Abstract
We employed multiphoton laser scanning microscopy (MPLSM) to image changes in mitochondrial distribution in living rhesus monkey embryos. This method of imaging does not impair development; thus, the same specimen can be visualized multiple times at various developmental stages. Not only does this increase the amount of information that can be gathered on a single specimen but it permits the correlation of early events with subsequent development in the same specimen. Here we demonstrate the utility of MPLSM for determining changes in mitochondrial organization at various developmental stages and show that rhesus zygotes possess a distinct accumulation of mitochondria between the pronuclei prior to syngamy. We present evidence that suggests that this pronuclear accumulation may be positively correlated with development to the blastocyst stage—in the same embryo—thereby illustrating how MPLSM can be used to correlate cellular dynamics of primate oocytes and early embryos with their developmental potential. Understanding the relationship between mitochondrial distribution and the subsequent development of mammalian embryos, particularly primates, will increase our ability to improve embryo culture technologies, including those used for human assisted reproduction.
Keywords: embryo, laser scanning microscopy, live imaging, mitochondria, multiphoton microscopy, primate
Introduction
One challenge of studying nonhuman primate embryos is gathering as much information as possible from each precious specimen. Primate oocytes and embryos are expensive to obtain, are in limited supply, and come from genetically diverse populations of females. Furthermore, to obtain large quantities for multiple and reliable studies, the oocytes and embryos are typically collected from hormonally superstimulated animals and this treatment may affect oocyte development, thereby exacerbating the heterogeneity of specimens available for study. To address these issues, it is necessary to utilize research techniques that permit the correlation of structure and developmental competence within the same specimen without compromising viability. This type of analysis has been conducted with video microscopy (Lehtonen, 1980; Bavister, 1988; Simerly et al., 1993; Gonzales et al., 1995). However, video microscopy does not permit the specific labeling of subcellular components. Epifluorescence and confocal microscopy, in conjunction with specific fluorescent labels, can be used for imaging subcellular components but require exposure to high-intensity light that may cause cellular damage (Terasaki and Dailey, 1995; Squirrell et al., 1999) and therefore limit the length of time available for imaging living embryos. On the other hand, multiphoton laser scanning microscopy (MPLSM) can also be used for imaging fluorescent markers in relatively thick specimens, such as mammalian embryos (Centonze and White, 1998), without detectably impairing development (Squirrell et al., 1999). With these advantages, MPLSM has the potential to provide important information about subcellular dynamics in living primate embryos that may enhance our knowledge of the effects of embryo culture. Such information will not only increase our knowledge of cell and embryo physiology, but also improve assisted reproductive technologies.
The role of mitochondria in the early mammalian embryo has elicited considerable interest in the past and there has been a recent resurgence of interest in this particular organelle and its role in the preimplantation embryo (Bavister and Squirrell, 2000; Van Blerkom et al., 2000). The early studies, mainly utilizing electron microscopy, revealed that the mitochondria are initially morphologically immature—usually spherical rather than elongated, with relatively few cristae—and undergo a maturation process prior to blastocoel formation. Although the details and timing differ among species, this general structural progression of mitochondria has been described for a number of species (mouse—Hillman and Tasca, 1983; sheep—Calarco and McLaren, 1976; pig—Hyttel and Niemann, 1990; bovine—Plante and King, 1994; primates—Panigel et al., 1975). However, the relationship between mitochondrial morphology and the functional efficiency of this organelle at different stages of embryo development remains to be determined.
Not only do changes in mitochondrial morphology occur, but the mitochondria also undergo a variety of species-specific reorganizations during early development. For example, mitochondria in mouse oocytes are asymmetrically localized but become homogeneously distributed during fertilization (Van Blerkom and Runner, 1984; Muggleton-Harris and Brown, 1988; Calarco, 1995). Similarly, organelles in rat oocytes exhibit a perinuclear aggregation which subsequently disperses into the cell cortex (Zernicka-Goetz et al., 1993). In contrast, mitochondria in hamster embryos undergo a reorganization from a homogeneous distribution in the oocyte and early pronucleate stage to a distinct perinuclear organization late in the pronucleate stage and at the two-cell stage (Barnett et al., 1996). In the bovine oocyte, the mitochondria exhibit a range of organizations, from a uniform distribution to a more cortical restriction. Following fertilization, regardless of the organization present in the oocyte, the cells exhibit an organelle-free zone at the cortex (Van Blerkom et al., 1990). During oocyte maturation in the pig, the majority of mitochondria are initially localized in the cortical region (Luoh and Wu, 1996; Sun et al., 2001) and subsequently show a perinuclear accumulation, both in the oocyte and the early embryo (Hyttel and Neimann, 1990; Sun et al., 2001). In human fertilized zygotes, a pronuclear accumulation of mitochondria has been described (Noto et al., 1993; Van Blerkom et al., 2000). Several studies indicate that mitochondrial organization is associated with developmental competence in rodents (hamster—Barnett et al., 1997; Lane and Bavister, 1998; Squirrell et al., 2001; mouse—Muggleton-Harris and Brown, 1988) and domestic species (pig—Hyttel and Niemann, 1990; Luoh and Wu, 1996; cattle—Van Blerkom et al., 1990). In the hamster, the mitochondria become dispersed away from the nuclei under culture conditions which disrupt development (Barnett et al., 1997; Lane and Bavister, 1998, Squirrell et al., 2001). In contrast, mouse embryos that exhibit a high level of developmental competence exhibit a homogeneous distribution of mitochondria in their cytoplasm whereas those embryos which are developmentally impaired show a more perinuclear organization (Muggleton-Harris and Brown, 1988). Bovine oocytes with the highest developmental potential show a homogenous distribution of mitochondria, whereas those with lower developmental potential exhibited vacuoles surrounded by mitochondria (Stojkovic et al., 2001). Similarly, a disruption of the organization of mitochondria into clumps around empty vesicles has been described for developmentally retarded pig embryos (Luoh and Wu, 1996).
Although elucidating the direct connection between cytoplasmic organization (including the localization of mitochondria) and subsequent embryo development is difficult, understanding this relationship is important for furthering our understanding of embryo physiology and how it is affected by culture conditions. This information is of paramount importance for determining the possible detrimental consequences of physically invasive reproductive technologies, such as oocyte and embryo freezing, nuclear transfer, ooplasm transfer, and intracytoplasmic sperm injection. Clearly, expanding our knowledge of mitochondrial and cytoplasmic organization in primates is critical for advancing such technologies. Thus, microscopic techniques which improve our ability to observe changes in cellular dynamics of living embryos and to follow the subsequent development of those embryos are important for elucidating such information.
In this study, we used MPLSM to observe living rhesus oocytes and embryos, labeled with a mitochondria-specific dye, in order to follow changes in mitochondrial organization in these specimens during early development. Furthermore, we present evidence showing that this technique can be used to assess the relationship between mitochondrial organization and developmental competence in primate embryos.
