ABSTRACT
Sorafenib is an oral anti-angiogenic multi-kinase inhibitor used for systemic therapy in patients with advanced hepatocellular carcinoma (HCC) who are not suitable candidates for surgery or liver transplantation. An earlier study conducted with HCC tumor tissue suggested that ERK phosphorylation (pERK), a downstream target of sorafenib, may serve as a potential biomarker for therapeutic efficacy of sorafenib. However, no study thus far has utilized a minimal invasive procedure to predict HCC patient responsiveness to sorafenib. We evaluated the biomarker utility of circulating endothelial progenitor cells (EPCs) frequency and intracellular pERK levels in EPCs in peripheral blood obtained pre- and post-sorafenib therapy or after transarterial chemoembolistaion (TACE). A statistically significant reduction in the level of ERK phosphorylation and in the absolute number of EPCs was detected following in vivo sorafenib treatment (p < 0 .01 for both). In contrast, the decrease in the level of ERK phosphorylation and EPC number was either marginally significant or insignificant in patients treated with TACE (p = 0.05 and 0.06, respectively). In vitro sorafenib treatment of pre- and post-samples from the same patient cohort inhibited ERK phosphorylation levels in EPCs and decreased the number of EPCs at all doses tested (p = 0.01). Our findings support that the evaluation of both the circulating EPC frequency and the level of ERK phosphorylation in EPCs may serve as potential non-invasive biomarkers of sorafenib efficacy, both as predictor of treatment outcome and efficacy during drug treatment.
KEYWORDS: Anti-VEGF therapy, biomarker, endothelial progenitor cells, ERK phosphorylation, hepatocellular carcinoma, sorafenib
Introduction
Hepatocellular carcinoma (HCC) is the fifth-most common cancer and the third leading cause of cancer-related mortality globally.1 Major etiological factors include chronic hepatitis, either due to chronic hepatitis B (HBV) or hepatitis C (HCV) viral infection, or excessive alcohol consumption. HCC patients are frequently diagnosed at a late stage of disease progression or with deteriorated liver function, thereby, precluding surgical intervention. Liver transplantation is indicated only for patients at the initial stage of disease development and its application is critically dependent on availability of donor liver. Treatment options for patients with advanced stage disease are limited to chemoembolization or systemic therapy with sorafenib, an oral anti-angiogenic agent which is the mainstay of treatment for these patients. Sorafenib was the first drug to show improvement in both progression free and overall survival among patients with advanced disease.2 Although these treatment approaches have led to improved clinical response rates, treatment associated toxicities are frequent, requiring dose interruptions and reductions for the majority of patients. Survival remains less than 1 y for patients with advanced stage disease who are eligible for such molecularly targeted therapies.
One characteristic feature of HCC is abundant angiogenesis and tumor neovascularization. Pro-angiogenic signaling through the RAF/MEK/ERK kinase cascade is critically important in the development and progression of HCC. Phosphorylated ERK (pERK) is the key downstream component of the RAF/MEK/ ERK signaling pathway, which serves as the main target for the multi-kinase inhibitor sorafenib. This drug has a dual mechanism of action; inhibition of tumor cell proliferation and angiogenesis. Sorafenib inhibits the RAF serine–threonine kinases and blocks signaling via the RAF/MEK/ERK pathway. A phase II study in HCC patients has shown that patients whose tumors expressed higher baseline ERK levels had a longer time to tumor progression following treatment with sorafenib.3 Thus, tumors containing higher levels of pERK were more responsive to sorafenib treatment, suggesting that levels of pERK may be a useful biomarker of efficacy of sorafenib treatment.
Tumor neovascularization is dependent on the recruitment and proliferation of endothelial cells. Several studies have demonstrated that endothelial progenitor cells (EPCs) are mobilized from the bone marrow by VEGF and home to the site of tumor neovascularization.4-6 Circulating EPCs have been detected in a wide variety of malignant diseases such as multiple myeloma,7 metastatic renal cell carcinoma,8 and non-small cell lung cancer.9 In patients with advanced-stage HCC, high levels of circulating EPCs were found to be associated with poor survival and elevated levels of circulating EPCs were noted in patients with un-resectable HCC as compared to patients with resectable HCC or those with liver cirrhosis.10 In patients with metastatic colorectal cancer, low levels of circulating EPCs correlated with treatment outcome with bevacizumab (anti-VEGF) in combination with chemotherapy.11 Bevacizumab reduces bone marrow-dependent tumor vasculogenesis by reducing EPC mobilization from bone marrow into peripheral blood and by reducing the proliferation of circulating EPCs.
