Abstract
The effect of low concentrations of inorganic phosphate (Pi) on development, metabolic activity, and mitochondrial organization in the same cohorts of cultured hamster embryos was evaluated. Two-cell embryos were collected from eCG-stimulated golden hamsters and cultured in HECM-10 with 0.0 (control), 1.25, 2.5, or 5.0 µM KH2PO4. Glucose utilization through the Embden-Meyerhof pathway (EMP) and tricarboxylic acid (TCA)-cycle activity were determined following 5 h of culture. Mitochondrial organization in living embryos was evaluated using multiphoton microscopy at 6 h of culture. Development was assessed at 27 h (on-time 8-cell stage) and 51 h (on-time blastocyst stage) of culture. Total cell numbers, as well as cell allocation to the trophectoderm and inner cell mass were determined for morula- and blastocyst-stage embryos. Culture with Pi did not alter TCA-cycle activity. However, culture with ≥2.5 µM Pi significantly increased (P < 0.01) EMP activity compared to control. Mitochondrial organization was significantly (P < 0.01) disrupted by Pi in a dose-dependent manner. Development to the 8-cell, morula/blastocyst, and blastocyst stages was significantly reduced (P < 0.05) in the presence of ≥2.5 µM Pi compared to both control and 1.25 µM Pi. This study clearly demonstrates that, for hamster embryos, inclusion of even exceptionally low concentrations of Pi in culture medium dramatically alters embryo physiology. Additionally, although 2-cell embryos can tolerate some structural disruption without concomitant, detrimental effects on development or metabolic activity, metabolic disturbance is associated with decreased developmental competence.
Keywords: developmental biology, early development, embryo
INTRODUCTION
The successful application of many new technologies, including genetic engineering, cloning, and xenotransplantation, are dependent, in large part, on our capacity to support the development of preimplantation mammalian embryos in culture. The ability to remove embryos from the female reproductive tract and to manipulate them in vitro has made these advances possible. Currently, a major limitation to widespread application of these technologies is our inability to consistently produce high-quality, developmentally competent embryos in vitro. The developmental competence (ability to produce a viable fetus) of cultured embryos is significantly reduced compared to in vivo-produced counterparts [1, 2]. In addition to enhancing our knowledge regarding basic developmental processes, increasing our understanding of how culture media components affect preimplantation embryos will facilitate the design of more suitable culture conditions, resulting in overall improved developmental competence of in vitro-cultured embryos. This, in turn, will allow us to maximize the benefit of technologies that are dependent on embryo culture.
An adequate supply of energy is vital for the developmental competence of preimplantation embryos. The dynamic period of development between formation of the zygote and of the blastocoele is metabolically very costly for the embryo [3]. Alterations in the metabolic profile could result in abnormal development or death of the embryo. Media components that alter the metabolic profile of the embryo can dramatically alter developmental competence of the embryo [4, 5]. Inclusion of oxidative phosphorylation inhibitors in the culture medium inhibits development of early cleavage stages in rats [4] and of all stages in mice [6]. Furthermore, studies of embryonic oxygen consumption in mice [7] and hamsters [8] show that conditions altering the rate of oxygen consumption also decrease the developmental competence of the embryo.
One pair of media components eliciting interest is glucose and inorganic phosphate (Pi). Glucose is an energy substrate that is present in the reproductive tract of most species [9–13], and Pi is essential for the production of ATP, the basic energy source for cell activities [14]. Considerable evidence, however, shows that inclusion of glucose/Pi in the culture medium is inhibitory to embryo development in a variety of species, including rodents [15–18], domestic animals [19, 20], and humans [21, 22]. In hamster embryos, glucose/Pi arrests development [23, 24], reduces respiration [8], and disrupts mitochondrial organization [25, 26].
Recent studies have shown, however, that glucose in the absence of Pi does not inhibit embryo development [27], suggesting that Pi alone may be responsible for the developmental inhibition previously attributed to the glucose/Pi combination. Whereas Pi alone is not inhibitory to hamster embryo development from the 8-cell stage [24], studies with earlier cleavage-stage embryos show that, even in the absence of glucose, Pi concentrations as low as 500 nM inhibit development in both hamsters and mice [23, 28–31]. Even with striking improvements in media formulations, a more recent report confirmed that concentrations of Pi as low as 2.5 µM alter ionic homeostasis and inhibit development of the embryo in culture [32]. The mechanism for this action remains to be elucidated.
It has been suggested that the mechanism for developmental inhibition in cultured hamster embryos may result from perturbed energy metabolism of the early embryo and/or disrupted mitochondrial organization, but such studies have only been performed in the presence of glucose [25]. Glucose can drive its own metabolism [14], so changes in metabolic profile in the presence and the absence of glucose in the medium are not surprising. Additionally, to our knowledge, only concentrations of Pi known to cause complete developmental inhibition (350 mM) have been examined. This makes it impossible to determine whether the observed developmental inhibition is the cause or the result of changes in the metabolic profile or mitochondrial organization of the embryo. Furthermore, although the developmental block in the presence of glucose/Pi is associated with disrupted mitochondrial organization [25], no study, to our knowledge, has examined the relationship between the mitochondrial organization and the metabolic profile of the early embryo. In the present study, we determined the effect of 0.0, 1.25, 2.5, and 5.0 µM Pi in the absence of glucose on development, mitochondrial organization, and metabolic profile within the same cohorts of embryos. Because this approach analyzed multiple parameters in the same experiment, it maximized the ability to correlate physiological parameters. Additionally, the use of multiple concentrations of Pi, including ones that are known not to cause developmental inhibition, permitted determination of a hierarchy and, thereby, answered the question of whether changes in developmental competence are preceded by alterations in metabolic profile or mitochondrial distribution.
MATERIALS AND METHODS
Culture Media
The base medium used in this study was a protein-free, chemically defined medium HECM-10 [33]. The formulation was as follows: 113.8 mM NaCl, 3.0 mM KCl, 1.0 mM CaCl2·2H2O, 2.0 mM MgCl2·6H2O, 25.0 mM NaHCO3, 4.50 mM dl-sodium lactate, 0.01 mM asparagine, 0.01 mM aspartate, 0.01 mM cysteine, 0.01 mM glutamate, 0.20 mM glutamine, 0.01 mM glycine, 0.01 mM histidine, 0.01 mM lysine, 0.01 mM proline, 0.01 mM serine, 0.5 mM taurine, 3 µM pantothenate, and 0.1 mg/ml of polyvinyl alcohol. Treatments were prepared by modifying the base medium to contain varying concentrations (0.0, 1.25, 2.5, or 5.0 µM) of Pi (KH2PO4). Media were prepared the day before use from stock solutions and stored at 4°C. All salts, carbohydrates, amino acids, and vitamins were purchased from Sigma Chemical Co. (St. Louis, MO).
Measuring pH and Osmolarity of the Media
Culture medium was divided into 5-ml aliquots in 10-ml culture tubes (Falcon Plastics, Becton-Dickinson, Franklin Lakes, NJ) and treated with 0.0 or 0.35 mM NaH2PO4 or 0.35 mM KH2PO4. Osmolarity of each medium was determined using an Osmette osmometer (Precision Scientific, Natick, MA). The tubes, with caps loosened, were equilibrated in a humidified atmosphere of 10% (v/v) CO2, 5% O2, and 85% N2 at 37.5°C for 2 h. The pH meter (Corning Life Sciences, Acton, MA) was adjusted and calibrated for reading 37°C solutions. Tubes were randomly removed from the incubator and placed into a 37°C hot block, and the pH measurement was obtained immediately.
