Abstract
Purpose
Ipilimumab is a standard treatment for metastatic melanoma, but immune-related adverse events (irAEs) are common and can be severe. We reviewed our large, contemporary experience with ipilimumab treatment outside of clinical trials to determine the frequency of use of systemic corticosteroid or anti-tumor necrosis factor α (anti-TNFα) therapy and the effect of these therapies on overall survival (OS) and time to treatment failure (TTF).
Patients and Methods
We reviewed retrospectively the medical records of patients with melanoma who had received treatment between April 2011 and July 2013 with ipilimumab at the standard dose of 3 mg/kg. We collected data on patient demographics, previous and subsequent treatments, number of ipilimumab doses, irAEs and how they were treated, and overall survival.
Results
Of the 298 patients, 254 (85%) experienced an irAE of any grade. Fifty-six patients (19%) discontinued therapy because of an irAE, most commonly diarrhea. Overall, 103 patients (35%) required systemic corticosteroid treatment for an irAE; 29 (10%) also required anti-TNFα therapy. Defining TTF as either starting a new treatment or death, estimated median TTF was 5.7 months. Twelve percent of patients experienced long-term disease control without receiving additional antimelanoma therapy. OS and TTF were not affected by the occurrence of irAEs or the need for systemic corticosteroids.
Conclusion
IrAEs are common in patients treated with ipilimumab. In our experience, approximately one-third of ipilimumab-treated patients required systemic corticosteroids, and almost one-third of those required further immune suppression with anti-TNFα therapy. Practitioners and patients should be prepared to treat irAEs and should understand that such treatment does not affect OS or TTF.
INTRODUCTION
Ipilimumab, an anti–cytotoxic T-cell lymphocyte-4 (anti–CTLA-4) antibody, has changed the treatment landscape for patients with metastatic melanoma. It was the first therapy shown to improve overall survival (OS) in melanoma. In the randomized trial with the longest follow-up time, the 2-year OS was 21% and the progression-free survival rate was ≤ 10%.1 However, ipilimumab also can result in activation of immune responses against normal tissues. The most common immune-related adverse events (irAEs) are diarrhea, rash, hepatitis, and hypophysitis. These irAEs can result in severe toxicity, although the majority of events are reversible with outpatient management, according to standard algorithmic guidelines.2–4 Over the years, investigators have learned to identify these irAEs early and treat them with immunosuppressive agents, most commonly corticosteroids.3 However, in some situations, corticosteroids are insufficient and additional immunosuppressive agents, such as anti-tumor necrosis factor α (anti-TNFα) monoclonal antibodies, are required.5
In the pivotal randomized trial in which patients received ipilimumab alone or with a gp100 peptide vaccine, the incidence of irAEs was 60%, although only 10% to 15% of patients had irAEs of grade 3 or greater.1 Eleven percent of patients received corticosteroids, and less than 1% received anti-TNFα therapy.1 In that trial, specific algorithms, mostly based on the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE; version 3.0) grade of toxicity, were used to guide the use of corticosteroids. Despite this, 1.3% of patients suffered a fatal irAE.1 The grading of irAEs can be problematic, however, because of the somewhat arbitrary distinction between CTCAE grade 2 and grade 3. For example, six loose stools per day above baseline is considered grade 2 diarrhea, whereas seven stools per day above baseline is considered grade 3. The delineation between the two grades therefore depends on patients' recall and does not necessarily reflect the severity of the toxicity. Further inaccuracies can arise in the ability to classify the same clinical event as either diarrhea or colitis, each having quite distinct grading schemes.
