Abstract
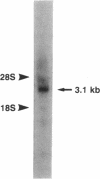
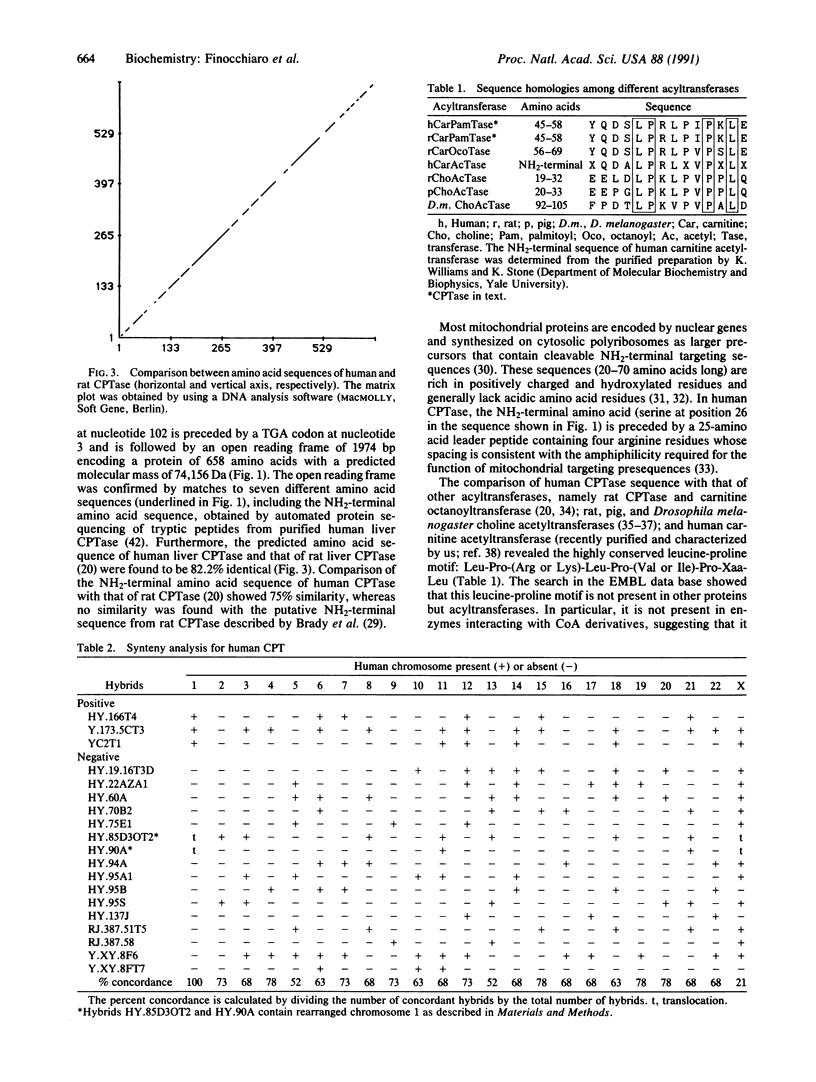
We have cloned and sequenced a cDNA encoding human liver carnitine palmitoyltransferase (CPTase; palmitoyl-CoA:L-carnitine O-palmitoyltransferase, EC 2.3.1.21), an inner mitochondrial membrane enzyme that plays a major role in the fatty acid oxidation pathway. Mixed oligonucleotide primers whose sequences were deduced from one tryptic peptide obtained from purified CPTase were used in a polymerase chain reaction, allowing the amplification of a 0.12-kilobase fragment of human genomic DNA encoding such a peptide. A 60-base-pair (bp) oligonucleotide synthesized on the basis of the sequence from this fragment was used for the screening of a cDNA library from human liver and hybridized to a cDNA insert of 2255 bp. This cDNA contains an open reading frame of 1974 bp that encodes a protein of 658 amino acid residues including 25 residues of an NH2-terminal leader peptide. The assignment of this open reading frame to human liver CPTase is confirmed by matches to seven different amino acid sequences of tryptic peptides derived from pure human CPTase and by the 82.2% homology with the amino acid sequence of rat CPTase. The NH2-terminal region of CPTase contains a leucine-proline motif that is shared by carnitine acetyl- and octanoyltransferases and by choline acetyltransferase. The gene encoding CPTase was assigned to human chromosome 1, region 1q12-1pter, by hybridization of CPTase cDNA with a DNA panel of 19 human-hamster somatic cell hybrids.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergstrom J. D., Reitz R. C. Studies on carnitine palmitoyl transferase: the similar nature of CPTi (inner form) and CPTo (outer form). Arch Biochem Biophys. 1980 Oct 1;204(1):71–79. doi: 10.1016/0003-9861(80)90008-9. [DOI] [PubMed] [Google Scholar]
- Berrard S., Brice A., Lottspeich F., Braun A., Barde Y. A., Mallet J. cDNA cloning and complete sequence of porcine choline acetyltransferase: in vitro translation of the corresponding RNA yields an active protein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9280–9284. doi: 10.1073/pnas.84.24.9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloisi W., Colombo I., Garavaglia B., Giardini R., Finocchiaro G., Didonato S. Purification and properties of carnitine acetyltransferase from human liver. Eur J Biochem. 1990 May 20;189(3):539–546. doi: 10.1111/j.1432-1033.1990.tb15520.x. [DOI] [PubMed] [Google Scholar]
- Bougnères P. F., Saudubray J. M., Marsac C., Bernard O., Odièvre M., Girard J. Fasting hypoglycemia resulting from hepatic carnitine palmitoyl transferase deficiency. J Pediatr. 1981 May;98(5):742–746. doi: 10.1016/s0022-3476(81)80834-7. [DOI] [PubMed] [Google Scholar]
- Brady P. S., Feng Y. X., Brady L. J. Transcriptional regulation of carnitine palmitoyltransferase synthesis in riboflavin deficiency in rats. J Nutr. 1988 Sep;118(9):1128–1136. doi: 10.1093/jn/118.9.1128. [DOI] [PubMed] [Google Scholar]
- Brice A., Berrard S., Raynaud B., Ansieau S., Coppola T., Weber M. J., Mallet J. Complete sequence of a cDNA encoding an active rat choline acetyltransferase: a tool to investigate the plasticity of cholinergic phenotype expression. J Neurosci Res. 1989 Jul;23(3):266–273. doi: 10.1002/jnr.490230304. [DOI] [PubMed] [Google Scholar]
