Significance
Transcription initiation by RNA polymerase (RNAP) is a highly regulated rate-limiting step in many genes and involves numerous intermediate states that remain incompletely understood. Here, we report the characterization of a previously hypothesized slow initiation pathway involving RNAP backtracking and pausing. This backtracked and paused state is observed when all nucleoside triphosphates (NTPs) are present at physiologically relevant concentrations, but becomes more prevalent with unbalanced NTP levels, which may occur in vivo under conditions of metabolic stress. Pausing and backtracking in initiation may play an important role in regulating RNAP transcription. Moreover, similar RNA backtracked states may contribute to promoter-proximal pausing among eukaryotic RNA polymerase II enzymes.
Keywords: transcription, pausing, backtracking, RNAP, RNA polymerase
Abstract
Initiation is a highly regulated, rate-limiting step in transcription. We used a series of approaches to examine the kinetics of RNA polymerase (RNAP) transcription initiation in greater detail. Quenched kinetics assays, in combination with gel-based assays, showed that RNAP exit kinetics from complexes stalled at later stages of initiation (e.g., from a 7-base transcript) were markedly slower than from earlier stages (e.g., from a 2- or 4-base transcript). In addition, the RNAP–GreA endonuclease accelerated transcription kinetics from otherwise delayed initiation states. Further examination with magnetic tweezers transcription experiments showed that RNAP adopted a long-lived backtracked state during initiation and that the paused–backtracked initiation intermediate was populated abundantly at physiologically relevant nucleoside triphosphate (NTP) concentrations. The paused intermediate population was further increased when the NTP concentration was decreased and/or when an imbalance in NTP concentration was introduced (situations that mimic stress). Our results confirm the existence of a previously hypothesized paused and backtracked RNAP initiation intermediate and suggest it is biologically relevant; furthermore, such intermediates could be exploited for therapeutic purposes and may reflect a conserved state among paused, initiating eukaryotic RNA polymerase II enzymes.
Transcription in Escherichia coli comprises three stages: initiation, elongation and termination. Initiation, which is typically the rate-limiting and the most regulated stage of transcription, is by itself a complex, multistep process consisting of the following successive steps (1, 2): (i) association of RNA polymerase (RNAP) core enzyme (subunit composition α2ββ′ω) with the promoter specificity factor σ (such as σ70 for transcription of housekeeping genes) to form RNAP holoenzyme; (ii) binding of holoenzyme to the −10 and −35 DNA elements in the promoter recognition sequence (PRS) upstream to the transcription start site (TSS) to form closed promoter complex (RPc); (iii) isomerization of RPc through multiple intermediates into an open promoter complex (RPo), in which a ∼12-bp DNA stretch (bases at registers −10 to +2) is melted to form a transcription bubble, the template DNA strand is inserted into RNAP major cleft positioning the base at register +1 of TSS at the active site, the nontemplate strand is tightly bound by σ70 and the downstream DNA duplex (bases +3 up to +20) is loaded into RNAP β′ DNA-binding clamp; (iv) an abortive initiation step (AI), where binding of nucleoside triphosphate (NTPs) and the start of RNA synthesis leads to formation of an initial transcribing complex (RPITC) followed by RNAP cycling through multiple polymerization trials via a DNA scrunching mechanism (3, 4), release of short “abortive transcripts” and repositioning itself in RPO for a new synthesis trial (5–7); and finally, (v) RNAP promoter escape, when enough strain is built in the enzyme, the σ70 undergoes structural transition to relieve blockage of the RNA exit channel in RNAP and loses its grip on the PRS, nascent RNA enters the RNA exit channel and transcription enters the elongation stage.
In AI, the interactions between σ70 and the PRS limit the lengths of abortive transcripts. The stronger these interactions are, the longer the time RNAP will spend cycling in AI (8) and the longer the lengths of abortive transcripts will be (9). For these reasons, after establishing tight promoter interactions, transcription initiation is rather slow. Transcription elongation, on the other hand, is a very efficient and fast process (10–13). However, in elongation, RNAP may encounter specific sequences that cause transcriptional pausing, sometimes accompanied by backtracking of the RNA chain pushing its 3′ end into the RNAP secondary channel (14), the site of NTPs entry (15). Pausing and pause release in elongation constitute additional steps (discussed below).
Whereas the basic biochemical steps of RNA polymerization (including NTP entrance and insertion, phosphodiester bond formation, pyrophosphate removal and RNAP translocation) are the same both in initiation and in elongation, their overall rates are far slower in initiation due to the structural differences between these states (16–18). In particular, the strong σ70–PRS interactions (absent in elongation) (19–21) and the interactions (steric and electrostatic) of the acidic tip of the σ70 region 3.2 (σR3.2, which blocks the RNA exit channel) and the 5′ end of the nascent transcript (22–25) contribute most to the slow rate of initiation. Nascent RNAs of up to 9 nt in length are stabilized by hybridization to the melted template DNA in the RNA:DNA hybrid (18, 26–28). Although RNAP can accommodate an RNA of up to 14 nt long (i.e., 9 nt in RNA:DNA hybrid + 5 nt in RNA exit channel) (29), the transition into elongation, whereby the RNA exit channel blockage by σR3.2 is relieved, begins when RNA reaches the length of 11 nt (16, 30–32). After removal of the exit channel blockage, RNAP undergoes a global conformational change, σ70 loses its grip on the PRS and RNAP processively and rapidly elongates until it reaches the termination signal (23).
In what ways can RNAP overcome the barrier between initiation and elongation? Previous experimental data suggested that in AI, RNAP scrunches downstream DNA into its active site (3, 4). Because σ70 holds the −10 and −35 DNA elements tightly, the dsDNA upstream to the transcription bubble stays as a duplex, whereas the 10 DNA bases in the noncoding part of the transcription bubble (bases −10 to −1 in both strands) become compressed. This results in a strain buildup into what is known as the “stressed intermediate” complex (5, 33). Additionally, the longer the initially transcribed nascent RNA is, the closer its negatively charged 5′ end will be to the acidic tip of σR3.2 (19, 24). Simultaneously, a longer nascent transcript makes more stable RNA:DNA hybrid, which, in turn, helps counteract strain from the compressed DNA bubble and electrostatic repulsion from the acidic tip of σR3.2. In AI, transcripts are held in RNAP until reaching a certain length, above which the strain is relieved either through RNA backtranslocation and its abortive release through the secondary channel (14, 34–36) or by pushing σR3.2 and unblocking the RNA exit channel (22–25).
The electrostatic repulsion between the acidic tip of σR3.2 and the negatively charged 5′ end of the nascent transcript should decrease with each backtranslocation step (i.e., the distance between the negatively-charged groups increases). Nascent transcripts >4 nt in length could be stabilized in a backtracked state resulting in initiation pausing without immediate abortive release. Similar situations in elongation stabilize the nascent transcript in the pretranslocated state (37–41), eventually causing the nascent RNA 3′ end to disengage from the active site, backtrack into the secondary channel and pause transcription. The backtracked RNA can be cleaved either by the intrinsic endonucleolytic activity of RNAP (very inefficient at physiological pH) or by the action of extrinsic transcript cleavage factors GreA and GreB (TFIIS in eukaryotic RNA polymerase II system (42)). This cleavage leads to realignment of the newly formed RNA 3′-terminus with the active site and reactivation of transcription (pause release) (43–45). Due to these parallels, we wanted to examine if backtracking and pausing occur in transcription initiation by RNAP as well.
Previously it has been shown that stalling of transcription initiation occurs in strong promoters (8, 46). In addition, it has been shown that GreA and GreB induce RNA cleavages in AI, reduce abortive cycling and stimulate RNAP transition to elongation in vitro and in vivo at certain promoters (5, 25, 47–55). Linking these biochemical studies to the aforementioned endonucleolytic activity that the GreA–RNAP complex has in elongation, it was hypothesized that RNAP backtracking may occur in initiation and that GreA factors, as in elongation, act upon the nascent RNA bases that enter into the secondary channel of RNAP through backtracking. To directly address this question and to study the mechanism of transcription initiation in greater detail, we developed a solution-based, single-round quenched kinetics transcription assay that measures the kinetics of runoff transcript production. This assay was initially used to assess the kinetics of exit out of NTP-starved RPITC states, using an E. coli transcription system reconstituted from native RNAP core enzyme and σ70. Such NTP-starvation experiments provided us with reliable means to interrogate RNAP transcription from specific initiation states and offered mechanistic insights that were further validated in the follow-up experiments with minimal or no starvation. In addition to quenched kinetics assays of runoff RNA, we performed gel-based in vitro transcription assays that focused on kinetics of abortive products. Finally, we carried out single-molecule magnetic tweezers experiments to monitor changes in transcription bubble size during transcription initiation by RNAP. Altogether, we report here the detection and direct observation of RNAP in a backtracked, paused state during early stages of transcription initiation. Although previously hypothesized, our results establish paused, backtracked RNAP as a bona fide initiation intermediate. This observation could have potential applications for molecular therapeutics and mechanistic implications for mammalian RNA polymerase II enzymes.
Results and Discussion
Single-Round Transcription Quenched Kinetics Assay.