The rationale of our investigation was to evaluate the predictive and prognostic changes in ERK phosphorylation levels and in the number of circulating EPCs in advanced HCC patients receiving sorafenib treatment by using an innovative and minimally invasive bench-to-bedside approach. We reasoned that the validation of reduction in the baseline pERK levels in EPCs following in vitro treatment with sorafenib could serve as a predictive biomarker of the in vivo response to sorafenib. Being a key downstream component for the RAF/MEK/ERK signaling pathway, changes in pERK levels would therefore reflect the functional activity of this signaling pathway as well as the extent of inhibition by sorafenib. Thus, decreased EPC ERK phosphorylation detected in the PBMC of patients following sorafenib treatment could be reflective of the in vivo efficacy of the therapy.
Results
Inhibition of ERK phosphorylation by sorafenib
We characterized live EPC by exclusion of a viability dye and a CD45lowCD146+CD133+CD31+CD34+ phenotype (Fig. 1). Representative plots demonstrating sorafenib (5 μM) or TACE specific inhibition of pERK levels in EPCs are shown in Fig. 1. Our in vitro dose response experiments demonstrated that sorafenib inhibited ERK phosphorylation in EPCs even at the lowest dose tested (5 µM). Additional inhibition was not achieved by increasing sorafenib concentration (Fig. 2A). The inhibitory effect of sorafenib on pERK was statistically significant at all the doses tested as compared to the patient's own baseline control (p < 0 .01 for all, Fig. 2A). These data demonstrate a cell specific target of sorafenib.
Figure 1.
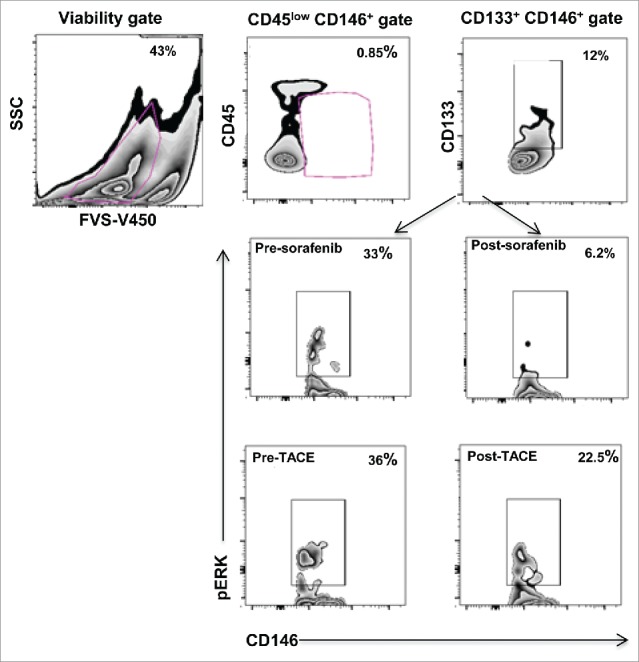
Characterization of endothelial progenitor cells (EPCs) from PBMC. Viable cells were gated based on the expression of CD45low CD146+, subsequently CD133+ cells were gated. Zebra plots showing reduction in ERK phosphorylation levels in EPCs of representative HCC patients after sorafenib/TACE treatment.
Figure 2.
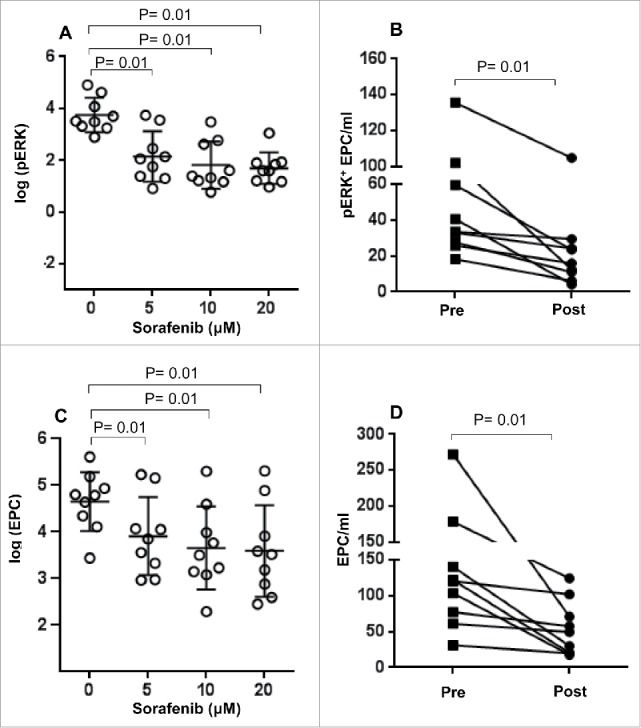
Effect of sorafenib treatment on ERK phosphorylation levels and absolute number of EPC. Pre-treatment samples of PBMC from HCC patients were treated with different concentrations of sorafenib (0–20 μM) in vitro and (A) the number of pERK+ EPC and (C) the absolute number of EPC were measured as described in Materials and Methods section. Each symbol ( ) represents an individual HCC patient and lines represent mean values for the group. (B) The number of pERK+ EPC/mL and (D) absolute number of EPC in HCC patients before (▪) or after treatment (•) with sorafenib (in vivo) was measured as described in the materials and methods.
