Significance
Symbioses between marine plankton species are diverse and widespread both spatially and taxonomically. However, the nature and function of such relationships in natural assemblages are severely underexplored due to technical challenges. Consequently, as an example, the relationship between the ciliate Mesodinium rubrum and its observed cryptophyte endosymbiont is varied and debated, from enslaving chloroplasts to exploiting an organelle complex. Applying environmental transcriptomics and other methods to a natural bloom of M. rubrum revealed an unsuspected relationship, “host farming symbiont,” in which the host helps to transport nutrients from the environment, promotes symbiont cell proliferation, and benefits from the symbiont’s photosynthesis.
Keywords: cryptophyte Teleaulax, Mesodinium rubrum, red-tide bloom, intact endosymbiont, metatranscriptome
Abstract
Protist–alga symbiosis is widespread in the ocean, but its characteristics and function in situ remain largely unexplored. Here we report the symbiosis of the ciliate Mesodinium rubrum with cryptophyte cells during a red-tide bloom in Long Island Sound. In contrast to the current notion that Mesodinium retains cryptophyte chloroplasts or organelles, our multiapproach analyses reveal that in this bloom the endosymbiotic Teleaulax amphioxeia cells were intact and expressing genes of membrane transporters, nucleus-to-cytoplasm RNA transporters, and all major metabolic pathways. Among the most highly expressed were ammonium transporters in both organisms, indicating cooperative acquisition of ammonium as a major N nutrient, and genes for photosynthesis and cell division in the cryptophyte, showing active population proliferation of the endosymbiont. We posit this as a “Mesodinium-farming-Teleaulax” relationship, a model of protist–alga symbiosis worth further investigation by metatranscriptomic technology.
Protist–alga symbiosis is much more widespread in the marine ecosystem than previously thought, both spatially and taxonomically (1). Such biotic relationships are ecologically important, because they either fix nitrogen to provide N nutrients to sustain host photosynthesis (in the case of eukaryotic plankton harboring diazotrophic cyanobacteria) or photosynthesize to provide the host with organic carbon (in the case of heterotrophic protist-harboring algae). Field and laboratory observations have documented a wide range of host integration of symbionts, from enslaving transiently kept chloroplasts (so-called kleptoplastids) to permanent intact-cell endosymbionts (2). However, the degree of host integration and function of endosymbionts in natural plankton assemblages in situ is poorly understood and severely understudied, because such studies are very challenging. One example is the ciliate Mesodinium rubrum, a widely distributed marine protist, known to feed upon cryptophyte algae and retain their chloroplasts for photosynthesis (3–12). M. rubrum can form massive blooms of up to 106 cells L−1 in coastal and estuarine waters, and the retained plastid contributes up to 70% of primary productivity under some conditions (5–7). Based on field observations and laboratory studies, a wide range of relationships between this ciliate and its cryptophyte symbiont has been reported, including retention of not only the cryptophyte chloroplast but also the nucleus (8–10), endoplasmic reticulum (10, 11), and plastid–mitochondrion complexes (9–12). Whole cryptophyte cells in the ciliate host have also been observed (2, 13, 14), although it is unclear whether they represent predigested prey algae. Applying environmental transcriptomics and other methods to a natural bloom of M. rubrum, we found that the ciliate population in this bloom hosted intact and actively reproducing cells of Teleaulax amphioxeia expressing genes of membrane transporters, nucleus-to-cytoplasm RNA transporters, and all major metabolic pathways, indicating an unsuspected relationship of “Mesodinium farming Teleaulax.”
Results and Discussion
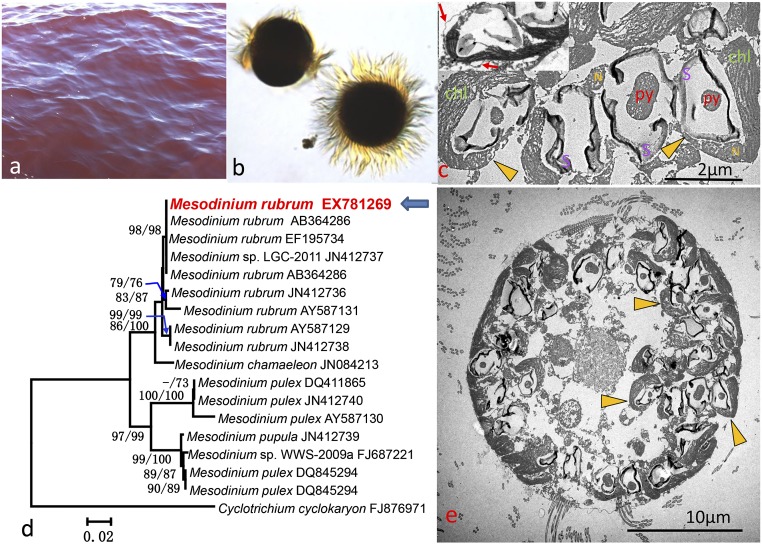
A red-tide bloom occurred in Long Island Sound in September of 2012 (Fig. 1A). Microscopic examination revealed M. rubrum as the causative species of the bloom, with no detectable cryptophytes and hardly any other organisms present in the bloom water (Fig. 1B). At 1.03 × 106 cells L−1, M. rubrum abundance in the bloom was over 100-fold higher than the annual peak in Long Island Sound (15). Each Mesodinium cell harbored 20 to 30 cryptophyte cells (n = 16), which packed the peripheral region of the M. rubrum cells (Fig. 1E), with complete cell structures, including cell membranes, nuclei, and chloroplasts (Fig. 1C). Taking advantage of the large cell size of Mesodinium spp. (width, 20 to 23 μm; length, 25 to 26 μm), we picked M. rubrum cells from samples under the microscope and obtained a 1,581-bp rRNA gene (rDNA) small subunit (SSU) sequence (GenBank accession no. KX781269) and plastid rDNA SSU sequences (GenBank accession nos. KX816859–KX816862), which phylogenetically verified the bloom organism as M. rubrum (Fig. 1D) and the endosymbiotic alga as T. amphioxeia (15). The endosymbiotic T. amphioxeia cells contained high amounts of phycoerythrin, indicating that they were photosynthetically active (15).
