Summary
While it has been clear that the brain regulates feeding behaviour and energy expenditure, the major determinants of energy balance and adiposity, roles for individual brain regions (and specific cell types within these regions) in the control of energy balance were not understood until very recently; these details continue to emerge rapidly. Much of what we now know flows from the discoveries of leptin and the hypothalamic melanocortin system, which define circuits crucial for the control of energy balance. Within the brain, hypothalamic circuits play a crucial role in the control of feeding and energy expenditure. Within the hypothalamus, the arcuate nucleus (ARC) functions as an entry point gateway for hormonal signals of energy balance, such as leptin; the ARC also contains the soma of melanocortinergic neurons. The paraventricular hypothalamic nucleus (PVH) receives direct melanocortin input, along with other integrated signals regarding energy balance, and mediates the majority of hypothalamic output to control feeding and energy expenditure. Herein, we review the structure and function of the ARC-PVH circuit in leptin action, in addition to it’s role in the control of feeding behavior and energy expenditure.
Keywords: Leptin, melanocortins, PVH, hypothalamus, brainstem, neuropeptides, NOS1, feeding, obesity
Obesity and the biology of energy balance
As the prevalence of obesity and its complications continue to rise worldwide, so too do their consequences in medical and financial terms. Obesity is not merely a cosmetic issue, but increases the incidence of type II diabetes, cardiovascular disease, cancer, sleep apnea, and a variety of mental health conditions, including depression. In addition to contributing to other economic and societal burdens, obesity-related illness underlies 10% of current annual health care expenditures in the United States(1). Thus, it is important that we understand the mechanisms that contribute to the development and maintenance of obesity and its complications, as well as potential points of therapeutic intervention for this disease.
The effort to study and cure obesity is complicated by common misperceptions: that obesity is a cosmetic rather than a medical concern and that obesity is the result of personal shortcomings (i.e., poor self-control), rather than the result of biologically-encoded processes. Much as high blood pressure and gastric ulcers (which were previously thought to result from psychic stressors) are now known to result from physiologic dysregulation and/or infectious agents, it is also now clear that the control of body weight is biologically encoded. Not only do a variety of single-gene mutations promote obesity in rodents, but also alterations in these genes (although generally rare) cause obesity in humans (2). However, most cases of “common” obesity likely result from more complicated alterations in multiple gene products (to date, GWAS have identified over 90 genetic regions that contain polymorphisms linked to the control of body weight) whose effects are unmasked by the ubiquitous availability of calories in modern society(3). The recent success of a number of weight-loss drugs that modulate systems known to participate in body weight control suggests the utility of more thoroughly understanding these systems to identify additional potential therapeutic targets, however.
The principles underlying the control of body weight are straightforward: simply stated, homeostasis is achieved when the amount of energy consumed (by eating) equals that expended (by the combination of basal metabolic rate and physical activity). Increasing energy intake relative to energy expenditure results in the storage of surplus calories as fat, while decreasing food intake relative to energy expenditure (as in dieting) decreases fat stores. Both energy intake and energy expenditure are largely governed by the central nervous system (CNS); this is supported by the fact that the obesity-linked genes identified in monogenetic syndromes and in human GWAS studies generally affect the CNS (3). Indeed, many of these genes have aided in the identification and analysis of the CNS circuits that control feeding and/or body weight(4). Furthermore, recent molecular genetic tools have made it possible to elucidate the neurobiological mechanisms underlying body weight regulation.
Leptin and the CNS melanocortin system represent crucial controllers of energy balance
Several spontaneously-arising mouse models of obesity not only revealed the importance of genetically-encoded biologic processes for the control of feeding, energy expenditure, and body weight, but also provided a starting entry point to explore this biology. Mice homozygous for the autosomal recessive obese (ob) and diabetes (db) mutations are hyperphagic and display decreased energy utilization, and are extremely obese and diabetic (5). Early experiments at the Jackson Laboratories suggested that the products of these genes were a hormone/receptor pair- a conclusion borne out by the subsequent cloning of the ob gene product (leptin), and its receptor (LepRb, encoded by db) (6, 7). Leptin is produced by adipose tissue in approximate proportion to triglyceride stores, thus serving as a signal of the repletion of long-term energy stores. Leptin administration to leptin-deficient (ob/ob) or normal mice decreases food intake and increases basal metabolic rate, along with activity(8–10). The lack of leptin during prolonged caloric restriction provides a powerful signal to increase hunger and decrease activity and overall energy expenditure, since leptin blunts the neuroendocrine changes and hunger associated with decreased fat mass in rodents and humans (11, 12).
Most leptin action is mediated by LepRb in the CNS(13). LepRb is expressed on specific populations of neurons located primarily in the hypothalamus and brainstem(14–16). These sets of neurons each presumably play distinct and specialized roles in the control of parameters relevant to energy balance. Thus, although human obesity resulting from disruption of leptin or LepRb (or other monogenic causes) is rare, leptin and the neural pathways it controls represent crucial players in the control of feeding and energy balance(17).
Mice containing the yellow mutation of the agouti locus (Ay) are notable for the dominant inheritance of a striking yellow coat color and obesity proportional to the intensity of the coat color(5). These characteristics were initially noted and maintained by European mouse fanciers beginning in the 1800s (5). The Ay allele represents a fusion of the constitutively active Raly promoter to the agouti coding sequences, resulting in the widespread ectopic expression of agouti. Agouti is normally expressed in the hair follicle, where it blocks the melanocortin 1 receptor (MC1R) to cause synthesis of phaeomelanin (yellow pigment) instead of eumelanin (black pigment), and thereby causing yellow coat color(18). Genetic expression or direct administration of agouti protein in the brain antagonizes central melanocortin receptors (Mc3R and Mc4R), which causes hyperphagia; thus this genetic model revealed the importance of CNS melanocortin action for feeding and bodyweight control(19, 20).
The subsequent development of mice lacking each of the CNS melanocortin receptors revealed relatively mild metabolic phenotypes in mice lacking Mc3R, but dramatic hyperphagia and obesity in Mc4R-null animals, thus demonstrating the crucial role played by CNS melanocortin action via Mc4R for the control of energy balance(21–23). Indeed, genetic alterations in Mc4R represent the most common monogenetic form of human obesity, underlying the phenotype of 1–2.5% of morbidly obese patients(24, 25). Hence, understanding the central melanocortin system is critical to unraveling the neurobiology of energy balance regulation.
