Abstract
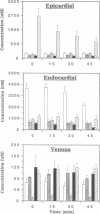
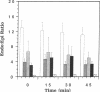
The purine adenosine appears to be involved in regulation of coronary vascular tone. Little is known concerning the levels and distribution of adenosine and related purines in the extracellular fluid of the heart. We have measured epicardial and endocardial levels of adenosine, inosine, hypoxanthine, AMP, and IMP in isolated constant flow perfused guinea pig hearts by using a recently developed technique with porous nylon sampling discs. Venous effluent purine levels were also measured. Concentrations of all purines measured, excluding IMP, were significantly higher in endocardial fluid samples than in epicardial fluid samples (P less than 0.05). Conversely, IMP levels were significantly lower in endocardial than in epicardial samples. The magnitude of the endocardial/epicardial ratios for adenosine, inosine, hypoxanthine, AMP, and IMP were approximately 12:1, 4:1, 5:1, 4:1, and 1:2, respectively. To assess cellular damage, lactate dehydrogenase activity was measured in all fluid samples and was not significantly different in endocardial and epicardial fluid. These data support the existence of significant transmural gradients for extracellular purine levels in crystalloid perfused guinea pig hearts. Transmural differences in vasoactive adenosine levels may be partially due to the greater endocardial oxygen consumption and metabolism and may be involved in maintaining relatively high subendocardial blood flows in the face of high intramyocardial pressures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Newsholme E. A. Activities and some properties of 5'-nucleotidase, adenosine kinase and adenosine deaminase in tissues from vertebrates and invertebrates in relation to the control of the concentration and the physiological role of adenosine. Biochem J. 1978 Sep 15;174(3):965–977. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardenheuer H., Schrader J. Relationship between myocardial oxygen consumption, coronary flow, and adenosine release in an improved isolated working heart preparation of guinea pigs. Circ Res. 1983 Mar;52(3):263–271. doi: 10.1161/01.res.52.3.263. [DOI] [PubMed] [Google Scholar]
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- De Tata V., Fierabracci V., Gori Z., Bergamini E. Metabolic heterogeneity of the muscle tissue: transmural distribution of glucose metabolizing enzymes across the left ventricular wall of control and hypertrophic rat heart. Biochem Int. 1988 Jan;16(1):119–126. [PubMed] [Google Scholar]
- De Tata V., Gini S., Simonetti I., Fierabracci V., Gori Z., Ipata P. L., Bergamini E. The regional distribution of adenosine-regulating enzymes in the left and right ventricle walls of control and hypertrophic heart. Basic Res Cardiol. 1989 Nov-Dec;84(6):597–605. doi: 10.1007/BF01906945. [DOI] [PubMed] [Google Scholar]
- Decking U. K., Juengling E., Kammermeier H. Interstitial transudate concentration of adenosine and inosine in rat and guinea pig hearts. Am J Physiol. 1988 Jun;254(6 Pt 2):H1125–H1132. doi: 10.1152/ajpheart.1988.254.6.H1125. [DOI] [PubMed] [Google Scholar]
- Deussen A., Borst M., Kroll K., Schrader J. Formation of S-adenosylhomocysteine in the heart. II: A sensitive index for regional myocardial underperfusion. Circ Res. 1988 Jul;63(1):250–261. doi: 10.1161/01.res.63.1.250. [DOI] [PubMed] [Google Scholar]
- Feigl E. O. Coronary physiology. Physiol Rev. 1983 Jan;63(1):1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- Fenton R. A., Dobson J. G., Jr Measurement by fluorescence of interstitial adenosine levels in normoxic, hypoxic, and ischemic perfused rat hearts. Circ Res. 1987 Feb;60(2):177–184. doi: 10.1161/01.res.60.2.177. [DOI] [PubMed] [Google Scholar]
- Fenton R. A., Tsimikas S., Dobson J. G., Jr Influence of beta-adrenergic stimulation and contraction frequency on rat heart interstitial adenosine. Circ Res. 1990 Feb;66(2):457–468. doi: 10.1161/01.res.66.2.457. [DOI] [PubMed] [Google Scholar]
- Gober J. R., Schaefer S., Camacho S. A., DeGroot M., Obregon R., Botvinick E. H., Weiner M., Massie B. Epicardial and endocardial localized 31P magnetic resonance spectroscopy: evidence for metabolic heterogeneity during regional ischemia. Magn Reson Med. 1990 Feb;13(2):204–215. doi: 10.1002/mrm.1910130204. [DOI] [PubMed] [Google Scholar]
- He M. X., Wangler R. D., Dillon P. F., Romig G. D., Sparks H. V. Phosphorylation potential and adenosine release during norepinephrine infusion in guinea pig heart. Am J Physiol. 1987 Nov;253(5 Pt 2):H1184–H1191. doi: 10.1152/ajpheart.1987.253.5.H1184. [DOI] [PubMed] [Google Scholar]