) represents an individual HCC patient and lines represent mean values for the group. (B) The number of pERK+ EPC/mL and (D) absolute number of EPC in HCC patients before (▪) or after treatment (•) with sorafenib (in vivo) was measured as described in the materials and methods.
The in vitro study was carried out to develop a model to predict in vivo response at the optimal range of sorafenib dose; sorafenib administration often necessitates drug de-escalation, thereby, minimizing long term efficacy. Based on the in vitro results, we predicted that sorafenib treatment will likely result in a decline in pERK levels of EPC in vivo. This prediction was confirmed by in our findings of significant decreases in the level of ERK phosphorylation detected in the samples collected after sorafenib therapy as compared to pre-treatment samples (p < 0 .01, Fig. 1, Fig. 2B). Although reduction in ERK phosphorylation correlated with overall survival of patients, it nevertheless did not reach statistical significance (Log-rank p = 0.20, Fig. S1). It is however worth noting that the four patients with greater than median drop of pERK+ EPC greatly exceeded (>30 months) the median survival expected for these patients (10.7 months). Collectively, these results suggest that functional in vitro monitoring of pERK levels in non-invasively collected blood bio-specimens may be an effective method to predict responsiveness or resistance to sorafenib.
Effect of sorafenib on the absolute number of EPC
Besides a decrease in pERK levels, in vitro exposure to the lowest dose of sorafenib, 5 µM, also resulted in a significant decrease in the absolute numbers of viable EPC (p < 0 .01 for all comparisons to 0 µM, Fig. 2C). Similar dose ranges of sorafenib have been used in previous studies in order to inhibit the induction of regulatory T cells or T cell proliferation in vitro.12,13 Sorafenib concentrations in patients normally range between 6 and 12 μM (pharmacologic concentration).14 Further reduction in EPC viability was not observed by escalating sorafenib dose beyond 5 μM (Fig. 2C). To our knowledge, this is the first study demonstrating that sorafenib-mediated abrogation of ERK signaling also directly impacts EPC viability, thereby, further reducing the pool of progenitor cells required to sustain tumor angiogenesis. This result suggested that in vivo therapy with sorafenib should be successful and result in similar declines in absolute numbers of EPC. This prediction was confirmed in measurements performed on patient samples collected post-sorafenib therapy. Not only does sorafenib treatment have a functional effect on the ERK signaling pathway, it also has a beneficial impact on the cell target (EPCs) often implicated in tumor neo-vascularization. Statistically significant decreases in the absolute numbers of EPCs were observed when pre- and post-sorafenib treatment samples were compared (Fig. 2D).
TACE decreases ERK phosphorylation in EPCs and reduces EPC number
Samples obtained from sorafenib naive patients scheduled for TACE treatment responded to sorafenib treatment (in vitro), with a significant reduction in the number of ERK+ EPCs (p < 0 .01 for all doses vs. 0 µM, Fig. 3A). To determine if TACE treatment impacts ERK phosphorylation in vivo, pERK levels in EPCs were compared in samples collected pre- and post-TACE treatment. A decrease in the level of ERK phosphorylation was observed in patients treated with TACE; however, the difference was only marginally significant (p = 0.05, Fig. 3B).
Figure 3.
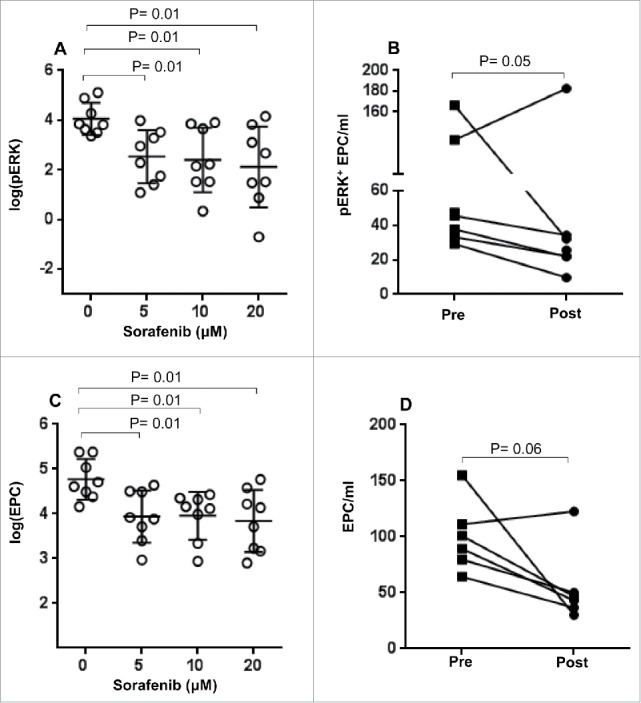
Effect of TACE on ERK phosphorylation levels and absolute number of EPC. Pre-treatment samples of PBMC from HCC patients (TACE) were treated with different concentrations of sorafenib (0–20 μM) in vitro and (A) the number of pERK+ EPC and (C) the absolute number of EPCs were measured as described in the Materials and Methods section. Each symbol ( ) represents an individual HCC patient and lines represent mean values for the group. (B) The number of pERK+ EPC/mL and (D) the absolute number of EPCs in the PBMC of HCC patients before (▪) or after TACE (•) were measured as described in the materials and methods.