Leptin and other signals converge with the melanocortin system in the ARC
A number of historical observations have pointed to an important role for the ventral medial portion of the hypothalamus (which contains the arcuate (ARC) and ventromedial (VMN) nuclei) in the restraint of feeding. A variety of chemical and electrolytic lesions of this brain region in animals results in hyperphagia and obesity(26, 27). Additionally, leptin fails to significantly alter food intake or body weight in ARC-lesioned animals, suggesting that neurons of the ARC are required for effective leptin action(28). Furthermore, the ARC represents the major inception point for the CNS melanocortin system: In addition to containing the major population of CNS cells that express proopiomelanocortin (POMC, the precursor for melanocortin peptides), the ARC contains a distinct set of neurons that express agouti-related peptide (AgRP), an antagonist of Mc3R and Mc4R(29).
The ARC lies in close proximity to the median eminence with its permeable vasclature, permitting rapid access of circulating factors to the ARC. Consistently, the ARC contains receptors for a variety of hormones that modulate energy balance, including leptin, insulin, and ghrelin(30). Indeed, the ARC contains perhaps the highest density of LepRb-expressing neurons in the brain, and many of these LepRb neurons express POMC or AgRP (Fig. 1) (31, 32). POMC cells, which produce (among other peptides) the melanocortin receptor agonist, αMSH, mediate important anorexigenic signals; leptin activates these neurons and increases their expression of Pomc(33). AgRP-containing neurons release not only this endogenous melanocortin receptor antagonist, but also the inhibitory neurotransmitters neuropeptide Y (NPY) and gamma aminobutyric acid (GABA)); these neurons promote food intake and are inhibited by leptin (34–37) (38). Consistent with the importance of the ARC for leptin action, leptin poorly suppresses food intake and body weight in animals containing lesions of the melanocortin system (including Ay, Pomc-null, and Mc4r-null animals), suggesting an important role for the melanocortinergic neurons in leptin action. Surprisingly, mouse models in which LepRb has been specifically deleted from POMC or AGRP neurons do not approach the obesity and metabolic dysregulation resulting from total loss of CNS LepRb, however(39, 40). This suggests that leptin mediates important melanocortin-independent effects and/or may control POMC and AgRP neurons by additional, indirect, mechanisms. Indeed, POMC and AgRP neurons together comprise only a small fraction of the total number of LepRb neurons in the brain. Furthermore, non-POMC, non-AgRP LepRb neurons that express Vgat or Nos1 each contribute to the modulation of POMC cells and play important roles in the control of feeding and body weight(41, 42).
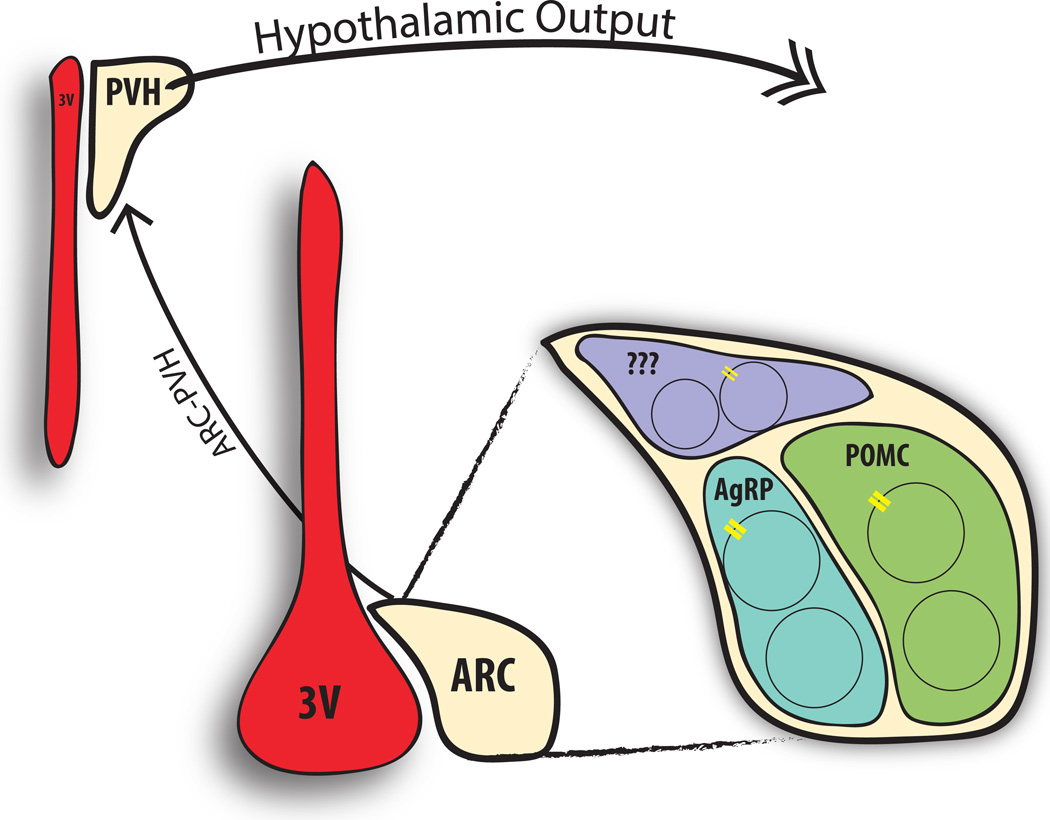
Figure 1.
The ARC-PVH circuit is an essential controller of energy balance. The arcuate nucleus (ARC) sits adjacent to the third ventricle (3V) and detects circulating signals of energy balance (including leptin via its receptor, LepRb (yellow receptors). A variety of ARC populations express LepRb, including distinct neurons that contain proopiomelanocortin (POMC) and agouti-related peptide (AgRP), which are important for the regulation of energy balance. Leptin activates the anorexigenic POMC neurons while inhibiting the orexigenic AgRP neurons; both send dense projections to the paraventricular nucleus of the hypothalamus (PVH), where they mediate crucial signals to control feeding and energy balance. The ARC also contains non-AgRP, non-POMC neurons (purple), some of which also express LepRb. The PVH integrates these ARC-derived signals with other information, and represents the primary hypothalamic output to other brain regions for the control of energy balance.
