Abstract
Joint pain is common in haemophilia and may be acute or chronic. Effective pain management in haemophilia is essential to reduce the burden that pain imposes on patients. However, the choice of appropriate pain-relieving measures is challenging, as there is a complex interplay of factors affecting pain perception. This can manifest as differences in patients’ experiences and response to pain, which require an individualized approach to pain management. Prophylaxis with factor replacement reduces the likelihood of bleeds and bleed-related pain, whereas on-demand therapy ensures rapid bleed resolution and pain relief. Although use of replacement or bypassing therapy is often the first intervention for pain, additional pain relief strategies may be required. There is an array of analgesic options, but consideration should be paid to the adverse effects of each class. Nevertheless, a combination of medications that act at different points in the pain pathway may be beneficial. Nonpharmacological measures may also help patients and include active coping strategies; rest, ice, compression, and elevation; complementary therapies; and physiotherapy. Joint aspiration may also reduce acute joint pain, and joint steroid injections may alleviate chronic pain. In the longer term, increasing use of prophylaxis or performing surgery may be necessary to reduce the burden of pain caused by the degenerative effects of repeated bleeds. Whichever treatment option is chosen, it is important to monitor pain and adjust patient management accordingly. Beyond specific pain management approaches, ongoing collaboration between multidisciplinary teams, which should include physiotherapists and pain specialists, may improve outcomes for patients.
Keywords: acute pain, bleed, chronic pain, haemophilia, pain management, quality of life
Introduction
Spontaneous bleeding into joints and muscles is the symptomatic hallmark of congenital haemophilia A and B [1–3]. Joint bleeds, which account for 70–80% of all bleeding episodes in patients with severe haemophilia [3], are extremely painful; repeated joint bleeds predispose to a vicious cycle of bleeding, synovitis, and more bleeding [1,4]. The joint pain associated with haemarthrosis causes flexion deformities, which become fixed over time [4]. Repeated haemarthrosis trigger progressive damage to the joint cartilage, which, in turn, results in haemophilic arthropathy [4]. This joint damage impacts on bone health, resulting in chronic pain and reduced quality of life (QoL) [4].
Pain in one or more joints is a daily reality for as many as two-thirds of patients with severe haemophilia [5]. Pain is therefore a critical aspect of haemophilia [3,6] and adds to the burden of the disease. Effective pain management is essential to improve patient outcomes and QoL; however, the selection and application of appropriate pain-relieving therapies in haemophilia remains a challenge [7].
Members of the Zürich Haemophilia Forum convened for their 14th meeting in October 2014 to discuss pain and pain management in haemophilia. This report summarizes our discussions and recommendations.
What is pain?
Pain can be defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage [8]. Human pain consists of three interacting dimensions: the sensory-discriminative dimension (identifies the location, timing, and physical characteristics of the painful stimulus); the affective-motivational dimension [closely linked with emotion and the triggering of defensive behaviours (e.g. escape and recuperation)]; and the cognitive-evaluative dimension (the meanings and consequences of pain) [9,10].
Cortical processing of the sensory-discriminative and affective-motivational pain dimensions consists of two separate but parallel neural systems (the lateral and medial pain systems, respectively) [10,11]. Both pathways synapse within the thalamus [10–12] (Fig. 1), with the lateral pain system projecting to the primary and secondary somatosensory cortices, and the medial pain system projecting to the anterior cingulate cortex and the limbic system [10,11] (Fig. 1).
Fig. 1.
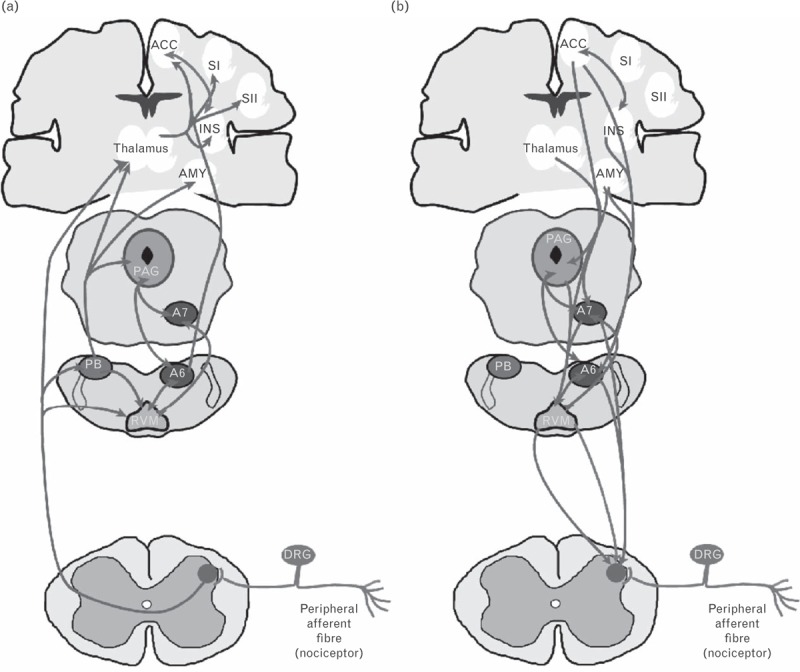
The major ascending (a) and descending (b) pain pathways. Painful (nociceptive) inputs enter the CNS at the spinal dorsal horn, where primary afferent terminals synapse with second-order projection neurons. The ascending tracts in (a) are in light grey, and the grey 2-headed arrows indicate bilateral communications. Descending projections in (b) are in grey, and the 2-headed arrows in dark grey indicate bilateral communications. The light grey and grey projections from the RVM to the spinal cord represent descending inhibition and facilitation. A6 and A7, noradrenergic nuclei; ACC, anterior cingulate cortex; AMY, amygdala; CNS, central nervous system; DRG, dorsal root ganglion; INS, insular cortex; PAG, periaqueductal grey matter; PB, parabrachial nuclei; RVM, rostroventromedial medulla; SI, primary somatosensory cortex; SII, secondary somatosensory cortex. Reproduced with permission from [11].