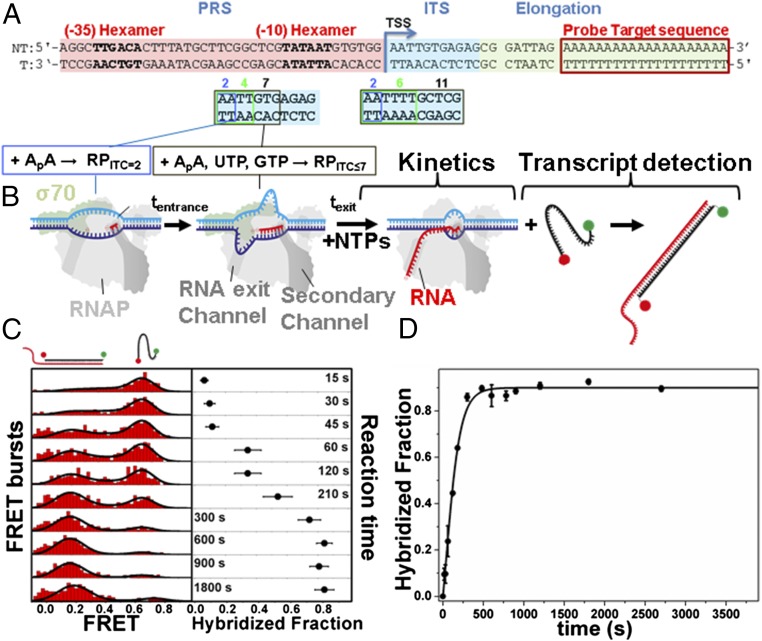
We developed a single-round quenched kinetics assay (Fig. 1) to probe the kinetics of transcription by directly counting the number of transcripts produced over time. Using this assay, we initially examined the kinetics of E. coli RNAP transcription from distinct RPITC states generated via NTP starvation (Fig. 1 A and B). The assay was based on quantification of single runoff transcripts by hybridization with a doubly labeled ssDNA probe (Fig. 1B). The number of hybridized probes (and hence the number of transcripts) was accurately determined using microsecond alternating-laser excitation (µsALEX)-based fluorescence-aided molecule sorting (ALEX-FAMS) (56, 57) (Fig. 1C). ALEX-FAMS is a method based on single-molecule Förster resonance energy transfer (smFRET) (58). A significant advantage of smFRET and ALEX-FAMS is the ability to identify distinct populations in a model-free manner, simply by counting single-molecule events of one sort (with a given FRET efficiency population) and comparing their number to single-molecule events of another sort (representing a distinct FRET efficiency population) (56, 57, 59, 60) (Fig. 1C). Hence, FAMS is a suitable method for the quantification of runoff transcripts at picomolar probe concentrations.
Fig. 1.
Single-round transcription quenched kinetics assay. (A) Representative promoter sequence used here (lacCONS promoter) to show how by changing the initially transcribed sequence (ITS; cyan), different NTP-starved states can be generated (RPITC=2, RPITC≤4,6,7, RDE=11). Other regions of the promoter include the promoter recognition sequence (PRS; pink) and the elongation sequence (yellow), including a probe target complementary sequence (red). All promoters measured are described in Fig. S1. (B) Schematic of RNAP runoff transcription starting from a particular NTP-starved state (incubation with a partial set of NTPs for tentrance). Upon supplementing all NTPs, transcription kinetics start and transcripts are quantified via hybridization to a ssDNA FRET probe for different incubation times (texit). (C) Example of quenched kinetics data generated from quantification of runoff transcripts. The example follows one repetition of the kinetics exiting from RPITC=2. (D) As an example for kinetic curve extraction, average runoff kinetics from various RPITC=2 are shown. The data points are averages of three repeats and the error bars are the SDs about these averages. The data are represented as points and the solid line represents the best-fit result to the model described in Methods. The best-fit values of the model parameters are shown in Table S1.
Our experimental design circumvented potential drawbacks of the quenched kinetics assay. Because we used low concentrations of RNAP and promoter DNA, the probability for RNAP reassembly/reinitiation was low, whereas single-round transcription reaction conditions were achieved kinetically and thermodynamically (61, 62) (details in Supporting Information, Rationale of the Single-Round Character of the Quenched Kinetics Transcription Assay). Furthermore, we designed the DNA template so that the probe hybridization sequence would anneal at the end of the fully elongated RNA (Fig. 1A); in this way, probe hybridization would not interfere with transcription initiation but instead report on successful promoter escape. Also, because transcription initiation in many genes is much slower than elongation (i.e., initiation is typically rate limiting) (63), the synthesis of relatively short, yet full-length RNA products (39-base and 41-base transcripts for the promoters studied here) reflected the rate of transcription initiation.
The ssDNA probe was doubly labeled with a FRET pair. When free in solution, the probe yields a single FRET population with a peak FRET efficiency of E ∼ 0.75 (Fig. 1C). Hybridization of the ssDNA FRET probe with the elongation part of the runoff transcript yields a FRET efficiency population with a lower peak value of E ∼ 0.3, due to the probe being stretched via hybridization to the 20A target sequence segment of the runoff transcript (Fig. 1 A and C). Correct assessment of transcription initiation rates by the quenched kinetics assay requires (i) formation of a stable initial state, (ii) addition of NTPs at t = 0, (iii) efficient and rapid quenching of the reaction at selected times, (iv) full hybridization of the ssDNA FRET probe to transcripts and (v) prevention of RNA degradation. As outlined below, our experiments were conducted under conditions that satisfied each of these requirements.
-
i)
The formation of a stable initial transcribing state was achieved by using partial sets of NTPs that limited transcript polymerization to a given maximal length, which was dependent on the initially transcribed sequence (ITS) of the promoter DNA. We used the ITS of either the lacCONS (64, 65) (Fig. 1A and Fig. S1) or T5N25 (4) (Fig. S1) promoters, which allowed production of abortive transcripts of varying maximal lengths upon addition of different partial NTP sets (Fig. S1). Stable open complexes were formed by adding an initiating dinucleotide to achieve RPITC=2 (control measurements in Figs. S2 and S3; detailed discussion in Supporting Information, Initiating Dinucleotide With/Without a 5′-Triphosphate Group). To stabilize an RPITC state (RPITC≤i, i ϵ [4,6,7]) or an RNAP–DNA elongation complex state (RDE=11), RPITC=2 was incubated for a given time, tentrance, in the presence of a partial set of NTPs (Fig. 1B).
-
ii)
In the absence of specific NTPs, NTP-starved RPITC or RDE states should not produce runoff transcripts. The stability of NTP-starved RPITC or RDE states was verified by negative control experiments, in which ssDNA FRET probe ALEX-FAMS measurements were performed with a limited set of NTPs, whereby hybridization of the FRET probe was not observed, and hence no runoff transcript was produced. Only after all four NTPs were added were runoff transcripts detected. Therefore, for the purposes of the assays described herein, the time at which all NTPs were added was set as t = 0.
-
iii)
The kinetics of runoff transcript production, starting from an NTP-starved state, were measured using a constant tentrance = 40 min, followed by a varying incubation time with all four NTPs (texit), followed by efficient quenching of the transcription reaction. tentrance = 40 min was selected to be in the plateau of the measured ∼7-min entrance kinetics (Fig. S4). Guanidinium hydrochloride (GndHCl; 0.5 M) served both as a reaction quencher (Fig. S5) and as an enhancer of hybridization of the ssDNA FRET probe to the runoff transcript (Fig. S6).
-
iv)
After quenching the transcription reaction, the 20-base ssDNA FRET probe was added. The hybridization of the ssDNA FRET probe to the elongation segment of the runoff transcript was probed by ALEX-FAMS (Fig. 1C). The number of runoff transcripts per time point was calculated from the results of a global fit to the ALEX-FAMS–derived FRET histograms to a two-Gaussian model (Fig. 1C and Supporting Information, μsALEX-smFRET Analysis for the Quantification of Transcription Kinetics). Then the fraction of events in the low-FRET subpopulation, “hybridized fraction” (the number of runoff transcripts produced, relative to known amount of ssDNA FRET probe) is plotted as a function of texit (Fig. 1C). Repeats of the same measurement are performed to produce reliable averaged kinetic curves (as in Fig. S7) that can be later used for curve fitting (Fig. 1D).
-
v)
Throughout the experiments, an RNase inhibitor was added to the reaction mixture to prevent degradation of RNA products.
Slower Transcription Initiation Kinetics from Select NTP-Starved States.
