Abstract
Green tea catechins and hydrolyzable tannins are gaining increasing attention as chemopreventive agents. However, their mechanism of action is poorly understood. We investigated the effects of four green tea catechins and two hydrolyzable tannins on microsome-induced benzo[a]pyrene (BP)–DNA adducts and the possible structure–activity relationship. BP (1 μM) was incubated with rat liver microsomes and DNA in the presence of the test compound (1–200 μM) or vehicle. The purified DNA was analyzed by 32P-postlabeling. The inhibitory activity of the catechins was in the following descending order: epigallocatechin gallate (IC50 = 16 μM) > epicatechin gallate (24 μM) > epigallocatechin (146 μM) > epicatechin (462 μM), suggesting a correlation between the number of adjacent aromatic hydroxyl groups in the molecular structure and their potencies. Tannic acid (IC50 = 4 μM) and pentagalloglucose (IC50 = 26 μM) elicited as much DNA adduct inhibitory activity as the catechins or higher presumably due to the presence of more functional hydroxyl groups. To determine if the activity of these compounds was due to direct interaction of phenolic groups with electrophilic metabolite(s) of BP, DNA was incubated with anti-benzo[a]pyrene-7,8-diol-9,10-epoxide (anti-BPDE) (0.5 μM) in the presence of test compounds (200 μM) or vehicle. Significant inhibition of DNA adduct formation was found (tannic acid > pentagalloglucose > epigallocatechin gallate > epicatechin gallate). This notion was confirmed by analysis of the reaction products of anti-BPDE with the catechins and pentagalloglucose by electrospray ionization mass spectrometry and liquid chromatography–mass spectrometry. In conclusion, our data demonstrate that green tea catechins and the hydrolyzable tannins are highly effective in inhibiting BP–DNA adduct formation at least, in part, due to direct interaction of adjacent hydroxyl groups in their structures and that the activity is higher with an increasing number of functional hydroxyl groups.
Introduction
Benzo[a]pyrene (BP)1 is a polycyclic aromatic hydrocarbon (PAH), which is present ubiquitously in tobacco smoke, automobile exhaust emissions, and grilled foods (1, 2). It is one of the most potent environmental carcinogens. Numerous studies have demonstrated the association of BP exposure and induction of carcinogensis in many organs including lung, skin, mammary gland, and others (3–5).
Enzymatic activation of BP by certain types of cytochrome P450s (CYPs) found in the subcellular microsomal fraction, especially CYP1A1, are needed to produce the ultimate carcinogen anti-benzo[a]pyrene-7,8-diol-9,10-epoxide (anti-BPDE) (6). anti-BPDE exerts its carcinogenic activity by alkylating nucleosides on DNA molecules at the bay region of anti-BPDE. The reaction primarily happens with the purine bases, deoxyguanosine and deoxyadenosine, in DNA (7). As a result, bulky stable and depurinating DNA adducts are formed (8, 9). Insufficient removal of these DNA adducts prior to replication creates hot spots in the gene and can result in deactivation of tumor suppressor genes or activation of oncogenes leading to tumor initiation (10).
Green tea is one of the most popular drinks in the world with some beneficial effects on cardiovascular (11, 12) and neuro-degenerative diseases (13). Green tea is now drawing increasing attention because of its possible application in cancer prevention (14, 15). Green tea preparations were found to decrease tumor incidence and tumor multiplicity in chemically-induced tumor models, including BP and other PAHs (16–22). Interestingly, green tea preparations were effective when administered to mice either during or after carcinogen exposure (16, 17), suggesting their chemopreventive effects in different phases of carcinogenesis.
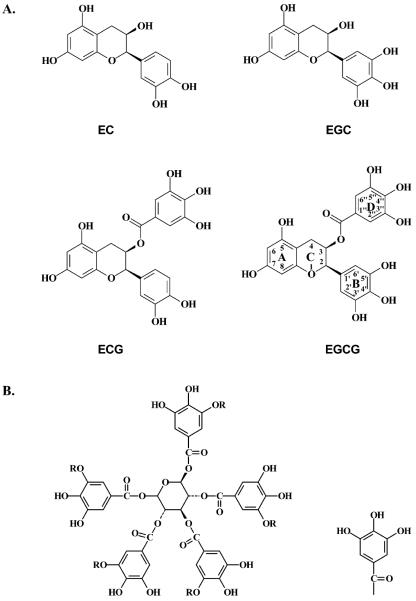
The catechins in green tea are thought to be the bioactive components, including (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), and (−)-epigallocatechin gallate (EGCG), which bear close structural similarities (Figure 1A). EGCG is the predominant catechin (23). Many mechanisms of action of green tea catechins have been proposed based on studies in vitro. Green tea catechins were found to induce apoptosis and inhibit the proliferation of a variety of cancer cell types (24–28). Green tea catechins can generate reactive oxygen species (ROS), including hydrogen peroxides, which are responsible for the death of cancer cells (29, 30). However, these mechanisms of action are more relevant to the chemotherapeutic effects of green tea catechins rather than their chemopreventive effects (31).
Figure 1.
Chemical structures of catechins and two hydrolyzable tannins. (A) EC, EGC, ECG, and EGCG. (B) Pentagalloylglucose (R = H) and TA (R = galloyl group as shown on the right).