) represents an individual HCC patient and lines represent mean values for the group. (B) The number of pERK+ EPC/mL and (D) the absolute number of EPCs in the PBMC of HCC patients before (▪) or after TACE (•) were measured as described in the materials and methods.
In vitro exposure to sorafenib using samples collected from patients prior to commencement of TACE therapy resulted in significant reduction in the EPC numbers (p = 0.01 for all doses vs. 0 µM, Fig. 3C). Similarly, patients treated with TACE also showed reduction in EPC numbers as compared to their pre-treatment sample; however, the reduction in EPC numbers did not achieve statistical significance in these patients (p = 0.06, Fig. 3D). Importantly, we also determined if patients who had received TACE retained the ability to respond to in vitro treatment with sorafenib. In vitro exposure to sorafenib of post-treatment samples from both sorafenib and TACE patients resulted in a significant reduction in pERK+ EPC (p = 0.01 for all doses vs. 0 µM, Fig. 4A, C respectively) and absolute numbers of EPCs (p = 0.01 for all doses vs. 0 µM, Fig. 4B and D, respectively). These results strongly suggest that patients treated with TACE may still be candidates for sorafenib therapy and therefore achieve clinical benefit in the event of a relapse.
Figure 4.
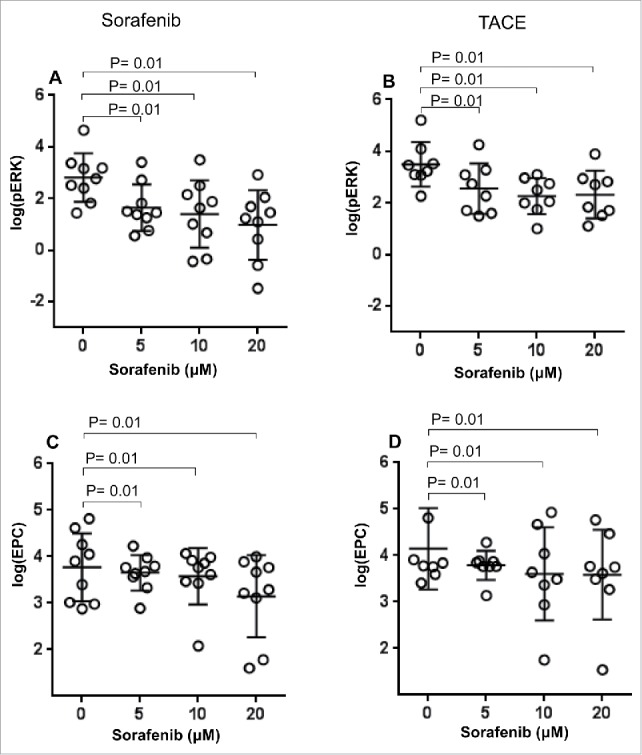
Effect of in vitro sorafenib treatment on ERK phosphorylation levels and absolute number of EPC. PBMC samples from HCC patients after treatment with sorafenib (A, C) or TACE (B, D) were treated with different concentrations of sorafenib (0–20 μM) in vitro and the number of pERK+ EPC and absolute number of EPC was measured as described in the Materials and Methods section. Each symbol ( ) represents an individual HCC patient and lines represent mean values for the group.
) represents an individual HCC patient and lines represent mean values for the group.
