Abstract
Objective:
To assess disease activity within 12 months after natalizumab (NZ) discontinuation in a large French postmarketing cohort.
Methods:
In France, patients exposed at least once to NZ were included in the TYSEDMUS observational and multicenter cohort, part of the French NZ Risk Management Plan. Clinical disease activity during the year following NZ discontinuation was assessed in this cohort. Time to first relapse after NZ stop was analyzed using Kaplan-Meier method and potentially associated factors were studied using a multivariate Cox model.
Results:
Out of the 4,055 patients with multiple sclerosis (MS) included in TYSEDMUS, 1,253 discontinued NZ and 715 of them had relevant data for our study. The probability of relapse within the year after NZ stop was estimated at 45% (95% confidence interval 0.41–0.49).
Conclusions:
This large and systematic survey of patients with MS after NZ withdrawal allows quantifying the risk of increased disease activity following treatment discontinuation. This study provides large-scale, multicenter, systematic data after NZ cessation in real-life settings.
Natalizumab (NZ) is a humanized anti-α-4 integrin monoclonal antibody that decreases lymphocyte trafficking across the blood–brain barrier. It has shown efficacy in preventing relapses and new MRI lesions.1 Its use has been restricted in Europe to the treatment of very active relapsing-remitting multiple sclerosis (MS), because of the risk of progressive multifocal leukoencephalopathy (PML). A risk stratification for PML has been developed over time, which relies upon NZ treatment duration, prior immunosuppressive therapy, JC virus (JCV) serology status, and more recently, anti-JCV antibody index.2–4 As a consequence, NZ is discontinued in high-risk patients after 2 years. Other reasons for stopping might be treatment failure, adverse event, or desire for pregnancy. However, this decision might be challenged by the relapse risk following NZ cessation. Phase II and III trials have suggested that disease activity after NZ interruption returned progressively to levels similar to those seen prior to treatment within 6 months.5,6 However, there has been growing evidence of relapses of unusual clinical severity with high number of new T2 and enhancing lesions following NZ interruption7–9 suggesting a rebound effect, possibly related to an immune reconstitution inflammatory syndrome–like syndrome.10 It was recently suggested that reducing the washout period between NZ discontinuation and onset of a new disease-modifying drug (DMD) might prevent this disease reactivation.11 To gain further insight into the relapse risk after NZ discontinuation, this study leaned on a phase IV national study implemented in France as part of the NZ Risk Management Plan, the TYSEDMUS study, and assessed the relapse risk after NZ discontinuation in the largest real-life population of patients with MS treated with NZ, systematically followed up during treatment and in the 12-month period after.
METHODS
Study population.
TYSEDMUS was an observational, prospective, multicenter, nationwide, longitudinal cohort of patients with MS treated with NZ (TYSABRI) in France, conducted from November 2007 to November 2012. For the first time in France, this postmarketing study was only sponsored by the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, formerly Agence Française de Sécurité Sanitaire des Produits de Santé[French Agency for the Safety of Health Products]), and was entrusted to the network of French neurologists using European Database for Multiple Sclerosis software12 in their daily practice. Their participation in the study was voluntary, but strongly supported and suggested by the French Medicines Agency, the French Neurological Society, and the French Neurological Federation.
A total of 4,055 patients were included in the TYSEDMUS study. They had definite MS and were exposed at least once to NZ in France.
Data collection.
An inclusion form was systematically filled in, listing the personal characteristics of the patient, including MS history, baseline disability status, previous and ongoing disease-modifying therapies, biological data of pretreatment tests, and date and results of last MRI. A monthly form was filled in after each NZ infusion to report any adverse events (AEs) and serious AEs (SAEs). An additional biannual follow-up form aimed at collecting information on the clinical evolution (relapses, disability), MRI, and biology (anti-NZ antibodies) during treatment. SAE and pregnancy were systematically collected on specific forms.
In the year following NZ discontinuation, neurologists were required to provide biannual information on the clinical evolution (relapses, disability, MRI) and DMD.
An MS relapse was defined as the occurrence, recurrence, or worsening of symptoms of neurologic dysfunction lasting over 24 hours and usually ending up in a partial or complete remission.12,13 Fatigue alone and transient fever-related worsening of symptoms were not considered as relapses. Symptoms occurring within a month were considered as part of the same relapse.
MS course was categorized according to Lublin and Reingold's 1996 classification.13 Patient disability was assessed using the Expanded Disability Status Scale (EDSS),14 which ranges from 0 (no disability) to 10 (death of the patient), with 0.5-point steps.
Among 4,055 patients included in the study, a permanent discontinuation has been reported in 1,253 during the study period (figure 1). Follow-up forms were available for 896 patients. Among these cases, women who became pregnant during the year after discontinuation were excluded from the analysis, due to the potential effect of pregnancy on the natural course of the disease. Patients treated for fewer than 3 infusions were also excluded. Finally, 715 files were analyzed.
Figure 1. Patient population included in the TYSEDMUS database.