Now that ipilimumab is approved for use in most parts of the world, there is extensive experience both in treating these irAEs outside of clinical trials and in evaluating the clinical activity of ipilimumab. Our institutional experience led us to suspect that the incidence of clinically significant irAEs might be higher than indicated by the incidence of CTCAE grade 3 irAEs and that a higher percentage of patients require immunosuppressive treatment. We also suspected that progression-free survival, as assessed with use of RECIST, underestimated the clinical benefit from ipilimumab. As a result, we evaluated the incidence of prolonged clinical benefit as measured by the time until subsequent therapy was required. Since we have a long experience with ipilimumab, we evaluated the incidence and treatment of irAEs in our patients treated with ipilimumab as a standard of care in which treatment decisions were dictated by the treating physician rather than by a clinical trial protocol.
PATIENTS AND METHODS
We conducted a retrospective analysis of all adult patients with melanoma who received ipilimumab (3 mg/kg) at Memorial Sloan Kettering Cancer Center between April 2011 and July 2013 and for whom there was adequate documentation of the clinical course on ipilimumab. Patients were excluded from our analysis if they were receiving ipilimumab as part of a clinical trial. Electronic medical records and pharmacy databases were used to obtain patient-specific information. Data collected included patient demographics, previous and subsequent treatments, the number of doses of ipilimumab that each patient received, ipilimumab-related irAEs (ie, diarrhea and/or colitis, hepatitis, dermatitis, hypophysitis, and uveitis), treatment of irAEs, and date of death or last follow-up. Toxicities were graded retrospectively by a single investigator (T.Z.H.) on the basis of chart review with use of CTCAE, version 4.0. The Memorial Sloan Kettering Cancer Center institutional review board reviewed the project and determined it to be exempt research. The primary objective of this analysis was to determine the incidence of irAEs associated with ipilimumab treatment, the incidence of systemic immunosuppression used to treat the irAEs, and the association of these factors with OS and time to treatment failure (TTF).
Statistical Analysis
Descriptive statistics were calculated for patient characteristics. OS was defined as the time from the first ipilimumab treatment until death. Patients still alive were censored at the time of last follow-up. TTF was defined as the time from first ipilimumab treatment until the patient was started on another line of systemic therapy or the patient died, whichever occurred first. Patients who were alive and did not start another therapy were censored at the time of last follow-up. OS and TTF were estimated by Kaplan-Meier methods; all patients were included in these analyses. We did not attempt to assess tumor responses retrospectively by RECIST criteria.
To analyze the effects of irAEs and corticosteroid use on OS and TTF, a landmark analysis was used. Since most irAEs and corticosteroid use have occurred by week 14, OS and TTF were defined beginning from the landmark of 14 weeks after the start of treatment. Patients who died before week 14 were not included in the irAE and corticosteroid analyses. Patients who changed therapy before week 14 were also not included in the TTF analyses of irAE and corticosteroid use. The log-rank test was used to analyze the differences in OS and TTF when stratifying by occurrence of irAE or corticosteroid use. All statistical tests were two-sided, and 5% was set as the level of significance. Statistical analyses were performed with use of R (version 3.0.1; R Development Core Team, Vienna, Austria), with the “survival” and “survcomp” packages.
RESULTS
Patients
Between April 1, 2011, and July 1, 2013, the 298 patients who were included in this analysis received a total of 1,133 doses of ipilimumab. All patients were treated by one or more of the coauthors. The complete baseline characteristics are shown in Table 1. The median age at the time of treatment was 65 years (range, 21 to 93 years), and 61% of the patients were men. Thirty-five percent of patients had received prior systemic therapy for melanoma; 19% had central nervous system metastases that had been, in most cases, previously treated with surgery and/or radiotherapy. Of the 298 patients, 210 patients (70%) completed all four doses of ipilimumab. In 27 patients, reinduction with ipilimumab was initiated at some point on the basis of published evidence that ipilimumab reinduction therapy led to restoration of disease control or even to partial or complete response at the time of progression.1 Of these 27 patients, 18 were able to complete all four doses of the reinduction course. Post-ipilimumab therapies included systemic chemotherapy (60 patients), anti-programmed death-1 (anti-PD-1) monoclonal antibodies (46 patients), RAF inhibitors (42 patients), MEK inhibitors (seven patients), anti-PD-L1 monoclonal antibodies (seven patients), and other investigational agents (14 patients).
