Abstract
Human chorionic gonadotropin (hCG) has been regarded as a biomarker for the diagnosis of pregnancy and some cancers. Because the currently used methods (e.g., disposable Point of Care Testing (POCT) device) for hCG detection require the use of many less stable antibodies, simple and cost-effective methods for the sensitive and selective detection of hCG have always been desired. In this work, we have developed a graphene oxide (GO)-based fluorescent platform for the detection of hCG using a fluorescein isothiocyanate (FITC)-labeled hCG-specific binding peptide aptamer (denoted as FITC-PPLRINRHILTR) as the probe, which can be manufactured cheaply and consistently. Specifically, FITC-PPLRINRHILTR adsorbed onto the surface of GO via electrostatic interaction showed a poor fluorescence signal. The specific binding of hCG to FITC-PPLRINRHILTR resulted in the release of the peptide from the GO surface. As a result, an enhanced fluorescence signal was observed. The fluorescence intensity was directly proportional to the hCG concentration in the range of 0.05–20 IU/mL. The detection limit was found to be 20 mIU/mL. The amenability of the strategy to hCG analysis in biological fluids was demonstrated by assaying hCG in the urine samples.
Keywords: graphene oxide, fluorescent biosensors, peptide aptamer, human chorionic gonadotropin, antibody-free
1. Introduction
Human chorionic gonadotropin (hCG) is a glycoprotein hormone produced by the embryo and presented in the blood and urine of pregnant women [1]. Recently, elevated levels of hCG were found in many cancerous tumors, such as prostate cancer, testicular cancer, trophoblastic cancer and gestational choriocarcinoma [2]. Thus, hCG can be regarded as a biomarker for the diagnosis of pregnancy and some cancers. Because the lateral-flow immunoassay (the most commonly used method for hCG detection) has trouble accurately quantifying the level of hCG, a few new techniques have been made recently to determine hCG in blood and urine, such as enzymelinked immunosorbent assay (ELISA) [3], fluorescent immunoassay [4], immunochromatography [5], photoluminescence [6,7], surface plasmon resonance (SPR) [8] and electrochemical immunosensors [9,10,11,12,13,14,15,16,17,18,19,20]. These methods are sensitive and selective, but they are usually expensive, time-consuming and labor intensive and require the use of less stable antibodies. Moreover, the drive to produce disposable Point of Care Testing (POCT) devices uses a lot of antibodies, much more than in test kits used in a medical laboratory. This is by virtue of the very nature of design, sample handling, and equipment used by the skilled laboratory technician, which is not available to the laboratory unskilled user of POCT devices. However, there is a question in manufacturing terms of the consistence of biologically produced antibody batches and supply to meet the demand for POCT devices.
Of the alternatives to antibody-based sensing techniques, aptamer-based methods have become popular over the past decade. Recently, peptide aptamers have attracted great attention as promising candidates to replace antibodies since they are more stable and resistant to harsh environments and can be readily prepared with the desired sequences to bind the specific targets. Using the in vitro screening techniques, a large number of engineered peptide aptamers have been found and used as the recognition elements for biosensing [21,22,23,24,25]. Also, with the phage display technique, Yang’s group found an hCG-binding peptide aptamer (KD = 0.9 nM) with a sequence of PPLRINRHILTR [2]. The findings gave the researchers a hint that the peptide could be used as an hCG-receptor for design of antibody-free biosensors. Typically, Lin and co-workers have developed two colorimetric biosensors based on the specific interaction between peptide aptamer and hCG and the good catalytic or optoelectronic properties of gold naoparticles (AuNPs) [26,27]. This AuNPs-based colorimetric sensing technique is simple and does not require modification of any analyte-binding molecules onto AuNPs. However, the unmodified AuNPs-based colorimetric assays show low sensitivity and poor anti-interference ability for protein assays in biological samples because the presence of some matrix components in biological fluids may protect or promote the aggregation of bare AuNPs [26,27].
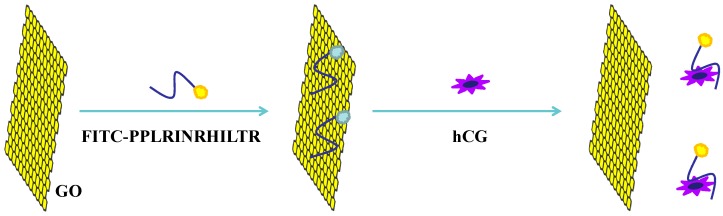
It has been suggested that graphene oxide (GO) exhibits extraordinarily high quenching ability toward fluorescently labeled (e.g., dye, quantum dots or metal nanoclusters) DNA and peptides due to the prominent nanoscale–surface energy transfer effect from the fluorophore to GO [28,29,30,31,32,33]. Thus, many GO-based fluorescent chem/bio-sensors have been developed for monitoring the enzymatic activities [34,35,36,37,38,39], measuring the levels of various analytes including nucleic acids, proteins, metal ions and small molecules [40,41,42,43,44], and imaging of cells as well as animals [45,46]. Based on the high quenching ability of GO and the specific aptamer–target interaction, several groups have reported the detection of proteins (e.g., thrombin, cyclin A2, amyloid-β oligomers, α-bungarotoxin and antibodies) with the dye-labeled DNA or peptide probes as the recognition elements [47,48,49,50,51]. In a typical detection model, the fluorescence of a dye-labeled probe would be quenched when it was absorbed onto the surface of GO. However, the specific binding of a target protein to the fluorescently labeled probe would induce the release of the probe from the GO surface, thus resulting in the fluorescence recovery. In the present work, we found that the fluorescently labeled hCG-binding peptide can adsorb onto the surface of GO (Scheme 1). Consequently, the fluorescence of the peptide was quenched effectively through the energy-transfer or electron-transfer processes. However, with the addition of hCG, the specific binding of hCG to the peptide probe resulted in the release of the peptide from the GO surface, thus leading to the recovery of the fluorescence signal. Based on this fact, we have developed a GO-based fluorescent platform for the detection of hCG in the urine samples.
