Abstract
The liver X receptor (LXR) functions as a receptor for oxysterols and plays a critical role in the regulation of glucose and lipid metabolism. We recently described a synthetic LXR inverse agonist that displayed efficacy in treatment of hepatic steatosis in a mouse model of non-alcoholic fatty liver disease (NAFLD). This compound, SR9238, was designed to display liver specificity so as to avoid potential detrimental effects on reverse cholesterol transport in peripheral tissues. Here, we examined the effects of a LXR antagonist/inverse agonist, GSK2033, which displays systemic exposure. Although GSK2033 performed as expected in cell-based models as a LXR inverse agonist, it displayed unexpected activity in the mouse NAFLD model. The expression of lipogenic enzyme genes such as fatty acid synthase and sterol regulatory binding protein 1c were induced rather than suppressed and no effect on hepatic steatosis was found. Further characterization of the specificity of GSK2033 revealed that it displayed a significant degree of promiscuity, targeting a number of other nuclear receptors that could clearly alter hepatic gene expression.
Keywords: Nuclear receptor, LXR, Transcription, NASH, Lipogenesis, Drug discovery
1. Introduction
The Liver X Receptor (LXR) serves as a receptor for oxysterols and regulates lipid and carbohydrate metabolism as well as immune function. Two LXRs exist, LXRα (NR1H3), which is expressed in liver, adipose tissue, and macrophages, and LXRβ (NR1H2) that is expressed ubiquitously. Both LXRs share a high degree of homology and act as cholesterol sensors, activated by cholesterol derivatives and regulate reverse cholesterol transport [1–7].
LXRs form heterodimers with the Retinoid X Receptor (RXR) and bind to LXR response elements (LXREs) and associate with coactivator or corepressor complexes to regulate the transcription of target genes. LXR agonists, including the well-characterized T0901317, are well characterized for their ability to suppress atherosclerosis in animal models and have also been shown to display anti-diabetic and anti-inflammatory activity [8–13] Although the LXR agonists display beneficial effects in these disease models, they also increase hepatic lipogenesis resulting in elevated plasma, which precludes them from further development towards clinical therapeutics [14–18]. Antagonists of LXR have also been described [3,19–23] and we recently described the action of a synthetic LXR inverse (SR9238) agonist that suppresses hepatic lipogenesis [24,25]. One might expect that LXR antagonists/inverse agonists would display proinflammatory effects as well as proatherogenic effects; however, SR9238 displays liver specificity. By limiting its extrahepatic exposure (SR9238), these untoward effects were eliminated [24,25]. SR9238 displays efficacy in reducing fatty liver in a mouse model of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis [24,25]. In this study, we examined the activity of a LXR antagonist that displays systemic exposure, GSK2033 [21].
2. Materials and methods
2.1. Cell lines and cotransfection assays
HEK293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS and antibiotics (penicillin and streptomycin; Invitrogen). HepG2 cells were maintained in minimal essential medium supplemented with 10% FBS and antibiotics. Transfection assays were carried out as previously described [24].
2.2. Pharmacokinetic studies
Pharmacokinetic studies were performed as previously described [24,26].
2.3. Animals and preparation of tissue samples
21-week old male C57BL6 DIO mice were purchased from Jackson Labs. All procedures were approved and conducted in accordance to the Scripps Florida Institutional Animal Use Committee. Animals were individually housed and fed a high fat diet (60% k cal/fat diet, 20% carbohydrate) for the duration of the experiment that included GSK2033 administration for 28 days (30 mg/kg, q. d, i. p.). Body weight and food intake was monitored daily. Animal feeding and collection procedures were carried out as previously described [24].
Quantitative Real Time PCR
RNA was isolated from HepG2 cells using the Qiagen RNeasy kit or from mouse tissues by Trizol preparation (Invitrogen). Total RNA was reverse transcribed using the iScript cDNA kit (BioRad). qRT-PCR analysis was performed using the SYBR green kit (Roche) with a 7900HT Fast Real Time PCR System (Applied Biosystems). Each sample was run in duplicate and the results were analyzed according to the ddCt method. For HepG2 cells, cyclophillin B was used as the reference gene. For mouse tissue, Gapdh was used as the reference gene.
