Abstract
Purpose
Obinutuzumab (GA101), a novel glycoengineered type II anti-CD20 monoclonal antibody, demonstrated responses in single-arm studies of patients with relapsed/refractory non-Hodgkin lymphoma. This is the first prospective, randomized study comparing safety and efficacy of obinutuzumab with rituximab in relapsed indolent lymphoma. The primary end point of this study was the overall response rate (ORR) in patients with follicular lymphoma after induction and safety in patients with indolent lymphoma.
Patients and Methods
A total of 175 patients with relapsed CD20+ indolent lymphoma requiring therapy and with previous response to a rituximab-containing regimen were randomly assigned (1:1) to four once-per-week infusions of either obinutuzumab (1,000 mg) or rituximab (375 mg/m2). Patients without evidence of disease progression after induction therapy received obinutuzumab or rituximab maintenance therapy every 2 months for up to 2 years.
Results
Among patients with follicular lymphoma (n = 149), ORR seemed higher for obinutuzumab than rituximab (44.6% v 33.3%; P = .08). This observation was also demonstrated by a blinded independent review panel that measured a higher ORR for obinutuzumab (44.6% v 26.7%; P = .01). However, this difference did not translate into an improvement in progression-free survival. No new safety signals were observed for obinutuzumab, and the incidence of adverse events was balanced between arms, with the exception of infusion-related reactions and cough, which were higher in the obinutuzumab arm.
Conclusion
Obinutuzumab demonstrated a higher ORR without appreciable differences in safety compared with rituximab. However, the clinical benefit of obinutuzumab in this setting remains unclear and should be evaluated within phase III trials.
INTRODUCTION
Rituximab, a chimeric type I anti-CD20 monoclonal antibody (mAb), has improved outcomes for patients with aggressive and indolent B-cell non-Hodgkin lymphoma (NHL), as well as chronic lymphocytic leukemia (CLL).1–9 Rituximab plus chemotherapy has improved response rates and progression-free survival (PFS) in both the first-line and relapsed/refractory settings compared with chemotherapy alone and has improved overall survival in previously untreated patients.1,7,10–12 Approximately 60% of patients with previously untreated indolent lymphoma and 40% to 50% of those with relapsed/refractory disease respond to rituximab monotherapy.13–17 Furthermore, maintenance therapy with rituximab in treatment responders significantly prolongs remission in patients with follicular lymphoma (FL).5,8,18,19 Despite these improvements, advanced-stage indolent lymphoma remains incurable, and attempts to improve the therapeutic benefit of targeting the CD20 surface antigen are rational.
Anti-CD20 mAbs are classified on the basis of their modes of action and CD20-binding properties.20–23 Type I mAbs such as rituximab induce complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity (ADCC), and direct cellular effects that lead to apoptosis. The extent to which each mode of action contributes to clinical efficacy is unclear. Conversely, type II mAbs such as obinutuzumab are potent inducers of ADCC but only weakly induce complement activation. Type II mAbs also induce nonapoptotic direct cell death,20,24–26 which is not observed with type I mAbs. Obinutuzumab is a novel type II anti-CD20 mAb that is designed to achieve enhanced therapeutic activity relative to rituximab.27
In preclinical studies, obinutuzumab exhibited superior activity relative to rituximab,26–28 with increased direct cell death induction, increased ADCC, enhanced B-cell depletion from whole human blood and lymphoid tissues of nonhuman primates,27,29 and increased antitumor activity in human xenograft models.27,28 A phase I study in patients with heavily pretreated lymphoma demonstrated that obinutuzumab (50 to 2,000 mg administered once every 3 weeks for eight cycles) had an acceptable safety profile, with responses in 43% of patients.30 The phase I component of the GAUSS study evaluated obinutuzumab monotherapy (200 to 2,000 mg administered as a once-per-week dose for four consecutive weeks, followed by maintenance treatment administered every 3 months for 2 years) in patients with heavily pretreated relapsed/refractory lymphoma or CLL.31 No maximum-tolerated dose was identified; an overall response rate (ORR) of 38% was observed, with a 15% response rate in rituximab-refractory patients.31 On the basis of pharmacokinetic (PK) data and results from phase I30,31 and II32,33 dose-finding studies, a flat dose of obinutuzumab 1,000 mg was selected for further evaluation. We performed a randomized phase II study to compare the safety and clinical activity of obinutuzumab with rituximab in patients with relapsed CD20+ indolent lymphoma.
PATIENTS AND METHODS
Study Design
This was an open-label, multicenter, randomized, phase II study comparing induction with obinutuzumab versus rituximab monotherapy in patients with relapsed CD20+ indolent lymphoma followed by 2 years of maintenance therapy in nonprogressing patients. The primary end point was investigator-assessed ORR to induction treatment in the FL population. Secondary end points included ORR in the non-FL population, complete response (CR) and partial response rates, PFS, and safety. The decision to incorporate a blinded response assessment by an independent review facility (IRF) was made shortly after trial initiation and before any response assessment to better guide phase III trial planning. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation guidelines for Good Clinical Practice. The protocol was approved by the ethics committees of participating centers and was registered at ClinicalTrials.gov.
Patient Eligibility
Eligible patients had histologically confirmed CD20+ indolent lymphoma (FL grade 1 to 3a, marginal zone lymphoma [MZL], lymphoplasmacytic lymphoma [LPL], or small lymphocytic lymphoma [SLL]) with one or more bidimensionally measurable lesions (> 1.5 cm) and an indication for therapy. Patients had received at least one previous rituximab-containing regimen with a documented response lasting ≥ 6 months after completion of the last rituximab dose. Additional key entry criteria included age ≥ 18 years, Eastern Cooperative Oncology Group performance status of 0 to 2, and life expectancy of more than 12 weeks. Key exclusion criteria were CNS lymphoma, evidence of transformation, hepatitis B or C or HIV positivity, and significantly abnormal laboratory values. All patients provided written informed consent.