Scheme 1.
Schematic illustration of the GO-based fluorescent method for hCG detection with a peptide of FITC-PPLRINRHILTR as the probe.
2. Materials and Methods
2.1. Reagents and Materials
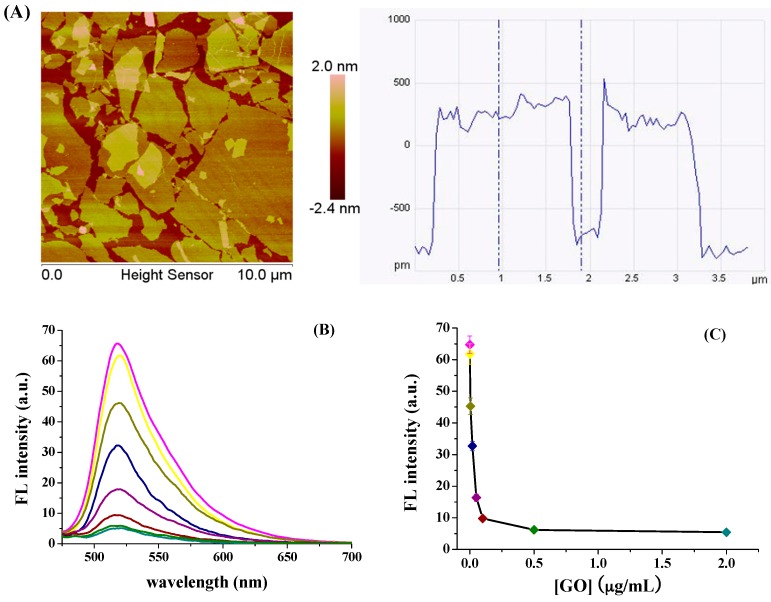
Bovine serum albumin (BSA), thrombin, immunoglobin G (IgG), hCG, KH2PO4, and K2HPO4 were purchased from Sigma-Aldrich (Shanghai, China). Recombinant human erythropoietin (rHuEPO) was provided by BioVision, Inc. (Milpitas, CA, USA). Beta-subunit of hCG (β-hCG) was obtained from YuduoBio Co., Ltd. (Shanghai, China). Single layer GO with oxygen content of 35%–40% was provided by Nanjing XFNANO Materials Tech Co., Ltd. (Nanjing, China). The Atomic Force Microscope (AFM) image shows that the thickness of GO is 0.8–1.2 nm and the lateral size is 0.5–5 μm (Figure 1A). The peptide probe labeled with fluorescein isothiocyanate (FITC) (a well-known fluorescent reporter for design of GO-based fluorescent chem/bio-sensors) in the N-terminal (FITC-PPLRINRHILTR) was obtained from Synpeptide Co., Ltd. (Shanghai, China). The serum sample containing 16.14 mIU/mL follicle-stimulating hormone (FSH), 11.82 mIU/mL luteotropic hormone (LH) and 3.41 mIU/mL thyroid stimulating hormone (TSH) from one woman donor (35 years old) and the urine samples from one woman donor (33 years old) were provided by Anyang Maternal and Child Heath Care Hospital (Anyang, China) and were stored at −18 °C for use. Amino acids used in this work were provided by Sangon Biotech. Co., Ltd. (Shanghai, China). The peptide at the concentration of 1 mM was dissolved with deionized-water and diluted to the desired concentration with a phosphate-buffered saline solution (PBS buffer, 20 mM, pH 7.2).
Figure 1.
(A) AFM image and lateral size of GO; (B) Fluorescence spectra of 50 nM FITC-PPLRINRHILTR in the presence of different concentrations of GO; (C) Fluorescence intensity of FITC-PPLRINRHILTR versus the concentration of GO.
2.2. Quenching Studies
To determine the quenching efficiency of GO to FITC-PPLRINRHILTR, 50 μL of GO at a given concentration was mixed with 100 μL of FITC-PPLRINRHILTR. Then, 50 μL of PBS was added to the mixed solution for fluorescence measurement. Fluorescence spectra was collected on a Varian Cary fluorescence spectrometer with an excitation wavelength of 470 nm. The emission wavelength was taken with a slit of 5 nm. To determine the influence of amino acid and protein on the quenching efficiency of GO to FITC-PPLRINRHILTR, 50 μL of amino acid or protein was added into 100 μL of FITC-PPLRINRHILTR, followed by the addition of 50 μL of GO for fluorescence measurement.
