ABSTRACT
Inflammation is one of the hallmarks of carcinogenesis. High mammographic density has been associated with increased risk of breast cancer but the mechanisms behind are poorly understood. We evaluated whether breasts with different mammographic densities exhibited differences in the inflammatory microenvironment.
Postmenopausal women attending the mammography-screening program were assessed having extreme dense, n = 20, or entirely fatty breasts (nondense), n = 19, on their regular mammograms. Thereafter, the women were invited for magnetic resonance imaging (MRI), microdialysis for the collection of extracellular molecules in situ and a core tissue biopsy for research purposes. On the MRI, lean tissue fraction (LTF) was calculated for a continuous measurement of breast density. LTF confirmed the selection from the mammograms and gave a continuous measurement of breast density. Microdialysis revealed significantly increased extracellular in vivo levels of IL-6, IL-8, vascular endothelial growth factor, and CCL5 in dense breast tissue as compared with nondense breasts. Moreover, the ratio IL-1Ra/IL-1β was decreased in dense breasts. No differences were found in levels of IL-1β, IL-1Ra, CCL2, leptin, adiponectin, or leptin:adiponectin ratio between the two breast tissue types. Significant positive correlations between LTF and the pro-inflammatory cytokines as well as between the cytokines were detected. Stainings of the core biopsies exhibited increased levels of immune cells in dense breast tissue.
Our data show that dense breast tissue in postmenopausal women is associated with a pro-inflammatory microenvironment and, if confirmed in a larger cohort, suggests novel targets for prevention therapies for women with dense breast tissue.
KEYWORDS: Cytokines, mammary gland, mammography, microdialysis, MRI
Abbreviations
- AI
aromatase inhibitors
- BI-RADS
breast imaging reporting and data system
- BMI
body mass index
- CCL
chemokine (C-C motif) ligand
- ER
estrogen receptor
- HRT
hormone replacement therapy
- IL
interleukin
- LTF
lean tissue fraction
- MRI
magnetic resonance imaging
- NSAID
non-steroidal anti-inflammatory drug
- PgR
progesterone receptor
- SERM
selective estrogen receptor modulator
Background
An inflammatory microenvironment has been associated with increased risk of several cancer forms including breast cancer and inflammation is one of the hallmarks of cancer.1 A reduced risk of breast cancer has been noticed in women who regularly use anti-inflammatory drugs.2,3 The inflammatory microenvironment is to a large extent regulated in the extracellular space where soluble factors including cytokines are key regulators of the cell–cell communication between different cell types. We have previously shown increased extracellular in vivo levels of several inflammatory mediators, such as interleukin (IL)-1, IL-8, chemokine (C-C motif) ligand (CCL) 2, CCL5, leptin, and vascular endothelial growth factor (VEGF) in breast cancers of women and an association with sex steroids of these factors in normal human breast tissue.4-12
Mammographic density has been associated with increased risk of breast cancer.13 In a meta-analysis women with >75% dense breast area have a 4-fold increased risk of developing breast cancer compared to women with <5% dense breast area.14 Mammographic density reflects variations in breast tissue composition; the stroma and epithelial cells appear white or light gray on a mammogram, whereas fat tissue appears dark. Dense breast tissue contains higher amounts of stroma, including collagen, and less fat tissue.15,16 The proportion of epithelial cells in normal breast tissue is only between 1% and 6%13,17,18 and conflicting data regarding the amount and proliferation rate of these cells in dense vs. nondense normal breast have been reported.15-17,19,20 No differences in the expression of estrogen receptor or progesterone receptor of epithelial cells have been found between dense and nondense breast tissues.17,21
Exposure to endogenous and exogenous sex steroids is an established risk factor for breast cancer22,23 but no associations have been found between circulating endogenous estrogen levels and breast density.13 Anti-estrogen therapies such as the selective estrogen receptor modulator (SERM) including tamoxifen or aromatase inhibitors (AI) have been shown to reduce the risk of breast cancer.24 The effects on mammographic density between these therapies are, however, somewhat different; tamoxifen seems to reduce mammographic density, whereas AI have lesser effect.25-28 Anti-estrogen therapies are associated with severe side-effects and decreased quality of life and the adherence of these therapeutics is very low.29,30 Hence, other less toxic prevention therapies, that may include anti-inflammatory treatments, need to be developed.
If the inflammatory microenvironment in vivo is different in dense vs. nondense breast tissue is not known. In an explorative study, we therefore recruited healthy postmenopausal women attending the mammography-screening program for determinations of the inflammatory microenvironment in women with either predominantly fatty breast tissue (nondense) or with extreme dense breast tissue (dense). Microdialysis for collection of extracellular molecules in vivo and breast tissue biopsies was obtained. Magnetic resonance imaging (MRI), for a continuous measurement of breast density, was performed and lean tissue fraction (LTF) was quantified. We found a pro-inflammatory profile of the in vivo levels of cytokines in women with dense breast tissue and this was positively correlated with increased LTF, whereas no correlation was detected with estradiol levels. Stainings of breast biopsies exhibited increased amounts of inflammatory cells in dense breast tissue. Our data suggest that dense breast tissue is associated with a pro-inflammatory microenvironment that may be important for preventive strategies, but our data need to be confirmed in larger cohorts of women.
Results
No differences in characteristics between the subjects
A total of 39 women were recruited into the study and their characteristics are summarized in Table 1. As shown no differences of age, years since menopause, BMI, plasma estradiol, and progesterone were found between the two cohorts. No differences in plasma levels of cytokines were detected between the two groups, Table 1.
Table 1.