The perception of pain is subjective, highly variable, and unique to each person [8,11,13], and certain factors (including stress, anxiety, attention, and distraction) influence the experience of pain. These findings suggest the presence of endogenous pain modulatory systems, the manipulation of which may lead to the development of more effective therapies for managing pain [11].
Pain in haemophilia is categorized as acute or chronic [10].
Acute pain in haemophilia
In haemophilia, bleeding episodes in joints and muscles cause acute pain. Therefore, pain can initially serve as an early warning sign of active joint (or other) bleeds [6,10].
What are the clinical features of acute joint bleeds?
According to recent consensus definitions from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis, outward signs of a joint bleed include pain combined with ‘aura’ (an unusual sensation in the joint), swelling, warmth, and/or decreased range of motion (ROM) [14] (Table 1). In infants and small children, decreased ROM may be the only indication that a joint bleed has occurred. The patient's response to certain trigger points being touched may also indicate a joint bleed.
Table 1.
Signs and symptoms of an acute joint bleed
| Early onset joint bleed [14,15] | Advanced joint bleed [14,15] | In infants and young children |
| • Pain | • Severe pain | • Decreased range of motion |
| • Tingling/unusual sensation (‘aura’) | • Swelling | • Reluctance to use an arm or leg [14] |
| • Limited range of motion | • Warmth of the skin over the joint | • Favouring one limb over another [16] |
| • Major decrease in ROM | • A limb that hurts when touched | |
| • Immobility | • Limping [16] | |
| • Walking slowly instead of running | ||
| • Abnormal swelling or stiffness in a joint |
| Acute joint bleed versus arthritic pain | ||
| Acute joint bleed | Pain resolves rapidly following infusion of factor concentrates | |
| Chronic arthritis | Pain related to physical activity soon improves after a period of rest | |
ROM, range of motion.
Pain is not always a reliable indicator of acute bleeding
Although acute pain in haemophilia can often be attributed to acute bleeds, it is important to remember that haemophilia patients can suffer injuries (e.g. fractures) that cause acute pain just as often as the general population. Additionally, patients with advanced arthropathy often find it difficult to distinguish between pain caused by an acute bleed and chronic arthritic pain [14]. If activity-related pain improves on resting, it is likely that the pain is arthritic and not bleed related.
Ceponis et al.[17] investigated whether pain was a reliable aetiological indicator of joint bleeding. Forty bleeding episodes in 30 consecutive patients with haemophilia A or B were evaluated for pain aetiology (joint or muscle bleeds versus arthritic or other musculoskeletal pain) using musculoskeletal ultrasound (MSKUS). Patient perceptions and physician assessments (based on physical examination and patient interview) of pain aetiology were also recorded. Following MSKUS, only 12 of 33 patient-reported ‘bleeding’ episodes were confirmed as bleeds by puncture and aspiration; only two of five patient-reported cases of ‘arthritic’ pain were confirmed as such. Three patient-reported cases of ‘arthritic’ pain were reclassified as joint bleeds, and MSKUS revealed bleeding and synovitis, respectively, in two patients who were unsure about their pain aetiology. Physician assessments were similarly incorrect, with MSKUS confirming physician diagnosis in only 18 out of 40 cases. All patients with pain had ROM deficits; swelling and warmth were present in ∼50% of confirmed and nonconfirmed bleeding episodes; these symptoms were, therefore, considered not to be clinically useful in diagnosing joint bleeds [17].
Overall, findings from the Ceponis study show that significant discrepancies exist between MSKUS, the findings on puncture and aspiration of joint fluid, and patient or physician-perceived pain classification [17]. However, the study included only adult patients (age ≥21 years), most of whom had some degree of arthropathy; this raises the possibility that the study results may have been different if children with pristine joints had also been included. Furthermore, it could be speculated that subclinical microbleeds [18] may have contributed to pain in those patients without MSKUS-confirmed macrobleeding.
When considering these findings, the forum members agreed that the future development of a simple ultrasound device providing patients with a ‘traffic light’ answer as to whether or not blood is present in the joint would be advantageous in guiding treatment decisions, particularly for inhibitor patients.
Chronic pain in haemophilia
Chronic pain in haemophilia is long lasting, recurrent, and usually results from arthropathy and/or other long-term complications of haemophilia (such as synovitis and arthritis) [6,19,20]. Chronic pain is more complicated than acute pain and is associated with neurobiological, psychological, and social changes that can maintain the pain [10]. Patients with chronic pain are more likely than those with acute pain to see a psychologist, either in a pain clinic or in therapy sessions to address issues arising from maladaptive pain-management strategies (e.g. substance abuse, relationship difficulties, and mood problems) [10].
It is vital to stress to patients and their families that pain can be managed, and that patients should not suffer in silence. The best treatment plan can be identified in partnership with the patient's comprehensive care team.
Physiotherapy and exercise in chronic pain
A physiotherapist with experience of haemophilia is a vital member of the comprehensive care team and should evaluate the patient's musculoskeletal status at least once or twice a year. Physical therapy in haemophilia aims to evaluate, diagnose, and treat any disability, while preventing injury or deterioration; this has helped to revolutionize the management of haemophilia patients in developed countries [21]. Specific treatment objectives of physical therapy include alleviation of pain, ROM recovery, prevention of muscle atrophy, as well as improvement in functional ability, reduction of joint bleed frequency, and improvement in QoL [21].
Exercise programmes for haemophilia patients consist of five individual components that are often used separately, but which may be most beneficial when applied together. These components are flexibility and stretching, strength, sensorimotor retraining (or proprioception), balance, and overall function [22] (Table 2).