Materials and Methods
Collection, Insemination, and Culture of Primate Oocytes and Embryos
Mature oocytes from female rhesus macaques (Macaca mulatta), hormonally stimulated as previously described (Schramm and Bavister, 1996; Schramm and Paprocki, 2000), were collected laparoscopically into 37°C TL-HEPES (Bavister, 1995) medium containing 5% bovine calf serum (Hyclone Laboratories Inc., Logan, UT) and 10 IU/ml heparin (Elkins-Sinn, Cherry Hill, NJ). Sperm capacitation and oocyte insemination were performed as previously described (Bavister et al., 1983; Boatman and Bavister, 1984). Capacitated spermatozoa (2 × 105/ml) were incubated with oocytes for 12–16 h at 37°C and 5% CO2 in 50 or 100 µl drops of TALP medium (Bavister and Yanagimachi, 1977) under mineral oil. Presumptive zygotes were cultured in 25 µl drops of modified Connaught Medical Research Laboratories Medium-1066 (CMRL) (Invitrogen, Carlsbad, CA; Boatman, 1998) under mineral oil (Sigma, St. Louis, MO), equilibrated in an atmosphere of 5% CO2 in air.
Microscopy
Living specimens were imaged with MPLSM. The imaging system used was designed and built at the University of Wisconsin–Madison and has been previously described (Wokosin et al., 1996; Wokosin and White, 1997; Squirrell et al., 1999). Briefly, the system consisted of a 1047-nm neodymium-doped, yittrium lithium fluoride-based ultrafast laser (Coherent-Scotland, Glasgow, Scotland), a Diaphot microscope (Nikon, Melville, NY) equipped with CF series oil immersion lenses (FLUOR 40×, 1.3 NA and Plan Apochromat 60×, 1.4 NA) attached to an MRC-600 scanhead (BioRad, Hercules, CA). The emission light was collected in a non-descanned light path at the bottom port of the microscope. The highly efficient light path provided less photon losses and rendered the instrument less sensitive to chromatic aberrations. The microscope was enclosed in a temperature-controlled Plexiglas chamber (Mohler and Squirrell, 2000) and the specimens were cultured within a minichamber on the stage (Bavister, 1988) supplied with humidified 5% CO2 in air.
Specimen Labeling, Image Collection, and Image Processing
Labeling
Living oocytes or presumptive zygotes were labeled with 500 nM Mitotracker-Rosamine (Molecular Probes, Eugene, OR) in 50 µl drops of CMRL under culture conditions for 30 min. Specimens were rinsed twice and placed into 50- or 25-µl drops of CMRL under oil in imaging dishes consisting of 60-mm culture dishes (Falcon #1007, Beckon Dickinson, Lincoln Park, NJ) with a siliconized coverslip covering a hole in the bottom of the dish. The hole was 1 cm in diameter and the round #1 coverslip (Bellco Glass, Vineland, NJ) was glued to the outside of the culture dish with 30-Minute Epoxy (SuperGlue Corp., Rancho Cucamonga, CA; Mohler and Squirrell, 2000). The epoxy was permitted to dry at least overnight, then the dishes were rinsed several times with sterile culture quality water and placed at 37°C to 40°C for several hours before they were stored ready for use. Although Mitotracker can be purchased with a variety of fluorescent tags, these authors have not determined the efficacy of other Mitotracker probes, either with mammalian embryo or with MPLSM.
Image Collection Experiment 1—Assessment of Mitochondrial Organization
Presumptive zygotes were labeled 14 to 16 h postinsemination (PI) and placed into a culture drop in the imaging dish, with 1 to 7 zygotes per drop. Optical sections (z-series) were collected every 5 µm through the entire volume of each specimen. The typical z-series was 21 planes through the embryo. Such z-series were collected from each specimen at intervals ranging from 3 min to 6 h, over a total period of at least 24 h.
Image Collection Experiment 2—Correlation of Mitochondrial Organization and Development
Oocytes were labeled with Mitotracker prior to insemination and each oocyte placed into separate, designated 20- or 25-µl drops of CMRL in imaging dishes. A single z-series, as described for Experiment 1, was collected of each oocyte. Following imaging, each oocyte was placed into a separate IVF drop and inseminated. At 16 h PI, presumptive zygotes were placed into 25-µl drops of either CMRL or G1/G2 media (Gardner and Lane, 1997) and imaged as described above (Experiment 1) for 24 h. Development to the blastocyst stage was assessed at 8 days PI.
Image Processing
Image data were collected as PIC files using either the COMOS (for single time points) or SOM (for time lapse recordings) software (BioRad, Hercules, CA). The digital images were prepared as figures using Adobe Photoshop (Adobe, San Jose, CA). Pseudocoloring and three-dimensional reconstruction were performed using NIH Image (NIH, Bethesda, MD).
Results
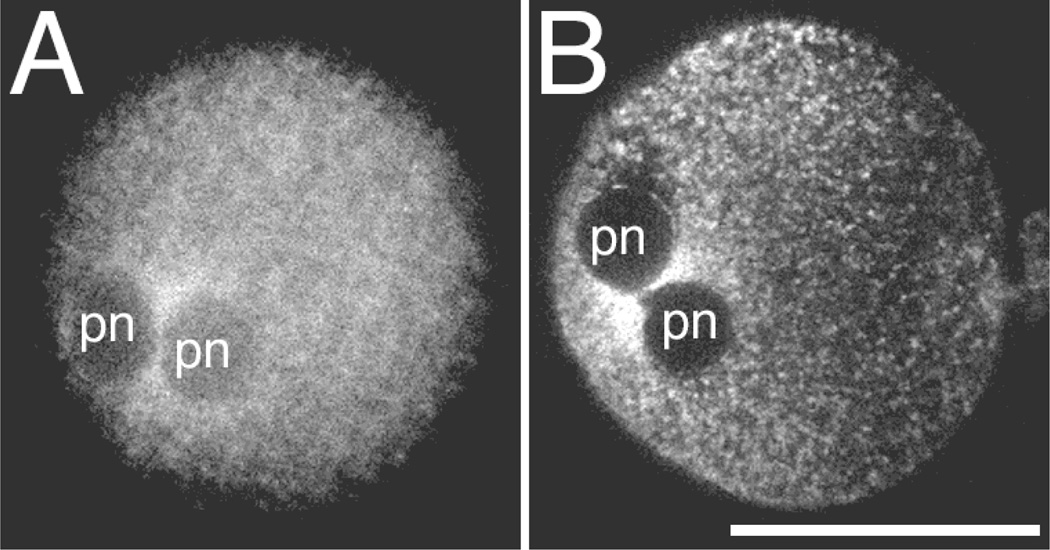
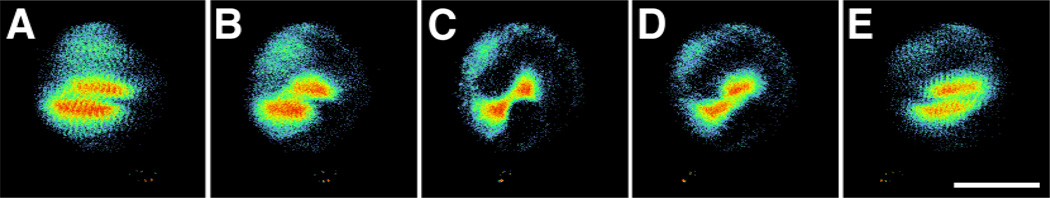
To assess the organization of mitochondria at different developmental stages, labeled presumptive zygotes were imaged for 24 h at various intervals after insemination so that information could be collected from multiple stages of the same specimens. From these images of living embryos, a time series of changes in mitochondrial organization during the first two cell cycles in the same specimens were constructed (Fig. 1). Oocytes undergoing fertilization exhibited an accumulation of mitochondria between the closely juxtaposed pronuclei (Fig. 2). In Experiment 1, this configuration of mitochondria was observed in 14 of the 18 pronucleate zygotes imaged from six different females. The apparent hour-glass shape of the mitochondria accumulation resulted from the mitochondria accumulating in two somewhat sausage-shaped structures along the region where the pronuclei meet, as revealed by three-dimensional reconstruction of a series of optical sections through this region (Fig. 3).
