenylated from cryptic polyadenylation signals (PAS) located in intron 1 or 2 of ∼1% of cellular genes. These previously undescribed prematurely cleaved and polyadenylated pre-mRNAs, some of which contain novel ORFs, were typically intronless, <2 Kb in length, expressed early during viral infection, and efficiently exported to cytoplasm. Sequence analysis revealed that ICP27-targeted genes are GC-rich (as are HSV genes), contain cytosine-rich sequences near the 5′ splice site, and have suboptimal splice sites in the impacted intron, suggesting that a common mechanism is shared between ICP27-mediated alternative polyadenylation and splicing. Optimization of splice site sequences or mutation of nearby cytosines eliminated ICP27-mediated splicing inhibition, and introduction of C-rich sequences to an ICP27-insensitive splicing reporter conferred this phenotype, supporting the inference that specific gene sequences confer susceptibility to ICP27. Although HSV is the first virus and ICP27 is the first viral protein shown to activate cryptic PASs in introns, we suspect that other viruses and cellular genes also encode this function.
Herpes simplex virus (HSV) infected cell culture polypeptide 27 (ICP27), an immediate early (IE) gene (among those first expressed after virus enters the cells) that is required for expression of some early and late viral genes as well as for virus growth, is highly conserved between HSV-1 and -2, two closely related neurotropic herpesviruses (1). ICP27 has a role in transcriptional regulation through association with the C-terminal domain of RNA polymerase II (2, 3), forms homodimers (4, 5), interacts with U1 small nuclear ribonucleoprotein (snRNP) through its C-terminal domain, and colocalizes with U1 and U2 snRNPs (6, 7). It also interacts with splicing factors such as SRSF3, SRSF1, SRSF7, and SRSF2 (8–11), and is involved in nuclear export of some viral transcripts (12, 13). The role of ICP27 in regulating pre-mRNA splicing remains controversial. Early studies indicated that, in an in vitro pre-mRNA splicing system, ICP27 may nonspecifically inhibit host pre-mRNA splicing, impairing spliceosome assembly as a result of interaction with SR protein kinase 1 (SRPK1) through ICP27’s N-terminal RGG RNA-binding motif and/or interaction with spliceosome-association protein 145 (SAP145 or SF3B2) through ICP27’s C-terminal domain (8, 11). A recent communication reported that HSV-1 does not inhibit cotranscriptional splicing and proposed that previous reports of ICP27-induced splicing inhibition were artifacts, due to misinterpretation of run-on transcription (14). Indeed, splicing of only a few viral and cellular pre-mRNAs have been reported to be inhibited by ICP27 in infected cell culture. For example, splicing of alpha-globin is inhibited by ICP27 when ICP4, another viral IE gene, is present (15). ICP27 also promotes expression of the full-length glycoprotein C protein (16, 17) and a truncated form of HSV-2 ICP34.5 (18, 19), the major viral neurovirulence factor, by inhibiting splicing of these genes. ICP27 inhibits splicing of only introns 7a and 8 of promyelocytic leukemia protein (PML) (20). We previously reported that ICP27 inhibits ICP34.5 splicing much more efficiently than other cotransfected splicing reporter genes in a way not fully dependent on the N-terminal RGG motif, suggesting that ICP27 may inhibit splicing in a gene- or sequence-specific manner (18) that cannot be completely explained by previously proposed mechanisms (1).
Results
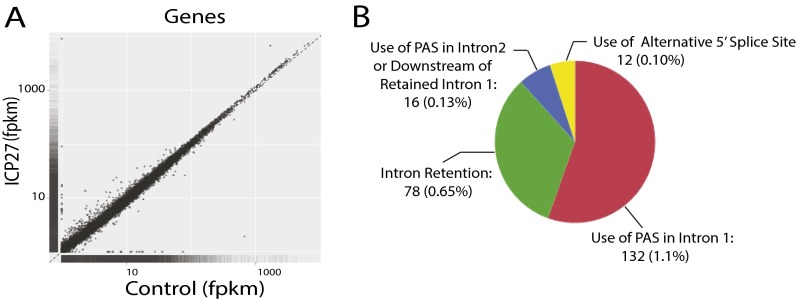
To further characterize the role of ICP27 in regulating host pre-mRNA processing, high-throughput RNA-sequencing (RNA-seq) data from poly-(A)-enriched RNA purified from HEK293 cells transiently transfected with or without ICP27 was analyzed. We narrowed our search from the 19,655 cellular genes with expression level ≥0.5 fragments per kilobase per million fragments mapped (fpkm) to the ∼12,000 highest-ranked genes [based on scores rating differences in expression in poly(A)-enriched RNA between ICP27-transfected and control samples] and visually examined gene expression profiles for differences in exon or intron use. ICP27 was associated with aberrant pre-mRNA processing in >200 genes (Fig. S1 A and B). Most frequently, this association was related to premature termination of pre-mRNA because of polyadenylation from a cryptic, previously undescribed, polyadenylation signal (PAS) in intron 1 (132 genes, ∼1.1%) or from a PAS in intron 2 or an alternative exon 2 in associated with retention of intron 1 (16 genes, ∼0.13%). ICP27 promoted use of a cryptic downstream 5′ splice site in 12 genes. Intron retention was identified in 78 genes (∼0.65%), consistent with our previous finding that ICP27 specifically inhibits the splicing only of certain genes (18). ICP27-targeted genes included genes that play roles in key cellular pathways, including transcription, DNA-damage response, stress and immunoregulation (including innate immunity), signal transduction, translation, the cell cycle, and metabolism (Table S1).
Fig. S1.
ICP27 causes aberrant mRNA processing in a small fraction of cellular genes. The 293 cells were transfected with an HSV-2 ICP27 expression vector (ICP27) or a control pFlag vector (control). High-throughput RNA-seq was performed by using poly(A)-enriched mRNA from the transfected cells. (A) Scatter plots of log10(fpkm) for ∼45,000 known genes expressed in cells transfected with pICP27 (ICP27) vs. cells transfected with vector control (control). (B) Aberrant mRNA processing was identified in only a very small number of genes. Approximately 2% of 12,000 genes that were highly ranked for differential expression and alternative splicing in the presence of ICP27 vs. control showed aberrant mRNA processing. Identified aberrant mRNA processing in cells expressing ICP27 included intron retention, use of intronic PASs, and use of cryptic 5′ splice sites.
Table S1.
ICP27-targeted genes identified by RNA-seq
| No. | ICP27-mediated polyadenylation in intron 1 (132) | ICP27-mediated polyadenylation in intron 2 or alternative exon 2 (16) | ICP27-mediated use of alternative 5′ ss (12) | ICP27-mediated intron retention (78) | ||||||||||||
| Gene | PAS sequence | PAS to TSS | PAS to 5′ ss | 5′ ss to TSS | Predicted size of novel ORF, aa | Gene | PAS sequence | PAS TO TSS | PAS TO 5′SS | Predicted size of novel ORF, aa | Gene | Affected 5′ ss to TSS | Predicted size of novel ORF, aa | Gene | Intron retained | |
| 1 | ABHD6 | AATAAA | 1,269 | 858 | 411 | None | CAD | AATAAA | 1,369 | 753 | <100 | DESI2 | 308 | <100 | AKT1S1 | Multiple introns |
| 2 | ACAD9 | AATAAA | 1,417 | 1,310 | 107 | <100 | CCT4 | AATAAA | 731 | 413 | <100 | HERPUD1 | 628 | 119 | ALAS1 | Intron 1 and 2 |
| 3 | ACTR3B | AATAAA | 1,696 | 1,518 | 178 | <100 | COQ4 | AATAAA | 1,046 | 410 | <100 | LEPR* | 89 | 113 | ALKBH6 | Multiple introns |
| 4 | ADCY1 | AATAAA | 2,375 | 1,717 | 658 | 249 | ELK1 | AATAAA | 1,847 | 1,163 | None | NSUN4 | 743 | <100 | ANKRD9 | Intron 1 and 2 |
| 5 | AEBP2 | ATTAAA | 1,404 | 525 | 879 | 227 | EIF4G3 | AATAAA | 1,605 | 459 | 144 | PIGG | 290 | <100 | ARFIP2 | Last intron |
| 6 | AFTPH | AATAAA | 2,176 | 1,868 | 308 | None | EPC1 | AATAAA | 1,944 | 1,636 | None | PPP1R8* | 151 | None) | ARMC5 | Last two introns |
| 7 | AGPAT5 | AATAAA | 2,197 | 1,666 | 531 | 134 | HOMEZ | AATAAA | 2,144 | 1,956 | None | RANGAP1 | 178 | None | ATPIF1 | Last intron |
| 8 | ALV9 | AATAAA | 1,241 | 864 | 377 | <100 | MDH2 | AATAAA | 1,147 | 939 | <100 | TACO1 | 492 | 122 | ATPIP | Multiple introns |
| 9 | AMMCR1 | AATAAA | 1,330 | 986 | 344 | None | MIB2 | AATAAA | 2,320 | 680 | 237 | TMEM5 | 346 | None | ATXN2L | Multiple introns |
| 10 | APPL1 | AATAAA | 860 | 659 | 201 | <100 | NFKBIL1 | ATTAAA | 2,002 | 413 | 121 | TRMU | 446 | 139 | BRD4 | Multiple introns |
| 11 | ARNTL | ATTAAA | 1,686 | 1,489 | 197 | None | SNX5 | AATAAA | 1,269 | 652 | 126 | UBE2J2 | 189 | None | CAND2 | Last two introns |
| 12 | ASB13 | AATAAA | 1,727 | 1,640 | 87 | <100 | STON1 | AATAAA | 886 | 225 | None | ZER1 | 349 | None | CC2D2A | Last intron |
| 13 | ATP6V0A2 | AATAAA | 719 | 354 | 365 | <100 | TMEM127 | AATAAA | 2,471 | 1,595 | 112 | CHMP2A | Intron 1 | |||
| 14 | ATR | AATAAA | 1053 | 872 | 181 | <100 | WWTR1 | ATTAAA | 1,855 | 627 | None | CHTF8 | Last intron | |||
| 15 | ATXN10 | AATAAA | 1134 | 752 | 382 | <100 | ZNF274 | AATAAA | 2,441 | 1,431 | None | CREBBP | Last intron | |||
| 16 | AVL9 | ATTAAA | 1,241 | 864 | 377 | <100 | ZNF778 | AATAAA | 554 | 347 | None | CRELD1 | Multiple introns | |||
| 17 | BRSK2 | AATAAA | 1,903 | 1,426 | 477 | 224 | CUL7 | Multiple introns | ||||||||
| 18 | BTBD2 | AATAAA | 3,542 | 3,135 | 407 | 205 | DAXX | Last intron | ||||||||