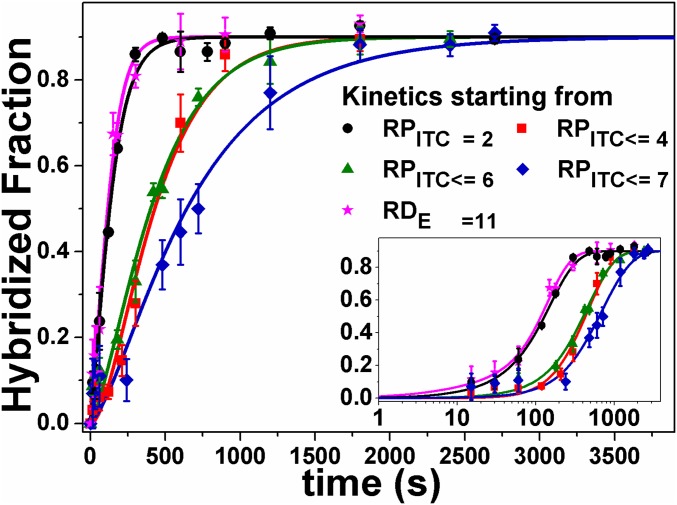
Because promoter escape is the rate-limiting step in initiation, we anticipated that RNAP transcription kinetics starting from different RPITC states (“exit kinetics”) would be similar. However, we found that exit kinetics from the RPITC≤4, RPITC≤6, or RPITC≤7 states were slower than from RPITC=2 state (Fig. 2). In fact, whereas exit kinetics from RPITC=2 were similar to those of RNAP already in the elongation state (RDE=11), the exit kinetics from RPITC≤7 were at least 3.5 times slower (Table S1). These results suggested the existence of a previously hypothesized paused state in RNAP transcription initiation (RPITC≤i*; see Fig. 5). Importantly, this state was transient and overall RNAP activity remained unchanged, given that all “delayed” RNAP complexes (RPITC≤4, RPITC≤6, or RPITC≤7) eventually transitioned to elongation (Fig. 2).
Fig. 2.
Quenched kinetics transcription results identify an initiation-related stalled state. Shown are runoff kinetics from various NTP-starved states. Kinetics starting from late initiation states (e.g., RPITC≤7, blue) are slower than from an earlier initiation state (e.g., RPITC=2, black). The data are represented as points and solid lines represent best-fit results to the model described in Methods. The best-fit values of the model parameters are shown in Table S1.
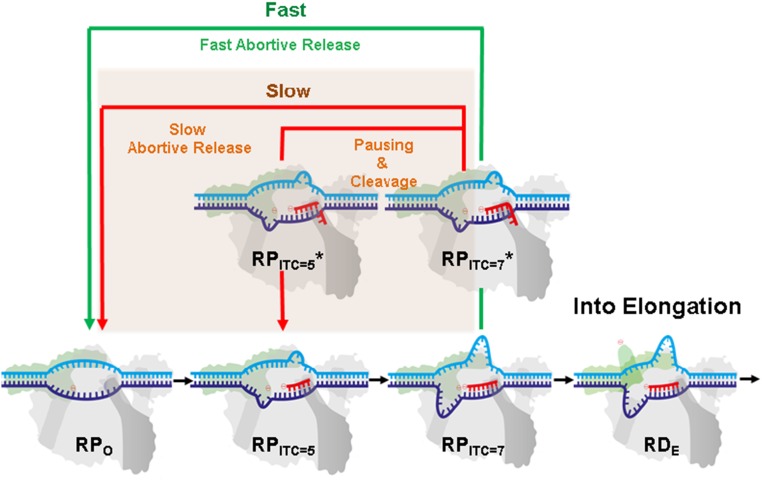
Fig. 5.
A modified transcription initiation model. RNAP transcription initiation branches to (i) promoter clearance and transitions into elongation (black arrows) or into (ii) release of abortive transcripts (green and red arrows). After initial backtracking steps (e.g., from RPITC=7 to RPITC=7*), the complex can continue with either (iii) fast abortive transcript release (classic model, green arrow) or (iv) transition into a paused and backtracked state. Exit from the paused–backtracked state can occur either by successive slow backtracking steps (red arrow) or through intrinsic cleavage of RNA bases that are in the secondary channel, which prepares RNAP in, e.g., the RPITC=5 state. Upon cleavage, the complex can release the abortive transcript or reestablish RNA polymerization (e.g., from the RPITC=5 state).
Delayed Initiation Kinetics Are Associated with Backtracking.
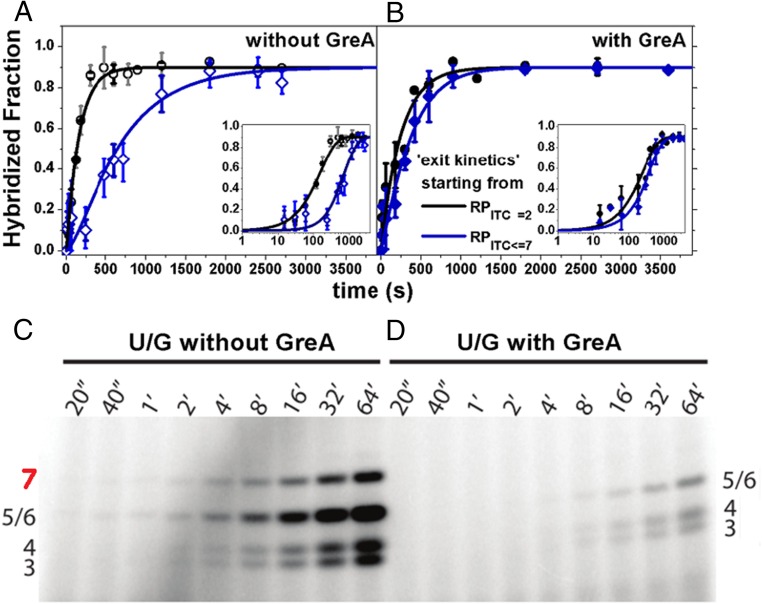
It is well established that elongating RNAP can backtrack and pause (44, 45). In such circumstances, the nascent RNA 3′ end back-translocates into the secondary channel, where it can undergo endonucleolytic cleavage by the GreA–RNAP complex (25, 44, 48, 66). To test whether delayed exit kinetics for RPITC≤7 were associated with backtracking during initiation, we assessed the effect of GreA, using our single-round quenched kinetics assay. As shown in Fig. 3 A and B, the addition of GreA at physiologically relevant concentration of 1 µM accelerated the exit kinetics from RPITC≤7 relative to the exit kinetics from RPITC=2 (∼50% recovery from the RPITC≤7* stalled state; Methods and Table S1). These results were consistent with GreA-dependent release of RNAP from a backtracked and paused state in elongation (44, 45, 47).
Fig. 3.
GreA suppresses the kinetic delay in transcription initiation. (A) Runoff transcription kinetics are slower when starting from RPITC≤7 (blue) than from RPITC=2 (black) (Fig. 2). (B) With 1 µM GreA, the delay in transcription initiation is reduced. The data are represented as points and solid lines represent best-fit results to the model described in Methods. The best-fit values of the model parameters are shown in Table S1. (C and D) Gel-based abortive initiation kinetics: Without GreA, NTP-starved RPITC≤7 produced abortive transcripts up to 7 bases long, whereas the 7-base product was not produced with 1 µM GreA, suggesting 2 bases of 3′-backtracked RNA were cleaved by RNAP in a GreA-catalyzed reaction during initiation. Band assignment is provided in Fig. S8A and the accompanying legend.
To further test the effect of GreA during RNAP transcription initiation, we performed in vitro transcription assays in which 32P-labeled abortive transcripts were quantified following polyacrylamide gel electrophoresis (PAGE). This enabled identification of various abortive transcripts (band assignment in Fig. S8A and accompanying legend) and thus provided a means to determine whether GreA catalyzed the cleavage of short transcripts during transcription initiation. As shown in Fig. 3 C and D, the production of the 7-base abortive RNA transcript was significantly suppressed in the presence of GreA. Because GreA stimulates cleavage only of “backtracked” RNA (i.e., when the RNA 3′ end is inserted into the secondary channel), these data, combined with our single-round kinetics data, confirmed that RNAP backtracks during transcription initiation. Because GreA predominantly induces cleavage of 2–3 bases from the RNA 3′- terminus (44, 45, 50–52), the lack of the 7-base abortive transcript is most likely due to GreA-induced shortening (compare Fig. 3 C and D) to a 5-mer, suggesting that RNAP in NTP-starved RPITC≤7 backtracks by 1 base.
RNAP Backtracking and Pausing in the Presence of All NTPs.
The data summarized in Figs. 2 and 3 studied pausing and backtracking in RNAP transcription initiation under conditions in which one or more NTPs were absent. Whereas the complete absence of select NTPs is highly unlikely in vivo, changes in pools of NTP levels may occur in bacteria in several conditions (e.g., metabolic stress, stages in cell growth; discussions in refs. 67–69). Accordingly, we examined RNAP transcription with the quenched kinetics assay with all NTPs present but under NTP concentration imbalance (UTP and GTP >> ATP and CTP at the lacCONS promoter; UTP and ATP >> CTP and GTP at the T5N25 promoter). According to the DNA sequences of these promoters (Fig. S1), these conditions were expected to temporarily populate delayed RPITC states (RPITC≤7 in lacCONS and RPITC≤8 in T5N25, respectively). Consistent with the results described above, we observed a delay in exit kinetics from the RPITC=2 state under conditions of NTP imbalance (Fig. S9, orange) compared with equimolar conditions (Fig. S9, black) at each of the two different tested promoter templates. In addition, the observed delay was smaller in magnitude than the delay achieved upon exit from an NTP-starved RPITC state (Fig. S9, blue). This result implies the kinetic delay inversely correlates with the abundance of the NTPs required for promoter clearance.
The single-round quenched transcription kinetics assay and transcription assays with 32P-labeled UTP are ensemble experiments and hence cannot reliably detect infrequent or transient events. Under conditions of NTP starvation, abortive transcripts are identifiable by the gel-based assay (Fig. 3B). However, in the presence of all NTPs we did not obtain radiolabeled bands with sufficient intensity for the quantification of abortive transcripts, due to a limited number of abortive transcripts produced under single-round transcription conditions (Fig. S8B). The single-round transcription kinetics starting from RPITC=2 may already be influenced by pausing and backtracking; however, in the quenched kinetics assay RPITC=2 serves as a reference point.