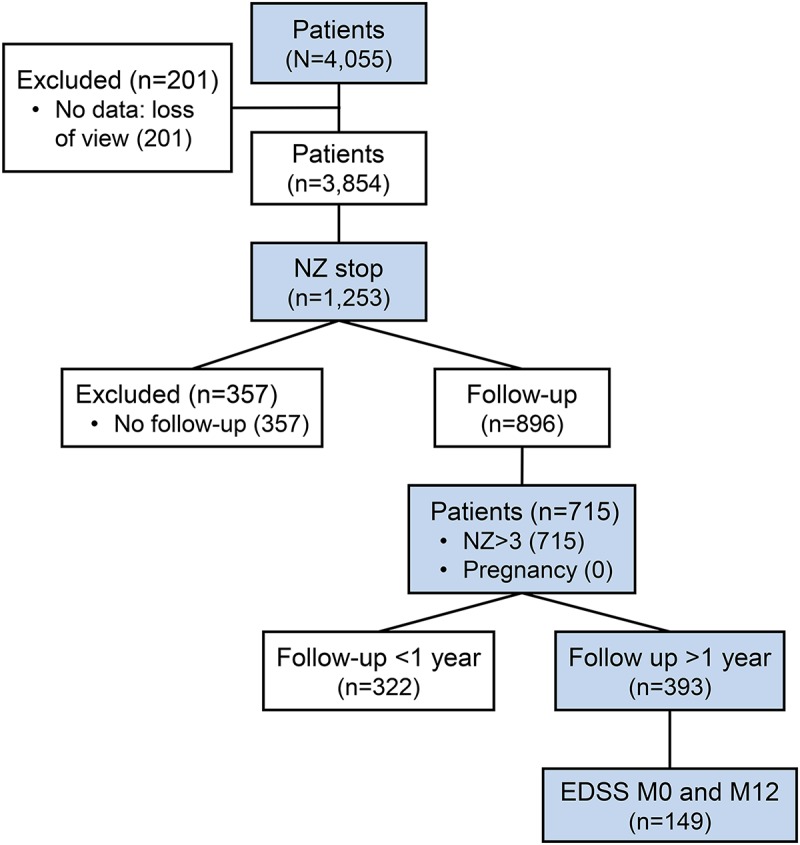
Natalizumab (NZ) >3: strictly more than 3 NZ infusions; pregnancy (0): no pregnancy during the year after NZ stop; Expanded Disability Status Scale (EDSS) M0 and M12: EDSS score available the month of the last NZ infusion and at 12 months after NZ discontinuation.
Study objectives.
The primary objective was to evaluate the time to the first relapse after NZ stop and to analyze the dynamic of the relapse occurrence over time.
The secondary objective was to determine the potential association of several factors with the occurrence of relapse after NZ stop: sex, number of relapses in the 12 months before inclusion, treatments received before NZ start, age at NZ withdrawal, relapse within 6 months before NZ withdrawal, treatment type after NZ withdrawal, duration of the washout period, disease course at NZ withdrawal, and EDSS score at time of NZ withdrawal.
The third objective was to evaluate the predictive factors of EDSS evolution between NZ discontinuation and 1-year follow-up.
Statistical analysis.
Characteristics of the study population at NZ initiation and at NZ stop was described together with NZ discontinuation reasons. Categorical variables are presented as number (%) and continuous variables as mean ± SD or median (lower quartile, upper quartile) when appropriate.
Time to first relapse was assessed using Kaplan-Meier survival analysis. Although the period of interest was the year following NZ stop, for this analysis, follow-up was considered up to 1.5 years.
The log-instantaneous relapse rate after NZ stop was modeled by a parametric cubic spline function with 2 knots at 0.5 and 1 year, using Poisson regression approach on finely split data.15 All relapses were taken into account, and follow-up was not censored after first relapse. Inflation of variance related to recurrent events was first evaluated and was finally neglected because it was very small. Factors associated with the occurrence of relapse after NZ stop were evaluated by proportional Cox regression model (univariate and multivariate). These factors were sex, number of relapses within 12 months before inclusion, treatments received before NZ start, age at NZ stop, relapse within 6 months before NZ stop, and disease course at NZ stop. All were included in the mutivariate model; continuous covariables were modeled with a linear effect (linearity was first tested in univariate analyses using likelihood-ratio test against a nonlinear effect modeled with a spline). In addition, new treatment initiation after NZ cessation was included in the model as a time-dependent variable. Duration of the washout period was taken into account in the model with an action time of 1 month for NZ and other immunosuppressive drugs (fingolimod, mitoxantrone, azathioprine, mycophenolate mofetil) and 3 months for immunomodulators (interferon, glatiramer acetate).
Concerning disability evaluation, EDSS scores were obtained at prespecified intervals, but systematic EDSS scores during and after a relapse were not collected systematically, therefore not allowing assessment of relapse severity directly. Disability progression was only assessed on patients followed up 1 year after NZ cessation and for whom an EDSS score was reported at the end of that 1-year period (n = 149). Potential factors associated with an increase of at least 1 point in EDSS between NZ stop and the end of the 1-year follow-up were evaluated by a logistic regression model. These factors were sex, disease course at NZ stop, occurrence of at least 1 relapse during the 1-year follow-up, and a new type of DMD treatment. All calculations were performed using R 3.1.1 software version. All p values were calculated using 2-tailed test, and values of 0.05 were considered significant.16
Ethical concern.