Initial Staging and Laboratory Assessments
Archival or fresh tissue was required at screening for central evaluation of histology and tumor CD20 expression. Staging investigations included history and physical examination; routine laboratory investigation; and computed tomography (CT) scanning of the neck, chest, abdomen, and pelvis. All radiologic images were collected for central review. A bone marrow biopsy was required unless performed in the previous 3 months. PK serum samples were obtained before and after each obinutuzumab infusion and for up to 1 year after the last obinutuzumab dose.
Induction and Maintenance Treatment
For induction, patients were stratified by country and histology and were randomly assigned to receive four once-per-week intravenous infusions of obinutuzumab (1,000 mg) or rituximab (375 mg/m2). Patients were premedicated with acetaminophen and an antihistamine 30 to 60 minutes before infusion. Pretreatment glucocorticoids were administered at the discretion of the treating physician. Obinutuzumab was administered at a graduated infusion rate, as previously published.30,31 Rituximab was administered per standard institutional practice. No dose reductions of obinutuzumab or rituximab were planned. If treatment was delayed for more than 4 weeks because of toxicity, the patient was withdrawn.
After induction, patients with CR, partial response, or stable disease were eligible to receive maintenance therapy (every 2 months for up to 2 years or until progression) with the same mAb and dose as administered during induction.
Safety and Response Assessments
Adverse events (AEs) and serious AEs were monitored according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0. Safety assessments were performed at screening and then continuously throughout the study. Patients were monitored for clinically relevant infections and grade 3 to 4 treatment-related AEs until 28 days after the last administration of study medication.
Initial response evaluation was performed 28 to 42 days after completing induction, and included CT scanning of the neck, chest, abdomen, and pelvis, and bone marrow biopsy (if previously positive and required to document CR). Patients were re-evaluated by CT scan every 3 months during maintenance, 28 days after the last maintenance dose, and every 6 months for up to 2 years or until progression.
Clinical responses were assessed using standardized lymphoma response criteria.34 [18F]fluorodeoxyglucose positron emission tomography scanning was performed at baseline and at initial induction response assessment but was considered exploratory and was not incorporated into response assessments. CT scans were examined by an IRF (consisting of two primary and one adjudicating radiologist), which independently and blindly reviewed all images.
Statistical Analysis
Patients who received at least one dose of obinutuzumab or rituximab were included in the safety analysis. All randomly assigned patients were analyzed for efficacy, but all statistical testing was performed on the FL population. With a sample size of 70 patients with FL in each arm, the one-sided χ2 test had approximately 80% power to detect a difference between the ORRs for the treatment groups of 15% when the α level was .20. Response rates and 95% Pearson-Clopper CIs were estimated for each group. PFS was defined as the time from treatment initiation to disease progression, relapse, or death from any cause. The survival distribution for PFS was calculated using Kaplan-Meier methodology, and comparisons were made using the log-rank test. P values were not adjusted for multiple comparisons.
Population PK modeling was performed using nonlinear mixed-effect modeling software (NONMEM VI; ICON, Dublin, Ireland). Concentration-time data were assessed using an open two-compartment model, and total clearance, central compartment volume, intercompartment clearance, and steady-state volume of distribution were investigated. B-cell depletion was defined as CD19+ cell counts < 0.07 × 109/L, and recovery was defined as counts ≥ 0.07 × 109/L after the completion of treatment in patients with previous B-cell depletion.
RESULTS
Patient Characteristics
Between July 2009 and August 2010, 175 patients with relapsed indolent lymphoma were enrolled at 74 sites across 15 countries; 149 patients had FL (obinutuzumab, n = 74; rituximab, n = 75; Fig 1). Of the 26 patients with non-FL, 14 were randomly assigned to receive obinutuzumab (MZL, n = 6; SLL, n = 7; LPL, n = 1), and 12 were randomly assigned to receive rituximab (MZL, n = 5; SLL, n = 5; LPL, n = 2).
Fig 1.
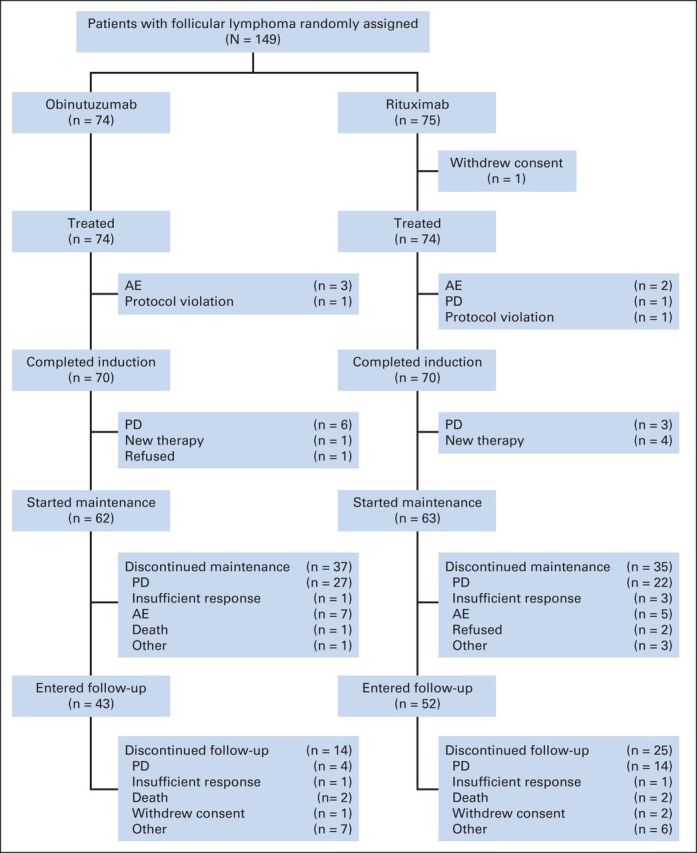
CONSORT diagram of patients with follicular non-Hodgkin lymphoma. AE, adverse event; PD, progressive disease.