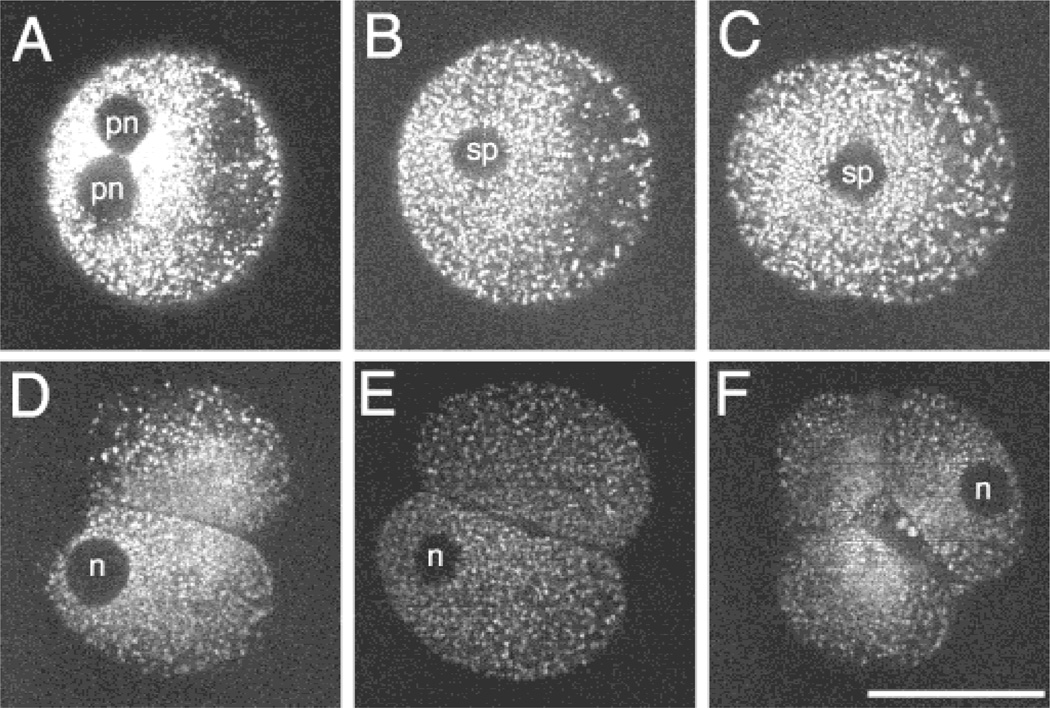
Figure 1.
Distribution of mitochondria in a living rhesus embryo over time. MPLSM images of single optical sections of the same Mitotracker-labeled embryo over time. The images were collected at several time points: (A) the pronucleate stage (12 h PI); (B, C) during the division from the one- to two-cell stage (205 h and 21 h PI, respectively); (D) early (235 h PI) and (E) late two-cell stage (265 h PI); (F) the four-cell stage (39 h PI). Spindle in B is at an oblique angle so that it appears essentially in cross section. pn: pronucleus; sp: spindle region; n: nucleus. Scale bar = 50 µm.
Figure 2.
Pronuclear accumulation of mitochondria in rhesus zygotes. In vivo matured, in vitro fertilized rhesus monkey pronucleate embryos labeled with Mitotracker and imaged with MPLSM at 12 to 16 h PI showing the accumulation of mitochondria between the pronuclei (pn). These images illustrate that although there were differences in the exact appearance of the mitochondrial labeling, due to variability in either embryos, staining, or imaging, the pronuclear accumulation was still observed. Apparent differences in size are due to differences in the level of optical section presented. Each embryo was from a different female. Scale bar = 50 µm.
Figure 3.
Three-dimensional reconstruction of optical sections through the pronuclear region. These rotations of a three-dimensional rendering of optical sections through a pronucleate region shows that the accumulation of mitochondria appears as two sausage-shaped structures along either side of the junction between the two pronuclei, connected by a thin, flat region. The angles of rotation are: (A) −60°, (B) −30°, (C) 0°, (D) 30°, (E) 60°. Images have been pseudocolored to reflect pixel intensity levels. Red indicates regions of high pixel intensity, yellow and green indicate regions of intermediate intensity, and blue indicates low pixel intensity. Scale bar = 50 µm.
The organization of mitochondria became more homogeneous after fertilization (Fig. 1). Of the 20 two-cell rhesus embryos (from nine females) imaged in both Experiment 1 and Experiment 2, none exhibited a perinuclear clustering of mitochondria at the two-cell stage. There may be some asymmetry of mitochondrial distribution, with preference in the early stages to the region around the pronuclei or nuclei, but this distribution was subtle and inconsistent among embryos.
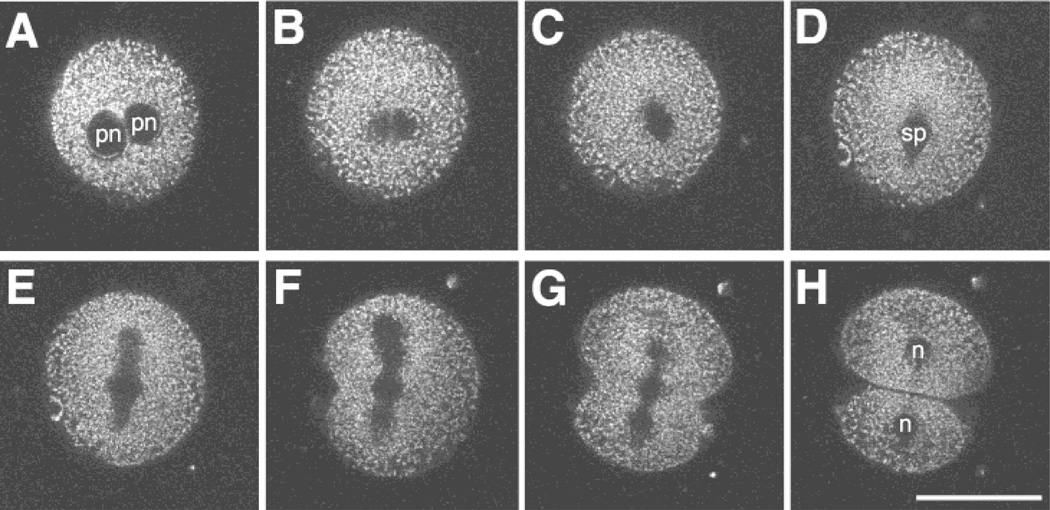
In addition to gathering information on the same specimen over relatively long periods of time (i.e., several hours), MPLSM can be used for the analysis of developmental events that require increased imaging frequency over shorter time intervals (on the order of minutes). The mitochondrial movements that occurred during the first cell division are highlighted in the selected image planes shown in Figure 4. Images were collected every 3 to 4 min, and illustrate the potential for imaging structural changes during dynamic cellular processes such as nuclear division and cytokinesis.