| 19 | CACHD1 | AATAAA | 1,952 | 1,802 | 150 | <100 | DMPK | Multiple introns | ||||||||
| 20 | CACNA2D1 | AATAAA | 813 | 371 | 442 | <100 | DLG3 | Intron 1 | ||||||||
| 21 | CAMSAP3 | AATAAA | 2,646 | 2,397 | 249 | 141 | DLX4 | Intron 1 | ||||||||
| 22 | CCDC85C | ATTAAA | 4,804 | 3,922 | 882 | 379 | DPP8 | Intron 1 | ||||||||
| 23 | CCDC113 | AATAAA | 2,073 | 1,893 | 180 | <100 | DYRK1B | Last intron | ||||||||
| 24 | CD99L2 | AATAAA | 1,071 | 776 | 295 | <100 | EMC6 | Intron 1 | ||||||||
| 25 | CEP85L | AATAAA | 2,586 | 1,925 | 661 | 240 | ERF | Multiple introns | ||||||||
| 26 | CLSTN1 | AATAAA | 2,122 | 1,238 | 884 | <100 | FBXO31 | Last intron | ||||||||
| 27 | CMTM4 | AATAAA | 1,715 | 1,347 | 368 | <100 | GTPBP3 | Intron 1 | ||||||||
| 28 | C6ORF89 | AATAAA | 2,775 | 2,611 | 164 | None) | IFT88 | Intron 1 | ||||||||
| 29 | CPSF6 | AATAAA | 1,100 | 930 | 170 | 127 | ILK | Intron 1 | ||||||||
| 30 | CTNNBIP1 | AATAAA | 1,274 | 1,100 | 174 | None | JRK | Intron 1 | ||||||||
| 31 | CYP2U1 | AATAAA | 1,160 | 521 | 639 | 205 | KLC2 | Last two introns | ||||||||
| 32 | DCAF10 | AATAAA | 979 | 339 | 640 | 180 | LINC00476 | Last intron | ||||||||
| 33 | DGCR2 | AATAAA | 1,188 | 861 | 327 | 185 | LPHN1 | Multiple introns | ||||||||
| 34 | DHRS4 | AATAAA | 1,113 | 931 | 182 | <100 | LPPR3 | Multiple introns | ||||||||
| 35 | DHX35 | AATAAA | 638 | 568 | 70 | 111 | LRFN4 | Intron 1 | ||||||||
| 36 | DUSP22 | AATAAA | 1,887 | 1,383 | 504 | <100 | MAP3K12 | Multiple introns | ||||||||
| 37 | DNMT3A | AATAAA | 5,653 | 5,492 | 161 | None | MFSD5 | Intron 1 | ||||||||
| 38 | DYNC1I1 | AATAAA | 1,547 | 1,279 | 268 | None) | MID1IP1 | Intron 1 | ||||||||
| 39 | EFCAB5 | AATAAA | 1,902 | 1,789 | 113 | None | MIDN | Multiple introns | ||||||||
| 40 | ENDOD1 | AATAAA | 1,085 | 667 | 418 | 157 | MIEF2 | Last intron | ||||||||
| 41 | EPN2 | ATTAAA | 2,214 | 2,059 | 155 | None | NADK | Multiple introns | ||||||||
| 42 | ERCC6 | AATAAA | 1,057 | 895 | 162 | None) | NFS1 | Intron1 | ||||||||
| 43 | ERLEC1 | AATAAA | 1,186 | 754 | 432 | 118 | PHF12 | Intron 1 and Last intron | ||||||||
| 44 | EXTL3 | AATAAA | 1,036 | 849 | 187 | None | P4HTM | Intron 1 | ||||||||
| 45 | FAM175A | AATAAA | 641 | 463 | 178 | 128 | PI4KB | Last intron | ||||||||
| 46 | FAM178A | AATAAA | 1,297 | 615 | 682 | <100 | PLEKH3 | Multiple introns | ||||||||
| 47 | FBXL5 | AATAAA | 1,952 | 1,743 | 209 | <100 | PMEL | Middle intron | ||||||||
| 48 | FBXW11 | AATAAA | 1,430 | 1,014 | 416 | 117 | PML | Multiple introns | ||||||||
| 49 | FNIP1 | AATAAA | 1,248 | 1,014 | 234 | <100 | POLR2A | Last intron | ||||||||
| 50 | FYCO1 | AATAAA | 1,520 | 1,417 | 103 | None | PPPDE1 | Intron 1 | ||||||||
| 51 | GIT1 | AATAAA | 3,257 | 2,991 | 266 | 205 | PPP1R7 | Last intron | ||||||||
| 52 | GLCCI1 | AATAAA | 2,978 | 1,921 | 1,057 | 249 | PRMT2 | Intron 1 | ||||||||
| 53 | GREB1l | AATAAA | 1,505 | 1,178 | 327 | None | RELA | Last intron | ||||||||
| 54 | GSKIP | AATAAA | 1,551 | 1,434 | 117 | None | RFT1 | Last intron | ||||||||
| 55 | GSR | AATAAA | 1,500 | 1,060 | 440 | 190 | SETD1A | Middle intron | ||||||||
| 56 | HDAC7 | AATAAA | 2,709 | 2,262 | 447 | 160 | SHISA5 | Last intron | ||||||||
| 57 | HILPDA | AATAAA | 581 | 387 | 194 | None | SLC10A3 | Last intron | ||||||||
| 58 | HNRNPLL | AATAAA | 1,728 | 1548 | 180 | None | SLC35A2 | Last intron | ||||||||
| 59 | HSDL1 | AATAAA | 1,040 | 924 | 116 | None | SPATA2 | Last intron | ||||||||
| 60 | IFNAR2 | AATAAA | 2,418 | 2,096 | 322 | None | STIM1 | Last intron | ||||||||
| 61 | INSR | AATAAA | 1,899 | 1,690 | 209 | 173 | STC2 | Last intron | ||||||||
| 62 | KLC1 | AATAAA | 1,060 | 753 | 307 | None | TAPBP | Intron 1 and 2 | ||||||||
| 63 | LEPR | AATAAA | 1,117 | 987 | 130 | 113 | TCF3 | Multiple introns | ||||||||
| 64 | LRRC47 | AATAAA | 1,751 | 1,108 | 643 | 234 | TMEM129 | Multiple introns | ||||||||
| 65 | MAML1 | AATAAA | 1,984 | 1,406 | 578 | 127 | TMEM173 | Intron 2 | ||||||||
| 66 | MAP3K3 | AATAAA | 1,581 | 1,026 | 555 | 183 | TJAP1 | Last intron | ||||||||
| 67 | MAP3K7 | AATAAA | 1,291 | 753 | 538 | <100 | TSC22D4 | Multiple introns | ||||||||
| 68 | MBP | AATAAA | 2,715 | 2,477 | 238 | None | VARS | First intron | ||||||||
| 69 | MCCC1 | AATAAA | 1,659 | 1,423 | 236 | <100 | WBP1L | Last intron | ||||||||
| 70 | MEGF8 | AATAAA | 1,564 | 742 | 822 | <100 | WDR5 | Intron 1 | ||||||||
| 71 | MKL1 | AATAAA | 2,983 | 2,741 | 242 | None | YPEL3 | Multiple introns | ||||||||
| 72 | MLLT1 | AATAAA | 5,612 | 5,436 | 176 | <100 | ZC3H10 | Intron 1 | ||||||||
| 73 | MOB1B | AATAAA | 1,586 | 1,378 | 208 | <100 | ZNF107 | Last intron | ||||||||
| 74 | NAA16 | AATAAA | 887 | 509 | 378 | 118 | ZNF200 | Intron 1 | ||||||||
| 75 | NFKB1 | AATAAA | 1,164 | 704 | 460 | None | ZNF416 | Last intron | ||||||||
| 76 | NUTM2B-AS1 | AATAAA | 1,804 | 1,407 | 397 | None | ZNF580 | Intron 1 | ||||||||
| 77 | PAFAH1B1 | AATAAA | 2,027 | 1,649 | 378 | None | ZNF598 | Multiple introns | ||||||||
| 78 | PFKFB3 | AATAAA | 4,212 | 4,078 | 134 | 203 | ZNF668 | Last intron | ||||||||
| 79 | PIP4K2B | ATTAAA | 3,766 | 3,066 | 700 | <100 | ||||||||||
| 80 | PLAG1 | AATAAA | 1,047 | 915 | 132 | None | ||||||||||
| 81 | PPM1E | AATAAA | 2,143 | 1,550 | 593 | 154 | ||||||||||
| 82 | PPP6C | AATAAA | 953 | 575 | 378 | 118 | ||||||||||
| 83 | PPP1R8 | AATAAA | 780 | 629 | 151 | None | ||||||||||
| 84 | PPTC7 | AATAAA | 1,351 | 900 | 451 | 291 | ||||||||||
| 85 | PRDM10 | AATAAA | 2,249 | 1917 | 332 | None | ||||||||||
| 86 | PRDM15 | AATAAA | 1,050 | 837 | 213 | 226 | ||||||||||
| 87 | PRKACB | AATAAA | 1,255 | 858 | 397 | None | ||||||||||
| 88 | QSOX2 | AATAAA | 2,650 | 2,284 | 366 | 186 | ||||||||||
| 89 | RABL6 | AATAAA | 2,076 | 1,671 | 405 | 148 | ||||||||||
| 90 | RBL1 | AATAAA | 891 | 663 | 228 | 133 | ||||||||||
| 91 | R3HDM1 | AATAAA | 1,096 | 917 | 179 | None | ||||||||||
| 92 | RFX2 | AATAAA | 2,944 | 2683 | 261 | None | ||||||||||
| 93 | RIC1 | AATAAA | 2,009 | 1585 | 424 | 220 | ||||||||||
| 94 | RMND5A | AATAAA | 1,285 | 766 | 519 | None | ||||||||||
| 95 | RRP1B | AATAAA | 3,066 | 2,822 | 244 | None | ||||||||||
| 96 | SCRN1 | AATAAA | 1,460 | 1,352 | 108 | <100 | ||||||||||
| 97 | SGSM2 | AATAAA | 1,155 | 921 | 234 | 180 | ||||||||||
| 98 | SLC4A4 | AATAAA | 2,154 | 2,036 | 118 | None | ||||||||||
| 99 | SLC22A5 | AATAAA | 1,115 | 453 | 662 | 139 | ||||||||||
| 100 | SLC46A3 | AATAAA | 1,235 | 717 | 518 | None | ||||||||||
| 101 | SNX25 | AATAAA | 754 | 528 | 226 | None | ||||||||||
| 102 | SP3 | ATTAAA | 912 | 454 | 458 | <100 | ||||||||||
| 103 | SPTAN1 | AATAAA | 759 | 620 | 139 | None | ||||||||||
| 104 | SSH2 | AATAAA | 1,069 | 767 | 302 | <100 | ||||||||||
| 105 | STXBP3 | AATAAA | 1,864 | 1,750 | 114 | <100 | ||||||||||
| 106 | SUPT16H | AATAAA | 1,640 | 862 | 778 | <100 | ||||||||||
| 107 | TBC1D2B | ATTAAA | 884 | 473 | 411 | 231 | ||||||||||
| 108 | TIAM1 | AATAAA | 2,475 | 2,425 | 50 | None | ||||||||||
| 109 | TIPRL | AATAAA | 815 | 478 | 337 | <100 | ||||||||||
| 110 | TMEM116 | AATAAA | 2,772 | 2,554 | 218 | None | ||||||||||
| 111 | TMEM170B | AATAAA | 2,283 | 2,186 | 97 | None | ||||||||||
| 112 | TMEM245 | AATAAA | 1,588 | 973 | 615 | 245 | ||||||||||
| 113 | TRAF6 | AATAAA | 1,047 | 781 | 266 | None | ||||||||||
| 114 | TSHZ3 | AATAAA | 1,054 | 949 | 105 | None | ||||||||||
| 115 | TTC13 | AATAAA | 1,039 | 726 | 313 | 166 | ||||||||||
| 116 | UBE2J1 | AATAAA | 1,117 | 755 | 362 | <100 | ||||||||||
| 117 | UNK | AATAAA | 1,535 | 1,389 | 146 | 139 | ||||||||||
| 118 | URI1 | AATAAA | 1,053 | 627 | 426 | None | ||||||||||
| 119 | USP31 | ATTAAA | 1,098 | 465 | 633 | 280 | ||||||||||
| 120 | WDYHV1 | AATAAA | 1,118 | 910 | 208 | None | ||||||||||
| 121 | YWHAG | AATAAA | 1,078 | 770 | 308 | 203 | ||||||||||
| 122 | ZAK | AATAAA | 1,459 | 1,293 | 166 | None | ||||||||||
| 123 | ZC3H6 | AATAAA | 909 | 441 | 468 | None | ||||||||||
| 124 | ZFP90 | AATAAA | 1,044 | 853 | 191 | None | ||||||||||
| 125 | ZKSCAN1 | AATAAA | 981 | 826 | 155 | None | ||||||||||
| 126 | ZMIZ2 | AATAAA | 4,051 | 3,667 | 384 | None | ||||||||||
| 127 | ZNF75A | AATAAA | 935 | 697 | 238 | None | ||||||||||
| 128 | ZNF250 | AATAAA | 2,426 | 1,869 | 557 | None | ||||||||||
| 129 | ZNF254 | AATAAA | 498 | 320 | 178 | None | ||||||||||
| 130 | ZNF273 | AATAAA | 537 | 359 | 178 | None | ||||||||||
| 131 | ZNF627 | AATAAA | 1,025 | 814 | 211 | None | ||||||||||
| 132 | ZSCAN32 | AATAAA | 452 | 360 | 92 | None | ||||||||||
Use of both alternative 5′ splice sites (ss) and intronic PAS were observed for these genes.
ICP27 Induces Expression of Previously Undescribed Cellular Pre-mRNAs Prematurely Cleaved and Polyadenylated from Cryptic PASs in Intron 1 or 2 or Immediately Downstream of Retained Intron 1.