Table 1.
Baseline Characteristics (N = 298)
| Characteristics | No. (%) |
|---|---|
| Age, years | |
| Median | 65 |
| Range | 21-93 |
| Male sex | 182 (61) |
| ECOG performance status | |
| 0 | 207 (70) |
| 1 | 84 (28) |
| 2 | 6 (2) |
| 3 | 1 (0.3) |
| 4 | 0 (0) |
| M stage | |
| M0 | 29 (10) |
| M1a | 34 (11) |
| M1b | 74 (25) |
| M1c | 161 (54) |
| Lactate dehydrogenase level | |
| ≤ Upper limit of normal range | 160 (54) |
| > Upper limit of normal range | 83 (28) |
| Unknown | 55 (18) |
| CNS metastases at baseline | 56 (19) |
| Previous systemic therapy | 105 (35) |
| No. of doses of ipilimumab | |
| Median | 4 |
| Range | 1-8 |
irAEs
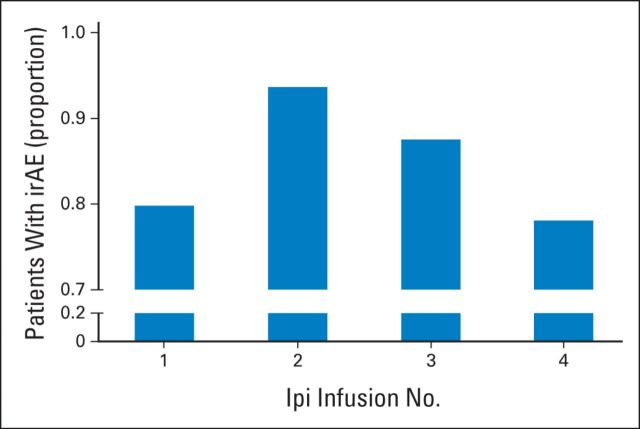
Of the 298 patients, 254 (85%) experienced an irAE of any grade. Grade 3, 4, and 5 irAEs (Table 2) were observed in 91 (31%), 20 (7%), and 1 patient, respectively. The most common irAE of grade 3 or greater was diarrhea, which occurred in 14% of patients. Although irAEs could be seen after any of the four doses of ipilimumab, dose number 2 was associated with a slightly higher incidence of irAEs than that seen with the other treatment cycles (Fig 1). Overall, 56 patients (19%) discontinued therapy because of an irAE, with the most common event being diarrhea (34 patients). Other irAEs that led to discontinuation of ipilimumab were hepatotoxicity (12 patients), hypophysitis (six patients), uveitis (two patients), neurotoxicity (one patient), and pneumonitis (one patient). Three of the 298 patients (1%) experienced bowel perforation from colitis. Two of the three underwent surgical management; the other patient, managed medically at an outside hospital, died as a result of colitis without having undergone surgery.
Table 2.
irAEs by Grade
| irAEs | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|---|
| Hepatotoxicity | 135 | 23 | 32 | 7 | 0 | 197 |
| Dermatitis | 69 | 36 | 18 | 0 | 0 | 123 |
| Diarrhea | 25 | 20 | 29 | 12 | 1 | 87 |
| Hypophysitis | 1 | 6 | 10 | 0 | 0 | 17 |
| Uveitis | 1 | 5 | 1 | 1 | 0 | 8 |
| Other | 1 | 6 | 8 | 0 | 0 | 15 |
| Total* | 232 | 96 | 98 | 20 | 1 | 447 |
Abbreviation: irAEs, immune-related adverse events.
Patients could have experienced more than one irAE. Therefore, the total number of irAEs is more than the total number of patients.
Fig 1.
Incidence of immune-related adverse events (irAEs) by dose of ipilimumab. y-axis indicates the proportion of patients experiencing an irAE of any grade after each of the ipilimumab (Ipi) infusions.