Fig. 1.
Red-tide bloom and the causative organisms. (A) Photograph of the red tide, which was detected on September 24, 2012, near the University of Connecticut’s oceanographic monitoring station (40° 57.01′ N, 73° 35.73′ W), when the water temperature was 21.9 °C and salinity was 27.9. (B) Light microscopic image of the causative ciliate, morphologically resembling M. rubrum. (C) TEM image showing T. amphioxeia cells within M. rubrum. chl, chloroplast; N, nuclei; py, pyrenoid; S, starch. Yellow arrowheads, T. amphioxeia cells; red arrows, cell membrane. (D) 18S rDNA phylogenetic tree verifying that the causative ciliate was M. rubrum. Values at the nodes are bootstrap values from maximum-likelihood/neighbor-joining analyses. (Scale bar, substitution rate per nucleotide site.) (E) TEM image of M. rubrum showing many T. amphioxeia cells packing the peripheral region of the M. rubrum cell.
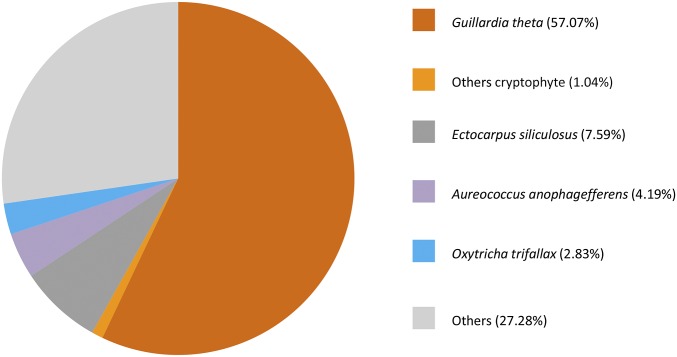
To investigate what biochemical activities were taking place in these cells, we extracted RNA from the bloom sample and conducted metatranscriptome sequencing using an Illumina HiSeq 2000, generating 39,488,639 raw reads (BioProject ID code PRJNA340945; Sequence Read Archive accession no. SRR4098290), which produced 297,537 unigenes (Table S1). About 164,199 (55.18%) of these unigenes were functionally annotated and the rest were novel genes, whose functions remain to be characterized. The annotated portion of the metatranscriptome was predominated by cryptophyte genes (cryptophyte subset), mainly matching Guillardia theta (16) but also Rhodomonas sp. (accounting for 58.11% of annotated reads and 11.36% of annotated unigenes), followed by the phaeophyte Ectocarpus siliculosus, the pelagophyte Aureococcus anophagefferens, and the ciliate Oxytricha trifallax (Fig. S1). Less than 1% of the unigenes (2,665) were annotated as ciliate genes, which are enriched in vital processes such as signal transduction, transport and catabolism, cell growth and death, and metabolism of carbohydrate, energy, and amino acids.
Table S1.
M. rubrum RNA sample sequencing and unigene information
| Name | Contents |
| Raw reads | 39,488,639 |
| Clean reads | 35,547,493 |
| Clean bases | 3.55G |
| GC, % | 53.19 |
| Total no. of unigenes | 297,537 |
| Range length of unigenes, bp | 201–31,002 |
| No. of unigenes in 200–500 bp | 213,620 |
| No. of unigenes in 500–1,000 bp | 54,348 |
| No. of unigenes in 1,000–2,000 bp | 23,624 |
| No. of unigenes in >2,000 bp | 5,945 |
| Mean length of unigenes, bp | 504 |
| N50 of unigenes | 614 |
| N90 of unigenes | 242 |
Fig. S1.
Taxonomic distribution of the annotated portion of the metatranscriptome (by read counts). The percentage represents the combination of total read count number and relative expression of these genes matching each group of organisms.
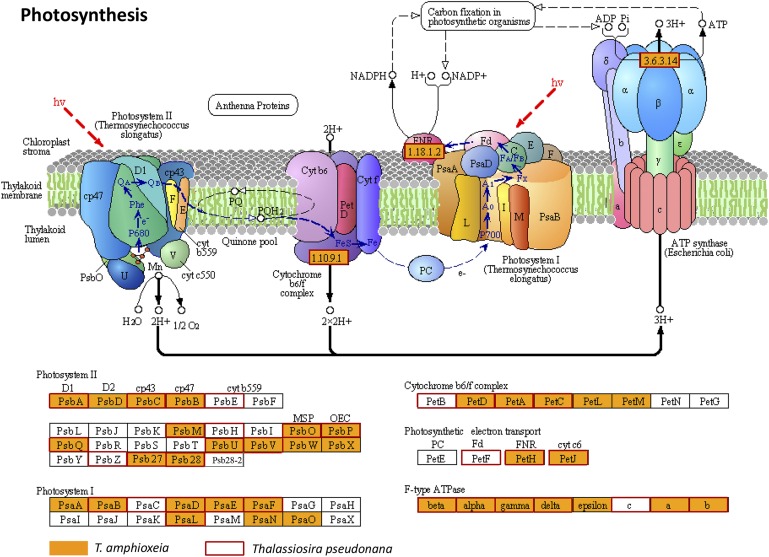
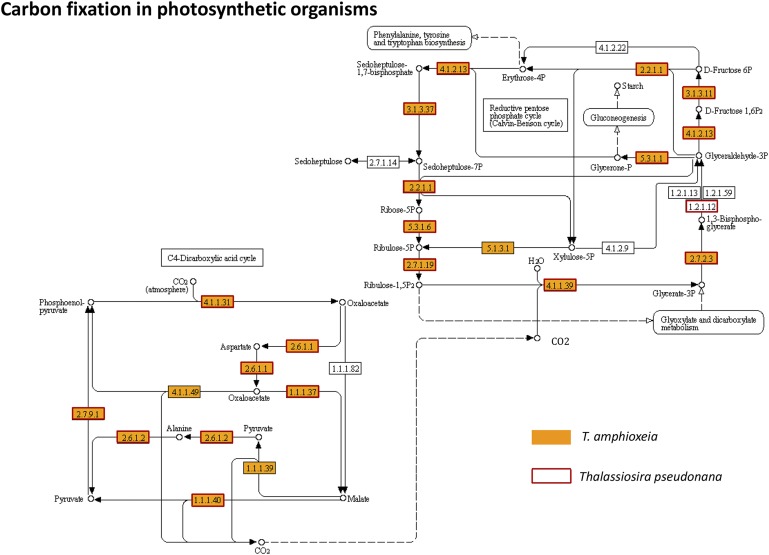
The cryptophyte subset of the metatranscriptome contained all major metabolic and regulatory pathways and plasma membrane transporters expected of an intact alga. For photosynthesis, we retrieved light-harvesting components, ATP synthases, electron transport components, and Calvin cycle and C4 pathways (Figs. S2 and S3 and Datasets S1 and S2), and compared them with those in the diatom Thalassiosira pseudonana (17). In particular, the phycoerythrin gene was expressed at an extremely high level (Table 1), consistent with the very strong orange fluorescence of phycoerythrin inside M. rubrum cells (15). Also highly expressed were chlorophyll a/b-binding proteins (Dataset S1), indicating that T. amphioxeia was actively absorbing light energy for photosynthesis and fighting for photoprotection (18, 19).
