ABSTRACT
Increasing transgene expression has been a major focus of attempts to improve DNA vaccine-induced immunity in both preclinical studies and clinical trials. Novel mini-intronic plasmids (MIPs) have been shown to cause elevated and sustained transgene expression in vivo. We sought to test the antitumor activity of a MIP, compared to standard DNA plasmid immunization, using the tumor-specific antigen SSX2 in an HLA-A2-restricted tumor model. We found that MIP vaccination elicited a greater frequency of antigen-specific CD8+ T cells when compared to conventional plasmid, and protected animals from subsequent tumor challenge. However, therapeutic vaccination with the MIP resulted in an inferior antitumor effect, and CD8+ tumor-infiltrating lymphocytes from these mice expressed higher levels of surface LAG3. Antitumor efficacy of MIP vaccination could be recovered upon antibody blockade of LAG3. In non-tumor bearing mice, MIP immunization led to a loss of epitope dominance, attenuated CD8+ cytokine responses to the dominant p103 epitope, and increased LAG3 expression on p103-specific CD8+ T cells. Further, LAG3 expression on CD8+ T cells was associated with antigen dose and persistence in spite of DNA-induced innate immunity. These data suggest that for antitumor immunization, approaches leading to increased antigen expression following vaccination might optimally be combined with LAG3 inhibition in human trials. On the other hand, mini-intronic vector approaches may be a superior means to elicit LAG3-dependent tolerance in the treatment of autoimmune diseases.
KEYWORDS: Antigen expression, DNA vaccine, LAG3, mini-intronic plasmid, tolerance
Introduction
DNA vaccines are bacterial plasmids encoding a protein or peptide antigen under a strong mammalian promoter. They are simple, safe, economical, and easy to mass produce. Though initially employed as vectors for in vivo transfer of a therapeutic gene,1,2 it was later observed that plasmid DNA encoding a viral protein was able to elicit antigen-specific cellular and humoral immune responses, and also protect against viral challenge.3,4 Follow-up studies showed preclinical efficacy of DNA vaccines in a large number of infectious and tumor disease models.3 However, results from human trials have been comparatively disappointing, with only modest immune responses observed in a majority of the cases.5
One of the major reasons cited for low immunogenicity of DNA vaccines in human subjects has been poor in vivo transfection efficiency and resultant low antigen/body mass ratio from plasmid DNA injections. Consequently, efforts to boost DNA vaccine efficacy have mainly focused on increasing antigen expression through either enhancing transfection efficiency (electroporation, gene guns, and similar particle bombardment type approaches) or optimization of the plasmid vector (strong promoters, Kozak sequences, codon optimization, and so on).5-18 Each of these techniques has achieved an incremental increase in DNA vaccine efficacy in preclinical models, setting the stage for an effort to further increase antigen expression using iterations or combinations of the above approaches.
Recent advances in the gene therapy field have shown that gene expression from traditional plasmid vectors is silenced rapidly in vivo, and this can be avoided by reducing the extragenic spacer length by either the removal of the bacterial backbone components (usually an origin of replication and an antibiotic resistance gene) as in DNA minicircles, or by inserting these elements necessary for plasmid propogation into an intron as in mini-intronic plasmids (MIPs).19-21 Thus, use of these vectors presents an opportunity to examine the effects of greatly increasing antigen expression without necessarily modifying the innate immune response to vaccination as in electroporation or similar approaches.22-24 Recently, DNA minicircles were reported to elicit greater frequencies of antigen-specific CD8+ T cells when compared to conventional plasmid immunization, and minicircle immunization was able to better protect against infection by an intracellular bacterium.25,26 However, these studies did not evaluate the efficacy of minicircle DNA immunization as an antitumor therapy. Further, to our knowledge, there have been no reports exploring the efficacy of MIP vaccination in any setting.
In the current study, we sought to evaluate whether DNA minicircle or MIP vectors provided sustained expression of a target tumor antigen, and whether this approach was superior relative to standard plasmid DNA in eliciting antigen-specific antitumor immunity. This was evaluated in a system we have previously reported using a plasmid DNA vaccine encoding the tumor associated cancer-testis antigen SSX2 (Synovial Sarcoma, X Breakpoint 2),27 relevant HLA-A2 transgenic mice that permit analysis of epitope specific immunity, and a syngeneic tumor cell line model engineered to express the antigen of interest.28-30 We found that MIP-SSX2 immunization elicited greater frequencies of tetramer positive CD8 T cells and antigen-specific IFNγ responses, as others have previously reported with minicircles. However, we observed that while MIP-SSX2 protected against tumor challenge in a prophylactic setting, there was a paradoxical loss of antitumor effect following treatment of established tumors. We found that this inferior response was associated with increased expression of the immune checkpoint molecule lymphocyte activation gene 3 (LAG3) on CD8+ tumor-infiltrating lymphocytes and on antigen-specific CD8+ T cells from the spleens of animals receiving the mini-intronic vaccine. Further, we demonstrated that LAG3 expression on CD8+ T cells was directly associated with antigen dose and persistence in a tolerizing environment both in vitro and in vivo. These data suggest the involvement of the LAG3 checkpoint pathway molecule in DNA vaccine-induced immunity, and that antitumor vaccine approaches designed to increase antigen dose or persistence might best be combined with LAG3 blockade in clinical trials.
Results
A mini-intronic plasmid encoding SSX2 provided sustained transgene expression in vitro and in vivo
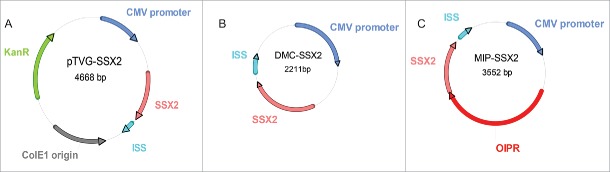
We first constructed a DNA minicircle (DMC-SSX2) by cloning the expression cassette of pTVG-SSX2 (a plasmid encoding the cancer-testis antigen SSX2 that we have previously described)30,31 into a minicircle production vector (Figs. 1A and B). We then used an optimized intron design (containing exonic splicing enhancers, a bacterial origin of replication modified to remove potential splice sites, and an RNA-based selection system) 32 to construct a MIP encoding SSX2 (MIP-SSX2, Fig. 1C).20 All plasmids were confirmed by sequencing (data not shown). We similarly generated a minicircle construct encoding enhanced green fluorescent protein (EGFP, DMC-EGFP, data not shown).
Figure 1.
Construction of plasmids used. (A) Conventional plasmid encoding cancer-testis antigen SSX2 (pTVG-SSX2), as previously described.27 (B) Minicircle DNA (DMC-SSX2) harboring an identical transgene expression cassette as pTVG-SSX2, created using the pMC.CMV-MCS-SV40polyA minicircle production vector and the ΦC31 integrase sensitive ZYCY10P3S2T E. coli strain (System Biosciences). (C) Mini-intronic plasmid encoding SSX2 (MIP-SSX2), obtained upon cloning of an OIPR intron containing a modified bacterial origin of replication, exonic splicing enhancers and a selectable RNA-OUT marker (Nature Technology Corporation), into a unique restriction site downstream of the CMV promoter in the DMC-SSX2 construct (as previously described).20
Transgene expression from these constructs was confirmed in vitro by transient transfection of equimolar amounts of pTVG-SSX2 and MIP-SSX2 into LNCaP prostate cancer cells cultured in low serum conditions. Two and seven days post-transfection, the cells were lysed and SSX2 expression was quantitatively assayed by ELISA. As shown in Fig. 2A, MIP-SSX2 transfection resulted in higher detectable levels of SSX2 on both day 2 (left) and day 7 (right). Similar results were obtained with minicircle (DMC-SSX2 and DMC-EGFP) constructs (Fig. S1), as expected.33 To test transgene persistence in vivo, we administered equimolar amounts of the different SSX2-expressing constructs into FVB mice by tail vein injection and assayed for SSX2 expression in harvested liver tissue by qRT-PCR. As shown in Fig. 2B, MIP-SSX2 delivery led to a near constant SSX2 expression in hepatocytes over a two-week period, whereas with pTVG-SSX2 delivery mRNA levels fell over 100-fold from the peak day 2 levels. These data correspond to previous studies showing that silencing of gene expression from conventional plasmid vectors is more pronounced in quiescent tissue in vivo.19,34,35 Given the similarity of gene expression from minicircle or MIP vectors, and given the ease of preparation of pure MIP vectors without contaminating DNA populations common with minicircle preparations (Fig. S2), subsequent studies focused on the use of the MIP construct.36
Figure 2.