Embryo Collection
These investigations were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals and in accordance with the local guidelines of the Research Animal Resources Center at the University of Wisconsin-Madison (Animal Use Protocol A-07-5600-A00502-3-03-97). Embryos were collected from 3- to 6-mo-old, cycling golden hamsters. Multiple ovulations were induced by an i.p. injection of 10–20 IU (indexed to body weight) of eCG (Gestyl, Houston, TX) on the morning of the postestrus discharge (Day 1 of cycle). Females were mated to fertile males on the evening of Day 4. Two-cell embryos were collected at 29 h post-egg activation (PEA) as described by Barnett and Bavister [34]. Embryos were flushed from the oviduct with warmed and equilibrated (10% CO2, 5% O2, and 85% N2) HECM-10 medium (no Pi). All embryos were then washed twice in HECM-10 and once in the appropriate treatment culture medium before being placed into culture.
Embryo Culture
All embryos were cultured in 35-µl drops of medium under mineral oil (Sigma) in groups of 5–12 embryos for either 5 h (for metabolic studies), 6 h (for cytoplasmic studies), or 51 h (for development to the blastocyst stage). Culture dishes, including media and oil overlay, were pre-equilibrated for at least 5 h before addition of embryos. Three replicates were performed. Each replicate assessed all three parameters in the same cohort of embryos. Embryos were cultured at 37.5°C in a humidified atmosphere of 10% CO2, 5% O2, and 85% N2. Embryos from individual females were distributed among treatments so that each treatment group (i.e., each concentration of Pi) for the assessment of each parameter (i.e., development, metabolism, and mitochondrial organization) contained an equivalent number of embryos from any given female. Embryos from multiple females were pooled in each culture drop [35].
Morphology Assessment
Development to the 8-cell stage was assessed after 27 h of culture, and morula and blastocyst development was assessed after 51 h of culture. Development to the morula and blastocyst stages was expressed as a single endpoint, in addition to blastocyst development, due to difficulties in accurately distinguishing morula- from blastocyst-stage hamster embryos. The hamster blastocyst in vitro undergoes repeated cycles of expansion and collapse [36], and following collapse, a blastocyst is indistinguishable from a morula using low-power microscopy. Therefore, embryos identified as morula and blastocyst (morula/blastocyst)-stage embryos include all postcompaction embryos, whereas embryos identified as blastocyst-stage embryos include only those embryos exhibiting a distinct blastocoele at the time of examination.
Differential Staining of Inner Cell Mass and Trophectoderm Cells
Embryo cell number and allocation of cells to the inner cell mass (ICM) and trophectoderm (TE) were determined by differentially staining the cell nuclei using the technique described by Lane et al. [33]. The base medium used for staining procedures was a modified BM-3 medium [35] in which 20 mM NaHCO3 was replaced with 20 mM Hepes (pH 7.4; HBM-3). Blastocysts were incubated in 0.5% (w/v) pronase in H-BM-3 for 30 sec to dissolve the zona pellucida. Embryos were then washed in H-BM-3 and incubated in 0.25% (v/v) picrylsulfonic acid (Sigma) for 10 min at 4°C before a further wash and 10-min incubation in 0.1 mg/ml of Anti-DNP BSA (ICN Technologies, Costa Mesa, CA) at 37°C. Following incubation with the antibody, embryos were again washed in H-BM-3 and incubated in a 1:5 (v/v) dilution of guinea pig serum (ICN Technologies) containing 25 µg/ml of propidium iodide (Sigma) in H-BM-3 for 5 min. Embryos were subsequently placed in 25 µg/ml of bisbenzimide (Hoechst 33258; Sigma) in ethanol overnight at 4°C. The following morning, differential staining of nuclei was examined using a Nikon optiphot epifluorescence microscope (Nikon, Melville, NY).
Metabolic Assessment
A modification of the hanging-drop technique [37, 38], as described by Krisher et al. [39], was used to determine simultaneously the metabolism of d-5[3H]glucose (specific activity, 15.9 Ci/mmol; Amersham Life Science, Buckinghamshire, England) and l-[14C(U)]glutamine (specific activity, 250 Ci/mol; ARC, St. Louis, MO). The production of 3H2O from glucose was used as a measure of glucose metabolism through the Embden-Meyerhof pathway (EMP), and the production of 14CO2 from glutamine was used to determine oxidation of glutamine through the tricarboxylic acid (TCA) cycle.
Assessment of Cytoplasmic Organization
Staining
Mitochondria of cultured embryos were labeled with the active mitochondria-specific dye Mitotracker-X-Rosamine (Molecular Probes, Eugene, OR) as described by Squirrell et al. [40]. Live embryos were incubated for 15 min in 330 nM Mitotracker in HECM-10 under culture conditions. Embryos were rinsed twice in 35-µl drops of HECM-10, then placed into 10-µl drops in imaging dishes [41] with up to six embryos per drop and one drop for each treatment in each dish. Because embryos had to be transported in culture drops to the imaging facility, all imaging drops were made with HECM-10 to eliminate the risk of contamination of medium from one treatment drop to another during transport. Embryos were transported in a warmed, gassed, covered chamber. The dishes were placed in an environmentally controlled minichamber on the microscope stage [42].
Multiphoton laser scanning microscopy
The multiphoton laser scanning microscopy (MPLSM) system, which was designed and used at the Integrated Microscopy Resource at the University of Wisconsin, has been previously described [43]. Briefly, the system utilized a 1047-nm fixed wavelength, femtosecond diode-pulsed, yttrium:lithium:fluoride laser (DPM 1000-PC; Microlase/Coherent, Santa Clara, CA) with a Nikon Eclipse microscope and a 100× oil-immersion objective. Digital images were recorded using the Lasersharp software (BioRad, Hercules CA). Single optical cross-sections through the nuclei were collected from each of the embryos in the control drop, then from each of the embryos in each of the Pi-containing drops. To confirm that changes observed in mitochondrial distribution were due to the experimental treatments and not an artifact of either the imaging itself or the time spent on the microscope stage, single optical sections were again collected from each control embryo. Embryos were returned to the incubator, and development to the morula and blastocyst stages was assessed at the same time point as the developmental study to confirm that no adverse effects from the transportation and imaging had occurred.
Quantitation of mitochondrial distribution
The pattern of distribution of mitochondria labeling in 2-cell embryos was quantified using the method developed by Barnett et al. [25] and described by Squirrell et al. [44]. All measurements were made on the MPLSM digital images using NIH Image software (Bethesda, MD). The data analyzed were the ratios of the average pixel intensity in a 5-µm diameter circle set 4 µm in from the cortex (intermediate region) and a 5-µm diameter circle adjacent to the nuclear membrane (perinuclear region). Two such ratios were collected from each blastomere. The location for collection of the regional pixel intensity was assigned using a straight line bisecting the nucleus and parallel to the junction of the two blastomeres. The circles for pixel intensity collection were centered on this line in their respective regions.
Statistical Analysis
A Student t-test was used to compare the pH of culture media. For development and metabolic experiments, data were subjected to least-squares analysis of variance (ANOVA) using the general linear models (GLM) procedure of the Statistical Analysis System (SAS Institute Inc., Cary, NC). Percentage data were arcsine transformed and weighted for the number of embryos in each experiment before analysis. The ANOVA was performed for a randomized, complete-block design structure, with block being the day on which the replicate was performed and treatment being increasing concentrations of Pi. All tests of hypotheses were performed using appropriate error terms according to the expectation of the mean squares. Differences were detected by least-squares means. For mitochondrial organization studies, data were compared using the GLM procedure in SAS [45], blocking on day, with embryo as the experimental unit. Treatment differences were compared using the least-squares means analysis.