According to the French legislation regarding observational studies, patients were informed of the study, but did not have to provide a formal and written informed consent to have their anonymized data collected.
Data confidentiality and safety were ensured in keeping with the recommendations of the French Commission Nationale Informatique et Libertés, which also provided approval.
RESULTS
Demographic and clinical characteristics of the patients.
Among the 4,055 patients analyzed from the TYSEDMUS cohort, 1,253 (32.5%, representing 990 women and 263 men) stopped NZ (figure 1). Mean age at first NZ infusion was 37.2 years (±9.7), mean NZ treatment duration was 1.7 years (±1.3), and mean EDSS score prior to NZ initiation was 3.7 (±1.8). All patients had relapsing-remitting MS with a mean annualized relapse rate (ARR) of 1.9 (±1.1) before NZ initiation. Fifty-four patients used NZ as first treatment (7.5%). Patients who stopped NZ significantly differed from patients maintained in NZ treatment: there were more women (sex ratio 3.76 and 2.59, respectively: p < 0.0001) and a higher EDSS score prior to NZ (EDSS 3.7 and 3.5, respectively: p = 0.0081). In contrast, age at first NZ infusion and number of relapses before NZ start were not significantly different between the 2 groups.
Among 1,253 patients who stopped NZ, 538 were excluded from the analysis: 357 lost to follow-up and 181 who either received less than 3 infusions of NZ (n = 31) or desire for pregnancy (n = 57) or pregnancy (n = 93). Therefore, 715 patients were finally included in our study (figure 1). These patients had similar characteristics to included patients in terms of sex, number of relapses before NZ start, EDSS at NZ stop, and treatment received before NZ. However, the reasons for stopping were different: excluded patients showed more intolerance (26.2% vs 15.8%) and a more frequent desire for pregnancy (16.2% vs 8.7%). In the subgroup of women who became pregnant, age at disease onset was 22.6 years (±4.5), and age at NZ start was 28.9 years (±4.8). Median follow-up after NZ discontinuation was 1.1 years (0.6–2.0), with data available for 393 at month 12. EDSS was available at month 0 and month 12 for 149 patients.
Reasons for NZ discontinuation.
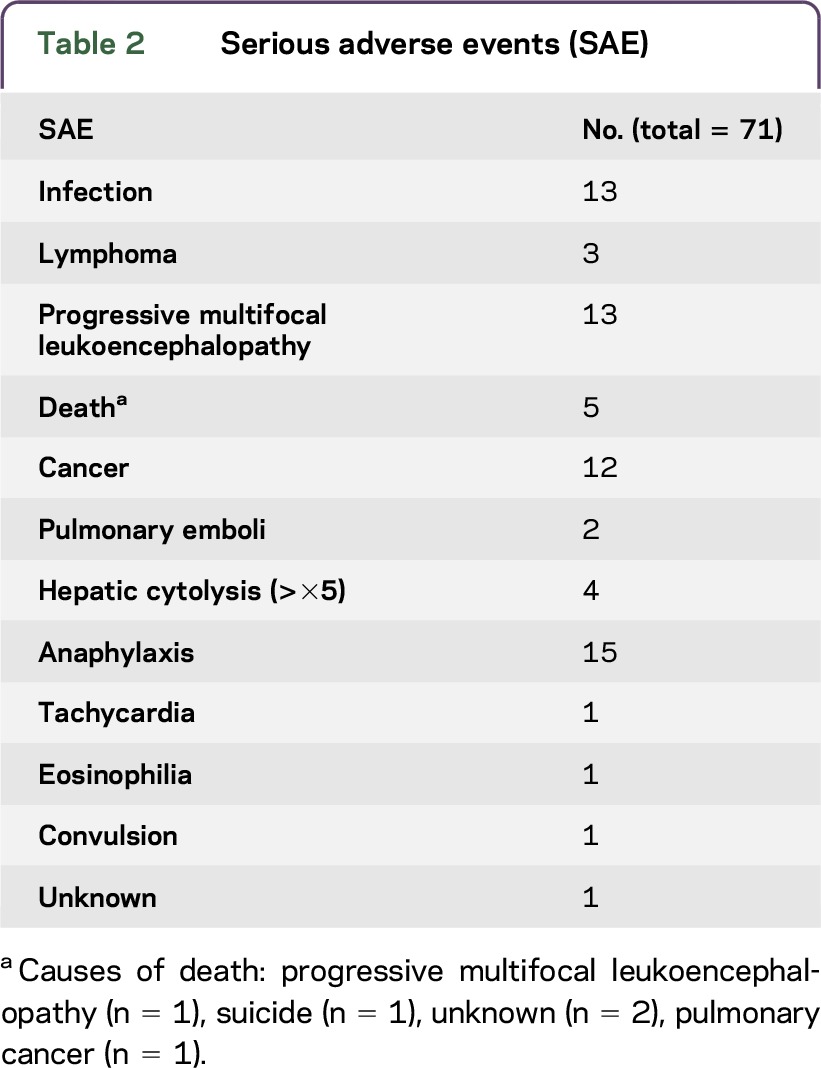
Reasons for NZ discontinuation were not exclusive (table 1), with several causes often being associated. The main reason for NZ withdrawal was scheduled stop or personal convenience (n = 436). These reasons represent discontinuations due to the risk of PML, although current risk stratification according to JCV status was not available at the time of the study. Other reasons were poor tolerance (n = 320), including 44 patients who discontinued due to persisting anti-NZ antibodies, and 71 patients for SAE (table 2), pregnancy (n = 93) or desire for pregnancy (n = 57), and lack of efficacy (n = 232), 130 because of a progressive evolution. Among the 715 patients analyzed, 83 patients discontinued NZ owing to progressive evolution. The other reasons are listed in table e-1 at Neurology.org/nn.