Figure 4.
Imaging the first cell division. MPLSM images of a Mitotracker-labeled pronucleate embryo undergoing the first cleavage division. The closely apposed pronuclei begin to break down (A—18.6 h PI) and lose their shape (B—19.1 h PI). Then a spindle begins to form (C—19.6 h PI) and exhibits a more distinct structure (D—20.25 h PI). The spindle elongates (E—20.4 h PI) and a cleavage furrow initiates (F—20.6 h PI). Finally, the cleavage furrow bisects the spindle (G—20.9 h PI) to form two daughter cells (H—21.0 h PI). Note: pronuclear accumulation of mitochondria was observed in this embryo prior to the time of the first image shown in this series. pn: pronucleus, sp: spindle, n: nucleus. Scale bar = 50 µm.
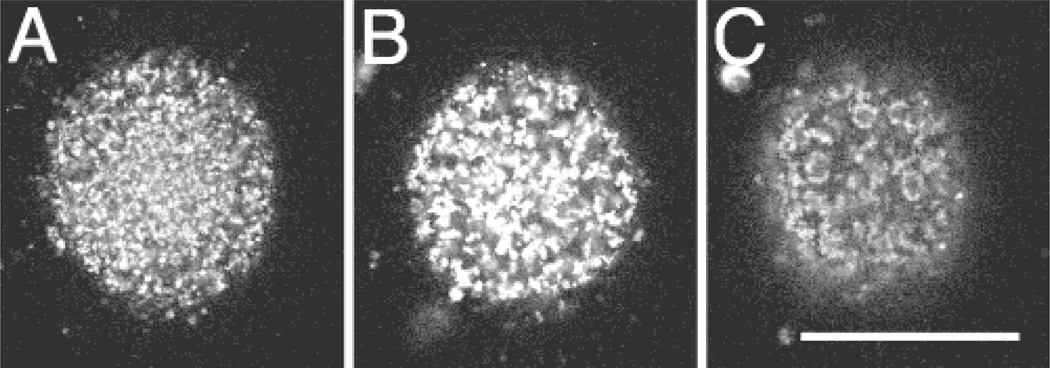
Multiphoton microscopy also permits the correlation of early cytological organization with subsequent development. To understand the possible relationship between mitochondrial organization, in particular their pronuclear accumulation, and subsequent preimplantation development, oocytes were labeled then imaged, inseminated, imaged again, and allowed to develop to the blastocyst stage in separate culture drops (Experiment 2). Data on oocytes/embryos from three females were collected in this fashion. The mitochondria in the cortical region of oocytes (Fig. 5) were organized in three major patterns: homogeneous, tiny clusters, or larger, distinct clusters. Of the oocytes imaged (n = 13), five showed the homogeneous pattern, three exhibited tiny clusters, and the remaining five had large clusters of mitochondria. Twelve hours following insemination, 40% of the oocytes with the homogeneous organization contained two pronuclei, all of the oocytes with tiny clusters of mitochondria contained two pronuclei, while 60% of those oocytes with large clusters of mitochondria exhibited two pronuclei.
Figure 5.
Variations in the distribution of mitochondria in rhesus oocytes. These representative images illustrate the three major patterns of mitochondrial organization observed at the cortex of in vivo matured oocytes. The patterns range from a fairly homogeneous distribution (A) to distinct clusters or islands of mitochondria (C). There are also intermediate patterns of distribution which include small clusters or groups of mitochondria (B). These oocytes are all from the same female. Scale bar = 50 µm.
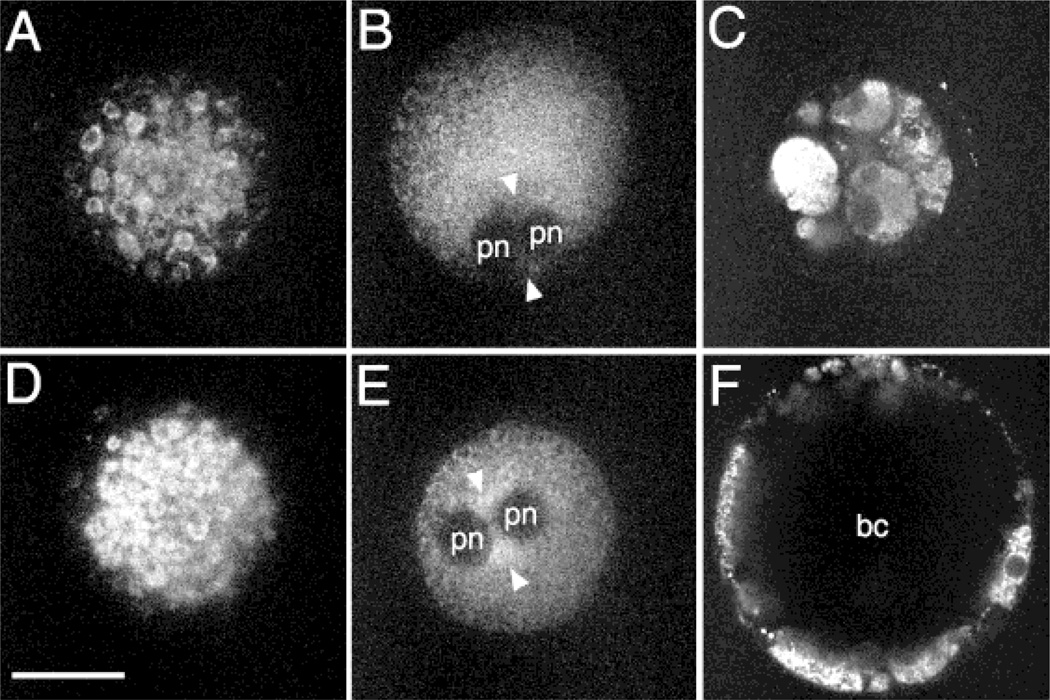
Of the eight pronucleate embryos observed, six exhibited pronounced pronuclear accumulation of mitochondria (Fig. 6E). Of these six embryos, on day 8, two had expanded blastocoel cavities, two had at least initiated blastocoel cavity formation (Fig. 6F), one was compacted, and one arrested with multiple cells but no indication of compaction or blastocoel formation. Both pronucleate ova in which the pronuclear accumulation of mitochondria was not observed (Fig. 6B) fragmented after the eight-cell stage (Fig. 6C).
Figure 6.
Possible relationship between cytoplasmic organization and developmental potential in the rhesus embryo. These are MPLSM images of living rhesus oocytes and embryos labeled with Mitotracker. A through C are images from one oocyte and the embryo derived from it while D through F are images from another oocyte and the embryo derived from it. (A, D) Two oocytes showing similar patterns of mitochondrial clustering. (B, E) The inseminated oocytes were imaged again beginning at 12 h PI. One pronucleate oocyte exhibits a pronuclear accumulation (E) of mitochondria (arrow) while the other does not (B). (C, F) These embryos were assessed for development at 8 days PI. pn: pronuclei; bc: blastocoel cavity. Scale bar = 50 µm.