Characteristic and plasma levels of hormones and cytokines of the participants. All values are mean ± SEM.
| Dense (n = 20) | Nondense(n = 19) | p-value | |
|---|---|---|---|
| Age (years) | 64 ± 1 | 66 ± 1 | 0.2 |
| Years since menopause | 13 ± 1 | 13 ± 1 | 0.7 |
| Body Mass Index (kg/m2) | 24 ± 0.7 | 25 ± 0.7 | 0.3 |
| P-Estradiol (pmol/L) | 120 ± 12 | 117 ± 11 | 0.8 |
| P-Progesterone (nmol/L) | 0.7 ± 0.2 | 0.4 ± 0.05 | 0.2 |
| IL-1β (pg/mL) | 2.1 ± 0.2 | 2.5 ± 0.5 | 0.9 |
| IL-1Ra (pg/mL) | 331 ± 40 | 483 ± 86 | 0.2 |
| IL-1Ra/IL-1β[{(βρ)}] | 206 ± 40 | 312 ± 106 | 0.5 |
| IL-6 (pg/mL) | 1.7 ± 0.2 | 2 ± 0.4 | 0.8 |
| IL-8 (pg/mL) | 5 ± 0.4 | 5.5 ± 0.5 | 0.4 |
| CCL2 (pg/mL) | 40 ± 8 | 36 ± 6.2 | 0.9 |
| CCL5 (pg/mL) | 11,606 ± 431 | 12,149 ± 449 | 0.3 |
| VEGF (pg/mL) | 49 ± 4 | 52 ± 5 | 0.7 |
| Leptin (pg/mL) | 20,845 ± 3,148 | 23,987 ± 3,827 | 0.7 |
| Adiponectin (ng/mL) | 16,013 ± 1,386 | 13,804 ± 1,600 | 0.1 |
| Leptin/Adiponectin | 0.002 ± 0.0003 | 0.004 ± 0.002 | 0.6 |
MRI quantification confirmed the categorization of breast density using mammography
Out of the 39 included women, three were not able to complete the MRI; one because of an allergy to Gd-contrast, which was used as part of the MRI-examination, and two because of anxiety attacks (claustrophobia) in the scanner. The MRI quantification LTF confirmed the mammographic selection of women with dense versus nondense breasts. There was a significantly higher LTF in the selected dense breasts compared with the nondense breasts on both left and right side, p < 0.0001, n = 16 in the nondense group and n = 20 in the dense group, Fig. 1. As expected the left and right breast exhibited similar density, Fig. 1. Typical images of the MRI from the two different groups used for the quantifications are depicted in Fig. 1.
Figure 1.
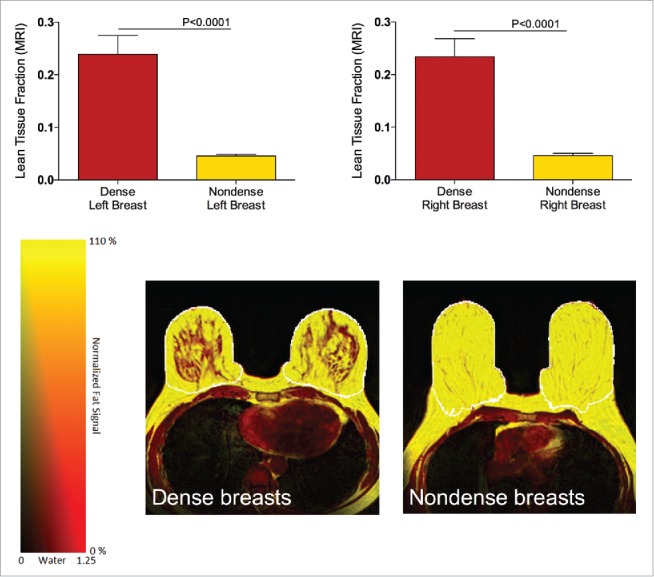
MRI quantification of lean tissue fraction (LTF) of postmenopausal women, attending the regular mammography-screening program and categorized as dense (n = 20) or nondense (n = 16) breasts on their regular screening mammograms underwent MRI for research purposes and LTF was quantified as described in Materials and Methods. Graphed data are presented as mean ± SEM. Representative MRIs of dense and nondense breasts. False color images of the lean LTF in a dense (lower left panel) and a nondense breast (lower right panel). The outer border of the segmented breast tissue is shown with a white line.
Pro-inflammatory cytokine profile in dense breast tissue
No complications after the microdialysis investigations were detected. Microdialysis for sampling of extracellular cytokines in vivo was performed in 39 women, n = 20 women with dense breasts and n = 19 women with nondense breasts.
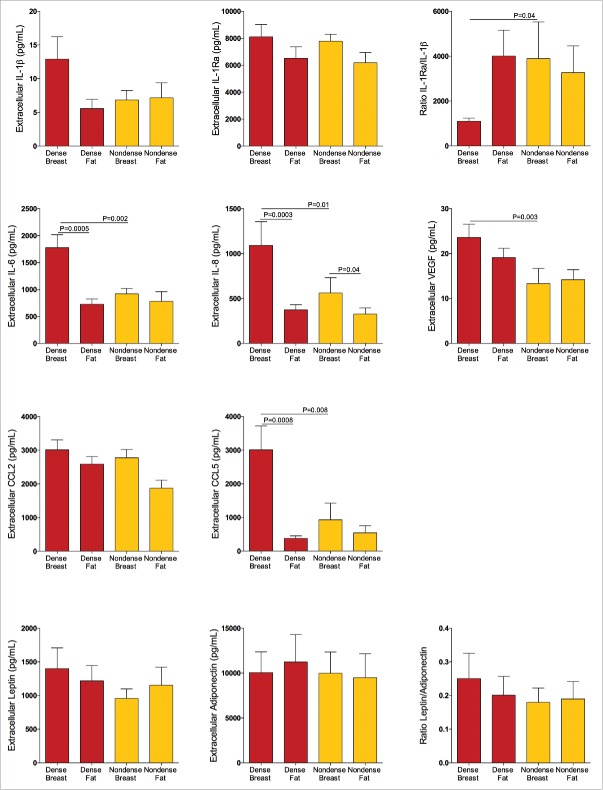
Significantly higher levels of the pro-inflammatory proteins IL-6, p = 0.002, IL-8, p = 0.01, CCL5, p = 0.008, and VEGF, p = 0.003 were detected in dense breast tissue compared with nondense breast tissue, Fig. 2. No differences were found between IL1β and IL-1Ra levels. However, as the pro-inflammatory IL1β is counteracted by the endogenous inhibitor IL-1Ra, the ratio between these proteins were calculated. The IL-1Ra/IL-1β ratio was significantly decreased in dense breasts, p = 0.04, suggesting a pro-inflammatory profile of these cytokines, Fig. 2. In addition, significant correlations between several cytokines per se were detected, as well as significant correlations with cytokine levels and LTF, further supporting a pro-inflammatory milieu in dense breast tissue, Tables 2 and 3. No significant differences were found in CCL2, leptin, adiponectin, or leptin/adiponectin ratio, Fig. 2. Similar to the IL-1 cytokine family, the effects of leptin are counteracted by adiponectin and the ratio between the two proteins may be a more physiologically relevant measurement than the absolute concentrations of either protein alone. There was no difference in local extracellular E2 between dense and nondense breast tissues, 40 ± 3.2 pmol/L in dense breasts and 41 ± 2.7 pmol/L in nondense breasts. TNF-α, IL-17, and GM-CSF were not detectable in the microdialysates.