Thus, while the ARC melanocortin system (POMC and AgRP cells) encompasses major controllers of feeding and body weight, and represents a crucial mediator of leptin action, other LepRb neurons play important roles in energy balance (potentially in part by indirectly controlling these ARC cells). Other modulators of feeding and body weight also contribute to the control of POMC and AgRP neurons, including hormones (insulin, ghrelin) and nutrients (glucose, lipids) from the circulation and neurotransmitters (serotonin, GABA) from elsewhere in the brain. ARC POMC and AgRP neurons can thus be thought of as sensors and integrators of a variety of peripheral and centrally-derived cues that signal energy status and affect feeding and energy expenditure. While leptin signals long-term energy stores and primarily modulates gene expression to control the overall sensitivity of the melanocortin system to other cues (hence, tending to suppress long-term food intake and permitting energy utilization), acute signals (such as ghrelin, which spikes in anticipation of a meal) change the activity of POMC and/or AgRP neurons more rapidly, and affect feeding and energy utilization over the short term.
Although leptin activates POMC neurons and inhibits AgRP neurons, these two populations do not comprise the entire LepRbARC population (Fig. 1). Some ARC neurons also express receptors for insulin (INSR), GLP-1 (GLP1R), ghrelin (GHSR), melanocortins (Mc3R), and serotonin (5-HT2CR, 5-HT1BR) (30). Specifically, ghrelin, an orexigenic peptide released from the stomach, stimulates AgRP neurons.(43) Mc3Rs on ARC POMC neurons are important for feedback inhibition of POMC neurons(32). Similar to leptin, serotonin increases the activity of POMC neurons and decreases AgRP neuronal activation(44, 45).
Control of feeding and energy balance downstream of the ARC
From the ARC, POMC and AgRP cells send dense projections to the paraventricular nucleus of the hypothalamus (PVH), as well as the dorsomedial hypothalamus (DMH) and other hypothalamic areas, and extra-hypothalamic sites such as the brainstem (e.g. NTS) and forebrain (e.g. BNST) (46–48) (49). Early on, it was apparent that POMC and AgRP projections to the PVH were likely important for the regulation of feeding for many reasons: 1) PVH lesions result in hyperphagic obesity, 2) Mc4R is densely expressed in the PVH, 3) the PVH sends dense projections to hindbrain regions important for the termination of feeding, and 4) deletion of Mc4R from PVH neurons increases feeding(50–56). While studies showing that the ablation of AgRP neurons suppresses food intake by inhibiting neurons in the hindbrain parabrachial nucleus (PBN), the activation of AgRP cells does not promote feeding via the PBN, but rather via actions in forebrain sites, including the PVH(57, 58). Indeed AgRP neuron-stimulated feeding requires their inhibition of PVH neurons(59). Additionally, activation of cells in the PVH acutely increases energy expenditure and decreases energy intake, further supporting a role for the PVH in the direct control of multiple energy balance parameters(60, 61). While the PVH responds only indirectly (i.e., downstream of neurons that respond directly) to peripheral signals of energy status (including leptin), it is crucial for leptin action and the overall control of energy balance, since it represents the major site of hypothalamic output to the hindbrain (Fig. 1).
Initial studies aimed at characterizing the role of the PVH in energy balance control identified the PVH as the primary hypothalamic site projecting to brainstem satiety regions, such as the NTS/DMV and parabrachial nucleus (PBN)(53). In general, the brainstem integrates neural (and, to a lesser extent, hormonal) signals from the periphery-especially those derived from the gut(62). The brainstem relays these signals to forebrain, hypothalamic, and cortical regions to coordinate appropriate behavioral and physiologic responses. For the regulation of satiety, projections from the PVH to the NTS are of particular interest, since the NTS is anatomically close to the area postrema (AP), a site adjacent to the fourth ventricle that lacks a blood brain barrier. The adjacent NTS receives both AP- and vagally-derived gut signals of acute and chronic nutritional state, which it passes on to the DMV to control gut motility and vagal reflexes(63). The NTS also receives descending signals from the hypothalamus that indicate energy status (many of these come from the PVH). The NTS is crucial for the satiety response that follows the ingestion of a meal, and at least part of this is conveyed via a direct glutamatergic projection to the PBN; connections to the PVH may also play a role(64) (65).
The PBN also integrates a variety of signals from the periphery and from other brainstem sites (e.g. PAG, NTS), as well as the spinal column(66). Also, direct projections from ARC AgRP neurons to the PBN can control anorexia(67). The PBN sends axonal projections to the hypothalamus, central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST) to modulate feeding behavior(58, 68) (69). Therefore, the dense innervation of both the PBN and NTS by the PVH suggests multiple pathways by which the PVH may modulate hindbrain responses to control feeding behavior.
In addition to the suggestive nature of PVH connections to the brainstem, studies modulating PVH function have revealed the crucial role of the PVH in feeding and body weight regulation. Indeed, mechanical or genetic disruption of the PVH in mice and rats results in hyperphagic obesity(50, 51, 70). For example, haploinsufficiency of Sim1, a transcription factor required for the development of the PVH (and the amygdala), results in hyperphagic obesity in mice and humans(71, 72). The importance of the melanocortinergic ARC-PVH circuit is demonstrated by the necessity and sufficiency of Mc4R signaling in the PVH for normal feeding behavior(56, 73). Indeed, Mc4R expression exclusively in Sim1 neurons normalizes feeding in an Mc4R-null background, reducing obesity by 60%. The persistence of modest obesity following restoration of Mc4R expression in PVH/Sim1 cells suggests that the melanocortin-dependent regulation of energy expenditure is likely carried out by non-PVH Mc4R-expressing neurons(56). Part of this effect is mediated by cholinergic neurons in the intermediolateral column of the spinal cord (IML)(74). More recent studies demonstrated that Mc4R signaling specifically in glutamatergic PVH neurons is sufficient to control feeding and overall energy balance(75).