Baseline demographic characteristics in the FL population were balanced between arms, and there were no significant differences in clinical characteristics (Table 1). Patients had received a median of two previous lines of therapy (obinutuzumab: range, 1 to 7; rituximab: range, 1 to 6).
Table 1.
Baseline Patient and Disease Characteristics of Patients With Follicular Lymphoma
| Characteristic | Obinutuzumab (n = 74) No. (%) | Rituximab (n = 75) No. (%) |
|---|---|---|
| Age, years | ||
| Median | 62 | 60 |
| Range | 33-84 | 38-80 |
| Male patients | 37 (50) | 39 (52) |
| Ann Arbor stage III/IV | 58 (78) | 63 (84) |
| ECOG PS | ||
| 0 | 61 (83) | 59 (79) |
| 1 | 11 (15) | 14 (18) |
| 2 | 1 (1) | 2 (3) |
| Unknown | 1 (1) | 0 |
| B symptoms | 9 (12) | 6 (8) |
| Bone marrow involvement | 20 (27) | 25 (33) |
| Lactate dehydrogenase > ULN | 34 (46) | 40 (53) |
| Bulky disease ≥ 7 cm | 10 (14) | 9 (12) |
| SPD at baseline, mm2 | ||
| Median | 2,397 | 1,934 |
| Range | 192-29,326 | 252-11,255 |
| FLIPI score at diagnosis | ||
| Low (0-1) | 17 (23) | 23 (31) |
| Intermediate (2) | 26 (35) | 18 (24) |
| High (3-5) | 18 (24) | 22 (29) |
| Missing | 13 (18) | 12 (16) |
| Previous treatment | ||
| No. of lines of therapy | ||
| Median | 2 | 2 |
| Range | 1-7 | 1-6 |
| Stem-cell transplantation | 8 (11) | 11 (15) |
| No. of lines of rituximab | ||
| Median | 1 | 1 |
| Range | 1-4 | 0-5 |
| Rituximab refractory in any previous regimen | 6 (8) | 8 (11) |
NOTE. No statistically significant differences between the two treatment groups were observed (ie, P values > .05, Wilcoxon rank sum test for continuous variables and χ2 test for the categorical variables).
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; FLIPI, Follicular Lymphoma International Prognostic Index; SPD, sum of the product of greatest diameters; ULN, upper limit of normal.
Treatment Received
Of the 149 patients with FL, 70 in each arm completed all four doses of induction therapy (Fig 1). In the obinutuzumab and rituximab arms, 62 and 63 patients, respectively, commenced maintenance therapy. At the time of analysis, 37 and 35 patients in the obinutuzumab and rituximab arms, respectively, had withdrawn during the maintenance phase. The median number of doses received was 10 for obinutuzumab and 12 for rituximab.
Efficacy
At the end of induction (week 8), the ORR (investigator assessed) for the 149 patients with FL seemed higher in the obinutuzumab arm than the rituximab arm (44.6% v 33.3%; difference, P = .08) and met the study objective, which required a P value of less than .2 for significance (Table 2). Nine patients in the obinutuzumab arm (12.2%) and four in the rituximab arm (5.3%) achieved CR or CR unconfirmed (CRu); this difference was not significant (P = .07). Independent review also found the ORR to be higher with obinutuzumab versus rituximab (44.6% v 26.7%; P = .01), but with no difference in CR/CRu rate (5.4 v 4.0; P = .34). At the end of induction, six patients in the obinutuzumab arm and three in the rituximab arm had progressive disease.
Table 2.
Response Rates in Patients With Follicular and Nonfollicular Lymphoma
| Response Rate | Investigator Assessed |
IRF Assessed |
||
|---|---|---|---|---|
| Obinutuzumab No. (%) | Rituximab No. (%) | Obinutuzumab No. (%) | Rituximab No. (%) | |
| Patients with follicular lymphoma | ||||
| No. of patients | 74 | 75 | 74 | 75 |
| Response at end of induction | ||||
| ORR | 33 (44.6) | 25 (33.3) | 33 (44.6) | 20 (26.7) |
| CR/CRu* | 9 (12.2) | 4 (5.3) | 4 (5.4) | 3 (4.0) |
| PR | 24 (32.4) | 21 (28.0) | 29 (39.2) | 17 (22.7) |
| Difference in ORR, % | 11.3 | 17.9 | ||
| 95% CI† | −5.1 to 27.6 | 2.0 to 33.8 | ||
| P, one-sided, χ2 test | .08 | .01 | ||
| Best response | ||||
| BOR | 49 (66.2) | 48 (64.0) | 47 (63.5) | 37 (49.3) |
| CR/CRu‡ | 31 (41.9) | 17 (22.7) | 28 (37.8) | 20 (26.7) |
| PR | 18 (24.3) | 31 (41.3) | 19 (25.7) | 17 (22.7) |
| Difference in BOR, % | 2.2 | 14.2 | ||
| 95% CI† | −13.9 to 18.3 | −2.4 to 30.7 | ||
| P, one-sided, χ2 test | .39 | .04 | ||
| Patients with nonfollicular indolent lymphoma | ||||
| No. of patients | 14 | 12 | 14 | 12 |
| Response at end of induction | ||||
| ORR | 6 (43) | 2 (17) | 6 (43) | 0 |
| CR/CRu | 1 (7) | 0 | 1 (7) | 0 |
| PR | 4 (29) | 2 (17) | 5 (36) | 0 |
| Best response | ||||
| BOR | 8 (57) | 3 (25) | 7 (50) | 2 (17) |
| CR/CRu | 4 (29) | 0 | 4 (29) | 1 (8) |
| PR | 4 (29) | 3 (25) | 3 (21) | 1 (8) |
Abbreviations: BOR, best overall response; CR, complete response; CRu, complete response unconfirmed; IRF, independent review facility; ORR, overall response rate; PR, progressive disease.