2.3. Detection of hCG
For the assay of hCG, FITC-PPLRINRHILTR was first mixed with GO for 10 min to form the FITC-PPLRINRHILTR/GO complex. Then, 50 μL of hCG at a given concentration was added to 150 μL of the prepared FITC-PPLRINRHILTR/GO solution. After incubation at room temperature for 15 min, the fluorescence of the mixed solution was collected as aforementioned method. For the assays of serum or hCG in urine, the samples were centrifuged at 1300 rpm for 5 min. Then, 50 μL of the supernatant were taken out and added to 50 μL of PBS buffer, followed by the addition of 100 μL of the prepared FITC-PPLRINRHILTR/GO solution. Other procedures for the determination of hCG in urine samples were the same as those for the assay of hCG in the blank PBS.
3. Results and Discussion
3.1. Quenching Efficiency of GO to FITC-PPLRINRHILTR
We first investigated the interaction between FITC-PPLRINRHILTR and GO by monitoring the fluorescence change of FITC-PPLRINRHILTR in the presence of various concentrations of GO. It can be seen that the fluorescence intensity of FITC-PPLRINRHILTR decreased with the increase of GO concentration and reached the minimum value beyond 0.5 μg/mL (Figure 1B,C). The result indicated that the fluorescence of FITC-PPLRINRHILTR was quenched efficiently by GO. It has been demonstrated that the adsorption of peptide onto the GO surface depends upon the electrostatic and π–π interactions between the negatively charged GO and the positively charged amino acid residues (Lys, His, and Arg) as well as the aromatic-ring-containing hydrophobic amino acids (Trp, Tyr, and Phe) [36,49,52]. Note that there are three Arg and one His amino-acid residues within the peptide probe (FITC-PPLRINRHILTR). Thus, the adsorption of the peptide onto the GO surface should be attributed to the electrostatic interaction. The quenching efficiency of 2 μg/mL GO to FITC-PPLRINRHILTR was found to be 89.2% ± 3.6% by the formula (1 = F/F0) × 100%, where F and F0 represent the fluorescence intensity at 518 nm with and without the addition of GO.
Some amino acids and proteins may also show the electrostatic and π–π interactions with GO. This maybe has a negative effect on the interaction of FITC-PPLRINRHILTR and GO. For this view, we studied the impact of hydrophilic or aromatic amino acids (Arg, His, Lys, Asp, Glu, Phe, Trp and Tyr) and proteins (BSA, IgG, rHuEPO and thrombin) on the quenching efficiency of GO to FITC-PPLRINRHILTR (Figure 2). Consequently, we found that Lys, His, Glu and Asp showed negligible effects on the quenching efficiency. However, the presence of Arg, Phe, Trp, Tyr and the four tested proteins weakened the interaction of GO to FITC-PPLRINRHILTR, thus decreasing the quenching efficiency in different degree. The use of excess concentrations of GO may reduce the interference of some matrix components existing in biological samples. Herein, we found that the fluorescence of FITC-PPLRINRHILTR could be quenched effectively by a high concentration of GO even in the presence of these tested interferences. Thus, in the following detection assays, GO at an excess concentration of 2 μg/mL was used for the detection assay.
Figure 2.
Effects of various amino acids and proteins on the quenching efficiency of 0.5 and 2 μg/mL GO to FITC-PPLRINRHILTR. The final concentrations of FITC-PPLRINRHILTR, amino acids and proteins were 50 nM, 50 μM and 10 ng/mL, respectively.
3.2. hCG Detection
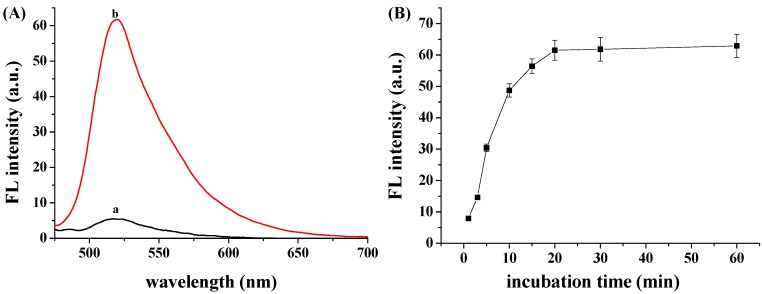
The fluorescence spectra of FITC-PPLRINRHILTR/GO in the absence (curve a) and presence (curve b) of hCG are shown in Figure 3A. It can be observed that the fluorescence signal increased in the presence of hCG. The result demonstrated that the specific binding of hCG to the peptide resulted in the desorption of FITC-PPLRINRHILTR from the GO surface. For three parallel experiments, the fluorescence intensities were found to be 59.2, 61.8 and 62.1, suggesting a good reproducibility. The result indicated that the FITC-PPLRINRHILTR/GO complex is a good probe for the detection of hCG. We also investigated the effect of the incubation time of hCG with the probe on the fluorescence intensity. As shown in Figure 3B, the fluorescence intensity increased gradually within 20 min incubation. The result indicated that the competitive binding of hCG with GO for FITC-PPLRINRHILTR diminished the GO and peptide contact, leading to the gradual desorption of peptide from the GO surface.
Figure 3.
(A) Fluorescence spectra of FITC-PPLRINRHILTR/GO in the absence (curve a) and presence (curve b) of hCG. The concentrations of FITC-PPLRINRHILTR and hCG were 50 nM and 50 IU/mL, respectively; (B) Fluorescence restoration of FITC-PPLRINRHILTR/GO by hCG as a function of time.