Fig. S2.
Photosynthetic proteins in Teleaulax amphioxeia inside M. rubrum (orange-filled boxes) in comparison with those in the diatom Thalassiosira pseudonana (red-outlined boxes).
Fig. S3.
Carbon fixation pathways in T. amphioxeia inside M. rubrum (orange-filled boxes) in comparison with those in the diatom Thalassiosira pseudonana (red-outlined boxes).
Table 1.
Key genes and their expression levels, indicated by RPKM, as evidence that T. amphioxeia within M. rubrum were intact and functionally active and exported photosynthate to M. rubrum
| Key enzyme/protein genes | Function | Subcellular localization | RPKM |
| Photosynthesis | |||
| Phycocyanin | Photosynthesis | Chloroplast | 377 |
| Phycoerythrin | Photosynthesis | Chloroplast | 39,153 |
| Ribulose-bisphosphate carboxylase large chain | Carbon fixation | Chloroplast | 12 |
| Cell-membrane transporters for N-nutrient import and photosynthate export | |||
| AmtB, NH3 channel protein, formerly NrgA | Ammonium transporter | Cell membrane | 142 |
| High-affinity electrogenic NH3/methylammonia transporter | Ammonium transporter | Cell membrane | 321 |
| Likely peptide exporter, ABC superfamily | Peptide exporter | Cell membrane | 25 |
| Exocyst complex component 7 | Exocyst exporter | Cell membrane | 11 |
| Cell division | |||
| DNA polymerase alpha subunit A | Eukaryote DNA replication | Nucleus | 17 |
| DNA primase | Chloroplast DNA replication | Chloroplast | 3 |
| Proliferating cell nuclear antigen | DNA replication and cell division | Nucleus | 90 |
| Cyclin A | Eukaryote cell growth and division | Nucleus | 21 |
| Cyclin B | Eukaryote cell growth and division | Nucleus | 25 |
| Cyclin-dependent kinase 2 | Eukaryote cell growth and division | Nucleus | 34 |
| Gene transcription and translation | |||
| DNA-directed RNA polymerases I, II, and III subunit RPABC5 | Eukaryote RNA polymerase | Nucleus | 34 |
| DNA-directed RNA polymerase subunit beta′ | Chloroplast RNA polymerase | Chloroplast | 5 |
| GTP-binding nuclear protein Ran | RNA transport | Nucleus | 135 |
| mRNA export factor | RNA transport | Nuclear pore | 37 |
| Snurportin-1 | RNA transport | Cytoplasm | 48 |
| Glutamyl-tRNA synthetase | Chloroplast aminoacyl-tRNA biosynthesis | Mitochondrion/chloroplast | 119 |
| Glutaminyl-tRNA synthetase | Eukaryote aminoacyl-tRNA biosynthesis | Eukaryote cytoplasm | 50 |
| 20S proteasome subunit beta 1 | Proteasome biosynthesis | Proteasome | 170 |
| Large subunit ribosomal protein L4 | Chloroplast ribosome biosynthesis | Mitochondrion/chloroplast | 24 |