Table 2.
The components of physical therapy in haemophilia management [22]
| Purpose | Techniques | Benefits | |
| Flexibility/stretching | • Improve performance | • Static (passive) stretching | • Sustained (up to 24 h) elongation of soft tissues and muscles |
| • Warm up before activity to reduce or prevent injury | • Ballistic (dynamic) stretching | • Reduced tension in skeletal muscles | |
| • Decrease muscle soreness | • PNF techniques | • Increased ROM (particularly with static stretching and PNF techniques) | |
| • Improve ROM | |||
| Strength | • Increase muscular strength, endurance, and power | • Isometric or isotonic strength training | • Increased joint and core muscle strength helps control exaggerated end-ROM joint movements and may therefore help prevent or decrease synovial impingement and associated haemarthroses or synovitis |
| • Improve motor performance | • To strike a balance between improving strength and avoiding joint injury, it is important to learn proper techniques and train at submaximal loads, at a lower velocity and in limited joint ranges (even isometrically at various joint angles) | ||
| • Increase cardiovascular fitness | |||
| • Increase lean body mass and tissue tensile strength | |||
| • Reduce pain | |||
| • Reduce psychological stress | |||
| Sensorimotor retraining | • Promote joint stability and function using four main stages of rehabilitation: | • Electromyographic feedback | Electromyographic feedback: |
| •Provide an optimal healing environment | • Hydrotherapy | • Trains the patient to produce greater amounts of force with static or dynamic exercise to elicit the same amount of sensory feedback | |
| •Restore muscle balance | • Various orthoses and footwear adaptations | Hydrotherapy: | |
| •Enhance motor function at the level of the brainstem | •Minimizes impact forces | ||
| •Restore and increase endurance and coordinated muscle patterns | •Minimizes pain | ||
| • Prevents rapid movement into ROM extremes where bleed risk is significant | |||
| Orthoses and footwear adaptation: | |||
| • Functional foot orthoses reduce pain and disability | |||
| Balance | • Treat balance impairments in haemophilia patients | • Start with simple exercises, such as lying on a hard floor, sitting on a rigid chair, kneeling, and standing | • Helps patients to perform daily activities and lead independent lives |
| • Balance impairments may result from one or more of the following: | • More progressive exercises include shifting weight from one leg to the other, trunk rotations, arm/leg movements, and blindfolding | ||
| •Degenerative joint disease (and repeated bleeds into joints and muscles) | • In later phases of rehabilitation, movable surfaces (e.g. steppers, rehabilitation balls, and balance boards) are added | ||
| •Age-related decline in vision, proprioception, and vestibular function | • Patients with significant balance impairments are encouraged to use assistive devices (e.g. crutches, walkers, or canes) | ||
| •Some medications (e.g. antidepressants) | |||
| Overall function | • Achieve the functional level the patient had before the last bleed | • Methods are similar to those used to learn a new sport, i.e. the patient practices the skill he wants to become proficient in performing | • Helps patients regain functional independence and maintain daily functioning |
| •For example, using the sport-specific activity of throwing darts acts as a functional exercise in rehabilitating an elbow joint | |||
| • Alternatively, occupational tasks can be used to achieve the same result | |||
| •For example, a patient with adaptive muscle shortening in the upper extremity could use reaching tasks at work to maintain function and therapeutically address the established pathology |
PNF, proprioceptive neuromuscular facilitation; ROM, range of motion.
When considering the financial cost of medical and pharmaceutical technologies used to treat haemophilia, physiotherapy is far less expensive than replacement therapy and may be more easily performed by patients and caregivers in the home setting [22]. Furthermore, the cost of regular physiotherapy may be offset by reduced factor consumption. This means that it is possible for haemophilia patients around the world to improve their joint and muscle status, even if they live in countries where resources are scarce [22].
Based on current evidence, the forum members agreed that all haemophilia patients, including those with inhibitors, may benefit from participation in sports, physical activity, and exercise [23,24]. The same principles and practical advice regarding physical activity apply to both noninhibitor and inhibitor patients [25]; however, for inhibitor patients, several caveats should be considered. As bypassing agents have lower efficacy and shorter half-lives than factor concentrates, exercise programmes should be tailored to the individual inhibitor patient and gradually extended in time and frequency. Inhibitor patients undergoing physical therapy should be monitored and assessed carefully by the physiotherapist, and all interventions should progress slowly.
When is orthopaedic surgery indicated?
Chronic pain is normally associated with joint degeneration or other long-term complications of haemophilia, which may require orthopaedic surgery to alleviate the pain. Orthopaedic surgery may be indicated for chronic synovitis that cannot be treated by adequate replacement therapy. Chronic synovitis is usually not associated with severe pain, but it does cause contractures. Additionally, so-called ‘silent’ synovitis may arise from ‘silent’ (subclinical) bleeds that lead to iron deposition and cartilage breakdown; trigger points can be very helpful in identifying potential silent synovitis, followed by confirmation using ultrasound or MRI. If chronic synovitis (silent or otherwise) persists, the dose and/or frequency of prophylaxis treatment should be increased. If this is unsuccessful, synovectomy should be considered.
Orthopaedic surgery may also be required for the following musculoskeletal problems: severe muscular contractures that cannot be resolved with tenotomy, capsulotomy, or osteotomy; bone deformities that require correction osteotomy; pseudotumours; and severe degenerative joint changes that cause immobilization and severe pain.
How does chronic pain affect quality of life?
Emotionally, chronic pain is associated with an increased incidence of depression, anxiety, irritability, anger, frustration, and even grief [26,27]. Intractable pain can also lead to chronic narcotic use and alcohol dependence in an attempt to improve pain relief [23].