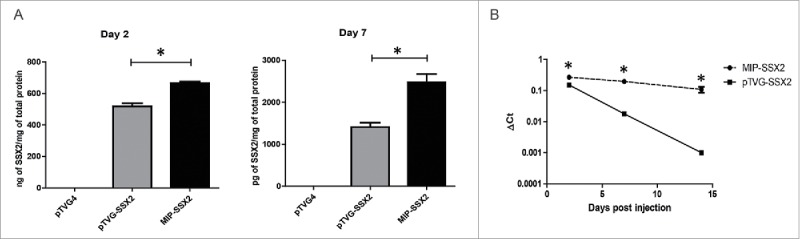
Delivery of MIP-SSX2 in vitro and in vivo resulted in elevated and sustained transgene expression. (A) Equimolar amounts of pTVG-SSX2 (2 µg) and MIP-SSX2 (1.6 µg) were used to transiently transfect LNCaP cells grown in low serum conditions. SSX2 production was assayed by quantitative ELISA on days 2 and 7 (n = 3 experimental replicates). pTVG4 = control plasmid vector not encoding SSX2. (B) Equimolar amounts of pTVG-SSX2 (30 µg) and MIP-SSX2 (24 µg) were administered to age-matched FVB mice (n = 3/group/time point) via tail vein injection. Livers were harvested on days 2, 7, and 14 and assayed for transgene expression using qRT-PCR. (* denotes a p-value < 0.05, two-sided t-test).
MIP immunization led to greater CD8+ T cell frequencies, but changes in cytokine secretion and elevated LAG3 expression on CD8+ T cells specific for the dominant epitope
In order to determine whether sustained expression through the use of a DNA MIP elicited greater CD8+ T cell responses, we vaccinated age-matched groups of HLA-A2/DRI expressing mice with either empty vector (pTVG4) or equimolar amounts of pTVG-SSX2 (100 µg) or MIP-SSX2 (83 µg) and assayed for Th1 biased immune responses after three biweekly intradermal immunizations. As shown in Fig. 3, we observed an increase in antigen-specific CD8+ T-cell mediated IFNγ secretion (Fig. 3A) and tetramer positive CD8 T cells (Fig. 3E) in MIP-SSX2 immunized animals compared to plasmid immunization. Of note, similar results were observed using a minicircle encoding SSX2 compared to conventional plasmid DNA (Fig. S3). However, we observed that while pTVG-SSX2 resulted in secretion of TNFα, IFNγ, and IL2 in a p103 epitope-specific fashion, MIP-SSX2 immunization resulted in p103 epitope-specific release of only IFNγ at levels above background (Fig. 3A). Conversely, only MIP-SSX2-treated animals had significant TNFα- and IFNγ-secreting CD8+ T cell responses to the subdominant p41 epitope (Fig. 3A). Representative flow plots for these data are shown in Fig. S4. Fig. 3B summarizes the difference in immune responses between the two treatment groups. As demonstrated in Fig. 3C, we did not detect a significant difference in the proportion of polyfunctional cells secreting more than one cytokine between the different treatment groups, rather differences in T cells responding to either epitope were largely due to distinct populations of CD8+ T cells secreting either TNFα, IFNγ, or IL2 alone.
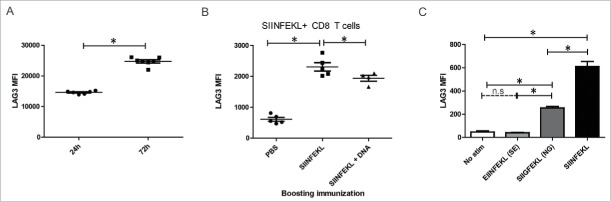
Figure 3.
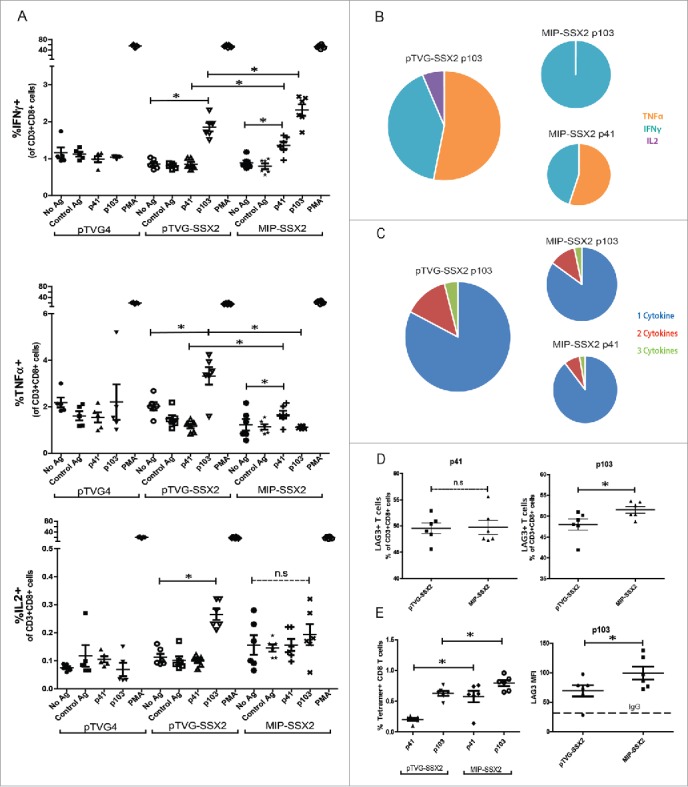
MIP immunization led to greater CD8+ T cell frequencies, but changes in cytokine secretion and elevated LAG3 expression on CD8+ T cells specific for the dominant epitope. HLA-A2/DRI mice (n = 6 per group) were immunized three times with either an empty vector control (pTVG4), or equimolar amounts of pTVG-SSX2 (100 µg) or MIP-SSX2 (83 µg). A) One week after the final immunization animals were euthanized and splenocytes were assayed for antigen-specific CD8+ responses by release of IFNγ, TNFα, or IL2 (panel A). (B) Averaged epitope-specific IFNγ (orange), TNFα (blue), or IL2 (violet) release for the two treatment groups over background levels. Diameter of the chart is proportional to the magnitude of total cytokine release. (C) Averaged proportions of epitope-specific polyfunctional CD8+ T cells. Diameter of the chart is proportional to the quantum of response, and each color is representative of the relative proportion of cells releasing either 1 (blue), 2 (red), or 3 (green) Th1 cytokines. (D) % of CD8+ T cells secreting at least 1 Th1 cytokine in response to either p41 or p103 restimulation that also expressed LAG3. (E) Frequencies of CD8+ T cells from animals receiving either pTVG-SSX2 or MIP-SSX2 immunizations that stained positive for p41 or p103 specific tetramers (left). Levels of LAG3 on p103 tetramer positive CD8+ T cells as measured by flow cytometry, with IgG fluorescent control MFI indicated by dotted line (right). These data are representative of three independent experiments. For all panels, * denotes a p-value < 0.05, two-sided t-test.
Lastly, we investigated whether SSX2 specific CD8+ T cells in each group differed with respect to surface expression of checkpoint molecules.37-39 As seen in Figs. 3D and E, p103 epitope-specific CD8+ T cells in the MIP-SSX2 treatment group had significantly higher expression of LAG3, whereas there was no detectable difference in CD8+ T cells responding to p41. No changes were observed in levels of PD1, TIM3, or CTLA4 (representative plots in Fig. S5) on these cell populations.
MIP-SSX2 immunization was inferior to standard plasmid DNA immunization as an antitumor therapy and resulted in elevated expression of LAG-3 on CD8+ tumor-infiltrating lymphocytes (TILs)
To evaluate MIP plasmids as antitumor vaccines, we first challenged HLA-A2/DRI expressing mice with syngeneic-SSX2 expressing tumor cells after four bi-weekly immunizations with either pTVG4 control vector, pTVG-SSX2 or MIP-SSX2. As seen in Fig. 4A (left), both pTVG-SSX2 and MIP-SSX2 protected animals against tumor cell challenge in an antigen-specific fashion, suggesting that functional CTL responses to the antigen were elicited by both constructs. Six weeks after tumor challenge, mice were evaluated for systemic SSX2-specific CD8+ T cells by tetramer staining. As shown in Fig. 4A (right), MIP-SSX2 vaccination again resulted in higher frequencies of p103 epitope-specific CD8+ T cells that persisted even 8 weeks after the final booster immunization. Splenocytes from animals immunized with MIP-SSX2 also had cytolytic activity to the SSX2-expressing tumor cell line in vitro (Fig. S6). Next, HLA-A2/DRI expressing mice were first inoculated with syngeneic sarcoma cells expressing SSX2, and then vaccinated with either pTVG4 (100 µg), pTVG-SSX2 (100 µg), or MIP-SSX2 (83 µg) on days 1 and 15. Tumor growth was monitored over time, and as seen in Fig. 4B and C, MIP-SSX2 unexpectedly failed to provide a significant antitumor response over vector control-treated animals. Given our recent findings that altering the plasmid sequence to encode an altered peptide epitope with enhanced HLA-A2 binding capacity could lead to increased expression of PD1 on vaccine elicited epitope-specific CD8+ T cells, and our current observations of elevated LAG3 expression following MIP vaccination (Figs 3D and E), we evaluated tumor cells and tumor-infiltrating lymphocytes (TIL) for differences in T-cell checkpoint molecules and ligands.29 As shown in Fig. 4D, we found that CD8+ TIL from animals treated with MIP-SSX2 had significantly higher levels of surface LAG3 expression than CD8+ TIL from pTVG-SSX2-treated animals. These CD8+ TIL also showed slightly reduced staining for PD1, albeit not significantly so, and no difference in TIM3 expression. Similarly, no changes were observed in CD8+ TIL expression of CD244 or CD160 (data not shown). Expression of checkpoint ligands HVEM, PD-L1, CD48, and Galectin-9 on tumor/tumor associated populations were also not statistically different among treatment groups (data not shown).