RESULTS
Effect of Pi on Osmolarity and pH of Medium
Previous work describing the developmental inhibitory effects of Pi was conducted with medium containing 0.35 mM Pi. Therefore, the pH of the equilibrated embryo culture medium, with or without 0.35 mM Pi, was measured. The pH of the equilibrated control medium was 7.26 ± 0.06. The addition of 0.35 mM Pi had no significant effect (P > 0.1) on the pH of the medium compared to control (NaH2PO4 = 7.24 ± 0.04, KH2PO4 = 7.20 ± 0.02). The osmolarity of the medium without Pi, with 0.35 mM NaH2PO4, or with 0.35 mM KH2PO4 was 310, 310, and 311 mOsm, respectively.
Effect of Low Levels of Pi on Development
Embryos were assessed at 27 h of culture to determine the effect of low levels of Pi on on-time development to the 8-cell stage. No effect of day on treatment was found. Culture with 1.25 µM Pi had no significant effect on mean cell number or percentage of embryos reaching the 8-cell stage at 27 h of culture (Table 1). However, culture with ≥2.5 µM Pi significantly decreased (P < 0.05) both the percentage of embryos reaching the 8-cell stage and the mean cell number at 27 h of culture in a dose-dependent manner. None of the embryos cultured with 5.0 µM Pi reached the 8-cell stage at this time point. Formation of a presumptive blastocoele cavity was observed in 4% of embryos cultured from the 2-cell stage for 27 h with 1.25 µM Pi.
TABLE 1.
Development of embryos cultured from the 2-cell stage in phosphate at 27 h of culture.*
| Concentration of Pi (µM) in HECM-10 |
% 8-cell embryos at 27 h of culture |
Mean cell number (± SEM) at 27 h of culture |
|---|---|---|
| 0.0 | 84.8a | 7.3 ± 0.2a |
| 1.25 | 80.4a | 7.4 ± 0.2a |
| 2.5 | 45.6b | 5.6 ± 0.2b |
| 5.0 | 0.0c | 3.0 ± 0.2c |
Values within a column with different superscript letters are significantly different (P < 0.05).
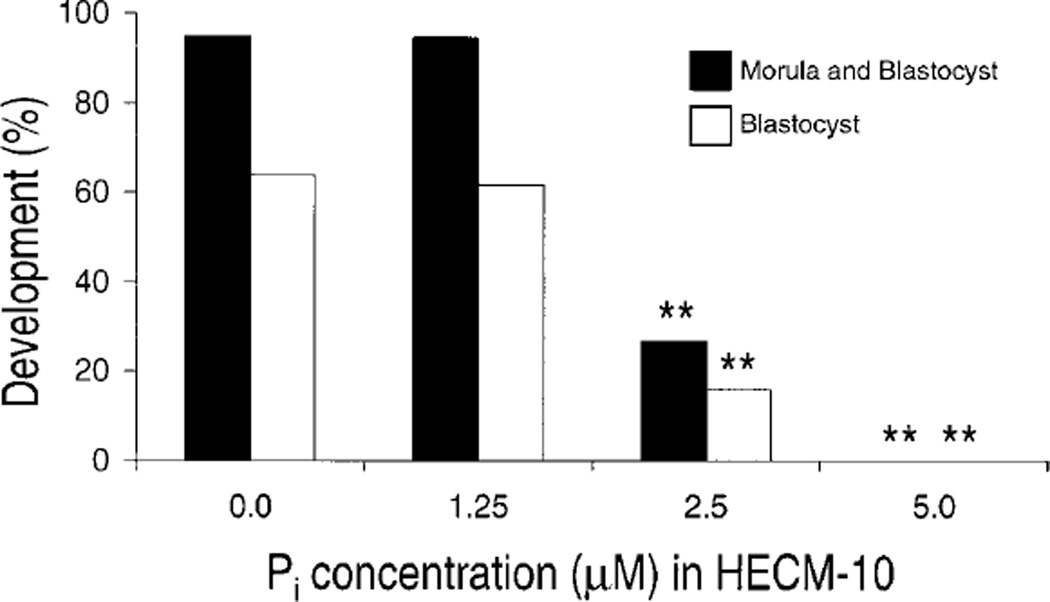
The effect of Pi on development to the morula/blastocyst stage was assessed at 51 h of culture. Culture of 29-h PEA, 2-cell embryos with 1.25 µM Pi had no significant effect on either morula/blastocyst or blastocyst development compared to control (Fig. 1). However, addition of ≥2.5 µM Pi significantly decreased morula/blastocyst and blastocyst development compared to control (P < 0.01). Additionally, culture with 5.0 µM Pi prevented development to the morula and blastocyst stages.
FIG. 1.
Effect of Pi on development to the morula and blastocyst stage in embryos cultured from the 2-cell stage (n = 12–24 embryos/treatment [depending on replicate], three replicates). **Significantly different from control (P < 0.01).
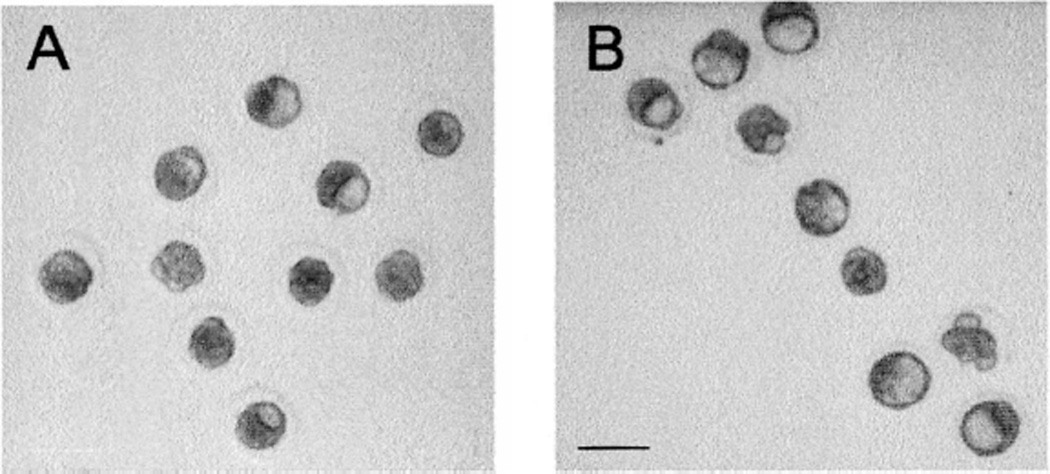
Whereas culture with 0.0 and 1.25 µM Pi resulted in statistically equivalent percentages of blastocysts, the morphology of the resulting blastocysts differed depending on the treatment. At visual examination, blastocysts cultured with 1.25 µM Pi had larger and more prominent blastocoele cavities than those cultured without Pi (Fig. 2A).
FIG. 2.
Morphological differences in blastocoele cavity formation caused by culture with Pi. These photographs show embryos cultured for 48 h from the 2-cell stage in A) HECM-10 or B) HECM 10 + 1.25 µM Pi. Bar = 100 µm. Note that blastocoele cavities were clearly larger and more prominent in the embryos cultured with low levels of Pi.
Cell counts were obtained to determine the effect of Pi on total mean cell number of morula/blastocyst-stage embryos as well as on cell allocation to the TE and ICM. In morulae and blastocysts derived from 2-cell embryos cultured without Pi, approximately 57% of the total mean cell number were TE cells, and 43% were ICM cells (Table 2). The presence of Pi significantly (P < 0.01) reduced total mean cell number and TE cell number in a dose-dependent manner. Culture with 2.5 mM Pi also significantly (P < 0.01) reduced ICM cell number compared to control. No morulae or blastocysts were obtained from culture of embryos with 5.0 mM Pi.