As noted above, DNA adduct formation is the initial key step in the BP-induced carcinogenic process. Because green tea manifests its chemopreventive effects in almost all of the animal studies conducted, we hypothesize that green tea should be able to inhibit the DNA adduct formation induced by BP. This notion has been supported by a limited number of studies, in which green tea components decreased BP-induced DNA damage in the Chang liver cell line evaluated by the comet assay (32) and EGCG inhibited the formation of [3H]-BP-derived DNA adducts in a cell-free system (33). However, the mechanism behind these effects is not known.
Back in the early 1980s, Conney and co-workers showed that the plant phenolic ellagic acid was highly potent in inhibiting the mutagenesis by anti-BPDE (34). Subsequently, this group demonstrated that this inhibition occurred due to covalent interaction of ellagic acid with anti-BPDE (35). This finding was later supported by inhibition of anti-BPDE-induced DNA adducts (36). Green tea extract, which contains several catechins with cis-diol groups, like in ellagic acid, was reported to decrease anti-BPDE-induced DNA strand breaks (37), presumably by the same mechanistic action of ellagic acid reported by Sayer et al. (35). Additionally, Bors and Michel (38) and Rice-Evans et al. (39) demonstrated that the cis-diol groups in green tea catechins could scavenge free radicals such as hydroxyl radicals, azide radicals, and superoxide anions, thus correlating with their antioxidant activities.
We hypothesized that green tea catechins will inhibit BP-induced DNA adduct formation by direct quenching of anti-BPDE produced in the metabolism of BP and that the potency of different catechins will vary with the number of their cis-diol groups. This structure–activity relationship (SAR) study will help to further identify the mechanism of action of green tea catechins. It might also be beneficial for drug modification and drug development based on catechins or compounds bearing similar groups.
This hypothesis cannot be readily tested in a whole cell system because many factors such as the lipid solubility of the catechins, etc., could bias the interpretation. We therefore used a microsomal system to assess the capacity of various catechins in green tea to inhibit DNA adduct formation and determine SAR. Two hydrolyzable tannins, pentagalloylglucose (5GG) and tannic acid (TA; penta-m-digalloyl-glucose), which have a higher number of cis-diols in their structures (Figure 1B) than the catechins, were also included to further test the SAR.
Experimental Procedures
Caution
Both BP and anti-BPDE are mutagenic and carcinogenic. Protective clothing should be worn, and appropriate safety procedures should be followed when working with these compounds.
Chemicals
EC, EGC, ECG, TA, glucose-6-phosphate, glucose-6-phosphate dehydrogenase from baker's yeast (G6PDH), NADP+, BP, and salmon testis (st)-DNA were purchased from Sigma-Aldrich (St. Louis, MO). EGCG was from LKT laboratories, Inc. (St. Paul, MN). 5GG was obtained from Sinova, Inc. (Bethesda, MD). Anti-BPDE was kindly provided by Dr. Subodh Kumar, State University of New York College at Buffalo. Chemicals used in 32P-postlabeling DNA adduct analysis were the same as described previously (40).
Microsomal BP-Induced DNA Adducts
Green tea catechins and hydrolyzable tannins were dissolved in Me2SO and prepared freshly. st-DNA (300 μg/mL) was preincubated with 50 mM Tris-HCl (pH 7.5), 1 mM MgCl2, 2.5 mM glucose-6-phosphate, 1 U/mL G6PDH, 0.5 mM NADP+, and β-naphthoflavone-induced microsomal proteins (1 mg/mL) in 1 mL for 10 min, in the presence of vehicle alone or green tea catechins or hydrolyzable tannins (1–200 μM). BP dissolved in Me2SO was added at a final concentration of 1 μM, and incubation was continued for another 30 min at 37 °C. The reaction was terminated by addition of EDTA and centrifugation (7500 rpm; 10 min). DNA was isolated from the supernatant by removal of RNA and proteins by digestions with RNases A and T1 and proteinase K and a series of extractions with phenol, phenol: Sevag (chloroform:isoamyl alcohol, 24:1), and Sevag, followed by precipitation of the DNA with ethanol (40). The DNA concentration was estimated spectrophotometrically.
Reaction of st-DNA with anti-BPDE
st-DNA (200 μg/mL) was preincubated with 50 mM Tris-HCl (pH 7.5) in a total of 0.2 mL of solution for 10 min, in the presence of vehicle or green tea catechins or hydrolyzable tannins. Then, anti-BPDE was added at a final concentration of 0.5 μM and incubated at 37 °C for another 30 min. The reaction was terminated by precipitating DNA with ethanol, and the DNA concentration was measured spectrophotometrically.
Analysis of DNA Adducts
DNA adducts were analyzed by 32P-postlabeling as described (40). Briefly, 10 μg of DNA was digested with micrococcal nuclease and spleen phosphodiesterase (MN/SPD). Before further treatment with nuclease P1 to enrich DNA adducts, an aliquot was used for evaluation of normal nucleotide levels. DNA adducts and normal nucleotides were labeled with [γ-32P]ATP and T4 polynucleotide kinase. Labeled DNA adducts were separated by multidirectional polyethyleneimine (PEI)-cellulose TLC in the following solvents: D1 = 1.0 M sodium phosphate, pH 6.0; D3 = 4 M lithium formate/7 M urea, pH 3.5; D4 = 4 M ammonium hydroxide/isopropanol (1.1:1); and D5 = 1.7 M sodium phosphate, pH 6.0. Normal nucleotides were resolved in 180 mM sodium phosphate, pH 6.0, by one-directional PEI-cellulose TLC. DNA adducts and normal nucleotides were detected and quantified by Packard InstantImager.