Figure 4.
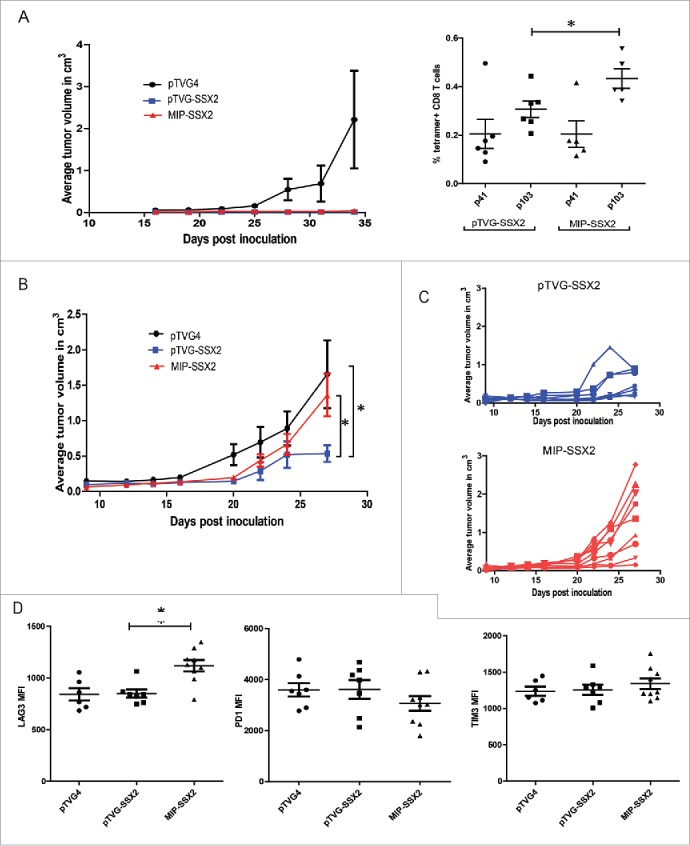
MIP-SSX2 immunization was inferior to standard plasmid DNA immunization as an antitumor therapy and resulted in elevated expression of LAG-3 on CD8+ tumor-infiltrating lymphocytes (TILs). A) HLA-A2/DR1 expressing mice were immunized four times bi-weekly (n = 6 per group) and subsequently challenged with a syngeneic sarcoma line engineered to overexpress SSX2. Tumor size was monitored over time (left). Splenocytes from tumor-challenged animals were analyzed for the frequency of SSX2-specific CD8+ T cells 35 d post tumor inoculation using tetramer staining specific for either p41 or p103 HLA-A2-restricted epitopes (right). (B) HLA-A2/DR1 expressing mice (n = 6–8 per group) were implanted with 5 × 104 syngeneic sarcoma cells overexpressing SSX2. On days 1 and 15 post-tumor inoculation, mice were immunized with either an empty vector control (pTVG4), or equimolar amounts of pTVG-SSX2 (100 µg) or MIP-SSX2 (83 µg), and tumor growth was monitored over time. Shown are the average tumor sizes per treatment group (B), or tumor growth curves for individual animals over time in the individual pTVG-SSX2 or MIP-SSX2 treatment groups (C). (D) CD8+ tumor-infiltrating lymphocytes from these animals were analyzed for surface expression of LAG3, PD1, and TIM3 checkpoint markers. MFI = median fluorescence intensity. For all panels, * denotes a p-value < 0.05, two-sided t-test. Similar results were observed in three independent studies.
Antitumor effects of MIP-SSX2 immunization can be rescued by combination with anti-LAG3
To evaluate whether the high LAG3 expression on CD8+ T cells was responsible for loss of antitumor efficacy in the MIP-SSX2-treated animals, we first implanted tumors and vaccinated animals as above. Animals then received either PBS vehicle control or 200 µg αLAG3 on days 1 and 3 after each immunization. As shown in Fig. 5A, LAG3 blockade was able to restore the antitumor activity of MIP-SSX2. Both DNA vaccines (pTVG-SSX2 or MIP-SSX2) when combined with αLAG3 were able to cause complete tumor regressions (CR) in several animals, not observed in the absence of LAG3 blockade (Fig. 5B). However, the improvement in the pTVG-SSX2 treatment upon addition of LAG3 blockade was not statistically significant. We further observed that LAG3 blockade along with MIP-SSX2 treatment increased CD8+ T cell infiltration into the tumors (Fig. 5C).
Figure 5.
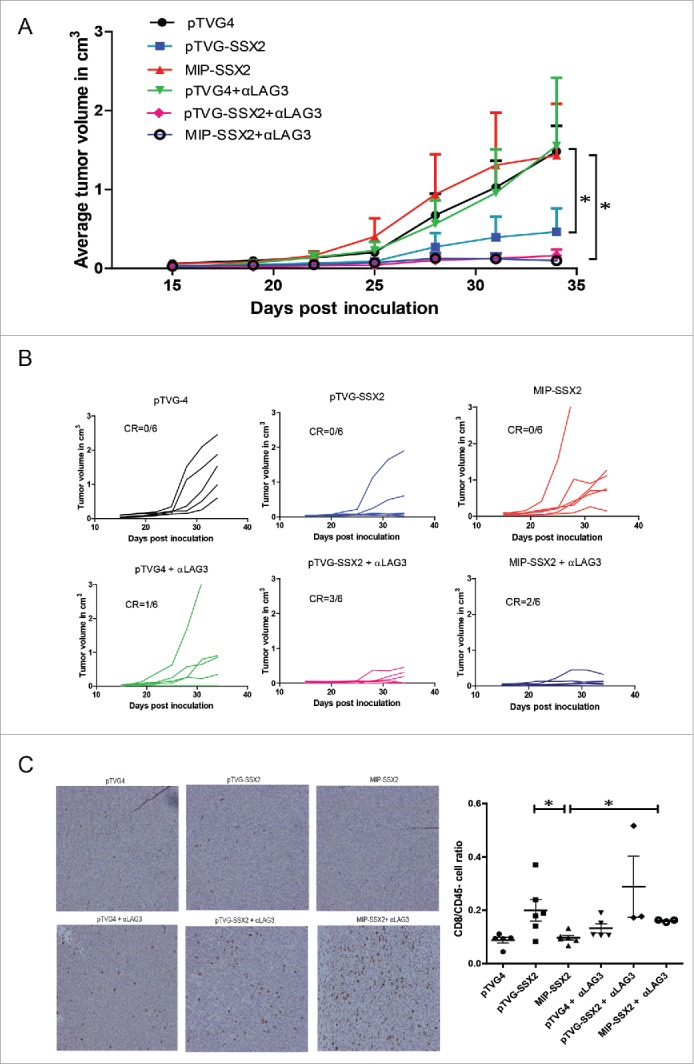
Antitumor effects of MIP-SSX2 immunization can be rescued by combination with anti-LAG3. (A) HLA-A2/DRI mice (n = 6 per group) were implanted with 5 × 104 syngeneic sarcoma cells overexpressing SSX2. Starting one day post tumor inoculation, mice were immunized with either an empty vector control, or equimolar amounts of pTVG-SSX2 (100 µg) or MIP-SSX2 (83 µg) on days 1 and 15. Animals from each treatment group were then administered either anti-LAG3 (αLAG3, 200 µg on days 2,4,16, and 19, clone C9B7W) or PBS (vehicle) control, and tumor growth was monitored over time. (A) The average tumor size per treatment group. (B) Tumor growth curves for individual animals over time in the separate treatment groups. CR = complete tumor response. (C) Representative immunohistochemistry of tumor sections from the different treatment groups for CD8α (left). Ratio of CD3+CD4-CD8+ TILs to CD45- cells as assessed by flow cytometry (right) in each of the treatment groups. Data are representative of two independent experiments. For all panels, * denotes a p-value < 0.05, two-sided t-test.
