Significance
Photosynthesis regulation is fundamental to responding to environmental dynamic changes and avoiding oxidative damage. These regulatory mechanisms were shaped during evolution from cyanobacteria to plants, as well as after the colonization of new habitats. Land colonization was a key phase in evolution with plants capable of adapting to drastically different conditions with respect to their aquatic ancestors. Valuable information on this adaptation can be obtained studying nonvascular plants, such as the moss Physcomitrella patens, that diverged from flowering plants early after land colonization. Here we show that in P. patens, Flavodiiron (FLV) proteins are acting as an electron sink to avoid photosynthetic electron transport chain over-reduction after any increase in illumination and are fundamental for protection under fluctuating light conditions.
Keywords: photosynthesis, electron transport, photoprotection, plant evolution, mosses
Abstract
Photosynthetic organisms support cell metabolism by harvesting sunlight to fuel the photosynthetic electron transport. The flow of excitation energy and electrons in the photosynthetic apparatus needs to be continuously modulated to respond to dynamics of environmental conditions, and Flavodiiron (FLV) proteins are seminal components of this regulatory machinery in cyanobacteria. FLVs were lost during evolution by flowering plants, but are still present in nonvascular plants such as Physcomitrella patens. We generated P. patens mutants depleted in FLV proteins, showing their function as an electron sink downstream of photosystem I for the first seconds after a change in light intensity. flv knock-out plants showed impaired growth and photosystem I photoinhibition when exposed to fluctuating light, demonstrating FLV’s biological role as a safety valve from excess electrons on illumination changes. The lack of FLVs was partially compensated for by an increased cyclic electron transport, suggesting that in flowering plants, the FLV’s role was taken by other alternative electron routes.
Life on Earth depends on oxygenic photosynthesis, which enables plants, algae, and cyanobacteria to convert light into chemical energy. Sunlight powers the transfer of electrons from water to NADP+ by the activity of two photosystems (PS), PSII and PSI, thus generating NADPH and ATP to sustain cell metabolism. Natural environmental conditions are highly variable, and sudden changes in irradiation can drastically affect the flow of excitation energy and electrons. At the same time, the ATP and NADPH consumption rate is also highly dynamic because of a continuous metabolic regulation (1–3). Photosynthetic organisms evolved several mechanisms to modulate the flow of excitation energy and electrons according to metabolic constraints, diverting/feeding electrons from/to the linear transport chain (3). These pathways modulate the ATP/NADPH ratio, as in the cyclic electron transport (CET) around PSI, where electrons are redirected from PSI to plastoquinone (PQ) or Cytb6f (4), contributing to proton translocation and ATP synthesis, but not to NADPH formation (5–9).
In cyanobacteria, the Flavodiiron proteins (known as FLV) have been identified as an additional component of electron transport chain (10–12). FLV proteins are constituted by three distinct domains: a N-terminal β-lactamase-like domain, a flavodoxin-like domain, and a C-terminal NAD(P)H-flavin reductase-like domain. The former two domains are also found in FLV proteins from archaea and anaerobic bacteria, where they are involved in O2 or NO reduction, whereas the latter is typical only of FLVs from oxygenic photosynthetic organisms (11–13). Recent studies showed that in cyanobacteria, the FLV1/FLV3 heterodimer catalyzes the light-dependent reduction of O2 to water, using NADPH as electron donor (10, 11), protecting PSI from light stress (10). Another FLVs heterodimer, FLV2/FLV4, instead, has been shown to be active in photo-protection of PSII (14–16). FLVs also were found expressed in green algae as Chlamydomonas reinhardtii (17, 18), and corresponding genes are present in nonvascular plants (16) and in gymnosperms (19), but they were lost during evolution by flowering plants. Heterologous expression of FLV from the moss Physcomitrella patens in Arabidopsis thaliana recently showed that these proteins can be functional in flowering plants (19), suggesting their loss is not a result of structural reasons but, rather, a change in strategy for regulation of photosynthetic electron flow during land colonization that made FLV activity superfluous, or possibly even detrimental.
In this article, we generated P. patens mutants depleted in FLVA or FLVB proteins, showing that they are active as an electron sink downstream of PSI, and that their role is prominent in the first seconds after a sudden increase of light intensity, acting as a safety valve for electrons. When exposed to a fluctuating light regime, flva or flvb knock-out (KO) mutants have impaired growth and suffer from severe PSI photoinhibition. In mutant plants, FLV absence was partially compensated for by an enhanced cyclic electron flow, suggesting this as a likely mechanism that took the biological role of FLV in angiosperms.
Results
Depletion of Either FLVA or FLVB Affects the Accumulation of Both Isoforms in Physcomitrella patens.
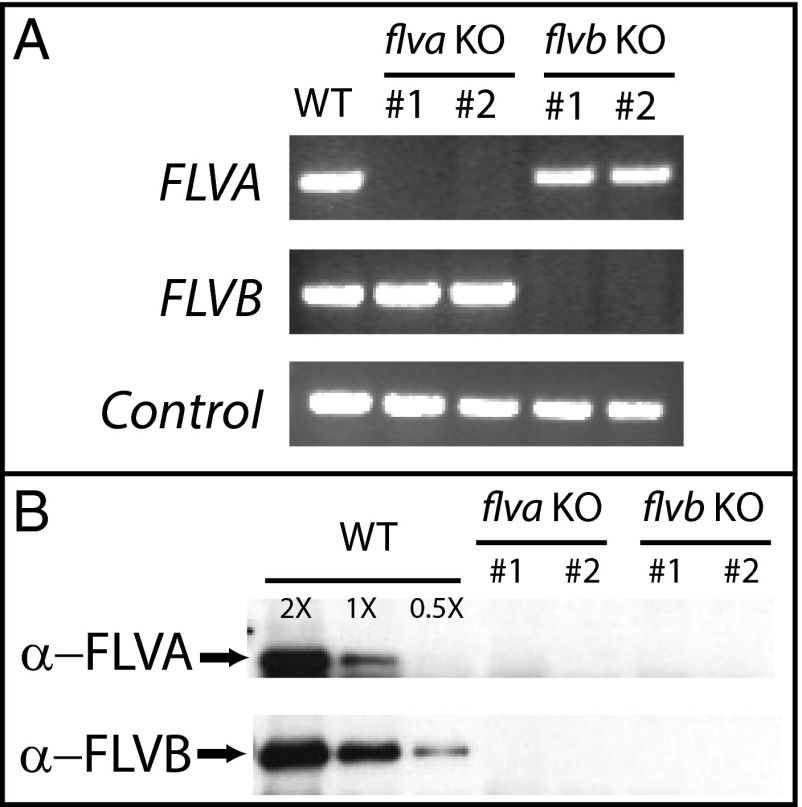
Two genes encoding the FLV proteins, named FLVA and FLVB, are found in the genome of the moss P. patens and show high similarity to cyanobacterial FLV1/FLV3 isoforms (SI Appendix, Fig. S1) (3, 11, 16). FLV biological function in P. patens was investigated by generating specific flva KO and flvb KO plants exploiting homologous recombination in the nuclear genome (20, 21) (SI Appendix, Fig. S2A). For each genotype, at least four independent lines were isolated, and they all showed indistinguishable phenotypes (SI Appendix, Fig. S3). In all lines, we first verified the disruption of the target gene by the insertion of the resistance cassette (SI Appendix, Figs. S2B and S3A). RT-PCR showed that FLVA and FLVB transcripts were missing in flva KO and flvb KO lines, respectively (Fig. 1A and SI Appendix, Fig. S3), whereas they were both detectable in WT plants, demonstrating that the isolated clones were indeed genuine KO lines.