While the PVH is critical for the regulation of energy balance, the specific PVH neurons that regulate feeding are not well defined. While almost entirely glutamatergic, the PVH is otherwise a heterogenous center that contains a variety of peptidergic cells (including those that contain oxytocin (OXT), vasopressin (AVP), corticotropin-releasing hormone (CRH), or thyrotropin-releasing hormone (TRH)) and non-peptidergic cells. The PVH contains magnocellular and parvocellular neurosecretory populations that primarily project to the median eminence to regulate pituitary function, as well as parvocellular populations that project within the brain (Fig. 2). Presumably, brainstem-projecting PVH neurons mediate important satiety signals, including those in response to melanocortins. Indeed, glutamatergic PBN-projecting PVH cells respond to melanocortin agonists(73). Moreover, stimulation of Mc4RPVH terminals projecting to the PBN, but not NTS/DMV, suppresses feeding, suggesting that Mc4RPVH neurons control satiety via the PBN (Fig. 2)(61). The neurochemical identity of these Mc4RPVH cells has yet to be established. Additionally, it is possible that non-Mc4RPVH neurons play a role in controlling feeding behavior (through connections to the PBN and/or other sites), and these cells will need to be identified, as well.
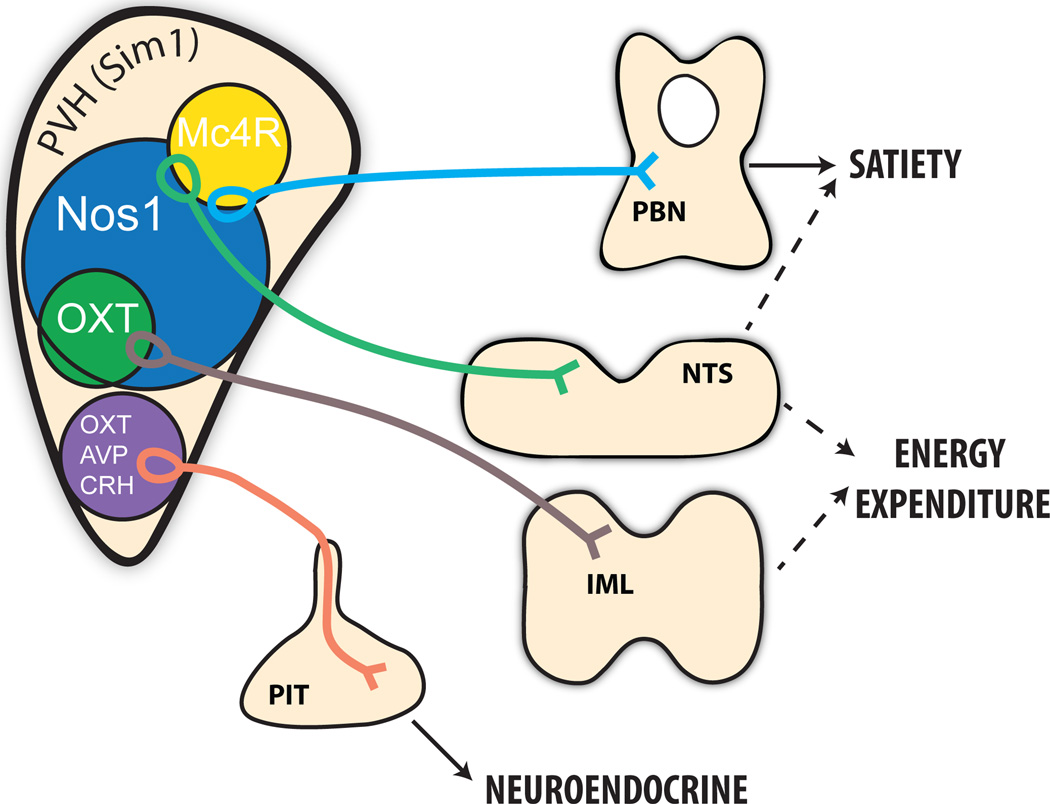
Figure 2.
Molecularly distinct subpopulations of PVH neurons control distinct aspects of energy balance. PVH neurons containing melanocortin 4 receptor (Mc4R) are distinct from the magnocellular neuroendocrine populations (purple) controlling pituitary function, including oxytocin (OXT), vasopressin (AVP), and corticotropin-releasing hormone (CRH) neurons. Instead, Mc4R neurons in the PVH regulate satiety via projections to hindbrain, including the parabrachial nucleus (PBN). PVH neurons containing neuronal nitric oxide synthase 1 (Nos1) comprise a large subpopulation of the entire PVH (identified by the developmental transcription factor Sim1) and control both feeding and energy expenditure, at least in part via the PBN and spinal cord, respectively. Parvocellular OXT neurons are a subpopulation of the Nos1PVH field and are modestly participate in the control of energy expenditure, but not feeding. The role of NTS projections, originating from non-OXT Nos1PVH and Mc4RPVH neurons, in regulating energy balance parameters remains unclear.
Retrograde tract tracing with cholera toxin B has been used to identify PVH neurons connected to the hindbrain, and presumably important for feeding. Initial studies using this technique suggested that PVH OXT (OXTPVH) cells project to the NTS in rats(76). Furthermore, NTS-projecting OXTPVH neurons are activated following the administration of leptin, suggesting a role for these cells in conveying the ARC-PVH signal to the NTS, and potentially for satiety signaling(55). Consistently, hyperphagic obese Sim1 haploinsufficient mice demonstrate decreased Oxt expression(77).
While these studies suggest the importance of OXTPVH neurons in the PVH-mediated control of energy balance, OXT knock-out and ablation studies do not support these findings. Ablation of OXT or OXT receptors in mice has little effect on body weight regulation until animals are placed on a high-fat diet, which promote only a slight enhancement of diet-induced obesity (due to decreased energy expenditure, not hyperphagia) (78, 79). This lack of phenotype is not a consequence of developmental compensation, since genetic ablation of OXT neurons using the Cre-dependent diphtheria toxin system in adult mice produces similar results(80). Furthermore, tracing studies using synaptic terminal-specific retrobeads identify few NTS-projecting OXTPVH neurons in mice; similarly, transgenic mouse models and viral vectors reveal that few OXTPVH neurons send projections to the NTS or PBN(60, 81). Moreover, activation of OXTPVH neurons does not alter feeding; nor does the re-expression of Mc4R in OXT neurons abrogate hyperphagia or obesity in Mc4R-null mice, suggesting that the control of feeding by the PVH and PVH melanocortin signaling is independent of OXT neurons. Consistently, OXTPVH neurons do not express Mc4R(73). Indeed, although activating Mc4RPVH neurons inhibits AgRP-mediated hyperphagia, activating OXTPVH neurons fails to suppress the feeding promoted by the activation of PVH-projecting AgRP neurons, (61).