Each patient experiences pain differently because of a variation in physical sensitivity, emotional and social factors, as well as strategies used to deal with pain [24]. Factors that exacerbate pain include stress, fatigue, and focussing on the pain. However, pain signals can be blocked by various factors, including exercise, massage, physiotherapy, a positive attitude, relaxation, and pain medication [24]. Active coping strategies to combat pain are also helpful and include distraction, trying to ignore the pain and remaining active despite the pain. The effectiveness of these active coping strategies is perhaps illustrated by the presence of an apparent ‘disability paradox’, which describes how patients with serious and persistent disabilities report good QoL despite all external appearances to the contrary [28]. The disability paradox is increasingly observed among older haemophilia patients, who report the best mental QoL even with significant orthopaedic complications and highly impaired physical QoL [29]. This may result from older patients’ ability to develop good adaptive coping strategies to deal with their pain. Forum members agreed that it is important to assess QoL in this population of older haemophilia patients; however, current QoL assessment tools might not be valid for younger patients who face different issues.
It is also important to educate children with haemophilia about their pain, the need for early treatment, and active pain-coping strategies. This education must begin with the child's parents: children often reflect their parents’ fears, so if a parent is afraid of bleeds and their treatment, then the child will learn to be fearful too. In this respect, peer support for parents (e.g. the ‘parents empowering parents’ programme) has been extremely valuable, as it encourages parents to allow their children to live normal lives and take small risks. Children with haemophilia also benefit from peer support through participation in activities such as summer camps, which allow children to learn from older peers.
The impact of pain on quality of life in inhibitor patients
Studies examining QoL and psychosocial issues in inhibitor patients are scarce [23]. However, patients with inhibitors face numerous challenges, have higher rates of morbidity than noninhibitor patients, and are more likely to experience hospitalizations, absenteeism, difficulty maintaining a job, and higher treatment costs [23]. As all these factors impact on psychosocial health, it is not surprising that QoL is worse for patients with inhibitors than for those without inhibitors [23,30]. A comprehensive, multidisciplinary assessment of the physical, emotional, and social status of inhibitor patients is critical for the development of individualized treatment strategies to meet the complex needs of inhibitor patients [23].
What are the strategies for managing pain in patients with haemophilia?
Rapid bleed control to minimize acute pain
Unrelieved pain can interfere with healing and turn acute pain into a chronic problem. Effective prophylaxis (where appropriate and available) is essential to prevent or reduce bleed frequency, whereas prompt on-demand treatment of acute bleeds ensures rapid bleed resolution and, in turn, relief from bleed-related pain. Together, these strategies help to reduce acute pain, arthropathy, and chronic pain, and therefore play a vital role in maintaining normal joint function.
When an acute bleed occurs, early treatment is crucial to resolve bleeding and reduce pain. Accordingly, the World Federation of Hemophilia recommends that acute bleeds are treated at home as soon as possible and preferably within 2 h of onset [3]. Although this is true for all haemophilia patients, it is particularly important for inhibitor patients, who have limited treatment options and who experience more joint pain and poorer outcomes than noninhibitor patients [30]. It is therefore essential that treatment be optimized to minimize pain and resolve bleeding quickly when an inhibitor is present. Early initiation of treatment with the bypassing agent recombinant activated factor VII (rFVIIa; NovoSeven, Novo Nordisk, Bagsværd, Denmark) in inhibitor patients has been shown to reduce rebleeding [31], produce better outcomes [32,33], significantly reduce the need for analgesics [34] and provide faster bleed resolution and faster pain relief [35] versus late treatment.
Initiating early treatment, however, is not always straightforward. For instance, patients who have experienced many bleeds in the same joint may no longer feel much pain with subsequent acute bleeds. Similarly, patients who have not experienced many bleeds may not recognize the onset of bleeding. In both instances, treatment initiation will be delayed. Additionally, one problem with initiating treatment as soon as an ‘aura’ is experienced (as recommended by the World Federation of Hemophilia [3]) is that a bleed may not have actually occurred, resulting in unnecessary factor usage. Forum members agreed that the timing of treatment initiation depends on a balance between the benefits of early treatment and the increased costs of unnecessary product use; however, if in doubt, treatment should be initiated.
Individual pharmacokinetics may help to determine the optimal on-demand dose for acute bleed treatment in each patient. The best pharmacokinetic parameter to individualize on-demand treatment is clearance, as it best expresses the total exposure. For new, extended half-life factor concentrates, the decay curve for each patient will also need to be determined to predict the dose required to treat any breakthrough bleeds. Pharmacokinetic simulations will be needed if standard (and therefore cheaper) factor concentrates are to be used instead.
Replacement therapy (or bypassing agent therapy for inhibitor patients) is often sufficient to relieve acute pain associated with a bleed. However, this is not always enough. In these circumstances – and for patients with chronic arthropathic pain – additional pain relief strategies are needed.
Analgesic use for acute and chronic pain
Little evidence exists on pharmacological pain management of patients with haemophilia [19,20,36,37], and clinical practice is largely empirical and varies widely [19,20]. From available data, however, it appears that there are varied pain management practices in current use in haemophilia. For example, in a European survey investigating pain management practices in haemophilia [19], paracetamol was the only analgesic regularly used by all 22 haemophilia treatment centres (HTCs) surveyed, and its use (with or without adjuvant treatment or weak opioids) was recommended for acute and chronic pain in both adults and children. Other recommended pharmacological analgesics were strong opioids, cyclooxygenase-2 (COX-2) inhibitors and nonselective NSAIDs [19] (Table 3). In contrast, a survey of HTCs in the United States showed that different pharmacological strategies for pain management were used, with the most commonly utilized medications being NSAIDs and acetaminophen [38] (Fig. 2). These observations highlight the lack of data and standardized guidelines for pain management in haemophilia.
Table 3.