| Large subunit ribosomal protein L4e | Eukaryote ribosome biosynthesis | Cytoplasm | 276 |
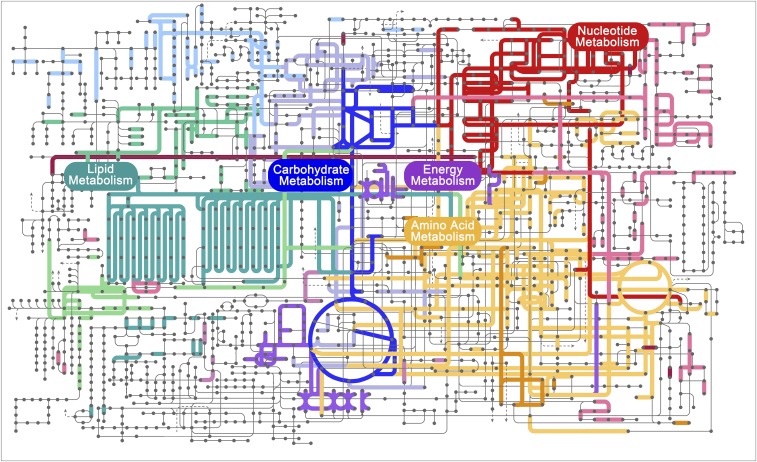
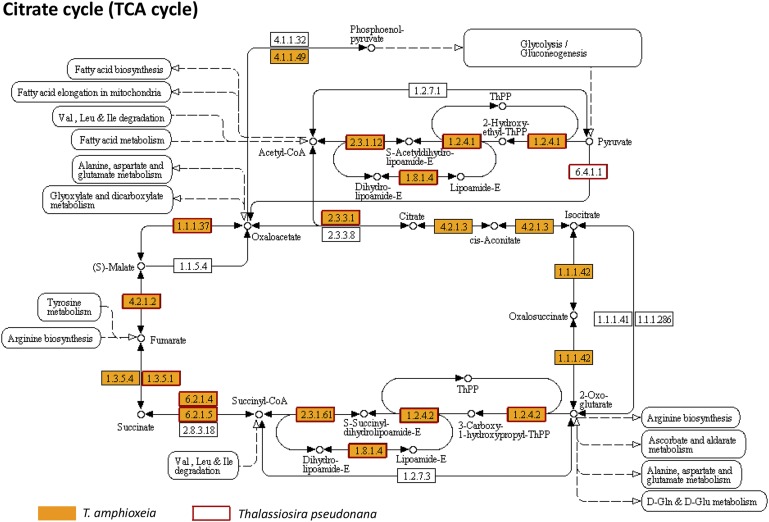
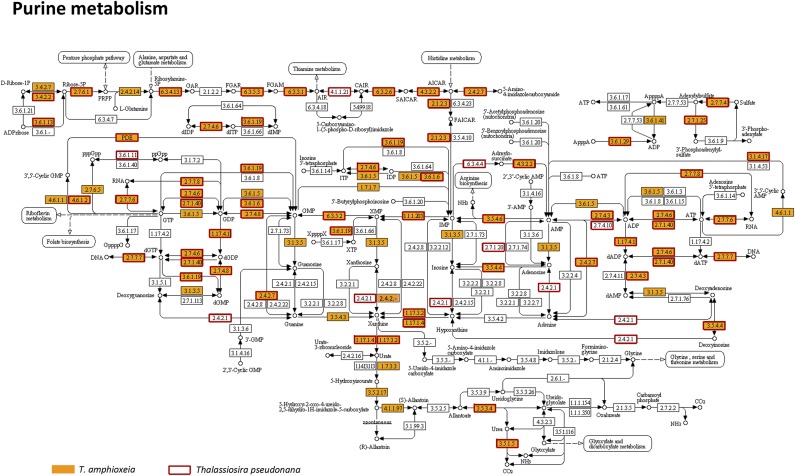
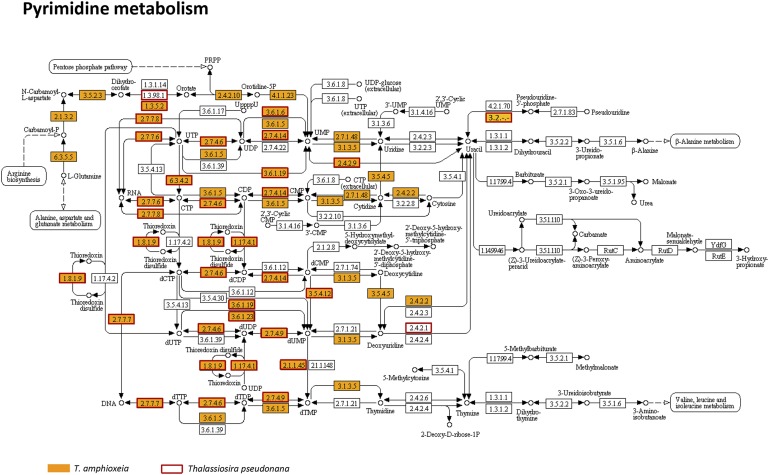
The metatranscriptome allowed us to provide detailed documentation of core metabolic pathways in a cryptophyte, including the tricarboxylic acid (TCA) cycle, pyruvate metabolism, glyoxylate metabolism, glycolysis/gluconeogenesis, pentose phosphate pathway, propanoate metabolism, fructose metabolism, and starch metabolism (Fig. 2, Fig. S4, and Dataset S3). The crucial connections from the TCA cycle to starch (amylose) and PRPP in the pentose phosphate pathway, and from carbohydrates, amino acids, and lipids to the TCA cycle, were uninterrupted (Fig. 2). Most of the linking enzymes were highly expressed in the bloom. In starch metabolism, we found evidence for synthesis of amylose in the chloroplasts from UDP-glucose and ADP-glucose, with abundant transcripts of granule-bound starch synthase and starch synthase 1. Additionally, we detected a cryptophyte starch-binding domain protein gene, consistent with a large starch structure noted in the periplastid of the endosymbiotic T. amphioxeia (Fig. 1C). Furthermore, we retrieved alpha-amylase, beta-amylase, and 4-alpha-glucanotransferase transcripts, evidence of alpha-d-glucose production from starch (Dataset S3). For nucleotide metabolism, almost complete cryptophyte biosynthetic and catabolic pathways of purine and pyrimidine nucleotides were identified, comparable to pathways in Thalassiosira pseudonana (Fig. 2, Figs. S5 and S6, and Dataset S4) (17).
Fig. 2.
Metabolic circuit map constructed from the cryptophyte subset of the metatranscriptome. Highlighted in this circuit are pathways of nucleotide metabolism (red), carbohydrate metabolism (blue), energy metabolism (purple), lipid metabolism (cyan), and amino acid metabolism (yellow) in cryptophytes.
Fig. S4.
Citrate cycle pathway in T. amphioxeia inside M. rubrum (orange-filled boxes) in comparison with that in the diatom Thalassiosira pseudonana (red-outlined boxes).
Fig. S5.
Pathway of purine metabolism in T. amphioxeia inside M. rubrum (orange-filled boxes) in comparison with that in the diatom Thalassiosira pseudonana (red-outlined boxes).
Fig. S6.
Pathway of pyrimidine metabolism in T. amphioxeia inside M. rubrum (orange-filled boxes) in comparison with that in the diatom Thalassiosira pseudonana (red-outlined boxes).