- Chatterjee B., Song C. S., Kim J. M., Roy A. K. Cloning, sequencing, and regulation of rat liver carnitine octanoyltransferase: transcriptional stimulation of the enzyme during peroxisome proliferation. Biochemistry. 1988 Dec 13;27(25):9000–9006. doi: 10.1021/bi00425a018. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clarke P. R., Bieber L. L. Isolation and purification of mitochondrial carnitine octanoyltransferase activities from beef heart. J Biol Chem. 1981 Oct 10;256(19):9861–9868. [PubMed] [Google Scholar]
- Cook G. A., Gamble M. S. Regulation of carnitine palmitoyltransferase by insulin results in decreased activity and decreased apparent Ki values for malonyl-CoA. J Biol Chem. 1987 Feb 15;262(5):2050–2055. [PubMed] [Google Scholar]
- Cook G. A., Khan B., Heimberg M. Feeding of lovastatin to rats increases the activity of the hepatic mitochondrial outer carnitine palmitoyltransferase. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1077–1082. doi: 10.1016/0006-291x(88)90739-5. [DOI] [PubMed] [Google Scholar]
- Cook G. A. The hypoglycemic sulfonylureas glyburide and tolbutamide inhibit fatty acid oxidation by inhibiting carnitine palmitoyltransferase. J Biol Chem. 1987 Apr 15;262(11):4968–4972. [PubMed] [Google Scholar]
- Couvreur J. M., Azuma T., Miller D. A., Rocchi M., Mohandas T. K., Boudi F. A., Taggart R. T. Assignment of cathepsin E (CTSE) to human chromosome region 1q31 by in situ hybridization and analysis of somatic cell hybrids. Cytogenet Cell Genet. 1990;53(2-3):137–139. doi: 10.1159/000132914. [DOI] [PubMed] [Google Scholar]
- Declercq P. E., Falck J. R., Kuwajima M., Tyminski H., Foster D. W., McGarry J. D. Characterization of the mitochondrial carnitine palmitoyltransferase enzyme system. I. Use of inhibitors. J Biol Chem. 1987 Jul 15;262(20):9812–9821. [PubMed] [Google Scholar]
- Declercq P. E., Venincasa M. D., Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and 2-tetradecylglycidyl-CoA with mitochondrial carnitine palmitoyltransferase I. J Biol Chem. 1985 Oct 15;260(23):12516–12522. [PubMed] [Google Scholar]
- Demaugre F., Bonnefont J. P., Mitchell G., Nguyen-Hoang N., Pelet A., Rimoldi M., Di Donato S., Saudubray J. M. Hepatic and muscular presentations of carnitine palmitoyl transferase deficiency: two distinct entities. Pediatr Res. 1988 Sep;24(3):308–311. doi: 10.1203/00006450-198809000-00006. [DOI] [PubMed] [Google Scholar]
- Di Donato S., Castiglione A., Rimoldi M., Cornelio F., Vendemia F., Cardace G., Bertagnolio B. Heterogeneity of carnitine-palmitoyltransferase deficiency. J Neurol Sci. 1981 May;50(2):207–215. doi: 10.1016/0022-510x(81)90167-2. [DOI] [PubMed] [Google Scholar]
- DiMauro S., DiMauro P. M. Muscle carnitine palmityltransferase deficiency and myoglobinuria. Science. 1973 Nov 20;182(4115):929–931. doi: 10.1126/science.182.4115.929. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Finocchiaro G., Colombo I., DiDonato S. Purification, characterization and partial amino acid sequences of carnitine palmitoyl-transferase from human liver. FEBS Lett. 1990 Nov 12;274(1-2):163–166. doi: 10.1016/0014-5793(90)81354-q. [DOI] [PubMed] [Google Scholar]
- Finocchiaro G., Ito M., Ikeda Y., Tanaka K. Molecular cloning and nucleotide sequence of cDNAs encoding the alpha-subunit of human electron transfer flavoprotein. J Biol Chem. 1988 Oct 25;263(30):15773–15780. [PubMed] [Google Scholar]