Figure 2.
Pro-inflammatory profile of extracellular cytokines in vivo in dense breast tissue of 39 postmenopausal women, attending the regular mammography-screening program who underwent microdialysis. On their regular screening mammograms the breast were categorized as either dense or nondense and thereafter the women were invited to participate in the study as described in the Materials and Methods. Women with dense breasts (n = 20) are depicted in red; one microdialysis catheter was inserted into the left breast (dense breast) and another into abdominal subcutaneous fat (dense fat). Women categorized as nondense (n = 19) are depicted in yellow; one microdialysis catheter was inserted into the left breast (nondense breast) and another into abdominal subcutaneous fat (nondense fat). Graphed data are presented as mean ± SEM.
Table 2.
Pro-inflammatory cytokines in breast tissue exhibit a significant positive correlation with breast density expresses as lean tissue fraction.
| Variable | IL1Ra/IL-1β | IL-6 | IL-8 | VEGF | CCL2 | CCL5 | Leptin/Adiponectin | E2 |
|---|---|---|---|---|---|---|---|---|
| LTF | −0.17 | 0.46** | 0.40** | 0.43* | 0.05 | 0.44** | −0.06 | −0.06 |
p < 0 .05,
p < 0 .01
Spearman's correlation coefficients of LTF calculated on breast magnetic resonance images and extracellular in vivo concentrations of cytokines and estradiol (E2) in breast tissue, n = 36. Significant values are in bold.
Table 3.
Pro-inflammatory cytokines in breast tissue exhibit significant correlations.
| Variable | IL1Ra/IL-1β | IL-6 | IL-8 | VEGF | CCL2 | CCL5 |
|---|---|---|---|---|---|---|
| IL-6 | −0.15 | |||||
| IL-8 | −0.59*** | 0.60*** | ||||
| VEGF | −0.51** | 0.56** | 0.73*** | |||
| CCL2 | 0.01 | 0.29 | 0.38* | 0.25 | ||
| CCL5 | −0.39* | 0.63*** | 0.60*** | 0.63*** | −0.08 | |
| Leptin/Adiponectin | −0.24 | 0.07 | 0.29 | 0.08 | 0.44** | −0.19 |
p < 0.05,
p < 0.001,
p < 0.0001
Spearman's correlation coefficients of extracellular in vivo concentrations of cytokines in breast tissue, n = 39. Significant values are in bold.
Significantly increased levels of immune cells in dense breast tissue
To investigate if the increased levels of pro-inflammatory cytokines in dense breast tissue were associated with an increase of inflammatory cells, two different leucocyte markers were used for stainings of the biopsies. As shown in Fig. 3, immunostaining with the leucocyte markers CD45 and CD68 revealed a significant increase of immune cells in dense tissue compared to nondense tissue. As expected, the dense breast tissue contained increased amounts of stroma and nondense breast increased amounts of fat. No difference in amount of epithelium was detected. Representative sections are shown in Fig. 3. No benign breast disease or atypia was found in the tissue biopsies.
Figure 3.