The pronuclear accumulation of mitochondria was not present throughout the entire period prior to syngamy. Because the image collection was intermittent rather than continuous, with relatively large intervals between time points, it was not possible to accurately define the time during which this pronuclear accumulation occurred. However, the data can be used to estimate the range of times during which the mitochondria exist in this particular configuration. Analysis of images collected from seven females (n = 16 embryos) from both Experiments 1 and 2 indicated that this accumulation was observable beginning between 11 and 20 h PI (mean = 16 h PI) and was no longer present after 15 to 34 h PI (mean = 21 h PI). The duration of the accumulation ranged from <1 h to 10 h, with a mean duration of 3 h.
Discussion
The use of multiphoton excitation for imaging cellular and subcellular dynamics during embryo development, although relatively new, is expanding, and now includes a variety of applications in several species. Not only has it been used for studying subcellular localization of mitochondria in mammalian embryos, as described here, but it has also been used to study vesicle dynamics in early nematode embryos (Skop et al., 2001) and the localization of symbiotic bacteria in wasp oocytes (Zchori-Fein et al., 1998). Green fluorescent protein (GFP; Chalfie, 1995) fused to proteins of interest, in conjunction with MPLSM, has been used to study various aspects of cell division in nematode embryos, such as spindle dynamics, utilizing a tubulin::GFP fusion (Strome et al., 2001) or chromosome dynamics (Siomos et al., 2001) utilizing a histone::GFP fusion protein. GFP fusion proteins have also been used to study cell dynamics during cell fusion in the hypodermis during the later stages of nematode embryo development (Mohler and White, 1998; Mohler et al., 1998). The use of MPLSM within the realm of developmental biology has not been limited to fluorescent imaging. It has been used to regionally uncage fluorescent dextrans in order to follow cell lineages in the sea urchin embryo (Summers et al., 1996). The near infrared light which passes through the specimen (that which is not incorporated into a multiphoton excitation event) can be used to generate a brightfield image (Wokosin et al., 2002) and three-photon excitation can be used to image the ultraviolet-excitable dyes, such as DAPI (Wokosin et al., 1996). Furthermore, optical workstations that incorporate MPLSM with other types of optical manipulations are being developed. With such multilaser systems, double-labeled imaging as well as laser microsurgery can be performed on a single microscope (Wokosin et al., 2002).
Our laboratory has previously shown that in developmentally competent hamster embryos, at the late one-cell and throughout the two-cell stage, the mitochondria become localized close to the nuclei (Barnett et al., 1996, 1997). In contrast, in polyspermic human oocytes, the mitochondria accumulate between the multiple pronuclei (Noto et al., 1993). However, it was unclear from that study whether the distribution of mitochondria was a normal phenomenon or a consequence of aberrant fertilization. Recently, it has been demonstrated that monospermic human oocytes also showed a similar pronuclear accumulation of mitochondria (Van Blerkom et al., 2000). The study by Van Blerkom et al. (2000) suggested that there is a positive relationship between the pronuclear accumulation observed in human oocytes and their developmental capacity, although the extent of the relationship remains to be determined.
In the current study, we show that although a slight graded increase in the perinuclear density of active mitochondria is observed in some rhesus embryos at and before the two-cell stage, the highly reproducible perinuclear ring described for the hamster embryo is absent. However, fertilized rhesus oocytes do exhibit a distinct organization of mitochondria—the mitochondria accumulate between the male and female pronuclei as these become closely apposed—similar to that described for human fertilized oocytes. Another marked difference is the heterogeneity of mitochondrial organization among oocytes, which may reflect the pronounced heterogeneity of oocyte quality and developmental competence in primates, unlike the striking homogeneity of hamster oocytes (Barnett et al., 1996). It is possible that the accumulation of mitochondria in rhesus oocytes is merely a biomechanical consequence of the pronuclei coming together, resulting in a compression of the cytoplasm with a concomitant increase in density of active mitochondria. However, the movement of the male and female pronuclei towards one another occurs in other mammalian oocytes during fertilization but such an accumulation of mitochondria has not been observed, even though the organization of mitochondria during the earliest stages of development has been documented in the hamster (Barnett et al., 1996), mouse (Batten et al., 1987; Muggleton-Harris and Brown, 1988), pig (Luoh and Wu, 1996; Sun et al., 2001), and cow (Van Blerkom et al., 1990). The particular pronuclear accumulation pattern of mitochondria shown in the present study may be unique to primates. The fact that this organization has been observed, to date, only in primates clearly indicates a need for further investigations into its significance for embryo development. Furthermore, studies on how this mitochondrial organization may be affected either by culture conditions or by mechanically invasive assisted reproductive techniques should be conducted using non-human primates and will depend on the efficient use of specimens obtained.
The mechanism by which the mitochondria change their location in early mammalian embryos has not yet been entirely elucidated. It is likely that the cytoskeleton, particularly microtubules, participate in the translocation of mitochondria in these embryos. The importance of the cytoskeleton to the movement of subcellular components has been shown in the embryos of a number of species (Bement et al., 1992). Microtubules are dramatically remodeled in mammalian embryos after insemination (Navara et al., 1995; Hewitson et al., 1997; reviewed in Lehtonen et al., 1988). This massive polymerization of microtubules has been described for mice (Schatten et al., 1985), cattle (Navara et al., 1995), rats (Zernicka-Goetz et al., 1993), pigs (Kim et al., 1996), rhesus monkeys (Wu et al., 1996), and humans (Van Blerkom et al., 2000). Microtubules are used to move mitochondria and other organelles in other cell types (Schroer and Kelly, 1985; Vale, 1987; Dabora and Sheetz, 1988; Goodson et al., 1997; Hirokawa, 1998). Human pronucleate embryos exhibit a microtubule network that radiates from the nuclear region (Van Blerkom et al., 2000). Although it has not yet been definitively demonstrated, it is possible that this microtubule network guides the mitochondria to their pronuclear location in primate embryos.
The pronuclear accumulation of mitochondria in rhesus oocytes is transient, on the order of a few hours, although the exact length of time that it is present is currently uncertain. The fact that the pronuclear accumulation was not observed in some of the fertilized oocytes may be explained by slight temporal variability in their development. Because this organization is present for only a short period of time, live imaging studies based on the results presented here are crucial in order to observe such transient changes, as well as to determine their functional significance and relevance to embryo viability. It remains to be determined exactly how changes in the location of mitochondria, in any mammalian species, enhance embryo survival. In fact, a direct link between cytoplasmic organization and development may not be present but rather cytoplasmic organization may influence, and be influenced by, other parameters which impinge on embryonic health. It is also possible that embryos use cytoplasmic organizations to promote embryo development for a variety of reasons, and these reasons may be species specific (Bavister and Squirrell, 2000). For example, reorganizations in mitochondria may be providing localized energy and/or metabolites (Barnett et al., 1996; Van Blerkom et al., 2000) or may be related to changes in ionic balance (Lane and Bavister, 1998; Squirrell et al., 2001).