2.4. Coregulator recruitment assay
A 33-mer corepressor peptide designated NCoR2 (Biotin-KGGFADPASNLGLEDIIRKALMGSFDDKVEDHG) and coactivator peptide SRC1-2 (Biotin-KGGGGSCPSSHSSLTERHKILHRLLQEGSPSDI) were synthesized by Anaspec, Inc. (San Jose, CA). Low-capacity strepavidin beads (Bead ID#24) were purchased from Radix Bio-solutions (Georgetown, TX). Fifty μg/ml working concentrations of the peptides were coupled to the beads overnight in 4C. The bead/peptide conjugates were subsequently washed twice in PBS/BSA buffer and resuspended in 600 μl of PBS/BSA buffer. PentaHis Alexa 532 antibody was purchased from Qiagen (Valencia, CA) and diluted to a final concentration of 0.8 μg/ml in Luminex buffer. Diluted antibody was added to 25X His-tagged LXR ligand binding domains in a 96-well round bottom plate and incubated at room temperature for 30 min. Peptide bead conjugates and 25X GSK2033 or T0901317 at each respective concentration were added to appropriate wells. LXR-Peptide interactions were allowed to proceed for 3 h at room temperature then read using the Bio-Plex 200 system with suspension array platform and the data was assessed with xMAP technology [27–29].
3. Results
Zuercher et al. previously identified GSK2033 (Fig. 1A) as an LXR antagonist that displayed high binding affinity for LXR while antagonizing LXR target gene expression in cell culture [21]. We confirmed this activity in cell based cotransfection assays where we assessed the ability of GSK2033 to suppress basal transcription LXRα and LXRβ as detected by luciferase reporters driven by either DR4 LXREs (Fig. 1B) or the ABCA1 promoter (Fig. 1C). As shown in Fig. 1B, GSK2033 dose-dependently suppressed basal transcription in full-length LXRα or full-length LXRβ cotransfection assays with IC50s of 17 nM and 9 nM, respectively. GSK2033 also effectively suppressed the transcription of an ABCA1 driven luciferase reporter dose-dependently displaying IC50s of 52 nM for LXRα and 10 nM for LXRβ (Fig. 1C). We also assessed the ability of GSK2033 to induce conformations in LXRα that result in recruitment of either a coactivator NR box peptide or a corepressor CoRNR box peptide. In Fig. 1D, recruitment of the SRC1 NR box protein fragment is clearly increased with the LXR agonist T0901317, but suppressed with addition of GSK2033. Consistent with function as an inverse agonist, GSK2033 induced recruitment of the NCoR CoRNR box peptide to LXR (Fig. 1E). Next, we assessed the ability of GSK2033 to suppress two well-characterized LXR target genes fatty acid synthase (FASN) and sterol regulatory binding protein 1c (SREBF1). HepG2 cells were treated for 24 h with GSK2033 followed by assessment of expression of these genes by qPCR. As illustrated in Fig. 1, GSK2033 suppressed the expression of both of FASN (Fig. 1F) and SREBP1 (Fig. 1G). These data clearly demonstrate that GSK2033 functions as a LXR inverse agonist causing recruitment of corepressor and suppression of basal transcription of LXR target genes.
Fig. 1.
Cotransfection assays in HEK293 cells demonstrate that GSK2033 is an LXR inverse agonist. (A) Structure of GSK2033. (B) Cell-based transfection assay using an LXRE-driven luciferase reporter demonstrates the ability of GSK2033 to reduce basal transcriptional expression of LXRα (IC50 = 17 nM) and LXRβ (IC50 = 9 nM). (C) Cotransfection assay illustrating the ability of GSK2033 to suppress transcriptional activity of LXRα (IC50 = 52 nM) and LXRβ (IC50 = 11 nM) in an ABCA1 driven luciferase reporter. (D) Luminex assay demonstrating that the SRC1 coactivator peptide is recruited to LXRα upon 10 μM treatment with T0901317, but suppressed when LXR is treated with 10 μM GSK2033 (E) GSK2033 (10 μM) induces recruitment of a CoRNR box-peptide of NCoR to LXRα. (F) GSK2033 functions as a LXR inverse agonist in HepG2 cells. GSK2033 suppresses the lipogenic genes fatty acid synthase (FASN) (F) and sterol regulatory binding protein 1c (SREBP1c) (E) with 24-h treatment at 10 μM in HepG2 cells. * indicates p < 0.05 using a Student's t-test.