3.3. Sensitivity to hCG
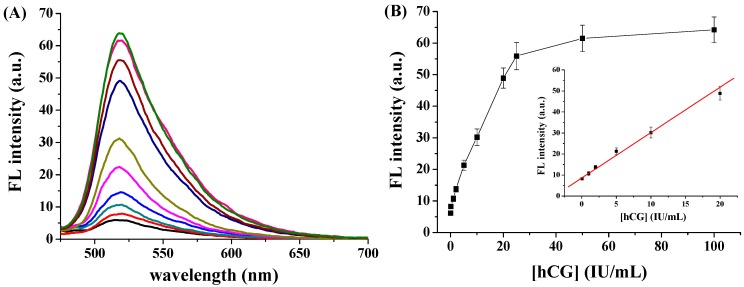
We determined the sensitivity and detection limit of this method. Figure 4A shows the fluorescence spectra of FITC-PPLRINRHILTR/GO in the presence of different concentrations of hCG. The fluorescence intensity increased with increasing hCG concentration. As shown in Figure 4B, the fluorescence intensity was linearly proportional to the hCG concentration in the range of 0.05–20 IU/mL. The linear regression equation is F = 8.6 + 2.2 [hCG] (IU/mL) (R = 0.993). The relative standard deviations (RSDs, shown as the error bars in Figure 4B) for assays of the different concentrations of hCG samples are all less than 8.5%, further indicating a good reproducibility of this method. The analytical performances of this method were compared to those achieved by other methods (Table 1). The detection limit of 20 mIU/mL was comparable to (or even lower than) that achieved by immunochromatography, photoluminescence, surface plasmon resonance, fluorescent immunoassays, liquid crystal-based assay and AuNPs-based colorimetric assays. Although the detection limit is higher than that achieved by ELISA and some electrochemical methods, our method obviates the pre-modification of nanomaterials, requires very simple sample handling, and does not need to use the relatively expensive and less stable antibodies.
Figure 4.
(A) Fluorescence spectra of FITC-PPLRINRHILTR/GO in the presence of different concentrations of hCG; (B) Dependence of fluorescence intensity on hCG concentration. The inset shows the linear segment in the range of 0.05 to 20 IU/mL.
Table 1.
Analytical performances of various methods for hCG detection.
| Materials | Methods | Detection Limit | Linear Range | Reference |
|---|---|---|---|---|
| SPAAB-HRP/anti-hCG | ELISA | 0.012 mIU/mL | - | [3] |
| anti-hCG/ZnO | PL | 2 ng/mL | 2–20 ng/mL | [6] |
| anti-hCG/CdSe-ZnS QDs | PL | 0.5 mIU/mL | - | [7] |
| anti-hCG/AuNPs | IGCA | 5 ng/mL | 10–600 ng/mL | [5] |
| anti-hCG/gold film | SPR | <500 ng/mL | - | [8] |
| peptide aptamer | LC | 1 IU/mL | 12.5–100 mIU/mL | [2] |
| PF@SiO2-Ab2 and Fe3O4@PANI-Ab1 | fluorescence | 3 pg/mL | 0.01–100 ng/mL | [4] |
| peptide aptamer/AuNPs/4-nitrophenol | colorimetry | 15 mIU/mL | 15–750 mIU/mL | [26] |
| peptide aptamer/AuNPs | colorimetry | 25 mIU/mL | 25–1000 mIU/mL | [27] |
| anti-hCG/Au-MWCNTs/GS/GCE | DPV | 0.0026 mIU/mL | 0.005–500 mIU/mL | [13] |
| anti-hCG/AuE | SWSV | 15 pM | 15–300 pM | [10] |
| HRP-Ab2/hCG/Ab1/nafion/GCE | CA | 11.2 mIU/mL | 200 mIU/mL | [9] |
| anti-hCG/CS/graphene-SPE | EIS | 0.016 ng/mL | 0.1–25 ng/mL | [19] |
| anti-HCG/ FPD/GCE | EIS | 0.03 ng/mL | 0.1–10 ng/mL | [14] |
| anti-hCG/Pd@SBA-15/TH/HSO3-GS/GCE | CV | 8.60 pg/mL | 0.01–16.00 ng/mL | [20] |
| GCE/GS/NPG/anti-hCG | CV | 0.034 ng/mL | 0.5–40.00 ng/mL | [11] |
| hCG/HRP-anti-hCG/sol–gel/GE | DPV | 0.3 mIU/mL | 0.5–50 mIU/mL | [17] |
| Peptide aptamer/GO | fluorescence | 20 mIU/mL | 0.05–20 IU/mL | This work |
SPAAB-HRP, horseradish peroxidase (HRP)-loaded nanospherical poly(acrylic acid) brushe (SPAAB); anti-hCG, human chorionic gonadotropin antibody; QDs, quantum dots; PF@SiO2, poly[(9,9-bis(3′-((N,Ndimethylamino)N-ethylammonium)propyl)-2,7-fluorene)-alt-2,7-(9,9-p-divinyl-benzene)-alt-fluorene)-alt-2,5-dimethyl-p-phenylenediamine] coated SiO2 nanoparticles; Fe3O4@PANI, polyaniline coated Fe3O4 nanoparticles; MWCNTs, multiwalled carbon nanotubes; CS, graphene sheets; GCE, glassy carbon electrode; AuE, gold electrode; Ab, antibody; SPE, screen printed electrode; FPD, 2-(4-Formylphenyl)[60]fulleropyrrolidine; HSO3-GS, Functionalized graphene nanomaterial with introduced -SO3 groups; SBA-15, one of mesoporous silicas with uniform tubular channels; TH, thionine; NPG, nanoporous gold; PL, photoluminescence; IGCA, immunogold chromatographic assay; LC, liquid crystal assay; DPV, differential pulse voltammetry; SWSV, square-wave stripping voltammetry; CA, chronoamperometry; EIS, electrochemical impedance spectroscopy.