Treatment of irAEs
Because grading the severity of an adverse event can be subjective, we analyzed the data on the basis of whether or not the patient required systemic immunosuppressive therapy as assessed by the treating physician. One hundred and three patients (35%) required systemic corticosteroid treatment for an irAE. The majority of these patients (n = 78) received corticosteroids for grade ≥ 3 irAEs. However, 25 patients received corticosteroids for grade 2 (23 patients) or grade 1 (two patients) irAEs. The reasons for corticosteroid initiation were diarrhea (50 patients), hepatitis (22 patients), dermatitis (21 patients), endocrinopathies (14 patients), uveitis (one patient), pneumonitis (one patient), seizure (one patient), arthritis (one patient), and hearing loss (one patient). Patients may have had multiple irAEs concurrently that led to corticosteroid initiation. Of the 25 patients who received systemic corticosteroids for grade 1 or grade 2 irAEs, 15 had failed to respond to milder treatment before receiving systemic corticosteroids. For example, of the 14 patients who received systemic corticosteroids for grade 1 or grade 2 diarrhea, 10 had failed to respond to budesonide or antidiarrheal medications. In most cases, these patients only required a short course of orally administered corticosteroids.
Of the 103 patients who were treated with systemic corticosteroids, 31 (10% of the entire study population) did not experience adequate resolution of their symptoms and were treated with additional systemic immunosuppressive therapy. Two patients received mycophenolate (1,000 mg, twice a day) for grade 3 and grade 4 immune-related hepatotoxicity, while the other 29 patients received infliximab (5 mg/kg) for immune-related diarrhea. These 29 patients had grade 2 (seven patients), grade 3 (15 patients), or grade 4 (seven patients) diarrhea and had been treated with systemic corticosteroids without resolution of symptoms. Twenty-six of the 29 patients (90%) had received at least one week of high-dose corticosteroids (1 mg/kg prednisone equivalent) without adequate improvement of symptoms. The other three patients were switched to infliximab because of worsening diarrhea, having received less than a week of corticosteroids. Twenty-one of the 29 patients (72%) responded to infliximab, most after a single dose, but three patients required a second dose of infliximab. Eight patients did not respond to infliximab and were managed with prolonged courses of corticosteroids. One of the eight patients who did not respond to the first dose of infliximab received adalimumab (80 mg) because of an infusion reaction during the second dose of infliximab. This patient did not respond to adalimumab and was managed on a prolonged course of corticosteroids.
OS and TTF
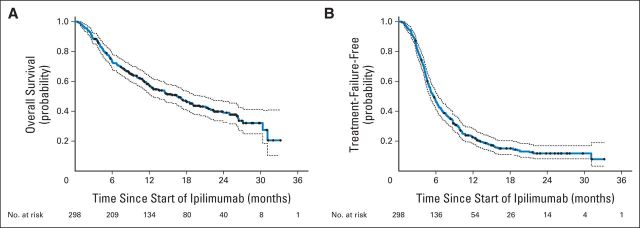
The estimated median OS was 16.5 months (95% CI, 12.6 to 21.1) with an estimated 2-year survival rate of 39% (95% CI, 33% to 46%) (Fig 2A). The estimated median TTF was 5.7 months (95% CI, 5.1 to 6.4) for all patients (Fig 2B). The TTF curve plateaued at approximately 12%, indicating that an estimated 88% of patients either progressed and required a change in therapy or died before a change in therapy could be initiated.
Fig 2.
Overall survival (OS) and time to treatment failure (TTF) in 298 patients treated with ipilimumab. (A) OS was defined as time from first dose of ipilimumab until death. (B) TTF was defined as time from first dose of ipilimumab until first dose of a subsequent therapy or death, whichever came first. Dashed lines indicate 95% CIs. Black dots represent censored patients.
