Magnetite is an iron-oxide mineral that occurs naturally on Earth. Because it is also an important component of many anthropogenic materials (e.g., coal fly ash) and synthetic products (e.g., black toner powders), magnetite can be released to the environment through human activities (1). In PNAS, Maher et al. (2) describe the abundant presence in the human brain of magnetite nanoparticles, some of which they attribute to air pollution. This finding could have major implications.
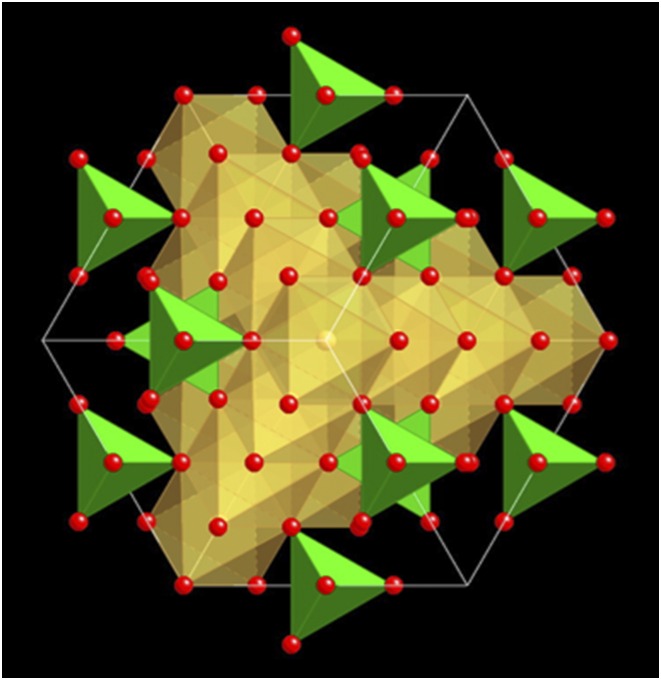
Magnetite belongs to the spinel group. It crystallizes in the cubic crystal system (Fig. 1) and can be described by the general formula Fe2+Fe3+2O4 (3). Magnetite is a common natural phase, occurring in various geological environments, ranging from igneous (e.g., layered ultrabasic rocks, basalts) to sedimentary (e.g., banded iron formations, beach sands) rocks, and to high-grade metamorphic rocks (e.g., schists, skarns), where it can be produced through a multitude of chemical reactions. Due to its tendency to react with oxygen to form hematite (Fe2O3) and various iron oxyhydroxides (e.g., ferrihydrite, goethite), magnetite can be used as a powerful tool to explore oxygen concentrations in rocks during geological processes, changes in the oxygen content of the atmosphere (e.g., early Earth), and redox conditions in near-surface environments (e.g., oxic-anoxic transition zone). Because magnetite is ferrimagnetic, it represents a phase that is essential for paleomagnetic investigations, which help in reconstructing plate tectonics through Earth’s history.
Fig. 1.
Crystal structure of magnetite as viewed along the [111] direction (diagonal through the cube). Green tetrahedra contain ferrous iron (Fe2+), yellowish octahedra contain ferric iron (Fe3+), and oxygen is shown as red spheres.
Biogenic, chemically pure magnetite crystals occur in the bodies of a wide range of organisms within the kingdoms of the Monera, Protista, and Animalia (e.g., magnetotactic microbes, insects, molluscs, fish, birds, mammals) (4). In these organisms, magnetite forms the basis for one type of biophysical mechanism of magnetic field detection, which facilitates orientation and navigation (5, 6). In the human brain, magnetite is also believed to precipitate biologically as part of the iron metabolism (7), but now, in PNAS, Maher et al. (2) suggest that it can originate from an external source.
Air pollution comprises not only gases (e.g., nitrogen oxides, ozone, sulfur dioxide) but also solid particles, which range in size from a few nanometers to several micrometers. These particles, known as particulate matter (PM), are generated through both natural processes and human activity, and are emitted directly into, or formed within, the atmosphere. As a result of atmospheric circulation, the airborne particles in a given environment can be derived from both local and distant sources, such as dry lakes, deserts, fires, smoke stacks, traffic, or mining operations. Magnetite is an abundant constituent of atmospheric PM pollution, especially in the urban environment (8), where it has been identified in diesel exhaust, as brake-abrasion particles, in the air of underground stations, along railway lines, at welding workplaces, and in the emissions from industrial combustion processes.