3.4. Selectivity and Real Sample Assay
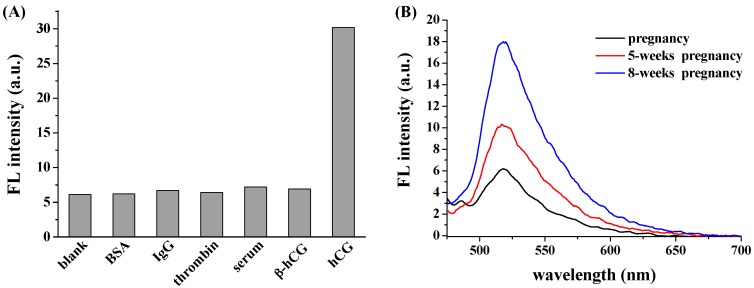
Biological fluids may contain many proteins (e.g., antibodies, proteases, glycoproteins and other common proteins). To explore the selectivity of the proposed method, three interfering proteins (BSA, IgG, thrombin) and serum sample containing FSH, LH and TSH were tested. Moreover, beta-subunit of hCG (β-hCG) is also abundant in pregnancy sample. Thus, the specificity of the aptamer to β-hCG was also investigated. As shown in Figure 5A, compared to the control, none of these interferences caused a significant increase in the fluorescence intensity, demonstrating that the established fluorescent platform showed extraordinary selectivity towards hCG. The high selectivity could be principally attributed to the strong and specific interaction between hCG and its binding peptide (KD = 0.9 nM) [2].
Figure 5.
(A) Selectivity of the proposed sensing strategy. The final concentrations of BSA, IgG, thrombin, β-hCG and hCG were 10 ng/mL, 10 ng/mL, 10 ng/mL, 25 ng/mL and 10 IU/mL, respectively; (B) Fluorescence spectra of FITC-PPLRINRHILTR/GO for the detection of hCG in urine samples.
The good selectivity and sensitivity of the method encouraged us to quantify hCG in the biological samples. Figure 5B shows the fluorescence spectra of FITC-PPLRINRHILTR/GO in the presence of urine samples provided by one female donor pregnant with different periods. It can be observed that the fluorescence signal is close to the background level for assay of the urine from the donor without pregnancy, demonstrating that no detectable hCG was found in the urine. Furthermore, we found that the recoveries for the added hCG with 1, 2 and 20 IU/mL were 97.4% ± 7.6%, 106.3% ± 8.1% and 96.6% ± 6.3%, respectively. The result also indicated that the matrix components in blank urine showed no or poor interaction with the aptamer. More interestingly, the fluorescence signal increased greatly at the case of 5 weeks and 8 weeks pregnancy. Based on the linear curve presented in Figure 4B, the concentrations of hCG in the two urine samples were calculated to be 2.54 ± 0.27 IU/mL and 16.7 ± 1.46 IU/mL, respectively. The result indicated that elevated level of hCG was presented in the urine with long-time pregnancy. Thus, the proposed GO-based biosensor may offer an alternative means for hCG detection in clinical investigations.
4. Conclusions
In conclusion, we presented an antibody-free GO-based fluorescent platform for the detection of hCG using a fluorescently labeled hCG-specific binding peptide as the probe, which can be manufactured cheaply and consistently. Compared with the previously reported optical and electronic immunoassays, our method requires simple operating procedure and obviates the use of less stable antibodies. The detection limit is comparable to that achieved by the AuNPs-based colorimetric assays with the hCG-specific binding peptide as the probe. However, our method shows good anti-interference ability for assays of biological samples, thus facilitating the detection of hCG in urine samples. Furthermore, this was coupled in a process format that directly links binding of hCG to aptamer to a colourimetric quenching competitive quantitative signal. This can form the basis of an inexpensive, quantitative hCG disposable POCT type device. This same format can also be used when the aptmer is replaced to quantitatively measure other important biomolecules such as carcino-embryonic antigen (CEA), cancer antigen 125 (CA-125) and alpha-fetoprotein (AFP) all in a POCT device.
Acknowledgments
Partial support of this work by the Joint Fund for Fostering Talents of National Natural Science Foundation of China and Henan Province (U1304205), the Program for Science and Technology Innovation Talents at the University of Henan Province (15HASTIT001) and the Science & Technology Foundation of Henan Province (17A150001) is gratefully acknowledged.