Fig. 1.
Genotype characterization of flva KO and flvb KO lines. Effective homologous recombination with the insertion of the resistance cassette in FLVA or FLVB loci was first verified by PCR to prove the integration of resistance cassette in the expected position of the genome (SI Appendix, Fig. S2). (A) FLVA and FLVB gene expression was assessed by RT-PCR in WT and selected flva KO and flvb KO lines grown in control light. Amplification of Actin transcript is also reported as control. (B) Western blotting against P. patens FLVA and FLVB proteins. In each lane, 100 µg total proteins from CL-grown samples were loaded. In the case of WT, 0.5X and 2X indicates lanes loaded with 50 or 200 µg, respectively. WT and two independent lines for each flva KO and flvb KO mutants are reported, but additional independent lines were analyzed with indistinguishable results (SI Appendix, Fig. S3).
FLV proteins accumulation was evaluated afterward, using specific antibodies raised against P. patens FLVA or FLVB polypeptides. In both cases, a band was detectable in WT, but not in the flva KO and flvb KO lines (Fig. 1B). Even if the depletion of a single flv gene did not affect the RNA accumulation of the other FLV gene, the protein accumulation of FLVA and FLVB showed instead a strong mutual dependence (Fig. 1). This suggests that FLVA and FLVB form a heterodimer, as their cyanobacteria homologs (10), and that each monomer is not stably accumulated if expressed alone.
FLV Proteins Are a Major Electron Sink at the Onset of Light.
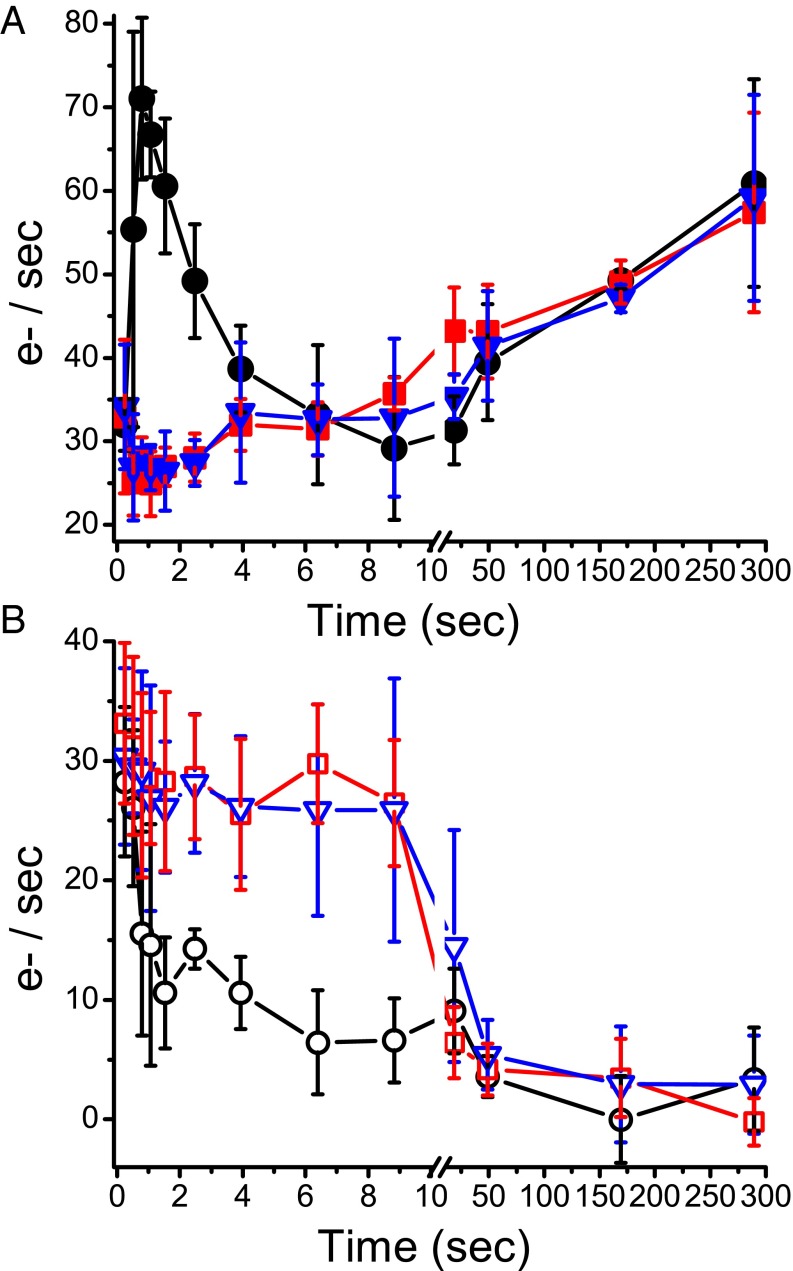
When grown in control conditions, mutants depleted in FLV proteins showed no significant differences in growth, pigment content, or photosynthetic quantum yield with respect to WT (SI Appendix, Table S1), suggesting the mutation had no major effect on the accumulation of protein complexes of the photosynthetic apparatus. Photosynthetic electron transport rate (ETR) in the different genotypes was then measured using electrochromic shift, following the approach used in different organisms, including P. patens (refs. 22–26; see also SI Appendix, Fig. S4). In dark-acclimated WT plants, approximately 1 s after the light was switched on, ETR showed a peak, the intensity of which depended on the illumination intensity (SI Appendix, Fig. S5A). After this peak, ETR slowly and steadily increased during the illumination, consistent with the increased consumption rate of ATP and NADPH on the activation of the Calvin-Benson cycle (SI Appendix, Fig. S5A) (25). flva KO and flvb KO showed significantly reduced ETR compared with WT either with a mild irradiation (SI Appendix, Fig. S5B) or with saturating light (Fig. 2A), where the transient peak in ETR was completely absent. This difference, however, was only detectable for a few seconds after the onset of illumination, whereas later ETR was indistinguishable between the three genotypes, and they all reached the same value of ∼ 60 e−/s after 5 min of light treatment (Fig. 2A).