Electrospray Ionization/Mass Spectrometry (ESI/MS) and ESI/Tandem Mass Spectrometry (MS/MS) Study
anti-BPDE and test compounds (green tea catechins and hydrolyzable tannins) were incubated at 37 °C at equimolar concentration (500 μM) in H2O and acetonitrile (9:1) for 40 min. Reaction products were diluted with 50% acetonitrile/0.1% formic acid and analyzed by ESI/MS in positive ion mode and a mass resolution of 10000 with a Q-TOF API-US mass spectrometer from Waters (Milford, MA). Samples were infused with a syringe pump at 1 μL/min. Data acquisition lasted for at least 1 min after the signal was stabilized, and the spectra were summed, smoothed, and stored. For MS/MS analysis, the collision energy was adjusted to a level such that the intensities of the precursor ions were decreased by 80–90%.
Liquid Chromatography/Mass Spectrometry (LC/MS) and LC/MS/MS Study
anti-BPDE and EGCG were incubated at 37 °C at equimolar concentration (100 μM) in H2O and acetonitrile (9:1) for 40 min. DNA adduct separation was performed by Accela LC from Thermo Scientific (San Jose, CA) with a Hypersil GOLD 50 mm × 2.1 mm C18 column. A 15 min gradient with 5% acetonitrile/0.1% formic acid (solvent A) and 95% acetonitrile/0.1% formic acid (solvent B) at 0.1 mL/min was used. The gradient started from 5% solvent B that increased linearly to 50% in 12 min and then increased linearly to 75% in 3 min. The elution from LC was coupled to a LTQ Orbitrap XL mass spectrometer from Thermo Scientific via an ESI source. MS and MS/MS spectra were acquired in positive ion mode at 30000 mass resolution.
Statistical Analysis
Results were reported as means ± SEMs. Student's t test was used for the determination of statistical significance between two individual groups. A p value less than 0.05 with a 95% confidence interval was considered to give the level of significance.
Results
Before we tested the efficacy of these various phenolic compounds, we first determined the lowest concentration of BP in a microsomal reaction that would produce measurable levels of DNA adducts detected by the highly sensitive 32P-postlabeling assay. Incubation of st-DNA with β-naphthoflavone-induced rat liver microsomes, which exhibit increased expression of CYP1A1 and CYP1B1, in the presence of varying concentrations of BP (0.5–10 μM) and cofactors resulted in the formation of two major DNA adducts (Figure 2). These DNA adducts have previously been characterized as the products of the interaction of anti-BPDE (DNA adduct 2) and 9-OH-benzo[a]pyrene-4,5-epoxide (9-OH-BPE) (DNA adduct 1) with dG (7, 41).
Figure 2.
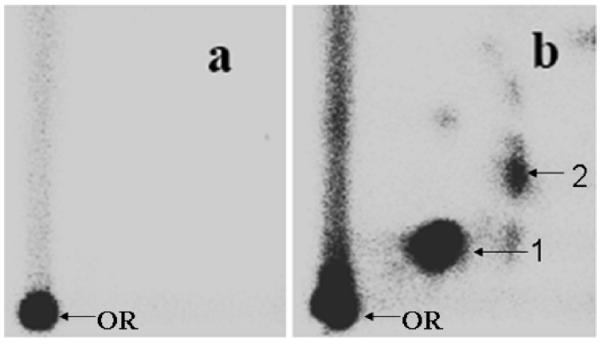
Representative autoradiographs of 32P-postlabeling analysis of microsome-mediated BP-induced DNA adducts. (a) Vehicle (2% DMSO) and (b) 1 μM BP. OR, origin. DNA adducts were resolved by multidirectional PEI-cellulose TLC using the following solvents: D1 = 1.0 M sodium phosphate, pH 6.0; D3 = 4 M lithium formate/7 M urea, pH 3.5; D4 = 4 M ammonium hydroxide/iso-propanol (1.1:1); and D5 = 1.7 M sodium phosphate, pH 6.0. DNA adducts were detected by Packard InstantImager.
Total DNA adduct levels increased with an increasing concentration of BP (15 ± 6 to 467 ± 49 DNA adducts/107 nucleotides). The relative levels of DNA adducts 1 and 2 varied with BP concentrations. At the highest concentration of BP (10 μM), 9-OH-BPE-dG levels were slightly greater than anti-BPDE-dG levels (9-OH-BPE-dG/anti-BPDE-dG = 1.6). However, the ratio of the two DNA adducts increased with decreasing BP concentrations (9-OH-BPE-dG/anti-BPDE-dG = 4.5 at 0.5 μM BP), indicating that metabolism of BP to DNA-reactive metabolites is dose-dependent and reflective of the relative amount of substrate. All subsequent reactions in the presence of the phenolic compounds were performed using a relatively low concentration of BP (1 μM).