In addition to having major atmospheric, environmental, and ecological impacts (8), airborne PM may have adverse health effects, both acute and chronic, because with each breath, millions of solid particles, including magnetite, can enter our respiratory system. Once inhaled, coarse particles (generally defined as particles with a diameter >2.5 μm) may be deposited on the surfaces of the conducting airways of the upper respiratory system, whereas smaller particles (<2.5 μm across, PM2.5) can migrate to the deepest parts of the lung where the gas exchange takes place (9). Ultrafine particles (<100 nm), or nanoparticles, may penetrate through the cell tissue that lines the respiratory tract and translocate into the blood circulation and into extrapulmonary organs, but also, via the olfactory nerve, into the central nervous system (10). In PNAS, Maher et al. (2) invoke this latter mechanism for the transfer of air pollution-derived magnetite nanoparticles to the brains of the studied individuals. These authors use the mostly spherical shapes of the magnetite as one of the main arguments for their hypothesis: Spherical shapes are typical of combustion-derived particles (e.g., in diesel exhaust) in contrast to abrasion-derived particles (e.g., brake-wear particles), which are typically irregularly shaped and angular, or to endogenous particles, which tend to be euhedral because they grew in situ (e.g., within the brain) (7). The electron microscope images presented by Maher et al. (2) document that two types of magnetite, spherical and euhedral, are present in the studied brains, suggesting that they were derived from two different sources, one external (from air pollution) and one internal (i.e., biogenic). This conclusion is further supported by the presence of other transition-metal nanoparticles, which are common in airborne PM from polluted areas.
One of the questions that arises from the discovery of externally derived magnetite in brain tissue is whether or not the abundant additional magnetite adversely affects human health. It is well known from epidemiological and toxicological studies that exposure to PM2.5 is linked to increases in mortality and hospital admissions due to respiratory and cardiovascular diseases (11). There is increasing evidence that coarser particles may also produce deleterious health effects (12). In addition to being dependent on size, however, the interactions are influenced by other particle characteristics, including structure, chemical composition, shape, surface area and reactivity, sorptive properties, and solubility. The adverse health effects include chronic bronchitis, exacerbation of asthma, fibrosis, and lung cancer (13). The mechanisms behind these diseases, as well as their dependence on particle properties, are still poorly known. The most likely mechanisms involve the excessive production of free radicals [e.g., reactive oxygen species (ROS)], which can lead to oxidative damage to cell membranes, proteins, and DNA, as well as to the release of chemical substances that trigger and perpetuate inflammation (14, 15).
In regard to the human health effects of magnetite, published data exist for both the brain and the respiratory system. For example, the presence in the brain of magnetite may be linked to several neurodegenerative diseases, including Alzheimer’s disease, and oxidative stress appears to play a key role in the pathogenesis (16, 17). In vitro experiments with human lung cells, which were exposed for 24 h to different magnetite size fractions (including nanoparticles) and doses, revealed that the studied particles, although being only slightly cytotoxic, led to increased ROS formation, mitochondrial damage, and genotoxic effects (18). The results allowed for the conclusion that ROS formation plays an important role in the genotoxicity of magnetite in lung cells. On the other hand, magnetite nanoparticles might be considerably less toxic when surface-modified (i.e., coated) (19).
The presence of magnetite in humans, however, also has other potential implications, including possible biological disorders linked to the weak magnetic fields generated by cellular phones, electric power lines, and appliances, or high-field saturation effects from exposure to strong magnetic fields during MRI procedures (7). At the same time, nanoparticles of magnetite are of special interest in the biomedical sciences, because they can be used as carriers for targeted drug delivery (20). Moreover, magnetite nanoparticles can be exploited for hyperthermia-based cancer therapy, where the heat induced by application of an alternating magnetic field causes necrosis of cancer cells but does not damage the surrounding normal tissue (21). Various researchers have further proposed that endogenous magnetite might play a key role in perception, transduction, and long-term storage of information in the human brain and in other organisms (22).
The occurrence of magnetite in cell tissues therefore represents an intriguing dichotomy: On the one hand, the mineral can play a key role in magnetoreception and navigation, and thus survival, of various types of organisms, and on the other hand, it can impart deleterious effects in humans, especially when they are exposed to high PM concentrations in polluted urban environments.
Footnotes
The author declares no conflict of interest.
See companion article on page 10797 in issue 39 of volume 113.