- Harano Y., Kashiwagi A., Kojima H., Suzuki M., Hashimoto T., Shigeta Y. Phosphorylation of carnitine palmitoyltransferase and activation by glucagon in isolated rat hepatocytes. FEBS Lett. 1985 Sep 2;188(2):267–272. doi: 10.1016/0014-5793(85)80385-9. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Fenton W. A., Furtak K., Pollock R. A., Rosenberg L. E. The ornithine transcarbamylase leader peptide directs mitochondrial import through both its midportion structure and net positive charge. J Cell Biol. 1987 Aug;105(2):669–677. doi: 10.1083/jcb.105.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide T., Ontko J. A. Increased secretion of very low density lipoprotein triglyceride following inhibition of long chain fatty acid oxidation in isolated rat liver. J Biol Chem. 1981 Oct 25;256(20):10247–10255. [PubMed] [Google Scholar]
- Itoh N., Slemmon J. R., Hawke D. H., Williamson R., Morita E., Itakura K., Roberts E., Shively J. E., Crawford G. D., Salvaterra P. M. Cloning of Drosophila choline acetyltransferase cDNA. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4081–4085. doi: 10.1073/pnas.83.11.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund H., Woldegiorgis G. Carnitine palmitoyltransferase: separation of enzyme activity and malonyl-CoA binding in rat liver mitochondria. Biochim Biophys Acta. 1986 Sep 12;878(2):243–249. doi: 10.1016/0005-2760(86)90152-9. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Woeltje K. F., Kuwajima M., Foster D. W. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev. 1989 May;5(3):271–284. doi: 10.1002/dmr.5610050305. [DOI] [PubMed] [Google Scholar]
- Miyazawa S., Ozasa H., Osumi T., Hashimoto T. Purification and properties of carnitine octanoyltransferase and carnitine palmitoyltransferase from rat liver. J Biochem. 1983 Aug;94(2):529–542. doi: 10.1093/oxfordjournals.jbchem.a134384. [DOI] [PubMed] [Google Scholar]
- Murthy M. S., Pande S. V. Malonyl-CoA binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc Natl Acad Sci U S A. 1987 Jan;84(2):378–382. doi: 10.1073/pnas.84.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. The mitochondrial protein import apparatus. Annu Rev Biochem. 1990;59:331–353. doi: 10.1146/annurev.bi.59.070190.001555. [DOI] [PubMed] [Google Scholar]
- Rocchi M., Roncuzzi L., Santamaria R., Archidiacono N., Dente L., Romeo G. Mapping through somatic cell hybrids and cDNA probes of protein C to chromosome 2, factor X to chromosome 13, and alpha 1-acid glycoprotein to chromosome 9. Hum Genet. 1986 Sep;74(1):30–33. doi: 10.1007/BF00278781. [DOI] [PubMed] [Google Scholar]
- Roise D., Theiler F., Horvath S. J., Tomich J. M., Richards J. H., Allison D. S., Schatz G. Amphiphilicity is essential for mitochondrial presequence function. EMBO J. 1988 Mar;7(3):649–653. doi: 10.1002/j.1460-2075.1988.tb02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein I., Cook G. A., Heimberg M. Regulation by oestrogen of carnitine palmitoyltransferase in hepatic mitochondria. Biochem J. 1986 Jul 15;237(2):593–596. doi: 10.1042/bj2370593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeltje K. F., Esser V., Weis B. C., Sen A., Cox W. F., McPhaul M. J., Slaughter C. A., Foster D. W., McGarry J. D. Cloning, sequencing, and expression of a cDNA encoding rat liver mitochondrial carnitine palmitoyltransferase II. J Biol Chem. 1990 Jun 25;265(18):10720–10725. [PubMed] [Google Scholar]
- Yamamoto M., Fukuda N., Triscari J., Sullivan A. C., Ontko J. A. Decreased hepatic production of very low density lipoproteins following activation of fatty acid oxidation by Ro 22-0654. J Lipid Res. 1985 Oct;26(10):1196–1204. [PubMed] [Google Scholar]