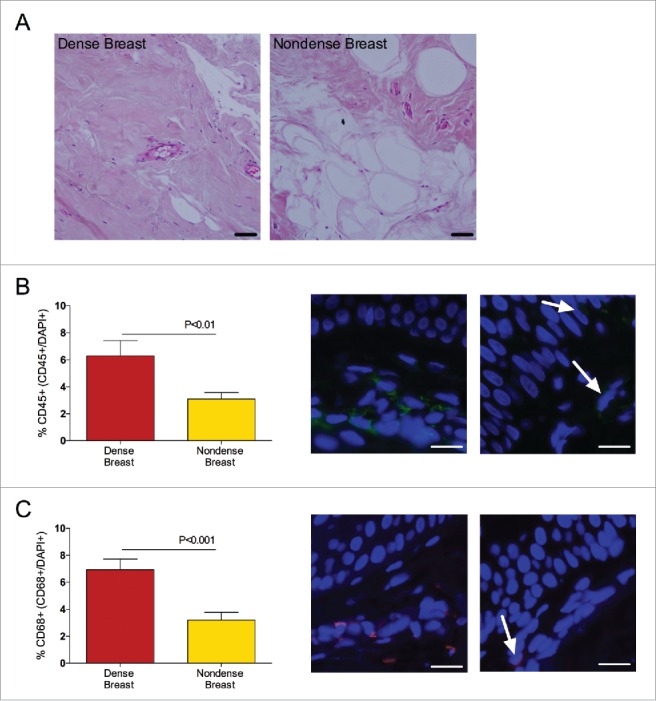
Dense breast tissue contains increased levels of inflammatory cells in 39 postmenopausal women, attending the regular mammography-screening program who underwent biopsies for research purposes. On their regular screening mammograms, the breast were categorized as either dense or nondense and thereafter the women were invited to participate in the study as described in the materials and methods section. Breast biopsies were obtained in the upper lateral quadrant of the left breast. Tissue sections were stained and quantified as described in Materials and Methods. Graphed data are presented as mean ± SEM. (A) Representative H&E-stained tissue sections from dense and nondense breast tissue, respectively. Scale bars = 50 μm. (B) Sections were stained with CD45 (green). Significantly increased levels of leucocytes were detected in dense breast tissue. Representative tissue sections from each group are shown. Arrows indicate CD45+ cells. Scale bars =100 μm. (C) Sections were stained with CD68 (red). Significantly increased levels of leucocytes were detected in dense breast tissue. Representative tissue sections from each group are shown. Arrows indicate CD68+ cells. Scale bars =100 μm
Discussion
Here, we show that dense breast tissue in postmenopausal women exhibited a pro-inflammatory breast microenvironment in vivo. The IL-1 family of cytokines showed a pro-inflammatory profile and significantly higher levels of IL-6, IL-8, CCL5, and VEGFs in dense breast tissue compared with nondense breast tissue were detected. In addition, a positive correlation between LTF and these cytokines was revealed. In line with the pro-inflammatory cytokine profile, dense breast tissue also contained significantly increased number of inflammatory cells. No correlations with plasma levels of cytokines and LTF were detected.
Stromal-epithelial interactions are critical for the progression of several cancers including breast cancer and it has recently been reported that the outcome of cancer therapy may be affected by tumor-stroma interactions.31-33 Normal breast tissue contains very low levels of epithelial cells, as the stroma is the main tissue component, and modifications in the stroma rather than epithelial changes seem to be related to mammographic density alterations.15-17 This emphasizes the need of investigations taking these interactions into consideration rather than focusing on epithelial cell alterations alone. All cell types such as fibroblasts, endothelial cells, immune cells, and adipocytes contribute to the total amount of biologically active molecules released in the extracellular space and thereby the regulation of the inter-cellular cross-talk in the microenvironment. Investigations of breast tissue biopsies with commonly used techniques such as immunohistochemistry may not reflect the extracellular soluble components. Sampling of extracellular molecules is possible by using microdialysis and this technique has been used to sample bioactive molecules present in situ in both normal and malignant human breast tissues.4,5,7,11,34 In the present study, we used microdialysis for sampling of cytokines and our data strongly suggest an inflammatory microenvironment in dense breast tissue. This was supported, not only by the significantly increased levels of individual cytokines in dense breast tissue, which may be released by all cell types in the microenvironment, but also by significant correlations between these inflammatory proteins in the breast. Stainings of the breast tissue biopsies, which revealed an increased amount of inflammatory cells in dense breasts, corroborated this finding further. Inflammation is considered as one of the hallmarks of cancer initiation and progression.1 The risk of breast cancer has been associated with circulating inflammatory markers and regular use of NSAIDs has been shown to reduce the risk of breast cancer.2,3,35,36 Thus, our results seem to be highly relevant.
Several inflammatory mediators have been linked to local estrogen production; IL-6 and TNF-α may increase the production of aromatase resulting in increased levels of estrogens in the tissue providing a link between inflammatory and estrogen pathways in the breast.37 It has been argued that after menopause, when the levels of circulating estradiol decrease, cytokines may induce local aromatase expression and thereby increase local estradiol levels.37 The majority of these studies have been performed using immunostainings rather than measuring the actual hormone levels.37 In addition, in the few studies where staining patterns have been combined with hormone tissue levels no correlations have been found.38,39 Measuring hormone levels in tissue homogenate has several methodological problems; the results obtained by this technique will reflect the intracellular levels rather than the extracellular levels, the effector for the inter-cellular communication.40 Our data do not support that the local extracellular estradiol production is different in dense and nondense breasts in postmenopausal women. As no correlations were found between hormone levels and extracellular cytokines in the breast, the regulation of these factors may be independent of local estrogen production. We have previously found an association of estrogen levels and the in vivo levels of several inflammatory biomarkers in human breast tissue.4,5,7,9,11,34 In these studies, both pre- and post-menopausal women and patients investigated before and after anti-estrogen treatment were included, thus reflecting cohorts of women with a wide range of hormone levels, whereas in the present study, postmenopausal women exhibiting minor variation of estradiol levels were included. Hence, correlations with estrogen may not be expected in this selected population. The lack of an association between leptin and adiponectin and breast density support previous results in pre-menopausal women where plasma levels of these adipokines were without association with mammographic breast density.41
The objective and continuous assessment of breast density by using MRI is a strong point of our study compared to BI-RADS categorization of the regular mammograms alone. In addition, measurements of breast density using high-resolution MRI imaging methods may be used to detect subtle changes in breast density that may not be visible on conventional mammograms. This may be especially important in postmenopausal women where it has been shown that mammographic density may not be a useful biomarker for evaluating the effects of AI treatment, the most potent therapeutic for breast cancer prevention.26 AIs exhibit a greater reduction in breast cancer risk and recurrence rates compared with tamoxifen; however, tamoxifen exerts a greater reduction of mammographic density compared to AIs.25-28 This suggests that a quantitative imaging technique with a direct measurement of the breast composition, as described in the present study, may be necessary as a surrogate in the development of breast cancer preventive therapeutics.
Although anti-estrogen therapies reduce the risk of breast cancer by 30% to 50%, the severe side effects such as thromboembolic events, osteoporosis (AIs only), endometrial cancer, and decreased quality of life in combination with the low compliance make these strategies unsuitable for a larger population.29,30 Studies of the biological basis and by which potential mechanism dense breast tissue exerts its tumorigenic risks are needed in developing preventive approaches against breast cancer.