TABLE 2.
Effect of phosphate on morula/blastocyst (M/B) mean cell number and allocation (± SEM) of embryos cultured from the 2-cell stage.*
| Concentration Pi (µM) in HECM-10 |
Total mean M/B cell number |
Mean TE cell number |
Mean ICM cell number |
Mean ICM % of total cells |
|---|---|---|---|---|
| 0.0 | 31.8 ± 1.6a | 18.4 ± 1.1a | 13.4 ± 1.0a | 42.9 ± 2.7ab |
| 1.25 | 26.4 ± 1.4b | 13.8 ± 1.0b | 12.6 ± 0.9a | 47.1 ± 2.3a |
| 2.5 | 17.0 ± 2.8c | 11.0 ± 2.0c | 6.0 ± 1.8b | 32.5 ± 4.8b |
Values within a column with different superscript letters are significantly different (P < 0.01).
Effect of Pi on Metabolic Profile
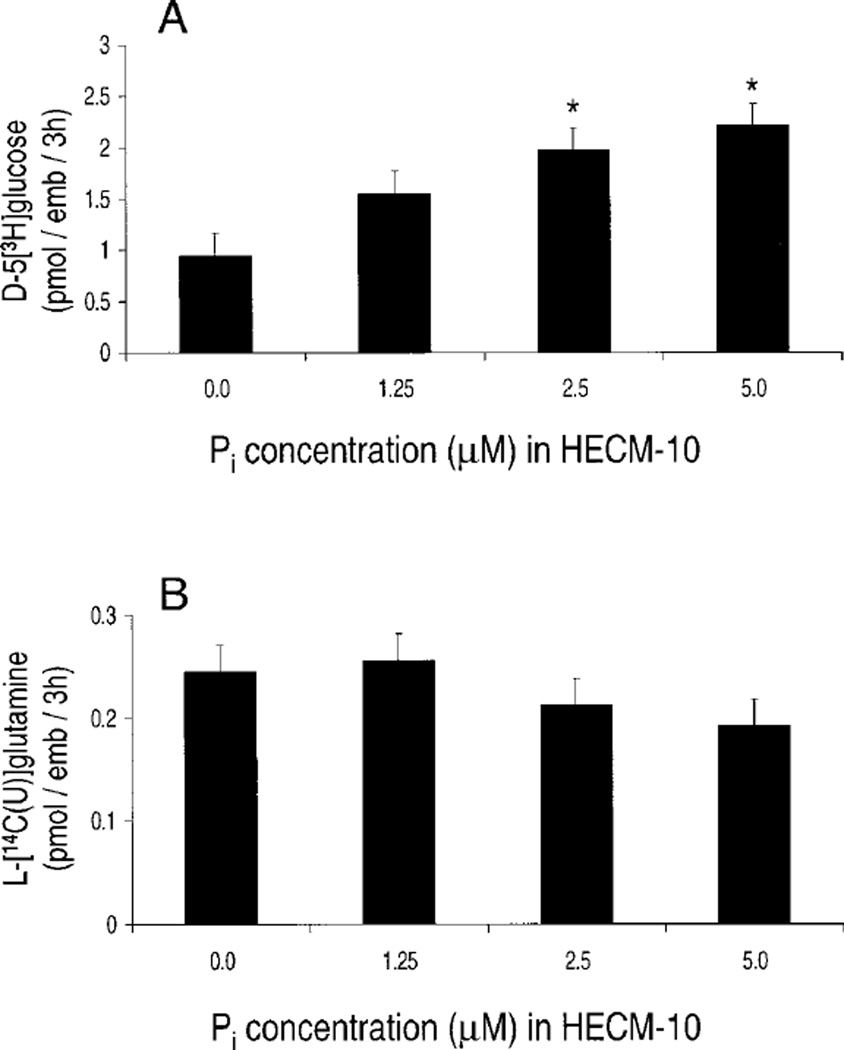
The effect of low Pi concentrations on the metabolic profile of 2-cell embryos was determined. Culture of 2-cell embryos with 1.25 µM Pi for 5 h had no significant effect on EMP activity as determined by utilization of d-5[3H]glucose (Fig. 3A). However, culture with both 2.5 and 5.0 µM Pi significantly (P < 0.01) increased EMP activity. In contrast, culture with up to 5 µM Pi had no observable effect on TCA-cycle activity as determined by utilization of l-[14C(U)]glutamine (Fig. 3B).
FIG. 3.
Effect of Pi on metabolism of A) d-5[3H]glucose through the EMP and B) l-[14C(U)]glutamine through the TCA cycle (n = 6–8 embryos/treatment [depending on replicate], three replicates). *Significantly different from control (P < 0.01).
Effect of Pi on Mitochondrial Organization
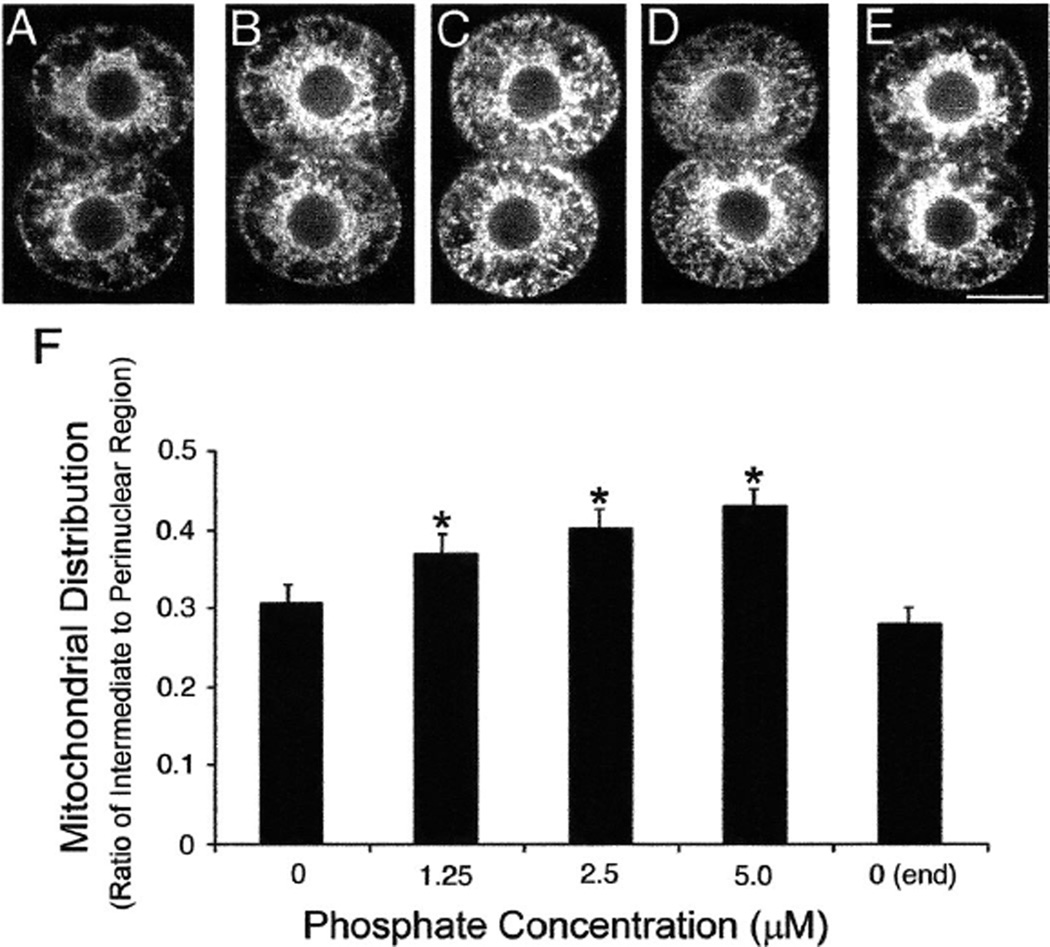
Two-cell embryos cultured in control medium exhibited a perinuclear distribution of mitochondria (Fig. 4A), as previously described [25, 26]. This pattern was maintained throughout the imaging period (Fig. 4E). With the addition of 1.25 µM Pi, the mitochondria were not only in the perinuclear region but also increasingly in the intermediate cytoplasm (Fig. 4B). This mitochondrial relocalization was more dramatic with increasing concentrations of Pi (Fig. 4, B–D). This observation was confirmed when the pattern of mitochondrial distribution was quantitated (see Materials and Methods). Culture with Pi resulted in a pattern of mitochondrial organization that was significantly (P < 0.05) different from the control (Fig. 4F). No difference was found in the pattern of mitochondria in the control embryos imaged at the beginning (Fig. 4A) and again at the end (Fig. 4E) of the imaging period.