European recommendations for the pharmacological management of acute and chronic pain [19]
| Children | Adults | ||
| Acute pain | Chronic pain | Acute pain | Chronic pain* |
| 1. Paracetamol† | 1. Paracetamol† ± adjuvant treatment | a. Without comorbidities | a. Without comorbidities |
| 2. Paracetamol† + weak opioid | 2. Paracetamol† + weak opioid | 1. Paracetamol† ± adjuvant treatment | 1. Paracetamol† |
| 3. Opioids in hospital setting | 3. Refer to pain specialist | 2. Paracetamol† + weak opioid or metamizol or COX-2-selective NSAID | 2. COX-2-selective NSAID or nonselective NSAID ± PPI or paracetamol + weak opioid |
| 3. Tramadol or strong opioids | 3. Tramadol or strong opioids ± nonopioid | ||
| b. With liver dysfunction | b. With mild-to-moderate liver dysfunction | ||
| 1. Liver function should be carefully surveyed when using paracetamol and metamizol | 1. Liver function should be carefully monitored when using paracetamol and metamizol | ||
| 2. Maximum doses should be reduced according to prescription guidelines | 2. Maximum doses should be reduced according to prescription guidelines | ||
| 3. Use COX-2-selective or nonselective NSAIDs ± PPI only in patients with mild chronic liver disease (monitor renal function) | |||
| c. With cardiovascular disease/risk | |||
| 1. Use all NSAIDs with caution | |||
| 2. If using NSAIDs, the least COX-2-specific drugs (e.g. naproxen, or ibuprofen) should be preferred. Consider coprescribing a PPI | |||
| 3. Avoid long-term NSAID use | |||
Nonvalidated recommendations based on general pain management guidelines, the experience of surveyed HTCs of the EHTSB, and consensus achieved following board discussion. The recommendations reflect the WHO pain ladder approach (i.e. subsequent steps are employed when the previous one has failed).
*Adjunctive antidepressants or anticonvulsants should be considered.
†Risk of acute hepatotoxicity with very high doses of paracetamol, especially in patients with liver disease.
COX-2, cyclooxygenase-2; EHTSB, European Haemophilia Therapy Standardisation Board; HTC, haemophilia centre; PPI, proton pump inhibitor.
Fig. 2.
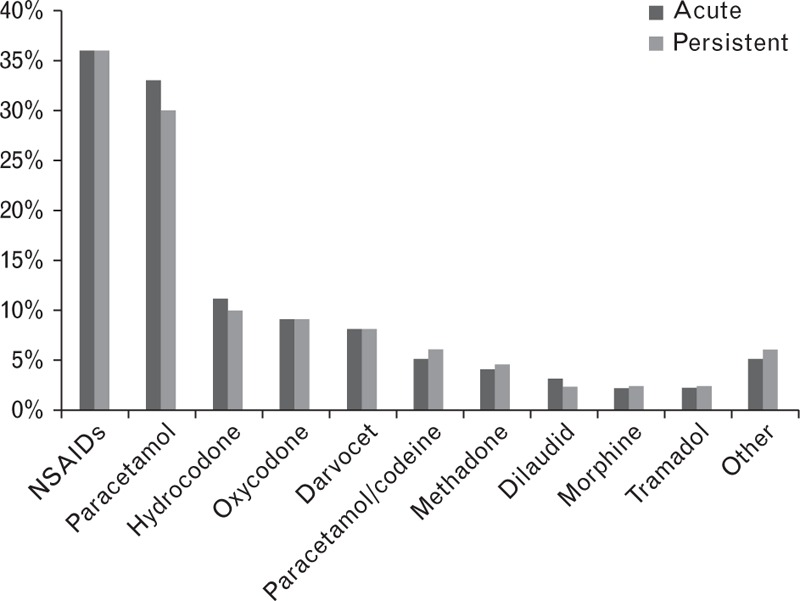
The most commonly used pain medications in three US states (Michigan, Ohio, and Indiana) [38].
Of note, patients often use replacement factor for pain relief, and an analgesic effect of factor VIII (FVIII) has been reported [39] that may not always be associated with a joint bleed (for example, in patients with chronic arthropathy). Although this finding is in keeping with psychological components of the pain experience, it has been suggested that further studies on the pathophysiological and pharmacological properties of replacement factor warrant further investigation [39].
Following a discussion of the above findings, the forum members agreed that while COX-2 inhibitors are useful for acute and chronic pain management, concerns remain regarding a potential increased risk of hypertension, and caution was therefore advised. It was also thought that a short course of steroids is sometimes necessary for major haemarthroses and that the use of anti-inflammatory drugs as soon as possible after a bleed might be useful; however, using this strategy to manage chronic pain may mask pain from acute bleeds. We also agreed that clinicians are not always aware of what haemophilia patients use to manage their pain at home, and that many may be using opiate pain relief. If used extensively, opioid use may expose patients to the risk of adverse events and dependency; such previous opiate use in the home setting may preclude further opiate use to manage pain from any future surgery. Instead, a combination of medications that act at different points of the pain pathway (Fig. 3) may be beneficial.
Fig. 3.
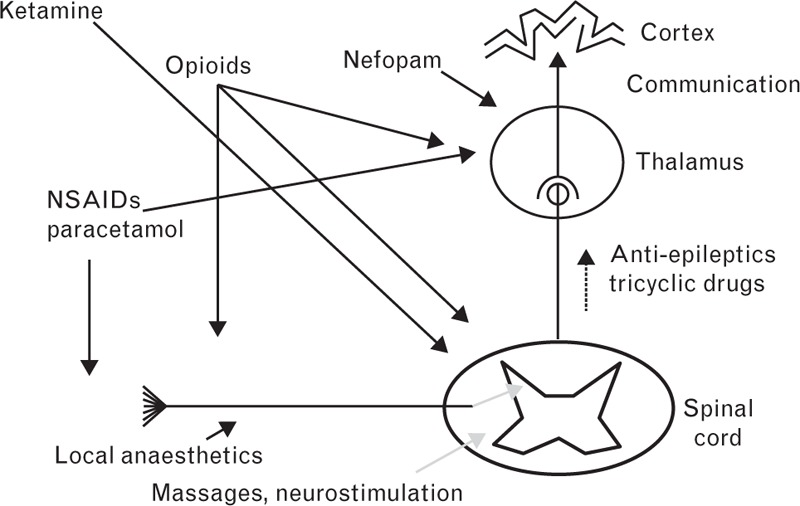
The pain pathway and various sites of action of analgesics.