The ability to image oocytes prior to insemination without compromising the fertilizability and developmental potential of the oocytes promises to illuminate the importance of the subcellular organization of the oocyte on embryo development under a variety of situations. Such studies have been conducted with bovine oocytes to compare the effects of maturation medium on the mitochondrial organization of oocytes and their subsequent development (Krisher and Bavister, 1997; Stojkovic et al., 2001). With the limited availability of primate oocytes and embryos, the ability to image the same specimens before and after insemination is essential, not only for maximizing the amount of information gathered from each specimen but also for strengthening the correlations among different physiological parameters in a heterogeneous population. Because the efficiency of in vitro fertilization and subsequent embryo transfer in humans is low (Scott et al., 2000), it is important that the oocytes and early embryos with the highest potential can be selected for transfer in order to increase the probability of a successful pregnancy. Morphological analysis, because it is relatively straightforward and minimally invasive, is frequently used to assess embryo potential. For example, nuclear morphology (specifically the size and location of nucleoli) has been used as a predictor of development to the blastocyct stage in humans (Scott et al., 2000; Salumet et al., 2001), while a centrally located granular region in the oocyte cytoplasm is correlated with an increase in chromosomal abnormalities and low pregnancy rates (Kahraman et al., 2000). However, to improve the accuracy of these morphological assessments it is important to determine the biological significance of the morphological changes observed. A more extensive discussion on the possible predictive properties of oocytes and early embryos, either with MPLSM or with other analytical methods, can be found in other sources (Van Soom and Boerjan, 2002).
It is clear that minimally invasive imaging techniques are important for the study of mammalian embryos, particularly when the specimens of interest are rare, difficult to obtain in large numbers, and exhibit a high degree of variability. An imaging technique such as MPLSM can be used to determine baseline information on the subcellular organization of oocytes and embryos from rare and endangered species. However, the arguably more significant application of this technique is to determine how cellular organization influences embryonic development. The work presented here, although it does not conclusively define the role of cytoplasmic organization in primate embryo development, provides evidence for a correlation between the presence of a particular mitochondrial accumulation pattern during fertilization and subsequent development to the blastocyst stage. To demonstrate a strong biological correlation, greater number of specimens would be necessary for establishing statistical significance. Mainly, this study illustrates the importance of live imaging for understanding the relationships between early cellular events and later development and as a method of extracting as much information as possible from a limited resource. For example, this type of imaging could be used to advance studies on changes in nucleoli profiles during fertilization (Scott et al., 2000). Additionally, live imaging is a significant tool for investigating how culture conditions and other oocyte and embryo manipulations alter proper cellular organization and concomitant development. Although in the study presented here we did not compare in vivo versus in vitro developed embryos, it does set the stage for similar studies which would investigate whether or not in vitro culture alters the cytoplasmic organization of mammalian embryos. Performing in vivo versus in vitro studies can be difficult since they require large numbers of animals for the collection of in vivo developed embryos at different stages. However, a few studies have shown that changes in culture conditions can alter the organization or structure of mitochondria in the oocytes or embryos of other species (mouse—Muggleton-Harris and Brown, 1988; hamster—Barnett et al., 1997; Lane and Bavister, 1998; Squirrell et al., 2000; Ludwig et al., 2001; bovine—Krisher and Bavister, 1997; Abe et al., 1999; pig—Hyttel and Neimann, 1990). The advantage of the type of study presented here is that the embryos are imaged live, so that the effects of alterations of mitochondrial organization on subsequent development can be assessed. For this reason, and because the imaging of the living embryos must, by the very nature of the instrumentation at this point in time, be performed in culture drops rather than in vivo, MPLSM lends itself well to the study the effects of different culture conditions on embryonic development. Such studies have been performed using MPLSM to investigate the effect of various culture conditions on the mitochondrial distribution in the hamster embryo (Ludwig et al., 2001; Squirrell et al., 2001), and it will be exciting to see what additional insight will be revealed when similar studies are performed with oocytes and embryos from other species. Live cell imaging studies will improve our ability to minimize the detrimental cellular effects of in vitro manipulations and thereby improve assisted reproductive techniques, and will also aid in the development of more accurate methods for identifying the most viable oocytes and embryos.
Acknowledgments
The authors thank Dr. Victoria Centonze-Frohlich for her assistance with the microscopy and Kevin Eliceiri for his technical expertise. The authors also thank Dr. John White for his suggestions on the manuscript. This work was performed as part of the National Cooperative Program on Non-Human In Vitro Fertilization and Preimplantation Development and was funded by the National Institute of Child Health and Human Development, NIH, through cooperative agreement HD-22023, grant R00167 to WRPRC and grant R01-RR00570 to the Laboratory for Optical and Computational Instrumentation (LOCI), UW–Madison.
References
- Abe H, Yamashita S, Itoh T, Satoh T, Hoshi H. Ultrastructure of bovine embryos developed from in vitro-matured and -fertilized oocytes: Comparative morphological evaluation of embryos cultured in either serum-free medium or serum-supplemented medium. Mol Reprod Dev. 1999;53:325–335. doi: 10.1002/(SICI)1098-2795(199907)53:3<325::AID-MRD8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Barnett D, Kimura J, Bavister BD. Translocation of active mitochondria during hamster preimplantation embryo development studied by confocal laser scanning microscopy. Dev Dyn. 1996;205:64–72. doi: 10.1002/(SICI)1097-0177(199601)205:1<64::AID-AJA6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Barnett DK, Clayton MK, Kimura J, Bavister BD. Glucose and phosphate toxicity in hamster preimplantation embryos involves disruption of cellular organization, including distribution of active mitochondria. Mol Reprod Dev. 1997;48:227–237. doi: 10.1002/(SICI)1098-2795(199710)48:2<227::AID-MRD10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Batten BE, Albertini DF, Ducibella T. Patterns of organelle distribution in mouse embryos during preimplantation development. Am J Anat. 1987;178:204–213. doi: 10.1002/aja.1001780212. [DOI] [PubMed] [Google Scholar]