Table 1.
Description of the reasons to stop natalizumab (NZ) (not exclusive; several reasons can be associated)
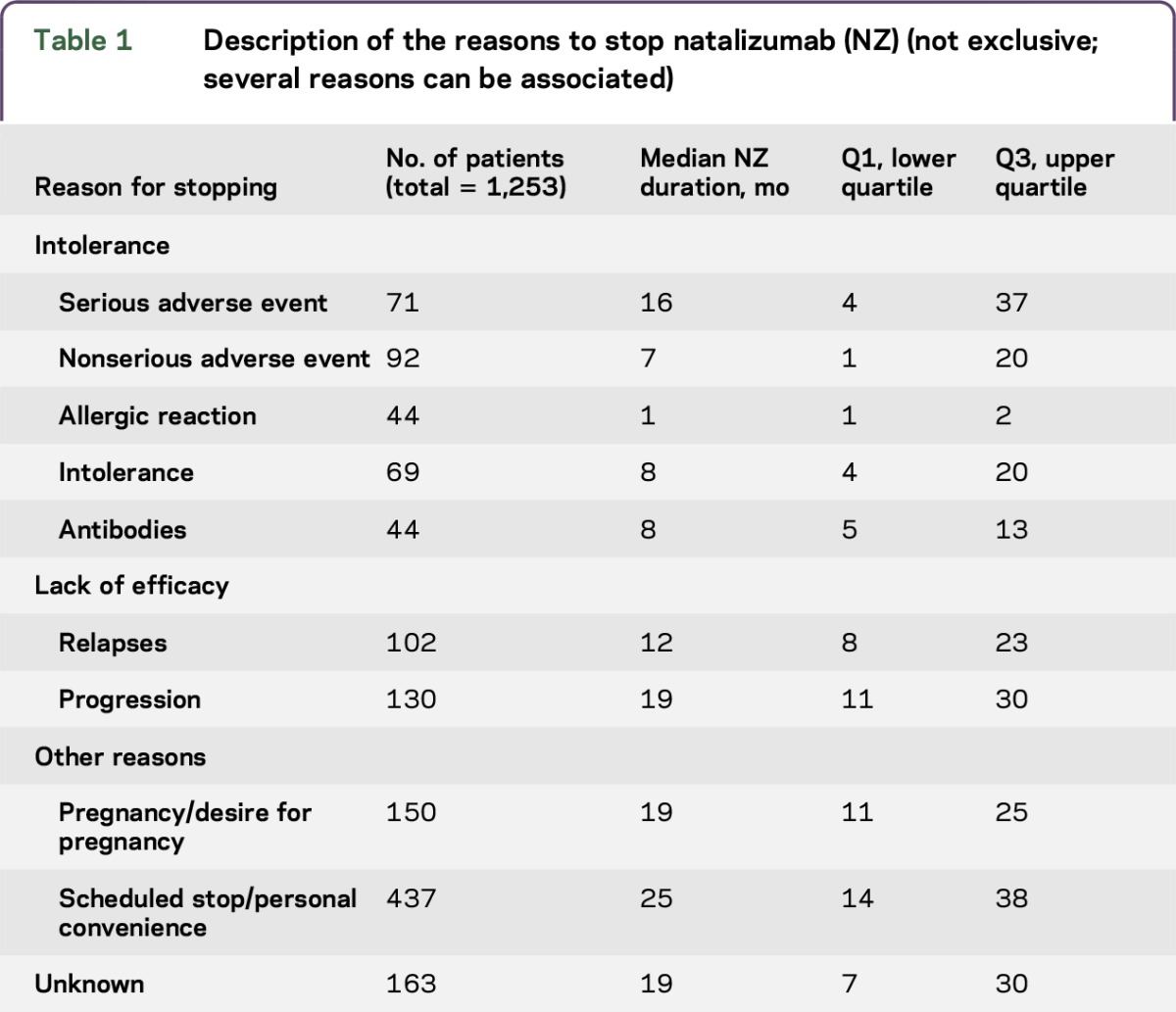
Table 2.
Serious adverse events (SAE)
DMD after NZ discontinuation.
Analysis of DMD treatment after NZ discontinuation was performed in the subgroup of patients with a follow-up of at least 1 year (n = 393). Among these 393 patients, 50% (n = 198) remained without DMD during the entire period after NZ cessation, 31% (n = 121) restarted NZ, 12% (n = 47) received an immunosuppressant (IS), and 7% (n = 27) received an immunomodulatory treatment (IM). The time to DMD start was shorter with IM initiation (median time 1.4 months) compared to IS (median time 4.08 months) and NZ (median time 5.40 months).
Description of relapses and time to first relapse after NZ discontinuation.