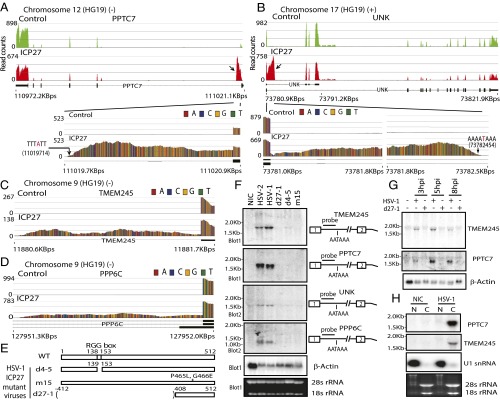
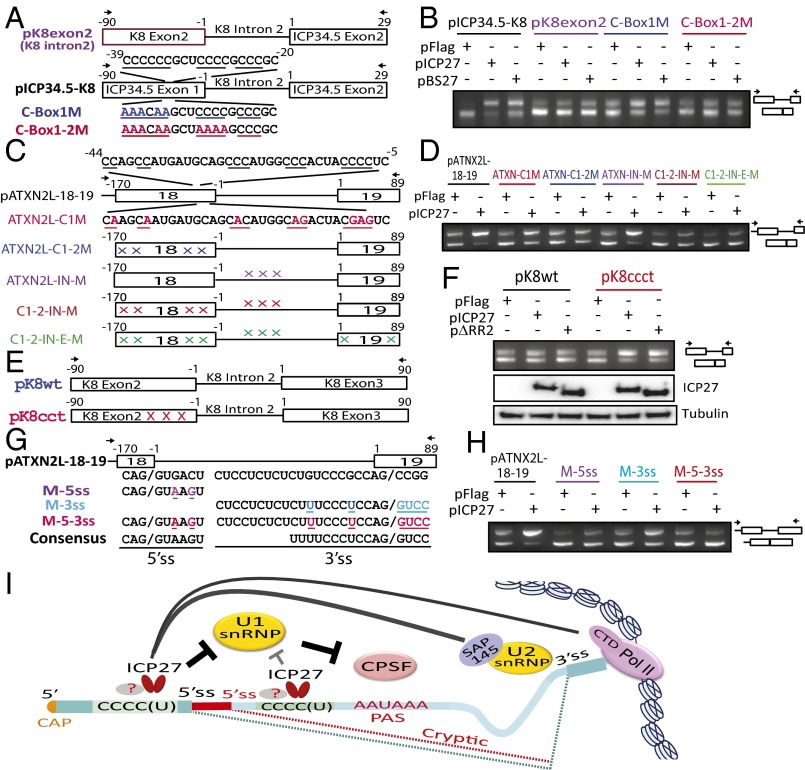
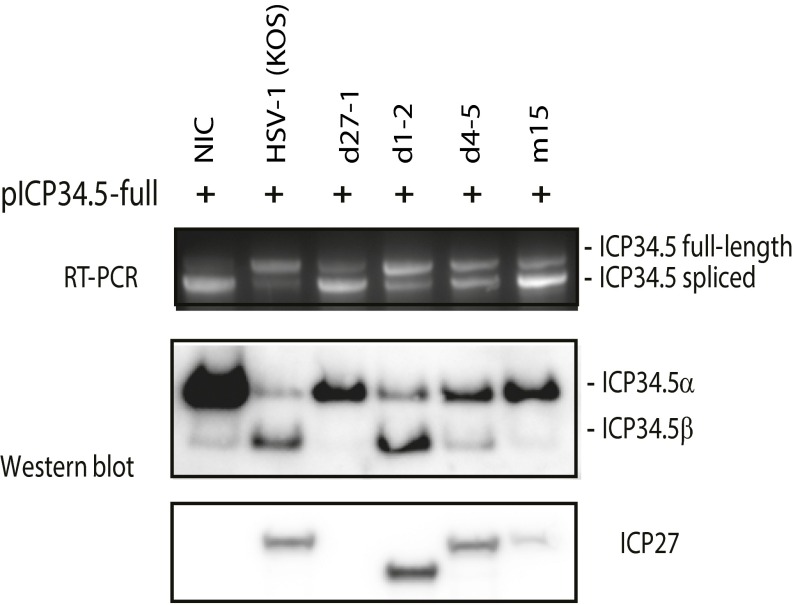
PPTC7 (Fig. 1A), a protein phosphatase gene, and UNK (Fig. 1B), an RNA-binding zinc finger protein implicated in the control of a neuronal morphology program (21), transmembrane protein TMEM245 (Fig. 1C), and the serine/threonine-protein phosphatase PPP6C (Fig. 1D) were among the 132 genes in which ICP27 induced partial retention of intron 1 with sharp decreases in read counts at a cryptic intron 1 PAS. A total of 48 (∼36%) of these intronless transcripts, including the 4 examples above, contain an ORF of >110 amino acids, with predicted molecular masses ranging from 11.5 to 39.9 kDa (Table S1), and none have been previously described. Northern hybridization of total RNA prepared from cells infected with wild-type HSV and ICP27 mutant viruses (Fig. 1E), using intron sequence-specific probes, revealed that both HSV-1 and -2 activated expression of cellular genes that use intronic PASs, based on detection of bands of sizes expected from the RNA-seq analysis (Fig. 1F). Mutant viruses with deletion of ICP27 (d27-1) or point mutations of amino acids 465 and 466 in the ICP27 C-terminal domain (m15) did not induce expression of these alternatively polyadenylated cellular transcripts. Deletion in mutant d4-5 of the RGG/SRPK-1 binding domain, which has been shown to interact with RNA and SRPK-1 (11, 22), also sharply reduced expression of alternatively polyadenylated PPTC7 and PPP6C and yielded only weak expression of alternatively polyadenylated TMEM245 and UNK, suggesting that this domain also plays an important role in the processing of these prematurely cleaved and polyadenylated intronless cellular transcripts. These alternatively polyadenylated transcripts were detectable as early as 3 h postinfection (hpi) and peaked at 5 hpi (Fig. 1G). Intronless PPTC7 was efficiently exported to the cytoplasm in infected cells (Fig. 1H).
Fig. 1.
HSV ICP27 activates expression of pre-mRNAs prematurely cleaved and polyadenylated from cryptic PASs in intron 1. (A and B) Read counts mapping to representative ICP27-targeted genes in poly(A)-selected RNA PPTC7 (negative strand; A) and UNK (positive strand; B). Control, pFlag vector-transfected cells; ICP27, HSV-2 ICP27-transfected cells. Previously described transcript variants (thick black lines denote exons) are shown underneath. Arrows indicate significant differences in intronic read counts in ICP27-expressing cells. Blowups showing intron 1 read counts are shown below. (C) Blowup showing intron 1 of TMEM245 (negative strand) read counts. (D) Blowup showing intron 1 of PPP6C (negative strand) read counts. (E) Domains and mutations in HSV-1 (WT) and ICP27 mutants d27-1, d4-5, and m15. (F) Northern hybridization of TMEM245, PPTC7, UNK, and PPP6C in HEK293 cells infected with HSV wild-type or ICP27 mutants at 8 hpi using intron-specific probes illustrated at Right to detect prematurely cleaved and polyadenylated pre-mRNAs. β-actin and ribosomal RNAs were used as loading controls. (G) ICP27-mediated prematurely cleaved and polyadenylated mRNAs are detectable during early infection. Northern hybridization for prematurely cleaved and polyadenylated TMEM245 and PPTC7 pre-mRNAs in HEK293 cells infected with HSV-1 KOS strain or d27-1 at 3, 5, and 8 hpi is shown. (H) ICP27-mediated prematurely cleaved and polyadenylated mRNAs can be efficiently exported to cytoplasm. Northern hybridization for prematurely terminated PPTC7 pre-mRNA of cytoplasmic (C) and nuclear (N) RNA fractions from HSV-1 infected at 5 hpi or uninfected (NIC) cells is shown. The same membrane was blotted with probes for PPTC7, TMEM245, and U1 snRNA. U1 snRNA and ribosomal RNAs indicate efficiency of cytoplasmic and nuclear fraction separation.
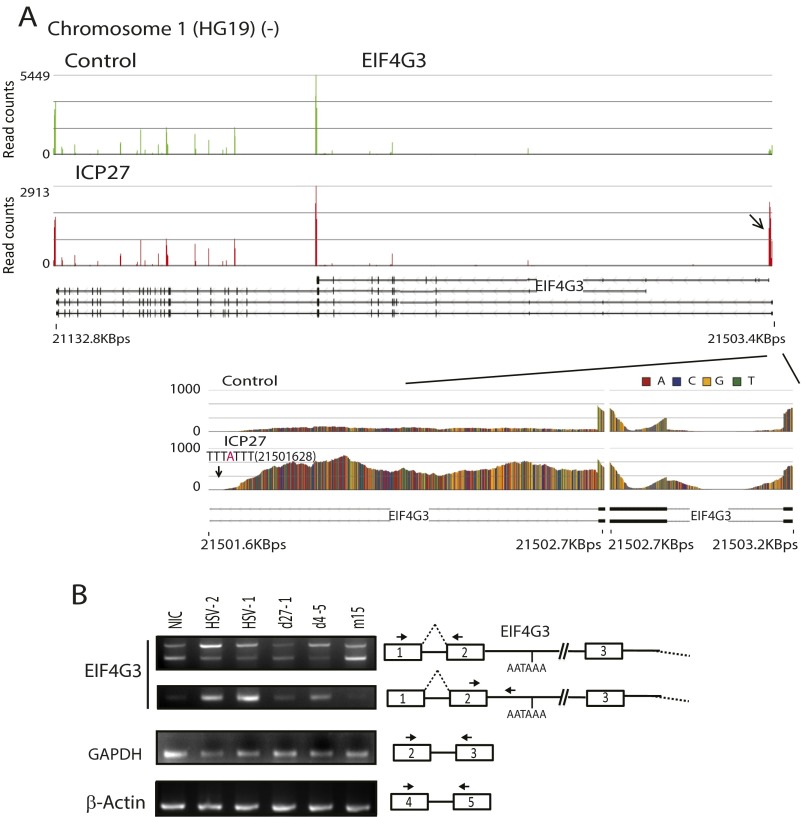
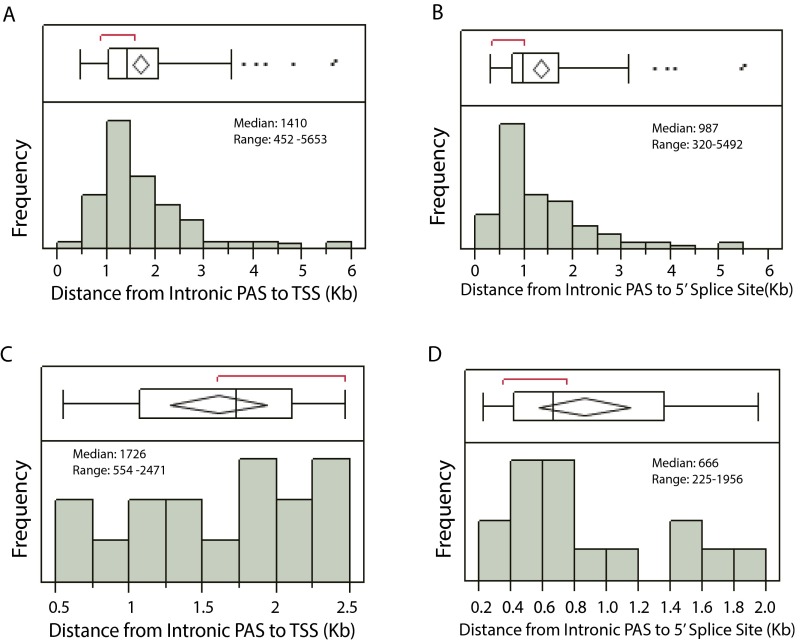
Eukaryotic translation initiation factor 4 gamma 3 (EIF4G3), a translation initiation factor targeted by vaccinia virus (23), was among the 16 genes for which ICP27 induced expression of pre-mRNAs polyadenylated from a PAS in intron 2 (frequently associated with retention of intron 1) or immediately downstream of retained intron 1 of the targeted gene (Fig. S2 and Table S1). As was observed for intron 1 alternative PASs, ICP27-activated PASs in intron 2 or downstream of retained intron 1 were typically within 1.7 Kb of the transcription start site (TSS) and within 0.7 Kb of the intron 2 5′ splice site (similar to the location of ICP27-facilitated intron 1 PASs, which were typically within 1.4 Kb of the TSS and within 1 Kb of the 5′ splice site, respectively) (Fig. S3).
Fig. S2.
ICP27 can inhibit the splicing of both a first short intron and a second intron, facilitating use of a PAS located in the second intron. (A) Read counts mapping to a representative ICP27-targeted gene, EIF4G3, eukaryotic translation initiation factor 4 gamma 3 (shown from 3′ to 5′ because it is expressed from the negative strand of chromosome 1). A close-up of the impacted region is shown in Lower. A downward-facing arrow shows the reads that are significantly different in ICP27-transfected cells compared with control. Reads mapping to the intronic PAS are also labeled in the close-up. (B) RT-PCR study of splicing inhibition of introns 1 and 2 and use of the PAS mapping to intron 2 of EIF4G3 by ICP27 in HSV-1 infected cells. cDNAs were obtained from 293 cells infected with HSV wild-type or ICP27 mutant viruses (as indicated) at a MOI of 3 and at 5 hpi. RT-PCR primers are shown using arrows in the diagram to the right.