While non-OXT PVH neurons clearly represent important regulators of feeding and energy balance in the PVH, the cell-specific genetic markers or neuropeptides important for regulating feeding remain largely undefined. Neither CRH nor AVP neurons appear to participate in the Mc4R null phenotype or play major roles in energy balance, either(73). In contrast, PVH neurons expressing neuronal nitric oxide synthase 1 (nNOS, Nos1; Nos1PVH cells) comprise a large population of PVH neurons and suffice to reduce feeding upon activation(60). However, given the number of Nos1PVH neurons, it is not clear whether this suppression results from the activation of a major portion of the PVH or, rather, reflects the importance of some smaller, as yet uncharacterized, subpopulation of these neurons. Additional genetic markers for specific PVH subpopulations would greatly improve our understanding of this nucleus.
Inputs to and regulation of the PVH
Since the PVH is the major output originating from the hypothalamus, it receives input from the majority of hypothalamic sites (ARC, VMH, DMH, etc.), including from neurons that express a variety of metabolically-important receptors (e.g., Mc3/4R, CB1R, CCK receptor, GLP1R), as well as receptors for peripheral signals (e.g., LepRb, insulin receptor) that are critical for feeding regulation (Fig. 3). The PVH also receives dense innervation from forebrain regions (i.e. BNST, POA) important for the stress response and anxiety with potential ties to the control of binge-drinking and even binge-eating. Many (mainly glutamatergic) brainstem sites (e.g., PAG, PBN, NTS) also project to the PVH and likely contribute to the control of feeding by the PVH(82–84). The PVH is also under tonic inhibitory control by the GABAergic shell that surrounds it(85). Furthermore, local, intra-PVH connectivity has been hypothesized, whereby PVH subpopulations communicate through dendritic (as well as axonal) neurotransmitter release(86, 87). Hence, it is conceivable that non-Mc4R PVH neurons that communicate with melanocortin-responsive cells play important roles in the control of energy balance by the ARC-PVH circuit.
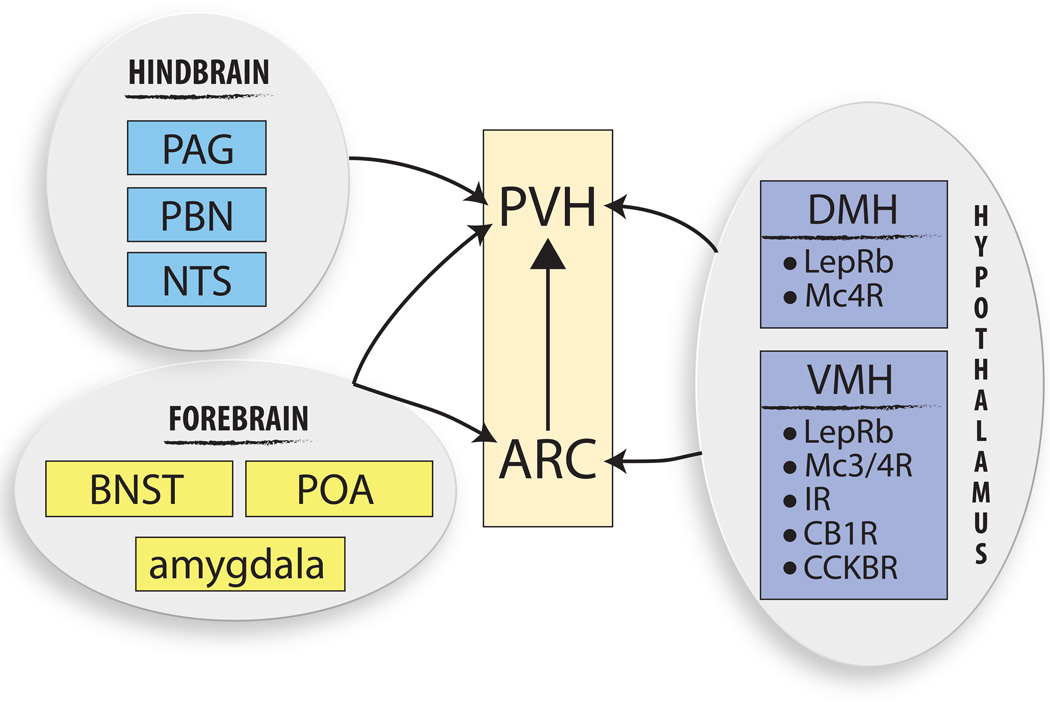
Figure 3.
Regulators of the ARC-PVH circuit. The ARC-PVH circuit is regulated at both levels by a variety of brain regions, some of which have been genetically characterized. Hypothalamic inputs to the ARC predominantly come from the ventromedial hypothalamus (VMH). Aside from the ARC, a major hypothalamic site of PVH regulation arises from the dorsomedial hypothalamus (DMH). Both the VMH and DMH contain a variety of receptor populations for both central and peripheral signals and are therefore poised to relay energy information to the ARC-PVH circuit in order to ultimately control energy balance. Concurrent with the idea of the PVH as an integrating center, there are many non-hypothalamic sites upstream of the PVH from both the forebrain (BNST, bed nucleus of the stria terminalis; POA, preoptic area; amygdala) and hindbrain (PAG, periacqueductal gray; PBN, parabrachial nucleus; NTS, nucleus of the solitary tract). These forebrain areas are also upstream of the ARC and are therefore theoretically able to modulate the ARC-PVH neuronal circuit and therefore control energy balance.
Other inputs to the ARC-PVH circuit
In addition to circulating signals that engage the ARC-PVH, there are also central neural populations upstream of the ARC capable of modulating this circuit. Specifically, the ARC receives dense innervation from the DMH and VMH, and minor innervation from the SON and PMV (Fig. 3) (88). Additionally, a small PACAP- and TRH-expressing PVH population directly projects to and increases the activity of ARC AgRP neurons, promoting feeding, revealing the existence of PVH populations capable of increasing hunger(88). Whether these PACAP/TRHPVH neurons are contained within the Nos1PVH population is not currently known.