The stroma plays a key role for breast carcinogenesis and various compositions of this compartment reflect differences in mammographic density. Here, we have shown that dense breast tissue contains a higher amount of inflammatory cells. To the best of our knowledge, this is also the first study to show a pro-inflammatory cytokine profile in the extracellular microenvironment in vivo in dense breast tissue. Due to the limitations of this study with only two extreme groups of breast densities and the low number of participant, our data need to be confirmed in larger cohorts of women, including women with intermediate breast density. If corroborated, the present data suggest that other targets than estrogen, such as anti-inflammatory approaches, may be considered for studies of breast cancer preventive strategies. A high-resolution imaging modality that facilitates tissue classification, as shown in this study, will be of significant value in these studies.
Materials and methods
The study was performed in accordance with the Declaration of Helsinki and the regional ethical review board of Linköping approved the study (#2013/476-31). All women gave informed written consent. A total of 39 postmenopausal women (55 y of age or older) were consecutively recruited from the screening mammography program at Linköping University Hospital during 10 mo (August 2014 to June 2015). Exclusion criteria were previous breast cancer or benign breast disease, current use of hormone replacement therapy (HRT), anti-estrogen therapies including SERMs or degraders, any clotting or metabolic disorder, or current use of non-steroidal anti-inflammatory drugs (NSAIDs). A wash out period of at least 3 mo was required if any of the above-mentioned drugs had been used. However, none of the women declared any such use within a 3-mo period including over the counter preparations. Characteristics of the women are summarized in Table 1.
Recruitment procedure
A poster in the waiting room at the mammography department informed that the study was ongoing. The women were informed that they could receive a letter of invitation to the study if they met the inclusion criteria. The regular mammograms were assessed by one experienced observer (AR) according to Breast Imaging Reporting and Data System (BI-RADS) density scale42 and breast densities were categorized as either BI-RADS A (entirely fatty nondense breasts) or BI-RADS D (extremely dense). A letter with information about the study was thereafter sent out to the two groups of women. The women were invited to undergo an MRI scan, microdialysis, and core biopsy investigations for research purposes. The recruitment was scheduled for 1 y excluding the summer holidays. 357 women were invited and 69 women responded to the letter and 39 were possible to include depending on the inclusion/exclusion criteria.
Microdialysis procedure
Prior insertion of the microdialysis catheters, 0.5 mL lidocain (10 mg/mL) was administrated intracutaneously. One microdialysis catheter was placed in the upper lateral quadrant of the left breast and directed toward the nipple as previously described5,7,8,34,43-48 and one reference catheter was placed in abdominal subcutaneous fat. Microdialysis catheters (71/M Dialysis AB, Stockholm, Sweden), which consists of a tubular dialysis membrane (length 20 mm, diameter 0.52 mm, and 100,000 atomic mass cut-off) glued to the end of a double-lumen tube (80 mm long × 0.8 mm in diameter), were inserted via a splitable introducer (M Dialysis AB), connected to a microinfusion pump (M Dialysis AB) and perfused with NaCl 154 mmol/L and hydroxyethyl starch 60 g/L (Voluven®, Fresenius Kabi, Uppsala, Sweden), at a perfusion rate of 0.5 µL/min. After a 60-min equilibration period, the outgoing perfusate was stored at –70°C for subsequent analysis. All microdialysis results in the present paper are given as raw data. EDTA plasma was collected at the time for microdialysis and stored at –70°C. After the microdialysis investigation an unguided breast biopsy was obtained from the upper lateral quadrant of the left breast (MAX-CORE, gauge 14, BARD Medical, Covington, GA, USA) and fixed in 4% paraformaldehyde.
Quantification of proteins and hormones
Microdialysates and plasma were analyzed with immunoassays from R&D Systems, Minneapolis, MN unless otherwise stated. Human IL-1β, IL-1Ra IL-6, IL-8, CCL-2, CCL-5, and VEGF were analyzed using Human Fluorokine MAP kits with corresponding beads and analyzed on a Luminex 200 System (Luminex, Austin, TX). Leptin and adiponectin were detected using ELISA kits. Estradiol and progesterone were quantified using EIA-kits (DRG Diagnostics, Marburg, Germany). For microdialysis analyses the kits were modified such that the standards for the assays were prepared in the same buffer that was used for sampling during microdialysis. All samples were analyzed in duplicates and in the same reagent batch.
Immunohistochemistry
Formalin-fixed, paraffin-embedded breast biopsies were cut in 4 μm sections, de-paraffinized, and exposed to anti-human CD45 antibody (SouthernBiotech) 1:100 and Alexa fluor 488 (Abcam) 1:200 or anti-human CD68 (Abcam) 1:100 and Alexa fluor 546 (Invitrogen) 1:200 and mounted using SlowFade Gold containing 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen) or hematoxylin and eosin (H&E). Three hot-spot areas on each section were visualized using a Olympus BX43 light/fluorescence microscope (×40/0.75 magnification) with excitation filters of 495–519 nm and 556–573 nm, equipped with a Olympus DP72 CCD camera and percent positive stained cells were calculated.