Fig. S3.
The intronic PASs activated by ICP27 are within a short distance of the TSS and the affected 5′ splice site. (A and C) The distributions of the distance from activated PASs in intron 1 (n = 132), or in intron 2 or downstream of retained intron 1 (n = 16) to the TSS are graphed in A and C. The mean distance from TSS to PAS for the 132 genes with activated PASs in intron 1, and in the 16 genes with activated PASs in intron 2 or downstream of exon 2 was 1,672 bp with 95% CI [1,512 bp, 1,834 bp] and 1,601 bp with 95% CI [1,273 bp, 1,931 bp], respectively. (B and D) The distributions of the distance from activated PASs in intron 1 (n = 132), or in intron 2 or downstream of exon 2 (n = 16) to affected 5′ splice sites are shown in B and D, respectively. The mean distance from TSS to PAS for activated PASs in intron 1, and in intron 2 or downstream of exon 2 was 1,338 bp with 95% CI [1,177 bp, 1,498 bp] and 856 bp with 95% CI [597 bp, 1,143 bp], respectively.
ICP27 Promotes Use of Cryptic 5′ Splice Sites.
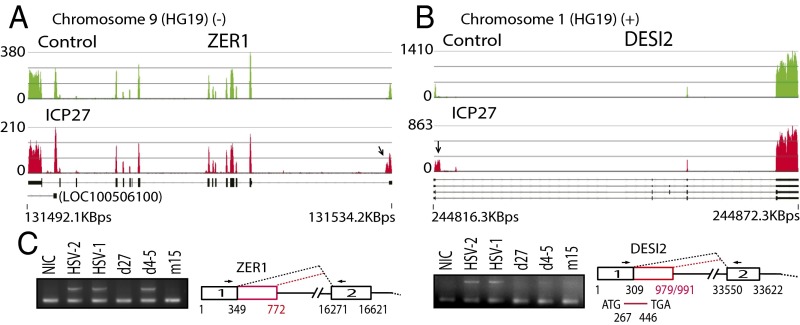
In 12 genes, including ZER1 (which encodes a subunit of an E3 ubiquitin ligase complex; Fig. 2A) and DESI2 (desumoylating isopeptidase 2; Fig. 2B), ICP27 induced partial retention of intron 1 with read counts declining abruptly not at PASs, but at potential 5′ splice site sequences. The sequences between the usual and the cryptic donor splice sequences encode alternative exons that have not been previously described. Two of these 12 genes, LEPR (a leptin receptor involved in fat metabolism) and PPP1R8 (an inhibitor subunit of the major nuclear protein phosphatase-1 required for cell proliferation), are sometimes prematurely terminated at a PAS downstream of the cryptic 5′ splice site (Table S1). In all 12 genes, the impacted 5′ splice sites were within a short distance of the TSS (<1 Kb). Use of these alternative 5′ splice sites was confirmed by RT-PCR and by sequencing of HEK293 cells infected with wild-type HSV-1 and ICP27 mutants (Fig. 2C), showing that the cryptic ZER1 splice site is at nucleotide 772 and that DESI2 has two downstream cryptic splice sites, at nucleotides 979 and 991 (used at a 7:1 ratio, consistent with the read counts shown in Fig. 2B). Use of the cryptic 5′ splice site at nucleotide 772 changes the 5′ UTR sequence of ZER1, whereas use of either cryptic 5′ splice sites changes the expected coding sequence for DESI2 (Fig. 2C). The ICP27 RGG domain-deleted HSV-1 mutant virus (d4-5) promoted the use of the alternative 5′ splice site in ZER1 more efficiently than that in DESI2, suggesting an additional role of the RGG RNA binding domain in regulating alternative splicing of DESI2.
Fig. 2.
ICP27 promotes use of cryptic 5′ splice sites. (A and B) Read counts mapping to ZER1 (negative strand; A) and DESI2 (positive strand; B). Previously described transcript variants (thick black lines denote exons) are shown below. Arrows denote differences in intron 1 between ICP27 and control-transfected cells. (C) RT-PCR for ZER1 and DESI2 of HSV-1–infected and mutant virus-infected (Fig. 1E) HEK293 cells. Cryptic 5′ splice sites were confirmed by sequencing of RT-PCR products and are illustrated in red. The same set of cDNAs were used for both Left and Right. NIC, noninfected control.
ICP27 Only Inhibits Splicing of Select Introns in Targeted Genes.
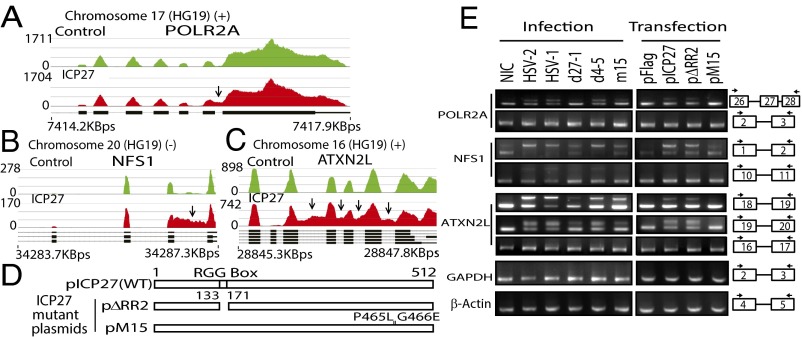
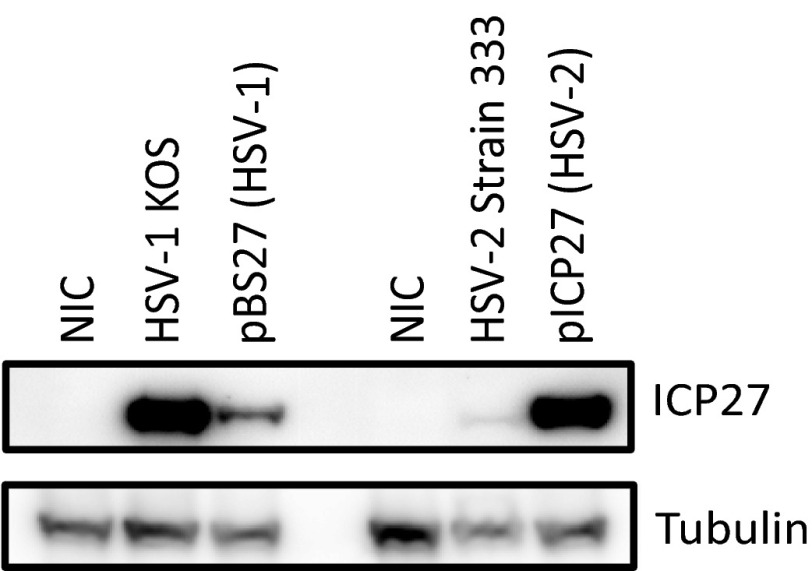
In the 78 genes in which ICP27 induced retention of one or more introns, the first and last introns appeared to be most susceptible. For example, ICP27 inhibited splicing of the last intron of POLR2A (encoding the large subunit of RNA polymerase II) (Fig. 3A), introducing a frameshift and a stop codon upstream of the final exon, which encodes a C-terminal domain previously described to interact with splicing factors, polyadenylation factors, and transactivating factors and with ICP27 itself (3, 24). Through retention of the last intron, ICP27 likely reduces functional POLR2A expression, and contributes to ICP27-mediated alteration of POLR2A functions (3, 25). ICP27 also promotes retention of the first intron of NFS1, a cysteine desulfurase related to protein dimerization activity (Fig. 3B). ICP27 induced retention of four introns (16–19) near the 3′ end of ATXN2L (ataxin-2-like), a regulator of stress granules that is also implicated in neurodegenerative disorders (26) (Fig. 3C). It appears that viral infection (vs. transfection of ICP27 alone) may be more efficient in inhibiting splicing, an observation that is not explained by differences in ICP27 protein levels between transfected vs. infected cells (Fig. S4), suggesting that other viral proteins or the microenvironment created by viral infection may facilitate ICP27’s function. Deletion of ICP27 (d27-1) or a two-amino-acid mutation in the C-terminal domain (m15 for the mutant virus or pM15 for the mutant plasmid) nearly abolished ICP27-mediated splicing inhibition of NFS1 in both virus infection and transfection experiments (Fig. 3 D and E). Deletion of the N-terminal RGG/SRPK-1 binding domain in viral mutant d4-5 reduced ICP27-mediated intron retention, but not to the extent of the C-terminal (m15) mutation.
Fig. 3.
ICP27-induced retention of specific introns in some host genes. (A–C) Read counts mapping to POLR2A (last intron retention; A), NFS1 (intron 1 retention; B), and ATXN2L (multiple internal intron retention; C). Arrows indicate significant differences in read counts. (D) Schematic diagrams of inserts in HSV-2 ICP27 expression plasmids. (E) Effect of ICP27 mutations in HSV-1 viruses (Fig. 1E) and HSV-2 plasmids on intron retention of cellular genes by RT-PCR of infected (8 hpi) or transfected HEK293 cells. Arrows denote RT-PCR primers (Table S2). Exons are numbered in boxes. NIC, noninfected control.
Fig. S4.
Comparison of ICP27 expression level in ICP27-transfected cells and HSV-infected cells by Western blot. The 293 cells were transfected with pBS27 (containing the entire HSV-1 ICP27 coding and promoter sequence) and pICP27 (containing HSV-2 ICP27 coding sequence under a CMV-IE promoter) for 24 h, respectively, or infected with HSV-1 KOS and HSV-2 strain 333 at a MOI of 3 for 5 h, respectively, before Western blotting for ICP27. A HSV-1/2 ICP27 specific antibody (Santa Cruz Biotechnology) was used to detect ICP27.
ICP27-Targeted Genes Are GC-Rich, with Suboptimal Splicing Sites and C-Rich Sequences Near the 5′ Splice Site.
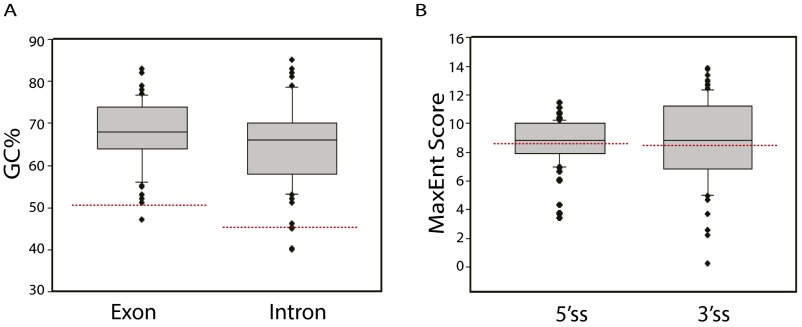
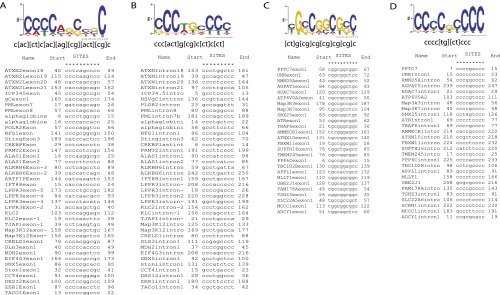
ICP27-mediated alternative pre-mRNA processing occurred only in relatively less abundant transcripts [based on fpkm (reads), the three most abundant ICP27-targeted mRNA transcripts in the RNA-seq experiment were ranked 283 for MDH2, 946 for YWHAH, and 1,882 for ZNF598). The GC content of analyzed ICP27-targeted host gene introns and exons near the impacted splice site averaged 64.5% and 68.0%, respectively, similar to that of HSV genes and much higher than that of typical human introns (46%) and exons (51%) (Fig. S5A; ref. 27). No example of a consensus 5′ or 3′ splice site was observed in an ICP27-targeted intron, suggesting that, although the average strength for both 5′ and 3′ splice sites was comparable to that of typical splice sites in human genes (Fig. S5B), ICP27-targeted splice sites are suboptimal (as are many human splicing sites). Indeed, we observed that ICP27-targeted introns are normally spliced efficiently when ICP27 is not present. Analysis using MEME GLAM2 software identified C-rich consensus sequences containing a stretch of cytosines such as CCCC(U) in exon (Fig. S6A) and/or intron (Fig. S6B) sequences near the 5′ splice site of genes for which splicing is inhibited by ICP27. In genes for which ICP27 activated intronic PAS, intronic cytosine stretches were more common (Fig. S6D) than were exonic cytosine stretches (Fig. S6C), suggesting that intronic cytosines may play a more important role in polyadenylation from intronic PAS of these transcripts in the presence of ICP27.