Discussion
It is well established that activation of the RAF/MEK/ERK signaling pathway is critically important in the development and progression of HCC and several other malignancies.15 RAF serine and threonine kinases phosphorylate and activate the MEK1/2 dual specificity protein kinases which in turn phosphorylate ERK1/2. Thus, activated ERK is the key downstream component of this signaling cascade which is being targeted by sorafenib.16 Earlier studies investigating the effect of sorafenib on HCC tumor cells have reported that a favorable response to sorafenib treatment is predominantly dependent on the baseline levels of phosphorylated ERK (pERK) present in the tumor cells prior to treatment.3,17 However, these earlier studies were carried out either using archived tumor tissues from HCC patients who were participants of phase II clinical trials on sorafenib therapy or using HCC cell lines.17 The authors suggested that pERK could be a potential biomarker which could predict the sensitivity of tumors to sorafenib therapy. However, measurement of changes in pERK levels utilizing HCC cell lines treated with sorafenib may not reliably predict patient responsiveness to sorafenib. Abou-Alfa et al. studied the gene expression profiles of HCC patients using RNA extracted from patient PBMCs; these studies could not elucidate any functional roles for the panel of genes that distinguished nonprogressors from progressors.3 Thus, their effort to identify a molecular signature of the tumor, based on the gene expression pattern of PBMCs that could predict the responsiveness to sorafenib treatment, was not successful. A recent study has reported a reduction in ERK phosphorylation levels using PBMCs from HCC patients treated with sorafenib plus octreotide, thus, demonstrating the feasibility of molecular screening studies using peripheral blood.18 Our study is distinguished by the fact that we measured pERK in EPCs and we utilized samples from patients prior to and post therapy with sorafenib or TACE. Since tumor neovascularization is dependent on the recruitment and proliferation of endothelial cells from bone marrow,4-6 quantification of the absolute number of circulating EPCs and pERK in EPCs may have greater relevance than studies based only on pre-treatment biopsy results. For our studies, PBMCs isolated from blood samples collected from patients with advanced HCC who were to commence sorafenib therapy were the source of EPCs. Our approach represents a surrogate and minimal invasive method compared to requirement for tumor biopsies from primary tumor or metastasis used in earlier studies.3 Additionally, this has clinical relevance as the standard of care for these advanced stage patients does not require the collection of tumor biopsy. By quantifying pERK levels in circulating EPCs using multi-color flow cytometry analysis, we have shown that treatment with sorafenib in vitro resulted in significant reduction in the levels of pERK on EPCs at all the concentrations tested as compared to untreated baseline controls. Furthermore, a significant decrease in the level of ERK phosphorylation was detected in the samples collected following in vivo sorafenib therapy as compared to the patients' pre-treatment samples. Since increased levels of circulating EPCs have been shown to be associated with poor clinical outcome in HCC, it is tempting to speculate that a decrease in the number of EPCs as well as the levels of EPC ERK phosphorylation in post sorafenib treatment sample may be indicative of a favorable response to sorafenib treatment in HCC patients. Even though the decline in EPC ERK phosphorylation levels after sorafenib therapy showed correlation with overall survival of patients, it did not achieve statistical significance. However, we have noted that the median survival of >30 months in two patients with greater than median drop in pERK+ EPCs far exceeds the expected median survival of patients treated with sorafenib (10.7 months). Sorafenib is a multikinase inhibitor which is able to antagonize different molecular mechanisms involved in the progression of HCC by inhibiting RAF and VEGF-R. Both the quantification of absolute number of EPC as well as the level of ERK phosphorylation on EPC could serve as potential biomarkers to predict the antitumor activity of sorafenib and their levels could be correlated with response to therapy. Our study is the first demonstration of this innovative bench-to-bedside assay with high translational potential.
Out of the eight TACE patients studied, one patient was resistant to sorafenib treatment in vitro. This patient displayed an inverse trend in pERK after sorafenib treatment. In this patient, increase in ERK phosphorylation following sorafenib treatment could be due to triggering of a feedback pathway that causes ERK phosphorylation. Protein kinase alpha C can induce RAF and RAS independent MEK/ERK activation in human hepatoma cells HepG2.19 A similar type of inverse trend in ERK phosphorylation has been reported in an earlier study investigating ERK phosphorylation in PBMCs of HCC patients treated with sorafenib.18 This indicates that the potential exists for ERK pathway to be augmented; thus, therapy that also reduces EPC numbers may be critical as this could overcome the potential activation of ERK phosphorylation through the feedback pathway.
In conclusion, reduced number of circulating EPCs after sorafenib therapy may be an indication of better clinical outcome. The ex vivo response to sorafenib following TACE that we have observed validates a role for combination therapy and may be a way to select patients most likely to benefit. Several trials of TACE plus anti-angiogenic therapy have thus far not shown significant clinical benefit; however, the optimal sequence and biomarkers to select patients and identify patients for such combined approaches are lacking. Further studies are justified to correlate the decline in EPC or ERK+EPC numbers following sorafenib treatment with survival of patients. Our results suggest that circulating EPC number and the levels of pERK on EPC have the potential to serve as a minimal invasive biomarker of sorafenib efficacy, both as a predictor of treatment outcome and efficacy during drug treatment. Further our data suggest that patients who received TACE may be benefited from future sorafenib therapy upon disease progression.