Fig. 2.
Photosynthetic electron transport in P. patens WT and flv KO lines grown in CL. (A) Total photosynthetic ETR measured in vivo in WT (black circles), flva KO (red squares), and flvb KO (blue triangles) at 940 μmol photons m−2⋅s−1 actinic light, calculated from electrochromic shift signal, as detailed in SI Appendix, Fig. S4. (B) Cyclic electron transport rate measured in the same samples treated with the PSII inhibitor DCMU. WT, empty black circles; flva KO, empty red squares; flvb KO, empty blue triangles. ETR values are normalized to xenon-induced PSI turnovers. Because of the presence of double PSI turnovers using a xenon lamp (24), ETR absolute values are underestimated by ∼40% (SI Appendix, Fig. S4). Data presented are averages ± SD of three to five independent biological replicates.
The same analyses were also performed in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), which inhibits PSII and therefore allows evaluating CET rate around PSI (22, 25) (Fig. 2B). In WT plants, CET is relatively low and stable at ∼ 5 e−/s, thus representing less than 10% of total electron transport (Fig. 2B), as reported in the literature for P. patens and other plant species (23, 25, 27). In contrast, flva KO and flvb KO plants showed a sustained cyclic electron transport activity for the first 10 s of illumination when it represents the major electron transport pathway (Fig. 2B). CET activity later decreased, and after 20 s of illumination, its contribution to electron transport in flv KO plants became indistinguishable from WT (Fig. 2B). Thus, about 1 s after the light was switched on, the major component of electron transport in the WT was FLV-dependent, whereas in flv KO mutants the cyclic electron flow was the main pathway activated (SI Appendix, Fig. S6).
The effect of FLV depletion was also assessed using chlorophyll (Chl) fluorescence. When dark-acclimated plants are exposed to illumination, Chl fluorescence signal is quenched as a result of the activation of both photosynthetic electron transport and mechanisms dissipating excess excited Chl singlet states as heat (nonphotochemical quenching; NPQ) (28). The induction of this Chl fluorescence quenching on illumination was slower in both flva KO and flvb KO with respect to WT (Fig. 3A). Moreover, both flva KO and flvb KO showed a higher QA redox state in the first few seconds after the light was switched on (Fig. 3B), indicating an over-reduction of the electron transport chain. Differences between the genotypes gradually disappeared during the illumination period, and all genotypes were indistinguishable after 1 min of light treatment. NPQ induction was also affected in both flv KOs, but again, differences with respect to WT were limited to the first minute after the light was switched on (Fig. 3C), and WT and flv KOs were indistinguishable at steady-state illumination (Fig. 3 and SI Appendix, Fig. S7). The same differences in both NPQ and QA redox state for the first minute after the light was switched on were present independent from the actinic light intensities used in the analysis (SI Appendix, Fig. S8).
Fig. 3.
Evaluation of PSII efficiency in P. patens flv KO lines. (A) Chl fluorescence kinetics, normalized to the dark acclimated maximum (Fm) value, of WT and flv KO lines. A representative kinetic for each genotype is reported (first 120 s after light is switched on). (B) QA relative reduction and (C) heat dissipation of excess energy (NPQ), calculated from fluorescence kinetics (complete measurements are reported in SI Appendix, Fig. S7). WT, shown in black; flva KO, red; flvb KO, blue; all plants were grown 10 d in control light. Upper yellow/black bars indicate when actinic light (175 µmol photons m−2⋅s−1) was on/off. Data are reported as average ± SD of four independent biological replicates. Asterisks indicate significant differences between WT and flv KOs (t test, *P = 0.05; **P = 0.01).
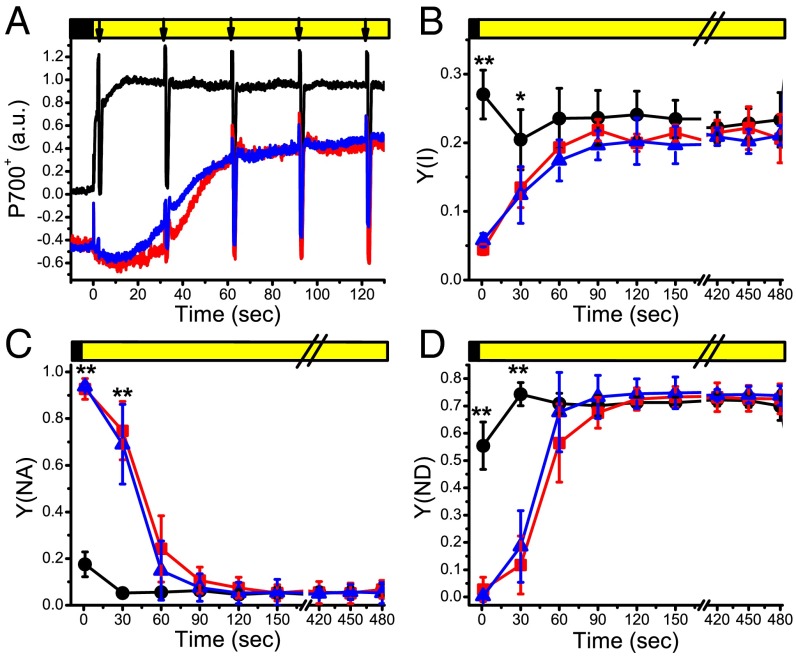
PSI efficiency was also monitored in the same experimental conditions, using the P700+ absorption signal (10, 29). In WT plants, when light was switched on, the P700+ signal immediately increased as a result of light-dependent PSI oxidation, then reaching a steady state (Fig. 4A). Both flv KO genotypes were slower in reaching this steady state, indicating a reduced efficiency in P700 oxidation in the mutants (Fig. 4A). Consistently, PSI quantum yield, Y(I), was reduced in flva KO and flvb KO for the first seconds after the light was switched on (Fig. 4B). This was the result of a strong acceptor side limitation in FLV-depleted strains [Y(NA); Fig. 4C], whereas both flv KO lines showed a smaller donor side limitation [Y(ND)] compared with WT (Fig. 4D). The alterations in PSI efficiency were only present in the first seconds after the light was switched on and progressively decreased during illumination (Fig. 4 and SI Appendix, Fig. S9). An analogous behavior was observed irrespective of the actinic light intensity used for the measurement, as observed earlier for PSII (SI Appendix, Fig. S8). The same analyses were performed in the presence of the artificial electron acceptor methylviologen (30), which, as demonstrated in SI Appendix, Fig. S10, rescued the phenotype of the flv KO plants, confirming that in these plants, PSI is limited on the acceptor side (SI Appendix, Fig. S10).