The PVH and energy expenditure: a new role for the PVH
While the PVH clearly plays a critical role in the regulation of feeding behavior, much less is known about the potential control of energy expenditure by the PVH. Certainly, energy expenditure is a critical component of homeostasis, since alterations in physical activity, thermogenesis, or BMR can affect adiposity. Defining the potential role for the PVH in the control of energy expenditure has been problematic, however, since many genetic PVH alterations lead to robust obesity from an early age, making it difficult to ascertain if the obesity caused alterations in energy expenditure, rather than the PVH lesion, itself. More recently, techniques have been established that allow for modifying neuronal populations in adult animals on both acute and chronic time scales in order to determine the control of distinct aspects of energy balance (e.g. food intake vs. energy expenditure) by different neuronal populations. Not surprisingly, ablation of Sim1PVH neurons in adult mice results in robust obesity; in addition to causing hyperphagia, ablation of the PVH decreased in activity, thermogenesis, and oxygen consumption prior to the onset of obesity(89, 90). Additional experiments aimed to test the sufficiency of neuronal populations to drive acute changes in energy expenditure also indicate that activation of the PVH drives increased activity and energy expenditure in the mouse (34, 60, 75). While these results suggest the importance of the PVH in driving negative energy balance through increased energy expenditure, separate experiments in rats support a role for the PVH in decreasing sympathetic outflow to brown adipose tissue, therefore decreasing thermogenesis.(91) Whether these discrepancies identify separate roles for distinct aspects of energy expenditure (e.g. thermogenesis vs. physical activity) or reflect differences between species has not yet been determined.
Little is known about the identity of PVH subpopulations that might control energy expenditure. Energy expenditure is unlikely to be controlled by the MC4RPVH neurons, since selective restoration of Mc4R expression in the PVH of Mc4R null mice fails to normalize energy expenditure, and acute activation of Mc4RPVH neurons does not effect energy expenditure (56, 61). Since activation of Nos1PVH and OXTPVH neurons only modestly increases activity and oxygen consumption, other cells must participate in an important manner(60). Consistently, the defects in sympathetic tone and energy expenditure in mice lacking OXT or OXT neurons are relatively mild. Identifying the other PVH populations that contribute to the control of sympathetic outflow and energy expenditure will be an important research goal for the future.
Insights into PVH populations that control energy expenditure can be aided by identifying projection targets within the CNS, with the idea that projections to sites important for sympathetic outflow (e.g. spinal cord) are likely functionally relevant as it pertains to energy expenditure regulation. Initial studies aiming to determine whether the PVH is relevant for the control of thermogenesis used transneuronal retrograde tracing reagents placed in peripheral tissues such as brown adipose tissue (BAT). Indeed, PVH populations are indirectly connected to BAT and therefore have the potential to regulate thermogenesis and overall energy expenditure in the Siberian hamster(92). Additional studies in multiple species identified direct connections between the PVH and the IML, a region in the thoracic spinal cord important for the regulation of sympathetic output(93). More recently, some of the PVH subtypes projecting to the IML were identified as OXT+, a subset of the Nos1PVH population (Fig. 2)(60). While it seems likely that the PVH is a regulator of thermogenesis via the spinal cord, whether direct PVH-IML projections contact ChAT+ neurons projecting to and regulating BAT has yet to be formally demonstrated. The PVH also has the potential to regulate energy expenditure via sites within the brain. Although PVH-NTS projections are primarily thought of in the regulation of feeding behavior, the NTS also has the potential to regulate sympathetic activity, since it also is indirectly connected to BAT(92). Indeed, the NTS has also been shown to control sympathetic outflow, and more recently sympathetic outflow specifically to BAT(94, 95). Additionally, PVH projections to the raphe pallidus (RPa) have been implicated in the regulation of BAT sympathetic nerve activity in the rat, specifically to inhibit BAT thermogenesis(91). This suggests multiple pathways for PVH regulation of thermogenesis, with potentially distinct roles for different sites.
Physical activity is an obvious contributor to overall energy expenditure. It is also a complex behavior influenced and controlled by a variety of CNS sites. In the hypothalamus, the LHA is the primary controller of spontaneous activity. PVH projections to the spinal cord also have the potential to regulate activity (presumably as a response to changes in sympathetic activity) though separating the contributions of thermogenesis and activity to overall energy expenditure remains difficult.
As with circuits controlling feeding regulation by the PVH, identifying sites upstream of PVH populations that control energy expenditure may provide valuable information regarding how the PVH regulates such a complex aspect of energy balance. While the ARC-PVH circuit is primarily thought of in terms of feeding behavior, it is also possible for this circuit to control energy expenditure. In fact, leptin-responsive ARC Rip+ neurons (which do not include POMC neurons) regulate thermogenesis via the release of GABA into the PVH. Importantly, this regulation does not involve GABA-releasing AgRP neurons, which act to decrease energy expenditure rather than increase it. Moreover, the RipARC-PVH regulation of energy expenditure appears to be modulated by NTS-projecting PVH neurons, therefore establishing at least one PVH circuit regulating thermogenesis via the ARC-PVH node that responds to leptin by a PVH projection site other than the spinal cord(96). This also establishes a connection from non-AgRP and non-POMC ARC neurons to the PVH that regulates energy expenditure via leptin, further supporting the idea that additional ARC populations are relevant in the regulation of energy balance (Fig. 1). Leptin-responsive POMC neurons are also important for the regulation of thermogenesis, and have the capability to promote increases in adipocyte “beiging,” although the downstream mediators important for this effect are not yet known(97).
Additional hypothalamic sites upstream of the PVH with the capability to regulate energy expenditure include both the DMH and VMH. In particular, LepRb+ DMH neurons mediate sympathetic outflow to BAT; the downstream mediators of this thermogenic response are unknown, but could potentially involve the PVH(98). Similarly, while the VMH, a critical site for the control of sympathetic tone, is known to send axonal projections to the PVH, the role of this projection in VMH-driven energy expenditure is not clear(99). Lastly, non-hypothalamic sites such as the NTS are also strong regulators of the PVH and are known to regulate sympathetic function and control sympathetic outflow.
Conclusions and directions forward: using novel technologies to dissect energy balance circuits
In an evolutionary sense, the control of the ARC-PVH circuit by a variety of circulating factors, including but not limited to leptin, in order to control both feeding and energy expenditure favors the maintenance of overall energy balance and therefore survival of a species. With this understanding, it follows that ARC populations involved in ARC-PVH circuit output as it relates to energy balance include both LepRb-containing and non-LepRb neurons. The peptidergic composition of these populations is not fully understood, but includes both POMC and AgRP populations. As the primary hypothalamic output, the PVH integrates information gathered from ARC subpopulations and is therefore the essential regulator of ARC-controlled feeding behavior.