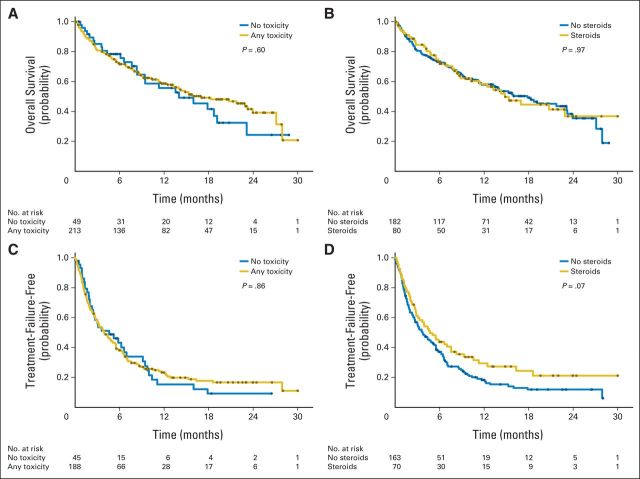
To assess the effect of irAEs on OS and TTF, a landmark analysis was performed. To ensure that all patients with irAEs were identified, patients who died or were lost to follow-up before week 14 were excluded. This resulted in the exclusion of only 36 patients. No difference in OS or TTF was detected when patients were stratified by the presence or absence of irAEs of any grade (Fig 3A, 3C). In addition, there was no difference in OS or TTF when patients were stratified by the administration of systemic corticosteroids to treat an irAE (Figs 3B and 3D).
Fig 3.
Landmark of correlates of overall survival (OS) and time to treatment failure (TTF) in patients treated with ipilimumab. OS shown after landmark analysis and stratifying by whether patients (A) had immune-related adverse event (irAE) or (B) required systemic corticosteroids. TTF shown after landmark analysis and stratifying by whether patients (C) had irAE or (D) required systemic corticosteroids. Black dots represent censored patients.
DISCUSSION
We analyzed 298 patients who received ipilimumab as a standard of care over a period of 27 months and were treated by a select group of physicians and nurses who were highly experienced in the use of ipilimumab. It should be noted that the clinical decisions regarding whether to start treatment with systemic corticosteroids were based on clinical judgment rather than strictly on the CTCAE grading system—a system that is necessarily arbitrary and not particularly suited to irAEs that result from immune checkpoint inhibitors. Although the Bristol-Myers Squibb guidelines for management of irAEs4 were generally followed, these are ultimately only guidelines and treatment decisions were always left with the treating physician. For example, we would aggressively treat a patient who is having five stools above baseline (grade 2 diarrhea) and has some blood in their stool (grade 2 colitis), even though these would be classified as grade 2 irAEs by CTCAE.
Regarding the grading of irAEs by CTCAE criteria, 31% of our patients were found to have grade ≥ 3 irAEs. In the Italian experience6, only 6% of patients were thought to have had a grade ≥ 3 irAE and there was no information reported on the use of corticosteroids. Intermediate between these two extremes is the pivotal phase-III trial1 in which 96 (19%) of 511 patients receiving ipilimumab were reported to have had an irAE of at least grade 3. Although this wide range of scoring might suggest that there are significant regional differences in how patients tolerate ipilimumab, we think it is more likely that this indicates that even with a standardized grading system, toxicity evaluations vary among investigators and global regions. Therefore, while treatment algorithms can be extremely useful as guidelines for treating irAEs, ultimately physicians should be guided by clinical judgment and experience. We agree with Ascierto et al, who pointed out that “… as physicians gain more experience of treating patients with ipilimumab, they are more familiar with its associated AEs, enabling earlier detection and timely intervention.”6(p6)
In our cohort, 35% of patients treated with ipilimumab required systemic corticosteroid treatment for irAEs. This is more than twice the rate of grade ≥ 3 toxicity reported in clinical trials with ipilimumab at the 3 mg/kg dose.1,6,7 This is probably explained by our group's experience regarding when to abandon less intensive therapies (eg, diet manipulation or budesonide for colitis) and our readiness to use systemic corticosteroids early in the course of irAEs. The higher rate of systemic corticosteroid use might also be related to treating some patients as a standard of care who would not have qualified for treatment on a clinical trial.