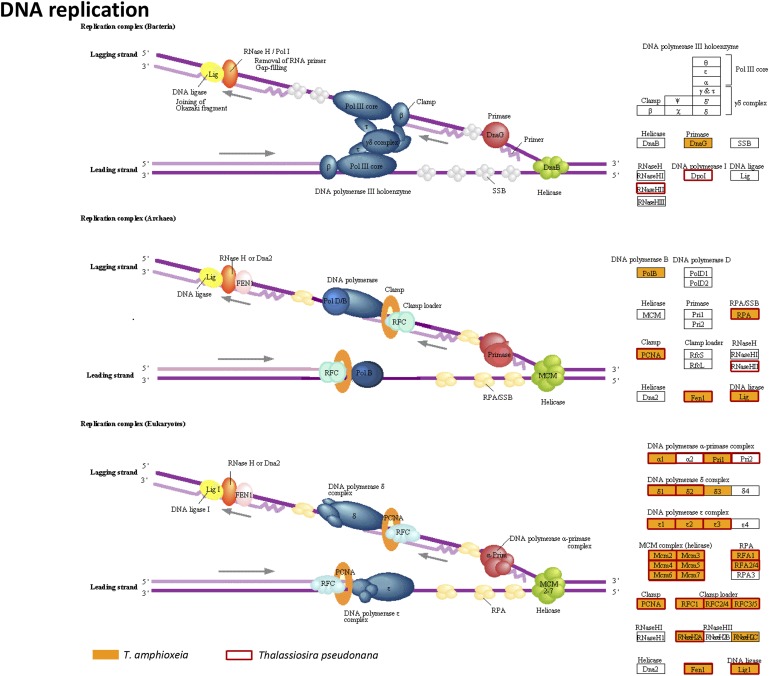
Proteins and enzymes required for key regulatory pathways, including those in DNA replication and repair, gene transcription, translation, folding, sorting, and degradation, were highly enriched in our cryptophyte submetatranscriptome (Datasets S5–S9). Nuclear gene transcripts responsible for DNA replication, mismatch repair, base excision repair, nucleotide excision repair, spliceosomes, ribosomal proteins, aminoacyl tRNA synthetases, RNA transport, and signal recognition were detected (Fig. S7 and Datasets S5–S7). Importantly, we retrieved transcripts of proliferating cell nuclear antigen, DNA polymerases, and other enzymes related to DNA replication, and also detected cyclin, cyclin-dependent kinase 2, and other genes related to eukaryotic cell growth and division (Table 1 and Datasets S5 and S6). This shows that the endosymbiotic T. amphioxeia cells were likely to be proliferating. Meanwhile, transcription and translation genes in the nucleolus, nucleus, nuclear pore, cytoplasm, and chloroplast/mitochondrion were also identified (Table 1 and Datasets S7 and S8).
Fig. S7.
Enzymes of DNA replication in T. amphioxeia inside M. rubrum (orange-filled boxes) in comparison with that in the diatom Thalassiosira pseudonana (red-outlined boxes).
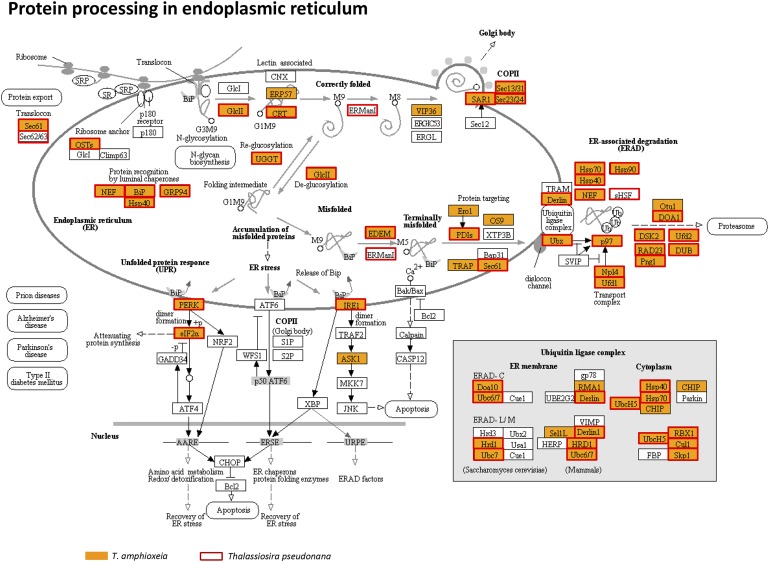
Furthermore, the majority of the proteins involved in protein processing in the endoplasmic reticulum were found (Fig. S8 and Dataset S9), including genes encoding protein modification and transport as well as endoplasmic reticulum-associated degradation and the ubiquitin pathway. We also found mRNAs encoding ubiquitin-mediated proteolysis and proteasome and RNA degradation (Dataset S9).
Fig. S8.
Enzymes involved in the protein processing pathway in T. amphioxeia inside M. rubrum (orange-filled boxes) in comparison with those in the diatom Thalassiosira pseudonana (red-outlined boxes).
The intactness of the plasma membrane and expression of its transporters are key to distinguishing the case of retaining chloroplasts or the organelle complex from that of adopting whole cells as symbionts. We not only observed the membrane around the endosymbiotic cells but also found cDNAs of plasma membrane transporters homologous to those in G. theta, indicating that these were genes expressed in the M. rubrum-harbored T. amphioxeia. These included transporters for inorganic nutrients (nitrate, ammonium, phosphate, and sulfate), organic nutrients (urea), amino acids, and metal ions (Mg2+, Zn2+, Mn2+, and Ca2+) (Table 1 and Dataset S10). Compared with the nitrate transporter [reads per kb of exon per million mapped reads (RPKM) 26; all being high-affinity types], ammonium transporters (RPKM 463) were expressed much more abundantly, including AmtB and a high-affinity electrogenic NH3/methylammonia transporter (Table 1). Meanwhile, we found very abundant transcripts of ammonium transporters (RPKM 695) and a high-affinity nitrate transporter (RPKM 38) that matched counterparts in alveolate species (O. trifallax, Perkinsus marinus, and Vitrella brassicaformis), indicating that the ciliate host was actively expressing these N-nutrient transporters. These results suggest that ammonium was the major form of N nutrient for the Mesodinium–Teleaulax system, and came directly (supplied by) or indirectly (acquired from the external environment) through the host. Because the bloom sample was nearly a T. amphioxeia-monospecific culture entirely “caged” in the cytoplasm of M. rubrum cells (Fig. 1 C and E) and showed a >20:1 T. amphioxeia-to-M. rubrum cell concentration ratio, these results indicate functional cell membranes in both the ciliate and symbiotic cryptophyte and a coordinated function in the endosymbiotic entity for nutrient uptake.