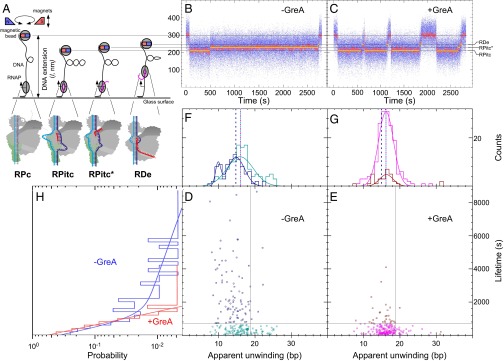
To detect the full initiation behavior on the lacCONS promoter in the presence of equimolar NTPs (100 μM each) with high sensitivity, we implemented magnetic tweezers experiments with positively supercoiled promoter templates (Fig. 4A). This assay allowed us to track individual RNAP complexes over time and simultaneously detect and identify distinct RPITC states, based upon well-established changes in DNA extension (4). In the absence of GreA, we observed short- and long-lived RPITC states (Fig. 4 B, D, F, and H). The lifetimes of RPITC states (n = 216) are summarized in a histogram fitted with a double exponential, in which ∼60% of events were short-lived (τ = 340 ± 60 s, SEM) and ∼40% were long-lived (τ = 4,600 ± 2,700 s, SEM; Fig. 4H, blue; fractions based on quadrant analysis as discussed in Fig. 3 legend). Correlating these lifetimes with DNA bubble sizes (representing distinct RPITC states; Fig. 4 A, D, and F and accompanying legend) revealed that short-lived RPITC states are characterized by a Gaussian distribution of transcription bubble sizes with a mean apparent unwinding of 15.9 ± 0.4 bp (SEM) and a SD of 3.6 ± 0.4 bp, whereas long-lived events display two distinct states of apparent unwinding: a 20% fraction with mean apparent unwinding of only 9.9 ± 0.3 bp (SEM) and a SD of 0.7 ± 0.3 bp (SEM) and an 80% fraction with mean apparent unwinding of 14.8 ± 0.4 bp (SEM) and a SD of 2.3 ± 0.4 bp (SEM).
Fig. 4.
Backtracking in initiation correlates with RNAP pausing in the presence of equimolar NTPs. (A) Schematics of the magnetic tweezer transcription assay (Methods). (B and C) Representative bead extension trajectories shown for single-molecule transcription experiments without (B) or with (C) 1 µM GreA. Unwinding levels (gray lines) are shown, indicating different bubble sizes imposed by different RNAP states (below). Yellow lines highlight typical lifetimes in each state. (D–H) Unwinding levels and RPITC and RPITC* lifetimes of individual initiation events (i.e., averaging over all RPITC and RPITC* states seen from initiation to promoter escape) are summarized into unwinding–lifetime scatter plots without (D) or with (E) 1 µM GreA; their 1D projections are shown in F–H. Quadrant structure is built as discussed in Methods, Illustrations. Lifetime data in the absence or presence of GreA were first fitted to single or double exponentials based on goodness-of-fit. Then, the 2D data were temporally separated into events shorter than (cyan, absence of GreA; magenta, presence of GreA) or longer than (dark blue, absence of GreA; dark red, presence of GreA) the fast timescale for promoter escape (∼300 s) plus 1 SD. Similarly, the 2D data were spatially separated into events with apparent unwinding amplitude smaller or larger than the mean unwinding observed during short-duration escape events plus 1 SD. Apparent unwinding data associated with short- or long-escape timescales were then fitted to single- or double-Gaussian distributions based on goodness-of-fit and according to the color code described above. Dotted vertical lines are visual guides to the maximum of the respective Gaussian distributions. Twenty to fifty DNA templates, carrying the lacCONS promoter sequence, were used for each condition, with 5–10 transcription pulses per template.
These data suggested that, in addition to the well-characterized RPITC state (Fig. 4A), a large fraction of RNAP complexes entered a distinct, long-lived state characterized by a smaller transcription bubble (denoted RPITC* in Fig. 4 A–C). We hypothesized that this long-lived initiation intermediate represented a backtracked RNAP that was characterized also in our quenched kinetics and gel-based transcription assays. If correct, the addition of GreA would be expected to markedly reduce the number of these long-lived events. In agreement, RPITC states (n = 209), observed in the presence of GreA, became uniformly short-lived (τ = 350 ± 30 s, SEM) and displayed a bubble size distribution similar to that observed for the short-lived RPITC states in the absence of GreA [16.2 ± 0.2 bp (SEM); SD 2 ± 0.2 bp (SEM); Fig. 4 C, E, G, and H]. The results shown in Fig. 4 followed transcription rounds of single RNAP molecules on immobilized DNA molecules carrying the lacCONS promoter sequence. These experiments were performed in the presence of the initiating dinucleotide used in the quenched kinetics assay (Figs. 1D, 2, and 3 and Figs. S3, S4, S8, and S9), ApA. Additional magnetic tweezer transcription experiments using the lacCONS promoter with all NTPs in the absence of ApA yielded similar trends to those in the presence of it (example of a trajectory in Fig. S10). Experiments conducted on a different promoter template (T5N25) at 100 μM each NTP also showed the same trends, which were furthermore independent of the presence (Fig. S11) or absence (Fig. S12) of the initiating dinucleotide of T5N25 promoter, ApU. Specifically, we observed both short-lived RPITC states lasting tens of seconds and long-lived RPITC* states lasting thousands of seconds, for which the RPITC* state systematically displays a smaller mean unwinding amplitude with narrower distribution than the RPITC state. As observed for the lacCONS promoter, addition of GreA once again abrogates the minimally unwound, long-lived RPITC* state in favor of short-lived RPITC states with extensive unwinding and leading to highly efficient promoter escape.
Finally, we performed magnetic tweezer transcription experiments on the lacCONS promoter and at higher NTP concentrations (1 mM each NTP; no initiating dinucleotide), which resemble the concentrations of NTPs under normal physiological conditions. The results obtained in the absence of GreA (Fig. S13) continue to show long-lived RPITC* events that resemble the ones measured under lower NTP concentrations. The transcription initiation complexes formed at the lacCONS promoter in these conditions continue to display double-exponential lifetimes before bona fide escape and regular processive elongation: 84% with a fast phase of only 91 ± 15 s (SEM) and 16% with a slow phase remaining in the thousands of seconds (Fig. S13).
In summary, the presence of GreA abrogates RPITC* states characterized by long lifetimes and smaller transcription bubbles in favor of RPITC states with short lifetimes and larger transcription bubbles, leading to highly efficient promoter escape (Figs. S11 and S12 D and E, quadrants, and S13C, quadrants). Hence, we conclude that GreA catalyzes the cleavage of nascent RNA transcript in backtracked complexes. The cleavage allows scrunching to resume so that the transcription bubble may reach its maximal size, efficiently driving the transition to a productive elongation. It also implies that the long-lived paused state is either caused by or stabilized through backtracking. These data further support the existence of a long-lived initiation state, in which the transcription bubble is smaller than its size in the presence of GreA, most likely due to backtracking. Altogether, the results provide evidence for the existence of the previously hypothesized paused and backtracked initiation state.
Conclusions
Our results support the existence of a previously hypothesized, yet uncharacterized, state in which RNAP backtracks and pauses during transcription initiation. We identified this paused–backtracked initiation intermediate under in vitro conditions with NTP concentrations that resemble physiological conditions (∼100 μM to 1 mM). Moreover, GreA and NTP availability appear to play key roles in regulating the flux in or out of this state. Based upon these results, we propose a modified transcription initiation model (Fig. 5). The model proposes that transcription initiation is rate limiting not only due to multiple abortive cycles that can occur before transitioning into elongation (5, 31, 63, 70), but also due to the existence of a backtracked, paused state. Thus, we hypothesize the presence of two initiation pathways. If nascent RNA backtracks into the secondary channel, either RNAP can swiftly release it as an abortive product, if the hybrid is short enough (14, 35, 36), or the backtracked complex can be stabilized. The prolonged association of the backtracked transcript may lead to intrinsic (or GreA-induced) RNA cleavage by RNAP. The backtracked-paused complex may also slowly release the nascent RNA through further backtracking. The model depicted in Fig. 5 adds an additional “slow” pathway (red arrows and beige rectangle background) to the conventional abortive initiation model.