We found that 30% of the patients who required systemic high-dose corticosteroid treatment did not have adequate resolution of irAEs and required additional immunosuppressive therapy. Most of these patients needed only a single dose of infliximab, although some patients required a second dose. The relatively high rate of infliximab use is a result of our clinical judgment that early treatment with infliximab is preferable to prolonged treatment with high-dose corticosteroids.
In our experience, if improvement in irAE symptoms is not evident early in the treatment with high-dose systemic corticosteroids, more prolonged treatment rarely leads to benefit and patients usually end up requiring infliximab anyway, independent of CTCAE grade. On the basis of our experience, we believe the overall risk-to-benefit ratio favors the early use of infliximab rather than prolonged treatment with corticosteroids. In general, we administer infliximab if symptoms do not clearly improve after one week of high-dose corticosteroid treatment. Despite aggressive management, we observed that three patients (1%) experienced a bowel perforation.
The median OS for the entire cohort was 16.5 months. This compares favorably with the recent pooled analysis of 1,861 patients treated with ipilimumab in which the median OS was 11.4 months.8 However, our follow-up is much shorter and effective treatment options after progression may have been more available for our patients than they were for most of the patients in the pooled analysis, as indicated by the 34% of our patients who went on to receive agents with a proven OS benefit after ipilimumab.
One of the unique characteristics of ipilimumab therapy is that it can result in prolonged, stable disease, which presumably contributes to the beneficial effect on OS. In many cases, distinguishing between stable disease and slight progression can be somewhat arbitrary, and even patients with slow progression may not require additional therapy. OS has become a problematic metric by which to assess melanoma therapies, as there are now several treatment options after relapse that have been shown to improve OS. For this reason, we assessed TTF in which an event was defined as starting a new treatment or death, whichever came first. When TTF is analyzed in this way, the TTF curve plateaus at 12% by 2 years. This indicates that 88% of patients receiving ipilimumab either required a subsequent treatment or died before receiving another form of treatment. This metric is not confounded by subsequent therapy as is OS. The observation that the OS curve is shifted to the right as compared with the TTF curve is consistent with the speculation that the subsequent therapies had a positive impact on OS.
An early report found that the objective response rates among patients who had experienced colitis were higher than the response rates of patients who had not.2 The investigators speculated that colitis could be a biomarker for response. Although we did not specifically measure tumor response, we used a landmark analysis to assess the effects of irAEs on OS and TTF. We found that neither the occurrence of irAEs (no matter the grade) nor the use of systemic corticosteroids was associated with OS or TTF, which is consistent with the observations from Ascierto et al6 However, we cannot rule out the possibility that a specific irAE might be associated with a worse (or better) OS or TTF. On the basis of our data and that of the Italian group, we believe that patients and physicians should not be concerned that irAEs requiring systemic immunosuppression will compromise the therapeutic benefit.
There are some limitations of our analysis. This is a single institution experience, albeit a relatively large one, and the data were not collected as part of a clinical trial. We did not grade toxicity prospectively, nor did we attempt to measure tumor responses formally. Our patients are perhaps less representative of prior clinical trial subjects and more representative of patients treated in clinical practice. We believe that the tumor response rate has been well established for ipilimumab and that the lack of a formal tumor response does not necessarily preclude a beneficial effect in individual patients.9
These data help guide expectations for both patients and practitioners. In our experience, one-third of patients receiving ipilimumab required systemic corticosteroid treatment and 10% required anti-TNFα immunosuppression. Practitioners should be prepared to treat irAEs aggressively and early. Our data indicate that the need for corticosteroid treatment is not associated with impaired TTF or OS. Overall, an estimated 12% of patients achieved long-term disease control and did not require subsequent therapy for melanoma.
Footnotes
P.B.C. was supported in part by the John K. Figge Fund.