LAG3 expression on CD8+ T cells increases with magnitude and duration of antigenic stimulation
LAG3 upregulation on CD8+ T cells has been detected as early as 24 h post-TCR activation, but kinetic studies have revealed that surface expression continues to rise and is maximal at 72 h.37,40 However, little is known about the effect of a pulse of antigen expression, as is likely with conventional plasmid immunization, versus sustained expression, as may be more likely with MIP immunization, on LAG3 expression during initial priming. It has been previously demonstrated that higher antigen dose can lead to higher LAG3 expression.37 To model this with DNA immunization in a separate model system, and be able to evaluate differences following a single priming immunization, we used the OT-1 murine model. First, we transfected B16 cells with low or high doses of plasmid DNA encoding secretory ovalbumin, and media from transfected cells was then used to stimulate OT-1 cells. As shown in Fig. 6A, increasing concentrations of antigen led to increased LAG3 expression. This could be replicated with peptide alone, as increasing concentrations of SIINFEKL peptide alone could lead to increased LAG3 expression on OT-1 cells stimulated in vitro (Fig. 6B). Next, OT-1 cells were transferred to naive C57/Bl6 mice and mice were then immunized with different doses of plasmid DNA. As shown in Fig. 6C, higher doses of plasmid DNA encoding ovalbumin with a single immunization led to increased LAG3 expression on activated antigen-specific CD8+ T cells. To then examine whether the duration of antigenic stimulation also affects LAG3 levels on CD8+ T cells, we stimulated naive OT1 splenocytes with the SIINFEKL peptide for either 24 h or 72 h. Samples stimulated for 24 h were washed and rested for 48 h. As shown in Fig. 7A, OT1 splenocytes that were stimulated continuously for 72 h had significantly higher surface LAG3 levels than those that received pulsatile stimulation of only 24 h. To evaluate this in an in vivo vaccination setting, C57BL6 were adoptively transferred with OT1 splenocytes (day 0). One day post transfer mice received an intradermal injection with 100 µg of SIINFEKL peptide along with 50 µg of empty plasmid vector pTVG4. Mice were then either boosted on day 5, given that intra-splenic peptide levels peak 125 h post-administration, with either PBS, 100 µg of SIINFEKL peptide, or 100 µg of SIINFEKL peptide + 50 µg pTVG4.41 With each of these treatments, we aimed to replicate the scenario of a single exposure to antigen (PBS boost), repeated exposure to high levels of antigen as with MIP immunization (peptide boost), or repeated exposure to antigen in the presence of an adjuvant as with traditional plasmid immunization (peptide + vector DNA). As seen in Fig. 7B, continuous antigen exposure caused elevated LAG3 expression on cognate CD8+ T cells that could be partially recovered with the provision of an adjuvant effect from co-administration of plasmid DNA. Either higher antigen dose or longer antigen exposure could result in greater signaling through the TCR. Therefore, in order to directly test whether increased TCR stimulation resulted in similar increases in LAG3, we employed previously reported variants of the SIINFEKL peptide, SIIGFEKL, and EIINFEKL, which have been demonstrated to bind H-2kb with similar affinity but have decreased affinity for the OT1 TCR.42 Consistent with our expectations, stimulation of naive OT1 splenocytes with the epitopte variants led to a decrease in LAG3 on CD8+ T cells after 24 h, in a manner proportional to the TCR affinity (Fig. 7C).
Figure 6.
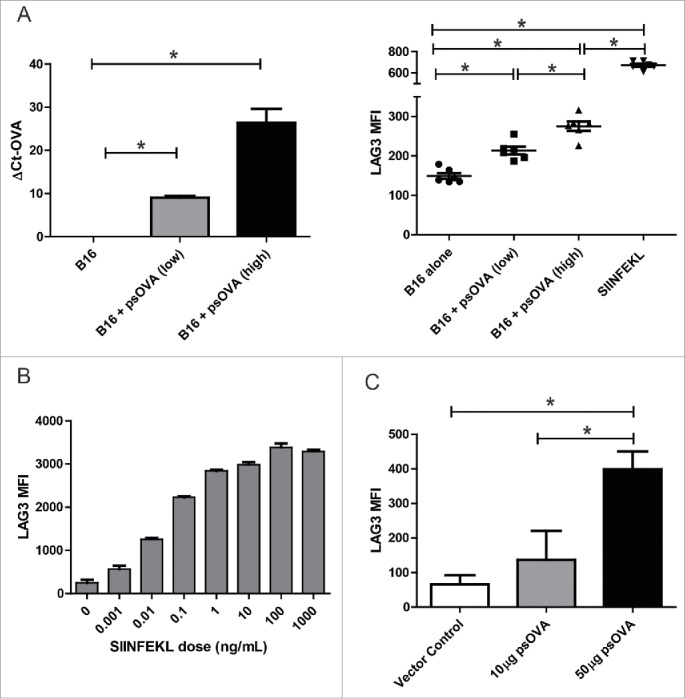
LAG3 expression on CD8+ T cells is linked to antigen dose in vitro and in vivo. (A) B16 melanoma cells were transfected with either a suboptimal (2 µg) or optimal (5 µg) amount of a plasmid expressing chicken ovalbumin (psOVA). One day later, the cells were assayed for levels of OVA mRNA by qPCR (left). Culture supernatant from the transfected cells was used to stimulate OT1 splenocytes in vitro. Levels of LAG3 on the CD3+CD8+ splenocytes stimulated with the different supernatants was assayed 24 h later by flow cytometry (right). (B) OT1 splenocytes were stimulated with different amounts of SIINFEKL peptide and assayed for LAG3 expression 24 h later as above (representative data from three independent experiments). (C) 106 OT1 splenocytes were adoptively transferred into wild type C57BL6 mice (n = 5 per group), and then immunized with either 10 µg or 50 µg of psOVA. Three days later, LAG3 levels on SIINFEKL-specific tetramer+CD3+CD8+ CD44+ cells were assayed by flow cytometry. Given the absence of CD44+ tetramer+ cells in animals given vector control, LAG3 expression on cells from control animals is shown for all SIINFEKL tetramer positive CD8+ T cells. For all panels, * denotes a p-value < 0.05, two-sided t-test.
Figure 7.
LAG3 expression on CD8+ T cells increases with length of antigenic stimulation. (A) Transgenic OT1 splenocytes were stimulated for either 24 h or 72 h with a fixed concentration of SIINFEKL peptide and assayed for LAG3 expression on 4-1BB+ CD8+ T cells by flow cytometry at 72 h (data is representative of three independent experiments, with 5–10 independent samples each). (B) Transgenic OT1 splenocytes were adoptively transferred into wild-type C57BL6 mice (n = 4 to 5 animals per group) and administered a priming immunization of SIINFEKL peptide (100 µg) and pTVG4 (50 µg) 24 h later. Four days after priming, animals were divided into groups and boosted with either PBS, SIINFEKL peptide (100 µg), or SIINFEKL peptide (100 µg) and pTVG4 (50µg). Two days after the booster immunization, levels of LAG3 were assayed on SIINFEKL tetramer+ CD8+ T cells from the spleens of treated mice by flow cytometry. (C) Transgenic OT1 splenocytes were stimulated with either SIINFEKL, EIINFEKL, or SIIGFEKL peptides at 2 µg/mL for 24 h and assayed for LAG3 expression by flow cytometry on CD8+ T cells. For all panels, * denotes a p-value < 0.05, two-sided t-test.
Discussion
Studies with DNA vaccines have demonstrated that detectable immune responses after a single immunization are infrequent, typically requiring multiple booster immunizations. This has led many groups to seek to increase the immunogenicity of DNA vaccines by improving DNA delivery and transgene expression. In preclinical studies, increasing the efficiency of DNA transfection in vivo through electroporation or optimization of the plasmid vector for expression in a mammalian host have led to increases in DNA vaccine immunogenicity and antitumor efficacy in viral models.23,43 Recently, novel minicircle and MIP vector configurations with reduced extra-genic spacer lengths have been reported to mediate greatly enhanced transgene expression in vivo. DNA minicircles have also been shown to elicit greater frequencies of antigen-specific CD8+ T cells when compared to conventional plasmid vaccination. Such an approach would be vastly simpler than employing different delivery agents or electroporation. In this report, we sought to evaluate, for the first time, whether a MIP encoding a tumor-associated antigen was able to elicit a stronger antitumor response relative to the native vaccine upon simple intradermal injection. We demonstrated that MIP vectors used for vaccination elicited: (1) persistent antigen expression in vitro and in vivo; (2) greater numbers of antigen-specific CD8+ T cells; (3) increased expression of LAG3 on cognate CD8+ T cells with attenuated cytokine responses and loss of epitope dominance; and (4) inferior antitumor responses in the absence of LAG3 blockade. Further, we show that LAG3 expression on CD8+ T cells following immunization is associated with antigen dose, length of antigenic stimulation, and possibly loss of an adjuvant effect.