P value (one-sided, χ2 test) comparing obinutuzumab versus rituximab for CR/CRu at end of induction by investigator assessment was .07 and by IRF was .34.
Approximate 95% CI for the difference of the two rates was based on Hauck-Anderson method.
P value (one-sided, χ2 test) comparing obinutuzumab versus rituximab for BOR CR/CRu by investigator assessment was .006 and by IRF was .07.
During the entire treatment period, response rates increased in both arms relative to the end of induction. The investigator-assessed best overall response (BOR) for obinutuzumab and rituximab was similar during or on completion of maintenance therapy (66.2% v 64.0%), but the proportion of patients achieving CR/CRu was higher in the obinutuzumab arm (41.9%) than the rituximab arm (22.7%; P = .006). IRF-assessed BOR was higher for obinutuzumab than rituximab (63.5% v 49.3%; P = .04), but CR/CRu was not different (37.8% v 26.7%; P = .07).
An exploratory analysis of the 26 patients without FL demonstrated higher investigator- and IRF-assessed ORR and CR/CRu rates for obinutuzumab versus rituximab (Table 2). Responses were seen in all non-FL subgroups.
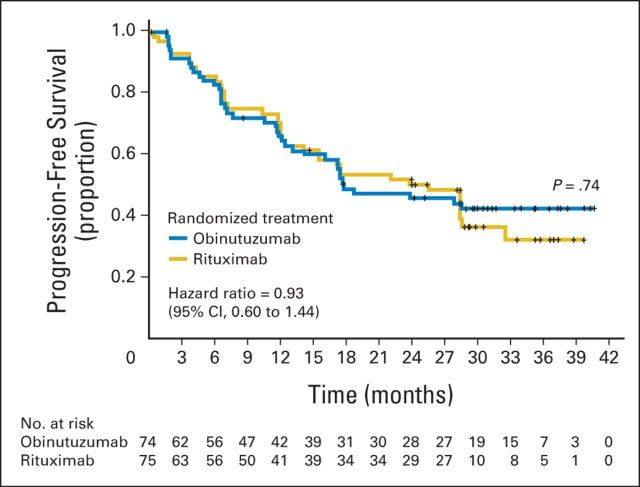
Among patients with FL, the median follow-up period was 32 months (range, 0.1 to 43 months). No difference in PFS was observed between the two arms (Fig 2) with a hazard ratio of 0.93 (95% CI, 0.60 to 1.44). The 2-year PFS was 45.8% (95% CI, 33.5% to 57.3%; median PFS, 17.6 months) for obinutuzumab and 50.3% (95% CI, 37.7% to 61.6%; median PFS, 25.4 months) for rituximab.
Fig 2.
Progression-free survival of patients with follicular lymphoma treated with obinutuzumab versus rituximab monotherapy.
Safety
The safety analysis population included 87 and 86 patients in the obinutuzumab and rituximab arms, respectively. Most AEs occurred at a similar incidence in each arm (Table 3). A higher rate of infusion-related reactions (IRRs; 74% v 51%) and cough (24% v 9%) was observed in the obinutuzumab versus the rituximab arm. IRRs were the most frequently observed AE, and most episodes were grade 1 to 2.
Table 3.
Adverse Events During the Entire Treatment Period for All Patients (those with follicular lymphoma and with nonfollicular lymphoma)*
| Adverse Event | All Grades |
Grade 3 to 4 |
||
|---|---|---|---|---|
| Obinutuzumab (n = 87) No. (%) | Rituximab (n = 86) No. (%) | Obinutuzumab (n = 87) No. (%) | Rituximab (n = 86) No. (%) | |
| Infusion-related reaction† | 64 (74) | 44 (51) | 10 (11) | 4 (5) |
| Fatigue | 23 (26) | 17 (20) | — | — |
| Cough‡ | 21 (24) | 8 (9) | — | — |
| Upper respiratory tract infection | 9 (10) | 9 (10) | — | — |
| Pyrexia | 6 (7) | 9 (10) | 1 (1) | — |
| Headache | 8 (9) | 7 (8) | — | — |
| Nausea | 8 (9) | 6 (7) | — | — |
| Diarrhea | 7 (8) | 7 (8) | — | — |
| Arthralgia | 4 (5) | 8 (9) | — | — |
| Decreased appetite | 8 (9) | 3 (3) | — | — |
| Asthenia | 6 (7) | 5 (6) | — | — |
| Neutropenia | 3 (3) | 7 (8) | 3 (3) | 6 (7) |
| Dizziness | 4 (5) | 6 (7) | — | — |
| Back pain | 7 (8) | 3 (3) | — | — |
| Bronchitis | 7 (8) | 3 (3) | — | — |
| Peripheral edema | 6 (7) | 4 (5) | — | — |
| Nasopharyngitis | 6 (7) | 4 (5) | — | — |
| Sinusitis | 6 (7) | 4 (5) | — | — |
| Hypertension | 2 (2) | 7(8) | — | — |
| Rash | 6 (7) | 3 (3) | — | — |
| Upper abdominal pain | 2 (2) | 6 (7) | — | — |
Occurring in more than five patients in either treatment arm. Unless otherwise noted, the adverse event proportions between the two treatment arms were not significantly different (Fisher's exact P value [two-sided] > .05).
Fisher's exact P value (two-sided) =.003
Fisher's exact P value (two-sided) =.013
Twenty-six patients (15% in each arm) experienced serious AEs (Table 4). Most grade 3 and 4 AEs during treatment were IRRs (obinutuzumab, 11%; rituximab, 5%) and occurred during or within 24 hours of the first infusion. No grade 3 or 4 IRRs were reported during maintenance therapy (Table 4). Treatment was discontinued because of AEs in 8% of patients in the obinutuzumab arm (three patients because of an IRR) and in 10% of patients in the rituximab arm (one patient because of an IRR).