Fig. S5.
ICP27-targeted genes are GC-rich and splice sites are comparable in strength to typical human gene splice sites. (A) The GC content of exon sequences (the intron-proximal 250 bp of the first affected exon or the entire first affected exon sequences if <250 bp), and the GC content of intron sequences (first 250 bp of the affected intron sequences or the entire intron sequences if <250 bp) of 58 ICP27-targeted genes including all genes listed in Table S1 and known ICP27-targeted genes (including ICP34.5, gC and alpha globin) were analyzed. For ICP27-mediated retention of multiple introns, only the most significantly impacted intron was included in the analysis. The median exon GC% and intron GC% was 68.0% and 66.0%, respectively, considerably higher than the GC% of typical human exons (51%) and introns (46%) (shown with red dashed lines). The average exon GC% and intron GC% was 68.0% with 95% CI [65.8%, 70.0%] and 64.5% with 95% CI [62.0%, 67.1%], respectively. (B) Splice sites of ICP27-targeted genes are comparable in strength to the median human gene splice site. The median MaxEnt Score of the 5′ splice site and 3′ splice site of the 58 ICP27 targeted genes was 8.81 and 8.85, respectively, which is comparable to the median MaxEnt score for human 5′ and 3′ splice sites of 8.54 and 8.85, respectively, which are indicated as red dashed lines.
Fig. S6.
Identification of consensus sequences in ICP27 targeted regions. Consensus sequences were identified in the ICP27 targeted genes listed in Table S1, using MEME GLAM2 motif identification and alignment software. (A) Consensus sequences identified from the 5′ exons of genes for which pre-mRNA splicing was inhibited by ICP27. (B) Motifs identified from the introns of genes for which pre-mRNA splicing was inhibited by ICP27. (C) Motifs identified from the 5′ exons of genes for which an intronic PAS was activated by ICP27. (D) Motifs identified from the introns of genes for which an intronic PAS was activated by ICP27. Consensus sequences and alignments are shown underneath the graph. Start and End indicate the relative position of the predicted sequence in the 250 bp sequences upstream or downstream of the 5′ splice site.
Splicing Inhibition Mediated by ICP27 and Cytosine-Rich Sequences Does Not Require the ICP27 N-Terminal RGG Motif.
ICP27 increased the unspliced to spliced ratio of a chimeric mRNA in which the C-rich HSV-2 ICP34.5 intron was replaced with the similarly sized intron 2 from the ICP27-insensitive KSHV K8 gene (Fig. 4 A and B), whereas neither HSV-1 nor HSV-2 ICP27 significantly inhibited splicing of mutant chimeric mRNAs in which ICP34.5 exon 1 was also replaced with corresponding KSHV K8 exon 2 sequences or in which point mutations of cytosines in ICP34.5 exon 1 were introduced (Fig. 4B). Mutation of ATXN2L exon 18 C-rich sequences, whether immediately upstream of the 5′ splice site or further upstream, sharply reduced ICP27-mediated intron 18 splicing inhibition in reporter assays, whereas mutation of C-rich sequences in intron 18 or in downstream exon 19 did not (Fig. 4 C and D). Together, these results indicate that exonic C-rich sequences near the 5′ splice site are more important for ICP27-mediated splicing inhibition than intronic sequences. KSHV K8 intron 2 is normally alternatively spliced and contains suboptimal splicing sites (28, 29). Splicing in a KSHV K8 splicing reporter containing both K8 introns 1 and 2 is not inhibited by ICP27 (18). Introduction of cytosines by G to C and A to C mutations in the K8 exon 2 sequence upstream of the 5′ splice site in pK8ccct (Fig. 4E), greatly increased its sensitivity to ICP27-mediated splicing inhibition (Fig. 4F), further confirming that C-rich sequences near the 5′ splice site are involved in ICP27-mediated splicing inhibition. Additionally, an ICP27-expressing plasmid mutant with deletion of the ICP27 N-terminal RGG/SRPK-1 motif and adjacent downstream potential RNA binding sequences was nearly as efficient as wild-type ICP27 in inhibiting pK8ccct mutant splicing, further indicating that ICP27 interactions with the RNA sequence and SRPK-1 through the RGG motif are not required for ICP27-mediated specific splicing inhibition.
Fig. 4.
Suboptimal splice sites and C-rich sequences mediate splicing inhibition by ICP27. (A, C, E, and G) Reporters used in B, D, F, and H, respectively, which show splicing analysis by RT-PCR of cells also transfected with HSV-2 ICP27 constructs (Fig. 3D) or HSV-1 ICP27 (pBS27). (B) Splicing analysis of KSHV K8/HSV-2 ICP34.5 constructs. ICP27-mediated splicing inhibition requires C-rich sequences in the 5′ exon of pICP34.5-K8. (D) Splicing analysis of ATXNL mutant constructs. ICP27-mediated splicing inhibition requires C-rich sequences (mutations shown by X) in the 5′ exon of pATXN2L-18-19. (F) Splicing analysis of KSHV K8 exon 2 mutations. Introducing C mutations (at X) in the 5′ exon of an ICP27-insensitive reporter enhances splicing inhibition by ICP27, independently of the N-terminal RGG motif. (H) Splicing analysis of ATXN2L mutants. Optimizing ATXN2L intron 18 splice sites abolishes ICP27-mediated splicing inhibition. (I) Proposed mechanism of ICP27-mediated cotranscriptional aberrant pre-mRNA processing. ICP27 (known to interact with U1 snRNP, U2 snRNP and the Pol II CTD) may prevent U1 binding to 5′ splice sites near C-rich sequences, causing inefficient spliceosome assembly and relief of U1-snRNP-mediated inhibition of CPSF binding to intronic PAS. CPSF, cleavage and polyadenylation specificity factor, Pol II CTD, RNA polymerase II C-terminal domain. Mutations are colored; arrows denote RT-PCR primers.
Suboptimal Splice Sites Contribute to ICP27-Mediated Splicing Inhibition.
Replacement of the suboptimal ATXN2L intron 18 5′ and 3′ splice sites with consensus sequences moderately increased basal splicing efficiency in the absence of ICP27, but nearly abolished ICP27-mediated splicing inhibition (Fig. 4 G and H). This finding suggests that the suboptimal splice sites that flank all of the identified ICP27-targeted introns are required for efficient ICP27-mediated splicing inhibition, which is also in agreement with a previous report that optimization of PML intron 7 splicing sites abolished its sensitivity to ICP27-mediated splicing inhibition (20).
Discussion
HSV-1 and -2 ICP27 modify the pre-mRNA processing of a select group of cellular genes, leading to use of cryptic intronic PAS, use of downstream cryptic 5′ splice sites, and retention of specific introns, reducing the expression of targeted genes while increasing the protein coding diversity of these genes. Both the N-terminal RGG domain and the C-terminal domain of ICP27 are required for efficient use of intronic PAS, with the C-terminal domain being apparently more important for regulating alternative splicing. Shared sequence elements (suboptimal splice sites and C-rich sequences near the 5′ splice site) and the reduced use of a specific 5′ splice site in all cases of these ICP27-mediated effects suggest that different forms of ICP27-mediated aberrant pre-mRNA processing likely have overlapping mechanisms.
Our results confirm ICP27’s role in cotranscriptional cellular pre-mRNA splicing and polyadenylation of specific transcripts, consistent with the results using splicing reporters (Figs. S7 and S8). Our findings, including identification of prematurely cleaved and polyadenylated transcripts by Northern hybridization in wild type, but not in ICP27 deletion mutant virus-infected cells, would not have been predicted by a recent report (14), which posited that ICP27 had no role in regulating cellular cotranscriptional pre-mRNA splicing or termination of cellular transcripts.
Fig. S7.
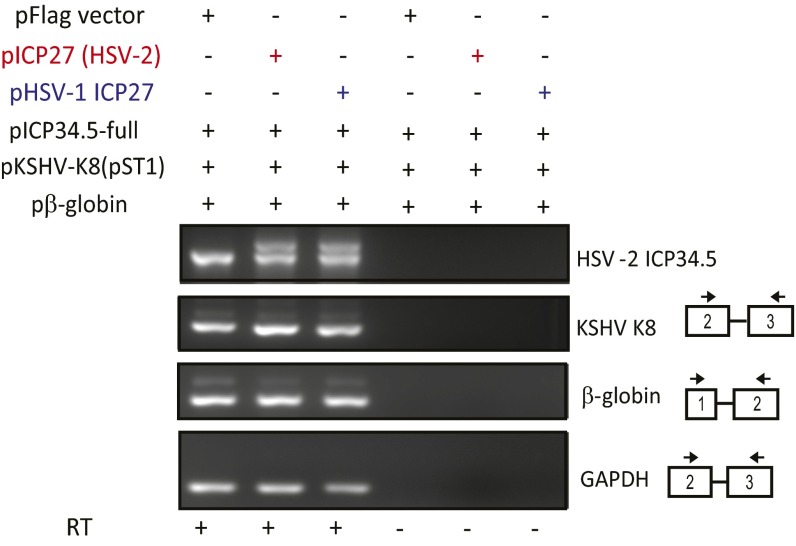
Cotranscriptional splicing of β-globin pre-mRNA is not inhibited by ICP27. The 293 cells were transfected with a β-globin expression plasmid (pβ-globin), ICP34.5 expression plasmid (pICP34.5-full), and KSHV K8 expression plasmid (pST1) together with the HSV-1 ICP27 expression plasmid (pHSV-1 ICP27), the HSV-2 ICP27 expression plasmid (pICP27), or pFlag vector. cDNAs were prepared from the total RNAs extracted from transfected cells with or without reverse transcription. RT-PCRs were performed by using specific primers (Table S2) using the same cDNA prepared from the total RNAs extracted from transfected cells.
Fig. S8.
HSV-1 inhibits cotranscriptional splicing of HSV-2 ICP34.5. The 293 cells were first transfected with the ICP34.5 expression plasmid (pICP34.5-full). Six hours after transfection, cells were infected with HSV-1 or ICP27 mutants. Both wild-type HSV-1 and d1-2, a HSV-1 mutant with deletion of the N-terminal acid region (with deletion of amino acids 12–63), efficiently inhibited ICP34.5 pre-mRNA splicing (Top) and promoted expression of ICP34.5β protein (Middle). Deletion of the RGG motif (d4-5) modestly reduced the efficiency of ICP27-mediated splicing inhibition but dramatically reduced the expression of ICP34.5 β protein. Mutation of two amino acids of the C-terminal domain (m15) nearly abolished ICP27-mediated splicing inhibition of ICP34.5 and expression of ICP34.5β protein.
In vitro polyadenylation experiments suggested that ICP27 is involved in promoting polyadenylation from “weak” PASs of late genes, including UL44 (glycoprotein C) (30–33), suggesting that ICP27 likely directly influences both polyadenylation and splicing. ICP27’s impact on polyadenylation from intronic PAS typically located within 1 kb of the 5′ splice site mirrors that recently observed when U1 snRNP’s binding to the 5′ splice site was inhibited, also relieving its inhibition of CPSF binding to the downstream PAS (34–36). We hypothesize that ICP27 may thus interfere with U1 snRNP’s binding to 5′ splice sites in the context of specific introns, through direct or indirect interaction with the C-rich sequences near the 5′ splice site (Fig. 4I). Recent crystal structure studies demonstrated that the structure of ICP27 does not have KH domains and that its C-terminal region does not fold into a potentially RNA-binding hnRNPK-like structure (4, 5). ICP27’s RGG motif has been shown to directly bind RNA (37, 38) and appears to play a significant role in alternative polyadenylation and a lesser role in splicing inhibition. However, our in vitro transfection experiments and previous reports (18, 20) showing that the RGG motif is not required for ICP27-mediated splicing inhibition suggest that there may be other RNA binding sites in ICP27 or that unknown adaptor proteins are involved in recognizing the C-rich sequences near the 5′ splice site. We also note that the precise nature of the C-rich sequences important for ICP27 effects has not yet been defined.
For LEPR and PPR1R8 (Table S1), some RNAs were alternatively polyadenylated using intronic PAS, and others used an alternative 5′ splice site, suggesting that the relative kinetics of splicing and polyadenylation are important for alternative polyadenylation, as has been hypothesized (39). Thus, it appears that the fate of ICP27-targeted pre-mRNA is determined by the strength and proximity of splice sites, availability of C-rich sequences near the 5′ splice site, availability and proximity of an intronic PAS, the size of the intron (with larger introns more likely to show use of an alternative 5′ splice site or intronic PAS, and with smaller introns more likely to be retained), and efficiency of RNA polymerase II transcription (i.e., reduced efficiency or “pausing” of RNA polymerase II at the TSS or at suboptimal 3′ splice sites favors alternative polyadenylation). Because ICP27 appears to target less abundant transcripts and expression of many genes is tissue-specific, it is possible that ICP27 has tissue-specific targets.