Fig. 4.
PSI efficiency in response to illumination. (A) Changes in P700+ absorption signal during a dark-to-light transition in WT (black), flva KO (red), and flvb KO (blue). For each genotype, a representative kinetic is shown. Signals are normalized to the steady state value, and flva KO and flvb KO kinetics are shifted (−0.5 on the y axis) with respect to WT for clarity. Arrows indicate saturation pulses. In B, C, and D, PSI quantum yield of energy conversion [Y(I)], acceptor [Y(NA)], and donor [Y(ND)] side limitation, respectively, are reported as calculated from the P700+ absorption kinetics. Complete kinetics are shown in SI Appendix, Fig. S9. WT, black; flva KO, red; flvb KO, blue; all plants were grown 10 d in control light. Upper yellow/black bars indicate the period where actinic light (540 µmol photons m−2⋅s−1) is on/off. Averages ± SD of four independent biological replicates are shown. Asterisks indicate values significantly different between WT and flv KOs (t test, *P = 0.05; **P = 0.01).
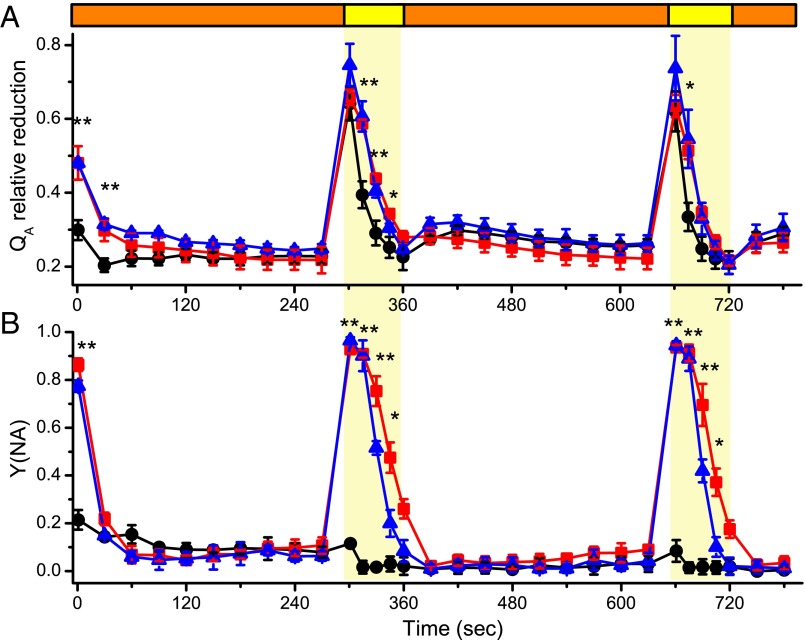
PSI and PSII functional measurements were also performed treating plants with cycles of dim/saturating actinic light, an illumination regime more representative of a natural environment than a dark-to-light transition (1). PSII and PSI responses observed after each increase in illumination were highly similar to the one detected in the dark-to-light switch presented earlier, with flv KOs showing increased relative QA reduction (Fig. 5A) and strong PSI acceptor side limitation (Fig. 5B), together with decreased PSI and PSII yields and a slower NPQ activation (SI Appendix, Fig. S11), every time the light intensity increased (yellow shading in Fig. 5 and in SI Appendix, Fig. S11).
Fig. 5.
Effect of FLV depletion on photosystems efficiencies upon light fluctuations. Dark-acclimated moss tissues grown for 10 d in control light were then exposed to cycles of 5 min of mild actinic illumination (50 µmol photons m−2⋅s−1, orange bars) and 1 min strong actinic light (540 µmol photons m−2⋅s−1, yellow bars and shading), monitoring PSII and PSI efficiencies simultaneously. QA relative reduction and Y(NA) parameter are reported in A and B, whereas additional parameters are shown in SI Appendix, Fig. S11. WT, flva KO, and flvb KO lines are shown in black, red, and blue, respectively. Data reported are average ± SD of three independent replicates, and asterisks indicate values significantly different between WT and flv KOs (t test, *P = 0.05; **P = 0.01).
flv KOs Show Increased PSI Photodamage in Fluctuating Light.
Results reported here clearly suggest that the FLV proteins have a significant influence on photosynthetic electron transport during the first seconds after a change in irradiation. To investigate the physiological relevance of this activity in vivo, WT, flva KO, and flvb KO were grown under a fluctuating light (FL) regime in which plants were treated with recurrent exposition to intense light (1 min at 800 µmol photons m−2⋅s−1 every 6 min). As shown in Fig. 6, in those dynamic light conditions, flva KO and flvb KO mutants showed a strong reduction in growth with respect to WT. Such a growth phenotype was not present in the case of constant illumination, even of high intensity (Fig. 6). This clearly showed that the flv KO mutants are not sensitive to intense irradiation per se but, rather, to fluctuations in light intensity.
Fig. 6.