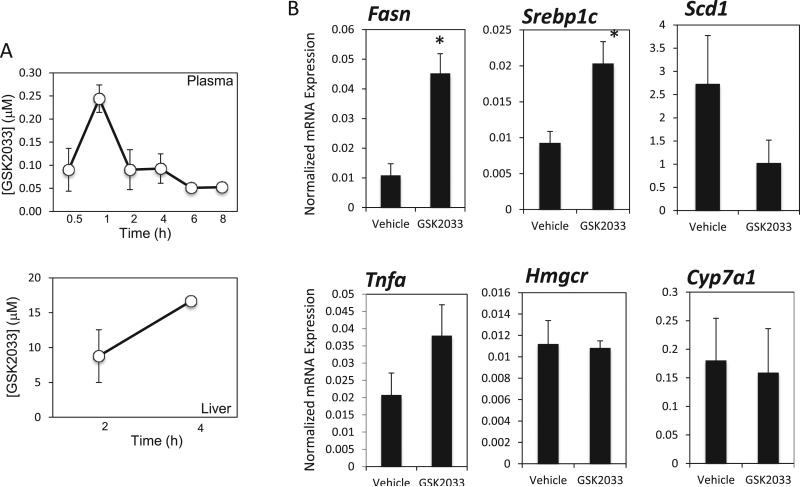
Prior to initiation of animal efficacy studies, exposure of the drug was determined for both plasma and liver samples. Over a time course of 8 h, a single i. p. injection of GSK2033 (30 mg/kg i. p.) resulted in sustained plasma levels of the compound (concentration range 50–250 nM) (Fig. 2A top panel). Additionally, compound levels 4 h post-injection were ~15 μM in the liver (Fig. 2A bottom panel) indicating that LXR in the liver should be saturated with GSK2033.
Fig. 2.
Pharmacological action of GSK2033 in vivo. (A) Pharmacokinetic data illustrating that GSK2033 is sustained in plasma over an 8-h time course following a single i. p. injection (30 mg kg). GSK2033 levels remain well above the IC50 in the liver 4-h post i. p. injection (30 mg/kg). (B) GSK2033 displays an unusual pharmacological profile in a mouse model of NAFLD. Following a once daily treatment with 30 mg/kg for 1 month, lipogenic enzyme and proinflammatory gene expression were assessed by qPCR. As illustrated above, expression of Fasn and SREBF1, lipogenic enzymes, appears to be significantly increased following the treatment, indicating that GSK2033 may have mixed agonist/antagonist properties in vivo. The proinflammatory cytokine TNFα displays a trend towards an increase in liver expression, but it is not significant after a 1-month treatment. Unlike SR9238, GSK2033 did not affect the expression of Hmgcr or Cyp7A1 in this study. N = 7, *, indicates p < 0.05 using a Student's t-test.
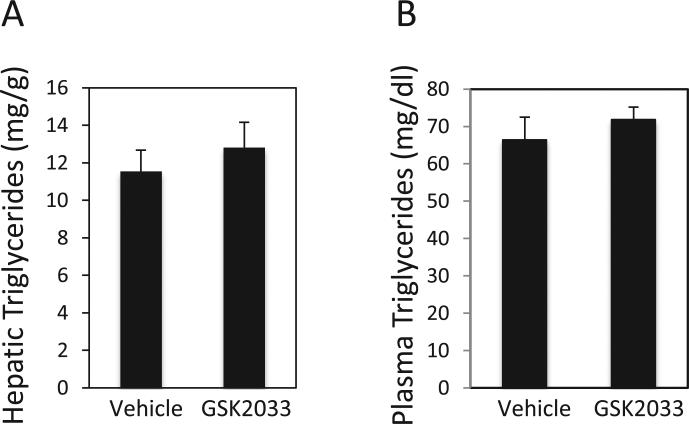
Diet-induced obese (DIO) were used as a model of NAFLD and were dosed once daily with 30 mg/kg GSK2033 (i.p.) for 1 month, an identical experimental design that we utilized to characterize the efficacy of SR9238 [24]. Following the dosing, livers and plasma were collected for gene expression and blood chemistry analysis. Interestingly, the liver gene expression data from these mice indicate that the effects of GSK2033 in this model were clearly distinct from what we previously observed with this compound in HepG2 cells (Fig. 1F and G) and with the LXR inverse agonist SR9238 in this identical animal model [24]. Quite unexpectedly, we actually observed a significant increase in the hepatic expression of Fasn and Srebp1c (Fig. 2B). We also failed to observe a decrease in the proinflammatory cytokine Tnfa as well as Hmgcr and Cyp7a1 as we had observed with SR9238 (Fig. 2B) [24]. Examination of hepatic lipid content indicated that there was no significant effect of treatment with GSK2033 (Fig. 3A) in contrast to the reduction in hepatosteatosis that we observed when mice were treated with SR9238 [24]. We also observed no effect on plasma triglycerides (TGs) in the treated mice (Fig. 3B). The lack of suppression of Tnfa expression, as we had observed with SR9238 treatment [24], is likely due to the fact that there was no decrease in steatosis, which is the inflammatory stimulus. These data indicated that GSK2033 displayed activity in vivo that was not consistent with an expected LXR antagonist profile.