Among the 715 included patients, 362 relapses were reported in 265 patients, corresponding to 37.1% of patients having at least 1 relapse during the year following NZ cessation; among them, 76 (10.3%) patients had more than 1 relapse. The demographic characteristics of these 76 patients did not differ from those of the 639 other patients but their clinical activity before NZ start was higher and a larger proportion was treated by IM before NZ (but a similar proportion by IS).
The probability of relapse within the year after NZ stop was estimated at 45% (95% confidence interval [CI] 0.41–0.49). Twenty-five percent of patients experienced a first relapse 4.9 months (4.2–5.5) after NZ stop (see figure e-1). The instantaneous relapse risk increased steadily in the first 3 months to reach a peak between months 4 and 6 and decreased thereafter (figure 2). For the whole cohort (n = 715), the ARR during the therapeutic window was 0.42 (±1.04) and during the year of follow-up was 0.65 (±1.05), therefore lower than the ARR pre-NZ (1.99 ± 1.03). In the subgroup of women who became pregnant (n = 93), ARR was 0.31 (±1.17).
Figure 2. Instantaneous relapse rate per person-year after first natalizumab (NZ) stop (year) (n = 715).
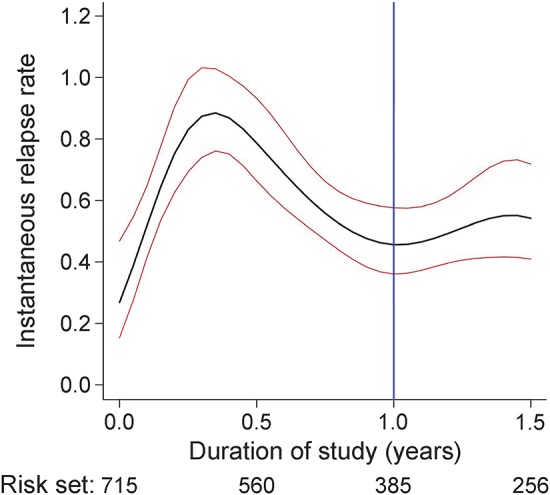
Black line: instantaneous relapse rate estimation. Red lines: instantaneous relapse rate 95% confidence interval. Blue line: the end of the study (1 year after NZ stop).
Factors associated with relapse.
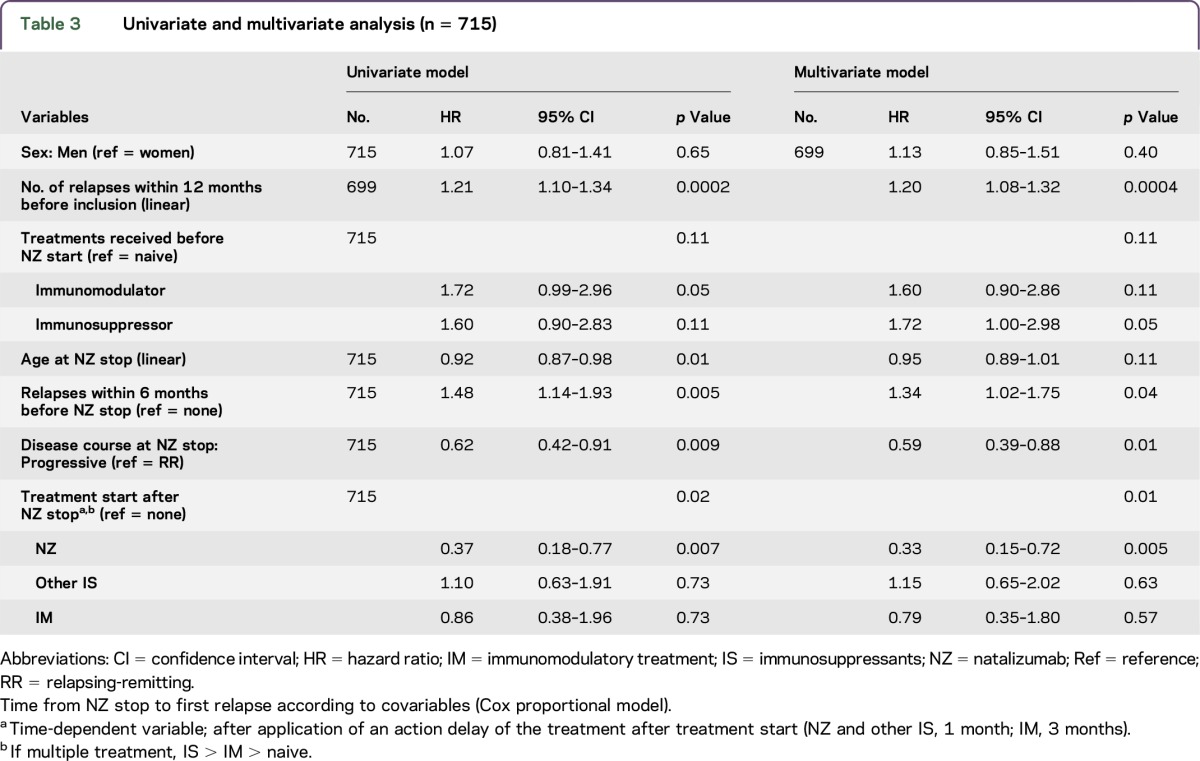
In the univariate analysis (table 3), the relapse risk was higher in patients who were younger at NZ onset and with a higher disease activity prior to NZ onset. As expected, patients who evolved to a secondary progressive course during NZ treatment had a lower relapse risk compared to patients who remained purely relapsing-remitting (hazard ratio [HR] 0.62, 95% CI 0.42–0.91). In the multivariate model, a higher relapse risk after NZ discontinuation remained associated with a higher disease activity during the year before NZ initiation (evaluated by the number of relapses during the year prior to NZ) and the occurrence of at least 1 relapse during the 6 months prior to NZ stop. In addition, after restart of DMD, the relapse risk was lower in patients who restarted NZ (HR 0.33 [0.15–0.72]) compared to other DMDs (IS [HR 1.1 (0.65–2.02)] and IM [HR 0.79 (0.35–1.80)]).