Although great progress has been made in determining the role of ARC and PVH subpopulations and the ARC-PVH circuit’s control of energy balance, much remains to be understood. Primarily, the composition (e.g. receptor expression, neuropeptide and/or neurotransmitter contents) of ARC neuronal populations that are directly capable of modulating PVH output as it relates to different energy balance parameters has not been determined. Furthermore, the downstream PVH populations vital for feeding suppression versus energy expenditure continue to be investigated. Certainly, understanding the genetic make-up of Mc4R-expressing PVH neurons has implications for pharmacologic obesity treatments via appetite suppression. Yet, given the neuroendocrine role of many PVH populations, drugs that change PVH output are likely to have side effects. Therefore, as genetic technologies continue to improve, it will be vital to determine upstream sites and distinct populations capable of regulating PVH output, specifically as it pertains to specific energy balance parameters.
Acknowledgments
We thank members of the Olson and Myers labs for helpful discussions. Supported by the Michigan Diabetes Research Center (NIH P30 DK020572), the American Diabetes Association, the Marilyn H. Vincent Foundation (MGM), XXXXX (DPO), and the NIH (MGM: DK056731; AS: NS082027; DPO: DKXXXXXX).
References
- 1.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28(5):w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 2.O'Rahilly S, Farooqi IS. The Genetics of Obesity in Humans. 2000 doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 3.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 5.Myers MG, Leibel RL. Lessons from Rodent Models of Obesity. 2000 [Google Scholar]
- 6.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J. Hered. 1950;41(12):317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 7.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 8.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 9.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 10.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Invest. 2005;115(12):3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 13.Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks AS, Myers MG. Regulation of Jak kinases by intracellular leptin receptor sequences. J. Biol. Chem. 2002;277(44):41547–41555. doi: 10.1074/jbc.M205148200. [DOI] [PubMed] [Google Scholar]
- 14.Patterson CM, Leshan RL, Jones JC, Myers MG. Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Research. 2011;1378:18–28. doi: 10.1016/j.brainres.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J. Comp. Neurol. 1998;395(4):535–547. [PubMed] [Google Scholar]
- 16.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, et al. Leptin targets in the mouse brain. J. Comp. Neurol. 2009;514(5):518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 18.Barsh GS, He L, Gunn TM. Genetic and biochemical studies of the Agouti-attractin system. J. Recept. Signal Transduct. Res. 2002;22(1–4):63–77. doi: 10.1081/rrs-120014588. [DOI] [PubMed] [Google Scholar]
- 19.Lu D, Willard D, Patel IR, Kadwell S, Overton L, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371(6500):799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 20.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, et al. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat. Genet. 1999;21(1):119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 21.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 22.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141(9):3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 23.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 2000;26(1):97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 24.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat. Genet. 1998;20(2):113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 25.Loos RJF, Lindgren CM, Li S, Wheeler E, Zhao JH, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat. Genet. 2008;40(6):768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat., vol. 41. Nutrition reviews. 1940:4. doi: 10.1111/j.1753-4887.1983.tb07169.x. [DOI] [PubMed] [Google Scholar]
- 27.Stevenson JA. VI. Sensory mechanisms and multi-factor models in the regulation of food and water intake. Mechanisms in the control of food and water intake. Ann. N. YAcad. Sci. 1969;157(2):1069–1083. doi: 10.1111/j.1749-6632.1969.tb12938.x. [DOI] [PubMed] [Google Scholar]
- 28.Choi S, Sparks R, Clay M, Dallman MF. Rats with hypothalamic obesity are insensitive to central leptin injections. Endocrinology. 1999;140(10):4426–4433. doi: 10.1210/endo.140.10.7064. [DOI] [PubMed] [Google Scholar]
- 29.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 30.Yeo GSH, Heisler LK. Unraveling the brain regulation of appetite: lessons from genetics. Nat. Neurosci. 2012;15(10):1343–1349. doi: 10.1038/nn.3211. [DOI] [PubMed] [Google Scholar]
- 31.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138(10):4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 32.Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 33.Thornton JE, Cheung CC, Clifton DK, Steiner RA. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology. 1997;138(11):5063–5066. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- 34.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24(1):155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 35.Tong Q, Ye C-P, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 2008;11(9):998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, et al. Long-term orexigenic effects of AgRP-(83--132) involve mechanisms other than melanocortin receptor blockade. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279(1):R47–R52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- 37.Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139(10):4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- 38.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23(4):775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 39.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42(6):983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 40.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149(4):1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE, Myers MG. Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat. Med. 2012;18(5):820–823. doi: 10.1038/nm.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vong L, Ye C, Yang Z, Choi B, Chua S, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71(1):142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 44.Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297(5581):609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- 45.Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51(2):239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Zheng H, Patterson LM, Rhodes CJ, Louis GW, Skibicka KP, et al. A potential role for hypothalamomedullary POMC projections in leptin-induced suppression of food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298(3):R720–R728. doi: 10.1152/ajpregu.00619.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proceedings of the National Academy of Sciences. 1998;95(25):15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang D, He X, Zhao Z, Feng Q, Lin R, et al. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat. 2015;9:40. doi: 10.3389/fnana.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouret SG, Draper SJ, Simerly RB. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 2004;24(11):2797–2805. doi: 10.1523/JNEUROSCI.5369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gold RM. Hypothalamic obesity: the myth of the ventromedial nucleus. Science. 1973;182(4111):488–490. doi: 10.1126/science.182.4111.488. [DOI] [PubMed] [Google Scholar]
- 51.Sims JS, Lorden JF. Effect of paraventricular nucleus lesions on body weight, food intake and insulin levels. Behav. Brain Res. 1986;22(3):265–281. doi: 10.1016/0166-4328(86)90071-9. [DOI] [PubMed] [Google Scholar]