Our studies demonstrate, as expected, that simple changes to the backbone structure of a DNA plasmid lead to an increase in the magnitude and length of transgene expression. Using this type of construct for immunization led to an increase in the magnitude of CD8+ T cells as determined by IFNγ secretion and tetramer staining (Figs. 3A and E). Importantly, we also showed that these CD8+ T cells elicited by MIP vaccination had cytotoxic capacity and protected against tumor cell line challenge when vaccines were administered prophylactically (Fig. 4). These data are consistent with previous reports using DNA minicircles.25,26 For example, Dietz and others have shown that a minicircle expressing ovalbumin produced greater SIINFEKL tetramer+ CD8+ T cells and protected against infection in a modified listeriosis model.26 Previous reports, however, have not examined regulatory marker expression or complete Th1 cytokine expression profiles of minicircle-elicited CD8+ T cell responses, both of which have been shown to be important for antitumor efficacy.29,39,44
Further analyses in our model revealed distinct differences in the “quality” of the immune response between traditional plasmid and MIP vaccination. Given persistent antigen expression, we examined whether there was a difference in expression of checkpoint proteins on antigen-specific CD8+ T cells. We found that a significantly greater proportion of p103 dominant epitope-specific CD8+ T cells from animals receiving the MIP vaccine expressed surface LAG3 (Figs. 3D and E). Interestingly, and unlike what has been observed in models of chronic viral infections, we found these cells to be exclusively LAG3hi and not expressing higher levels of PD1 or TIM3. We also observed increased cytokine responses to the subdominant p41 epitope and attenuated cytokine responses to the dominant p103 epitope, suggesting a loss of epitope dominance (Fig. 3B). On the other hand, MIP vaccination, and not native plasmid immunization, resulted in detectable responses to the subdominant p41 epitope (Fig. 3A). These differences suggest that persistent expression of the antigen led to a LAG3-expressing tolerant phenotype to the dominant epitope, permitting the generation of immunity to a subdominant epitope, in a manner similar to changes in immunodominance patterns that have been observed in viral epitopes upon chronic viral infections.45,46 For example, Wherry and others have shown that chronic lymphocytic choriomeningitis virus infection led to disruption of the normal immunodominance patterns and resulted in hierarchical CD8+ T cell dysfunction.46
We also examined the antitumor efficacy of immune responses elicited by MIP vaccination, the first evaluation of antitumor immunity using minicircle- or MIP-type DNA constructs as vaccines. While MIP vaccination could protect animals from subsequent tumor challenge, it was inferior to conventional plasmid DNA in treating animals with pre-existing tumors (Fig. 4). Upon profiling CD8+ TILs from these animals, we correspondingly found elevated LAG3 expression on their surface. We have similarly reported recently that antigen-specific upregulation of PD1 on CD8+ T cells occurred as a result of epitope optimization, and this led to lower antitumor efficacy due to preferential dysregulation of PD1high CD8+ T cells in the tumor microenvironment.29 This suggests that contrary to the prevailing model about CD8+ T cell exhaustion in which sequential expression of multiple checkpoint proteins is proposed, different CD8+ T cell regulatory pathways can be preferentially induced depending on the magnitude and duration of antigenic stimulation. Further, this study, along with our previous report, highlight that activation- or priming-induced checkpoint marker expression on CD8+ T cells is also important and can significantly influence efficacy in the presence of a pre-existing tumor expressing the cognate inhibitory ligand. In fact, it has recently been shown that galectin-3 binds to LAG3 on terminally differentiated CD8+ T cells only in the tumor microenvironment to cause their dysregulation.47 Similarly, it is conceivable that the LAG3 binding MHCIIhigh CD45+ haematopoietic cells recruited only after tumor formation are primarily responsible for suppression of LAG3 expressing CD8+ T cells. In our model, we have found both high levels of MHCII and galectin-3 in the tumor microenvironment (Fig. 5D and data not shown). Further mechanistic studies to elucidate the precise mechanisms of tumor-mediated dysregulation of LAG3-expressing CD8+ T cells are underway.
Other groups have previously shown that antibody-mediated LAG3 blockade can reverse CD8+ T cell tolerance, either alone or in combination with other checkpoint inhibitors.39,48 Consequently, we examined whether blockade of LAG3 could rescue the antitumor response of MIP vaccination. As seen in Fig. 6, LAG3 blockade was sufficient to restore MIP vaccine efficacy and increased CD8+ T cell infiltration of tumors. LAG3 blockade without antigen-specific vaccination (in combination with pTVG4 vector control) had modest antitumor effects. This is interesting, suggesting that LAG3 blockade might alter the fate of the CD8+ T cell during the initial priming in a manner dissimilar to PD1.40,49 Furthermore, LAG3 blockade in combination with pTVG-SSX2 vaccination also resulted in improved antitumor efficacy as measured by the number of complete tumor regressions, perhaps by improving the function of even lower LAG3-expressing CD8+ TIL in this model. This suggests that DNA vaccination could be generally combined with concurrent LAG3 blockade for improved antitumor benefit. To our knowledge, ours is the first report showing enhancement of the antitumor effect of a vaccine looking to elicit CD8+ T cell responses de novo upon combination with LAG3 blockade alone. However, our observations correspond with a previous report demonstrating that LAG3 blockade mediated enhanced tumor infiltration and effector function of adoptively transferred clonal CD8+ T cells when combined with a viral vaccine.48
Studies of LAG3 induction on CD8+ T cells have previously reported a link between antigen dose and LAG3 levels following peptide immunization, and the activation of innate immunity.37 In Fig. 6, we show that treatment with increasing doses of ovalbumin encoding plasmid DNA in both an in vitro model of cross priming and an in vivo immunization setting similarly resulted in higher LAG3 expression on cognate CD8+ T cells. The duration of antigen stimulation (Fig. 7) similarly contributed to persistent LAG3 expression. Moreover, we found that boosting rapidly with SIINFEKL peptide without an adjuvant resulted in high LAG3 expression on adoptively transferred OT1 CD8+ T cells that had been previously primed with antigen. We also found that provision of plasmid DNA itself as an adjuvant caused a modest but significant reduction of LAG3 levels on CD8+ T cells. These data suggest that both antigen levels and duration of antigenic stimulation determine LAG3 expression, which in turn can be modulated by the presence of an adjuvant. Whether stronger adjuvants would be more effective in reducing LAG3 expression is currently unknown, but these findings suggest that LAG3 expression could be associated with inflammation-induced production of ADAM proteins that can cleave surface expression of LAG3 on T cells.37,50
While our findings are consistent with the well documented role of LAG3 as a negative regulator of CD8+ T cell function as described above, it could be viewed to be in contrast to reports describing the strong adjuvant activity of soluble LAG3 fused to the Fc portion of IgG molecules (LAG3-Ig).51,52 In combination with LAG3-Ig, a DNA vaccine encoding Her-2/neu was able to better protect against an autochthonous breast tumor.51 Similarly, Kano et al. demonstrated the utility of LAG3-Ig as an adjuvant in enhancing antitumor responses upon peptide vaccination in the presence of Poly I:C.52 Mechanistic studies have found that LAG3-Ig is able to mediate DC activation and maturation through its binding to MHC-II, and it is currently assumed that this is the primary mechanism of immune stimulation.53 Our studies suggest that an alternative mechanism may be that LAG3 signaling on antigen primed CD8+ T cells is inhibited by saturation of LAG3 binding partners following LAG3-Ig administration, similar to the use of a LAG3-specific blocking antibody. This hypothesis is further strengthened by observations that LAG3-Ig is able to bind more strongly to MHC-II than native LAG3.54,55
Combining our findings with those from relevant previous reports, we propose a model (Fig. 8) for induction of antigen-specific LAG3, and its effects on antigen-specific tolerance after vaccination. As has been previously reported, LAG3 expression is proportional to the magnitude of antigen presented, and a short pulse of antigen expression (Fig. 8A) is likely to cause only transient expression of LAG3.37 Combining vaccines with certain adjuvants is likely to further reduce the magnitude of this initial LAG3 upregulation, and the increased benefit obtained from combining even traditional plasmid vaccination with LAG3 blockade suggests that this reduction might be beneficial for induction of cellular immunity. Further, the success of electroporation approaches with plasmid DNA vaccines are probably due to such pulsatile antigen expression along with a strong adjuvant effect, with electroporation causing an increase in magnitude but not the duration of antigen expression.23 As demonstrated in our results, however, vaccination schemes leading to persistence of antigen expression in a non-inflammatory environment (Fig. 8B) are likely to cause prolonged LAG3 expression on antigen-specific CD8+ T cells that ultimately leads to tolerance. Periodic administration of an adjuvant in such a setting might help elucidate whether antigen persistence in an inflammatory environment could delay or abrogate CD8+ T cell tolerance. However, in studies involving chronic infection with an influenza virus, Bucks and others have found that chronic antigen exposure is alone sufficient to drive CD8+ T cell exhaustion in a PD1-independent manner.38 Hailemichel and others have similarly reported that persistent antigen exposure at the site of peptide vaccination through the use of incomplete Freund's adjuvant (IFA), a common formulation that has been extensively tested in human trials, causes CD8+ T cell dysfunction and sequestration.56 It is conceivable that LAG3 blockade in such settings might restore effective Th1 immunity.
Figure 8.
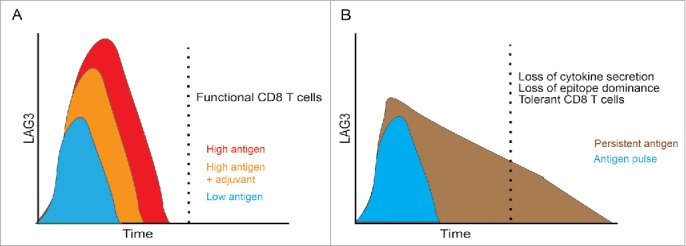
Model: Increased antigen dose and duration of presentation lead to increased LAG3 expression and a tolerogenic phenotype. (A) Traditional model in which LAG3 expression is transient with vaccination, and increases with high antigen dose. Addition of an appropriate adjuvant may reduce the initial upregulation of LAG3. (B) Vaccination schemes that mediate persistent expression of antigen in a non-inflammatory environment (such as mini-intronic plasmids) result in sustained LAG3 expression on CD8+ T cells, which is in turn associated with loss of effector function and a tolerant phenotype.