Trafficking between organelles would also indicate an endosymbiont beyond an organelle complex arrayed in the ciliate host. We detected cDNAs encoding transporters in the chloroplast membrane, including glucose-6-phosphate/phosphate and phosphoenolpyruvate/phosphate translocators, which facilitate export of photosynthetically produced carbohydrates from the chloroplast to the cytoplasm in exchange for phosphorus import into the chloroplast. Also retrieved were cDNAs of transporters for phosphate, S-adenosylmethionine, and aspartate/glutamate; of ABC transporters; of sodium/potassium/calcium exchangers in mitochondria; of an acetyl-CoA transporter in the endoplasmic reticulum; and of transporters for nucleotide sugars (GDP-fucose and UDP-galactose) in the Golgi apparatus (Dataset S10).
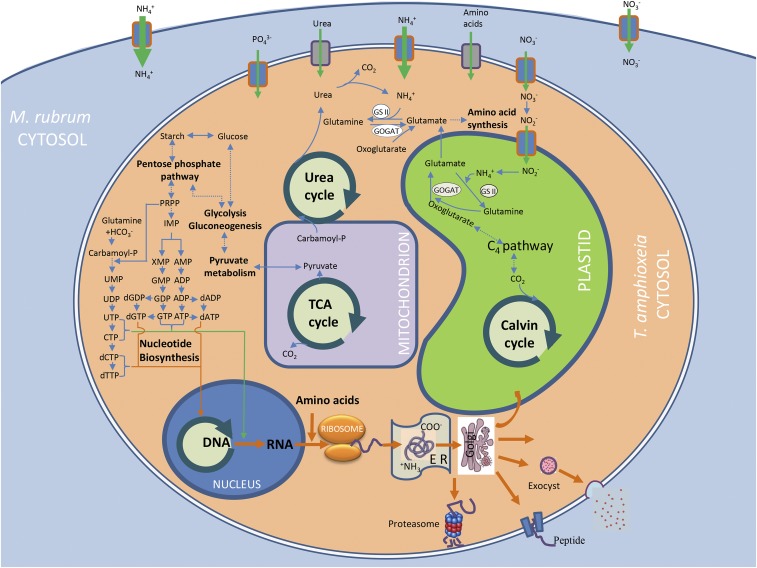
Furthermore, we looked for evidence that the symbiont benefited the host. mRNAs were found that encode likely peptide exporter and exocyst complex components (Table 1), which can transport the peptide or other materials from T. amphioxeia to M. rubrum (Fig. 3). Endomembrane-based protein import and export have been reported in Geminigera cryophila as an endosymbiont within M. rubrum (20). These findings offer evidence that M. rubrum receive photosynthetic products from symbiotic T. amphioxeia or G. cryophila cells.
Fig. 3.
Schematic summary of key components of nutrient transport and metabolism in T. amphioxeia inside M. rubrum as well as photosynthate export from the cryptophyte endosymbiont to the ciliate host. Solid arrows indicate direct steps in a pathway, dashed arrows indicate omission of multiple steps in a pathway, and thick arrows indicate genetic information processing and cargo transport.
All our data in concert demonstrate that the T. amphioxeia plasma membrane, plastid, nucleus, mitochondrion, Golgi apparatus, endoplasmic reticulum, and proteasome were retained intact and metabolically active within M. rubrum during the bloom (Fig. 3 and Table 1). These endosymbiotic T. amphioxeia cells were expressing genes that regulate photosynthesis (including photoprotection), cytoplasmic metabolic pathways, and cell division. More importantly, the two parties of the symbiotic entity seemed to cooperate in nutrient uptake and enabled translocation of photosynthetic products to the host. The necessary regulation and orchestration of all these algal cellular functions would be too extensive for the host ciliate to execute as implied in the model in which only organelles are retained. Rather, our data indicate that the M. rubrum population kept intact the T. amphioxeia cells they ingested, and were promoting their ability to photosynthesize and reproduce and in return benefited from the photosynthetic products (Fig. 3). Consistent with the possibility that the cryptophyte endosymbiont reproduced while in the host, we found that the bloom was equivalent to a bloom of T. amphioxeia at a concentration of 2 to 3 × 107 cells L−1, almost 100 times higher than the highest Teleaulax spp. concentrations we have ever seen in our past 5 y monitoring data in Long Island Sound (∼4 × 105 cells L−1; Dataset S11). However, this cryptophyte was undetectable in ambient water (Fig. 1). Therefore, M. rubrum behaved like a farmer of the cryptophyte.
The Mesodinium-farming-Teleaulax model not only distinguishes itself from the current model of Mesodinium enslaving cryptophyte chloroplasts or the organelle complex, but by promoting the proliferation of the endosymbiotic cells also differs from the conventional whole-cell endosymbiont models in which only binary division synchronous with host division is expected. The disparity from previous studies on Mesodinium–cryptophyte symbiosis mentioned earlier might have arisen from the pairing of different M. rubrum strains [at least five clades have been identified (21)] with different species or strains of the cryptophyte. The difference can be a result of spatially separated host–symbiont coevolution, as predicted by the geographic mosaic of coevolution theory (22). This and the ecological significance in each case should be further studied in the future.
Materials and Methods
Sampling.
Ten liters of surface water was collected in Long Island Sound on September 24, 2012 during a red-tide event, which covered about 20 km2, an estimation based on remote sensing data (15). The sample was transported to the laboratory and kept in a wide-open bucket to allow ample air exchange overnight; two samples (each 50 mL) were collected by centrifugation for 10 min at 5,000 × g (4 °C). The cell pellets were suspended in TRIzol solution and stored at −80 °C until RNA extraction. Another sample was preserved with neutral Lugol’s solution and stored in the dark at 4 °C for microscopic analysis.
Analysis for Identities of the Causative Species and Its Plastids.