Beyond medication
It is important to remember that nonpharmacological interventions may also be used to help manage pain. These include rest, ice, compression, and elevation; complementary therapies (such as acupuncture, hypnosis); physiotherapy (see section entitled ‘Physiotherapy and exercise in chronic pain’) and religious faith [38,40]. Lifestyle changes may also be beneficial. Further to the use of analgesics and other noninvasive methods to treat pain, joint aspiration may reduce acute joint pain and steroid injections may alleviate chronic pain. Surgery may also be considered for chronic pain (see section entitled ‘When is orthopaedic surgery indicated?’).
What are the challenges associated with pain assessment and management?
No validated pain assessment scales for haemophilia
Ultimately, the patient himself is the ‘expert’ on (and the best evaluator of) his own pain. However, objective pain assessment methods aim to give visibility to a subjective phenomenon and provide a scale for treatment evaluation. Multiple instruments have been applied extensively to (nonhaemophilia) pain assessment in adults and children [6]: visual analogue scale; numerical rating scale; McGill pain questionnaire; behavioural observational pain scale; scales used as part of QoL assessment (e.g. health-related QoL); Wong-Baker Faces rating scale (children); and the Face, Legs, Activity, Cry and Consolability scale (children) [41]. However, although many of these scales have also been used in haemophilia, few pain instruments have been validated in haemophilia (especially with regard to use in children) [6,39]. Therefore, pain instruments should be developed and validated for use in haemophilia, particularly for paediatric cohorts, to allow standardized assessment.
Lack of collaboration with pain specialists
A lack of collaboration with pain specialists is evident among haemophilia patients themselves and the HTCs providing their care. Patients learn to manage their own pain and rarely consult a pain specialist; furthermore, most HTCs do not arrange regular consultations with pain specialists or involve them in prescribing pain medication [19], even when pain consultants are available. Many HTCs use only a verbal description for pain assessment, rather than a defined scale, and only a minority have guidelines for pain management [19]. Pain management practices, therefore, vary widely, and patients may only see pain specialists when chronic pain has already become established. As pain is such a critical aspect of haemophilia, these issues clearly need to be addressed.
Forum members agreed that increased collaboration and consultation with pain specialists may be beneficial to patients by helping to identify both the cause of pain and the best treatment. To this end, it is crucial to educate HTC healthcare providers about pain management, and the clinical care of haemophilia patients should be adjusted to include regular assessment and documentation of their pain. Pain specialists could perhaps be used more if it was known in advance when patients would be visiting the HTC. It is also important to attract more pain specialists to the field of haemophilia. Finally, we believe that studies to determine what haemophilia patients are looking for in pain medications could be beneficial; a survey may be helpful in identifying patient needs and fears, but conducting such a study might not be feasible.
How does the experience of bleeding and the perception of pain change over time?
Data on bleed experience and pain perception according to age are scarce. Flood et al.[27] used thematic analysis of patient interviews from 10 adults (mean age: 41 years; range 19–52) and 10 adolescents (mean age: 13 years; range 12–17) to try to better understand adult and adolescent patients’ bleeding experiences. Adolescents were found to often feel frustrated that they are unable to participate in sports because of risk of bleeding [27]. Interestingly, adolescents appeared to experience pain relief faster than adults. In this respect, it is noteworthy that adults sometimes delayed treatment of bleeds, whereas adolescents generally treated immediately.
Recently, Teyssler et al.[42] used mechanical pain thresholds (MPT) in 23 haemophilia patients [13 adult and 10 paediatric patients (aged ≤15 years)] to characterize pain perception in the different age groups. Unfortunately, the main group comparisons performed were between haemophilia patients and matched healthy controls and the authors did not comment on differences between adult and paediatric patients with haemophilia. Athough the MPT results did not correlate well with other pain assessment tools, the MPT was thought to be potentially valuable as an objective measure of pain.
Although age-specific data on clinical presentation, experiences, and pain perception/management are limited, some general conclusions can be drawn. When compared with adults, adolescent patients have preserved joints, easily recognize acute bleeds that are treated earlier and resolved more quickly, and experience acute and transient pain. Finally, it is important to note that infants in the first few days of life do experience and react to pain, despite some assertions to the contrary [43]. Care must be taken with young infants and children to avoid causing pain, and pain relief should be used if necessary. Further studies are needed in this area.
Conclusion
Pain is a critical aspect of haemophilia, forming part of daily reality for many patients and significantly increasing the burden of the disease. The perception of pain – and how it is treated – are very personal and can greatly influence QoL. Effective pain management in haemophilia is therefore essential and behavioural, sociological and psychosocial aspects of a patient's life are as important as the medical/clinical aspects when determining an individual's best pain management strategy.
Replacement or bypassing therapy is generally the first response to pain, although it is not necessarily the only (or best) solution. There are numerous pharmacological and other options, both invasive and noninvasive, for pain management; however, in the longer term, increasing prophylaxis or performing surgery (especially synovectomy) may be necessary to reduce the burden of pain caused by the degenerative effects of repeated bleeds. Whichever treatment option is chosen, it is important to monitor pain and adjust patient management if necessary. Ongoing collaboration between haematologists and pain specialists is crucial.