FIG. 4.
Distribution of mitochondria in 2-cell embryos cultured with increasing concentrations of Pi. These MPLSM micrographs illustrate the mitochondrial organization in 2-cell embryos labeled with Mitotracker and cultured for 5.5 h in HECM-10 containing different concentrations of Pi: A) 0 µM, B) 1.25 µM, C) 2.5 µM, D) 5.0 µM, and E) a second image of the same embryo shown in A collected following the imaging of all other embryos in all other treatments. All embryos shown are from the same replicate; bar = 25 µm. F) The graph shows the quantitation of the mitochondrial distribution (see Materials and Methods for explanation of quantitation). *Significantly different from control (P < 0.01).
DISCUSSION
The developmental competence of the embryo is related to its metabolic profile [4, 5, 15, 46] and, possibly, to its subcellular organization [25, 47–49]. Any specific relationship or hierarchy has remained unclear, however, because no single study has examined all parameters within the same embryos. Therefore, the present study was designed to allow direct comparisons to be made of the changes in different physiological parameters within one experiment. To our knowledge, this is the first time that the same cohort of embryos has been used to simultaneously assess developmental competence, metabolic profile, and mitochondrial organization. Culture with 350 mM Pi is known to halt development [23, 28, 29, 50], to have dramatic effects on mitochondrial organization [25], and to reduce oxygen consumption [8] in hamster embryos. Therefore, we used Pi to analyze the relationship between these physiological parameters. By using lower concentrations of Pi, including those that do not cause severe developmental inhibition, we were able to analyze subtle but potentially important changes in the developing embryo. Because equal numbers of embryos were taken from donor females to assess each parameter, and because these assessments were run concurrently, variation due to donor was eliminated within each replicate. This allowed us to be confident that any effects seen within a replicate were due to the treatment and, thus, increased the strength of the conclusions drawn regarding the interaction between development, metabolism, and cytoplasmic organization. Similar to genetic epistatic studies, use of this approach also allowed us to determine a hierarchy of changes.
Although embryo physiology can be affected by changes in the pH or osmolarity of the medium [51–53], neither the pH nor the osmolarity of HECM-10 was altered by the inclusion of even relatively high levels of Pi. Furthermore, the early hamster embryo can compensate for small changes in external pH [54]. Therefore, the mechanism of action for Pi on embryo physiology as described in the experiments presented here is not due to alterations in the pH or osmolarity of the culture medium.
Mitochondrial organization was significantly affected by treatment with Pi. Even concentrations of Pi that did not alter development to the blastocyst stage or the metabolic profile significantly disrupted mitochondrial organization in the 2-cell embryo. Subcellular localization is important for the function of a number of somatic cell types, and cytoplasmic reorganization is an important and necessary part of embryonic development in invertebrates and lower vertebrates [55–58]. Although much remains to be learned concerning the significance of changes in cytoplasmic organization during early mammalian development, a number of studies have suggested a relationship with embryo developmental competence [47–49, 59]. In cultured hamster embryos, perturbed mitochondrial distribution has been associated with developmental block, and considerable evidence suggests that the spatial positioning of mitochondria may be a prerequisite for normal development [25, 26, 44, 60]. The present study marks the first instance, to our knowledge, that the disruption of mitochondrial organization in hamster embryos was not associated with a developmental block, and it shows that embryos can compensate for some level of disruption in mitochondrial organization without concomitant developmental inhibition. However, disturbances in cytoplasmic organization may be a precursor to developmental and/or metabolic disruption and, therefore, could be an important marker. Our data indicate that cytoplasmic organization is more sensitive than enzymatic pathways to culture conditions, because effects on mitochondrial distribution occurred at lower Pi concentrations than did detectable effects on embryo metabolism. Furthermore, this study makes the important observation that subcellular organization of embryos can change dramatically and is exquisitely sensitive to culture conditions. A more thorough understanding of the subcellular organization of mammalian embryos in culture may be particularly important for determining the effects of physically invasive procedures such as intra cytoplasmic sperm injection (ICSI) and nuclear transfer.
The metabolic profile of 2-cell hamster embryos was also altered following culture with Pi. Although no detectable difference in TCA-cycle activity of embryos treated with up to 5 µM Pi was found, treatment with as little as 2.5 µM caused a significant increase in EMP activity (a measure of glycolytic activity). Previous studies have shown that changes occur in the metabolic profile of the embryo in the presence of Pi. Seshagiri and Bavister [8] noted what they termed a “Crabtree-like effect,” referring to a decrease in oxygen consumption in the presence of Pi (and glucose) at the 8-cell stage [8]. However, this does not appear to be the case in the hamster 2-cell embryo, which is not surprising. Although some limited TCA-cycle activity occurs at this stage, mitochondria are probably immature at this point [59, 61, 62] and, therefore, potentially unable to perform additional oxidative phosphorylation. Low TCA-cycle activity at this stage may not be critical to appropriate embryo development, because studies that have omitted Pi from the culture media for embryo development [22, 35] suggest that the early embryo is able to recycle its intracellular Pi to meet its energy demands. However, if the end product of the increased glycolysis (pyruvic acid) seen in the presence of Pi is not being processed through the TCA cycle, then we can assume that it is, instead, being shunted into lactate production [63]. This would result in a decrease of ATP production (2 ATPs vs. 36 ATPs per glucose molecule) rather than the increase that might be expected to result from increased glycolysis. A significant shift in the amount of lactic acid being produced could also alter the intracellular ionic homeostasis and decrease the viability of the cell. Previous studies have demonstrated that “blocking strains” of mice (i.e., those in which embryos exhibit a 2-cell block in culture) have increased lactate production [64]. Furthermore, alterations in intracellular pH disrupt mitochondrial organization [44] and embryo development [65].