Author Contributions
Ning Xia oversaw all of the research (collecting urine samples, performing the fluorescent detection of hCG in urine and revising the paper). Xin Wang conducted part of the fluorescent experiments. Lin Liu designed the experiments and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Yeh C.-H., Zhao Z.-Q., Shen P.-L., Lin Y.-C. Optimization of an optical inspection system based on the taguchi method for quantitative analysis of point-of-care testing. Sensors. 2014;14:16148–16158. doi: 10.3390/s140916148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding X., Yang K.-L. Antibody-free detection of human chorionic gonadotropin by use of liquid crystals. Anal. Chem. 2013;85:10710–10716. doi: 10.1021/ac400732n. [DOI] [PubMed] [Google Scholar]
- 3.Qu Z., Xu H., Xu P., Chen K., Mu R., Fu J., Gu H. Ultrasensitive ELISA using enzyme-loaded nanospherical brushes as labels. Anal. Chem. 2014;86:9367–9371. doi: 10.1021/ac502522b. [DOI] [PubMed] [Google Scholar]
- 4.Chu C., Li L., Li S., Li M., Ge S., Yu J., Yan M., Song X. Fluorescence-based immunoassay for human chorionic gonadotropin based on polyfluorene-coated silica nanoparticles and polyaniline-coated Fe3O4 nanoparticles. Microchim. Acta. 2013;180:1509–1516. doi: 10.1007/s00604-013-1067-7. [DOI] [Google Scholar]
- 5.Su J., Zhou Z., Li H., Liu S. Quantitative detection of human chorionic gonadotropin antigen via immunogold chromatographic test strips. Anal. Methods. 2014;6:450–455. doi: 10.1039/C3AY41708E. [DOI] [Google Scholar]
- 6.Yan X., Huang Z., He M., Liao X., Zhang C., Yin G., Gu J. Detection of HCG-antigen based on enhanced photoluminescence of hierarchical ZnO arrays. Colloids Surf. B Biointerface. 2012;89:86–92. doi: 10.1016/j.colsurfb.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C., Yuan H., Shen H., Guo Y., Li X., Liu D., Xu L., Ma L., Li L.S. Synthesis of size-tunable photoluminescent aqueous CdSe/ZnS microspheres via a phase transfer method with amphiphilic oligomer and their application for detection of HCG antigen. J. Mater. Chem. 2011;21:7393–7400. doi: 10.1039/c1jm10090d. [DOI] [Google Scholar]
- 8.Piliarik M., Vaisocherová H., Homola J. A new surface plasmon resonance sensor for high-throughput screening applications. Biosens. Bioelectron. 2005;20:2104–2110. doi: 10.1016/j.bios.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Chetcuti A.F., Wong D.K.Y. An indirect perfluorosulfonated ionomer-coated electrochemical immunosensor for the detection of the protein human chorionic gonadotrophin. Anal. Chem. 1999;71:4088–4094. doi: 10.1021/ac981216a. [DOI] [PubMed] [Google Scholar]
- 10.Kerman K., Nagatani N., Chikae M., Yuhi T., Takamura Y., Tamiya E. Label-free electrochemical immunoassay for the fetection of human chorionic gonadotropin hormone. Anal. Chem. 2006;78:5612–5616. doi: 10.1021/ac051762l. [DOI] [PubMed] [Google Scholar]
- 11.Li R., Wu D., Li H., Xu C., Wang H., Zhao Y., Cai Y., Wei Q., Du B. Label-free amperometric immunosensor for the detection of human serum chorionic gonadotropin based on nanoporous gold and graphene. Anal. Biochem. 2011;414:196–201. doi: 10.1016/j.ab.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Lim S.A., Yoshikaw H., Tamiy E., Yasin H.M., Ahmed M.U. A highly sensitive gold nanoparticle bioprobe based electrochemical immunosensor using screen printed graphene biochip. RSC Adv. 2014;4:58460–58466. doi: 10.1039/C4RA11066H. [DOI] [Google Scholar]
- 13.Lu J.J., Liu S.Q., Ge S.G., Yan M., Yu J.H., Hu X.T. Ultrasensitive electrochemical immunosensor based on Au nanoparticles dotted carbon nanotube–graphenecomposite and functionalized mesoporous materials. Biosens. Bioelectron. 2012;33:29–35. doi: 10.1016/j.bios.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 14.Qiu W., Gao F., Chen J., Xie L., Wang Q. Application of 2-(4-Formylphenyl) [60]Fulleropyrrolidine as anelectrode matrix for cross linker-free immobilization of HCG-antibodyand the sensing analysis. Sens. Actuators B Chem. 2016;231:376–383. doi: 10.1016/j.snb.2016.03.047. [DOI] [Google Scholar]
- 15.Roushani M., Valipour A. Voltammetric immunosensor for human chorionic gonadotropin using a glassy carbon electrode modified with silver nanoparticles and a nanocomposite composed of graphene, chitosan and ionic liquid, and using riboflavin as a redox probe. Microchim. Acta. 2016;183:845–853. doi: 10.1007/s00604-015-1731-1. [DOI] [Google Scholar]
- 16.Roushani M., Valipour A. Using electrochemical oxidation of Rutin in modeling a novel andsensitive immunosensor based on Pt nanoparticle and graphene–ionicliquid–chitosan nanocomposite to detect human chorionicgonadotropin. Sens. Actuators B Chem. 2016;222:1103–1111. doi: 10.1016/j.snb.2015.08.031. [DOI] [Google Scholar]