References
- 1.Gminski R, et al. Genotoxic effects of three selected black toner powders and their dimethyl sulfoxide extracts in cultured human epithelial A549 lung cells in vitro. Environ Mol Mutagen. 2011;52(4):296–309. doi: 10.1002/em.20621. [DOI] [PubMed] [Google Scholar]
- 2.Maher BA, et al. Magnetite pollution nanoparticles in the human brain. Proc Natl Acad Sci USA. 2016;113(39):10797–10801. doi: 10.1073/pnas.1605941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowles JFW, Howie RA, Vaughan DJ, Zussman J. Non-Silicates: Oxides, Hydroxides and Sulphides. 2nd Ed The Geological Society; Bath, UK: 2011. [Google Scholar]
- 4.Kirschvink JL, Walker MM, Diebel CE. Magnetite-based magnetoreception. Curr Opin Neurobiol. 2001;11(4):462–467. doi: 10.1016/s0959-4388(00)00235-x. [DOI] [PubMed] [Google Scholar]
- 5.Mouritsen H, Ritz T. Magnetoreception and its use in bird navigation. Curr Opin Neurobiol. 2005;15(4):406–414. doi: 10.1016/j.conb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Kirschvink JL, Winklhofer M, Walker MM. Biophysics of magnetic orientation: Strengthening the interface between theory and experimental design. J R Soc Interface. 2010;7(Suppl 2):S179–S191. doi: 10.1098/rsif.2009.0491.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirschvink JL, Kobayashi-Kirschvink A, Woodford BJ. Magnetite biomineralization in the human brain. Proc Natl Acad Sci USA. 1992;89(16):7683–7687. doi: 10.1073/pnas.89.16.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gieré R, Querol X. Solid particulate matter in the atmosphere. Elements. 2010;6:215–222. [Google Scholar]
- 9.Plumlee GS, Morman SA, Ziegler TL. The toxicological geochemistry of Earth materials: An overview of processes and the interdisciplinary methods used to understand them. Rev Mineral Geochem. 2006;64:5–57. [Google Scholar]
- 10.Oberdörster G, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16(6-7):437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 11.Englert N. Fine particles and human health--a review of epidemiological studies. Toxicol Lett. 2004;149(1-3):235–242. doi: 10.1016/j.toxlet.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Brunekreef B, Forsberg B. Epidemiological evidence of effects of coarse airborne particles on health. Eur Respir J. 2005;26(2):309–318. doi: 10.1183/09031936.05.00001805. [DOI] [PubMed] [Google Scholar]
- 13.Fubini B, Fenoglio I. Toxic potential of mineral dusts. Elements. 2007;3:407–414. [Google Scholar]
- 14.Donaldson K, Tran CL. Inflammation caused by particles and fibers. Inhal Toxicol. 2002;14(1):5–27. doi: 10.1080/089583701753338613. [DOI] [PubMed] [Google Scholar]
- 15.Schoonen MAA, et al. Mineral-induced formation of reactive oxygen species. Rev Mineral Geochem. 2006;64:179–221. [Google Scholar]
- 16.Pankhurst Q, Hautot D, Khan N, Dobson J. Increased levels of magnetic iron compounds in Alzheimer’s disease. J Alzheimers Dis. 2008;13(1):49–52. doi: 10.3233/jad-2008-13105. [DOI] [PubMed] [Google Scholar]
- 17.Tabner BJ, Mayes J, Allsop D. Hypothesis: Soluble aβ oligomers in association with redox-active metal ions are the optimal generators of reactive oxygen species in Alzheimer’s disease. Int J Alzheimers Dis. 2010;2011:546380. doi: 10.4061/2011/546380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Könczöl M, et al. Cytotoxicity and genotoxicity of size-fractionated iron oxide (magnetite) in A549 human lung epithelial cells: Role of ROS, JNK and NF-κB. Chem Res Toxicol. 2011;24:1460–1475. doi: 10.1021/tx200051s. [DOI] [PubMed] [Google Scholar]
- 19.Mesárošová M, et al. The role of reactive oxygen species in the genotoxicity of surface-modified magnetite nanoparticles. Toxicol Lett. 2014;226(3):303–313. doi: 10.1016/j.toxlet.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Duguet E, Vasseur S, Mornet S, Devoisselle JM. Magnetic nanoparticles and their applications in medicine. Nanomedicine (Lond) 2006;1(2):157–168. doi: 10.2217/17435889.1.2.157. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol J. 2011;6(11):1342–1347. doi: 10.1002/biot.201100045. [DOI] [PubMed] [Google Scholar]
- 22.Banaclocha MAM, Bókkon I, Banaclocha HM. Long-term memory in brain magnetite. Med Hypotheses. 2010;74(2):254–257. doi: 10.1016/j.mehy.2009.09.024. [DOI] [PubMed] [Google Scholar]