The increased glycolytic activity observed in this study was associated with decreased developmental competence. Although culture with 1.25 µM Pi altered mitochondrial organization, only those concentrations of Pi with a significant effect on glycolytic activity altered on-time development to the 8-cell and the morula/blastocyst stages. Development timing to both the 8-cell and blastocyst stages has been correlated with fetal viability [66–68]. Surprisingly, the percentages of embryos developing to the morula/blastocyst stage were the same in the 1.25 µM Pi treatment and the control, but the mean cell numbers of these morulae and blastocysts was not. Culture with 1.25 µM Pi decreased total mean cell number and TE cell number at the morula/blastocyst stage compared to controls, indicating that some developmental differences occur even at the lowest concentrations of Pi examined. Cell number further decreased as the Pi concentration in the medium increased, concomitant with a decrease in development, confirming that the effect of Pi on the developing hamster embryo is dose dependent.
The decrease in cell number in the presence of Pi is especially interesting considering the presence of precocious blastocoele cavities at the 8-cell stage (blastocoele cavities have not previously been noted at this early time point in any medium or in vivo) and the visual difference in the embryos at the blastocyst stage when compared to control embryos. One generally expects that formation of earlier and larger blastocysts translates into increased cell numbers, but this clearly was not the case in the present study. The presence of precocious blastocysts at the 8-cell stage and larger blastocoele cavities at the morula/blastocyst stage, despite reduced cell numbers, may provide insight regarding the mechanism of action for Pi in the preimplantation embryo. As the embryo clearly is not developing faster in the presence of Pi, the difference in visual morphology is probably due to an increase in fluid transport into the blastocoele cavity. Formation of the blastocoele cavity is dependent on the Na/K-ATPase [69]. Because the control medium already contains 3.0 mM KCl, it seems highly unlikely that the minute increase in K+ contributed by the addition of 1.25 µM KH2PO4 would result in the precocious blastulation that was observed. More likely, this effect is due to increased intracellular calcium ([Ca2+]i). In his 1988 review, Yingst [70] noted that changes in [Ca2+]i can affect the function of the Na/K-ATPase, with the specific effect (increase or decrease) being dependent on the cell type. Previous reports from our laboratory demonstrate that levels of [Ca2+]i increased in the presence of Pi [32]. Therefore, Pi may work to enhance blastocoele formation in preimplantation hamster embryos by increasing [Ca2+]i and, thereby, stimulating Na/K-ATPase in blastocysts. Additionally, changes in [Ca2+]i can affect numerous cellular events, including protein synthesis, DNA replication, mitochondrial function, and cell-to-cell communication [71– 74], all of which are necessary for normal development and appropriate metabolic profile. Therefore, the results presented here, in addition to previous work in our laboratory [32], increasingly suggest that the deleterious effect of Pi on preimplantation hamster embryos may be mediated by increasing levels of [Ca2+]i.
In summary, inclusion of even exceptionally low concentrations of Pi dramatically alters the physiology of cultured hamster embryos. Mitochondrial distribution is most sensitive to changes in media composition and may be an important marker for assessing developmental competence. Finally, whereas embryos can tolerate some level of organizational disruption without concomitant changes in blastocyst development or metabolism, increased glycolytic activity caused by Pi is associated with decreased developmental competence. This observation may have implications in other species for embryos developing in culture.
Acknowledgments
This work was done as part of the National Cooperative Program on Non-Human In Vitro Fertilization and Preimplantation Embryo Development and was funded by the National Institute of Child Health and Human Development, National Institutes of Health, through cooperative agreement HD-22023.
The authors wish to thank Dr. John White for use of his MPLSM system (funded by National Institutes of Health R01 RR00570-29A1) to analyze the mitochondrial distribution. We are also indebted to Marchel Hill for her expert technical assistance and to Susan McKiernan for her review of this manuscript.
REFERENCES
- 1.Thompson JG. In vitro culture and embryo metabolism of cattle and sheep embryos—a decade of achievement. Anim Reprod Sci. 2000;60–61:263–275. doi: 10.1016/s0378-4320(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 2.Bavister BD. Culture of preimplantation embryos: facts and artifacts. Hum Reprod Update. 1995;1:91–148. doi: 10.1093/humupd/1.2.91. [DOI] [PubMed] [Google Scholar]
- 3.Brinster RL. Embryo development. J Anim Sci. 1974;38:1003–1012. doi: 10.2527/jas1974.3851003x. [DOI] [PubMed] [Google Scholar]
- 4.Brison DR, Leese HJ. Blastocoele cavity formation by preimplantation rat embryos in the presence of cyanide and other inhibitors of oxidative phosphorylation. J Reprod Fertil. 1994;101:305–309. doi: 10.1530/jrf.0.1010305. [DOI] [PubMed] [Google Scholar]
- 5.Thompson JG, Partridge RJ, Houghton FD, Cox CI, Leese HJ. Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J Reprod Fertil. 1996;106:299–306. doi: 10.1530/jrf.0.1060299. [DOI] [PubMed] [Google Scholar]
- 6.Thompson JL. Effect of inhibitors of carbohydrate metabolism on the development of preimplantation mouse embryos. Exp Cell Res. 1967;41:411–427. doi: 10.1016/0014-4827(67)90063-8. [DOI] [PubMed] [Google Scholar]
- 7.Houghton FD, Thompson JG, Kennedy CJ, Leese HJ. Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev. 1996;44:476–485. doi: 10.1002/(SICI)1098-2795(199608)44:4<476::AID-MRD7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Seshagiri PB, Bavister BD. Glucose and phosphate inhibit respiration and oxidative metabolism in cultured hamster 8-cell embryos: evidence for the “Crabtree effect”. Mol Reprod Dev. 1991;30:105–111. doi: 10.1002/mrd.1080300206. [DOI] [PubMed] [Google Scholar]
- 9.Nichol R, Hunter RHF, Gardner DK, Leese HJ, Cooke GM. Concentrations of energy substrates in oviductal fluid and blood plasma of pigs during the peri-ovulatory period. J Reprod Fertil. 1992;96:699–707. doi: 10.1530/jrf.0.0960699. [DOI] [PubMed] [Google Scholar]
- 10.Wales RG. The uterus of the ewe. II. Chemical analysis of the uterine fluid collected by cannulation. Aust J Biol Sci. 1973;26:947–959. doi: 10.1071/bi9730947. [DOI] [PubMed] [Google Scholar]
- 11.Leese H. The formation and function of oviduct fluid. J Reprod Fertil. 1988;82:843–856. doi: 10.1530/jrf.0.0820843. [DOI] [PubMed] [Google Scholar]
- 12.Carlson D, Black DL, Howe GR. Oviduct secretion in the cow. J Reprod Fertil. 1970;22:549–552. doi: 10.1530/jrf.0.0220549. [DOI] [PubMed] [Google Scholar]