Quantification of lean tissue fraction using MRI
The LTF of each breast was computed as the ratio of its lean tissue volume to its total volume. The LTF ratio has been shown to be a variable that strongly correlate with the categorical BI-RADS classification of breast density.49 The lean and total volumes were computed based on manual whole-breast segmentation of quantitative water-fat MRI images. The quantitative fat images were computed by calibrating the original fat images using adipose tissue as an internal intensity reference, thus, allowing adipose tissue volume to be quantified within segmentation.50,51 The lean tissue volume of each breast was determined by subtracting its adipose tissue volume from its total volume.52
Water- and fat-separated MR images were computed, as previously described,53 from axial 3D 6-echo turbo field echo MRI images acquired on a 1.5 T Achieva (Philips Healthcare, Best, Netherlands) with a seven-element breast coil, anterior-posterior frequency encoding, first TE at 2.3 ms and ΔTE of 2.3 ms, TR 15.4 ms, flip angle 10°, 300 × 300 × 150 mm3 field of view, 200 × 200 scan matrix and 3 mm slice thickness. The left and right breasts were manually segmented using SmartPaint.54 The posterior boundary of the breast segmentation was medially set in the adipose tissue anterior of the pectoral muscle and laterally by a line crossing the pectoral muscle and sternum. The lateral, medial, superior, and inferior boundaries were defined by following the contour of the breast and its continuation toward the posterior boundary. The nipple was excluded from the segmentation.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software 6.0. For paired data, two-tailed Wilcoxon signed rank test was used and for unpaired data two-tailed Mann–Whitney U-test. Correlations were calculated with two-tailed Spearman's rank correlation test. Data are expressed as mean ± SEM. A p < 0.05 was considered as statically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
The authors thank RN Ann-Christine N Andersson for excellent assistance in the recruitment of the women.
Funding
This work was supported by grants to C.D. from the Swedish Cancer Society (2015/309), the Swedish Research Council (2013-2457), LiU-Cancer, and Research Funds of Linköping University Hospital.
Author contributions
All authors collaborated on the study conception, study design, and interpretation of data. TR, ODL, and MB performed the LTF calculations. CD performed all microdialysis experiments and core biopsies. AA and CD carried out the cytokine analyses and immunohistochemistry. JK and PL coordinated the patients. AR assessed the mammographic density. AA and CD performed the data analysis and drafted the manuscript. All authors have read and approved the final manuscript.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:21376230; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 2.Harris RE, Chlebowski RT, Jackson RD, Frid DJ, Ascenseo JL, Anderson G, Loar A, Rodabough RJ, White E, McTiernan A. Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women's Health Initiative. Cancer Res 2003; 63:6096-101; PMID:14522941 [PubMed] [Google Scholar]
- 3.Khuder SA, Mutgi AB. Breast cancer and NSAID use: a meta-analysis. Br J Cancer 2001; 84:1188-92; PMID:11336469; http://dx.doi.org/ 10.1054/bjoc.2000.1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrahamsson A, Morad V, Saarinen NM, Dabrosin C. Estradiol, tamoxifen, and flaxseed alter IL-1beta and IL-1Ra levels in normal human breast tissue in vivo. J Clin Endocrinol Metab 2012; 97:E2044-54; PMID:22930784; http://dx.doi.org/ 10.1210/jc.2012-2288 [DOI] [PubMed] [Google Scholar]
- 5.Bendrik C, Dabrosin C. Estradiol increases IL-8 secretion of normal human breast tissue and breast cancer in vivo. J Immunol 2009; 182:371-8; PMID:19109168; http://dx.doi.org/ 10.4049/jimmunol.182.1.371 [DOI] [PubMed] [Google Scholar]
- 6.Dabrosin C. Variability of Vascular Endothelial Growth Factor in Normal Human Breast Tissue in Vivo during the Menstrual Cycle. J Clin Endocrinol Metab 2003; 88:2695-8; PMID:12788875; http://dx.doi.org/ 10.1210/jc.2002-021584 [DOI] [PubMed] [Google Scholar]
- 7.Dabrosin C. Positive correlation between estradiol and vascular endothelial growth factor but not fibroblast growth factor-2 in normal human breast tissue in vivo. Clin Cancer Res 2005; 11:8036-41; PMID:16299233; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-0977 [DOI] [PubMed] [Google Scholar]
- 8.Garvin S, Dabrosin C. In vivo measurement of tumor estradiol and vascular endothelial growth factor in breast cancer patients. BMC Cancer 2008; 8:73; PMID:18366667; http://dx.doi.org/ 10.1186/1471-2407-8-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindahl G, Saarinen N, Abrahamsson A, Dabrosin C. Tamoxifen, flaxseed, and the lignan enterolactone increase stroma- and cancer cell-derived IL-1Ra and decrease tumor angiogenesis in estrogen-dependent breast cancer. Cancer Res 2011; 71:51-60; PMID:21097717; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-2289 [DOI] [PubMed] [Google Scholar]
- 10.Svensson S, Abrahamsson A, Rodriguez GV, Olsson AK, Jensen L, Cao Y, Dabrosin C.. CCL2 and CCL5 Are Novel Therapeutic Targets for Estrogen-Dependent Breast Cancer. Clin Cancer Res 2015; 21:3794-805; PMID:25901081; http://dx.doi.org/ 10.1158/1078-0432.CCR-15-0204 [DOI] [PubMed] [Google Scholar]
- 11.Morad V, Abrahamsson A, Dabrosin C. Estradiol affects extracellular leptin:adiponectin ratio in human breast tissue in vivo. J Clin Endocrinol Metab 2014; 99:3460-7; PMID:24796929; http://dx.doi.org/ 10.1210/jc.2014-1129 [DOI] [PubMed] [Google Scholar]