Live and fixed samples were observed under an Olympus BX51 epifluorescence microscope. Microscopic observation of the species was done following Garcia-Cuetos et al. (21). M. rubrum cells were isolated with Pasteur pipets, and rinsed carefully with glass fiber membrane (0.7-μm pore size) filtered seawater for subsequent DNA extraction. Twenty of these cells of M. rubrum were resuspended in 0.5 mL DNA lysis buffer and incubated for 48 h at 55 °C. DNA extraction, PCR amplification [using a pair of rDNA SSU primers (23, 24) and a pair of plastid rDNA SSU primers (25) (Table S2)], cloning, and gene sequencing for rDNA SSU gene and plastid rDNA SSU gene were conducted with methods outlined previously (25). Sequence alignment and phylogenetic analyses were conducted as previously reported (15).
Table S2.
Primers used in the present study
Observation of Cell Structures of M. rubrum and Its Symbionts.
Fifty milliliters of bloom sample fixed in 5% (vol/vol) Lugol’s was centrifuged at 5,000 × g for 10 min and resuspended in 2 mL of 5% (wt/vol) buffered glutaraldehyde fixative and fixed at 4 °C for 48 h. The refixed cells were recentrifuged at 5,000 × g for 10 min and resuspended in 0.5 mL of 2% (wt/vol) osmium tetraoxide solution prepared in cacodylate buffer as a poststain and fixative at 4 °C for 12 h. The sediment of osmium-fixed cells was washed in PBS buffer, dehydrated in a graded ethanol/aqueous series and acetone, and embedded in Spurr's resin (SPI-Chem). Ultrathin sectioning, poststaining, and transmission electron microscope (TEM) observation (JEM-100CX II; JEOL) were done as described previously (26).
RNA Isolation and Transcriptome Sequencing.
Total RNA was extracted from the collected cells using a TRIzol Reagent Kit (ThermoFisher Scientific) essentially as reported (27), treated with RNase-free DNase I (TaKaRa) to eliminate residual genomic DNA if any, and concentrated and repurified using the RNeasy MinElute Kit (Qiagen). Then, the extracted RNA was determined using the Qubit RNA Assay Kit by a Qubit 2.0 fluorometer (Life Technologies) and NanoPhotometer spectrophotometer (IMPLEN). RNA integrity was verified (6.4 on a scale of 1 to 10) using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies).
A total of 10 μg RNA was used as input material for Illumina high-throughput sequencing (RNA-seq). The sequencing library was prepared using the NEBNext Ultra Directional RNA Library Prep Kit for Illumina (NEB) following the manufacturer’s recommendations (28). Library quality was assessed on the Agilent Bioanalyzer 2100 system. The library preparation was then sequenced on an Illumina HiSeq 2000 platform and paired-end reads were generated.
Bioinformatic Analysis.
Transcriptome assembly was accomplished using Trinity (29). The resultant nonredundant unigenes were used for BLAST search and annotation against the NCBI nr database and Swiss-Prot database with a 1e-5 value cutoff, euKaryotic Ortholog Groups (KOG) database with a 1e-3 value cutoff, and Protein family (Pfam) Kyoto Encyclopedia of Genes and Genomes (KEGG) database (30–32). Functional annotation by Gene Ontology (GO) terms was analyzed using the Blast2GO program (33). Genes associated with the major pathways and functions elaborated in this paper were manually reanalyzed using BLAST to verify the functional prediction. The metabolic circuit (Fig. 2) was manually checked to ensure the completeness of the pathways and the connectivity of the major nodes. Furthermore, the expression level of each gene was quantified as reads per kb of exon per million mapped reads to the transcriptome (34). Based on the KEGG Orthology (KO), KOG, and other databases, metabolic network pathways in T. amphioxeia were further analyzed using iPath2.0 (pathways.embl.de/iPath2.cgi) (35).
Supplementary Material
Acknowledgments
We thank Kay Howard-Strobel, Huan Zhang, and Brittany Sprecher from the University of Connecticut for taking bloom photos and collecting samples, Teleaulax spp. cell-count data and advice on sample preparation, and help with English, respectively. Yu Zhong from the South China Sea Institute of Oceanology, Chinese Academy of Sciences helped with TEM sample preparation. We are indebted to Diane K. Stoecker from the University of Maryland and George McManus from the University of Connecticut for insightful discussions. Novogene Bioinformatics Technology Co. Ltd. provided transcriptome sequencing services. The project was supported by the International Collaborative Program of the Natural Science Foundation of China (Grant 41129001 to S.L.), National Science Foundation of the United States (Grant OCE-1212392 to S.L.), Science Technology Program Guangzhou of China (Grant 201607010289 to D.Q.), and Natural Science Foundation of China (Grant 41130855 to L.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the National Center for Biotechnology Information (NCBI) BioProject database (ID code PRJNA340945) and Sequence Read Archive (accession no. SRR4098290).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612483113/-/DCSupplemental.