- Bavister BD. A minichamber device for maintaining a constant carbon dioxide in air atmosphere during prolonged culture of cells on the stage of an inverted microscope. In Vitro Cell Dev Biol. 1988;24:759–763. doi: 10.1007/BF02623645. [DOI] [PubMed] [Google Scholar]
- Bavister BD. Culture of preimplantation embryos: Facts and artifacts. Hum Reprod Update. 1995;1:91–148. doi: 10.1093/humupd/1.2.91. [DOI] [PubMed] [Google Scholar]
- Bavister BD, Boatman DE, Leibfried L, Loose M, Vernon MW. Fertilization and cleavage of rhesus monkey oocytes in vitro. Biol Reprod. 1983;28:983–999. doi: 10.1095/biolreprod28.4.983. [DOI] [PubMed] [Google Scholar]
- Bavister BD, Squirrell JM. Mitochondrial distribution and function in oocytes and early embryos. Hum Reprod. 2000;15(suppl. 2):189–198. doi: 10.1093/humrep/15.suppl_2.189. [DOI] [PubMed] [Google Scholar]
- Bavister BD, Yanagimachi R. The effects of sperm extracts and energy sources on the motility and acrosome reaction of hamster spermatozoa in vitro. Biol Reprod. 1977;16:228–237. doi: 10.1095/biolreprod16.2.228. [DOI] [PubMed] [Google Scholar]
- Bement W, Gallicano G, Capco D. Role of the cytoskeleton during early development. Micro Res Tech. 1992;22:23–48. doi: 10.1002/jemt.1070220105. [DOI] [PubMed] [Google Scholar]
- Boatman DE. In vitro growth of non-human primate pre- and peri-implantation embryos. In: Bavister BD, editor. The Mammalian Pre-implantation Embryo. New York: Plenum Press; 1998. pp. 273–308. [Google Scholar]
- Boatman DE, Bavister BD. Stimulation of rhesus monkey sperm capacitation by cyclic nucleotide mediators. J Reprod Fertil. 1984;71:357–366. doi: 10.1530/jrf.0.0710357. [DOI] [PubMed] [Google Scholar]
- Calarco PG. Polarization of mitochondria in the unfertilized mouse oocyte. Dev Genet. 1995;16:36–43. doi: 10.1002/dvg.1020160108. [DOI] [PubMed] [Google Scholar]
- Calarco PG, McLaren A. Ultrastructural observations of preimplantation stages of the sheep. J Embryol Exp Morph. 1976;36:609–622. [PubMed] [Google Scholar]
- Centonze VE, White JG. Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging. Biophys J. 1998;75:2015–2024. doi: 10.1016/S0006-3495(98)77643-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M. Green fluorescent protein. Photochem Photobiol. 1995;62:651–656. doi: 10.1111/j.1751-1097.1995.tb08712.x. [DOI] [PubMed] [Google Scholar]
- Dabora SL, Sheetz MP. Cultured cell extracts support organelle movement on microtubules in vitro. Cell Motil Cytosk. 1988;10:482–495. doi: 10.1002/cm.970100405. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M. Culture and selection of viable blastocysts: A feasible proposition for human IVF? Hum Reprod Update. 1997;3:367–382. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- Gonzales DS, Pinheiro JC, Bavister BD. Prediction of the developmental potential of hamster embryos in vitro by precise timing of the third cell cycle. J Reprod Fertil. 1995;105:1–8. doi: 10.1530/jrf.0.1050001. [DOI] [PubMed] [Google Scholar]
- Goodson HV, Valetti C, Kreis TE. Motors and membrane traffic. Curr Opin Cell Biol. 1997;9:18–28. doi: 10.1016/s0955-0674(97)80147-0. [DOI] [PubMed] [Google Scholar]
- Hewitson L, Haavisto A, Simerly C, Jones J, Schatten G. Microtubule organization and chromatin configurations in hamster oocytes during fertilization and parthenogenetic activation, and after insemination with human sperm. Biol Reprod. 1997;57:967–975. doi: 10.1095/biolreprod57.5.967. [DOI] [PubMed] [Google Scholar]
- Hillman N, Tasca R. Ultrastructural and autoradiographic studies of mouse cleavage stages. Am J Anat. 1983;126:151–174. doi: 10.1002/aja.1001260203. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Hyttel P, Niemann H. Ultrastructure of porcine embryos following development in vitro versus in vivo. Mol Reprod Dev. 1990;27:136–144. doi: 10.1002/mrd.1080270208. [DOI] [PubMed] [Google Scholar]
- Kahraman S, Yakin K, Donmez E, Samli H, Bahce M, Cengiz G, Sertyel S, Samli M, Imirzahoglu N. Relationship between granular cytoplasm of oocytes and pregnancy outcome following intracytoplasmic sperm injection. Hum Reprod. 2000;15:2390–2393. doi: 10.1093/humrep/15.11.2390. [DOI] [PubMed] [Google Scholar]
- Kim NH, Simerly C, Funahashi H, Schatten G, Day BN. Microtubule organization in porcine oocytes during fertilization and parthenogenesis. Biol Reprod. 1996;54:1397–1404. doi: 10.1095/biolreprod54.6.1397. [DOI] [PubMed] [Google Scholar]
- Krisher RL, Bavister BD. Correlation of mitochondrial organization with developmental competence in bovine oocytes matured in vitro. Biol Reprod. 1997;56:602. [Google Scholar]
- Lane M, Bavister BD. Calcium homeostasis in early hamster preimplantation embryos. Biol Reprod. 1998;59:1000–1007. doi: 10.1095/biolreprod59.4.1000. [DOI] [PubMed] [Google Scholar]
- Lehtonen E. Changes in cell dimensions and intercellular contacts during cleavage-stage cell cycles in mouse embryonic cells. J Embryol Exp Morphol. 1980;58:231–249. [PubMed] [Google Scholar]
- Lehtonen E, Ordonez G, Reima I. Cytoskeleton in preimplantation mouse development. Cell Diff. 1988;24:165–178. doi: 10.1016/0045-6039(88)90048-6. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Squirrell JM, Palmenber AC, Bavister BD. Relationship between development, metabolism, and mitochondrial organization in 2-cell hamster embryos in the presence of low levels of phosphate. Biol Reprod. 2001;65:1648–1654. doi: 10.1095/biolreprod65.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoh Y, Wu M. Mitochondrial distribution of swine ova and embryos analyzed by rhodamine 123 fluorescent assay. J Chin Soc Anim Sci. 1996;25:53–66. [Google Scholar]
- Mohler WA, Simske JS, Williams-Masson EM, Hardin JD, White JG. Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Curr Biol. 1998;8:1087–1090. doi: 10.1016/s0960-9822(98)70447-6. [DOI] [PubMed] [Google Scholar]
- Mohler WA, Squirrell JM. Multiphoton imaging of embryonic development. In: Yuste R, Lanni F, Konnerth A, editors. Imaging Neurons: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2000. pp. 21.1–21.11. [Google Scholar]
- Mohler WA, White JG. Stereo-4-D reconstruction and animation from living fluorescent specimens. Biotechniques. 1998;24:1006–1010. [PubMed] [Google Scholar]
- Muggleton-Harris AL, Brown JJ. Cytoplasmic factors influence mitochondrial reorganization and resumption of cleavage during culture of early mouse embryos. Hum Reprod. 1988;3:1020–1028. doi: 10.1093/oxfordjournals.humrep.a136815. [DOI] [PubMed] [Google Scholar]
- Navara CS, Wu GJ, Simerly C, Schatten G. Mammalian model systems for exploring cytoskeletal dynamics during fertilization. Curr Top Dev Biol. 1995;31:321–342. doi: 10.1016/s0070-2153(08)60232-x. [DOI] [PubMed] [Google Scholar]