Table 4.
Summary of Adverse Events During Induction and Maintenance for All Patients (those with follicular lymphoma and with nonfollicular lymphoma)*
| Patients | Induction |
Maintenance |
||
|---|---|---|---|---|
| Obinutuzumab (n = 87) No. (%) | Rituximab (n = 86) No. (%) | Obinutuzumab (n = 73) No. (%) | Rituximab (n = 72) No. (%) | |
| ≥ 1 AE† | 75 (86) | 58 (67) | 61 (84) | 58 (81) |
| Grade 3 to 4 AE | 15 (17) | 13 (15) | 13 (18) | 15 (21) |
| SAE | 5 (6) | 7 (8) | 8 (11) | 6 (8) |
| Grade 3 to 4 IRR | 10 (11) | 4 (5) | 0 | 0 |
| Grade 3 to 4 infection | 0 | 4 (5) | 3 (4) | 2 (3) |
| Grade 3 to 4 neutropenia | 0 | 1 (1) | 3 (4) | 6 (8) |
Abbreviations: AE, adverse event; IRR, infusion-related reaction; SAE, serious adverse event.
Unless otherwise noted, the AE proportions between the two treatment arms were not significantly different (Fisher's exact P [two-sided] > .05).
Fisher's exact P (two-sided) = .03.
Twenty-nine deaths occurred (18 patients in the obinutuzumab and 11 patients in the rituximab arm) during the entire study, and of these, fifteen deaths were a result of disease progression (10 patients in the obinutuzumab arm and five patients in the rituximab arm).
PKs
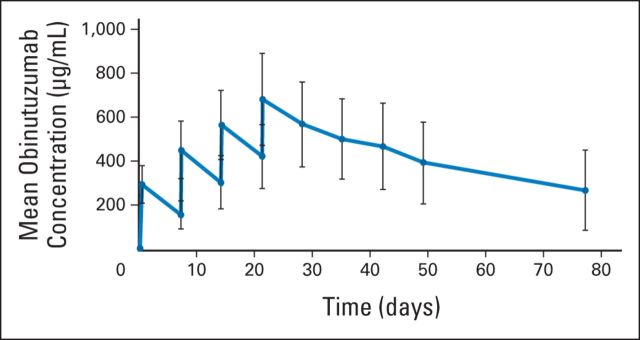
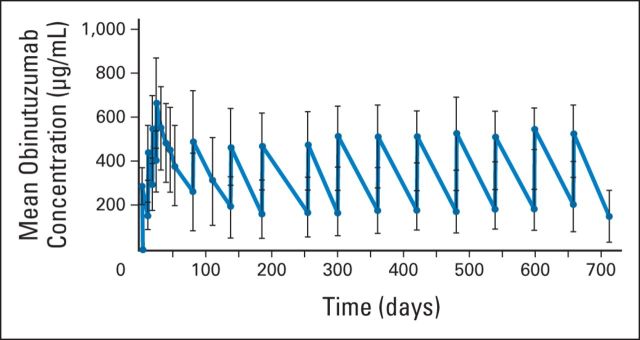
The PK analysis revealed that systemic exposure to obinutuzumab increased during the once-per-week infusions of the induction period (Appendix Fig A1, online only). During the maintenance phase, the maximum concentration of obinutuzumab did not increase compared with the previous infusion, and mean serum trough concentration values (170 to 209 μg/mL) were maintained over the course of maintenance treatment (Appendix Fig A2, online only).
Pharmacodynamics/Biomarkers
B-cell depletion in peripheral blood was observed in 96% and 95% of patients receiving obinutuzumab and rituximab, respectively. B-cell recovery could not be assessed in most patients because of the development of progressive disease or initiation of new antilymphoma therapies. Median interleukin-6 (IL-6), IL-8, and IL-10 levels increased during the first cycle in both arms, with greater increases for obinutuzumab versus rituximab. Levels remained close to baseline for subsequent infusions in both cohorts during the induction period (Appendix Fig A3, online only). Similarly, both tumor necrosis factor–alpha (TNF-α) and interferon-gamma levels increased during the first infusion, with a greater increase noted in the obinutuzumab arm. Levels returned to baseline and remained stable during subsequent infusions (data not shown).
DISCUSSION
The availability of rituximab for the treatment of indolent NHL has resulted in a significant improvement in patient outcomes. Novel anti-CD20 mAbs are under evaluation in an attempt to further improve efficacy. This study is the first randomized trial comparing a novel anti-CD20 mAb with rituximab in patients with indolent lymphoma.
On the basis of investigator and IRF assessments, obinutuzumab resulted in a higher ORR (a difference of > 11.3% per investigator and > 17.9% per IRF) at the end of induction, which was the primary end point of the trial. Investigator-assessed BORs further improved during and after maintenance but were similar for obinutuzumab (64.0%) and rituximab (66.2%). However, the quality of remissions was better with obinutuzumab, with an almost two-fold higher CR/CRu rate (41.9% v 22.7%; P = .006). On the basis of IRF assessment, the BOR was better in the obinutuzumab arm (P = .04), but the CR/CRu rate was not different. Although limited by small sample size, results in patients with nonfollicular indolent lymphoma demonstrated a favorable response with obinutuzumab.
Obinutuzumab has been engineered to potentiate ADCC and direct cell death compared with rituximab and has lower potential for complement-dependent cytotoxicity. Although the predominant mechanism of action in vivo continues to be debated, and may vary on the basis of histology or the patient's immune status, both ADCC and direct cell death seem instrumental to biologic activity.35,36 Preclinical data have demonstrated the activity of obinutuzumab,26–28 and the current study provides comparative clinical evidence that supports this. Results seen in the rituximab arm (ORR of 33%, as assessed by investigators) is slightly lower than the ORR of approximately 40% that was reported in previous studies evaluating rituximab retreatment in patients with FL.15,37 However, in the current study, patients had a much higher level of previous rituximab exposure, receiving up to five previous regimens.15 In comparison, the end-of-induction ORR of 44.6% for obinutuzumab in such highly pretreated patients with FL was encouraging. However, it should be stressed that the higher ORR seen with obinutuzumab in this trial did not ultimately translate into an improvement in PFS (median follow-up of 32 months).