- Headrick J. P., Berne R. M. Endothelium-dependent and -independent relaxations to adenosine in guinea pig aorta. Am J Physiol. 1990 Jul;259(1 Pt 2):H62–H67. doi: 10.1152/ajpheart.1990.259.1.H62. [DOI] [PubMed] [Google Scholar]
- Headrick J. P., Matherne G. P., Berr S. S., Han D. C., Berne R. M. Metabolic correlates of adenosine formation in stimulated guinea pig heart. Am J Physiol. 1991 Jan;260(1 Pt 2):H165–H172. doi: 10.1152/ajpheart.1991.260.1.H165. [DOI] [PubMed] [Google Scholar]
- Headrick J. P., Willis R. J. Adenosine formation and energy metabolism: a 31P-NMR study in isolated rat heart. Am J Physiol. 1990 Mar;258(3 Pt 2):H617–H624. doi: 10.1152/ajpheart.1990.258.3.H617. [DOI] [PubMed] [Google Scholar]
- Holtz J., Grunewald W. A., Manz R., von Restorff W., Bassenge E. Intracapillary hemoglobin oxygen saturation and oxygen consumption in different layers of the left ventricular myocardium. Pflugers Arch. 1977 Sep 16;370(3):253–258. doi: 10.1007/BF00585535. [DOI] [PubMed] [Google Scholar]
- Matherne G. P., Headrick J. P., Coleman S. D., Berne R. M. Interstitial transudate purines in normoxic and hypoxic immature and mature rabbit hearts. Pediatr Res. 1990 Oct;28(4):348–353. doi: 10.1203/00006450-199010000-00010. [DOI] [PubMed] [Google Scholar]
- Olsson R. A., Bünger R. Metabolic control of coronary blood flow. Prog Cardiovasc Dis. 1987 Mar-Apr;29(5):369–387. doi: 10.1016/0033-0620(87)90003-x. [DOI] [PubMed] [Google Scholar]
- Poole D. C., Mathieu-Costello O. Analysis of capillary geometry in rat subepicardium and subendocardium. Am J Physiol. 1990 Jul;259(1 Pt 2):H204–H210. doi: 10.1152/ajpheart.1990.259.1.H204. [DOI] [PubMed] [Google Scholar]
- Robitaille P. M., Lew B., Merkle H., Sublett E., Lindstrom P., From A. H., Garwood M., Bache R. J., Uğurbil K. Transmural metabolite distribution in regional myocardial ischemia as studied with 31P NMR. Magn Reson Med. 1989 Apr;10(1):108–118. doi: 10.1002/mrm.1910100110. [DOI] [PubMed] [Google Scholar]
- Schrader J., Haddy F. J., Gerlach E. Release of adenosine, inosine and hypoxanthine from the isolated guinea pig heart during hypoxia, flow-autoregulation and reactive hyperemia. Pflugers Arch. 1977 May 6;369(1):1–6. doi: 10.1007/BF00580802. [DOI] [PubMed] [Google Scholar]
- Sparks H. V., Jr, Bardenheuer H. Regulation of adenosine formation by the heart. Circ Res. 1986 Feb;58(2):193–201. doi: 10.1161/01.res.58.2.193. [DOI] [PubMed] [Google Scholar]
- Stein P. D., Marzilli M., Sabbah H. N., Lee T. Systolic and diastolic pressure gradients within the left ventricular wall. Am J Physiol. 1980 May;238(5):H625–H630. doi: 10.1152/ajpheart.1980.238.5.H625. [DOI] [PubMed] [Google Scholar]
- Stein P. D., Sabbah H. N., Marzilli M., Blick E. F. Comparison of the distribution of intramyocardial pressure across the canine left ventricular wall in the beating heart during diastole and in the arrested heart. Evidence of epicardial muscle tone during diastole. Circ Res. 1980 Aug;47(2):258–267. doi: 10.1161/01.res.47.2.258. [DOI] [PubMed] [Google Scholar]
- Tietjan C. S., Tribble C. G., Gidday J. M., Phillips C. L., Belardinelli L., Rubio R., Berne R. M. Interstitial adenosine in guinea pig hearts: an index obtained by epicardial disks. Am J Physiol. 1990 Nov;259(5 Pt 2):H1471–H1476. doi: 10.1152/ajpheart.1990.259.5.H1471. [DOI] [PubMed] [Google Scholar]
- Van Wylen D. G., Willis J., Sodhi J., Weiss R. J., Lasley R. D., Mentzer R. M., Jr Cardiac microdialysis to estimate interstitial adenosine and coronary blood flow. Am J Physiol. 1990 Jun;258(6 Pt 2):H1642–H1649. doi: 10.1152/ajpheart.1990.258.6.H1642. [DOI] [PubMed] [Google Scholar]
- Visser M. P., Krill M. T., Muijtjens A. M., Willems G. M., Hermens W. T. Distribution of enzymes in dog heart and liver; significance for assessment of tissue damage from data on plasma enzyme activities. Clin Chem. 1981 Nov;27(11):1845–1850. [PubMed] [Google Scholar]
- Weiss H. R., Neubauer J. A., Lipp J. A., Sinha A. K. Quantitative determination of regional oxygen consumption in the dog heart. Circ Res. 1978 Mar;42(3):394–401. doi: 10.1161/01.res.42.3.394. [DOI] [PubMed] [Google Scholar]
- Wienen W., Kammermeier H. Intra- and extracellular markers in interstitial transudate of perfused rat hearts. Am J Physiol. 1988 Apr;254(4 Pt 2):H785–H794. doi: 10.1152/ajpheart.1988.254.4.H785. [DOI] [PubMed] [Google Scholar]
- van der Vusse G. J., Arts T., Glatz J. F., Reneman R. S. Transmural differences in energy metabolism of the left ventricular myocardium: fact or fiction. J Mol Cell Cardiol. 1990 Jan;22(1):23–37. doi: 10.1016/0022-2828(90)90969-9. [DOI] [PubMed] [Google Scholar]