ICP27-induced aberrant pre-mRNA processing likely leads to reduced expression of many affected cellular genes and alteration in the UTR sequence of other cellular transcripts that may alter mRNA stability. ICP27-induced aberrant pre-mRNA processing likely also leads to expression of novel truncated or frameshifted host cell proteins, expanding the genomic material available to the virus. Although aberrant pre-mRNAs containing premature termination codons (PTCs) are often subjected to degradation via nonsense-mediated decay (NMD) (40), at least some ICP27-mediated aberrant pre-mRNAs contain PTCs are able to escape NMD and express proteins, including full-length glycoprotein C and HSV-2 ICP34.5β (16, 18). It thus seems likely that at least some of these host transcripts can also express novel proteins. Recent studies suggested that the virion host shutoff-RNase (vhs) protein, previously thought to nonspecifically degrade host and viral mRNAs, more selectively targets specific host mRNAs, but not GC-rich viral mRNAs (41, 42). Because the GC content of ICP27-targeted genes is similar to that of HSV genes, they also likely escape selective degradation by vhs. Thus, by specifically modifying pre-mRNA processing of HSV-like GC-rich transcripts that are likely spared by the virion host shutoff protein, ICP27 contributes to virus-induced host shutoff required for efficient viral growth.
ICP27 affects pre-mRNA processing of >200 genes in ICP27-transfected cells involved in important cellular pathways, implying a broad program of ICP27-mediated cellular modification to favor the virus, and helping to explain the observation that ICP27 expression is toxic to the cell and is both required for efficient virus growth and for severe symptoms (43–45). Of the affected genes, >30, including PML, STING, TRAF6, PPP6C, MAP3K7, FBXW11, IFNAR2, NFKB1, RELA, and CREBP, are related to innate immunity pathways, which is consistent with ICP27’s known role in regulating innate immunity (46–49). Although it would not be practical to separately examine these effects in each of these genes, it seems likely that the combined effect of these alterations exceeds that of any one. It has been reported that ICP27-induced intron retention in PML appears to alter viral growth (20), that alternative splicing in viral gC plays an important role in viral immune evasion by regulating the relative expression of full-length and secreted forms of gC (16), and that ICP27 alters viral neurovirulence through inhibition of HSV-2 ICP34.5 splicing (18).
Although HSV is the first virus and ICP27 is the first viral or cellular protein shown to promote expression of pre-mRNAs prematurely cleaved and polyadenylated from intronic PAS, we suspect that other viruses or unidentified cellular genes also encode this function. Further investigation will likely yield insight both into mechanisms of viral pathogenesis, potentially leading to identification of new targets for antiviral strategies, and into the mechanisms by which the cell itself controls alternative polyadenylation and splicing of selected genes. ICP27 could also potentially be used as a template for future design of proteins that influence cellular gene expression in this manner.
Methods
HEK293 cells were transfected with pICP27 or pFlag vector by using Lipofectamine 2000. More than 95% transfection efficiency was achieved, as determined by fluorescence microscopy of cells transfected with the same amount of pEGFP-C1 (Clontech). At 48 h after transfection, total RNAs were purified with the All-Prep DNA/RNA Kit (Qiagen). cDNA libraries were prepared from polyadenylated RNA by using the Truseq RNA Sample Kit V2 (Illumina) and were sequenced on the HiSeq 2500 according to the manufacturer’s instructions (Illumina). The two samples shared a single sequencer lane. The resulting paired-end sequencing data were first aligned to the HG19 reference human genome by using Partek Flow and then further analyzed by using the Partek Genomics Suite according to the software instructions. A total of 19,655 genes were selected after applying expression-level filters (≥0.5 fpkm) for both the control (pFlag vector-transfected sample) and the ICP27 (ICP27-transfected sample) from a total of 45,000 identified genes. Genes were ranked by scores of differential expression. The expression profile of each of the first 12,000 genes for both control and ICP27 samples was visually examined. Other methods are described in SI Materials and Methods.
Supporting information includes SI Materials and Methods, Figs. S1–S8, and Tables S1 and S2.
Table S2.
Sequences of oligonucleotide primers, probes and point mutations for splicing reporter genes
| Gene and relative location | Name/additional details | Sequence |
| ATXN2L exon 17 forward | oST919 | GCTCGGACCAACACCAGCCAG |
| ATXN2L exon 18 backward | oST920 | GGTAGAGGATGACACGATGGCCTG |
| ATXN2L exon 19 forward | oST921 | CCAGGCCATCGTGTCATCCTCTAC |
| ATXN2L exon 20 backward | oST922 | TGCGGCTGGCTTCCAGTAGG |
| ATNX2L exon 16 forward | oST949 | CCATCCCGGTGCTGACAGCAG |
| ATNX2L exon 17 backward | oST950 | CCAGGCACTGAATTGGATACAGGAT |
| pFlag vector specific primer forward | oST973 | TTTGTAGTCAGCCCGGGATCCTC |
| pFlag vector specific primer backward | oST995 | AGAGCTCGTTTAGTGAACCGTCAGA |
| POLR2 exon 26 forward | oST909 | TGCCATGACACCTTGGAACCA |
| POLR2 exon 28 backward | osT910 | GCAGGTGACGTTGGCGAGTAG |
| POLR2 exon 2 forward | oST917 | ACTGGCCGCTGCCAAACATGTG |
| POLR2 exon 3 backward | oST918 | AGAGTCCACAAGCAGTTTGGAGCA |
| NFS1 exon 1 forward | oST961 | GGCGGTGACAGCGGCTCCAG |
| NFS1 exon 2 backward | oST962 | CATCCATATAGAGAGGTCGCAGCAC |
| NFS1 exon 10 forward | oST981 | TATGTGGAAGGGGAAAGTCTGCTGA |
| NFS1 exon 11 backward | oST982 | CTGATAGAAGAGTGCGCTAAATCCTC |
| ZER1 exon 1 forward | oST989 | CTTAGAAGCTCAAGGACGACTTGGA |
| ZER1 exon 2 backward | oST990 | CAGTACAGAGGGCCATCAGCGA |
| DESI2 exon 1 forward | oST985 | GACGCTCCGGTGAACCCAGT |
| DESI2 exon 2 backward | oST986 | CTCCAATTCCAATGGATGAGGTATAT |
| EIF4G3 exon 1 forward | oST945 | GACTGCTGGAGGCGGCCACA |
| EIF4G3 exon 2 backward | oST946 | GCCGGGTCCGGTTCCTGCTG |
| EIF4G3 exon 2 forward | oST947 | AGCAGGAACCGGACCCGGCA |
| EIF4G3 Intron 2 backward | oST948 | CTGTCCCTTTCCTGGCTGGGCT |
| ICP34.5 exon 1 (-90) forward | oST708 | CTGCGCACCACGACGGAGTA |
| ICp34.5 exon 2 (29) backward | oST430 | CCGCGCGTGCAGGTGCG |
| KSHV K8 exon 2 (-90) forward | oST838 | GAAGTATGTGATCAGTCACATTCT |
| GAPDH exon 2 forward | oST726 | TACATGTTCCAATATGATTC |
| GAPDH exon 3 backward | oST727 | GTGGACTCCACGACGTACTC |
| β-actin exon 4 forward | oST728 | GACCTGTACGCCAACACAG |
| β-actin exon 4 backward | oST729 | TCGTCATACTCCTGCTTGC |
| pATXN2L-C1-2M | Mutations in exon 17 C-rich sequence (-170-1) | gctcggaccaacaccagccagcaacagcgacgccgatgatgcaggcagcggcggctgctggcacgcagatggtggctgccacgcaagattctgactacatccaatacaacaatcagcagttaacaggccagcaagcaatgatgcagcacatggcagactacgagacacag |
| pATXN2L-IN-M | Mutations in intron 17 C-rich sequence (1-364) | gtgactgcggcccaggagggcagtgaggatacagggcaactgctagggataactctaaaccagagacttgggagctggctaggggtggcaggcagtgttgtaggtgggatcggcaatctgtggtattggcggtgtcagacttgggcttgagcaatggctctggtggtacctgtaacaaggcattggacatctgtatctctgaagtgtagagaaaatagtgtctgctgggtgggatcgttatgaatgttgaatcaatagggtgattgtgaggaggcccaagcggtgctgtgcacgcagtgactggcaggaggacaccttcccagctggcggctgtgccaaccactcctctctctgtcccgccag |
| Mutations in C1-2-IN-M | Contains combined mutations from both pATXN2L-C1-2M and pATNX2L-IN-M | See above |
| Mutations in C1-2-IN-E-M | Contains mutations in C1-2-IN-M and additional mutations (shown) of C-rich sequences in exon 18 | ccggtgtttgcaaacatgcttcagagcaacccacgcatgctgacgtcgggcagccatcaacaggccatcgtgtcataatctacc |
| KSHV K8 exon 2 (-90) | oST1010, primer for pK8wt and pK8cct | AAGCTT/gaagtatgtgatcagtcacattct |
| KSHV K8 exon 3 (-90) | oST1011, backward primer | CAGCATGTCGCGAAGGAAAATAATC |
| Synthesized insertion for pK8wt | Contains partial wild-type sequence of KSHV K8 exon2, intron 2 and exon 3. | aattAAGCTT/Gaagtatgtgatcagtcacattctcccacgcgaaagcaaggcagatacggccgcgtgtcatcgaaagcatacacaagacagctgcagcag/gtatagacgggaaacaggtgtctatcttggccggctggttactcaaatgggaacaatggcgccaccttgctgtctttgtag/gcattagaagaaaaggatgcacaactatgtttcctagcggcgagattggaggcacataaggaacagattattttccttcgcgacatgctg/GAATTCtaa |
| Synthesized insertion for pK8ccct | Contains mutations upstream of 5′ss | aattAAGCTT/GaagtatgtgatcagtcacattctcccacgcgaaagcaaggcagatacggccCcCtgtcCtccccaCcCtCcCcaagacCCctCcCgcag/gtatagacgggaaacaggtgtctatcttggccggctggttactcaaatgggaacaatggcgccaccttgctgtctttgtag/gcattagaagaaaaggatgcacaactatgtttcctagcggcgagattggaggcacataaggaacagattattttccttcgcgacatgctg/GAATTCtaa |
| PPTC7 | Synthesized DNA template used to make Northern blot probe. | acacctgcaggctcctaacctcgttctggttccctctccgcatgccccggggatccctgcctcttcgcgtgctcccgacaccacagcccggcccaggctgcgggatcgccggccgacaggcagtcgtgagcccccagacagcccggccgtattctttcgccgcctggatggtaaccaaatcttcacccttttcggagtgtggcggtgggggctgctggcttggcgaaaaaaccctcacagcgttttctcccaaattgatcttgtccacttgcggtcacttgggggttggcagagtctccttgatccaaaatagaatggtcgagcctacttgtagattgcagccggtaaagctctgaggattggtcacagccttttcaggccacaggtgctccctgaggcctattccagttataccttttgggggtgggagactacggagagagctggcagaaagggctaaatatagttcacaagcagtcagaaactttaccataatgta |
| TMEM245 | Synthesized DNA template used to make Northern blot probe. | caggaatttggcggacattcttccaaaaagggggcgggcgtgatttgaaacagcctccctgcttttcgccttgtttcaatcctaataggccctcttagggggacagtgccctggattgagaatcgttatttcgctttgcagagagaggcctcagagcatggcagtggggcttggttttacttctggttggagagggcgggggtgcttcggccaccctcagttctgcgcatctgaatgtcagagctggaagggaccttagagattctaggccaacttgccatcctgcagataggaaaggtgacctcttaccaggctagggctacttagggacaaagaggggtttgggtgtcttagatttcttacaagtcctaaaacaaagcttgcttcactggctggtgctgcctctcagttcaggtgattcctgtataatttgtgcaaggcactgggtgctgagctgcctgtgcttcttcctctccacgctttacttttaggtaactgc |
| UNK | Synthesized DNA template used to make Northern blot probe. | tcctcagaccataaaaccacagtagtaggctcctttggggcagtgagaagtccaagacacaaccaagaggagttccgtggggtccagccccagaacccacctatgaatgatgattaacgtgaagtcctgtcaggagattccgtgcagtttgcgcaggaagccttttgtgtccctaaggctcgtaggttgtccacgagggccacacttgcttgaattcacagtcgacgtagataaacatgcaaagatggaaacgtgtccgtgcgtgcccatgaccgtttccctcgcgggctgtgcggagcctgagtctgggagcccgaaggtgatgggttccaagtatgtctgcaggctcagcagaaatgtagcattagagggagccccaaatactgacactacaggagtccagcaggatgggaaaacctcttcctggaagtcttgcatctgtgtgcttttaggtcgctgtcttcatttccttcaagccacgttaagcattccctagagt |
| PPP6C | Synthesized DNA template used to make Northern blot probe. | agggcctcccccattgccagtcgacccggtcctacccggcgtcacgccgagggtctcgcgagccgagtcgtcgaatgctgtcccggttgcctcagtggggatcccgagggatgtcgcggcctctgtcccagggtcgccccttctggccttcgggctgccccaggacccgcagctgtaacagctttatttagaaggctcggctccccaccccatccctgggtctcccgcagtcggagccgagcccccgcggcagtgccctcgggatggggtcgcctccaccggaggtgacaggagcgcggagtggccggcgtctgacaggagatgccaccggctaccggaacgcggcctcagtgtgtttaggtctctgaggaaggggaaggcggtgggcccgagtggtttggtactggcgggagcgaacgtcaggtcgtccctcttcattgccactcctccaaattgctttttaggatcgtcggttttgctaattcagaggtgaatcccg |
| β-actin | Synthesized DNA template used to make Northern blot probe. | accatggatgatgatatcgccgcgctcgtcgtcgacaacggctccggcatgtgcaaggccggcttcgcgggcgacgatgccccccgggccgtcttcccctccatcgtggggcgccccaggcaccagggcgtgatggtgggcatgggtcagaaggattcctatgtgggcgacgaggcccagagcaagagaggcatcctcaccctgaagtaccccatcgagcacggcatcgtcaccaactgggacgacatggagaaaatctggcaccacaccttctacaatgagctgcgtgtggctcccgaggagcaccccgtgctgctgaccgaggcccccctgaaccccaaggccaaccgcgagaagatgacccagatcatgtttgagaccttcaacaccccagccatgtacgttgctatccaggctgtgctatccctgtacgcctctggccgtaccactggcatcgtgatggactccggtgacggggtcacccacactgtgcccatc |
| β-globin | oST821, forward primer for cloning and RT-PCR | cacc/atggtgcatctgactcctgagga |
| β-globin | oST822, backward primer for cloning the 1.6 Kb β-globin gene | gtgatacttgtgggccagggc |
| β-globin | oST823, backward primer for RT-PCR | cctgaagttctcaggatccacgt |
Mutations are shown underlined and in boldface type.