- 52.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J. Comp. Neurol. 2003;457(3):213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 53.Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Research. 1982;257(3):275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- 54.Fulwiler CE, Saper CB. Cholecystokinin-immunoreactive innervation of the ventromedial hypothalamus in the rat: possible substrate for autonomic regulation of feeding. Neurosci. Lett. 1985;53(3):289–296. doi: 10.1016/0304-3940(85)90553-1. [DOI] [PubMed] [Google Scholar]
- 55.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287(1):R87–R96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 56.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 57.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137(7):1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503(7474):111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488(7410):172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sutton AK, Pei H, Burnett KH, Myers MG. Control of Food Intake and Energy Expenditure by Nos1 Neurons of the Paraventricular Hypothalamus. The Journal of …. 2014 doi: 10.1523/JNEUROSCI.0226-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garfield AS, Li C, Madara JC, Shah BP, Webber E, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci. 2015;18(6):863–871. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16(3):296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berthoud HR, Blackshaw LA, Brookes SJH, Grundy D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol. Motil. 2004;16(Suppl 1(s1)):28–33. doi: 10.1111/j.1743-3150.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 64.Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience. 1978;3(2):207–218. doi: 10.1016/0306-4522(78)90102-1. [DOI] [PubMed] [Google Scholar]
- 65.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J. Comp. Neurol. 1990;293(4):540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 66.Becskei C, Grabler V, Edwards GL, Riediger T, Lutz TA. Lesion of the lateral parabrachial nucleus attenuates the anorectic effect of peripheral amylin and CCK. Brain Research. 2007;1162:76–84. doi: 10.1016/j.brainres.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 67.Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483(7391):594–597. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D'Hanis W, Linke R, Yilmazer-Hanke DM. Topography of thalamic and parabrachial calcitonin gene-related peptide (CGRP) immunoreactive neurons projecting to subnuclei of the amygdala and extended amygdala. J. Comp. Neurol. 2007;505(3):268–291. doi: 10.1002/cne.21495. [DOI] [PubMed] [Google Scholar]
- 69.Schwaber JS, Sternini C, Brecha NC, Rogers WT, Card JP. Neurons containing calcitonin gene-related peptide in the parabrachial nucleus project to the central nucleus of the amygdala. J. Comp. Neurol. 1988;270(3):416–426. 398–399. doi: 10.1002/cne.902700310. [DOI] [PubMed] [Google Scholar]
- 70.Shor-Posner G, Azar AP, Insinga S, Leibowitz SF. Deficits in the control of food intake after hypothalamic paraventricular nucleus lesions. Physiology & Behavior. 1985;35(6):883–890. doi: 10.1016/0031-9384(85)90255-0. [DOI] [PubMed] [Google Scholar]
- 71.Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 1998;12(20):3264–3275. doi: 10.1101/gad.12.20.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holder JL, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum. Mol. Genet. 2000;9(1):101–108. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- 73.Shah BP, Vong L, Olson DP, Koda S, Krashes MJ, et al. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc. Natl. Acad. Sci. U.S.A. 2014;111(36):13193–13198. doi: 10.1073/pnas.1407843111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13(2):195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Y, Wu Z, Sun H, Zhu Y, Kim ER, et al. Glutamate mediates the function of melanocortin receptor 4 on sim1 neurons in body weight regulation. Cell Metab. 2013;18(6):860–870. doi: 10.1016/j.cmet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Research. 2003;993(1–2):30–41. doi: 10.1016/j.brainres.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 77.Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol. Endocrinol. 2008;22(7):1723–1734. doi: 10.1210/me.2008-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19(9):951–955. doi: 10.1097/WNR.0b013e3283021ca9. [DOI] [PubMed] [Google Scholar]
- 79.Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver Spring) 2009;17(5):980–984. doi: 10.1038/oby.2009.12. [DOI] [PubMed] [Google Scholar]
- 80.Wu Z, Xu Y, Zhu Y, Sutton AK, Zhao R, et al. An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS ONE. 2012;7(9):e45167. doi: 10.1371/journal.pone.0045167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, et al. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J. Comp. Neurol. 2012;520(1):6–33. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sawchenko PE, Swanson LW. The organization and biochemical specificity of afferent projections to the paraventricular and supraoptic nuclei. Prog. Brain Res. 1983;60:19–29. doi: 10.1016/S0079-6123(08)64371-X. [DOI] [PubMed] [Google Scholar]
- 83.Ziegler DR, Edwards MR, Ulrich-Lai YM, Herman JP, Cullinan WE. Brainstem origins of glutamatergic innervation of the rat hypothalamic paraventricular nucleus. J. Comp. Neurol. 2012;520(11):2369–2394. doi: 10.1002/cne.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ulrich-Lai YM, Jones KR, Ziegler DR, Cullinan WE, Herman JP. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J. Comp. Neurol. 2011;519(7):1301–1319. doi: 10.1002/cne.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol. Biochem. Behav. 2002;71(3):457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 86.Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J. Neurophysiol. 1997;77(6):3396–3400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- 87.Ziegler DR, Herman JP. Local integration of glutamate signaling in the hypothalamic paraventricular region: regulation of glucocorticoid stress responses. 2000;141(12):4801–4804. doi: 10.1210/endo.141.12.7949. [DOI] [PubMed] [Google Scholar]
- 88.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507(7491):238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xi D, Gandhi N, Lai M, Kublaoui BM. Ablation of Sim1 neurons causes obesity through hyperphagia and reduced energy expenditure. PLoS ONE. 2012;7(4):e36453. doi: 10.1371/journal.pone.0036453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tolson KP, Gemelli T, Meyer D, Yazdani U, Kozlitina J, Zinn AR. Inducible neuronal inactivation of Sim1 in adult mice causes hyperphagic obesity. Endocrinology. 2014:en20132125. doi: 10.1210/en.2013-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296(3):R831–R843. doi: 10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol. 1999;276(6 Pt 2):R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 93.Caverson MM, Ciriello J, Calaresu FR. Paraventricular nucleus of the hypothalamus: an electrophysiological investigation of neurons projecting directly to intermediolateral nucleus in the cat. Brain Research. 1984;305(2):380–383. doi: 10.1016/0006-8993(84)90447-5. [DOI] [PubMed] [Google Scholar]
- 94.Cao W-H, Madden CJ, Morrison SF. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299(1):R277–R290. doi: 10.1152/ajpregu.00039.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19(5):741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kong D, Tong Q, Ye C, Koda S, Fuller PM, et al. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151(3):645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160(1–2):88–104. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Enriori PJ, Sinnayah P, Simonds SE, Garcia Rudaz C, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 2011;31(34):12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lindberg D, Chen P, Li C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. J. Comp. Neurol. 2013;521(14):3167–3190. doi: 10.1002/cne.23338. [DOI] [PubMed] [Google Scholar]