Finally, further study is needed to improve our understanding of the complex interplay of factors influencing pain perception and management in haemophilia patients across the age spectrum.
Acknowledgements
Novo Nordisk provided financial support for the 14th Zürich Haemophilia Forum and for medical writing assistance, provided by Sharon Eastwood of PAREXEL, in compliance with international guidelines for good publication practice.
Conflicts of interest
G.A. has received reimbursement for attending symposia/congresses, and/or honoraria for speaking and/or consulting, and/or funds for research from Baxter, Bayer, CSL Behring, Grifols, Novo Nordisk, Biotest and Pfizer. G.D. has received from Novo Nordisk honoraria for speaking and participating on advisory boards. A.D. will receive honoraria payment from Novo Nordisk for reviewing Research Grant applications this year. C.H. has acted as a consultant and been a board member for Bayer, Baxter, Pfizer, Sobi Biogen, CSL Behring, LFB, Octapharma, Novo Nordisk and CAF-DCF, and has received grants from Bayer, Baxter and Pfizer. V.Y. has received reimbursement for attending symposia/congresses, and/or honoraria for speaking and/or consulting, and/or funds for research from Baxter, Bayer, CSL Behring, Grifols, Novo Nordisk, Octapharma and Pfizer. R.L. has received consultancy or speaker fees from Bayer, Baxter, Novo Nordisk, Biogen and Octapharma during the past 5 years. M.M. has acted as a paid consultant to Bayer, Baxter and Novo Nordisk, and has served as a consultant on Pfizer Advisory Boards. He has received speaker fees from CSL Behring, Octapharma, Bayer and Novo Nordisk, and has received unrestricted research grants from Bayer, Pfizer and Baxter. T.L. has received reimbursement for attending symposia/congresses, and/or honoraria for consulting, and/or funds for research from Baxter (Baxalta), Bayer, CSL Behring, LFB, Novo Nordisk, Octapharma, Pfizer, Roche and Sobi. S.Z.S. has received reimbursement for attending symposia and congresses, and honoraria payment for speaking from Novo Nordisk, Baxter and Octapharma.
References
- 1.Bolton-Maggs PH, Pasi KJ. Haemophilias A and B. Lancet 2003; 361:1801–1809. [DOI] [PubMed] [Google Scholar]
- 2.Gringeri A, Ewenstein B, Reininger A. The burden of bleeding in haemophilia: is one bleed too many? Haemophilia 2014; 20:459–463. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava A, Brewer AK, Mauser-Bunschoten EP, Key NS, Kitchen S, Llinas A, et al. Treatment guidelines working group on behalf of the world federation of hemophilia. Guidelines for the management of hemophilia. Haemophilia 2013; 19:e1–e47. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Merchan EC. Prevention of the musculoskeletal complications of hemophilia. Adv Prev Med 2012; 2012:201271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Genderen FR, Fischer K, Heijnen L, De Kleijn P, van den Berg HM, Helders PJ, et al. Pain and functional limitations in patients with severe haemophilia. Haemophilia 2006; 12:147–153. [DOI] [PubMed] [Google Scholar]
- 6.Humphries TJ, Kessler CM. The challenge of pain evaluation in haemophilia: can pain evaluation and quantification be improved by using pain instruments from other clinical situations? Haemophilia 2013; 19:181–187. [DOI] [PubMed] [Google Scholar]
- 7.Kalnins W, Schelle G, Jost K, Eberl W, Tiede A. Pain therapy in haemophilia in Germany. Patient survey (BESTH study). Hamostaseologie 2015; 35:167–173. [DOI] [PubMed] [Google Scholar]
- 8.Merskey H, Bogduk N. International Association for the Study of Pain. Part III: pain terms, a current list with definitions and notes on usage. Classification of Chronic Pain International Association for the Study of Pain, 2nd edSeattle, WA: 1994. [Google Scholar]
- 9.Liu MG, Chen J. Roles of the hippocampal formation in pain information processing. Neurosci Bull 2009; 25:237–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, et al. Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol 2011; 67:942–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ossipov MH. The perception and endogenous modulation of pain. Scientifica (Cairo) 2012; 2012:561761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yen CT, Lu PL. Thalamus and pain. Acta Anaesthesiol Taiwan 2013; 51:73–80. [DOI] [PubMed] [Google Scholar]
- 13.Pedrosa DF, Siqueira HB, Gomez RR, Saltareli S, da Silva Tde C, Sousa FA. Evaluation of the perception of chronic ischemic pain in humans with peripheral arterial disease. J Vasc Nurs 2014; 32:82–87. [DOI] [PubMed] [Google Scholar]
- 14.Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost 2014; 12:1935–1939. [DOI] [PubMed] [Google Scholar]
- 15.American Thrombosis and Hemostasis Network. Emergency care for patients with haemophilia. http://athn.org/files/resources/athnready/Emergency%20Care%20for%20Patients%20with%20Hemophilia%20FINAL.pdf [Accessed 24 August 2015] [Google Scholar]
- 16.Clement P, Evangelos SP. Understanding your child's pain. http://www.kelleycom.com/pen/november2008pen.pdf [Accessed 24 August 2015] [Google Scholar]
- 17.Ceponis A, Wong-Sefidan I, Glass CS, von Drygalski A. Rapid musculoskeletal ultrasound for painful episodes in adult haemophilia patients. Haemophilia 2013; 19:790–798. [DOI] [PubMed] [Google Scholar]