Recently, the CLL11 trial demonstrated a significant improvement in CR rate (21% v 7%; P < .001) and median PFS (26.7 v 15.2 months; P < .001) with obinutuzumab and chlorambucil compared with rituximab and chlorambucil in patients with previously untreated CLL with coexisting comorbidities.38 The large difference in PFS observed in CLL11 is in contrast with what was seen in the current randomized phase II study, which may reflect the limitation of monotherapy to achieve prolonged control within this highly pretreated patient population, or the added benefit of maintenance therapy. Alternatively, the advantage of obinutuzumab may be augmented within the context of combined chemoimmunotherapy in view of its higher capacity to induce direct cell death and to synergize with chemotherapy. Currently, it remains unknown whether a differential benefit between mAbs with respect to histologic subtype will be observed as a result of underlying differences in molecular mechanisms or immunologic interactions. Although the absence of improvement in PFS in this phase II study may partly reflect the limited power to detect such a difference, demonstration of a clinically meaningful benefit such as PFS will be required to confirm the efficacy of obinutuzumab in indolent NHL.
The recommended dose of obinutuzumab has been optimized on the basis of PK and early-phase data.30–33 It is unknown whether differences in doses administered within this study may have affected outcome, but in the preclinical setting, the superiority of obinutuzumab has been demonstrated when compared with rituximab at identical doses. The bimonthly maintenance schedule of obinutuzumab was selected on the basis of preclinical and clinical PK data.26–28,31 In the phase I precursor to this study, maintenance was administered every 3 months; however, trough levels were below the desired target threshold.31 Thus, in this phase II study, maintenance was administered every 2 months to maintain plasma obinutuzumab trough concentrations of more than 25 μg/mL, a level that has been recommended and achievable with bimonthly rituximab dosing.39
A similar safety profile was observed in each arm, although the incidence of IRRs and cough was higher for obinutuzumab than rituximab. Release of various cytokines after rituximab infusion, including TNF-α and IL-6, has been found to be the likely cause of IRR clinical signs and symptoms.40,41 In this study, peak cytokine levels of IL-6, IL-8, IL-10, TNF-α, and interferon-gamma were notably higher during the first infusion of obinutuzumab compared with rituximab, but then returned to baseline without an increase in subsequent infusions. Most IRRs occurred with the first infusion of obinutuzumab, which provides further support for a role of cytokine release in the development of IRRs. The etiology of the increased incidence of cough is not fully understood. Although a proportion of patients experienced cough as a result of IRRs, many cases of cough were independent of IRRs or infection and were largely self-limited events. There were no significant differences in treatment discontinuation rates between the two cohorts.
In conclusion, this study demonstrates that in patients with relapsed FL, obinutuzumab induces higher response rates relative to rituximab while exhibiting an acceptable safety profile. However, this did not translate into an improvement in PFS; therefore, the clinical value of obinutuzumab in this setting remains unclear. Results are eagerly awaited from the ongoing GADOLIN trial that is comparing bendamustine plus obinutuzumab with bendamustine monotherapy in patients with rituximab-refractory, indolent NHL (NCT01059630) and the GALLIUM study that is comparing obinutuzumab plus chemotherapy followed by obinutuzumab maintenance with rituximab plus chemotherapy followed by rituximab maintenance in patients with previously untreated indolent NHL (NCT01332968).
Acknowledgment
Support for third-party writing assistance for this article was provided by F. Hoffmann-La Roche.
Appendix
Fig A1.
Mean (± standard deviation) obinutuzumab serum concentrations in patients with indolent non-Hodgkin lymphoma after once-per-week administration of 1,000 mg obinutuzumab during the four-cycle induction period. Error bars represent the standard deviation.
Fig A2.
Mean (± standard deviation) obinutuzumab serum concentrations in patients with indolent non-Hodgkin lymphoma after once-per-week administration of 1,000 mg obinutuzumab during the four-cycle induction period and during 11 cycles of maintenance regimen, which consisted of once-every-2-months administration of 1,000 mg obinutuzumab. Error bars represent the standard deviation.
Fig A3.
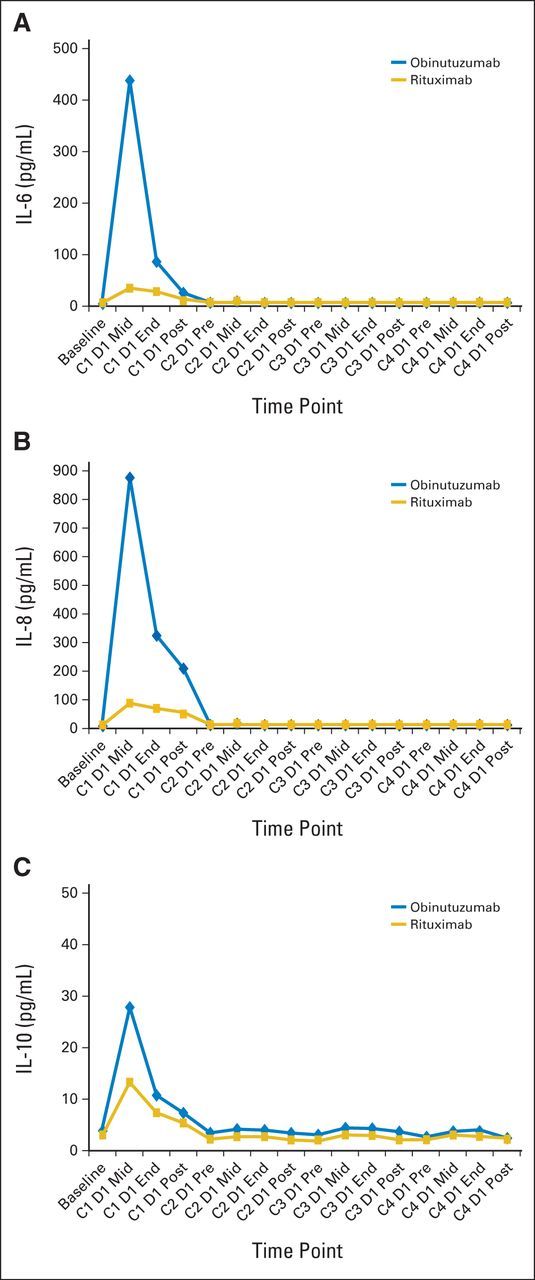
Median serum concentrations of (A) interleukin-6 (IL-6), (B) IL-8, and (C) IL-10 in patients with indolent non-Hodgkin lymphoma after once-per-week administration of 1,000 mg obinutuzumab or 375 mg/m2 rituximab during the four-cycle induction period. C, cycle; D, day; End, end of infusion; Mid, midinfusion; Post, postinfusion; Pre, preinfusion.