Fig. 3.
Triglyceride levels in GSK2033-treated DIO mice are unaltered. (A) One month treatment of GSK2033 does not have significant effects on hepatic triglyceride levels. (B) Plasma triglyceride levels were also unaffected by treatment with GSK2033. No significant differences between groups were detected by ANOVA (A) or Student's t-test (B).
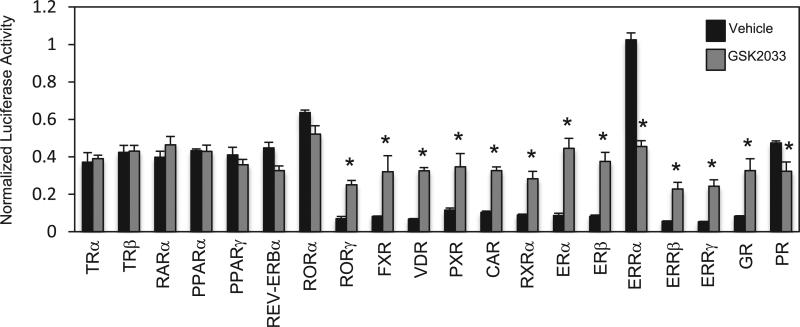
The unusual pharmacological profile displayed by GSK2033 suggested that it may display either LXR “modulator” activity where it functions as a context dependent agonist/antagonist or that it may display promiscuity with respect to targeting various receptors. We assessed the second possibility by testing GSK2033's activity at a range of nuclear receptors in a Gal4-LBD cotransfection assay format as we have previously used to characterize novel nuclear receptor ligands [24,30–32]. Within this assay panel, we observed that GSK2033 activated several nuclear receptors including RORγ, FXR, VDR, PXR, CAR, ERα, ERβ, GR, ERRβ, and ERRγ while suppressing the activity of ERRα and PR (Fig. 4). Given that many of these receptors are expressed in the liver, this could clearly underlie the unexpected results we observed on gene expression in this tissue.
Fig. 4.
Nuclear receptor specificity assay demonstrates that GSK2033 is promiscuous. Cotransfection assay in which Gal4-LBDs of various nuclear receptors were treated with 10 μM GSK2033 in HEK293 cells. As shown above, GSK2033 appears to activate a number of receptors, while also repressing ERRα and PR, indicating the promiscuity of this compound. *, indicates p < 0.05 using a Student's t-test.