Although the paused–backtracked initiation intermediate was observed frequently even at high NTP concentration, its effect on transcription and promoter escape was most pronounced under limiting NTP concentrations. These findings could have direct implications for in vivo conditions. First, the intracellular concentrations of NTPs can vary by severalfold, even during normal bacterial growth from midlog to early stationary phase (68). Additionally, nutritional limitation (e.g., carbon sources) can lead to a significant decrease of intracellular NTPs that directly affect the efficiency of transcription initiation and start-site selection at promoters of ribosomal and pyrimidine biosynthetic operons [reviewed by Turnbough (69)]. Moreover, decreases in nucleotide pools become even more dramatic during metabolic stress induced by antibiotics, oxidative stress, etc. (67). Therefore, under certain growth and environmental conditions, NTP concentrations may become limiting and thus are likely to affect abortive synthesis, early transcription, pausing, and promoter escape. Second, a number of in vitro studies have shown that the rate-limiting steps in transcription initiation include promoter DNA recognition/binding, open complex formation, abortive RNA synthesis/release, and promoter escape, all of which are targets for regulation (53). In vivo analysis of the chromosomal distribution of bacterial RNAPs by ChIP-chip, ChIP-seq, and DNA footprinting demonstrated that even under normal growth conditions (without NTP deprivation), a substantial fraction of RNAP accumulated at promoters of both transcriptionally active and inactive regions, engaged in either “poised” or open promoter complexes or in initial transcribing and paused complexes (43, 71–74). These results indicate that abortive initiation and promoter escape are the major rate-limiting steps in vivo.
Mechanisms of transcription by cellular RNA polymerases are broadly conserved (75). For example, scrunching (3, 4, 76) and trigger loop function during catalysis (37, 77–80) are similar, as are some of the mechanistic roles of σ70 and TFIIB (1, 81). It will be important to determine whether similar backtracked states are adopted during transcription initiation by eukaryotic RNA polymerases. Mammalian RNA polymerase II (Pol II) pauses during early stages of transcription, and this represents a common regulatory intermediate (82). Potentially, a mechanistic intermediate of paused mammalian Pol II enzymes may involve RNA backtracking; such backtracked intermediates may help explain why TFIIS, a eukaryotic convergently evolved analog of GreA, has been linked to transcription initiation and assembles with Pol II at the promoter (42, 83, 84). Finally, we emphasize that, because RNAP transcription initiation is rate limiting at many genes and highly regulated in vivo, this previously hypothesized yet uncharacterized backtracked-paused RNAP state may lead to potential new strategies for molecular therapeutics and to the development of novel antibiotics.
Methods
Transcription Quenched Kinetics Assay.
Preparation of a stable RPO, RPITC=2.
RPO solution is prepared with 3 μL E. coli RNAP holoenzyme (NEB, M0551S; 1.6 μM), 10 μL 2× transcription buffer [80 mM Hepes KOH, 100 mM KCl, 20 mM MgCl2, 2 mM dithiotreitol (DTT), 2 mM 2-mercaptoethylamine-HCl (MEA), 200 μg/mL BSA, pH 7], 1 μL of 1 μM lacCONS (65) or T5N25 promoter (5) (sequence in Fig. 1A and Fig. S1), and 6 μL of water. RPO is then incubated in solution at 37 °C for 30 min. To remove unreacted and nonspecifically bound RNAP, 1 μL of 100 mg/mL Heparin-Sepharose CL-6B beads (GE Healthcare) is added to RPO solution together with 10 μL of prewarmed 1× transcription buffer [Heparin challenge (3, 65)]. The mixture is incubated for 1 min at 37 °C and centrifuged for at least 45 s at 6,000 rpm. A total of 20 μL of the supernatant containing RPO is transferred into a new tube with initiating dinucleotide, for sP-DNA complexes (40–42). In this context, the Heparin-challenged RPO solution is incubated with 1.5 μL of 10 mM adenylyl(3′–5′) adenosine or adenylyl(3′–5′)uridine (ApA or ApU; Ribomed) at 37 °C for 20 min to form stable RPITC=2 solution. This RPITC=2 solution is used as a stock for all transcription reactions (for more information regarding the use of initiating dinucleotides, see Supporting Information, Initiating Dinucleotide With/Without a 5′-Triphosphate Group). A total of 2 μL of RNase inhibitor (NEB; M0314S) is added into the RPITC=2 solution to prevent degradation of newly transcribed RNA molecules.
Design and measurement of the transcription kinetics.
To produce runoff transcripts, high-purity ribonucleotide triphosphates (NTPs) (GE Healthcare) were used in all transcription reactions at 100 μM each. To obtain a specific initiation or elongation state, only a partial set of NTPs was used. The choice of the partial set of NTPs depended on the sequence of ITSs being used (Fig. 1A and Fig. S1). To exit from the initiation/elongation NTP-starved state the reaction mixture was complemented with all four NTPs. The nontemplate strands of all promoter DNAs have the sequence of 20 dA (20 consecutive A), the complementary sequence of ssDNA FRET probe (20 dT), at the end (Fig. 1A and Fig. S1 for probe target sequence). The ssDNA FRET probe is doubly end labeled with a pair of fluorophores suitable for smFRET: a donor, tetramethylrhodamine, at the 5′ end (5′ TAMRA modification) and an acceptor, Alexa Fluor 647, at the 3′ end (3′ Alexa Fluor 647 modification) [ordered from IDT (65)].
For kinetics, the reaction mixture is incubated with the partial set of NTPs for a constant duration of 40 min at 37 °C. All four NTPs are then added to the reaction mixture and incubated for various times, texit, at 37 °C. The reaction is quenched by addition of 0.5 M GndHCl. Subsequently, a ssDNA FRET probe is added to the quenched reaction mixture and incubated for 20 min at room temperature to detect runoff RNA transcripts produced during texit (see Fig. S6 for hybridization kinetics in 0.5M GndHCl). The quenched-probed reaction mixtures were then used for μsALEX measurements. An example of the quenched kinetic assay FRET results is shown in Fig. 1 C and D.
Each kinetic curve was measured in two or three separate repeats to show reproducibility (Fig. S7). The concentration of the ssDNA FRET probe in each experimental repeat was designed so that the steady-state level of a kinetic curve would be 0.9 ± 0.05. To make all repeats of kinetic curves reach a steady-state level of exactly 0.9 and not close to it, these repeats had to be rescaled (as shown in Fig. S14 and a detailed explanation in Supporting Information, RNAP–DNA Complex Concentration Adjustment and Rescaling for Transcription Kinetics Assays).
Each time point in the quenched kinetics assay is measured for duration of 10–15 min, using a setup described in Panzeri et al. (85), using Perkin-Elmer SPADs and 532-nm and 638-nm CW lasers operating at powers of 170 μW and 80 μW, respectively.
For transcription kinetics experiments with GreA, 1 μM GreA is added to transcription complexes in NTP-starved initiation or elongation states and incubated for 15 min before adding all four NTPs to initiate transcription reactions for texit.
Each kinetic measurement was performed at least in duplicates, using different preparations obtained on different days (Fig. S7). For each batch, we made sure of the following:
-
i)
The FRET probe in the presence of transcription complexes without NTPs yielded a large high-FRET population with no or a negligible amount of low-FRET population (no NTP, negative control).
-
ii)
After 20 min incubation of RPITC=2 with all four NTPs (steady state for RPITC=2; Figs. 1D, 2, and 3 A and B and Figs. S3, S4A, S7, S9, and S14), the fraction of hybridized probe reaches 90 ± 5% (positive control). This control is performed daily on the same batch used to prepare NTP-starved RNAP states.
-
iii)
With an extralong incubation time (typically several hours) after quenching the reaction, the measurement yielded the same hybridized fraction as measured without the extra incubation (quenching does work).
The results of the negative control (no NTP) serve as the “t = 0” time point. The positive control shows that the prepared transcription complexes are active and produce a lower amount of transcripts than the detection limit of the assay (overall amount of transcripts is lower than the amount of ssDNA FRET probe) at very long times, “t = ∞.”
All NTP-starved states were prepared from RPITC=2 stock solution. Because the other experimental conditions (concentrations, temperature, etc.) are identical, any changes in activity that may be caused solely due to the starvation of NTPs will show a change in the hybridized fraction in long time points of the kinetic trace. Such comparisons were routinely performed and have never shown a difference in the long time point baseline between the kinetics from RPITC=2 and from NTP-starved states (within 5% error). Therefore, we conclude that our experimental conditions (e.g., NTP starvation) did not alter the transcription activity but only the kinetics. In this regard, the same quantity of the stock solution could be used for kinetics assays for NTP-starved states as the one used for the abovementioned positive control.
μsALEX-smFRET data were analyzed as described in Supporting Information, μsALEX-smFRET Analysis for the Quantification of Transcription Kinetics.
Transcription Assays Visualizing Abortive Product Formation Using Urea-Denaturing PAGE Analysis of [32P]-Radiolabeled RNA Products.
Abortive transcription assays were run using the lacCONS promoter, having its probe target 20A sequence replaced by the WT lacUV5 sequence at registers from +20 to +39 (Fig. S8). Three units of RNAP holoenzyme (NEB; M0551S) was mixed with 50 nM promoter DNA in 1× transcription buffer in a final volume of 20 μL. The reaction was then incubated at 37 °C for 20 min to form RPO, followed by addition of 1 μL of 100 mg/mL Heparin-Sepharose beads and 10 μL of transcription buffer. The mixture was incubated for ∼1 min and centrifuged, and 20 μL of the supernatant was removed and added to 10 μL prewarmed transcription buffer. After incubating an additional 10 min, ApA was added at a final concentration of 1.3 mM and incubated for 30 min to form the RPITC=2. The RPITC=2 was then diluted to 400 μL with transcription buffer containing SUPERaseIN (AM2696; Thermo Fisher Scientific) to final concentrations of 1.7 nM template, 112 µM ApA, and 0.3 units/μL SUPERaseIN. This solution was stored at room temperature and used as a stock for each time course.