- 17.Tan F., Yan F., Ju H. Sensitive reagentless electrochemical immunosensor based on an ormosil sol–gel membrane for human chorionic gonadotrophin. Biosens. Bioelectron. 2007;22:2945–2951. doi: 10.1016/j.bios.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira S., Conlan R.S., Guy O.J., Goreti M., Sales F. Label free human chorionic gonadotropin detection at picogram levels using oriented antibodies bound to graphene screen-printed electrodes. J. Mater. Chem. B. 2014;2:1852–1865. doi: 10.1039/c3tb21235a. [DOI] [PubMed] [Google Scholar]
- 19.Teixeira S., Ferreira N.S., Conlan R.S., Guy O.J., Sales M.G.F. Chitosan/AuNPs modified graphene electrochemical sensor for label-free human chorionic gonadotropin detection. Electroanalysis. 2014;26:2591–2598. doi: 10.1002/elan.201400311. [DOI] [Google Scholar]
- 20.Wu D., Zhang Y., Shi L., Cai Y., Ma H., Du B., Wei Q. Electrochemical immunosensor for ultrasensitive detection of human chorionic gonadotropin based on Pd@SBA-15. Electroanalysis. 2013;25:427–432. doi: 10.1002/elan.201200237. [DOI] [Google Scholar]
- 21.Cohen B.A., Colas P., Brent R. An artificial cell-cycle inhibitor isolated from a combinatorial Library. Proc. Natl. Acad. Sci. USA. 1998;95:14272–14277. doi: 10.1073/pnas.95.24.14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis J.J., Tkac J., Laurenson S., Ferrigno P.K. Peptide aptamers in label-free protein detection: 1. Characterization of the immobilized scaffold. Anal. Chem. 2007;79:1089–1096. doi: 10.1021/ac061863z. [DOI] [PubMed] [Google Scholar]
- 23.Li H., Xie H., Cao Y., Ding X., Yin Y., Li G. A general way to assay protein by coupling peptide with signal reporter via supermolecule formation. Anal. Chem. 2013;85:1047–1052. doi: 10.1021/ac302906c. [DOI] [PubMed] [Google Scholar]
- 24.Xie H.N., Li H., Huang Y., Wang X.Y., Yin Y.M., Li G.X. Combining peptide and DNA for protein assay: CRIP1 detection for breast cancer staging. ACS Appl. Mater. Interface. 2014;6:459–463. doi: 10.1021/am404506g. [DOI] [PubMed] [Google Scholar]
- 25.Xu X., Zhou J., Liu X., Nie Z., Qing M., Guo M., Yao S. Aptameric peptide for one-step detection of protein kinase. Anal. Chem. 2012;84:4746–4753. doi: 10.1021/ac3001918. [DOI] [PubMed] [Google Scholar]
- 26.Chang C.-C., Chen C.-P., Lee C.-H., Chen C.-Y., Lin C.-W. Colorimetric detection of human chorionic gonadotropin using catalytic gold nanoparticles and a peptide aptamer. Chem. Commun. 2014;50:14443–14446. doi: 10.1039/C4CC06366J. [DOI] [PubMed] [Google Scholar]
- 27.Chang C.-C., Chen C.-Y., Chen C.-P., Lin C.-W. Facile colorimetric detection of human chorionic gonadotropin based on the peptide-induced aggregation of gold nanoparticles. Anal. Methods. 2015;7:29–33. doi: 10.1039/C4AY01429D. [DOI] [Google Scholar]
- 28.Chen Y., Star A., Vidal S. Sweet carbon nanostructures: Carbohydrate conjugates with carbon nanotubes and graphene and their applications. Chem. Soc. Rev. 2012;42:4532–4542. doi: 10.1039/C2CS35396B. [DOI] [PubMed] [Google Scholar]
- 29.Loh K.P., Bao Q., Eda G., Chhowalla M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010;2:1015–1024. doi: 10.1038/nchem.907. [DOI] [PubMed] [Google Scholar]
- 30.Chung C., Kim Y.-K., Shin D., Ryoo S.-R., Hong B.H., Min D.-H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013;46:2211–2224. doi: 10.1021/ar300159f. [DOI] [PubMed] [Google Scholar]
- 31.Feng L., Wu L., Qu X. New horizons for diagnostics and therapeutic applications of graphene and graphene oxide. Adv. Mater. 2013;25:168–186. doi: 10.1002/adma.201203229. [DOI] [PubMed] [Google Scholar]
- 32.Lee J., Yim Y., Kim S., Choi M.-H., Choi B.-S., Lee Y., Min D.-H. In-depth investigation of the interaction between DNA and nano-sized graphene oxide. Carbon. 2016;97:92–98. doi: 10.1016/j.carbon.2015.07.093. [DOI] [Google Scholar]
- 33.Wick P., Louw-Gaume A.E., Kucki M., Krug H.F., Kostarelos K., Fadeel B., Dawson K.A., Salvati A., Vzquez E., Ballerini L., et al. Classification framework for graphene-based materials. Angew. Chem. Int. Ed. 2014;53:2–7. doi: 10.1002/anie.201403335. [DOI] [PubMed] [Google Scholar]
- 34.Tian J., Ding L., Wang Q., Hu Y., Jia L., Yu J.-S., Ju H. Folate receptor-targeted and cathepsin B-activatable nanoprobe for in situ therapeutic monitoring of photosensitive cell death. Anal. Chem. 2015;87:3841–3848. doi: 10.1021/acs.analchem.5b00429. [DOI] [PubMed] [Google Scholar]