- 12.Morad V, Abrahamsson A, Kjolhede P, Dabrosin C. Adipokines and vascular endothelial growth factor in normal human breast tissue in vivo - correlations and attenuation by dietary flaxseed. J Mammary Gland Biol Neoplasia 2016; 21:69-76; PMID:27059487; http://dx.doi.org/ 10.1007/s10911-016-9352-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyd NF, Martin LJ, Bronskill M, Yaffe MJ, Duric N, Minkin S. Breast tissue composition and susceptibility to breast cancer. J Natl Cancer Inst 2010; 102:1224-37; PMID:20616353; http://dx.doi.org/ 10.1093/jnci/djq239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006; 15:1159-69; PMID:16775176; http://dx.doi.org/ 10.1158/1055-9965.EPI-06-0034 [DOI] [PubMed] [Google Scholar]
- 15.Lin SJ, Cawson J, Hill P, Haviv I, Jenkins M, Hopper JL, Southey MC, Campbell IG, Thompson EW. Image-guided sampling reveals increased stroma and lower glandular complexity in mammographically dense breast tissue. Breast Cancer Res Treat 2011; 128:505-16; PMID:21258862; http://dx.doi.org/ 10.1007/s10549-011-1346-0 [DOI] [PubMed] [Google Scholar]
- 16.Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res 2003; 5:R129-35; PMID:12927043; http://dx.doi.org/ 10.1186/bcr622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh K, Brandt KR, Reynolds C, Scott CG, Pankratz VS, Riehle DL, Lingle WL, Odogwu T, Radisky DC, Visscher DW et al.. Tissue composition of mammographically dense and non-dense breast tissue. Breast Cancer Res Treat 2012; 131:267-75; PMID:21877142; http://dx.doi.org/ 10.1007/s10549-011-1727-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang JM, Byrne DJ, Takano EA, Jene N, Petelin L, McKinley J, Poliness C, Saunders C, Taylor D, Mitchell G et al.. Breast tissue composition and immunophenotype and its relationship with mammographic density in women at high risk of breast cancer. PLoS One 2015; PMID:26110820; http://dx.doi.org/16646977 10.1371/journal.pone.0128861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawes D, Downey S, Pearce CL, Bartow S, Wan P, Pike MC, Wu AH. Dense breast stromal tissue shows greatly increased concentration of breast epithelium but no increase in its proliferative activity. Breast Cancer Res 2006; 8:R24; PMID:16646977; http://dx.doi.org/ 10.1186/bcr1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan QJ, Kimler BF, O'Dea AP, Zalles CM, Sharma P, Fabian CJ. Mammographic density does not correlate with Ki-67 expression or cytomorphology in benign breast cells obtained by random periareolar fine needle aspiration from women at high risk for breast cancer. Breast Cancer Res 2007; 9:R35; PMID:17537236; http://dx.doi.org/ 10.1186/bcr1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verheus M, Maskarinec G, Erber E, Steude JS, Killeen J, Hernandez BY, Cline JM. Mammographic density and epithelial histopathologic markers. BMC Cancer 2009; 9:182; PMID:19523235; http://dx.doi.org/ 10.1186/1471-2407-9-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PH, Biessy C, Dossus L, Lukanova A, Bingham S, Khaw KT et al.. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer 2005; 12:1071-82; PMID:16322344; http://dx.doi.org/ 10.1677/erc.1.01038 [DOI] [PubMed] [Google Scholar]
- 23.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003; 362:419-27; PMID:12927427; http://dx.doi.org/ 10.1016/S0140-6736(03)14596-5 [DOI] [PubMed] [Google Scholar]
- 24.Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, Davidson NE, Decensi A, Fabian C, Ford L et al.. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2013; 31:2942-62; PMID:23835710; http://dx.doi.org/ 10.1200/JCO.2013.49.3122 [DOI] [PubMed] [Google Scholar]
- 25.Huo CW, Chew GL, Britt KL, Ingman WV, Henderson MA, Hopper JL, Thompson EW. Mammographic density-a review on the current understanding of its association with breast cancer. Breast Cancer Res Treat 2014; 144:479-502; PMID:24615497; http://dx.doi.org/ 10.1007/s10549-014-2901-2 [DOI] [PubMed] [Google Scholar]
- 26.Vachon CM, Suman VJ, Brandt KR, Kosel ML, Buzdar AU, Olson JE, Wu FF, Flickinger LM, Ursin G, Elliott CR et al.. Mammographic breast density response to aromatase inhibition. Clin Cancer Res 2013; 19:2144-53; PMID:23468058; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst 2011; 103:744-52; PMID:21483019; http://dx.doi.org/ 10.1093/jnci/djr079 [DOI] [PubMed] [Google Scholar]
- 28.van Nes JG, Beex LV, Seynaeve C, Putter H, Sramek A, Lardenoije S, Duijm-de Carpentier M, Van Rongen I, Nortier JW, Zonderland HM et al.. Minimal impact of adjuvant exemestane or tamoxifen treatment on mammographic breast density in postmenopausal breast cancer patients: a Dutch TEAM trial analysis. Acta Oncol 2015; 54:349-60; PMID:25383451; http://dx.doi.org/ 10.3109/0284186X.2014.964809 [DOI] [PubMed] [Google Scholar]
- 29.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 2006; 99:215-20; PMID:16541307; http://dx.doi.org/ 10.1007/s10549-006-9193-0 [DOI] [PubMed] [Google Scholar]
- 30.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 2008; 26:556-62; PMID:18180462; http://dx.doi.org/ 10.1200/JCO.2007.11.5451 [DOI] [PubMed] [Google Scholar]