For time-course experiments, 90 µL of the stock solution was briefly incubated to bring it to 37 °C. To analyze the production kinetics of abortive products from RPITC≤7, stock solution was mixed with 10 µL of 200 µM UTP+GTP mixture supplemented with ∼10 µCi [α32P]UTP. At each time point, 10 µL aliquot was then removed and mixed with an equal volume of formamide gel loading buffer. To analyze abortive product formation from RNAP that was not stalled, the UTP+GTP mixture was replaced by a complete set of NTPs. In experiments looking at the effects of GreA on abortive product formation, additional 15-min incubation at 37 °C was performed before the addition of NTPs, either in the presence or in the absence of 1 µM GreA. The stopped reaction aliquots were stored at −20 °C until running the urea-denaturing PAGE.
Samples were heated for 3 min at 90 °C and loaded on a 23% (wt/vol, 19:1 acrylamide:bis-acrylamide), 0.4-mm-thick urea-denaturing polyacrylamide gel. The gel usually ran for 5–6 h at 1,500 V in 1× TBE with an additional 0.3 M sodium acetate in the bottom well. The gels were then removed, dried, and exposed on a phosphor-storage screen about 2 d. Screens were visualized using a Typhoon PhosporImager.
Magnetic Trapping Assay.
Single-molecule experiments.
For the single-molecule experiments we used the DNA constructs described in Supporting Information, DNA Constructs for the Magnetic Trapping Assay.
Functionalized 2.2-kbp DNA molecules were first attached to 1-µm-diameter streptavidin-coated magnetic beads (MyOne Streptavidin C1; Life Technologies) and then tethered to a modified glass capillary surface coated with anti-digoxigenin (Roche) (86). Experiments were carried out on a homemade magnetic tweezer microscope to extend and supercoil the DNA, running the PicoTwist software suite to track and analyze the position of the magnetic bead. This position marks the free end and thus the extension of the functionalized DNA. Data were analyzed using custom routines in the Xvin software suite. Experiments were carried out in standard buffer at 34 °C, using 100 pM RNAP saturated with σ70 (prepared as in ref. 87) and 100 µM ApA (for experiments on lacCONS promoter) (we used 100 μM ApU for experiments on T5N25 promoter) and 100 µM each of ATP, UTP, GTP, and CTP. When added, GreA is at 1 µM.
Raw time traces were filtered (∼1 s) for analysis. The lifetime of the initiation state is defined as the total time elapsed between initial promoter unwinding and the transition to promoter escape and formation of the elongation complex (RDE). The extent of apparent unwinding is determined by averaging the DNA extension over the entire lifetime of the initiation state as defined above and taking the difference between this average extension and that observed in the baseline high-extension state that separates the succession of transcription events. Importantly, our analysis excluded unproductive transcription initiation events in which RNAP spontaneously dissociated from DNA without forming the elongation state RDE. Finally, we note that all elongation complexes formed, whether from RPITC or RPITC*, displayed the expected elongation rate, namely ∼12 bases/s for 100 μM NTPs at 34 °C.
Interpretation of apparent DNA unwinding during scrunching and backtracking and its relation to the length of transcribed RNA as seen in the quenched kinetics assay.
In the supercoiling transcription assay where plectonemic supercoils are present (+4 positive supercoils throughout), the extension changes of the DNA construct report on the number of supercoils. Specifically, the DNA typically contracts by ∼55 nm for every additional supercoil when extended at low force (F = 0.3 pN) as in these experiments. DNA unwinding by RNAP is sensitively reported via its effect on overall DNA supercoiling: Conservation of linking number means that topological unwinding of 10.5 bp results in an ∼55-nm decrease in DNA extension.
During initial transcription and scrunching, an “N-2” rule has been observed (88), relating the length of the RNA and the extent of DNA unwinding in the bubble. Because an RNA 2-mer can be formed in RPo without a need for additional DNA unwinding and scrunching, an RNA N-mer can be formed by additional unwinding and scrunching of N-2 bases. However, during backtracking, this linear relationship is lost, although the simplest hypothesis is that backtracking by 1 bp dehybridizes 1 bp of the RNA–DNA hybrid and reduces the bubble size by the same amount. Thus, in this work the single-molecule assay can report only on the apparent bubble size, which results from the final backtracked state. This measurement provides information complementary to that obtained from the quenched kinetics assay, which instead provides insight into the relation between accessible RNA lengths and likelihood of entry into a state that is not competent for promoter escape.
Separation of correlative lifetime/amplitude data into quadrants.
We separate short- and long-lifetime initiation events by setting a boundary corresponding to the short mean lifetime plus 1 SD (as it is a single-exponential distribution this essentially means two times the short lifetime). We separate small and large bubbles by setting a boundary corresponding to the mean unwinding amplitude for the short-lifetime state in the absence of GreA plus 1 SD. Similar results are obtained if one uses the mean and SDs of amplitude data obtained in the +GreA condition and for which lifetime is homogeneously short. These boundaries determine the fractions of events categorized and cited as short or long lived and displaying small or large bubble sizes.
Illustrations.
All illustrations of RNAP transcription initiation and elongation states have been prepared in Adobe Illustrator CC 2015.
Note Added in Proof.
A study by Duchi et al. (89) that describes similar findings (but with much shorter paused initiation state), using somewhat different methodologies, was published while this manuscript was under final revisions.
Supplementary Material
Acknowledgments
We thank Prof. William Gelbart, Prof. Charles Knobler, Dr. Cathy Yan Jin, and Xiyu Yi for fruitful discussions and Maya Lerner for preparation of illustrations. We thank Prof. Richard Ebright for generously providing us with the triphosphate dinucleotide pppApA, as a gift. The T.R.S. laboratory wishes to thank the CNRS, the University of Paris Diderot, and the Programme Equipe Labellisees of the French Ligue Contre le Cancer for core funding; this work was further made possible by ANR Grant “RepOne”. This work was funded by the National Institutes of Health (Grant GM069709 to S. Weiss and Grant GM095904 to X.M. and S. Weiss) and the National Science Foundation (Grant MCB-1244175 to S. Weiss and D.J.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605038113/-/DCSupplemental.
References
- 1.Murakami KS, Darst SA. Bacterial RNA polymerases: The wholo story. Curr Opin Struct Biol. 2003;13(1):31–39. doi: 10.1016/s0959-440x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 2.Young BA, Gruber TM, Gross CA. Views of transcription initiation. Cell. 2002;109(4):417–420. doi: 10.1016/s0092-8674(02)00752-3. [DOI] [PubMed] [Google Scholar]
- 3.Kapanidis AN, et al. Initial transcription by RNA polymerase proceeds through a DNA-scrunching mechanism. Science. 2006;314(5802):1144–1147. doi: 10.1126/science.1131399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Revyakin A, Liu C, Ebright RH, Strick TR. Abortive initiation and productive initiation by RNA polymerase involve DNA scrunching. Science. 2006;314(5802):1139–1143. doi: 10.1126/science.1131398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu LM. Promoter clearance and escape in prokaryotes. Biochim Biophys Acta. 2002;1577(2):191–207. doi: 10.1016/s0167-4781(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 6.Luse DS, Jacob GA. Abortive initiation by RNA polymerase II in vitro at the adenovirus 2 major late promoter. J Biol Chem. 1987;262(31):14990–14997. [PubMed] [Google Scholar]
- 7.Carpousis AJ, Gralla JD. Cycling of ribonucleic acid polymerase to produce oligonucleotides during initiation in vitro at the lac UV5 promoter. Biochemistry. 1980;19(14):3245–3253. doi: 10.1021/bi00555a023. [DOI] [PubMed] [Google Scholar]
- 8.Ellinger T, Behnke D, Bujard H, Gralla JD. Stalling of Escherichia coli RNA polymerase in the +6 to +12 region in vivo is associated with tight binding to consensus promoter elements. J Mol Biol. 1994;239(4):455–465. doi: 10.1006/jmbi.1994.1388. [DOI] [PubMed] [Google Scholar]