Effect of Green Tea Catechins on Microsomal BP–DNA Adducts
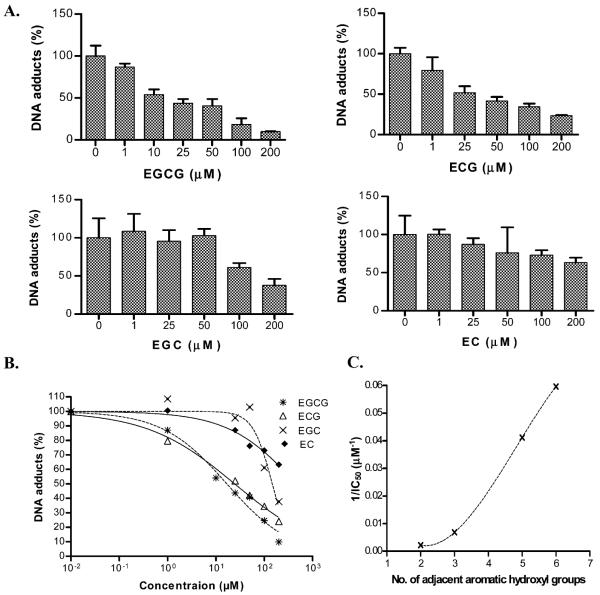
Incubation of st-DNA with BP (1 μM) in the absence or presence of varying concentrations (1ȁ200 μM) of EC, EGC, ECG, and EGCG produced qualitatively the same DNA adduct profile (data not shown). Quantitatively, however, BP–DNA adduct levels varied with the type of catechin (Figure 3A). As compared with BP alone (25.2 ± 1.8 DNA adducts/107 nucleotides; n = 4), each catechin tested (100 μM) resulted in significant inhibition of BP-induced DNA adducts, with EGCG (75%) > ECG (66%) > EGC (39%) > EC (27%). The DNA adduct inhibition observed with each compound was dose-dependent (Figure 3A).
Figure 3.
Effect of indicated green tea catechins on microsomal BP–DNA adducts. (A) Dose response of test catechins. Data are expressed as means ± SEMs (n = 4). (B) Estimation of IC50 of green tea catechins. (C) The correlation between 1/IC50 with the number of adjacent aromatic hydroxyl groups.
When the percent DNA adduct inhibition was plotted against the various catechin concentrations, a clear dose response was observed in the form of a sigmoid curve (Figure 3B). EGCG and ECG were the most potent components of green tea catechins, with half maximal inhibitory concentration (IC50) values of 16 and 24 μM, respectively. The other two compounds, EGC and EC, were least effective showing IC50 values of 146 and 462 μM, respectively.
To determine the SAR, 1/IC50 was plotted against the number of adjacent OH groups in their molecular structure. A clear relationship was evident (Figure 3C), suggesting that the activity may reside in the cis-diol groups.
Effect of Hydrolyzable Tannins on Microsomal BP–DNA Adducts
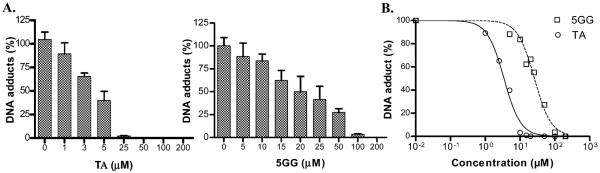
To further prove that the activity lies in the cis-diol groups, we investigated the effect of 5GG and TA, which are hydrolyzable tannins. The rationale for the use of these compounds is that they contain a higher number of adjacent OH groups in their molecular structure, as compared to the green tea catechins, 15 for 5GG and 25 for TA (see Figure 1B). Therefore, it is reasonable to expect that these compounds may be more efficacious than green tea catechins against BP–DNA adduction. As shown in Figure 4A,B, both of these compounds showed effective inhibition of microsomal BP–DNA adducts, and the inhibition was dose-dependent. Both 5GG and TA elicited almost complete DNA adduct inhibition, with TA being much more potent than 5GG (IC50 = 4 and 26 μM, respectively). It is also interesting to note that the dose–response sigmoid curves were parallel to each other presumably due to their extreme structural similarities.
Figure 4.
Effect of TA and 5GG on microsomal BP–DNA adducts. (A) Data are expressed as means ± SEMs (n = 4). (B) Denotes estimated IC50 of TA and 5GG.
Effect of Green Tea Catechins and Hydrolyzable Tannins on anti-BPDE–DNA Adducts
To determine the mechanism by which the test cis-diol-containing green tea catechins and the hydrolyzable tannins inhibit microsomal BP–DNA adduction, these compounds were studied in a nonenzymatic reaction; that is, anti-BPDE (0.5 μM), the ultimate carcinogenic metabolite of BP was incubated with st-DNA (200 μg/mL) in the presence of vehicle alone or EGCG, ECG, 5GG, and TA (200 μM each), followed by analysis of the DNA adduct levels by 32P-postlabeling. As shown in Figure 5, all compounds showed effective inhibition of anti-BPDE-dG. However, the degree of inhibition with the test compounds varied as follows: TA (98% inhibition) > 5GG (68%) > EGCG (64%) > ECG (39%). These data further support our earlier conclusion that the higher the number of adjacent OH groups is, the greater the DNA adduct inhibition is. These data also suggest that inhibition of microsomal BP–DNA adducts by the catechins and test hydrolyzable tannins is at least, in part, due to their direct interaction with the electrophilic metabolites of BP.
Figure 5.