Table 3.
Univariate and multivariate analysis (n = 715)
Evaluation of EDSS progression.
Evaluation of EDSS progression after NZ discontinuation was performed on a subgroup of 149 patients (see Methods). In 23% of these patients (n = 35), EDSS increased by at least 1 point at 1 year post-NZ stop. Among these 35 patients, 20 patients (57.1%) experienced at least 1 relapse vs 15 patients who did not (42.9%). In the multivariate logistic model, the risk of EDSS increase was associated with disease course (secondary progressive) at NZ stop (OR 4.07 [1.38–11.98]; p = 0.02) and occurrence of at least 1 relapse during the year following NZ stop (OR 2.32 [0.98–5.09]; p = 0.06). Initiation of a new DMD treatment (NZ, other IS, or IM) was not associated with an EDSS score increase (p = 0.52).
DISCUSSION
In this French cohort, the largest reported to date, 37.1% of patients who discontinued NZ experienced a relapse within 12 months after NZ discontinuation. Most of these relapses occurred between 3 and 5 months after NZ discontinuation. The ARR following NZ discontinuation (0.65 ± 1.05) was lower than the ARR before NZ (1.99 ± 1.03), therefore not supporting the hypothesis of a rebound effect defined as an increase of relapse rate. The high level of relapse rate pre-NZ treatment, as compared with pivotal essay,1 is explained by the restriction of NZ by French authorities to patients with very active disease. Relapse risk was associated with young age and disease activity before NZ initiation. These results are in accordance with the registration clinical trials on NZ where drug withdrawal was associated with a recurrence of disease activity to levels similar to those seen prior to treatment.5
The time to first clinical relapse (3–5 months) was consistent with the natural course of elimination of the drug, and in agreement with previous small studies. Stüve et al.17 reported on a small group of patients (n = 23) followed with immunologic, clinical, and MRI evaluations during 14 months after discontinuation of NZ that the effect of NZ decreased over a 3- to 6-month period. Similarly, a North American study18 based on 175 patients with MS treated with NZ for at least 1 year showed that gadolinium-enhancing lesions were first detected 3 months after the last NZ infusion, and authors19 reported in a small group of patients (n = 32) a reactivation of disease activity between 3 and 9 months after NZ interruption, independently of an alternative treatment prescribed.
Our study supports a relapse rate reduction related to DMD initiation. These results are consistent with some other reported data: regarding immunomodulators, among the 1,615 patients from the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM), Safety and Efficacy of Natalizumab in Combination with Interferon β-1a in Patients with Relapsing-Remitting Multiple Sclerosis (SENTINEL), and Glatiramer Acetate and Natalizumab Combination Evaluation (GLANCE) trials enrolled in the NZ safety extension study,5 13% received immunomodulators after NZ cessation (interferon 9.9%; glatiramer acetate 2.4%), and had a reduced relapse rate compared to untreated patients (0.43 [95% CI 0.36–0.52] vs 0.63 [95% CI 0.37–1.01]). Similarly, in a short study on 40 patients with active relapsing-remitting MS who started glatiramer acetate 4 weeks after NZ discontinuation, authors20 reported that 60% of patients were relapse-free 12 months after the DMD initiation. Regarding IS, in our cohort, few patients received fingolimod after NZ stop because this study was conducted from November 2007 to November 2012 and fingolimod has been only available from December 2011. However, recent results suggest that early initiation (less than 3 months) of fingolimod after NTZ interruption reduces the relapse risk (odds ratio 0.23) compared to delayed treatment.11,21
Our study underlines the efficacy of NZ restart. In the same way, the randomized, partially placebo-controlled study to evaluate the effect on MS disease activity during a 24-week period after natalizumab discontinuation (RESTORE) study22 showed a restart of disease activity 3 months after NZ discontinuation in 167 of the 175 randomized patients in all therapeutic subgroups, except for patients who restarted NZ.
Although this observational study was not initially designed to address relapse severity, and although EDSS data during and after the relapses were not systematically collected, an increase of 1 point in EDSS was detected at the end of the follow-up period in 23% of patients compared to the last NZ infusion. Although this analysis was only performed on a small population (149 patients), this worsening of EDSS tends to suggest that NZ cessation increases the risk of severe relapse, most probably related to inflammatory reactivation within the CNS, accordingly with several monocentric open studies and case reports that reported severe relapses and high inflammatory MRI activity after stopping NZ.23–27
Despite some limitations of this real-life study due to missing data (only 57% of the files were analyzed; 31% had clinical data available at 12 months [12% had an EDSS score reported]; no MRI data), it is important to emphasize that results are based on one of the largest cohorts of patient after NZ withdrawal.