Our results further open the possibility for minicircle and mini-intronic plasmid vectors as a means of inducing antigen-specific tolerance. In fact, repeated administration of low doses of plasmid DNA encoding myelin basic protein (MBP) as a means of inducing antigen-specific tolerance has been evaluated in patients with multiple sclerosis in a phase II clinical trial.57 Use of MIP constructs encoding relevant antigens might therefore create opportunities for improved tolerance induction in autoimmune disease settings such as multiple sclerosis or type I diabetes.
Together, our results suggest that the LAG3 receptor may be involved in attenuation of functional Th1 responses upon antitumor vaccination, especially when employing methods that increase antigen dose and persistence. This is of high relevance to the design of antitumor vaccines, as well as the use of gene delivery approaches to treat autoimmune diseases. In the case of antitumor DNA vaccines, vaccination in combination with LAG3 blockade is likely to improve immunogenicity and antitumor efficacy, and combination approaches in human clinical trials are eagerly anticipated.
Materials and methods
Mice and cell lines
HLA-A2.01/HLA-DRI expressing, murine MHC class I/II knockout, mice (HHDII-DRI, HLA-A2/DR1) on a C57/BL6 background were obtained from Charles River Labs courtesy of Dr Francois Lemonnier (Institut Pasteur, Paris, France). Mice were maintained under aseptic conditions and all experiments were conducted under an IACUC-approved protocol. Male HHDII-DRI mice were bred with female C57/BL6J mice (Jackson Laboratories) to give heterologous mice with a full complement of immune receptors that were used in all the studies described. Wild-type FVB and OT1 CD8+ transgenic mice were obtained from Jackson laboratories.
The A2/Sarcoma cell line expressing SSX2 (MCA-SSX2) was generated as previously described.29 LNCaP and COS7 cells were grown in RPMI 1640 media supplemented with 2% fetal calf serum (FCS; Invitrogen). Cell lines were obtained from American Type Culture Collection (ATCC), verified using polyphasic testing to confirm identity (DDC Biomedical, Fairfleid, OH), and passaged for less than 6 mo.
DNA constructs
A DNA vaccine encoding SSX2 (pTVG-SSX2) was constructed as previously described.27,28 A minicircle encoding SSX2 (DMC-SSX2) was prepared from a parent minicircle-producing vector, pMC-SSX2, using standard methods as per the manufacturer's instructions (System Biosciences). pMC-SSX2 itself was prepared by cloning the complete transgene expression cassette from pTVG-SSX2 into the MN501A1 pMC.CMV-MCS-SV40polyA vector (System Biosciences) containing the minicircle production and bacterial backbone elements using the Polymerase Incomplete Primer Extension cloning method using the following primer pairs: TCAATTCACTAGTCGCGCCCG (vector forward), CTCTAGCTATCCCGCCCCTAACT (vector reverse), and GCGCGACTAGTGAATTGACGTTACATAACTTACGGTAAATGGCCCG (insert forward), GGGCGGGATAGCTAGAGTTTACGGTTCCTGGCCTTTTGCTG (insert reverse).58 MIP-SSX2 was constructed and propagated by the inclusion of an optimized intron (OIPR) in an Eco-RV restriction site upstream of the transgene coding region.20 DMC-EGFP was produced from a parent pMC-EGFP vector as above.
Plasmid encoding full length secreted chicken egg ovalbumin (pCI-neo-sOVA (psOVA)) was a gift from Maria Castro (Addgene plasmid #25098, Cambridge, MA).
Endotoxin free DNA constructs for transfection and immunization were isolated using the ZymoPURE Giga Plasmid Prep Kits (Zymo Research Corporation, Irvine, CA).
Immunization and transgene expression studies
Six- to ten-week old HLA-A2/DR1 mice were immunized with plasmid DNA intradermally in the ear pinna as previously reported.27,28 For tumor protection studies, mice were immunized four times biweekly with the different constructs: pTVG4 (100 µg), pTVG-SSX2 (100 µg), or MIP-SSX2 (83 µg) and challenged with 105 A2/Sarc SSX2 cells administered subcutaneously.29 For tumor therapy studies, animals were first inoculated with 5 × 104 tumor cells followed by bi-weekly immunizations beginning day 1 after tumor implantation. In studies using LAG3 blockade, 200 µg of the antibody (clone C9B7W; BioXCell, West Lebanon, NH) was administered intraperitoneally on days 1 and 3 post-immunization.
For in vivo transgene expression studies, age-matched groups of three female FVB mice (per construct, per time point) were injected with either pTVG-SSX2 (50 µg/mouse) or MIP-SSX2 (41.5 µg/mouse) via tail vein injection in 2.0 mL of 0.9% NaCl solution. RNA from liver tissue was extracted using the RNA DirectZol Mini-Prep Plus kit (Zymo Research) and qPCR (SSoFast qPCR mix, BioRad, Hercules, CA) for SSX2 mRNA was performed using the primer pair: CATCTTCCTCAGGGTCGCTGATCTC (forward) and CCACAAAATGATGGGAAAGAGCTGTG (reverse). For in vitro expression studies, the indicated cell lines were transfected using Lipofectamine LTX with PLUS reagent (Life Technologies, Carlsbad, CA) and analyzed by the indicated method—fluorescence microscopy, flow cytometry or ELISA.
For adoptive transfer experiments, age-matched female C57BL6 mice were inoculated with 106 OT1 splenocytes intraperitoneally and treated as described. SIINFEKL peptide for immunization was obtained from Lifetein (Somerset, NJ).
Immunological analyses
Tumor cell suspensions were obtained as previously described, 29 and stained with anti-CD3 (17A2; BD Biosciences), anti-CD8+ (53-6.7; BD Biosciences), anti-PD1 (J43; BD Biosciences), anti-LAG3 (C9B7W; eBioscience (San Diego, CA)), anti-TIM3 (8B.2C12; eBioscience), anti-CD45 (30-F11; BD Biosciences), anti-I-Ab (HM-48.1; BD Biosciences), and live-dead ghost 780 (Tonbo biosciences, San Diego, CA). For intracellular cytokine staining, splenocytes were stimulated with the relevant peptide for 2 h, followed by treatment with monensin (GolgiStop, BD Biosciences) for 4 h. Cells were then stained for surface markers as above, followed by fixation, permeabilization (Cytofix/Cytoperm, BD Biosciences) and cytokine staining: IFNγ (XMG1.2; BD Biosciences), TNFα (MP6-XT22; BD Biosciences), IL2 (JES6-5H4; eBioscience). Where indicated, CD8+ T cells were stained with tetramers specific for the p41, p103, or SIINFEKL epitopes (NIH Tetramer Core Facility, Emory, GA).
For in vitro stimulation experiments, wild-type or OTI transgenic splenocytes were incubated with either SIINFEKL, EIINFEKL, or SIIGFEKL (2 µg/mL; Lifetein) peptide for 24 h or 72 h. Samples were stained and analyzed as above. Additional CD44 staining in in vivo experiments involving OT1 adoptive transfer was performed with clone IM7 (BD Biosciences).
Interferon (IFN)-γ enzyme linked immunosorbent spot (ELISPOT) assays were performed according to the manufacturer's instructions (R&D Systems, Minneapolis, MN) as previously described.27,28,59 Enzyme linked immunosorbent assay (ELISA) for SSX2 was performed using standard methods, and the following reagents: 10 antibody 1A4 (Abnova, Heidelberg, Germany), 20 antibody Rabbit polyclonal (Abnova).
Immunohistochemistry for CD8α was performed on FFPE tumor tissue after antigen retrieval in citrate buffer (pH 6.0) prior to staining with a 10 rat-anti-mouse CD8α antibody (4SM15; eBioscience) and a 20 anti-rat IgG (MP-444; Vector, Burlingame, CA). Standard development with DAB and hematoxylin counter-staining was performed.
In vitro cytotoxicity assays were performed using ex vivo restimulated splenocytes and the CytoTox 96® Non-Radioactive Cytotoxicity Assay Kit (Promega, Madison, WI) as per the manufacturer's instructions.
Statistical analysis
All data are representative of at least three independent experiments, with data in each figure from one representative experiment. No outliers were excluded from analyses. The Mann–Whitney test was used for comparing group MFI values for flow cytometry data. For all other analyses, comparisons were made using a two-sided t test, assuming unequal variance. In all studies, a p < 0.05 was used to define a significant difference. All statistical analyses were performed using GraphPad Prism (version 5.0).
Supplementary Material
Disclosure of potential conflicts of interest
DGM has ownership interest, receives research support, and serves as consultant to Madison Vaccines, Inc., that has licensed material related to this report.
Acknowledgments
The authors thank the NIH Tetramer Facility (Atlanta, GA) for tetramer reagents, the UWCCC Flow Cytometry core facility (and NIH small instrument grants 1S10RR025483-01 and 1S100OD018202-01), the UWCCC Experimental Pathology facility for technical support, Mr. Reinier Hernandez for assistance with tail-vein injections, and Mr. Adam Bayless for assistance with PIPE cloning.
Funding
This work was supported by the Prostate Cancer Foundation 2014 Movember-PCF Global Treatment Sciences Challenge Award and by NIH R01-CA142608.