Footnotes
Presented in part at the American Society of Hematology 53rd Annual Meeting, San Diego, CA, December 10-13, 2011.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00576758
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Laurie H. Sehn
Provision of study materials or patients: Fritz C. Offner, Oliver W. Press
Collection and assembly of data: Laurie H. Sehn, Andre Goy, Fritz C. Offner, Giovanni Martinelli, M. Dolores Caballero, Ole Gadeberg, Tara Baetz, Andrew D. Zelenetz, Gianluca Gaidano, Luis E. Fayad
Data analysis and interpretation: Laurie H. Sehn, Andre Goy, Fritz C. Offner, Rena Buckstein, Jonathan W. Friedberg, Michael Crump, Branimir Jaksic, Pier Luigi Zinzani, Swaminathan Padmanabhan Iyer, Deniz Sahin, Akiko Chai, Günter Fingerle-Rowson, Oliver W. Press
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase II Trial Comparing Obinutuzumab (GA101) With Rituximab in Patients With Relapsed CD20+ Indolent B-Cell Non-Hodgkin Lymphoma: Final Analysis of the GAUSS Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Laurie H. Sehn
Honoraria: Roche/Genentech, Amgen, Janssen, Seattle Genetics, Lundbeck, Celgene
Consulting or Advisory Role: Roche/Genentech, Amgen, Janssen, Seattle Genetics, Lundbeck, Celgene
Research Funding: Roche/Genentech (Inst)
Andre Goy
Consulting or Advisory Role: Celgene, Johnson & Johnson/Pharmacyclics
Speakers' Bureau: Celgene, Johnson & Johnson/Pharmacyclics, Takeda
Research Funding: Celgene (Inst)
Fritz C. Offner
No relationship to disclose
Giovanni Martinelli
No relationship to disclose
M. Dolores Caballero
Honoraria: Roche
Consulting or Advisory Role: Celgene, Gilead Sciences, Janssen-Cilag
Ole Gadeberg
No relationship to disclose
Tara Baetz
Honoraria: Amgen
Consulting or Advisory Role: Bristol-Myers Squibb, Lundbeck, Gilead Pharmaceuticals
Andrew D. Zelenetz
Honoraria: Celgene, Gilead Sciences
Consulting or Advisory Role: Amgen, Boehringer Ingelheim, Celgene, Dr. Reddy's Laboratories, Genentech/Roche, Gilead Sciences, Hospira, Novartis, Quintiles, Sanofi, TRM Oncology
Research Funding: Genentech/Roche, Bristol-Myers Squibb
Gianluca Gaidano
No relationship to disclose
Luis E. Fayad
Research Funding: Roche/Genentech
Rena Buckstein
Honoraria: Celgene
Consulting or Advisory Role: Celgene
Research Funding: Celgene
Jonathan W. Friedberg
Consulting or Advisory Role: Trubion, Bayer, Kite Pharmaceuticals
Research Funding: Seattle Genetics (Inst), Genentech (Inst), Millennium (Inst), Janssen Pharmaceuticals (Inst)
Michael Crump
Research Funding: Roche, Millenium, Novartis
Branimir Jaksic
Honoraria: F. Hoffmann-LaRoche
Research Funding: F. Hoffmann-LaRoche
Pier Luigi Zinzani
No relationship to disclose
Swaminathan Padmanabhan Iyer
No relationship to disclose
Deniz Sahin
Employment: Roche
Stock or Other Ownership: Roche
Akiko Chai
Employment: Genentech
Stock or Other Ownership: Roche
Günter Fingerle-Rowson
Employment: F. Hoffmann-LaRoche
Stock or Other Ownership: F. Hoffmann-LaRoche
Oliver W. Press
Stock or Other Ownership: PhaseRx, Emergent BioSolutions
Consulting or Advisory Role: BIND Biosciences, Algeta
Research Funding: Genentech (Inst), Presage (Inst)
Travel, Accommodations, Expenses: Algeta
REFERENCES
- 1.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 2.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 4.Robak T, Dmoszynska A, Solal-Céligny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–1765. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 5.van Oers MH, Van Glabbeke M, Giurgea L, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin's lymphoma: Long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol. 2010;28:2853–2858. doi: 10.1200/JCO.2009.26.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 7.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26:4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 8.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 9.Salles G, Mounier N, de Guibert S, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: Results of the GELA-GOELAMS FL2000 study. Blood. 2008;112:4824–4831. doi: 10.1182/blood-2008-04-153189. [DOI] [PubMed] [Google Scholar]
- 10.Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:3064–3071. doi: 10.1182/blood-2004-04-1323. [DOI] [PubMed] [Google Scholar]
- 11.Herold M, Haas A, Srock S, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: An East German Study Group Hematology and Oncology Study. J Clin Oncol. 2007;25:1986–1992. doi: 10.1200/JCO.2006.06.4618. [DOI] [PubMed] [Google Scholar]
- 12.Schulz H, Bohlius JF, Trelle S, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: A systematic review and meta-analysis. J Natl Cancer Inst. 2007;99:706–714. doi: 10.1093/jnci/djk152. [DOI] [PubMed] [Google Scholar]
- 13.Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: A phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21:1746–1751. doi: 10.1200/JCO.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Davis TA, White CA, Grillo-López AJ, et al. Single-agent monoclonal antibody efficacy in bulky non-Hodgkin's lymphoma: Results of a phase II trial of rituximab. J Clin Oncol. 1999;17:1851–1857. doi: 10.1200/JCO.1999.17.6.1851. [DOI] [PubMed] [Google Scholar]