- Noto V, Campo R, Roziers P, Swinnen K, Vercruyssen M, Gordts S. Mitochondrial distribution after fast embryo freezing. Hum Reprod. 1993;8:2115–2118. doi: 10.1093/oxfordjournals.humrep.a137992. [DOI] [PubMed] [Google Scholar]
- Panigel M, Kraemer DC, Kalter SS, Smith GC, Heberling RL. Ultrastructure of cleavage stages and pre-implantation embryos of the baboon. Anat Embr. 1975;147:45–62. doi: 10.1007/BF00317963. [DOI] [PubMed] [Google Scholar]
- Plante L, King WA. Light and electron microscopic analysis of bovine embryos derived by in vitro and in vivo fertilization. J Assist Reprod Genet. 1994;11:515–529. doi: 10.1007/BF02216032. [DOI] [PubMed] [Google Scholar]
- Salumet A, Hyden-Grankog C, Suikkari A, Tiitinen A, Tuuri T. The predictive value of pronuclear morphology of zygotes in the assessment of human embryo quality. Hum Reprod. 2001;16:2177–2181. doi: 10.1093/humrep/16.10.2177. [DOI] [PubMed] [Google Scholar]
- Schatten G, Simerly C, Schatten H. Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubule-mediated motility during mammalian fertilization. Proc Natl Acad Sci USA. 1985;82:4152–4156. doi: 10.1073/pnas.82.12.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm RD, Bavister BD. Development of in-vitro-fertilized primate embryos into blastocysts in a chemically defined, protein-free culture medium. Hum Reprod. 1996;11:1690–1697. doi: 10.1093/oxfordjournals.humrep.a019471. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Paprocki AM. Birth of rhesus monkey infant after transfer of embryos derived from in-vitro matured oocytes. Hum Reprod. 2000;15:2411–2414. doi: 10.1093/humrep/15.11.2411. [DOI] [PubMed] [Google Scholar]
- Schroer TA, Kelly RB. In vitro translocation of organelles along microtubules. Cell. 1985;40:729–730. doi: 10.1016/0092-8674(85)90329-0. [DOI] [PubMed] [Google Scholar]
- Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod. 2000;15:2394–2403. doi: 10.1093/humrep/15.11.2394. [DOI] [PubMed] [Google Scholar]
- Simerly CR, Hecht NB, Goldberg E, Schatten G. Tracing the incorporation of the sperm tail in the mouse zygote and early embryo using an anti-testicular alpha-tubulin antibody. Dev Biol. 1993;158:536–548. doi: 10.1006/dbio.1993.1211. [DOI] [PubMed] [Google Scholar]
- Siomos M, Badrinath A, Pasierbek P, Livingstone D, White JG, Glotzer M, Nasmyth K. Separase is required for chromosome segregation during meiosis I in Caenorhabditis elegans. Curr Biol. 2001;11:1825–1835. doi: 10.1016/s0960-9822(01)00588-7. [DOI] [PubMed] [Google Scholar]
- Skop AR, Bergmann D, Mohler WA, White JG. Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr Biol. 2001;11:735–746. doi: 10.1016/s0960-9822(01)00231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squirrell JM, Lane M, Bavister BD. Altering intracellular pH disrupts development and cellular organization in preimplantation hamster embryos. Biol Reprod. 2001;64:1845–1854. doi: 10.1095/biolreprod64.6.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squirrell JM, Wokosin DL, White JG, Bavister BD. Long-term two-photon fluorescence imaging of mammalian embryos without compromising viability. Nat Biotechnol. 1999;17:763–767. doi: 10.1038/11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, Wolf E. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: Correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64:904–909. doi: 10.1095/biolreprod64.3.904. [DOI] [PubMed] [Google Scholar]
- Strome S, Powers J, Dunn M, Reese K, Malone CJ, White J, Seydoux G, Saxton W. Spindle dynamics and the role of gamma-tubulin in early Caenorhabditis elegans embryos. Mol Biol Cell. 2001;12:1751–1764. doi: 10.1091/mbc.12.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers RG, Piston DW, Harris KM, Morrill JB. The orientation of first cleavage in the sea urchin embryo, Lytechinus variegatus, does not specify the axes of bilateral symmetry. Dev Biol. 1996;175:177–183. doi: 10.1006/dbio.1996.0105. [DOI] [PubMed] [Google Scholar]
- Sun QY, Wu GM, Lai L, Park KW, Cabot R, Cheong HT, Day BN, Prather RS, Schatten H. Translocation of active mitochondria during pig oocyte maturation, fertilization, and early embryo development in vitro. Reprod. 2001;122:155–163. [PubMed] [Google Scholar]
- Terasaki M, Dailey ME. Confocal microscopy of living cells. In: Pawley JB, editor. Handbook of Biological Confocal Microscopy. New York: Plenum Press; 1995. pp. 327–346. [Google Scholar]
- Vale RD. Intracellular transport using microtubule-based motors. Ann Rev Cell Biol. 1987;3:347–378. doi: 10.1146/annurev.cb.03.110187.002023. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Bell H, Weipz D. Cellular and developmental biological aspects of bovine meiotic maturation, fertilization, and preimplantation embryogenesis in vitro. J Elect Micro Tech. 1990;16:298–323. doi: 10.1002/jemt.1060160404. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Davis P, Alexander S. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: Relationship to microtubular organization, ATP content and competence. Hum Reprod. 2000;15:2621–2633. doi: 10.1093/humrep/15.12.2621. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Runner MN. Mitochondrial reorganization during resumption of arrested meiosis in the mouse oocyte. Am J Anat. 1984;171:335–355. doi: 10.1002/aja.1001710309. [DOI] [PubMed] [Google Scholar]
- Van Soom A, Boerjan M. Mammalian Embryo Quality: Invasive and Non-Invasive Techniques. Dordrecht, The Netherlands: Kluwer Academic Publisher; 2002. [Google Scholar]
- Wokosin DL, Centonze VE, White JG, Hird SN, Sepsenwol S, Malcolm GPA, Ferguson AL. Multiple-photon excitation imaging with an all-solid-state laser. SPIE. 1996;2678:38–49. [Google Scholar]
- Wokosin DL, Squirrell JM, Eliceiri KW, White JG. An optical workstation with concurrent, independent multiphoton imaging and experimental laser microbeam capabilities. Rev Sci Inst. 2002;74:1–9. doi: 10.1063/1.1524716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wokosin DL, White JG. Optimization of the design of a multiple-photon excitation laser scanning fluoresence imaging system. In: Cogswell CJ, Conchello J-A, Wilson T, editors. Three-Dimensional Microscopy: Image Acquisition and Processing. Vol. 2984. Bellingham, WA: SPIE; 1997. pp. 24–29. [Google Scholar]
- Wu GJ, Simerly C, Zoran SS, Funte LR, Schatten G. Microtubule and chromatin dynamics during fertilization and early development in rhesus monkeys, and regulation by intracellular calcium ions. Biol Reprod. 1996;55:260–270. doi: 10.1095/biolreprod55.2.260. [DOI] [PubMed] [Google Scholar]
- Zchori-Fein E, Roush RT, Rosen D. Distribution of parthenogenesis-inducing symbionts in ovaries and eggs of Aphytis (Hymentoptera: Aphelinidae) Curr Microbiol. 1998;36:1–8. doi: 10.1007/s002849900270. [DOI] [PubMed] [Google Scholar]
- Zernicka-Goetz M, Kubiak JZ, Antony C, Maro B. Cytoskeletal organization of rat oocytes during metaphase II arrest and following abortive activation: A study by confocal laser scanning microscopy. Mol Reprod Dev. 1993;35:165–175. doi: 10.1002/mrd.1080350210. [DOI] [PubMed] [Google Scholar]