Growth phenotype of WT and flv KO plants in different light conditions. P. patens WT, flva KO, and flvb KO plants were grown for 2 weeks either under constant illumination at 50, 150, or 500 µmol photons m−2⋅s−1, or in fluctuating light (FL, cycles of 5 min at 25 µmol photons m−2⋅s−1 followed by 1 min at 800 µmol photons m−2⋅s−1). It is noteworthy that the FL regime is characterized by the same integrated light intensity as constant illumination at 150 µmol photons m−2⋅s−1.
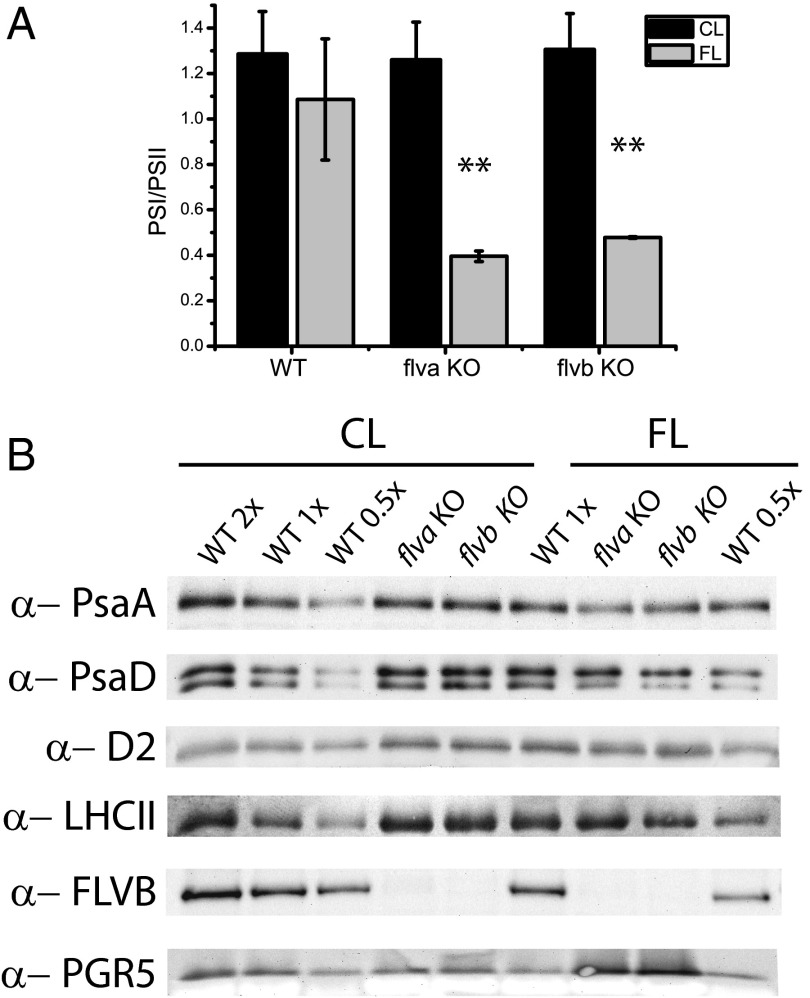
When grown under FL conditions, flva KO and flvb KO plants showed lower PSII efficiency compared with WT (SI Appendix, Table S1), but the strongest effect was on PSI, as evidenced by the drastic decrease in PSI/PSII ratio (Fig. 7A). flv KO mutants treated with FL also showed a strong reduction in maximum P700+ signal (Pm, SI Appendix, Table S1), suggesting the altered PSI/PSII ratio was the result of an inactivation of PSI, rather than an increase in PSII. Western blotting analyses consistently showed no significant difference in PSII subunit accumulation between WT and the flv KO plants (Fig. 7B). Conversely, a decrease in PSI subunits was detected in flv KO compared with WT after FL growth (Fig. 7B), which further increased when the treatment in FL was prolonged (SI Appendix, Fig. S12), suggesting PSI was first inactivated and then slowly degraded. Western blotting with the PGR5 antibody demonstrated, especially in FL conditions, an accumulation of PGR5 in flv KO plants (Fig. 7B). The PGR5 protein is known to be involved in cyclic electron transport (8).
Fig. 7.
Long-term effects of fluctuating light on PSI and PSII in flv KOs. (A) PSI/PSII ratio was determined as detailed in SI Appendix, Fig. S4A in dark-acclimated plants either grown in constant illumination (CL, 50 µmol photons m−2⋅s−1) or in fluctuating light (FL, cycles of 5 min at 25 µmol photons m−2⋅s−1, followed by 1 min at 800 µmol photons m−2⋅s−1) conditions. PSI content is over-estimated because of xenon-induced double turnovers (24), and therefore the PSI/PSII ratios are underestimated by the same extent (∼40%). Asterisks (**) indicate values significantly different from WT (t test, P = 0.01, n = 3). (B) Western blotting analysis on CL and FL grown plants of WT and flva KO and flvb KO using antibodies against FLVB, PGR5, PSI (PsaA and PsaD), and PSII (D2 and LHCII) subunits. Whereas 0.5 Chl µg thylakoid extracts were used for LHCII and 1 Chl µg thylakoid extracts for all of the other cases, 5 Chl µg total protein extracts were loaded for each sample for FLVB, 4 Chl µg for PGR5 immunodetection. For WT, 0.5X and 2X indicates lane loaded with half or double those Chl µg amounts, respectively.
Discussion
The ability of photosynthetic organisms to convert light into chemical energy requires the generation of pigment excited states and multiple electron transport reactions. This process thus inevitably involves the formation of instable molecules that must be readily consumed to avoid the formation of undesired side products such as reactive oxygen species. As a consequence, all oxygenic photosynthetic organisms must find an optimal compromise between maximizing light use efficiency and maintaining an effective protection from eventual excess of absorbed energy or excited electrons. Keeping such a balance is particularly complex in a highly variable environment in which the energy availability (light) and its use (carbon fixation and metabolism in general) are continuously changing, and it thus requires a constant modulation of photosynthetic efficiency (2, 5, 6, 31–34).