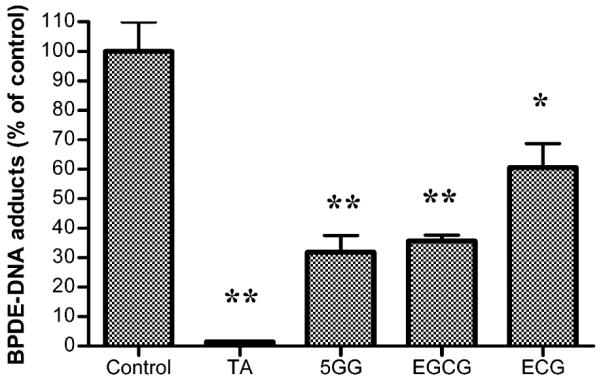
Effect of TA, 5GG, EGCG, and ECG (200 μM) on anti-BPDE (0.5 μM)-induced DNA adduct formation. Data are expressed as means ± SEMs (n = 4) (**p < 0.01, and *p < 0.05).
Detection of anti-BPDE–Catechin Complex by ESI/MS/MS
This analysis was performed to detect the reaction products in the reaction mixtures of anti-BPDE and test compounds (green tea catechins and hydrolyzable tannins). All showed clear peaks with the expected mass for the complexes formed. For example, a peak with a m/z ratio of 761 corresponding to anti-BPDE-EGCG complex was found in the anti-BPDE–EGCG reaction mixture; the MS/MS spectrum of the complex further suggested a direct covalent interaction of anti-BPDE and EGCG (data not shown).
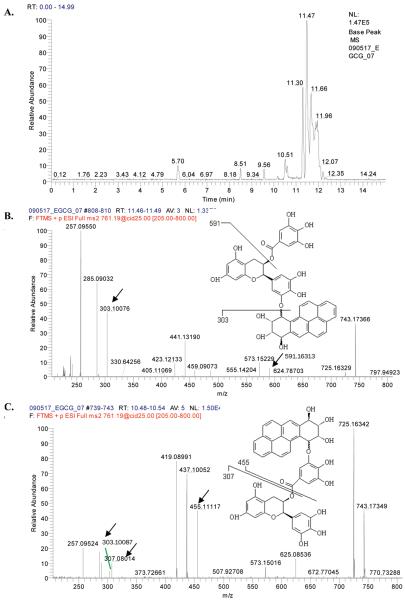
Investigation of Fragmentation Pattern of anti-BPDE–EGCG Complex by LC/MS/MS
LC/MS/MS analysis was performed to further rule out the possibility of noncovalent interaction between EGCG and anti-BPDE and also to investigate the fragmentation pattern of anti-BPDE–EGCG complex. Several peaks with m/z ratio of 761 in the spectrum, which correspond to the anti-BPDE–EGCG complex, were found. Their retention times were 5.70, 8.51, 9.56, 10.17, 10.51, 10.59, 11.30, 11.47, 11.66, and 11.96 min (Figure 6A). The MS/MS studies of each peak were performed, and two major fragmentation patterns were found in the spectrum. The peaks with retention times of 8.51, 9.56, 10.17, 11.30, 11.47, 11.66, and 11.96 all generated a fragmentation pattern in which a fragment with a m/z ratio of 591 and another fragment with a m/z ratio of 303 exist, suggesting that anti-BPDE attacks the hydroxyl groups on the B ring of EGCG molecules (Figure 6B). The peaks with retention times of 10.51 and 10.59 min generated a different fragmentation pattern in which a fragment with a m/z ratio of 455 and another fragment with a m/z ratio of 307 exist, suggesting that anti-BPDE attacks the hydroxyl groups on the D ring of EGCG molecules (Figure 6C). On the basis of the data gathered, two possible anti-BPDE–EGCG complexes are proposed (Figure 6B,C).
Figure 6.
LC/MS and LC/MS/MS spectra. (A) LC/MS spectrum of anti-BPDE–EGCG complex. (B and C) LC/MS/MS spectrum of anti-BPDE–EGCG complex.
Discussion
In this study, we used a range of EGCG (1–200 μM) and other catechins and hydrolyzable tannins to show dose-dependent inhibition of microsome-mediated BP-induced DNA adducts. Some of the catechins (e.g., EGCG) and hydrolyzable tannins (TA) showed nearly 50% inhibition of the adduct formation at as low as 16 and 4 μM concentrations, respectively. The higher concentrations of test agents were necessary to combat the somewhat high concentration of BP used to be able to reliably quantify the resultant DNA adducts. The plasma concentration of EGCG in rodents and in human volunteers is reported to vary with the dosing of green tea extracts. For example, when high pharmacological doses of EGCG was given to mice (2000 mg/kg) (42) or polyphenon E (containing 1200 mg of EGCG) given to human volunteers (43) orally, peak plasma concentrations found were approximately 9 and 7.5 μM in mice and humans, respectively. A typical achievable plasma EGCG submicromolar concentration has also been reported after two or three cups of tea consumption in humans (44).