4. Discussion
LXR is ligand-activated nuclear receptor that plays a role in cholesterol, fatty acid, and glucose homeostasis and is also a potential drug target for the treatment of several diseases including atherosclerosis [11,9,12], Alzheimer's Disease [33] and NAFLD/NASH [24,25]. Several studies have previously shown the beneficial effects on atherosclerosis when treating with LXR agonists such as T0901317 or GSK3965 [8,12,34–36], however the development of LXR agonists for cardiovascular disease has been limited since these compounds induce hepatic lipogenesis and hypertriglyceridemia [3]. Although the effects on hepatic lipogenesis limit the utility of LXR agonists in treatment of atherosclerosis, we recently utilized these hepatic effects to investigate the effects of a liver specific LXR inverse agonist that suppressed lipogenic enzyme gene expression. Lipogenesis is elevated in NAFLD and suppression of this pathway may be useful in treatment of this disease as well as its progression to non-alcoholic steatohepatitis. We found that SR9238, a liver selective LXR inverse agonist, effectively suppressed lipogenesis in a mouse model of NAFLD and effectively blocked hepatic steatosis even though the mice remained on a high fat diet [24]. Here, we assessed the effects of GSK2033 in the identical mouse model of NAFLD. GSK2033 is described as a LXR antagonist [21] although we observed clear inverse agonist activity as well. GSK2033 display systemic exposure and we believed it might be a good comparison to SR9238, which displays liver specificity. However, we observed unexpected effects of GSK2033 on lipogenic gene expression when it was administered to the mice. Fasn and Srebp-1c expression was increased while Scd-1 expression displayed a clear trend towards repression. There was also no effect on hepatic or plasma triglycerides in contrast to what we previously observed with SR9238 treatment [24]. This led us to the hypothesis that either GSK2033 displayed LXR modulator agonist/antagonist activity or that GSK2033 might target additional receptors. After analysis of GSK2033 specificity, we found that GSK2033 was quite promiscuous and targeted a number of nuclear receptors, such as the glucocorticoid receptor, pregnane X receptor, farnesoid X receptor, among others that are expressed in the liver that could clearly alter metabolic gene expression profiles in the tissue.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M. A novel orphan receptor specific for a subset of thyroid hormone responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol. Cell. Biol. 1994;14:7025–7035. doi: 10.1128/mcb.14.10.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michael LF, Schkeryantz JM, Burris TP. The pharmacology of LXR. Mini Rev. Med. Chem. 2005;5:729–740. doi: 10.2174/1389557054553767. [DOI] [PubMed] [Google Scholar]
- 4.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. Lxr, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 5.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 6.Peet DJ, Janowski BA, Mangelsdorf DJ. The LXRs: a new class of oxysterol receptors. Curr. Opin. Genet. Dev. 1998;8:571–575. doi: 10.1016/s0959-437x(98)80013-0. [DOI] [PubMed] [Google Scholar]
- 7.Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 2000;275:28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 8.Terasaka N, Hiroshima A, Koieyama T, Ubukata N, Morikawa Y, Nakai D, et al. T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 2003;536:6–11. doi: 10.1016/s0014-5793(02)03578-0. [DOI] [PubMed] [Google Scholar]
- 9.Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 11.Joseph SB, McKilligin E, Pei LM, Collins AR, Laffitte BA, Tangirilla RK, et al. The nuclear receptor LXR inhibits development of atherosclerosis. Circulation. 2002;106:121. [Google Scholar]
- 12.Joseph SB, McKilligin E, Pei LM, Watson MA, Collins AR, Laffitte BA, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao GQ, Liang Y, Broderick CL, Oldham BA, Beyer TP, Schmidt RJ, et al. Antidiabetic action of a liver X receptor agonist mediated by inhibition of hepatic gluconeogenesis. J. Biol. Chem. 2003;278:1131–1136. doi: 10.1074/jbc.M210208200. [DOI] [PubMed] [Google Scholar]
- 14.Cao G, Liang Y, Broderick CL, Oldham BA, Beyer TP, Schmidt RJ, et al. Antidiabetic action of a liver x receptor agonist mediated by inhibition of hepatic gluconeogenesis. J. Biol. Chem. 2003;278:1131–1136. doi: 10.1074/jbc.M210208200. [DOI] [PubMed] [Google Scholar]