References
- 1.de Vargas C, et al. Tara Oceans Coordinators Ocean plankton. Eukaryotic plankton diversity in the sunlit ocean. Science. 2015;348(6237):1261605. doi: 10.1126/science.1261605. [DOI] [PubMed] [Google Scholar]
- 2.Johnson MD. Acquired phototrophy in ciliates: A review of cellular interactions and structural adaptations. J Eukaryot Microbiol. 2011;58(3):185–195. doi: 10.1111/j.1550-7408.2011.00545.x. [DOI] [PubMed] [Google Scholar]
- 3.Taylor FJR, Blackbourn DJ, Blackbourn J. The red-water ciliate Mesodinium rubrum and its “incomplete symbionts”: A review including new ultrastructural observations. J Fish Res Board Can. 1971;28(3):391–407. [Google Scholar]
- 4.Gustafson DE, Jr, Stoecker DK, Johnson MD, Van Heukelem WF, Sneider K. Cryptophyte algae are robbed of their organelles by the marine ciliate Mesodinium rubrum. Nature. 2000;405(6790):1049–1052. doi: 10.1038/35016570. [DOI] [PubMed] [Google Scholar]
- 5.Crawford DW. Mesodinium rubrum: The phytoplankter that wasn’t. Mar Ecol Prog Ser. 1989;58:161–174. [Google Scholar]
- 6.Stoecker DK, Putt M, Davis LH, Michaels AE. Photosynthesis in Mesodinium rubrum: Species-specific measurements and comparison to community rates. Mar Ecol Prog Ser. 1991;73:245–252. [Google Scholar]
- 7.Lindholm T. Mesodinium rubrum—A unique photosynthetic ciliate. Adv Aquat Microbiol. 1985;3:1–48. [Google Scholar]
- 8.Hibberd DJ. Observations on the ultrastructure of the cryptomonad endosymbiont of the red-water ciliate Mesodinium rubrum. J Mar Biol Assoc U.K. 1977;57:45–61. [Google Scholar]
- 9.Oakley BR, Taylor FJR. Evidence for a new type of endosymbiotic organization in a population of the ciliate Mesodinium rubrum from British Columbia. Biosystems. 1978;10(4):361–369. doi: 10.1016/0303-2647(78)90019-9. [DOI] [PubMed] [Google Scholar]
- 10.Johnson MD, Tengs T, Oldach D, Stoecker DK. Sequestration, performance, and functional control of cryptophyte plastids in Myrionecta rubra. J Phycol. 2006;42(6):1235–1246. [Google Scholar]
- 11.Johnson MD, Oldach D, Delwiche CF, Stoecker DK. Retention of transcriptionally active cryptophyte nuclei by the ciliate Myrionecta rubra. Nature. 2007;445(7126):426–428. doi: 10.1038/nature05496. [DOI] [PubMed] [Google Scholar]
- 12.Taylor FJR, Blackbourn DJ, Blackbourn J. Ultrastructure of the chloroplasts and associated structures within the marine ciliate Mesodinium rubrum (Lohmann) Nature. 1969;224:819–821. [Google Scholar]
- 13.Lindholm T, Lindroos P, Mörk AC. Ultrastructure of the photosynthetic ciliate Mesodinium rubrum. Biosystems. 1988;21(2):141–149. doi: 10.1016/0303-2647(88)90007-x. [DOI] [PubMed] [Google Scholar]
- 14.Hansen JP, Fenchel T. The bloom-forming ciliate Mesodinium rubrum harbours a single permanent endosymbiont. Mar Biol Res. 2006;2(3):169–177. [Google Scholar]
- 15.Dierssen H, et al. Space station image captures a red tide ciliate bloom at high spectral and spatial resolution. Proc Natl Acad Sci USA. 2015;112(48):14783–14787. doi: 10.1073/pnas.1512538112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis BA, et al. Algal genomes reveal evolutionary mosaicism and the fate of nucleomorphs. Nature. 2012;492(7427):59–65. doi: 10.1038/nature11681. [DOI] [PubMed] [Google Scholar]
- 17.Armbrust EV, et al. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science. 2004;306(5693):79–86. doi: 10.1126/science.1101156. [DOI] [PubMed] [Google Scholar]
- 18.Peterson RB, Schultes NP. Light-harvesting complex B7 shifts the irradiance response of photosynthetic light-harvesting regulation in leaves of Arabidopsis thaliana. J Plant Physiol. 2014;171(3–4):311–318. doi: 10.1016/j.jplph.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, et al. The global phosphoproteome of Chlamydomonas reinhardtii reveals complex organellar phosphorylation in the flagella and thylakoid membrane. Mol Cell Proteomics. 2014;13(9):2337–2353. doi: 10.1074/mcp.M114.038281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lasek-Nesselquist E, Wisecaver JH, Hackett JD, Johnson MD. Insights into transcriptional changes that accompany organelle sequestration from the stolen nucleus of Mesodinium rubrum. BMC Genomics. 2015;16:805. doi: 10.1186/s12864-015-2052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Cuetos L, Moestrup Ø, Hansen PJ. Studies on the genus Mesodinium II. Ultrastructural and molecular investigations of five marine species help clarifying the taxonomy. J Eukaryot Microbiol. 2012;59(4):374–400. doi: 10.1111/j.1550-7408.2012.00630.x. [DOI] [PubMed] [Google Scholar]
- 22.Thompson JN. The Coevolutionary Process. Univ of Chicago Press; Chicago: 1994. [Google Scholar]
- 23.McManus GB, Xu D, Costas BA, Katz LA. Genetic identities of cryptic species in the Strombidium stylifer/apolatum/oculatum cluster, including a description of Strombidium rassoulzadegani n. sp. J Eukaryot Microbiol. 2010;57(4):369–378. doi: 10.1111/j.1550-7408.2010.00485.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Lin S. Detection and quantification of Pfiesteria piscicida by using the mitochondrial cytochrome b gene. Appl Environ Microbiol. 2002;68(2):989–994. doi: 10.1128/AEM.68.2.989-994.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu D, Huang L, Liu S, Lin S. Nuclear, mitochondrial and plastid gene phylogenies of Dinophysis miles (Dinophyceae): Evidence of variable types of chloroplasts. PLoS One. 2011;6(12):e29398. doi: 10.1371/journal.pone.0029398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu D, Huang L, Liu S, Zhang H, Lin S. Apical groove type and molecular phylogeny suggests reclassification of Cochlodinium geminatum as Polykrikos geminatum. PLoS One. 2013;8(8):e71346. doi: 10.1371/journal.pone.0071346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin S, Zhang H, Zhuang Y, Tran B, Gill J. Spliced leader-based metatranscriptomic analyses lead to recognition of hidden genomic features in dinoflagellates. Proc Natl Acad Sci USA. 2010;107(46):20033–20038. doi: 10.1073/pnas.1007246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen KD, Brenner SE, Dudoit S. Biases in Illumina transcriptome sequencing caused by random hexamer priming. Nucleic Acids Res. 2010;38(12):e131. doi: 10.1093/nar/gkq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grabherr MG, et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanehisa M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36(Database issue):D480–D484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finn RD, et al. The Pfam protein families database. Nucleic Acids Res. 2008;36(Database issue):D281–D288. doi: 10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Götz S, et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36(10):3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat Methods. 2008;5(7):621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 35.Yamada T, Letunic I, Okuda S, Kanehisa M, Bork P. iPath2.0: Interactive pathway explorer. Nucleic Acids Res. 2011;39(Suppl 2):W412–W415. doi: 10.1093/nar/gkr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.