A study by Bors and Michel found that the reaction rates of green tea catechins and gallate esters against hydroxyl radicals, azide radicals, or superoxide anions correlate with catechol and pyrogallol groups in their molecular structures (38), which may explain the antioxidant properties of these compounds. In this study, we demonstrate a clear correlation of adjacent aromatic hydroxyl groups in the molecular structure of green tea catechins and hydrolyzable tannins with the inhibitory effects of these compounds on DNA adduct formation induced by BP. Interestingly, it is nearly an exponential relationship between the number of adjacent aromatic hydroxyl groups and the IC50 of these catechins. There are at least two possible mechanisms through which these compounds can decrease BP–DNA adduct formation, through either interacting with reactive intermediates or interfering with microsomal enzyme activities (e.g., CYP1A1). Green tea catechins have inhibitory effects on CYP1A1 activity with the following descending order: ECG ≈ EGCG > EC ≈ EGC (45). In our study, all of the catechins interacted with anti-BPDE directly, indicating an exponential relationship with EGCG and ECG being much more potent than the other two catechins studied.
The higher efficacy of the two hydrolyzable tannins is due to a greater number of functional hydroxyl groups in their molecular structures. With regards to the potency, the more functional hydroxyl groups in green tea catechins correspond to lower IC50 values. This conclusion also holds true in hydrolyzable tannins with TA being more potent than 5GG. However, the problem arises when we compare the IC50 values of EGCG and 5GG, which are about 16 and 26 μM, respectively, while apparently 5GG has more functional hydroxyl groups than EGCG. This is probably because the potency of the compounds could also be affected by the basic structures. The molecular structures of green tea catechins and hydrolyzable tannins are quite different although some similarities exist, so the comparison of these two compounds may not be appropriate.
In this study, the covalent reaction of anti-BPDE and EGCG was demonstrated through ESI/MS/MS and LC/MS/MS. The hydroxyl groups on either the B ring or the D ring of EGCG molecules (Figure 6B,C), but not both, were found to react with anti-BPDE, thus sequestering anti-BPDE. This finding suggests that EGCG shares the same mechanism of action with ellagic acid, which also interacts directly with anti-BPDE and leads to sequestration of anti-BPDE (35).
Our MS studies on the anti-BPDE–EGCG complex did not provide information on the exact position of the hydroxyl groups that react with anti-BPDE. The hydroxyl group on EGCG could be the 3′, 4′, or 5′ on the B ring or the 3″, 4″, or 5″ on the D ring. NMR studies may be necessary to address this question. It is interesting to note that there are several peaks corresponding to the m/z ratio of 761 in LC/MS spectrum in the anti-BPDE–EGCG reaction (Figure 6A). This is probably because anti-BPDE has two optical enantiomers, and also, anti-BPDE can attack different positions on the B ring or D ring of EGCG molecules, which produce different complexes with different retention times as shown in our results.
The significance of the present study is to demonstrate a new mechanism of action of test catechins. The SAR of green tea catechins and hydrolyzable tannins illustrated in this study may help us discover other chemopreventive reagents. It will also be useful in drug modification and development based on these compounds or compounds with similar molecular structures.
In conclusion, our data demonstrate that green tea catechins and the hydrolyzable tannins are highly effective in inhibiting BP–DNA adduct formation at least, in part, due to direct interaction of adjacent hydroxyl groups in their structures and that the activity is higher with an increasing number of functional hydroxyl groups.
Acknowledgment
This work was supported from the US-PHS Grant CA-118114, Kentucky Lung Cancer Research Program grant, and Agnes Brown Duggan Endowment. We thank Dr. Wendy Spencer for her constructive comments, Gilandra Russells for her initial assistance with 32P-postlabeling, and Dr. Gavin Arteel for suggesting the use of 5GG. R.C.G. holds the Agnes Brown Duggan Endowed Chair in Oncological Research.
Footnotes
Abbreviations: BP, benzo[a]pyrene; anti-BPDE, anti-benzo[a]pyrene-7,8-diol-9,10-epoxide; 9-OH-BPE, 9-OH-benzo[a]pyrene-4,5-epoxide; EC, (−)-epicatechin; EGC, (−)-epigallocatechin; EGC, (−)-epicatechin gallate; EGCG, (−)-epigallocatechin gallate; 5GG, pentagalloylglucose; TA, tannic acid
References
- (1).Schoket B. DNA damage in humans exposed to environmental and dietary polycyclic aromatic hydrocarbons. Mutat. Res. 1999;424:143–153. doi: 10.1016/s0027-5107(99)00015-9. [DOI] [PubMed] [Google Scholar]