In this article, we demonstrate that in the moss P. patens, FLV proteins are active as electron sinks downstream of PSI and are a major component of the photosynthesis regulation machinery, with a seminal role in fluctuating light conditions. FLV activity is prominent for a few seconds after an increase in illumination, being responsible for a transient peak in ETR of WT plants, which is completely missing from the flv KO mutants (Fig. 2A). This reduction in electron transport ability in flv KO caused a strong acceptor side limitation in PSI (Figs. 4 and 5) and over-reduction of electron transporters (Figs. 3 and 5) after any increase in illumination. FLV effect on electron transport is, however, transient and after 1 min of light exposure, all of the effects on PSI, QA redox state, or ETR are absent, making the flv KO mutants and WT indistinguishable. This observation suggests that during prolonged illumination with various constant light intensities, the role of FLVs role is negligible, and other metabolic pathways (e.g., carbon fixation reactions) consume the NADPH/ATP produced, therefore protecting the photosynthetic chain from over-reduction in steady state illumination, even in the presence of strong light (Fig. 6).
The biological significance for this transient, FLV-dependent increase in ETR on a sudden increase in light intensity is in accordance with the general knowledge that light absorption and electron transfer are immediately affected by any change in illumination conditions. In contrast, regulation of metabolism and carbon fixation have slower kinetics, and thus they are not immediately capable of consuming all the ATP and reducing power produced, generating a dangerous imbalance between production of excited states and consumption of the final products of photosynthetic electron transport. FLV proteins, in accepting electrons from PSI (Fig. 2), consume a significant fraction of the extra reducing power available, and thus avoid over-reduction of electron transport chain. In this context, it should also be considered that oxidized P700 (P700+) is a very good quencher of Chl excited states (35–38). PSI is tolerant to excess excitation energy, being able to induce nonphotochemical energy dissipation even when damaged (39), but instead it is extremely sensitive to excess electrons on the donor side of the complex (36, 37). In WT, FLV activity as electron sink maintains PSI in a donor state limitation (Fig. 4D and SI Appendix, Fig. S9), thus keeping it in a more stable state, effectively protecting it from light-induced damage as a result of excess electrons. Conversely, in flv KO mutants, PSI activity is not limited at the donor side (Fig. 4D and SI Appendix, Fig. S9), and the resulting electron pressure toward PSI caused the drastic PSI inactivation in flv KOs (Fig. 7 and SI Appendix, Table S1).
An additional beneficiary effect of FLV activity is a result of its contribution to the total electron transport (Fig. 2) that supports proton translocation into the lumen, as confirmed by the observation that flv KO mutants show an alteration of total transmembrane potential and ΔpH formation (SI Appendix, Fig. S13). Lumen acidification is well known to be a major signal for the activation of several photo-protection mechanisms such as NPQ, xanthophyll cycle, and cytb6f regulation (6, 32, 33, 40–42). FLV activity thus allows for a faster activation of these mechanisms (Fig. 3), leading to a decreased excitation pressure by activation of heat dissipation of excess absorbed energy, and thus contributing to protection of whole photosynthetic apparatus, including PSII (SI Appendix, Table S1). The biological relevance of FLV activity is well visualized when plants are exposed to a fluctuating light regime, conditions in which its activity is continuously required. FLV-depleted mutants show strong growth defects and PSI photo-damage (Figs. 6 and 7), whereas an even stronger illumination does not affect FLV mutants if provided constantly (Fig. 6).
Considering such a strong effect on photosynthesis regulation, it is surprising that FLVs are not conserved in angiosperms (3, 12, 16, 19). A recent report showed that FLVs from P. patens are capable of accepting electrons from PSI when expressed in A. thaliana (19), and thus this loss was not a result of mechanistic or structural reasons but, rather, to a change in regulatory strategies that made FLV activity superfluous or even detrimental. A likely explanation can be found by observing that in flv KO mutants, cyclic electron transport partially compensates for the absence of FLV (Figs. 2 and 7B), clearly suggesting CET could have taken over the FLV function in protecting PSI from over-reduction in plants. This hypothesis is consistent with the observations that CET is transiently activated on dark-to-light transitions in vascular plants (6, 26), and that A. thaliana CET mutants also show PSI photosensitivity when exposed to light fluctuations (8, 29).
Materials and Methods
Plant Material and Light Treatments.
Protonemal tissue of P. patens, Gransden WT strain, flva and flvb single KO lines were grown on minimum PpNO3 media in controlled conditions: 24 °C, 16 h light/8 h dark photoperiod and a light intensity of 50 µmol photons m−2⋅s−1 (control light, CL), and analyzed after 10 d of growth. For fluctuating light treatment (FL), 3-d-old plates grown in CL were moved to a light regime with cycles of 5 min at 25 µmol photons m−2⋅s−1 and 1 min at 800 µmol photons m−2⋅s−1 during the light phase of photoperiod and analyzed after 7 d in FL (10-d-old plates).
The growth phenotype of flv KO lines was evaluated on spots of tissue grown in different constant light intensities [50 (CL), 150, or 500 µmol photons m−2⋅s−1], or FL. It should be mentioned that the plants grown at 150 µmol photons m−2⋅s−1 received almost the same total amount of photons of FL conditions.
flva and flvb KO Constructs Design, Moss Transformation, and Screening of Resistant Lines.
Selected upstream and downstream homologous recombination regions from FLVA (locus XP_001759251.1) and FLVB (XP_001756079.1) genes were amplified by PCR from WT genomic DNA and cloned respectively into BHRf and BNRf plasmids (kindly provided by F. Nogue, INRA Versailles, France), for targeted KO generation of FLVA or FLVB genes, respectively, as detailed in SI Appendix, Table S2 and Fig. S2A.
WT Gransden strain was then used as genetic background to obtain flva KO and flvb KO single KO lines. P. patens transformation was performed as in ref. 20, with minor modifications (21). After two rounds of selection, genomic DNA from WT and resistant lines was obtained with EuroGOLD Plant DNA mini kit (EuroClone) and used as templates to confirm DNA insertion by PCR (see SI Appendix, Table S2 for the primers list). RNA was afterward purified with RNeasy Plant Mini Kit (Qiagen) and used as a template for cDNA synthesis with RevertAid Reverse Transcriptase (Thermo Scientific) to verify FLVA/B gene expression in WT, flva KO, and flvb KO.
Thylakoid and Total Protein Extracts.