SI Materials and Methods
Cells, Viruses, and Antibodies.
HSV-2 strain HG52 (GenBank accession no. NC_001798) and HSV-1 strain 17syn+ (GenBank accession no. NC_001806) genomic sequences were used as reference sequences. Vero and HEK 293 cell lines were obtained from ATCC. HSV-2 strain 333 was obtained from Gary Hayward, Johns Hopkins University, Baltimore. HSV-1 strain KOS, HSV-1 mutant viruses including d27-1 (ICP27 deletion mutant with deletion from the ICP27 promoter starting -412 bp relative to the ATG to amino acids 408), d1-2 (with deletion of amino acids 12–63), d4-5 (with deletion of amino acids 139–153), m15 (with substitution of amino acids 465 and 466), the V27 ICP27-complementing Vero cell line used to grow ICP27 mutant viruses, and HSV-1 ICP27 expressing plasmid pBS27 containing the entire HSV-1 ICP27 sequence with the ICP27 promoter were obtained from Stephen Rice, University of Minnesota, Minneapolis (37). Anti–HSV-1/2 ICP27 (Santa Cruz Biotechnology), anti-Flag antibody (Sigma-Aldrich), and anti–beta-tubulin antibody (Santa Cruz Biotechnology) were sourced commercially. Anti–HSV-2 ICP34.5 antibody has been reported (18).
Plasmids, Primers, and Probes.
pICP34.5-full, containing the 5′ UTR, the entire HSV-2 ICP34.5 coding region and its stop codon, and pICP27, containing the HSV-2 ICP27 coding region under a CMV IE promoter, have been reported (6). HSV-2 ICP27 mutant plasmids including pΔRR2 (with deletion of RGG and the adjacent RNA binding sequence from aa 133–171) and pM15 (with a two amino acid mutation, Pro-466–Leu and Gly-467–Glu) at the previously predicted KH3 domain were obtained from Masatoshi Hagiwara and Takayuki Nojima, Tokyo Medical and Dental University, Tokyo (18). pKSHV-K8 (pST1) was obtained from Zhiming Zheng, National Institutes of Health, Bethesda (28). Primers, mutations, and probes used are listed in Table S2. pATXN2L-18-19 was constructed by inserting a synthesized DNA fragment containing partial ATNX2L exon 18 sequences (170 bp), intron 18 sequences (364 bp), and partial exon 19 sequences (89 bp) into the pFlag vector at the EcoRI site. Similarly, ATXN2L-C1M and ATXN2L-C1-2M were also constructed by cloning the synthesized DNA fragment with mutations in C-rich regions into the pFlag vector. Mutations made in ATXN2L-C1M are shown in Fig. 4C. Additional mutations in the upstream C-rich sequences of exon 18 were introduced in ATXN2L-C1-2M. ATXN2L-IN-M containing mutations in C-rich regions in intron 18, C1-IN-M containing mutations made in ATXN2L-C1-2M and ATXN2L-IN-M, and C1-2-IN-E-M containing mutations made in C1-2-IN-M and additional mutations in the C-rich region in exon 19 were constructed similarly (for details, see Table S2). Similarly, mutant plasmid M-5ss with optimization of the 5′ splice site, M-3ss with optimization of the 3′ splice site and M-5–3ss with optimization of both 5′ and 3′ splice sites were also constructed by cloning the synthesized DNA fragment into the pFlag vector. The exact mutations are labeled in Fig. 4E. pICP34.5(−90+29) was cloned by inserting the ICP34.5 sequence amplified by oST708 and oST430 into pFlag vector at the EcoRI site. pICP34.5-K8 was constructed by synthesizing (Origene) 90 bp HSV-2 ICP34.5 exon 1 sequences, the KSHV K8 exon 2 sequences and 29 bp of ICP34.5 exon 2 sequences and insertion into the pFlag vector at the EcoRI site. pK8exon2, containing 90 bp of K8 exon2, K8 intron 2 and 29 bp of ICP34.5 exon 2 was constructed analogously. C-Box1M and C-Box1-2M containing mutations (shown in Fig. 4A) in a C-rich region of ICP34.5 exon 1 were also constructed similarly. pK8wt and pK8ccct were constructed by cloning a synthesized insert (IDT DNA) containing 90 bp of the KSHV K8 exon 2 sequence (also including G to C and A to C mutations as shown in Table S2 for pK8ccct), K8 intron 2 and 90 bp of exon 3 sequences, into pFlag vector using the Hind III and EcoR I sites. A PCR product containing full-length β-globin coding sequences including introns was cloned into the pFlag vector to make pβ-globin.
Transfection, Viral Infection, and RT-PCR.
A total of 1.5 million 293 cells were transfected in six-well plates with various plasmids by using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Similarly, 1.5 million 293 cells were infected with wild-type or HSV mutants at a MOI of 3.0. cDNA was made by using the first-strand cDNA kit (Invitrogen) using 2 μg of total RNA prepared by the All-Prep DNA/RNA Kit (Qiagen) from transfected or infected cells. RT-PCR using the same set of cDNAs and the primers indicated in the figures were performed with the Hot-Star High-fidelity Taq polymerase Kit (Qiagen). Oligonucleotides used in RT-PCR reactions to amplify specific genes are listed in Table S2.
RNA-Seq and Data Analysis.
The 293 cells were transfected with pICP27 or pFlag vector by using Lipofectamine 2000. More than 95% transfection efficiency was achieved, as determined by fluorescence microscopy of cells transfected with the same amount of pEGFP-C1 (Clontech). At 48 h posttransfection, total RNAs were purified with the All-Prep DNA/RNA Kit (Qiagen). cDNA libraries were prepared from polyadenylated RNA by using the Truseq RNA Sample Kit V2 (Illumina) and sequenced on the HiSeq 2500 according to the manufacturer’s instructions (Illumina). The two samples shared a single sequencer lane. The resulting paired-end sequencing data were first aligned to the HG19 reference human genome by using Partek Flow and then further analyzed by using the Partek Genomics Suite according to the software instructions (Partek). A total of 19,655 genes were selected after applying expression level filters (≥0.5 fpkm) for both the control (pFlag Vector-transfected sample) and the ICP27 (ICP27-transfected sample) from a total of 45,000 identified genes. Genes are ranked by scores of differential expression. The expression profile of each of the first 12,000 genes for both control and ICP27 samples was visually examined.
Detection of Cellular RNAs Polyadenylated from Intronic PASs by Northern Hybridization.
Total RNA from 293 cells either uninfected or infected with HSV was purified by TRIzol according to the manufacturer’s instructions (Invitrogen). The cytoplasmic and nuclear fractions were prepared by using the PARIS Kit for the isolation of nuclear and cytoplasmic RNA (Invitrogen) according to the manufacturer’s instructions. Fifteen micrograms of total RNA and the entire amount of cytoplasmic fraction, nuclear fraction, or oligo-dT enriched RNAs were resolved in a formaldehyde denaturing 1.5% (wt/vol) agarose gel. After transfer to GeneScreen Plus hybridization transfer membrane (PerkinElmer), the membrane was UV–cross-linked and incubated in Hybrisol containing 50% (vol/vol) Formamide and 6× SSC (EMD Millipore) at 43 °C overnight with gene-specific probes labeled with [α-32P]-dCTP using a random priming kit (Promega). The sequences of specific DNA probe templates (synthesized by IDT DNA) are shown in Table S2. Some membranes were reprobed after stripping twice in boiling water with 0.1% SDS. For example, after hybridization with the TMEM245 intron 1 specific probe (blot 1) in Fig. 1E, the same membrane was rehybridized with a PPTC7 intron 1 specific probe, and subsequently with a β-actin probe. After hybridization with the UNK probe (blot 2), the other membrane rehybridized with a PPP6C intron 1-specific probe. The bottom shows the ethidium bromide-stained gel, including 28S and 18S rRNA for the first described gel, which appeared identical to that of the second gel. Similarly, after hybridization with the TMEM245 intron 1-specific probe (Fig. 2G), the same membrane was rehybridized with the PPTC7 intron 1-specific probe, and subsequently with the β-actin probe. After hybridization with the PPTC7 intron 1-specific probe (Fig. 2H), the same membrane was rehybridized with an oligonucleotide probe specific to U1 (labeled using T4 polynucleotide kinase and [γ-32P]-ATP) at 43 °C overnight using PerfectHyb Plus hybridization buffer (Sigma-Aldrich) as described (18).
Splice Site Strength Determination and Sequence Analysis Near the 5′ Splice Site.
The strength of the splice site strength of impacted introns was evaluated by using the MaxEntScan program, an online splice site strength analysis tool (genes.mit.edu/burgelab/maxent/Xmaxentscan_scoreseq_acc.html) (50). GLAM2 (Gapped Local Alignment of Motifs) from the MEME Suite (51), an alignment-based motif analysis tools (meme-suite.org/), was used to analyze the sequence of ICP27-targeted genes. A total of 200 bp of the exon upstream of the targeted 5′ splice site or the entire upstream exon for exons <200 bp were used. A total of 250 bp of the intron sequences starting from the 5′ splice site or the entire intron sequences for introns <250 bp were used in the analysis. If the intron sequences included the polypyrimidine tract sequences near the 3′ splice site, 30 bp of sequence from the 3′ splice site were excluded from the intron sequences in the analysis.
Acknowledgments
We thank Drs. Keith Peden and Haruhiko Murata for critical reading of the manuscript; Dr. Rong Wang for performing the RNA-seq; Dr. Haiyan Lei for help with Partek Flow software; Dr. Stephen Rice for providing the HSV-1 ICP27 mutant viruses and expression plasmids and HSV-1 strain KOS; Dr. Masatoshi Hagiwara for providing the HSV-2 ICP27 expression plasmids; and Dr. Zhiming Zheng for providing the KSHV K8 expression plasmid. This study was supported by the Center for Biologics Evaluation and Research’s intramural research program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence data reported in this paper have been deposited in the NCBI Sequence Read Archive (SRA), www.ncbi.nlm.nih.gov/sra (accession no. PRJNA343110).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609695113/-/DCSupplemental.