Materials and methods
The study is based on samples from 17 HCC patients treated at the Roswell Park Cancer Institute (RPCI) between 3/1/12 and 9/17/2013. The study protocol (I 67809) was approved by the RPCI Institutional Review Board. Informed consent was obtained in a manner consistent with the World Medical Association Declaration of Helsinki and RPCI standards. Clinical characteristics of patients including their liver function, stage, and etiology of cirrhosis are summarized in Table 1. All 17 HCC patients analyzed in this study had locally advanced or metastatic disease and none had early stage surgically resectable or transplantable disease. Approximately 30% of patients had received prior therapies. By radiographic criteria, 7/17 patients showed no signs of cirrhosis. The remaining 10/17 patients had some evidence radiographically (CT, MRI, USG. or more than one) of cirrhosis and none were deemed as surgical candidates. Eleven of seventeen patients were classified as Child Pugh score class A, whereas five patients were class B and one was class C. Partial response was observed in five patients. Another four had stable disease. The three sorafenib patients with missing response information died of liver failure before having the requisite response scan. Of the 17 patients, four were still alive at the last follow up. Median time to death from any cause was 23 months (95% CI: 7–40).
Table 1.
Clinical characteristics of patients.
| Sorafenib | TACE | ALL | |
|---|---|---|---|
| (n = 9) | (n = 8) | (n = 17) | |
| Age | |||
| Median (Min,Max) | 64 (53, 81) | 69.5 (53, 84) | 67 (53, 84) |
| Sex | |||
| Female | 1 (11.1%) | 1 (12.5%) | 2 (11.8%) |
| Male | 8 (88.9%) | 7 (87.5%) | 15 (88.2%) |
| Race | |||
| White | 7 (77.8%) | 7 (87.5%) | 14 (82.4%) |
| Black | 1 (11.1%) | 0 | 1 (5.9%) |
| Am Indian, Aleutian, Eskimo | 1 (11.1%) | 0 | 1 (5.9%) |
| Asian Indian | 0 | 1 (12.5%) | 1 (5.9%) |
| Hepatitis B | |||
| No | 9 (100%) | 7 (87.5%) | 16 (94.1%) |
| Yes | 0 | 1 (12.5%) | 1 (5.9%) |
| Hepatitis C | |||
| No | 4 (44.4%) | 4 (50%) | 8 (47.1%) |
| Yes | 5 (55.6%) | 4 (50%) | 9 (52.9%) |
| Alcohol Use | |||
| No | 5 (55.6%) | 6 (75%) | 11 (64.7%) |
| Yes | 4 (44.4%) | 2 (25%) | 6 (35.3%) |
| Cirrhosis | |||
| No | 3 (33.3%) | 4 (50%) | 7 (41.2%) |
| Yes | 6 (66.7%) | 4 (50%) | 10 (58.8%) |
| Previous Treatment | |||
| No | 6 (66.7%) | 6 (75%) | 12 (70.6%) |
| Yes | 3 (33.3%) | 2 (25%) | 5 (29.4%) |
| ECOG | |||
| 0 = Normal, no complaints | 6 (66.7%) | 4 (50%) | 10 (58.8%) |
| 1 = Normal with effort | 3 (33.3%) | 4 (50%) | 7 (41.2%) |
| AJCC Stage | |||
| 1 | 2 (22.2%) | 1 (12.5%) | 3 (17.6%) |
| 2 | 1 (11.1%) | 3 (37.5%) | 4 (23.5%) |
| 3 | 6 (66.7%) | 2 (25%) | 8 (47.1%) |
| 4A | 0 | 2 (25%) | 2 (11.8%) |
| BCLC Stage | |||
| B | 8 (88.9%) | 6 (75%) | 14 (82.4%) |
| C | 1 (11.1%) | 2 (25%) | 3 (17.6%) |
Heparinized peripheral blood samples were obtained from HCC patients before the initiation of treatment and after four weeks of sorafenib or TACE treatment, through RPCI Data Bank and Biorepository. Clinical therapy and baseline demographic data were recorded. PBMCs were isolated by Ficoll-PaqueTM PLUS density gradient centrifugation of blood samples (GE Healthcare, Uppsala) as described elsewhere.20
Sorafenib
Sorafenib tosylate (Nexavar, [N-(3-trifluoromethyl-4-chlorophenyl)-N-(4-(2-methylcarbamoylyridin-4-yl) oxyphenyl) urea]) (Bayer HealthCare Pharmaceuticals, Inc.) was dissolved in 100% dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO, USA) and diluted with RPMI 1640 to the desired concentration with a final DMSO concentration of 0.1% (v/v) for in vitro studies.