Author contributions
VTC conceived the project, designed and performed the experiments, analyzed the data, and prepared the manuscript. CDZ assisted in experiment design and execution. DGM supervised the concept, experimental design, data analysis, and manuscript preparation.
References
- 1.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science 1990; 247:1465-8; PMID:1690918; http://dx.doi.org/ 10.1126/science.1690918 [DOI] [PubMed] [Google Scholar]
- 2.Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther 1999; 10:1735-7; PMID:10428218; http://dx.doi.org/ 10.1089/10430349950017734 [DOI] [PubMed] [Google Scholar]
- 3.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev 2011; 239:62-84; PMID:21198665; http://dx.doi.org/ 10.1111/j.1600-065X.2010.00980.x [DOI] [PubMed] [Google Scholar]
- 4.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 1993; 259:1745-9; PMID:8456302; http://dx.doi.org/ 10.1126/science.8456302 [DOI] [PubMed] [Google Scholar]
- 5.Colluru VT, Johnson LE, Olson BM, McNeel DG. Preclinical and clinical development of DNA vaccines for prostate cancer. Urol Oncol Semin Orig Investig 2016; 34:193-204; PMID:24332642; http://dx.doi.org/ 10.1016/j.urolonc.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowler V, Robinson L, Bankowski B, Cox S, Parida S, Lawlor C, Gibson D, O'Brien F, Ellefsen B, Hannaman D et al.. A DNA vaccination regime including protein boost and electroporation protects cattle against foot-and-mouth disease. Antiviral Res 2012; 94:25-34; PMID:22330893; http://dx.doi.org/ 10.1016/j.antiviral.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 7.Vandermeulen G, Richiardi H, Escriou V, Ni J, Fournier P, Schirrmacher V, Scherman D, Préat V. Skin-specific promoters for genetic immunisation by DNA electroporation. Vaccine 2009; 27:4272-7; PMID:19450641; http://dx.doi.org/ 10.1016/j.vaccine.2009.05.022 [DOI] [PubMed] [Google Scholar]
- 8.Hon H, Oran A, Brocker T, Jacob J. B lymphocytes participate in cross-presentation of antigen following gene gun vaccination. J Immunol 2005; 174:5233-42; PMID:15843519; http://dx.doi.org/ 10.4049/jimmunol.174.9.5233 [DOI] [PubMed] [Google Scholar]
- 9.Ito K, Ito K, Shinohara N, Kato S. DNA immunization via intramuscular and intradermal routes using a gene gun provides different magnitudes and durations on immune response. Mol Immunol 2003; 39:847-54; PMID:12686500; http://dx.doi.org/ 10.1016/S0161-5890(03)00024-5 [DOI] [PubMed] [Google Scholar]
- 10.Lauterbach H, Gruber A, Ried C, Cheminay C, Brocker T. Insufficient APC capacities of dendritic cells in gene gun-mediated DNA vaccination. J Immunol 2006; 176:4600-7; PMID:16585550; http://dx.doi.org/ 10.4049/jimmunol.176.8.4600 [DOI] [PubMed] [Google Scholar]
- 11.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med 1998; 188:1075-82; PMID:9743526; http://dx.doi.org/ 10.1084/jem.188.6.1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen-Hoai T, Kobelt D, Hohn O, Vu MD, Schlag PM, Dörken B, Norley S, Lipp M, Walther W, Pezzutto A et al.. HER2/neu DNA vaccination by intradermal gene delivery in a mouse tumor model: Gene gun is superior to jet injector in inducing CTL responses and protective immunity. Oncoimmunology 2012; 1:1537-45; PMID:23264900; http://dx.doi.org/ 10.4161/onci.22563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amante DH, Smith TRF, Mendoza JM, Schultheis K, McCoy JR, Khan AS, Sardesai NY, Broderick KE. Skin transfection patterns and expression kinetics of electroporation-enhanced plasmid delivery using the CELLECTRA-3P, a Portable next-generation dermal electroporation device. Hum Gene Ther Methods 2015; 26:134-46; PMID:26222896; http://dx.doi.org/ 10.1089/hgtb.2015.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi T, Huang M, Gorman C, Jaenisch R. A generic intron increases gene expression in transgenic mice. Mol Cell Biol 1991; 11:3070-4; PMID:2038318; http://dx.doi.org/ 10.1128/MCB.11.6.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.André S, Seed B, Eberle J, Schraut W, Bültmann A, Haas J. Increased Immune Response Elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usages. J Virol 1998; 72:1497-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagata T, Uchijima M, Yoshida A, Kawashima M, Koide Y. Codon optimization effect on translational efficiency of DNA vaccine in mammalian cells: analysis of plasmid DNA encoding a CTL epitope derived from microorganisms. Biochem Biophys Res Commun 1999; 261:445-51; PMID:10425204; http://dx.doi.org/ 10.1006/bbrc.1999.1050 [DOI] [PubMed] [Google Scholar]
- 17.Jechlinger W. Optimization and delivery of plasmid DNA for vaccination. Expert Rev Vaccines 2006; 5:803-25; PMID:17184219; http://dx.doi.org/ 10.1586/14760584.5.6.803 [DOI] [PubMed] [Google Scholar]
- 18.Xu Z-L, Mizuguchi H, Ishii-Watabe A, Uchida E, Mayumi T, Hayakawa T. Optimization of transcriptional regulatory elements for constructing plasmid vectors. Gene 2001; 272:149-56; PMID:11470520; http://dx.doi.org/ 10.1016/S0378-1119(01)00550-9 [DOI] [PubMed] [Google Scholar]
- 19.Chen Z-Y, He C-Y, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther J Am Soc Gene Ther 2003; 8:495-500; PMID:12946323; http://dx.doi.org/21649506 10.1016/S1525-0016(03)00168-0 [DOI] [PubMed] [Google Scholar]
- 20.Lu J, Zhang F, Kay MA. A Mini-intronic Plasmid (MIP): A novel robust transgene expression vector in vivo and in vitro. Mol Ther [Internet] 2013. [cited 2013March6]; Available from: http://www.nature.com/mt/journal/vaop/ncurrent/full/mt201333a.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu J, Zhang F, Xu S, Fire AZ, Kay MA. The extragenic spacer length between the 5|[prime]| and 3|[prime]| Ends of the transgene expression cassette affects transgene silencing from plasmid-based vectors. Mol Ther [Internet] 2012. [cited 2012September21]; Available from: http://www.nature.com/mt/journal/vaop/ncurrent/full/mt201265a.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roos A-K, Eriksson F, Timmons JA, Gerhardt J, Nyman U, Gudmundsdotter L, Bråve A, Wahren B, Pisa P. Skin electroporation: Effects on transgene expression, DNA persistence and local tissue environment. PLoS ONE [Internet] 2009. [cited 2016March14]; 4 Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2748717/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee IH, Park J-B, Cheong M, Choi YS, Park D, Sin J-I. Antitumor therapeutic and antimetastatic activity of electroporation-delivered human papillomavirus 16 E7 DNA vaccines: a possible mechanism for enhanced tumor control. DNA Cell Biol 2011; 30:975-85; PMID:21649506; http://dx.doi.org/ 10.1089/dna.2011.1266 [DOI] [PubMed] [Google Scholar]
- 24.Stacey KJ, Ross IL, Hume DA. Electroporation and DNA-dependent cell death in murine macrophages. Immunol Cell Biol 1993; 71 (Pt 2):75-85; PMID:8486399; http://dx.doi.org/ 10.1038/icb.1993.8 [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Jiang W, Chen Y, Liu P, Sheng C, Chen S, Zhang H, Pan C, Gao S, Huang W. In Vivo Electroporation of Minicircle DNA as a Novel Method of Vaccine Delivery To Enhance HIV-1-Specific Immune Responses. J Virol 2014; 88:1924-34; PMID:24284319; http://dx.doi.org/24492013 10.1128/JVI.02757-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietz WM, Skinner NEB, Hamilton SE, Jund MD, Heitfeld SM, Litterman AJ, Hwu P, Chen Z-Y, Salazar AM, Ohlfest JR et al.. Minicircle DNA is Superior to Plasmid DNA in Eliciting Antigen-specific CD8+ T-cell Responses. Mol Ther [Internet] 2013. [cited 2013May22]; Available from: http://www.nature.com/mt/journal/vaop/ncurrent/full/mt201385a.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith HA, McNeel DG. Vaccines targeting the cancer-testis antigen SSX-2 elicit HLA-A2 epitope-specific cytolytic T cells. J Immunother Hagerstown Md 1997 2011; 34:569-80; PMID:21904219; http://dx.doi.org/24492013 10.1097/CJI.0b013e31822b5b1d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith HA, Rekoske BT, McNeel DG. DNA vaccines encoding altered peptide ligands for SSX2 enhance epitope-specific CD8+ T-cell immune responses. Vaccine 2014; 32:1707-15; PMID:24492013; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG. PD-1 or PD-L1 blockade restores antitumor efficacy following SSX2 Epitope-modified DNA vaccine immunization. Cancer Immunol Res 2015; 3:946-55; PMID:26041735; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith HA, Cronk RJ, Lang JM, McNeel DG. Expression and immunotherapeutic targeting of the SSX family of cancer-testis antigens in prostate cancer. Cancer Res 2011; 71:6785-95; PMID:21880588; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2127 [DOI] [PubMed] [Google Scholar]