- 13.Lippes J, Enders RG, Pragay DA, Bartholomew WR. The collection and analysis of human fallopian tubal fluid. Contraception. 1972;5:85–103. doi: 10.1016/0010-7824(72)90021-2. [DOI] [PubMed] [Google Scholar]
- 14.Stryer L. Biochemistry. New York: W.H. Freeman and Co; 1995. [Google Scholar]
- 15.Barnett DK, Bavister BD. What is the relationship between the metabolism of preimplantation embryos and their developmental competence? Mol Reprod Dev. 1996;43:105–133. doi: 10.1002/(SICI)1098-2795(199601)43:1<105::AID-MRD13>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Lawitts JA, Biggers JD. Overcoming the 2-cell block by modifying standard components in a mouse culture medium. Biol Reprod. 1991;45:245–251. doi: 10.1095/biolreprod45.2.245. [DOI] [PubMed] [Google Scholar]
- 17.Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- 18.Kishi J, Noda Y, Narimoto K, Umaoka Y, Mori T. Block to development in cultured rat one-cell embryos is overcome using medium HECM-1. Hum Reprod. 1991;6:1445–1448. doi: 10.1093/oxfordjournals.humrep.a137286. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Niwa K, Lim JM, Okuda K. Effects of phosphate, energy substrates and amino acids on development of in vitro-matured, in vitro-fertilized bovine oocytes in a chemically defined, protein-free culture medium. Biol Reprod. 1993;48:1320–1325. doi: 10.1095/biolreprod48.6.1320. [DOI] [PubMed] [Google Scholar]
- 20.Thompson JG, Simpson AC, Pugh PA, Tervit HR. Requirement for glucose during in vitro culture of sheep preimplantation embryos. Mol Reprod Dev. 1992;31:253–257. doi: 10.1002/mrd.1080310405. [DOI] [PubMed] [Google Scholar]
- 21.Conaghan J, Handyside AH, Winston RM, Leese HL. Effects of pyruvate and glucose on the development of human preimplantation embryos in vitro. J Reprod Fertil. 1993;99:87–95. doi: 10.1530/jrf.0.0990087. [DOI] [PubMed] [Google Scholar]
- 22.Quinn P. Enhanced results in mouse and human embryo culture using a modified human tubal fluid medium lacking glucose and phosphate. J Assist Reprod Genet. 1995;12:97–105. doi: 10.1007/BF02211377. [DOI] [PubMed] [Google Scholar]
- 23.Schini SA, Bavister BD. Two-cell block to development of cultured hamster embryos is caused by phosphate and glucose. Biol Reprod. 1988;39:1183–1192. doi: 10.1095/biolreprod39.5.1183. [DOI] [PubMed] [Google Scholar]
- 24.Seshagiri PB, Bavister BD. Glucose inhibits development of hamster 8-cell embryos in vitro. Biol Reprod. 1989;40:599–606. doi: 10.1095/biolreprod40.3.599. [DOI] [PubMed] [Google Scholar]
- 25.Barnett DK, Clayton MK, Kimura J, Bavister BD. Glucose and phosphate toxicity in hamster preimplantation embryos involves disruption of cellular organization, including distribution of active mitochondria. Mol Reprod Dev. 1997;48:227–237. doi: 10.1002/(SICI)1098-2795(199710)48:2<227::AID-MRD10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 26.Barnett DK, Kimura J, Bavister BD. Translocation of active mitochondria during hamster preimplantation embryo development studied by confocal laser scanning microscopy. Dev Dyn. 1996;205:64–72. doi: 10.1002/(SICI)1097-0177(199601)205:1<64::AID-AJA6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig TE, Lane M, Bavister BD. Differential effect of hexoses on hamster embryo development in culture. Biol Reprod. 2001;64:1366–1374. doi: 10.1095/biolreprod64.5.1366. [DOI] [PubMed] [Google Scholar]
- 28.Meyer M, Seshagiri PB. Analysis of phosphate-inhibition of development of hamster 2-cell embryos in vitro. Biol Reprod. 1990;42:56. [Google Scholar]
- 29.Monis H, Bavister BD. Analysis of the inhibitory effect of inorganic phosphate on development of 4-cell hamster embryos in vitro. J Exp Zool. 1990;256:75–83. doi: 10.1002/jez.1402560110. [DOI] [PubMed] [Google Scholar]
- 30.Monis H, Bavister BD. Development of 4-cell hamster embryos to the blastocyst stage in vitro and its regulation by components of the culture milieu. Reprod Fertil Dev. 1990;2:1–9. doi: 10.1071/rd9900001. [DOI] [PubMed] [Google Scholar]
- 31.Lawitts JA, Biggers JD. Optimization of mouse embryo culture media using simplex methods. J Reprod Fertil. 1991;91:543–556. doi: 10.1530/jrf.0.0910543. [DOI] [PubMed] [Google Scholar]
- 32.Lane M, Ludwig TE, Bavister BD. Phosphate induced developmental arrest of hamster 2-cell embryos is associated with disrupted ionic homeostasis. Mol Reprod Dev. 1999;54:410–417. doi: 10.1002/(SICI)1098-2795(199912)54:4<410::AID-MRD12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 33.Lane M, Boatman DE, Albrecht RM, Bavister BD. Intracellular divalent cation homeostasis and developmental competence in the hamster preimplantation embryo. Mol Reprod Dev. 1998;50:443–450. doi: 10.1002/(SICI)1098-2795(199808)50:4<443::AID-MRD8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 34.Barnett DK, Bavister BD. Hypotaurine requirement for in vitro development of golden hamster 1-cell embryos into morulae and blastocysts, and production of term offspring from in vitro-fertilized ova. Biol Reprod. 1992;47:297–304. doi: 10.1095/biolreprod47.2.297. [DOI] [PubMed] [Google Scholar]
- 35.McKiernan SH, Bavister BD, Tasca RJ. Energy substrate requirements for in-vitro development of hamster 1- and 2-cell embryos to the blastocyst stage. Hum Reprod. 1991;6:64–75. doi: 10.1093/oxfordjournals.humrep.a137260. [DOI] [PubMed] [Google Scholar]
- 36.Gonzales DS, Pinheiro JC, Bavister BD. Prediction of the developmental potential of hamster embryos in vitro by precise timing of the third cell cycle. J Reprod Fertil. 1995;105:1–8. doi: 10.1530/jrf.0.1050001. [DOI] [PubMed] [Google Scholar]
- 37.O’Fallon JV, Wright RW., Jr Quantitative determination of the pentose phosphate pathway in preimplantation mouse embryos. Biol Reprod. 1986;34:58–64. doi: 10.1095/biolreprod34.1.58. [DOI] [PubMed] [Google Scholar]
- 38.Rieger D, Luskutoff NM, Betteridge KJ. Developmentally related changes in the uptake and metabolism of glucose, glutamine and pyruvate by cattle embryos produced in vitro. Reprod Fertil Dev. 1992;4:547–557. doi: 10.1071/rd9920547. [DOI] [PubMed] [Google Scholar]
- 39.Krisher RL, Lane M, Bavister BD. Developmental competence and metabolism of bovine embryos cultured in semi-defined and defined culture media. Biol Reprod. 1999;60:1345–1352. doi: 10.1095/biolreprod60.6.1345. [DOI] [PubMed] [Google Scholar]
- 40.Squirrell JM, Wokosin DL, White JG, Bavister BD. Long-term two-photon fluorescence imaging of mammalian embryos without compromising viability. Nat Biotechnol. 1999;17:763–767. doi: 10.1038/11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohler WA, Squirrell JM. Multiphoton imaging of embryonic development. In: Yuste R, Lanni F, Konnerth A, editors. Imaging Neurons: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 21.21–21.11. [Google Scholar]
- 42.Bavister BD. A minichamber device for maintaining a constant carbon dioxide in air atmosphere during prolonged culture of cells on the stage of an inverted microscope. In Vitro Cell Dev Biol. 1988;24:759–763. doi: 10.1007/BF02623645. [DOI] [PubMed] [Google Scholar]