Cell culture
PBMC were plated at a concentration of 2.5×105 cells per well in 96-well microtiter plates. Cells were stimulated with PMA (20 ng/mL) and 1 µL of 1 mM ionomycin/mL (both Sigma-Aldrich) and simultaneously different concentrations of sorafenib ranging from 5 µM to 20 µM were added to the wells. Plates were incubated at 37°C in a humidified incubator containing 5% CO2. Reactions were stopped by fixing the cells at different time points by addition of 50 µL of cytofix (BD Biosciences, San Jose, CA). DMSO was added to control wells at 0.1% (v/v) as a solvent control. Additional controls included cultures incubated in cell culture medium without PMA/ionomycin and sorafenib or with PMA/ionomycin alone. Cells were washed and processed for flow cytometry staining.21
Endothelial progenitor cells (EPCs)
FACS analysis was performed to measure the frequencies of bone marrow derived circulating EPCs and pERK2 positive EPCs were identified using an eight-color staining protocol. The following markers were used: APC-H7 CD45, V450 fixable viability dye, PE pERK, FITC CD31, PE-CF594 CD34, PerCP/CY5.5 VEGEF-R2, PE-Cy7 CD146 (BD Biosciences, CA), and APC CD133 (Miltenyi Biotech, CA). Cells were washed and stained with surface antibody for 30 min at 4°C. After fixation and permeabilization, intracellular staining for pERK2 was performed according to the manufacturer's instructions using intracellular staining kit (eBiosciences, CA). Multi-color flow cytometry was performed using an LSR-II flow cytometer and FCS-3 data files were analyzed using Flowjo-10.02 software (Tree Star, Inc.).22
Statistical analysis
The effect of in vitro sorafenib exposure on EPC and pERK outcomes was quantified using Linear Mixed models with a random patient effect and fixed effects for in vivo treatment received (sorafenib ref: TACE) and the in vitro sorafenib dose. The outcomes were log-transformed to satisfy modeling assumptions. Sidak multiple testing adjustments were applied to p values for pairwise comparisons of mean outcome variables across all in vitro dose levels. Adjusted p values less than 0.05 were considered statistically significant. The primary model in vitro dose model was supported by the pre-treatment samples only. Models on pre-treatment samples from the in vivo sorafenib patients gave similar results. Similar models were specified to assess the effect of in vivo treatment on EPC and pERK levels. The effects of changes in pERK levels and EPC on overall survival of patients were explored using Kaplan Meier methods. The sorafenib and TACE treatments caused a decline in EPC and pERK for most patients. The patient set was bifurcated at the median of the observed post–pre differences. The Log-rank test was used to compare survival in patients classified as greater decrease to those with lesser decrease.
All data analyses were generated using SAS/STAT software, Version 9.4. Copyright 2012, SAS Institute Inc. SAS is a registered trademark of SAS Institute Inc., Cary, NC, USA.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Dr. Paul Wallace for his help with FACS experiments.
Funding
This study was supported by funding from American College of Gastroenterology grant to Dr. Thanavala. The Roswell Park Cancer Institute Biostatistics, Data Bank and Biorepository, and Flow Cytometry facilities are Cancer Center Support Grant (CCSG) Shared Resources supported, in part, by the National Cancer Institute (NCI) Core Grant to Roswell Park Cancer Institute (NIH P30 CA016056-27).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277-300; PMID:20610543; http://dx.doi.org/ 10.3322/caac.20073 [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A et al.. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359:378-90; PMID:18650514; http://dx.doi.org/ 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 3.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B et al.. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006; 24:4293-300; PMID:16908937; http://dx.doi.org/ 10.1200/JCO.2005.01.3441 [DOI] [PubMed] [Google Scholar]
- 4.Rafii S. Circulating endothelial precursors: mystery, reality, and promise. J Clin Invest 2000; 105:17-9; PMID:10619857; http://dx.doi.org/ 10.1172/JCI8774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275:964-7; PMID:9020076; http://dx.doi.org/ 10.1126/science.275.5302.964 [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 1999; 5:434-8; PMID:10202935; http://dx.doi.org/ 10.1038/8462 [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Vakil V, Braunstein M, Smith EL, Maroney J, Chen L, Dai K, Berenson JR, Hussain MM, Klueppelberg U et al.. Circulating endothelial progenitor cells in multiple myeloma: implications and significance. Blood 2005; 105:3286-94; PMID:15618473; http://dx.doi.org/ 10.1182/blood-2004-06-2101 [DOI] [PubMed] [Google Scholar]