- 18.Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007; 357:535–544. [DOI] [PubMed] [Google Scholar]
- 19.Holstein K, Klamroth R, Richards M, Carvalho M, Pérez-Garrido R, Gringeri A. Pain management in patients with haemophilia: a European survey. Haemophilia 2012; 18:743–752. [DOI] [PubMed] [Google Scholar]
- 20.Riley RR, Witkop M, Hellman E, Akins S. Assessment and management of pain in haemophilia patients. Haemophilia 2011; 17:839–845. [DOI] [PubMed] [Google Scholar]
- 21.De la Corte-Rodriguez H, Rodriguez-Merchan EC. The role of physical medicine and rehabilitation in haemophiliac patients. Blood Coagul Fibrinolysis 2013; 24:1–9. [DOI] [PubMed] [Google Scholar]
- 22.Blamey G, Forsyth A, Zourikian N, Short L, Jankovic N, De Kleijn P, et al. Comprehensive elements of a physiotherapy exercise programme in haemophilia—a global perspective. Haemophilia 2010; 16 Suppl 5:136–145. [DOI] [PubMed] [Google Scholar]
- 23.duTreil S. Physical and psychosocial challenges in adult hemophilia patients with inhibitors. J Blood Med 2014; 5:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Federation of Hemophilia. The pain management book for people with hemophilia and related bleeding disorders. Hemophilia Foundation Australia World Federation of Hemophilia 2000; 22:2–7. [Google Scholar]
- 25.Heijnen L. The role of rehabilitation and sports in haemophilia patients with inhibitors. Haemophilia 2008; 14 Suppl 6:45–51. [DOI] [PubMed] [Google Scholar]
- 26.Boerner H. How to manage chronic pain. HemAware. http://www.hemaware.org/story/how-manage-chronic-pain [Accessed 4 February 2015] [Google Scholar]
- 27.Flood E, Pocoski J, Michaels LA, McCoy A, Beusterien K, Sasanè R. Patient-reported experience of bleeding events in haemophilia. Eur J Haematol 2014; 93 Suppl 75:19–28. [DOI] [PubMed] [Google Scholar]
- 28.Albrecht GL, Devlieger PJ. The disability paradox: high quality of life against all odds. Soc Sci Med 1999; 48:977–988. [DOI] [PubMed] [Google Scholar]
- 29.Stieltjes N, Torchet MF, Misrahi L, Roussel-Robert V, Lambert T, Guérois C, et al. Epidemiological survey of haemophiliacs with inhibitors in France: orthopaedic status, quality of life and cost—the ‘Statut Orthopedique des Patients Hemophiles’ avec Inhibiteur study. Blood Coagul Fibrinolysis 2009; 20:4–11. [DOI] [PubMed] [Google Scholar]
- 30.Morfini M, Haya S, Tagariello G, Pollmann H, Quintana M, Siegmund B, et al. European study on orthopaedic status of haemophilia patients with inhibitors. Haemophilia 2007; 13:606–612. [DOI] [PubMed] [Google Scholar]
- 31.Salaj P, Brabec P, Penka M, Pohlreichova V, Smejkal P, Cetkovsky P, et al. Effect of rFVIIa dose and time to treatment on patients with haemophilia and inhibitors: analysis of HemoRec registry data from the Czech Republic. Haemophilia 2009; 15:752–759. [DOI] [PubMed] [Google Scholar]
- 32.Key NS, Aledort LM, Beardsley D, Cooper HA, Davignon G, Ewenstein BM, et al. Home treatment of mild to moderate bleeding episodes using recombinant factor VIIa (Novoseven) in haemophiliacs with inhibitors. Thromb Haemost 1998; 80:912–918. [PubMed] [Google Scholar]
- 33.Santagostino E, Gringeri A, Mannucci PM. Home treatment with recombinant activated factor VII in patients with factor VIII inhibitors: the advantages of early intervention. Br J Haematol 1999; 104:22–26. [DOI] [PubMed] [Google Scholar]
- 34.Salaj P, Ovesna P, Penka M, Hedner U. Analyses of recombinant activated factor VII treatments from clinical practice for rapid bleeding and acute pain control in haemophilia patients with inhibitors. Haemophilia 2012; 18:e409–e411. [DOI] [PubMed] [Google Scholar]
- 35.Pan-Petesch B, Laguna P, Mital A, Stanley J, Torchet MF, Salek SZ, et al. Single-dose (270 microg kg(-1)) recombinant activated factor VII for the treatment and prevention of bleeds in haemophilia A patients with inhibitors: experience from seven European haemophilia centres. Haemophilia 2009; 15:760–765. [DOI] [PubMed] [Google Scholar]
- 36.Elander J, Barry T. Analgesic use and pain coping among patients with haemophilia. Haemophilia 2003; 9:202–213. [DOI] [PubMed] [Google Scholar]
- 37.Humphries TJ, Kessler CM. Managing chronic pain in adults with haemophilia: current status and call to action. Haemophilia 2015; 21:41–51. [DOI] [PubMed] [Google Scholar]
- 38.Witkop M, Lambing A, Kachalsky E, Divine G, Rushlow D, Dinnen J. Assessment of acute and persistent pain management in patients with haemophilia. Haemophilia 2011; 17:612–619. [DOI] [PubMed] [Google Scholar]
- 39.Wallny T, Hess L, Seuser A, Zander D, Brackmann HH, Kraft CN. Pain status of patients with severe haemophilic arthropathy. Haemophilia 2001; 7:453–458. [DOI] [PubMed] [Google Scholar]
- 40.Young G, Tachdjian R, Baumann K, Panopoulos G. Comprehensive management of chronic pain in haemophilia. Haemophilia 2014; 20:e113–e120. [DOI] [PubMed] [Google Scholar]
- 41.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs 1997; 23:293–297. [PubMed] [Google Scholar]
- 42.Teyssler P, Kolostova K, Bobek V. Assessment of pain threshold in haemophilic patients. Haemophilia 2014; 20:207–211. [DOI] [PubMed] [Google Scholar]
- 43.Rodkey EN, Pillai RR. The infancy of infant pain research: the experimental origins of infant pain denial. J Pain 2013; 14:338–350. [DOI] [PubMed] [Google Scholar]