- 9.Hsu LM. Monitoring abortive initiation. Methods. 2009;47(1):25–36. doi: 10.1016/j.ymeth.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbondanzieri EA, Shaevitz JW, Block SM. Picocalorimetry of transcription by RNA polymerase. Biophys J. 2005;89(6):L61–L63. doi: 10.1529/biophysj.105.074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbondanzieri EA, Greenleaf WJ, Shaevitz JW, Landick R, Block SM. Direct observation of base-pair stepping by RNA polymerase. Nature. 2005;438(7067):460–465. doi: 10.1038/nature04268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adelman K, et al. Single molecule analysis of RNA polymerase elongation reveals uniform kinetic behavior. Proc Natl Acad Sci USA. 2002;99(21):13538–13543. doi: 10.1073/pnas.212358999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang MD, et al. Force and velocity measured for single molecules of RNA polymerase. Science. 1998;282(5390):902–907. doi: 10.1126/science.282.5390.902. [DOI] [PubMed] [Google Scholar]
- 14.Epshtein V, et al. Swing-gate model of nucleotide entry into the RNA polymerase active center. Mol Cell. 2002;10(3):623–634. doi: 10.1016/s1097-2765(02)00640-8. [DOI] [PubMed] [Google Scholar]
- 15.Zhang G, et al. Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 A resolution. Cell. 1999;98(6):811–824. doi: 10.1016/s0092-8674(00)81515-9. [DOI] [PubMed] [Google Scholar]
- 16.Hansen UM, McClure WR. Role of the sigma subunit of Escherichia coli RNA polymerase in initiation. II. Release of sigma from ternary complexes. J Biol Chem. 1980;255(20):9564–9570. [PubMed] [Google Scholar]
- 17.Kapanidis AN, et al. Retention of transcription initiation factor sigma70 in transcription elongation: Single-molecule analysis. Mol Cell. 2005;20(3):347–356. doi: 10.1016/j.molcel.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Korzheva N, Mustaev A, Nudler E, Nikiforov V, Goldfarb A. Mechanistic model of the elongation complex of Escherichia coli RNA polymerase. Cold Spring Harb Symp Quant Biol. 1998;63:337–345. doi: 10.1101/sqb.1998.63.337. [DOI] [PubMed] [Google Scholar]
- 19.Zuo Y, Steitz TA. Crystal structures of the E. coli transcription initiation complexes with a complete bubble. Mol Cell. 2015;58(3):534–540. doi: 10.1016/j.molcel.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campagne S, Marsh ME, Capitani G, Vorholt JA, Allain FH. Structural basis for -10 promoter element melting by environmentally induced sigma factors. Nat Struct Mol Biol. 2014;21(3):269–276. doi: 10.1038/nsmb.2777. [DOI] [PubMed] [Google Scholar]
- 21.Campbell EA, et al. Structure of the bacterial RNA polymerase promoter specificity sigma subunit. Mol Cell. 2002;9(3):527–539. doi: 10.1016/s1097-2765(02)00470-7. [DOI] [PubMed] [Google Scholar]
- 22.Mekler V, et al. Structural organization of bacterial RNA polymerase holoenzyme and the RNA polymerase-promoter open complex. Cell. 2002;108(5):599–614. doi: 10.1016/s0092-8674(02)00667-0. [DOI] [PubMed] [Google Scholar]
- 23.Murakami KS, Masuda S, Campbell EA, Muzzin O, Darst SA. Structural basis of transcription initiation: An RNA polymerase holoenzyme-DNA complex. Science. 2002;296(5571):1285–1290. doi: 10.1126/science.1069595. [DOI] [PubMed] [Google Scholar]
- 24.Pupov D, Kuzin I, Bass I, Kulbachinskiy A. Distinct functions of the RNA polymerase σ subunit region 3.2 in RNA priming and promoter escape. Nucleic Acids Res. 2014;42(7):4494–4504. doi: 10.1093/nar/gkt1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samanta S, Martin CT. Insights into the mechanism of initial transcription in Escherichia coli RNA polymerase. J Biol Chem. 2013;288(44):31993–32003. doi: 10.1074/jbc.M113.497669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DN, Landick R. Structure of RNA and DNA chains in paused transcription complexes containing Escherichia coli RNA polymerase. J Mol Biol. 1992;228(3):759–777. doi: 10.1016/0022-2836(92)90862-e. [DOI] [PubMed] [Google Scholar]
- 27.Nudler E, Avetissova E, Markovtsov V, Goldfarb A. Transcription processivity: Protein-DNA interactions holding together the elongation complex. Science. 1996;273(5272):211–217. doi: 10.1126/science.273.5272.211. [DOI] [PubMed] [Google Scholar]
- 28.Sidorenkov I, Komissarova N, Kashlev M. Crucial role of the RNA:DNA hybrid in the processivity of transcription. Mol Cell. 1998;2(1):55–64. doi: 10.1016/s1097-2765(00)80113-6. [DOI] [PubMed] [Google Scholar]
- 29.Komissarova N, Kashlev M. Functional topography of nascent RNA in elongation intermediates of RNA polymerase. Proc Natl Acad Sci USA. 1998;95(25):14699–14704. doi: 10.1073/pnas.95.25.14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Travers AA, Burgessrr Cyclic re-use of the RNA polymerase sigma factor. Nature. 1969;222(5193):537–540. doi: 10.1038/222537a0. [DOI] [PubMed] [Google Scholar]
- 31.Straney DC, Crothers DM. Intermediates in transcription initiation from the E. coli lac UV5 promoter. Cell. 1985;43(2 Pt 1):449–459. doi: 10.1016/0092-8674(85)90175-8. [DOI] [PubMed] [Google Scholar]
- 32.Krummel B, Chamberlin MJ. RNA chain initiation by Escherichia coli RNA polymerase. Structural transitions of the enzyme in early ternary complexes. Biochemistry. 1989;28(19):7829–7842. doi: 10.1021/bi00445a045. [DOI] [PubMed] [Google Scholar]
- 33.Straney DC, Crothers DM. A stressed intermediate in the formation of stably initiated RNA chains at the Escherichia coli lac UV5 promoter. J Mol Biol. 1987;193(2):267–278. doi: 10.1016/0022-2836(87)90218-x. [DOI] [PubMed] [Google Scholar]
- 34.Hsu LM, et al. Initial transcribed sequence mutations specifically affect promoter escape properties. Biochemistry. 2006;45(29):8841–8854. doi: 10.1021/bi060247u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weixlbaumer A, Leon K, Landick R, Darst SA. Structural basis of transcriptional pausing in bacteria. Cell. 2013;152(3):431–441. doi: 10.1016/j.cell.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez-Rucobo FW, Cramer P. Structural basis of transcription elongation. Biochim Biophys Acta. 2013;1829(1):9–19. doi: 10.1016/j.bbagrm.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Bar-Nahum G, et al. A ratchet mechanism of transcription elongation and its control. Cell. 2005;120(2):183–193. doi: 10.1016/j.cell.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 38.Foster JE, Holmes SF, Erie DA. Allosteric binding of nucleoside triphosphates to RNA polymerase regulates transcription elongation. Cell. 2001;106(2):243–252. doi: 10.1016/s0092-8674(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 39.Holmes SF, Erie DA. Downstream DNA sequence effects on transcription elongation. Allosteric binding of nucleoside triphosphates facilitates translocation via a ratchet motion. J Biol Chem. 2003;278(37):35597–35608. doi: 10.1074/jbc.M304496200. [DOI] [PubMed] [Google Scholar]
- 40.Nedialkov YA, et al. NTP-driven translocation by human RNA polymerase II. J Biol Chem. 2003;278(20):18303–18312. doi: 10.1074/jbc.M301103200. [DOI] [PubMed] [Google Scholar]
- 41.Sousa R. A new level of regulation in transcription elongation? Trends Biochem Sci. 2001;26(12):695–697. doi: 10.1016/s0968-0004(01)02000-x. [DOI] [PubMed] [Google Scholar]
- 42.Guglielmi B, Soutourina J, Esnault C, Werner M. TFIIS elongation factor and Mediator act in conjunction during transcription initiation in vivo. Proc Natl Acad Sci USA. 2007;104(41):16062–16067. doi: 10.1073/pnas.0704534104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reppas NB, Wade JT, Church GM, Struhl K. The transition between transcriptional initiation and elongation in E. coli is highly variable and often rate limiting. Mol Cell. 2006;24(5):747–757. doi: 10.1016/j.molcel.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 44.Borukhov S, Lee J, Laptenko O. Bacterial transcription elongation factors: New insights into molecular mechanism of action. Mol Microbiol. 2005;55(5):1315–1324. doi: 10.1111/j.1365-2958.2004.04481.x. [DOI] [PubMed] [Google Scholar]
- 45.Toulmé F, et al. GreA and GreB proteins revive backtracked RNA polymerase in vivo by promoting transcript trimming. EMBO J. 2000;19(24):6853–6859. doi: 10.1093/emboj/19.24.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brodolin K, Zenkin N, Mustaev A, Mamaeva D, Heumann H. The sigma 70 subunit of RNA polymerase induces lacUV5 promoter-proximal pausing of transcription. Nat Struct Mol Biol. 2004;11(6):551–557. doi: 10.1038/nsmb768. [DOI] [PubMed] [Google Scholar]
- 47.Shaevitz JW, Abbondanzieri EA, Landick R, Block SM. Backtracking by single RNA polymerase molecules observed at near-base-pair resolution. Nature. 2003;426(6967):684–687. doi: 10.1038/nature02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stepanova E, et al. Analysis of promoter targets for Escherichia coli transcription elongation factor GreA in vivo and in vitro. J Bacteriol. 2007;189(24):8772–8785. doi: 10.1128/JB.00911-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stepanova E, Wang M, Severinov K, Borukhov S. Early transcriptional arrest at Escherichia coli rplN and ompX promoters. J Biol Chem. 2009;284(51):35702–35713. doi: 10.1074/jbc.M109.053983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng GH, Lee DN, Wang D, Chan CL, Landick R. GreA-induced transcript cleavage in transcription complexes containing Escherichia coli RNA polymerase is controlled by multiple factors, including nascent transcript location and structure. J Biol Chem. 1994;269(35):22282–22294. [PubMed] [Google Scholar]