This is a large-scale, multicenter, systematic study evaluating MS activity after NZ cessation in real-life settings. It showed that NZ withdrawal results in MS activity recurrence, with a relapse risk depending on age and disease activity before NZ initiation. These results have to be taken into account when a decision to stop NZ is planned. If discontinuation is needed, it should be considered to treat these patients with IS or IM within 3 months after NZ stop.
Supplementary Material
GLOSSARY
- AE
adverse event
- ARR
annualized relapse rate
- CI
confidence interval
- DMD
disease-modifying drug
- EDSS
Expanded Disability Status Scale
- HR
hazard ratio
- IM
immunomodulatory treatment
- IS
immunosuppressants
- JCV
JC virus
- MS
multiple sclerosis
- NZ
natalizumab
- PML
progressive multifocal leukoencephalopathy
- SAE
serious adverse event
Footnotes
Supplemental data at Neurology.org/nn
Contributor Information
Collaborators: TYSEDMUS and OFSEP Group, Sandra Vukusic, Michel Clanet, Bertrand Fontaine, Thibault Moreau, Bruno Brochet, Jean Pelletier, Jerome de Seze, Francois Cotton, Vincent Dousset, David Laplaud, Marc Debouvery, Gilles Edan, Caroline Papeix, Bruno Stankoff, Alain Créange, Yann Mickaeloff, Kumaran deiva, Olivier Gout, Christine Lebrun Frenay, Patrick, vermersch, Giulles Defer, Eric Berger, Eric Thouvenot, Philippe CabreMD, Pierre Labauge, William Camu, Bertrand Bourre, Jean Philippe Candessanche, Laurent Magy, Abdellatif Al-Khedr, Ayman Tourbah, Olivier Casez, Anne-Marie Guennoc, and Jonathan Ciron
AUTHOR CONTRIBUTIONS
Caroline Papeix: study concept and design, acquisition of data, analysis and interpretation, writing, rewriting. Sandra Vukusic: study concept and design, acquisition of data, analysis and interpretation, writing, rewriting. Romain Casey: analysis and interpretation, writing, rewriting. Nadine Passante: acquisition of data, critical revision of the manuscript for important intellectual content. Bruno Stankoff: acquisition of data, critical revision of the manuscript for important intellectual content. Serge Mrejen: acquisition of data, critical revision of the manuscript for important intellectual content. Zoe Uhry: analysis and interpretation, writing, rewriting. Eric Van Ganse: study concept and design. Anne Castot: study concept and design, critical revision of the manuscript for important intellectual content. Michel Clanet: study concept and design, critical revision of the manuscript for important intellectual content. Catherine Lubetzki: study concept and design, acquisition of data, analysis and interpretation, writing, rewriting, critical revision of the manuscript for important intellectual content, study supervision. Christian Confavreux: study concept and design, acquisition of data, analysis and interpretation, study supervision.
STUDY FUNDING
No targeted funding.
DISCLOSURE
C. Papeix served on the scientific advisory board for Bayer Schering, Novartis, and Teva and was employed for educational activities from Bayer Schering, Sanofi-Genzyme, Biogen Idec, Novartis, Merck Serono, and Roche. S. Vukusic served on the scientific advisory board for Biogen Idec, Geneuro, Genzyme, Merck-Serono, Novartis Pharma, Roche, Sanofi Aventis, and Teva Pharma; and received research support from Biogen Idec, Genzyme, Merck-Serono, Novartis Pharma, Roche, Sanofi Aventis, and Teva Pharma. R. Casey and N. Debard report no disclosures. B. Stankoff served on the scientific advisory board for Biogen Idec, Novartis, Genzyme, Teva Pharma, and Roche; received travel funding and/or speaker honoraria from Teva, Novartis, Biogen, and Genzyme; has a patent pending for the identification of a compound that stimulates demyelination; and received research support from Merck-Serono, Genzyme, ANR, PHRC, Foundation ARSEP, and Progressive MS Alliance. S. Mrejen and Z. Uhry report no disclosures. E. Van Ganse received grants and personal fees from ALK ABELLO, Bayer, BMS, GlaxoSmithKline, and Merck Sharp & Dohme, and personal fees from AstraZeneca, Boehringer Ingelheim, IMS, and LASER. A. Castot reports no disclosures. M. Clanet served on the scientific advisory board for Biogen Idec, Genzyme, Merck Serono, and Sanofi Teva; received travel funding from ECTRIMS; served on the editorial board for Multiple Sclerosis; and received research support from ARSEP, EDMUS, and Eugene Devic Foundation. C. Lubetzki served on the scientific advisory board for Bertex, Biogen, Novartis, and Genzyme; served on the editorial board for MSJ and MSJ and Related Disorders; was associate editor for Brain; consulted for Biogen, Roche, and Genzyme; and had a scientific collaboration with EMD Serono. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. [DOI] [PubMed] [Google Scholar]