Presented at the Society for Melanoma Research International Conference, Zurich, Switzerland, November 13, 2014.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Troy Z. Horvat, Thu-Oanh Dang, Paul B. Chapman
Financial support: Paul B. Chapman
Provision of study materials or patients: Margaret K. Callahan, Richard D. Carvajal, Mark A. Dickson, Sandra P. D'Angelo, Jedd D. Wolchok, Paul B. Chapman
Collection and assembly of data: Troy Z. Horvat, Nelly G. Adel, Margaret K. Callahan, Richard D. Carvajal, Mark A. Dickson, Sandra P. D'Angelo, Jedd D. Wolchok, Paul B. Chapman
Data analysis and interpretation: Troy Z. Horvat, Thu-Oanh Dang, Parisa Momtaz, Michael A. Postow, Kaitlin M. Woo, Katherine S. Panageas, Paul B. Chapman
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Troy Z. Horvat
No relationship to disclose
Nelly G. Adel
No relationship to disclose
Thu-Oanh Dang
No relationship to disclose
Parisa Momtaz
No relationship to disclose
Michael A. Postow
Consulting or Advisory Role: Bristol-Myers Squibb, Amgen
Research Funding: Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Margaret K. Callahan
Employment: Bristol-Myers Squibb (I)
Consulting or Advisory Role: Kiowa Hakko Kiran, GlaxoSmithKline
Research Funding: Bristol-Myers Squibb
Richard D. Carvajal
Consulting or Advisory Role: Aura Biosciences, AstraZeneca, Novartis, Biogen Idec
Research Funding: GlaxoSmithKline (Inst), Bristol-Myers Squibb (Inst), Novartis (Inst), AstraZeneca (Inst), Daiichi Sankyo (Inst), Incyte (Inst), Amgen (Inst), Bayer (Inst), Celldex (Inst), Pfizer (Inst)
Mark A. Dickson
No relationship to disclose
Sandra P. D'Angelo
No relationship to disclose
Kaitlin M. Woo
No relationship to disclose
Katherine S. Panageas
Travel, Accommodations, Expenses: Astra Zeneca
Jedd D. Wolchok
Honoraria: EMD Serono, Janssen Oncology
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, MedImmune, Ziopharm, Polynoma, Polaris, Jounce, GlaxoSmithKline
Research Funding: Bristol-Myers Squibb (Inst), MedImmune (Inst), GlaxoSmithKline (Inst), Merck (Inst)
Patents, Royalties, Other Intellectual Property: I am a co-inventor on an issued patent (patent no. 7556805) for DNA vaccines for treatment of cancer in companion animals
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Paul B. Chapman
Honoraria: Bristol-Myers Squibb, GlaxoSmithKline, Genentech/Roche, Provectus, Momenta Pharmaceuticals, Daiichi Sankyo
Consulting or Advisory Role: Bristol-Myers Squibb, GlaxoSmithKline, Genentech/Roche, Daiichi Sankyo, Provectus, Momenta Pharmaceuticals
Research Funding: GlaxoSmithKline, Genentech/Roche, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb
REFERENCES
- 1.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 4.Princeton, NJ: Bristol-Myers Squibb Pharmaceuticals; 2012. Yervoy (ipilimumab) Immune-Mediated Adverse Reaction Management Guide. [Google Scholar]
- 5.Johnston RL, Lutzky J, Chodhry A, et al. Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab. Dig Dis Sci. 2009;54:2538–2540. doi: 10.1007/s10620-008-0641-z. [DOI] [PubMed] [Google Scholar]
- 6.Ascierto PA, Simeone E, Sileni VC, et al. Clinical experience with ipilimumab 3 mg/kg: Real-world efficacy and safety data from an expanded access programme cohort. J Transl Med. 2014;12:116. doi: 10.1186/1479-5876-12-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 8.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015 Feb 9;pii doi: 10.1200/JCO.2014.56.2736. JCO.2014.56.2736. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]