- 51.Hsu LM, Vo NV, Chamberlin MJ. Escherichia coli transcript cleavage factors GreA and GreB stimulate promoter escape and gene expression in vivo and in vitro. Proc Natl Acad Sci USA. 1995;92(25):11588–11592. doi: 10.1073/pnas.92.25.11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borukhov S, Sagitov V, Goldfarb A. Transcript cleavage factors from E. coli. Cell. 1993;72(3):459–466. doi: 10.1016/0092-8674(93)90121-6. [DOI] [PubMed] [Google Scholar]
- 53.Hsu LM. Promoter escape by Escherichia coli RNA polymerase. Ecosal Plus. 2008;3(1) doi: 10.1128/ecosalplus.4.5.2.2. [DOI] [PubMed] [Google Scholar]
- 54.Goldman SR, Ebright RH, Nickels BE. Direct detection of abortive RNA transcripts in vivo. Science. 2009;324(5929):927–928. doi: 10.1126/science.1169237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skancke J, Bar N, Kuiper M, Hsu LM. Sequence-dependent promoter escape efficiency is strongly influenced by bias for the pretranslocated state during initial transcription. Biochemistry. 2015;54(28):4267–4275. doi: 10.1021/acs.biochem.5b00272. [DOI] [PubMed] [Google Scholar]
- 56.Kapanidis AN, et al. Fluorescence-aided molecule sorting: Analysis of structure and interactions by alternating-laser excitation of single molecules. Proc Natl Acad Sci USA. 2004;101(24):8936–8941. doi: 10.1073/pnas.0401690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kapanidis AN, et al. Alternating-laser excitation of single molecules. Acc Chem Res. 2005;38(7):523–533. doi: 10.1021/ar0401348. [DOI] [PubMed] [Google Scholar]
- 58.Förster T. Zwischenmolekulare Energiewanderung Und Fluoreszenz. Ann Phys. 1948;2(1-2):55–75. [Google Scholar]
- 59.Deniz AA, et al. Single-pair fluorescence resonance energy transfer on freely diffusing molecules: Observation of Förster distance dependence and subpopulations. Proc Natl Acad Sci USA. 1999;96(7):3670–3675. doi: 10.1073/pnas.96.7.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deniz AA, et al. Ratiometric single-molecule studies of freely diffusing biomolecules. Annu Rev Phys Chem. 2001;52:233–253. doi: 10.1146/annurev.physchem.52.1.233. [DOI] [PubMed] [Google Scholar]
- 61.Mekler V, Pavlova O, Severinov K. Interaction of Escherichia coli RNA polymerase σ70 subunit with promoter elements in the context of free σ70, RNA polymerase holoenzyme, and the β′-σ70 complex. J Biol Chem. 2011;286(1):270–279. doi: 10.1074/jbc.M110.174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mekler V, Minakhin L, Kuznedelov K, Mukhamedyarov D, Severinov K. RNA polymerase-promoter interactions determining different stability of the Escherichia coli and Thermus aquaticus transcription initiation complexes. Nucleic Acids Res. 2012;40(22):11352–11362. doi: 10.1093/nar/gks973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Margeat E, et al. Direct observation of abortive initiation and promoter escape within single immobilized transcription complexes. Biophys J. 2006;90(4):1419–1431. doi: 10.1529/biophysj.105.069252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukhopadhyay J, et al. Translocation of sigma(70) with RNA polymerase during transcription: Fluorescence resonance energy transfer assay for movement relative to DNA. Cell. 2001;106(4):453–463. doi: 10.1016/s0092-8674(01)00464-0. [DOI] [PubMed] [Google Scholar]
- 65.Kim S, et al. High-throughput single-molecule optofluidic analysis. Nat Methods. 2011;8(3):242–245. doi: 10.1038/nmeth.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laptenko O, Lee J, Lomakin I, Borukhov S. Transcript cleavage factors GreA and GreB act as transient catalytic components of RNA polymerase. EMBO J. 2003;22(23):6322–6334. doi: 10.1093/emboj/cdg610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belenky P, et al. Bactericidal antibiotics induce toxic metabolic perturbations that lead to cellular damage. Cell Reports. 2015;13(5):968–980. doi: 10.1016/j.celrep.2015.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buckstein MH, He J, Rubin H. Characterization of nucleotide pools as a function of physiological state in Escherichia coli. J Bacteriol. 2008;190(2):718–726. doi: 10.1128/JB.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turnbough CL., Jr Regulation of bacterial gene expression by the NTP substrates of transcription initiation. Mol Microbiol. 2008;69(1):10–14. doi: 10.1111/j.1365-2958.2008.06272.x. [DOI] [PubMed] [Google Scholar]
- 70.Tang GQ, Roy R, Ha T, Patel SS. Transcription initiation in a single-subunit RNA polymerase proceeds through DNA scrunching and rotation of the N-terminal subdomains. Mol Cell. 2008;30(5):567–577. doi: 10.1016/j.molcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herring CD, et al. Immobilization of Escherichia coli RNA polymerase and location of binding sites by use of chromatin immunoprecipitation and microarrays. J Bacteriol. 2005;187(17):6166–6174. doi: 10.1128/JB.187.17.6166-6174.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grainger DC, Hurd D, Harrison M, Holdstock J, Busby SJ. Studies of the distribution of Escherichia coli cAMP-receptor protein and RNA polymerase along the E. coli chromosome. Proc Natl Acad Sci USA. 2005;102(49):17693–17698. doi: 10.1073/pnas.0506687102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kusuya Y, Kurokawa K, Ishikawa S, Ogasawara N, Oshima T. Transcription factor GreA contributes to resolving promoter-proximal pausing of RNA polymerase in Bacillus subtilis cells. J Bacteriol. 2011;193(12):3090–3099. doi: 10.1128/JB.00086-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hatoum A, Roberts J. Prevalence of RNA polymerase stalling at Escherichia coli promoters after open complex formation. Mol Microbiol. 2008;68(1):17–28. doi: 10.1111/j.1365-2958.2008.06138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Werner F, Grohmann D. Evolution of multisubunit RNA polymerases in the three domains of life. Nat Rev Microbiol. 2011;9(2):85–98. doi: 10.1038/nrmicro2507. [DOI] [PubMed] [Google Scholar]
- 76.Fazal FM, Meng CA, Murakami K, Kornberg RD, Block SM. Real-time observation of the initiation of RNA polymerase II transcription. Nature. 2015;525(7568):274–277. doi: 10.1038/nature14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allison LA, Moyle M, Shales M, Ingles CJ. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985;42(2):599–610. doi: 10.1016/0092-8674(85)90117-5. [DOI] [PubMed] [Google Scholar]
- 78.Hekmatpanah DS, Young RA. Mutations in a conserved region of RNA polymerase II influence the accuracy of mRNA start site selection. Mol Cell Biol. 1991;11(11):5781–5791. doi: 10.1128/mcb.11.11.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thuillier V, Brun I, Sentenac A, Werner M. Mutations in the alpha-amanitin conserved domain of the largest subunit of yeast RNA polymerase III affect pausing, RNA cleavage and transcriptional transitions. EMBO J. 1996;15(3):618–629. [PMC free article] [PubMed] [Google Scholar]
- 80.Weilbaecher R, Hebron C, Feng G, Landick R. Termination-altering amino acid substitutions in the beta’ subunit of Escherichia coli RNA polymerase identify regions involved in RNA chain elongation. Genes Dev. 1994;8(23):2913–2927. doi: 10.1101/gad.8.23.2913. [DOI] [PubMed] [Google Scholar]
- 81.Sainsbury S, Niesser J, Cramer P. Structure and function of the initially transcribing RNA polymerase II-TFIIB complex. Nature. 2013;493(7432):437–440. doi: 10.1038/nature11715. [DOI] [PubMed] [Google Scholar]
- 82.Adelman K, Lis JT. Promoter-proximal pausing of RNA polymerase II: Emerging roles in metazoans. Nat Rev Genet. 2012;13(10):720–731. doi: 10.1038/nrg3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim B, et al. The transcription elongation factor TFIIS is a component of RNA polymerase II preinitiation complexes. Proc Natl Acad Sci USA. 2007;104(41):16068–16073. doi: 10.1073/pnas.0704573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stepanova EV, Shevelev AB, Borukhov SI, Severinov KV. Mechanisms of action of RNA polymerase-binding transcription factors that do not bind to DNA. Biofizika. 2009;54(5):773–790. [PubMed] [Google Scholar]
- 85.Panzeri F, et al. 2013. Single-molecule FRET experiments with a red-enhanced custom technology SPAD. Proc SPIE Int Soc Opt Eng, 8590.
- 86.Revyakin A, Ebright RH, Strick TR. Single-molecule DNA nanomanipulation: Improved resolution through use of shorter DNA fragments. Nat Methods. 2005;2(2):127–138. doi: 10.1038/nmeth0205-127. [DOI] [PubMed] [Google Scholar]
- 87.Howan K, et al. Initiation of transcription-coupled repair characterized at single-molecule resolution. Nature. 2012;490(7420):431–434. doi: 10.1038/nature11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Revyakin A, Ebright RH, Strick TR. Promoter unwinding and promoter clearance by RNA polymerase: Detection by single-molecule DNA nanomanipulation. Proc Natl Acad Sci USA. 2004;101(14):4776–4780. doi: 10.1073/pnas.0307241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duchi D, et al. RNA polymerase pausing during initial transcription. Mol Cell. 2016;63(6):939–950. doi: 10.1016/j.molcel.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.