- 31.Smith HA, McNeel DG. Vaccines targeting the cancer-testis antigen SSX-2 elicit HLA-A2 epitope-specific cytolytic T cells. J Immunother Hagerstown Md 1997 2011; 34:569-80; PMID:21904219; http://dx.doi.org/19559109 10.1097/CJI.0b013e31822b5b1d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luke J, Carnes AE, Hodgson CP, Williams JA. Improved antibiotic-free DNA vaccine vectors utilizing a novel RNA based plasmid selection system. Vaccine 2009; 27:6454-9; PMID:19559109; http://dx.doi.org/ 10.1016/j.vaccine.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maniar LEG, Maniar JM, Chen Z-Y, Lu J, Fire AZ, Kay MA. Minicircle DNA vectors achieve sustained expression reflected by active chromatin and transcriptional level. Mol Ther [Internet] 2012. [cited 2012November29]; Available from: http://www.nature.com/mt/journal/vaop/ncurrent/full/mt2012244a.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobelt D, Schleef M, Schmeer M, Aumann J, Schlag PM, Walther W. Performance of high quality minicircle DNA for in vitro and in vivo gene transfer. Mol Biotechnol 2013; 53:80-9; PMID:22467123; http://dx.doi.org/ 10.1007/s12033-012-9535-6 [DOI] [PubMed] [Google Scholar]
- 35.Darquet AM, Rangara R, Kreiss P, Schwartz B, Naimi S, Delaére P, Crouzet J, Scherman D. Minicircle: an improved DNA molecule for in vitro and in vivo gene transfer. Gene Ther 1999; 6:209-18; PMID: 10435105; AMBIGUOUShttp://dx.doi.org/10.1038/sj.gt.3300816 [DOI] [PubMed] [Google Scholar]
- 36.Alves CPA, Šimčíková M, Brito L, Monteiro GA, Prazeres DMF. Development of a nicking endonuclease-assisted method for the purification of minicircles. J Chromatogr A [Internet] [cited 2016March28]; Available from: http://www.sciencedirect.com/science/article/pii/S0021967316303004; PMID:AMBIGUOUS [DOI] [PubMed] [Google Scholar]
- 37.Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen H-R, Pyle KJ, Hipkiss E, Vignali DAA, Pardoll DM, Drake CG. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol 2009; 182:6659-69; PMID:19454660; http://dx.doi.org/ 10.4049/jimmunol.0804211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bucks CM, Norton JA, Boesteanu AC, Mueller YM, Katsikis PD. Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. J Immunol Baltim Md 1950 2009; 182:6697-708; PMID:1945466; http://dx.doi.org/19043418 10.4049/jimmunol.0800997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DAA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009; 10:29-37; PMID:19043418; http://dx.doi.org/ 10.1038/ni.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldberg MV, Drake CG. LAG-3 in Cancer Immunotherapy. Curr Top Microbiol Immunol 2011; 344:269-78; PMID:21086108; http://dx.doi.org/ 10.1007/82_2010_114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weijzen S, Meredith SC, Velders MP, Elmishad AG, Schreiber H, Kast WM. Pharmacokinetic differences between a T Cell-Tolerizing and a T cell-activating peptide. J Immunol 2001; 166:7151-7; PMID:11390461; http://dx.doi.org/ 10.4049/jimmunol.166.12.7151 [DOI] [PubMed] [Google Scholar]
- 42.Denton AE, Wesselingh R, Gras S, Guillonneau C, Olson MR, Mintern JD, Zeng W, Jackson DC, Rossjohn J, Hodgkin PD et al.. Affinity thresholds for naive CD8+ CTL activation by peptides and engineered influenza a viruses. J Immunol 2011; 187:5733-44; PMID:22039305; http://dx.doi.org/ 10.4049/jimmunol.1003937 [DOI] [PubMed] [Google Scholar]
- 43.Kim MS, Sin J-I. Both antigen optimization and lysosomal targeting are required for enhanced anti-tumour protective immunity in a human papillomavirus E7-expressing animal tumour model. Immunology 2005; 116:255-66; PMID:16162274; http://dx.doi.org/ 10.1111/j.1365-2567.2005.02219.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med 2005; 11:1113-7; PMID:16186817; http://dx.doi.org/ 10.1038/nm1293 [DOI] [PubMed] [Google Scholar]
- 45.van der Most RG, Murali-Krishna K, Lanier JG, Wherry EJ, Puglielli MT, Blattman JN, Sette A, Ahmed R. Changing immunodominance patterns in antiviral CD8 T-cell responses after loss of epitope presentation or chronic antigenic stimulation. Virology 2003; 315:93-102; PMID:14592762; http://dx.doi.org/ 10.1016/j.virol.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 46.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 2003; 77:4911-27; PMID:12663797; http://dx.doi.org/ 10.1128/JVI.77.8.4911-4927.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kouo T, Huang L, Pucsek AB, Cao M, Solt S, Armstrong T, Jaffee E. Galectin-3 shapes antitumor immune responses by suppressing CD8+ T Cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol Res 2015; 3:412-23; PMID:25691328; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC et al.. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest 2007; 117:3383-92; PMID:17932562; http://dx.doi.org/ 10.1172/JCI31184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sierro S, Romero P, Speiser DE. The CD4-like molecule LAG-3, biology and therapeutic applications. Expert Opin Ther Targets 2011; 15:91-101; PMID:21142803; http://dx.doi.org/ 10.1517/14712598.2011.540563 [DOI] [PubMed] [Google Scholar]
- 50.Li N, Wang Y, Forbes K, Vignali KM, Heale BS, Saftig P, Hartmann D, Black RA, Rossi JJ, Blobel CP et al.. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J 2007; 26:494-504; PMID:17245433; http://dx.doi.org/ 10.1038/sj.emboj.7601520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cappello P, Triebel F, Iezzi M, Caorsi C, Quaglino E, Lollini P-L, Amici A, Carlo ED, Musiani P, Giovarelli M et al.. LAG-3 enables DNA vaccination to persistently prevent mammary carcinogenesis in HER-2/neu transgenic BALB/c Mice. Cancer Res 2003; 63:2518-25; PMID:12750275 [PubMed] [Google Scholar]
- 52.Kano Y, Iguchi T, Matsui H, Adachi K, Sakoda Y, Miyakawa T, Doi S, Hazama S, Nagano H, Ueyama Y et al.. Combined adjuvants of poly(I:C) plus LAG-3-Ig improve antitumor effects of tumor-specific T cells, preventing their exhaustion. Cancer Sci 2016; 107:398-406; PMID:27079438; http://dx.doi.org/ 10.1111/cas.12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andreae S, Buisson S, Triebel F. MHC class II signal transduction in human dendritic cells induced by a natural ligand, the LAG-3 protein (CD223). Blood 2003; 102:2130-7; PMID:12775570; http://dx.doi.org/ 10.1182/blood-2003-01-0273 [DOI] [PubMed] [Google Scholar]
- 54.Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, Flores M, Li N, Schweighoffer E, Greenberg S et al.. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol 2008; 180:5916-26; PMID:18424711; http://dx.doi.org/ 10.4049/jimmunol.180.9.5916 [DOI] [PubMed] [Google Scholar]
- 55.Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DAA. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3). Eur J Immunol 2002; 32:2255-63; PMID:12209638; http://dx.doi.org/ 10.1002/1521-4141(200208)32:8%3c2255::AID-IMMU2255%3e3.0.CO;2-A [DOI] [PubMed] [Google Scholar]
- 56.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang X-F, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W et al.. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med [Internet] 2013. [cited 2013March11]; advance online publication. Available from: http://www.nature.com/nm/journal/vaop/ncurrent/abs/nm.3105.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bar-Or A, Vollmer T, Antel J, Arnold DL, Bodner CA, Campagnolo D, Gianettoni J, Jalili F, Kachuck N, Lapierre Y et al.. INduction of antigen-specific tolerance in multiple sclerosis after immunization with dna encoding myelin basic protein in a randomized, placebo-controlled phase 1/2 trial. Arch Neurol 2007; 64:1407-15; PMID:17698695; http://dx.doi.org/ 10.1001/archneur.64.10.nct70002 [DOI] [PubMed] [Google Scholar]
- 58.Klock HE, Koesema EJ, Knuth MW, Lesley SA. Combining the polymerase incomplete primer extension method for cloning and mutagenesis with microscreening to accelerate structural genomics efforts. Proteins Struct Funct Bioinforma 2008; 71:982-994; PMID:18004753; http://dx.doi.org/20140431 10.1002/prot.21786 [DOI] [PubMed] [Google Scholar]
- 59.Olson BM, Frye TP, Johnson LE, Fong L, Knutson KL, Disis ML, McNeel DG. HLA-A2-restricted T-cell epitopes specific for prostatic acid phosphatase. Cancer Immunol Immunother CII 2010; 59:943-53; PMID:20140431; http://dx.doi.org/ 10.1007/s00262-010-0820-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.