- 15.Davis TA, Grillo-López AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's lymphoma: Safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135–3143. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 17.Piro LD, White CA, Grillo-López AJ, et al. Extended rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin's lymphoma. Ann Oncol. 1999;10:655–661. doi: 10.1023/a:1008389119525. [DOI] [PubMed] [Google Scholar]
- 18.Ghielmini M, Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood. 2004;103:4416–4423. doi: 10.1182/blood-2003-10-3411. [DOI] [PubMed] [Google Scholar]
- 19.Hochster H, Weller E, Gascoyne RD, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: Results of the randomized phase III ECOG1496 study. J Clin Oncol. 2009;27:1607–1614. doi: 10.1200/JCO.2008.17.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan HT, Hughes D, French RR, et al. CD20-induced lymphoma cell death is independent of both caspases and its redistribution into triton X-100 insoluble membrane rafts. Cancer Res. 2003;63:5480–5489. [PubMed] [Google Scholar]
- 21.Cragg MS, Glennie MJ. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood. 2004;103:2738–2743. doi: 10.1182/blood-2003-06-2031. [DOI] [PubMed] [Google Scholar]
- 22.Glennie MJ, French RR, Cragg MS, et al. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 23.Niederfellner G, Lammens A, Mundigl O, et al. Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies. Blood. 2011;118:358–367. doi: 10.1182/blood-2010-09-305847. [DOI] [PubMed] [Google Scholar]
- 24.Ivanov A, Beers SA, Walshe CA, et al. Monoclonal antibodies directed to CD20 and HLA-DR can elicit homotypic adhesion followed by lysosome-mediated cell death in human lymphoma and leukemia cells. J Clin Invest. 2009;119:2143–2159. doi: 10.1172/JCI37884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alduaij W, Illidge TM. The future of anti-CD20 monoclonal antibodies: Are we making progress? Blood. 2011;117:2993–3001. doi: 10.1182/blood-2010-07-298356. [DOI] [PubMed] [Google Scholar]
- 26.Alduaij W, Ivanov A, Honeychurch J, et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood. 2011;117:4519–4529. doi: 10.1182/blood-2010-07-296913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mössner E, Brünker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalle S, Reslan L, Besseyre de Horts T, et al. Preclinical studies on the mechanism of action and the anti-lymphoma activity of the novel anti-CD20 antibody GA101. Mol Cancer Ther. 2011;10:178–185. doi: 10.1158/1535-7163.MCT-10-0385. [DOI] [PubMed] [Google Scholar]
- 29.Patz M, Isaeva P, Forcob N, et al. Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br J Haematol. 2011;152:295–306. doi: 10.1111/j.1365-2141.2010.08428.x. [DOI] [PubMed] [Google Scholar]
- 30.Salles G, Morschhauser F, Lamy T, et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119:5126–5132. doi: 10.1182/blood-2012-01-404368. [DOI] [PubMed] [Google Scholar]
- 31.Sehn LH, Assouline SE, Stewart DA, et al. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012;119:5118–5125. doi: 10.1182/blood-2012-02-408773. [DOI] [PubMed] [Google Scholar]
- 32.Salles GA, Morschhauser F, Solal-Céligny P, et al. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin lymphoma: Results from the phase II GAUGUIN study. J Clin Oncol. 2013;31:2920–2926. doi: 10.1200/JCO.2012.46.9718. [DOI] [PubMed] [Google Scholar]
- 33.Morschhauser FA, Cartron G, Thieblemont C, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large B-cell lymphoma or mantle-cell lymphoma: Results from the phase II GAUGUIN study. J Clin Oncol. 2013;31:2912–2919. doi: 10.1200/JCO.2012.46.9585. [DOI] [PubMed] [Google Scholar]
- 34.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 35.Klein C, Lammens A, Schäfer W, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs. 2013;5:22–33. doi: 10.4161/mabs.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanco-Toribio A, Sainz-Pastor N, Álvarez-Cienfuegos A, et al. Generation and characterization of monospecific and bispecific hexavalent trimerbodies. MAbs. 2013;5:70–79. doi: 10.4161/mabs.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston A, Bouafia-Sauvy F, Broussais-Guillaumot F, et al. Retreatment with rituximab in 178 patients with relapsed and refractory B-cell lymphomas: A single institution case control study. Leuk Lymphoma. 2010;51:399–405. doi: 10.3109/10428190903503404. [DOI] [PubMed] [Google Scholar]
- 38.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 39.Gordan LN, Grow WB, Pusateri A, et al. Phase II trial of individualized rituximab dosing for patients with CD20-positive lymphoproliferative disorders. J Clin Oncol. 2005;23:1096–1102. doi: 10.1200/JCO.2005.12.171. [DOI] [PubMed] [Google Scholar]
- 40.Maloney DG, Grillo-López AJ, Bodkin DJ, et al. IDEC-C2B8: Results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol. 1997;15:3266–3274. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 41.Winkler U, Jensen M, Manzke O, et al. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8) Blood. 1999;94:2217–2224. [PubMed] [Google Scholar]