- 2.Gorelik L, Lerner M, Bixler S, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol 2010;68:295–303. [DOI] [PubMed] [Google Scholar]
- 3.Plavina T, Subramanyann M, Blogren G, et al. Anti-JC virus antibody levels in serum or plasma further define risk of NZ associated PML. Ann Neurol 2014;76:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2006;354:911–923. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor PW, Goodman A, Kappos L, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 2011;76:1858–1865. [DOI] [PubMed] [Google Scholar]
- 6.Miller DH, Khan OA, Sheremato WA, et al. A controlled trial of NZ for relapsing multiple sclerosis. N Engl J Med 2003;348:15–23. [DOI] [PubMed] [Google Scholar]
- 7.Killenstein J, Vennegoor A, Strijbis EM, et al. Natalizumab drug holiday in multiple sclerosis: poorly tolerated. Ann Neurol 2010;68:392–395. [DOI] [PubMed] [Google Scholar]
- 8.Vellinga MM, Castelijns JA, Barkhof F, Uitdehaag BM, Polman CH. Postwithdrawal rebound increase in T2 lesional activity in natalizumab-treated MS patients. Neurology 2008;70:1150–1151. [DOI] [PubMed] [Google Scholar]
- 9.Schiess N, Calabresi PA. Natalizumab: bound to rebound? Neurology 2009;72:392–393. [DOI] [PubMed] [Google Scholar]
- 10.Lenhard T, Biller A, Mueller W, Metz I, Schonberger J, Wildemann B. Immune reconstitution inflammatory syndrome after withdrawal of natalizumab? Neurology 2010;75:831–833. [DOI] [PubMed] [Google Scholar]
- 11.Cohen C, Maillart E, Tourbah A, et al. Switching from natalizumab to fingolimod in multiple sclerosis: a French prospective study. JAMA Neurol 2014;71:436–441. [DOI] [PubMed] [Google Scholar]
- 12.Confavreux C, Compston DA, Hommes OR, McDonald WI, Thompson AJ. EDMUS, a European database for multiple sclerosis. J Neurol Neurosurg Psychiatry 1992;55:671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey: National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trial of New Agents in Multiple Sclerosis. Neurology 1996;46:907–911. [DOI] [PubMed] [Google Scholar]
- 14.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 15.Laird N, Olivier D. Covariance analysis of censored survival data using log-linear analysis techniques. J Am Stat Assoc 1981;76:231–240. [Google Scholar]
- 16.R Core Team. R: A Language and Environment for Statistical Computing [R-project.org/]. Vienna: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 17.Stüve O, Cravens PD, Frohman EM, et al. Immunologioc, clinical, and radiologic status 14 months after cessation of NZ therapy. Neurology 2009;72:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cree B, de Seze J, Fox R, et al. Natalizumab effects during a 6 month dose Interruption: relationship of pharmacokinetic (PK), pharmacodynamic (PD) and MRI measurement. Neurology 2013;80(meeting abstract 1):S41.003.23479543 [Google Scholar]
- 19.Guegen A, Roux P, Deschamps R, et al. Abnormal inflammatory activity returns after natalizumab cessation in multiple sclerosis. J Neurol Neurosurg Psychiatry 2014;85:1038–1040. [DOI] [PubMed] [Google Scholar]
- 20.Rossi S, Motra C, Struder V, et al. Effect of glatiramer acetate on disease reactivation in MS patients discontinuing natalizumab. Eur J Neurol 2013;20:87–94. [DOI] [PubMed] [Google Scholar]
- 21.Jokubaitis VG, Li V, Kalincik T, et al. Fingolimod after natalizumab and the risk of short term relapse. Neurology 2014;82:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox R, Kappos L, Cree B et al. Effects of a 24-week natalizumab treatment interruption on clinical and radiologic parameters of multiple sclerosis disease activity: the RESTORE Study. ECTRIMS and ACTRIMS, October 19–22, 2011, Amsterdam, the Netherlands.
- 23.Marousi S, Giannouli E, Karkanis I, et al. Immune reconstitution inflammatory syndrome after withdrawal of natalizumab? Neurology 2011;76:1362–1363. [PubMed] [Google Scholar]
- 24.Miravalle A, Jensen R, Kinkel RP. Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of NZ therapy. Arch Neurol 2011;68:186–191. [DOI] [PubMed] [Google Scholar]
- 25.Papeix C, Depaz R, Tourbah A, Stankoff B, Lubetzki C. Dramatic worsening following plasma exchange in severe post-natalizumab withdrawal multiple sclerosis relapse. Mult Scler 2011;17:1520–1522. [DOI] [PubMed] [Google Scholar]
- 26.Daelman L, Maitrot A, Maarouf A, Chaunu MP, Papeix C, Tourbah A. Severe multiple sclerosis reactivation under fingolimod 3 months after natalizumab withdrawal. Mult Scler 2012;18:1647–1649. [DOI] [PubMed] [Google Scholar]
- 27.Kerbrat A, Le Page E, Leray E, et al. Natalizumab and drug holiday in clinical practice: an observational study of very active relapsing remitting multiple sclerosis patients. J Neurol Sci 2011;308:98–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