- 8.Farace F, Gross-Goupil M, Tournay E, Taylor M, Vimond N, Jacques N, Billiot F, Mauguen A, Hill C, Escudier B. Levels of circulating CD45(dim)CD34(+)VEGFR2(+) progenitor cells correlate with outcome in metastatic renal cell carcinoma patients treated with tyrosine kinase inhibitors. Br J Cancer 2011; 104:1144-50; PMID:21386843; http://dx.doi.org/ 10.1038/bjc.2011.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vroling L, Lind JS, de Haas RR, Verheul HM, van Hinsbergh VW, Broxterman HJ, Smit EF. CD133+ circulating haematopoietic progenitor cells predict for response to sorafenib plus erlotinib in non-small cell lung cancer patients. Br J Cancer 2010; 102:268-75; PMID:20010948; http://dx.doi.org/ 10.1038/sj.bjc.6605477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho JW, Pang RW, Lau C, Sun CK, Yu WC, Fan ST, Poon RT. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology 2006; 44:836-43; PMID:17006919; http://dx.doi.org/ 10.1002/hep.21353 [DOI] [PubMed] [Google Scholar]
- 11.Matsusaka S, Mishima Y, Suenaga M, Terui Y, Kuniyoshi R, Mizunuma N, Hatake K. Circulating endothelial progenitors and CXCR4-positive circulating endothelial cells are predictive markers for bevacizumab. Cancer 2011; 117:4026-32; PMID:21858803; http://dx.doi.org/ 10.1002/cncr.25977 [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Yu T, Yuan Y, Zhuang H, Wang Z, Liu X, Feng M. Sorafenib reduces hepatic infiltrated regulatory T cells in hepatocellular carcinoma patients by suppressing TGF-beta signal. J Surg Oncol 2013; 107:422-7; PMID:22833259; http://dx.doi.org/ 10.1002/jso.23227 [DOI] [PubMed] [Google Scholar]
- 13.Cabrera R, Ararat M, Xu Y, Brusko T, Wasserfall C, Atkinson MA, Chang LJ, Liu C, Nelson DR. Immune modulation of effector CD4+ and regulatory T cell function by sorafenib in patients with hepatocellular carcinoma. Cancer Immunol Immunother 2013; 62:737-46; PMID:23223899; http://dx.doi.org/ 10.1007/s00262-012-1380-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanchet B, Billemont B, Cramard J, Benichou AS, Chhun S, Harcouet L, Ropert S, Dauphin A, Goldwasser F, Tod M. Validation of an HPLC-UV method for sorafenib determination in human plasma and application to cancer patients in routine clinical practice. J Pharm Biomed Anal 2009; 49:1109-14; PMID:19278805; http://dx.doi.org/ 10.1016/j.jpba.2009.02.008 [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M et al.. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004; 64:7099-109; PMID:15466206; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-1443 [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 2006; 66:11851-8; PMID:17178882; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-1377 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Zhou X, Shen H, Wang D, Wang Y. Phosphorylated ERK is a potential predictor of sensitivity to sorafenib when treating hepatocellular carcinoma: evidence from an in vitro study. BMC Med 2009; 7:41; PMID:19698189; http://dx.doi.org/ 10.1186/1741-7015-7-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caraglia M, Giuberti G, Marra M, Addeo R, Montella L, Murolo M, Sperlongano P, Vincenzi B, Naviglio S, Prete SD et al.. Oxidative stress and ERK1/2 phosphorylation as predictors of outcome in hepatocellular carcinoma patients treated with sorafenib plus octreotide LAR. Cell Death Dis 2011; 2:e150; PMID:21525937; http://dx.doi.org/ 10.1038/cddis.2011.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen-Sheng W. Protein kinase C α trigger Ras and Raf-independent MEK/ERK activation for TPA-induced growth inhibition of human hepatoma cell HepG2. Cancer Lett 2006; 239:27-35; PMID:16169661; http://dx.doi.org/ 10.1016/j.canlet.2005.07.034 [DOI] [PubMed] [Google Scholar]
- 20.Suresh K, Fraser G, Scheid E, Leber B, Gauldie J, Foley R. Generation of in vitro B-CLL specific HLA class I restricted CTL responses using autologous dendritic cells pulsed with necrotic tumor lysate. Leuk Lymphoma 2006; 47:297-306; PMID:16321861; http://dx.doi.org/ 10.1080/10428190500301231 [DOI] [PubMed] [Google Scholar]
- 21.Kalathil S, Lugade AA, Miller A, Iyer R, Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res 2013; 73:2435-44; PMID:23423978; http://dx.doi.org/24825462 10.1158/00008-5472.CAN-12-3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalathil SG, Lugade AA, Pradhan V, Miller A, Parameswaran GI, Sethi S, Thanavala Y. T-regulatory cells and programmed death 1+ T cells contribute to effector T-cell dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 190:40-50; PMID:24825462; http://dx.doi.org/ 10.1164/rccm.201312-2293OC [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