- (2).Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat. Res. 1999;443:139–147. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- (3).Mumford JL, Li X, Hu F, Lu XB, Chuang JC. Human exposure and dosimetry of polycyclic aromatic hydrocarbons in urine from Xuan Wei, China with high lung cancer mortality associated with exposure to unvented coal smoke. Carcinogenesis. 1995;16:3031–3036. doi: 10.1093/carcin/16.12.3031. [DOI] [PubMed] [Google Scholar]
- (4).Hecht SS. Tobacco smoke carcinogens and breast cancer. Environ. Mol. Mutagen. 2002;39:119–126. doi: 10.1002/em.10071. [DOI] [PubMed] [Google Scholar]
- (5).el-Bayoumy K, Chae YH, Upadhyaya P, Rivenson A, Kurtzke C, Reddy B, Hecht SS. Comparative tumorigenicity of benzo[a]pyrene, 1-nitropyrene and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine administered by gavage to female CD rats. Carcinogenesis. 1995;16:431–434. doi: 10.1093/carcin/16.2.431. [DOI] [PubMed] [Google Scholar]
- (6).Conney AH, Chang RL, Jerina DM, Wei SJ. Studies on the metabolism of benzo[a]pyrene and dose-dependent differences in the mutagenic profile of its ultimate carcinogenic metabolite. Drug Metab. Rev. 1994;26:125–163. doi: 10.3109/03602539409029788. [DOI] [PubMed] [Google Scholar]
- (7).Ross J, Nelson G, Kligerman A, Erexson G, Bryant M, Earley K, Gupta R, Nesnow S. Formation and persistence of novel benzo(a)pyrene adducts in rat lung, liver, and peripheral blood lymphocyte DNA. Cancer Res. 1990;50:5088–5094. [PubMed] [Google Scholar]
- (8).Chen L, Devanesan PD, Higginbotham S, Ariese F, Jankowiak R, Small GJ, Rogan EG, Cavalieri EL. Expanded analysis of benzo[a]pyrene-DNA adducts formed in vitro and in mouse skin: Their significance in tumor initiation. Chem. Res. Toxicol. 1996;9:897–903. doi: 10.1021/tx960004a. [DOI] [PubMed] [Google Scholar]
- (9).Chakravarti D, Venugopal D, Mailander PC, Meza JL, Higginbotham S, Cavalieri EL, Rogan EG. The role of polycyclic aromatic hydrocarbon-DNA adducts in inducing mutations in mouse skin. Mutat. Res. 2008;649:161–178. doi: 10.1016/j.mrgentox.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Baird WM, Hooven L, Mahadevan B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ. Mol. Mutagen. 2005;45:106–114. doi: 10.1002/em.20095. [DOI] [PubMed] [Google Scholar]
- (11).Babu PV, Liu D. Green tea catechins and cardiovascular health: an update. Curr. Med. Chem. 2008;15:1840–1850. doi: 10.2174/092986708785132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Jochmann N, Baumann G, Stangl V. Green tea and cardiovascular disease: From molecular targets towards human health. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:758–765. doi: 10.1097/MCO.0b013e328314b68b. [DOI] [PubMed] [Google Scholar]
- (13).Rezai-Zadeh K, Arendash GW, Hou H, Fernandez F, Jensen M, Runfeldt M, Shytle RD, Tan J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177–187. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- (14).Stoner GD, Mukhtar H. Polyphenols as cancer chemopreventive agents. J. Cell. Biochem. Suppl. 1995;22:169–180. doi: 10.1002/jcb.240590822. [DOI] [PubMed] [Google Scholar]
- (15).Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;269:269–280. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Katiyar SK, Agarwal R, Zaim MT, Mukhtar H. Protection against N-nitrosodiethylamine and benzo[a]pyrene-induced forestomach and lung tumorigenesis in A/J mice by green tea. Carcinogenesis. 1993;14:849–855. doi: 10.1093/carcin/14.5.849. [DOI] [PubMed] [Google Scholar]
- (17).Wang ZY, Hong JY, Huang MT, Reuhl KR, Conney AH, Yang CS. Inhibition of N-nitrosodiethylamine- and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced tumorigenesis in A/J mice by green tea and black tea. Cancer Res. 1992;52:1943–1947. [PubMed] [Google Scholar]
- (18).Xu Y, Ho CT, Amin SG, Han C, Chung FL. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992;52:3875–3879. [PubMed] [Google Scholar]
- (19).Liao J, Yang GY, Park ES, Meng X, Sun Y, Jia D, Seril DN, Yang CS. Inhibition of lung carcinogenesis and effects on angiogenesis and apoptosis in A/J mice by oral administration of green tea. Nutr. Cancer. 2004;48:44–53. doi: 10.1207/s15327914nc4801_7. [DOI] [PubMed] [Google Scholar]
- (20).Lu G, Liao J, Yang G, Reuhl KR, Hao X, Yang CS. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006;66:11494–11501. doi: 10.1158/0008-5472.CAN-06-1497. [DOI] [PubMed] [Google Scholar]
- (21).Yang CS, Lee MJ, Chen L, Yang GY. Polyphenols as inhibitors of carcinogenesis. Environ. Health Perspect. 1997;105(Suppl. 4):971–976. doi: 10.1289/ehp.97105s4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lubet RA, Yang CS, Lee MJ, Hara Y, Kapetanovic IM, Crowell JA, Steele VE, Juliana MM, Grubbs CJ. Preventive effects of polyphenon E on urinary bladder and mammary cancers in rats and correlations with serum and urine levels of tea polyphenols. Mol. Cancer Ther. 2007;6:2022–2028. doi: 10.1158/1535-7163.MCT-07-0058. [DOI] [PubMed] [Google Scholar]