- 35.Feng D., Zhang Y., Feng T., Shi W., Li X., Ma H. A graphene oxide-peptide fluorescence sensor tailor-made for simple and sensitive detection of matrix metalloproteinase 2. Chem. Commun. 2011;47:10680–10682. doi: 10.1039/c1cc13975d. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M., Yin B.-C., Wang X.-F., Ye B.-C. Interaction of peptides with graphene oxide and its application for real-time monitoring of protease activity. Chem. Commun. 2011;47:2399–2401. doi: 10.1039/C0CC04887A. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J., Xu X., Liu W., Liu X., Nie Z., Qing M., Nie L., Yao S. Graphene oxide−peptide nanocomplex as a versatile fluorescence probe of protein kinase activity based on phosphorylation protection against carboxypeptidase digestion. Anal. Chem. 2013;85:5746–5754. doi: 10.1021/ac400336u. [DOI] [PubMed] [Google Scholar]
- 38.Liu L., Xia N., Yu J. A graphene oxide-based fluorescent scheme for the determination of the activity of the β-site amyloid precursor protein (BACE1) and its inhibitors. Microchim. Acta. 2016;183:265–271. doi: 10.1007/s00604-015-1647-9. [DOI] [Google Scholar]
- 39.Jang H., Kim Y.-K., Kwon H.-M., Yeo W.-S., Kim D.-E., Min D.-H. A graphene-based platform for the assay of duplex-DNA unwinding by helicase. Angew. Chem. Int. Ed. 2010;49:5703–5707. doi: 10.1002/anie.201001332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding S., Cargill A.A., Das S.R., Medintz I.L., Claussen J.C. Biosensing with förster resonance energy transfer coupling between fluorophores and nanocarbon allotropes. Sensors. 2015;15:14766–14787. doi: 10.3390/s150614766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Z., Hu J., He Z. A split G-quadruplex and graphene oxide-based low-background platform for fluorescence authentication of pseudostellaria heterophylla. Sensors. 2014;14:22971–22981. doi: 10.3390/s141222971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z., Liu B., Ding J., Liu J. Fluorescent sensors using DNA-functionalized graphene oxide. Anal. Bioanal. Chem. 2014;406:6885–6902. doi: 10.1007/s00216-014-7888-3. [DOI] [PubMed] [Google Scholar]
- 43.Tan X., Chen T., Xiong X., Mao Y., Zhu G., Yasun E., Li C., Zhu Z., Tan W. Semiquantification of ATP in live cells using nonspecific desorption of DNA from graphene oxide as the internal reference. Anal. Chem. 2012;84:8622–8627. doi: 10.1021/ac301657f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong B.J., An Z., Compton O.C., Nguyen S.T. Tunable biomolecular interaction and fluorescence quenching ability of graphene oxide: Application to “turn-on” DNA sensing in biological media. Small. 2012;8:2469–2476. doi: 10.1002/smll.201200264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y., Chen J.-T., Yan X.-P. Fabrication of transferrin functionalized gold nanoclusters/graphene oxide nanocomposite for turn-on near-infrared fluorescent bioimaging of cancer cells and small animals. Anal. Chem. 2013;85:2529–2535. doi: 10.1021/ac303747t. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Li Z., Hu D., Lin C.-T., Li J., Lin Y. Aptamer/graphene oxide nanocomplex forin situ molecular probing in living cells. J. Am. Chem. Soc. 2010;132:9274–9276. doi: 10.1021/ja103169v. [DOI] [PubMed] [Google Scholar]
- 47.Wang X., Wang C., Qu K., Song Y., Ren J., Miyoshi D., Sugimoto N., Qu X. Ultrasensitive and selective detection of a prognostic indicator in early-stage cancer using graphene oxide and carbon nanotubes. Adv. Funct. Mater. 2010;20:3967–3971. doi: 10.1002/adfm.201001118. [DOI] [Google Scholar]
- 48.Lu C.-H., Li J., Zhang X.-L., Zheng A.-X., Yang H.-H., Chen X., Chen G.-N. General approach for monitoring peptide-protein interactions based on graphene-peptide complex. Anal. Chem. 2011;83:7276–7282. doi: 10.1021/ac200617k. [DOI] [PubMed] [Google Scholar]
- 49.Feng B., Guo L., Wang L., Li F., Lu J., Ga O.J., Fan C., Huang Q. A graphene oxide-based fluorescent biosensor for the analysis of peptide-receptor interactions and imaging in somatostatin receptor subtype 2 overexpressed tumor cells. Anal. Chem. 2013;85:7732–7737. doi: 10.1021/ac4009463. [DOI] [PubMed] [Google Scholar]
- 50.Long F., Zhu A., Shi H., Wang H. Hapten-grafted graphene as a transducer for homogeneous competitive immunoassay of small molecules. Anal. Chem. 2014;86:2862–2866. doi: 10.1021/ac500347n. [DOI] [PubMed] [Google Scholar]
- 51.Liu L., Xia N., Zhang J., Mao W., Wu Y., Ge X. A graphene oxide-based fluorescent platform for selective detection of amyloid-β oligomers. Anal. Methods. 2015;7:8727–8732. doi: 10.1039/C5AY02018B. [DOI] [Google Scholar]
- 52.Gu X., Yang G., Zhang G., Zhang D., Zhu D. A new fluorescence turn-on assay for trypsin and inhibitor screening based on graphene oxide. ACS Appl. Mater. Interface. 2011;3:1175–1179. doi: 10.1021/am2000104. [DOI] [PubMed] [Google Scholar]