References
- 1.Roizman B, Knipe DM, Whitley RJ. Herpes simplex viruses. In: Knipe D, Howley PM, editors. Fields Virology. 6th ed. vol 2. Lippincott; Philadelphia: 2013. pp. 1823–1897. [Google Scholar]
- 2.Zhou C, Knipe DM. Association of herpes simplex virus type 1 ICP8 and ICP27 proteins with cellular RNA polymerase II holoenzyme. J Virol. 2002;76(12):5893–5904. doi: 10.1128/JVI.76.12.5893-5904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai-Ju JQ, Li L, Johnson LA, Sandri-Goldin RM. ICP27 interacts with the C-terminal domain of RNA polymerase II and facilitates its recruitment to herpes simplex virus 1 transcription sites, where it undergoes proteasomal degradation during infection. J Virol. 2006;80(7):3567–3581. doi: 10.1128/JVI.80.7.3567-3581.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tunnicliffe RB, et al. The structure of the folded domain from the signature multifunctional protein ICP27 from herpes simplex virus-1 reveals an intertwined dimer. Sci Rep. 2015;5:11234. doi: 10.1038/srep11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel V, et al. Structure of the C-terminal domain of the multifunctional ICP27 protein from herpes simplex virus 1. J Virol. 2015;89(17):8828–8839. doi: 10.1128/JVI.00441-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandri-Goldin RM, Hibbard MK. The herpes simplex virus type 1 regulatory protein ICP27 coimmunoprecipitates with anti-Sm antiserum, and the C terminus appears to be required for this interaction. J Virol. 1996;70(1):108–118. doi: 10.1128/jvi.70.1.108-118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phelan A, Carmo-Fonseca M, McLaughlan J, Lamond AI, Clements JB. A herpes simplex virus type 1 immediate-early gene product, IE63, regulates small nuclear ribonucleoprotein distribution. Proc Natl Acad Sci USA. 1993;90(19):9056–9060. doi: 10.1073/pnas.90.19.9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant HE, Wadd SE, Lamond AI, Silverstein SJ, Clements JB. Herpes simplex virus IE63 (ICP27) protein interacts with spliceosome-associated protein 145 and inhibits splicing prior to the first catalytic step. J Virol. 2001;75(9):4376–4385. doi: 10.1128/JVI.75.9.4376-4385.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escudero-Paunetto L, Li L, Hernandez FP, Sandri-Goldin RM. SR proteins SRp20 and 9G8 contribute to efficient export of herpes simplex virus 1 mRNAs. Virology. 2010;401(2):155–164. doi: 10.1016/j.virol.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandri-Goldin RM, Hibbard MK, Hardwicke MA. The C-terminal repressor region of herpes simplex virus type 1 ICP27 is required for the redistribution of small nuclear ribonucleoprotein particles and splicing factor SC35; however, these alterations are not sufficient to inhibit host cell splicing. J Virol. 1995;69(10):6063–6076. doi: 10.1128/jvi.69.10.6063-6076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sciabica KS, Dai QJ, Sandri-Goldin RM. ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation. EMBO J. 2003;22(7):1608–1619. doi: 10.1093/emboj/cdg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koffa MD, et al. Herpes simplex virus ICP27 protein provides viral mRNAs with access to the cellular mRNA export pathway. EMBO J. 2001;20(20):5769–5778. doi: 10.1093/emboj/20.20.5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mears WE, Rice SA. The herpes simplex virus immediate-early protein ICP27 shuttles between nucleus and cytoplasm. Virology. 1998;242(1):128–137. doi: 10.1006/viro.1997.9006. [DOI] [PubMed] [Google Scholar]
- 14.Rutkowski AJ, et al. Widespread disruption of host transcription termination in HSV-1 infection. Nat Commun. 2015;6:7126. doi: 10.1038/ncomms8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellison KS, Rice SA, Verity R, Smiley JR. Processing of alpha-globin and ICP0 mRNA in cells infected with herpes simplex virus type 1 ICP27 mutants. J Virol. 2000;74(16):7307–7319. doi: 10.1128/jvi.74.16.7307-7319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sedlackova L, et al. Herpes simplex virus type 1 ICP27 regulates expression of a variant, secreted form of glycoprotein C by an intron retention mechanism. J Virol. 2008;82(15):7443–7455. doi: 10.1128/JVI.00388-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park D, Lalli J, Sedlackova-Slavikova L, Rice SA. Functional comparison of herpes simplex virus 1 (HSV-1) and HSV-2 ICP27 homologs reveals a role for ICP27 in virion release. J Virol. 2015;89(5):2892–2905. doi: 10.1128/JVI.02994-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang S, Guo N, Patel A, Krause PR. Herpes simplex virus 2 expresses a novel form of ICP34.5, a major viral neurovirulence factor, through regulated alternative splicing. J Virol. 2013;87(10):5820–5830. doi: 10.1128/JVI.03500-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis KL, Korom M, Morrison LA. Herpes simplex virus 2 ICP34.5 confers neurovirulence by regulating the type I interferon response. Virology. 2014;468-470:330–339. doi: 10.1016/j.virol.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Nojima T, et al. Herpesvirus protein ICP27 switches PML isoform by altering mRNA splicing. Nucleic Acids Res. 2009;37(19):6515–6527. doi: 10.1093/nar/gkp633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murn J, et al. Control of a neuronal morphology program by an RNA-binding zinc finger protein, Unkempt. Genes Dev. 2015;29(5):501–512. doi: 10.1101/gad.258483.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mears WE, Rice SA. The RGG box motif of the herpes simplex virus ICP27 protein mediates an RNA-binding activity and determines in vivo methylation. J Virol. 1996;70(11):7445–7453. doi: 10.1128/jvi.70.11.7445-7453.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcet-Palacios M, et al. Granzyme B inhibits vaccinia virus production through proteolytic cleavage of eukaryotic initiation factor 4 gamma 3. PLoS Pathog. 2011;7(12):e1002447. doi: 10.1371/journal.ppat.1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsin JP, Manley JL. The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev. 2012;26(19):2119–2137. doi: 10.1101/gad.200303.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spencer CA, Dahmus ME, Rice SA. Repression of host RNA polymerase II transcription by herpes simplex virus type 1. J Virol. 1997;71(3):2031–2040. doi: 10.1128/jvi.71.3.2031-2040.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaehler C, et al. Ataxin-2-like is a regulator of stress granules and processing bodies. PLoS One. 2012;7(11):e50134. doi: 10.1371/journal.pone.0050134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu L, et al. Patterns of exon-intron architecture variation of genes in eukaryotic genomes. BMC Genomics. 2009;10:47. doi: 10.1186/1471-2164-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang S, Zheng ZM. Kaposi’s sarcoma-associated herpesvirus K8 exon 3 contains three 5′-splice sites and harbors a K8.1 transcription start site. J Biol Chem. 2002;277(17):14547–14556. doi: 10.1074/jbc.M111308200. [DOI] [PubMed] [Google Scholar]
- 29.Yamanegi K, Tang S, Zheng ZM. Kaposi’s sarcoma-associated herpesvirus K8beta is derived from a spliced intermediate of K8 pre-mRNA and antagonizes K8alpha (K-bZIP) to induce p21 and p53 and blocks K8alpha-CDK2 interaction. J Virol. 2005;79(22):14207–14221. doi: 10.1128/JVI.79.22.14207-14221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobrikova E, Shveygert M, Walters R, Gromeier M. Herpes simplex virus proteins ICP27 and UL47 associate with polyadenylate-binding protein and control its subcellular distribution. J Virol. 2010;84(1):270–279. doi: 10.1128/JVI.01740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGregor F, Phelan A, Dunlop J, Clements JB. Regulation of herpes simplex virus poly (A) site usage and the action of immediate-early protein IE63 in the early-late switch. J Virol. 1996;70(3):1931–1940. doi: 10.1128/jvi.70.3.1931-1940.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins KD, Gregonis J, Borge S, Rice SA. Transactivation of a viral target gene by herpes simplex virus ICP27 is posttranscriptional and does not require the endogenous promoter or polyadenylation site. J Virol. 2003;77(18):9872–9884. doi: 10.1128/JVI.77.18.9872-9884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hann LE, Cook WJ, Uprichard SL, Knipe DM, Coen DM. The role of herpes simplex virus ICP27 in the regulation of UL24 gene expression by differential polyadenylation. J Virol. 1998;72(10):7709–7714. doi: 10.1128/jvi.72.10.7709-7714.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaida D, et al. U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature. 2010;468(7324):664–668. doi: 10.1038/nature09479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg MG, et al. U1 snRNP determines mRNA length and regulates isoform expression. Cell. 2012;150(1):53–64. doi: 10.1016/j.cell.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almada AE, Wu X, Kriz AJ, Burge CB, Sharp PA. Promoter directionality is controlled by U1 snRNP and polyadenylation signals. Nature. 2013;499(7458):360–363. doi: 10.1038/nature12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corbin-Lickfett KA, Souki SK, Cocco MJ, Sandri-Goldin RM. Three arginine residues within the RGG box are crucial for ICP27 binding to herpes simplex virus 1 GC-rich sequences and for efficient viral RNA export. J Virol. 2010;84(13):6367–6376. doi: 10.1128/JVI.00509-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corbin-Lickfett KA, Chen IH, Cocco MJ, Sandri-Goldin RM. The HSV-1 ICP27 RGG box specifically binds flexible, GC-rich sequences but not G-quartet structures. Nucleic Acids Res. 2009;37(21):7290–7301. doi: 10.1093/nar/gkp793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elkon R, Ugalde AP, Agami R. Alternative cleavage and polyadenylation: Extent, regulation and function. Nat Rev Genet. 2013;14(7):496–506. doi: 10.1038/nrg3482. [DOI] [PubMed] [Google Scholar]
- 40.Lykke-Andersen S, Jensen TH. Nonsense-mediated mRNA decay: An intricate machinery that shapes transcriptomes. Nat Rev Mol Cell Biol. 2015;16(11):665–677. doi: 10.1038/nrm4063. [DOI] [PubMed] [Google Scholar]
- 41.Shu M, Taddeo B, Zhang W, Roizman B. Selective degradation of mRNAs by the HSV host shutoff RNase is regulated by the UL47 tegument protein. Proc Natl Acad Sci USA. 2013;110(18):E1669–E1675. doi: 10.1073/pnas.1305475110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taddeo B, Zhang W, Roizman B. The herpes simplex virus host shutoff RNase degrades cellular and viral mRNAs made before infection but not viral mRNA made after infection. J Virol. 2013;87(8):4516–4522. doi: 10.1128/JVI.00005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu N, Watkins SC, Schaffer PA, DeLuca NA. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J Virol. 1996;70(9):6358–6369. doi: 10.1128/jvi.70.9.6358-6369.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gillis PA, Okagaki LH, Rice SA. Herpes simplex virus type 1 ICP27 induces p38 mitogen-activated protein kinase signaling and apoptosis in HeLa cells. J Virol. 2009;83(4):1767–1777. doi: 10.1128/JVI.01944-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolb AW, Lee K, Larsen I, Craven M, Brandt CR. Quantitative trait locus based virulence determinant mapping of the HSV-1 genome in murine ocular infection: Genes involved in viral regulatory and innate immune networks contribute to virulence. PLoS Pathog. 2016;12(3):e1005499. doi: 10.1371/journal.ppat.1005499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson KE, Song B, Knipe DM. Role for herpes simplex virus 1 ICP27 in the inhibition of type I interferon signaling. Virology. 2008;374(2):487–494. doi: 10.1016/j.virol.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melchjorsen J, Sirén J, Julkunen I, Paludan SR, Matikainen S. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-kappaB and IRF-3. J Gen Virol. 2006;87(Pt 5):1099–1108. doi: 10.1099/vir.0.81541-0. [DOI] [PubMed] [Google Scholar]
- 48.Stingley SW, et al. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J Virol. 2000;74(21):9916–9927. doi: 10.1128/jvi.74.21.9916-9927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JC, et al. HSV-1 ICP27 suppresses NF-kappaB activity by stabilizing IkappaBalpha. FEBS Lett. 2008;582(16):2371–2376. doi: 10.1016/j.febslet.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 50.Yeo G, Burge CB. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J Comput Biol. 2004;11(2-3):377–394. doi: 10.1089/1066527041410418. [DOI] [PubMed] [Google Scholar]
- 51.Frith MC, Saunders NF, Kobe B, Bailey TL. Discovering sequence motifs with arbitrary insertions and deletions. PLoS Comput Biol. 2008;4(4):e1000071. doi: 10.1371/journal.pcbi.1000071. [DOI] [PMC free article] [PubMed] [Google Scholar]