- 15.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li LP, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen GX, Liang GS, Ou JF, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chisholm JW, Hong J, Mills SA, Lawn RM. The LXR ligand T0901317 induces severe lipogenesis in the db/db diabetic mouse. J. Lipid Res. 2003;44:2039–2048. doi: 10.1194/jlr.M300135-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipo-protein particles. J. Biol. Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 19.Motoshima K, Noguchi-Yachide T, Sugita K, Hashimoto Y, Ishikawa M. Separation of alpha-glucosidase-inhibitory and liver X receptor-antagonistic activities of phenethylphenyl phthalimide analogs and generation of LXRalpha-selective antagonists. Bioorg Med. Chem. 2009;17:5001–5014. doi: 10.1016/j.bmc.2009.05.066. [DOI] [PubMed] [Google Scholar]
- 20.Jiao X, Kopecky DJ, Fisher B, Piper DE, Labelle M, McKendry S, et al. Discovery and optimization of a series of liver X receptor antagonists. Bioorg. Med. Chem. Lett. 2012;22:5966–5970. doi: 10.1016/j.bmcl.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 21.Zuercher WJ, Buckholz RG, Campobasso N, Collins JL, Galardi CM, Gampe RT, et al. Discovery of tertiary sulfonamides as potent liver X receptor antagonists. J. Med. Chem. 2010;53:3412–3416. doi: 10.1021/jm901797p. [DOI] [PubMed] [Google Scholar]
- 22.Tamehiro N, Sato Y, Suzuki T, Hashimoto T, Asakawa Y, Yokoyama S, et al. Riccardin C: a natural product that functions as a liver X receptor (LXR)alpha agonist and an LXRbeta antagonist. FEBS Lett. 2005;579:5299–5304. doi: 10.1016/j.febslet.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 23.Thomas J, Bramlett KS, Montrose C, Foxworthy P, Eacho PI, McCann D, et al. A chemical switch regulates fibrate specificity for peroxisome proliferator-activated receptor alpha (PPAR alpha) versus liver X receptor. J. Biol. Chem. 2003;278:2403–2410. doi: 10.1074/jbc.M209629200. [DOI] [PubMed] [Google Scholar]
- 24.Griffett K, Solt LA, El-Gendy Bel D, Kamenecka TM, Burris TP. A liver-selective LXR inverse agonist that suppresses hepatic steatosis. ACS Chem. Biol. 2013;8:559–567. doi: 10.1021/cb300541g. [DOI] [PubMed] [Google Scholar]
- 25.Griffett K, Welch RD, Flaveny CA, Kolar GR, Neuschwander-Tetri BA, Burris TP. The LXR inverse agonist SR9238 suppresses fibrosis in a model of non-alcoholic steatohepatitis. Mol. Metab. 2015;4:353–357. doi: 10.1016/j.molmet.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flaveny CA, Griffett K, El-Gendy Bel D, Kazantzis M, Sengupta M, Amelio AL, et al. Broad anti-tumor activity of a small molecule that selectively targets the warburg effect and lipogenesis. Cancer Cell. 2015;28:42–56. doi: 10.1016/j.ccell.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, et al. Identification of heme as the ligand for the orphan nuclear receptors REVERBalpha and REV-ERBbeta. Nat. Struct. Mol. Biol. 2007;14:1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar N, Solt LA, Wang Y, Rogers PM, Bhattacharyya G, Kamenecka TM, et al. Regulation of adipogenesis by natural and synthetic REV-ERB ligands. Endocrinology. 2010;151:3015–3025. doi: 10.1210/en.2009-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kojetin D, Wang Y, Kamenecka TM, Burris TP. Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB. ACS Chem. Biol. 2011;6:131–134. doi: 10.1021/cb1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, et al. Regulation of circadian behavior and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solt LA, Kumar N, Nuhant P, Wang YJ, Lauer JL, Liu J, et al. Suppression of T(H)17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, et al. The benzenesulfonamide T0901317 is a novel ROR{alpha}/{gamma} Inverse Agonist. Mol. Pharmacol. 2010;77:228–236. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koldamova RP, Lefterov IM, Staufenbiel M, Wolfe D, Huang S, Glorioso JC, et al. The liver X receptor ligand T0901317 decreases amyloid beta production in vitro and in a mouse model of Alzheimer's disease. J. Biol. Chem. 2005;280:4079–4088. doi: 10.1074/jbc.M411420200. [DOI] [PubMed] [Google Scholar]
- 34.Claudel T, Leibowitz MD, Fievet C, Tailleux A, Wagner B, Repa JJ, et al. Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2610–2615. doi: 10.1073/pnas.041609298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sparrow CP, Baffic J, Lam MH, Lund EG, Adams AD, Fu XA, et al. A potent synthetic LXR agonist is more effective than cholesterol loading at inducing ABCA1 mRNA and stimulating cholesterol efflux. J. Biol. Chem. 2002;277:10021–10027. doi: 10.1074/jbc.M108225200. [DOI] [PubMed] [Google Scholar]
- 36.Levin N, Bischoff ED, Daige CL, Thomas D, Vu CT, Heyman RA, et al. Macrophage liver x receptor is required for antiatherogenic activity of LXR agonists. Arterioscler. Thromb.Vasc. Biol. 2005;25:135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]