Thylakoids from protonemal tissue grown in CL or FL were prepared by means of an Arabidopsis protocol with minor modifications, as in ref. 43. Total extracts were instead obtained by grinding tissues in sample buffer before SDS/PAGE. For immunoblotting analysis, after SDS/PAGE, proteins were transferred to nitro-cellulose membranes (Pall Corporation) and detected with specific commercial (anti-PsaA and anti-PsaD, Agrisera, catalog numbers AS06 172 and AS09 461, respectively), custom-made (anti-FLVA, anti-FLVB and anti-PGR5, from Agrisera) or homemade polyclonal antibodies (D2 and LHCII). Chl a/b and Chl/Car ratios were obtained by fitting the spectrum of 80% acetone pigment extracts with spectra of the individual purified pigments, as in ref. 44.
Fluorescence and P700 Measurement with Dual-PAM.
In vivo chlorophyll fluorescence and oxidized P700+ absorption signal were monitored simultaneously at room temperature with a Dual PAM-100 fluorometer (Walz) in P. patens WT, flva KO, and flvb KO tissues grown for 10 d in minimum medium (PpNO3) in control growth conditions (CL). Before measurements, plates were dark-acclimated for 40 min. For induction/recovery kinetics, actinic light was set to 540 (saturating actinic light), 175, or 50 µmol photons m−2⋅s−1. For fluctuating light mimicking kinetics, actinic light was set at 50 µmol photons m−2⋅s−1 for mild illumination and 540 µmol m−2⋅s−1 for the saturating light steps. PSII and PSI parameters were calculated as following: Fv/Fm as (Fm − Fo)/Fm, Y(II) as (Fm′ − F)/Fm′, relative QA reduction as F′/Fm, NPQ as (Fm − Fm′)/Fm′, Y(I) as 1 − Y(ND) − Y(NA), Y(NA) as (Pm − Pm')/Pm, Y(ND) as (1 − P700 red). Data are presented as mean ± SD of at least 3 independent experiments.
Spectroscopic Analyses with Joliot-Type Spectrometer (JTS).
Spectroscopic analysis on WT, flva KO and flvb KO lines was performed in vivo on 10-d-old intact tissues, using a JTS-10 spectrophotometer (Biologic). Relative amount of functional photosynthetic complexes and electron transport rates were evaluated measuring the electrochromic shift spectral change, using a protocol already used in refs. 22, 24–26 as detailed in SI Appendix, Fig. S4.
Supplementary Material
Acknowledgments
C.G. acknowledges financial support by the University of Padova (Grant GRIC13V6YZ) and by the Ingegner Aldo Gini Foundation (Padova). T.M. received financial support from the European Research Council (BIOLEAP Grant 309485), and E.-M.A. received financial support from the Academy of Finland (Grants 271832 and 273870).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606685113/-/DCSupplemental.
References
- 1.Külheim C, Agren J, Jansson S. Rapid regulation of light harvesting and plant fitness in the field. Science. 2002;297(5578):91–93. doi: 10.1126/science.1072359. [DOI] [PubMed] [Google Scholar]
- 2.Allahverdiyeva Y, Suorsa M, Tikkanen M, Aro EM. Photoprotection of photosystems in fluctuating light intensities. J Exp Bot. 2015;66(9):2427–2436. doi: 10.1093/jxb/eru463. [DOI] [PubMed] [Google Scholar]
- 3.Peltier G, Tolleter D, Billon E, Cournac L. Auxiliary electron transport pathways in chloroplasts of microalgae. Photosynth Res. 2010;106(1-2):19–31. doi: 10.1007/s11120-010-9575-3. [DOI] [PubMed] [Google Scholar]
- 4.Arnon DI, Chain RK. Regulation of ferredoxin-catalyzed photosynthetic phosphorylations. Proc Natl Acad Sci USA. 1975;72(12):4961–4965. doi: 10.1073/pnas.72.12.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shikanai T. Central role of cyclic electron transport around photosystem I in the regulation of photosynthesis. Curr Opin Biotechnol. 2014;26:25–30. doi: 10.1016/j.copbio.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Joliot P, Johnson GN. Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci USA. 2011;108(32):13317–13322. doi: 10.1073/pnas.1110189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peltier G, Aro EM, Shikanai T. NDH-1 and NDH-2 plastoquinone reductases in oxygenic photosynthesis. Annu Rev Plant Biol. 2016;67:55–80. doi: 10.1146/annurev-arplant-043014-114752. [DOI] [PubMed] [Google Scholar]
- 8.Munekage Y, et al. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell. 2002;110(3):361–371. doi: 10.1016/s0092-8674(02)00867-x. [DOI] [PubMed] [Google Scholar]
- 9.Peng L, Fukao Y, Fujiwara M, Takami T, Shikanai T. Efficient operation of NAD(P)H dehydrogenase requires supercomplex formation with photosystem I via minor LHCI in Arabidopsis. Plant Cell. 2009;21(11):3623–3640. doi: 10.1105/tpc.109.068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allahverdiyeva Y, et al. Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc Natl Acad Sci USA. 2013;110(10):4111–4116. doi: 10.1073/pnas.1221194110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helman Y, et al. Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr Biol. 2003;13(3):230–235. doi: 10.1016/s0960-9822(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 12.Allahverdiyeva Y, Isojärvi J, Zhang P, Aro E-M. Cyanobacterial oxygenic photosynthesis is protected by Flavodiiron proteins. Life (Basel) 2015;5(1):716–743. doi: 10.3390/life5010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vicente JB, Carrondo MA, Teixeira M, Frazão C. Structural studies on flavodiiron proteins. Methods Enzymol. 2008;437:3–19. doi: 10.1016/S0076-6879(07)37001-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhang P, et al. Operon flv4-flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. Plant Cell. 2012;24(5):1952–1971. doi: 10.1105/tpc.111.094417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bersanini L, et al. Flavodiiron protein Flv2/Flv4-related photoprotective mechanism dissipates excitation pressure of PSII in cooperation with phycobilisomes in Cyanobacteria. Plant Physiol. 2014;164(2):805–818. doi: 10.1104/pp.113.231969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P, Allahverdiyeva Y, Eisenhut M, Aro E-M. Flavodiiron proteins in oxygenic photosynthetic organisms: Photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS One. 2009;4(4):e5331. doi: 10.1371/journal.pone.0005331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jokel M, et al. Chlamydomonas Flavodiiron proteins facilitate acclimation to anoxia during sulfur deprivation. Plant Cell Physiol. 2015;56(8):1598–1607. doi: 10.1093/pcp/pcv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dang K-V, et al. Combined increases in mitochondrial cooperation and oxygen photoreduction compensate for deficiency in cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell. 2014;26(7):3036–3050. doi: 10.1105/tpc.114.126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto H, Takahashi S, Badger MR, Shikanai T. Artificial remodelling of alternative electron flow by Flavodiiron proteins in Arabidopsis. Nat Plants. 2016;2(3):16012. doi: 10.1038/nplants.2016.12. [DOI] [PubMed] [Google Scholar]