- 31.Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, Becette V, André S, Piccart M, Campone M, Brain E et al.. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med 2009; 15:68-74; PMID:19122658; http://dx.doi.org/ 10.1038/nm.1908 [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436-44; PMID:18650914; http://dx.doi.org/ 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 33.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008; 27:5904-12; PMID:18836471; http://dx.doi.org/ 10.1038/onc.2008.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aberg UW, Saarinen N, Abrahamsson A, Nurmi T, Engblom S, Dabrosin C. Tamoxifen and flaxseed alter angiogenesis regulators in normal human breast tissue in vivo. PLoS One 2011; 6:e25720; PMID:21984941; http://dx.doi.org/ 10.1371/journal.pone.0025720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cotterchio M, Kreiger N, Sloan M, Steingart A. Nonsteroidal anti-inflammatory drug use and breast cancer risk. Cancer Epidemiol Biomarkers Prev 2001; 10:1213-7; PMID:11700271 [PubMed] [Google Scholar]
- 36.Gunter MJ, Wang T, Cushman M, Xue X, Wassertheil-Smoller S, Strickler HD, Rohan TE, Manson JE, McTiernan A, Kaplan RC et al.. Circulating adipokines and inflammatory markers and postmenopausal breast cancer risk. J Natl Cancer Inst 2015; 107; PMID:26185195; http://dx.doi.org/12398994 10.1093/jnci/djv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purohit A, Reed MJ. Regulation of estrogen synthesis in postmenopausal women. Steroids 2002; 67:979-83; PMID:12398994; http://dx.doi.org/ 10.1016/S0039-128X(02)00046-6 [DOI] [PubMed] [Google Scholar]
- 38.van Landeghem AA, Poortman J, Nabuurs M, Thijssen JH. Endogenous concentration and subcellular distribution of estrogens in normal and malignant human breast tissue. Cancer Res 1985; 45:2900-6; PMID:3986816 [PubMed] [Google Scholar]
- 39.Vermeulen A, Deslypere JP, Paridaens R, Leclercq G, Roy F, Heuson JC. Aromatase, 17 β-hydroxysteroid dehydrogenase and intratissular sex hormone concentrations in cancerous and normal glandular breast tissue in postmenopausal women. Eur J Cancer Clin Oncol 1986; 22:515-25; PMID:3015631; http://dx.doi.org/ 10.1016/0277-5379(86)90121-5 [DOI] [PubMed] [Google Scholar]
- 40.Geisler J. Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators. J Steroid Biochem Mol Biol 2003; 86:245-53; PMID:14623518; http://dx.doi.org/ 10.1016/S0960-0760(03)00364-9 [DOI] [PubMed] [Google Scholar]
- 41.Maskarinec G, Woolcott C, Steude JS, Franke AA, Cooney RV. The relation of leptin and adiponectin with breast density among premenopausal women. Eur J Cancer Prev 2010; 19:55-60; PMID:19927000; http://dx.doi.org/ 10.1097/CEJ.0b013e328333fb0e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sickles E, D'Orsi C, Bassett L et al.. ACR BI-RADS® mammography In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology, 2013 [Google Scholar]
- 43.Dabrosin C. Increase of free insulin-like growth factor-1 in normal human breast in vivo late in the menstrual cycle. Breast Cancer Res Treat 2003; 80:193-8; PMID:12908822; http://dx.doi.org/ 10.1023/A:1024575103524 [DOI] [PubMed] [Google Scholar]
- 44.Dabrosin C. Increased extracellular local levels of estradiol in normal breast in vivo during the luteal phase of the menstrual cycle. J Endocrinol 2005; 187:103-8; PMID:16214945; http://dx.doi.org/ 10.1677/joe.1.06163 [DOI] [PubMed] [Google Scholar]
- 45.Dabrosin C. Microdialysis—an in vivo technique for studies of growth factors in breast cancer. Front Biosci 2005; 10:1329-35; PMID:15769628; http://dx.doi.org/ 10.2741/1622 [DOI] [PubMed] [Google Scholar]
- 46.Dabrosin C. Sex steroid regulation of angiogenesis in breast tissue. Angiogenesis 2005; 8:127-36; PMID:16211362; http://dx.doi.org/ 10.1007/s10456-005-9002-0 [DOI] [PubMed] [Google Scholar]
- 47.Nilsson UW, Abrahamsson A, Dabrosin C. Angiogenin regulation by estradiol in breast tissue: tamoxifen inhibits angiogenin nuclear translocation and antiangiogenin therapy reduces breast cancer growth in vivo. Clin Cancer Res 2010; 16:3659-69; PMID:20501617; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-0501 [DOI] [PubMed] [Google Scholar]
- 48.Abrahamsson A, Dabrosin C. Tissue specific expression of extracellular microRNA in human breast cancers and normal human breast tissue in vivo. Oncotarget 2015; 6:22959-69; PMID:26008976; http://dx.doi.org/ 10.18632/oncotarget.4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petridou E, Kibiro M, Gladwell C, Malcolm P, Juette A, Toms A, Borga M, Dahlqvist Leinard O, Denton E.. Breast fat volume measurement in a wide-bore 3T MR: comparison of traditional mammographic density evaluation with MR density measurements using automatic segmentation. EPOSTM: European Society of Radiology's online database for electronic scientific exhibits 2015; http://dx.doi.org/ 10.1594/ecr2015/C-1801 [DOI] [Google Scholar]
- 50.Peterson P, Romu T, Brorson H, Dahlqvist Leinhard O, Mansson S. Fat quantification in skeletal muscle using multigradient-echo imaging: Comparison of fat and water references. J Magn Reson Imaging 2016; 43:203-12; PMID:26095018; http://dx.doi.org/ 10.1002/jmri.24972 [DOI] [PubMed] [Google Scholar]
- 51.Dahlqvist Leinard O, Johansson A, Rydell J, Smedby Ö. Quantitative abdominal fat estimation using MRI International Conference on Pattern Recognition. Tampa, FL: IEEE Computer Society, 2008:1-4 [Google Scholar]
- 52.Karlsson A, Rosander J, Romu T, Tallberg J, Gronqvist A, Borga M, Dahlqvist Leinhard O. Automatic and quantitative assessment of regional muscle volume by multi-atlas segmentation using whole-body water-fat MRI. J Magn Reson Imaging 2015; 41:1558-69; PMID:25111561; http://dx.doi.org/ 10.1002/jmri.24726 [DOI] [PubMed] [Google Scholar]
- 53.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 2008; 60:1122-34; PMID:18956464; http://dx.doi.org/ 10.1002/mrm.21737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malmberg F, Nordenskjöld R, Strand R, Kullberg J. SmartPaint: a tool for interactive segmentation of medical volume images. Comput Methods Biomech Biomed Eng: Imag Vis 2014; 1-9; http://dx.doi.org/ 10.1080/21681163.2014.960535 [DOI] [Google Scholar]