- (23).Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: Molecular mechanisms and human relevance. Toxicol. Appl. Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Qin J, Xie LP, Zheng XY, Wang YB, Bai Y, Shen HF, Li LC, Dahiya R. A component of green tea, (−)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochem. Biophys. Res. Commun. 2007;354:852–857. doi: 10.1016/j.bbrc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- (25).Sadava D, Whitlock E, Kane SE. The green tea polyphenol, epigallocatechin-3-gallate inhibits telomerase and induces apoptosis in drug-resistant lung cancer cells. Biochem. Biophys. Res. Commun. 2007;360:233–237. doi: 10.1016/j.bbrc.2007.06.030. [DOI] [PubMed] [Google Scholar]
- (26).Albrecht DS, Clubbs EA, Ferruzzi M, Bomser JA. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chem.-Biol. Interact. 2008;171:89–95. doi: 10.1016/j.cbi.2007.09.001. [DOI] [PubMed] [Google Scholar]
- (27).Raza H, John A. In vitro effects of tea polyphenols on redox metabolism, oxidative stress, and apoptosis in PC12 cells. Ann. N. Y. Acad. Sci. 2008;1138:358–365. doi: 10.1196/annals.1414.037. [DOI] [PubMed] [Google Scholar]
- (28).Umeda D, Yano S, Yamada K, Tachibana H. Involvement of 67-kDa laminin receptor-mediated myosin phosphatase activation in antiproliferative effect of epigallocatechin-3-O-gallate at a physiological concentration on Caco-2 colon cancer cells. Biochem. Biophys. Res. Commun. 2008;371:172–176. doi: 10.1016/j.bbrc.2008.04.041. [DOI] [PubMed] [Google Scholar]
- (29).Lee KW, Hur HJ, Lee HJ, Lee CY. Antiproliferative effects of dietary phenolic substances and hydrogen peroxide. J. Agric. Food Chem. 2005;53:1990–1995. doi: 10.1021/jf0486040. [DOI] [PubMed] [Google Scholar]
- (30).Yang GY, Liao J, Li C, Chung J, Yurkow EJ, Ho CT, Yang CS. Effect of black and greent ea polyphenols on c-jun phosphorylation and H(2)O(2) production in transformed and non-transformed human bronchial cell lines: Possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis. 2000;21:2035–2039. doi: 10.1093/carcin/21.11.2035. [DOI] [PubMed] [Google Scholar]
- (31).Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Yen GC, Ju JW, Wu CH. Modulation of tea and tea polyphenols on benzo(a)pyrene-induced DNA damage in Chang liver cells. Free Radical Res. 2004;38:193–200. doi: 10.1080/10715760310001638001. [DOI] [PubMed] [Google Scholar]
- (33).Krishnan R, Maru GB. Inhibitory effect(s) of polymeric black tea polyphenol fractions on the formation of [(3)H]-B(a)P-derived DNA adducts. J. Agric. Food Chem. 2004;52:4261–4269. doi: 10.1021/jf049979o. [DOI] [PubMed] [Google Scholar]
- (34).Wood AW, Huang MT, Chang RL, Newmark HL, Lehr RE, Yagi H, Sayer JM, Jerina DM, Conney AH. Inhibition of the mutagenicity of bay-region diol epoxides of polycyclic aromatic hydrocarbons by naturally occurring plant phenols: Exceptional activity of ellagic acid. Proc. Natl. Acad. Sci. U.S.A. 1982;79:5513–5517. doi: 10.1073/pnas.79.18.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Sayer JM, Yagi H, Wood AW, Conney AH, Jerina DM. Extremely facile reaction between the ultimate carcinogen benzo[a]pyrene-7,8-diol 9,10-epoxide and ellagic acid. J. Am. Chem. Soc. 1982;104:5562–5564. [Google Scholar]
- (36).Smith WA, Gupta RC. Use of a microsome-mediated test system to assess efficacy and mechanisms of cancer chemopreventive agents. Carcinogenesis. 1996;17:1285–1290. doi: 10.1093/carcin/17.6.1285. [DOI] [PubMed] [Google Scholar]
- (37).Zhang H, Spitz MR, Tomlinson GE, Schabath MB, Minna JD, Wu X. Modification of lung cancer susceptibility by green tea extract as measured by the comet assay. Cancer Detect. Prev. 2002;26:411–418. doi: 10.1016/s0361-090x(02)00127-7. [DOI] [PubMed] [Google Scholar]
- (38).Bors W, Michel C. Antioxidant capacity of flavanols and gallate esters: Pulse radiolysis studies. Free Radical Biol. Med. 1999;27:1413–1426. doi: 10.1016/s0891-5849(99)00187-2. [DOI] [PubMed] [Google Scholar]
- (39).Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- (40).Gupta RC. 32P-postlabeling for detection of DNA adducts. In: Pfeifer GP, editor. Technologies for Detection of DNA Damage and Mutations. Plenum Press; New York: 1996. pp. 45–61. [Google Scholar]
- (41).Fang AH, Smith WA, Vouros P, Gupta RC. Identification and characterization of a novel benzo[a]pyrene-derived DNA adduct. Biochem. Biophys. Res. Commun. 2001;281:383–389. doi: 10.1006/bbrc.2000.4161. [DOI] [PubMed] [Google Scholar]
- (42).Lambert JD, Lee MJ, Diamond L, Ju J, Hong J, Bose M, Newmark HL, Yang CS. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab. Dispos. 2006;34:8–11. doi: 10.1124/dmd.104.003434. [DOI] [PubMed] [Google Scholar]
- (43).Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, Alberts DS. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin. Cancer Res. 2005;11:4627–4633. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- (44).Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol. Nutr. Food Res. 2008;52(Suppl. 1):S139–S151. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- (45).Muto S, Fujita K, Yamazaki Y, Kamataki T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat. Res. 2001;479:197–206. doi: 10.1016/s0027-5107(01)00204-4. [DOI] [PubMed] [Google Scholar]