- 43.Wokosin DL, Centonze V, White JG, Armstrong D, Robertson G, Ferguson AI. All-solid-state ultrafast lasers facilitate multiphoton excitation fluorescence imaging. IEEE J Select Topics Quant Electr. 1996;2:1051–1065. [Google Scholar]
- 44.Squirrell JM, Lane M, Bavister BD. Altering intracellular pH disrupts development and cytoplasmic organization in preimplantation hamster embryos. Biol Reprod. 2001;64:1845–1854. doi: 10.1095/biolreprod64.6.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.SAS OnlineDoc. Version 7–1. Cary, NC: SAS Institute Inc; [Google Scholar]
- 46.Krisher RL, Bavister BD. Enhanced glycolysis after maturation of bovine oocytes in vitro is associated with increased developmental competence. Mol Reprod Dev. 1999;53:19–26. doi: 10.1002/(SICI)1098-2795(199905)53:1<19::AID-MRD3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 47.Muggleton-Harris AL, Brown JJ. Cytoplasmic factors influence mitochondrial reorganization and resumption of cleavage during culture of early mouse embryos. Hum Reprod. 1988;3:1020–1028. doi: 10.1093/oxfordjournals.humrep.a136815. [DOI] [PubMed] [Google Scholar]
- 48.Noto V, Campo R, Roziers P, Swinnen K, Vercruyssen M, Gordts S. Mitochondrial distribution after fast embryo freezing. Hum Reprod. 1993;8:2115–2118. doi: 10.1093/oxfordjournals.humrep.a137992. [DOI] [PubMed] [Google Scholar]
- 49.Krisher RL, Bavister BD. Responses of oocytes and embryos to the culture environment. Theriogenology. 1998;49:103–114. doi: 10.1016/s0093-691x(97)00405-6. [DOI] [PubMed] [Google Scholar]
- 50.Seshagiri PB, Bavister BD. Phosphate is required for inhibition by glucose of development of hamster 8-cell embryos in vitro. Biol Reprod. 1989;40:607–614. doi: 10.1095/biolreprod40.3.607. [published erratum appears in Biol Reprod 1989; 40:1130] [DOI] [PubMed] [Google Scholar]
- 51.Brinster RL, Troike DE. Requirements for blastocyst development in vitro. J Anim Sci. 1979;49:26–34. doi: 10.1093/ansci/49.supplement_ii.26. [DOI] [PubMed] [Google Scholar]
- 52.Lane M. Mechanisms for managing cellular and homeostatic stress in vitro. Theriogenology. 2001;55:225–236. doi: 10.1016/s0093-691x(00)00456-8. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Y, Chauvet PJ, Alper SL, Baltz JM. Expression and function of bicarbonate/chloride exchangers in the preimplantation mouse embryo. J Biol Chem. 1995;270:24428–24434. doi: 10.1074/jbc.270.41.24428. [DOI] [PubMed] [Google Scholar]
- 54.Lane M, Baltz JM, Bavister BD. Regulation of intracellular pH in hamster preimplantation embryos by the sodium hydrogen (Na+/H+) antiporter. Biol Reprod. 1998;59:1483–1490. doi: 10.1095/biolreprod59.6.1483. [DOI] [PubMed] [Google Scholar]
- 55.Hird SN, Paulsen JE, Strome S. Segregation of germ granules in living Caenorhabditis elegans embryos: cell-type-specific mechanisms for cytoplasmic localization. Development. 1996;122:1303–1312. doi: 10.1242/dev.122.4.1303. [DOI] [PubMed] [Google Scholar]
- 56.Strome S, Hill DP. Early embryogenesis in Caenorhabditis elegans: the cytoskeleton and spatial organization of the zygote. Bioessays. 1988;8:145–149. doi: 10.1002/bies.950080504. [DOI] [PubMed] [Google Scholar]
- 57.Schatten G, Schatten H, Spector I, Cline C, Paweletz N, Simerly C, Petzelt C. Latrunculin inhibits the microfilament-mediated processes during fertilization, cleavage and early development in sea urchins and mice. Exp Cell Res. 1986;166:191–208. doi: 10.1016/0014-4827(86)90519-7. [DOI] [PubMed] [Google Scholar]
- 58.Micklem DR. mRNA localization during development. Dev Biol. 1995;172:377–395. doi: 10.1006/dbio.1995.8048. [DOI] [PubMed] [Google Scholar]
- 59.Hyttel P, Niehmann H. Ultrastructure of porcine embryos following development in vitro vs. in vivo. Mol Reprod Dev. 1990;27:136–144. doi: 10.1002/mrd.1080270208. [DOI] [PubMed] [Google Scholar]
- 60.Bavister BD, Squirrell JM. Mitochondrial distribution and function in oocytes and early embryos. Hum Reprod. 2000;15(suppl 2):189–198. doi: 10.1093/humrep/15.suppl_2.189. [DOI] [PubMed] [Google Scholar]
- 61.Plante L, King WA. Light and electron microscopic analysis of bovine embryos derived by in vitro and in vivo fertilization. J Assist Reprod Genet. 1994;11:515–529. doi: 10.1007/BF02216032. [DOI] [PubMed] [Google Scholar]
- 62.Calarco PG, McLaren A. Ultrastructural observations of the preimplantation stages of the sheep. J Embryol Exp Morphol. 1976;36:609–622. [PubMed] [Google Scholar]
- 63.Gott AL, Hardy K, Winston RM, Leese HJ. Non-invasive measurement of pyruvate and glucose uptake and lactate production by single human preimplantation embryos. Hum Reprod. 1990;5:104–108. doi: 10.1093/oxfordjournals.humrep.a137028. [DOI] [PubMed] [Google Scholar]
- 64.Gardner D, Lane M. The 2-cell block in CF1 mouse is associated with an increase in glycolysis and a decrease in tricarboxylic acid (TCA) cycle activity: alleviation of the 2-cell block is associated with the restoration of in vivo metabolic pathway activities. Biol Reprod. 1993;48(suppl 1):152. [Google Scholar]
- 65.Leclerc C, Becker D, Buehr M, Warner A. Low intracellular pH is involved in the early embryonic death of DDK mouse eggs fertilized by alien sperm. Dev Dyn. 1994;200:257–267. doi: 10.1002/aja.1002000307. [DOI] [PubMed] [Google Scholar]
- 66.McKiernan SH, Bavister BD. Timing of development is a critical parameter for predicting successful embryogenesis. Hum Reprod. 1994;9:2123–2129. doi: 10.1093/oxfordjournals.humrep.a138403. [DOI] [PubMed] [Google Scholar]
- 67.Hasler JF. The current status of oocyte recovery, in vitro embryo production, and embryo transfer in domestic animals, with an emphasis on the bovine. J Anim Sci. 1998;76:52–74. [Google Scholar]
- 68.Racowsky C, Jackson KV, Cekleniak NA, Fox JH, Hornstein MD, Ginsburg ES. The number of 8-cell embryos is a key determinant for selecting day 3 or day 5 transfer. Fertil Steril. 2000;73:558–564. doi: 10.1016/s0015-0282(99)00565-8. [DOI] [PubMed] [Google Scholar]
- 69.Watson AJ. The cell biology of blastocyst development. Mol Reprod Dev. 1992;33:492–504. doi: 10.1002/mrd.1080330417. [DOI] [PubMed] [Google Scholar]
- 70.Yingst DR. Modulation of the Na, K-ATPase by Ca and intracellular proteins. Annu Rev Physiol. 1988;50:291–303. doi: 10.1146/annurev.ph.50.030188.001451. [DOI] [PubMed] [Google Scholar]
- 71.Ishide N. Intracellular calcium modulators for cardiac muscle in pathological conditions. Jpn Heart J. 1996;37:1–17. doi: 10.1536/ihj.37.1. [DOI] [PubMed] [Google Scholar]
- 72.Lazrak A, Peracchia C. Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells. Biophys J. 1993;65:2002–2012. doi: 10.1016/S0006-3495(93)81242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herman B. Regulation of calcium metabolism in cells. In: Anderson JJB, Gardner SC, editors. Calcium and Phosphorus in Health and Disease. Boca Raton: CRC Press; 1995. pp. 83–93. [Google Scholar]
- 74.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian mitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]