- 20.Schaefer DG, Zrÿd JP. Efficient gene targeting in the moss Physcomitrella patens. Plant J. 1997;11(6):1195–1206. doi: 10.1046/j.1365-313x.1997.11061195.x. [DOI] [PubMed] [Google Scholar]
- 21.Alboresi A, Gerotto C, Giacometti GM, Bassi R, Morosinotto T. Physcomitrella patens mutants affected on heat dissipation clarify the evolution of photoprotection mechanisms upon land colonization. Proc Natl Acad Sci USA. 2010;107(24):11128–11133. doi: 10.1073/pnas.1002873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allorent G, et al. Adjustments of embryonic photosynthetic activity modulate seed fitness in Arabidopsis thaliana. New Phytol. 2015;205(2):707–719. doi: 10.1111/nph.13044. [DOI] [PubMed] [Google Scholar]
- 23.Kukuczka B, et al. Proton gradient regulation5-like1-mediated cyclic electron flow is crucial for acclimation to anoxia and complementary to nonphotochemical quenching in stress adaptation. Plant Physiol. 2014;165(4):1604–1617. doi: 10.1104/pp.114.240648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailleul B, Cardol P, Breyton C, Finazzi G. Electrochromism: A useful probe to study algal photosynthesis. Photosynth Res. 2010;106(1-2):179–189. doi: 10.1007/s11120-010-9579-z. [DOI] [PubMed] [Google Scholar]
- 25.Joliot P, Joliot A. Cyclic electron transfer in plant leaf. Proc Natl Acad Sci USA. 2002;99(15):10209–10214. doi: 10.1073/pnas.102306999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joliot P, Béal D, Joliot A. Cyclic electron flow under saturating excitation of dark-adapted Arabidopsis leaves. Biochim Biophys Acta. 2004;1656(2-3):166–176. doi: 10.1016/j.bbabio.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Bendall DS, Manasse RS. Cyclic photophosphorylation and electron transport. Biochim Biophys Acta - Bioenerg. 1995;1229(1):23–38. [Google Scholar]
- 28.Baker NR. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu Rev Plant Biol. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- 29.Suorsa M, et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell. 2012;24(7):2934–2948. doi: 10.1105/tpc.112.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schansker G, Tòth SZ, Strasser RJ. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim Biophys Acta - Bioenerg. 2005;1706(3):250–261. doi: 10.1016/j.bbabio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Rochaix J-D. Regulation and dynamics of the light-harvesting system. Annu Rev Plant Biol. 2014;65:287–309. doi: 10.1146/annurev-arplant-050213-040226. [DOI] [PubMed] [Google Scholar]
- 32.Li Z, Wakao S, Fischer BB, Niyogi KK. Sensing and responding to excess light. Annu Rev Plant Biol. 2009;60:239–260. doi: 10.1146/annurev.arplant.58.032806.103844. [DOI] [PubMed] [Google Scholar]
- 33.Niyogi KK, Truong TB. Evolution of flexible non-photochemical quenching mechanisms that regulate light harvesting in oxygenic photosynthesis. Curr Opin Plant Biol. 2013;16(3):307–314. doi: 10.1016/j.pbi.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Tikkanen M, Aro E-M. Integrative regulatory network of plant thylakoid energy transduction. Trends Plant Sci. 2014;19(1):10–17. doi: 10.1016/j.tplants.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Shubin VV, Terekhova IN, Kirillov BA, Karapetyan NV. Quantum yield of P700+ photodestruction in isolated photosystem I complexes of the cyanobacterium Arthrospira platensis. Photochem Photobiol Sci. 2008;7(8):956–962. doi: 10.1039/b719122g. [DOI] [PubMed] [Google Scholar]
- 36.Sonoike K. Photoinhibition of photosystem I. Physiol Plant. 2011;142(1):56–64. doi: 10.1111/j.1399-3054.2010.01437.x. [DOI] [PubMed] [Google Scholar]
- 37.Chaux F, Peltier G, Johnson X. A security network in PSI photoprotection: Regulation of photosynthetic control, NPQ and O2 photoreduction by cyclic electron flow. Front Plant Sci. 2015;6:875. doi: 10.3389/fpls.2015.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caffarri S, Tibiletti T, Jennings RC, Santabarbara S. A comparison between plant photosystem I and photosystem II architecture and functioning. Curr Protein Pept Sci. 2014;15(4):296–331. doi: 10.2174/1389203715666140327102218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiwari A, et al. Photodamage of iron–sulphur clusters in photosystem I induces non-photochemical energy dissipation. Nat Plants. 2016;2(4):16035. doi: 10.1038/nplants.2016.35. [DOI] [PubMed] [Google Scholar]
- 40.Niyogi KK, Grossman AR, Björkman O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell. 1998;10(7):1121–1134. doi: 10.1105/tpc.10.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li XP, et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature. 2000;403(6768):391–395. doi: 10.1038/35000131. [DOI] [PubMed] [Google Scholar]
- 42.Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J. Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot. 2012;63(4):1637–1661. doi: 10.1093/jxb/ers013. [DOI] [PubMed] [Google Scholar]
- 43.Gerotto C, Alboresi A, Giacometti GM, Bassi R, Morosinotto T. Coexistence of plant and algal energy dissipation mechanisms in the moss Physcomitrella patens. New Phytol. 2012;196(3):763–773. doi: 10.1111/j.1469-8137.2012.04345.x. [DOI] [PubMed] [Google Scholar]
- 44.Croce R, Canino G, Ros F, Bassi R. Chromophore organization in the higher-plant photosystem II antenna protein CP26. Biochemistry. 2002;41(23):7334–7343. doi: 10.1021/bi0257437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.