Abstract
Background
We sought to identify donor characteristics influencing long-term graft survival, expressed by a novel measure, kidney life years (KLYs), in living donor kidney transplantation (LDKT).
Methods
Cox and multiple regression analyses were applied to data from the Scientific Registry for Transplant Research from 1987 to 2015. Dependent variable was KLYs.
Results
Living donor kidney transplantation (129 273) were performed from 1987 to 2013 in the United States. To allow sufficient time to assess long-term results, outcomes of LDKTs between 1987 and 2001 were analyzed. After excluding cases where a patient died with a functioning graft (8301) or those missing HLA data (9), 40 371 cases were analyzed. Of 18 independent variables, the focus became the 4 variables that were the most statistically and clinically significant in that they are potentially modifiable in donor selection (P <0.0001; ie, HLA match points, donor sex, donor biological sibling and donor age). HLA match points had the strongest relationship with KLYs, was associated with the greatest tendency toward graft longevity on Cox regression, and had the largest increase in KLYs (2.0 year increase per 50 antigen Match Points) based on multiple regression.
Conclusions
In cases when a patient has multiple potential donors, such as through paired exchange, graft life might be extended when a donor with favorable matching characteristics is selected.
With increasing numbers of patients awaiting kidney transplant, many consider living donor kidney transplantation (LDKT) as an option. Patients rely on transplant centers for direction, but centers lack information on which living donor characteristics predict the best long-term results. Introduction of an allocation system for deceased donation is an attempt at “longevity matching” using the Kidney Donor Profile Index, though it is not directly applicable to LDKT.1 More recently, a Living Kidney Donor Risk Index was published.2
For practical purposes, it is difficult to conceptualize how indices such as those above translate into measurable long-term graft survival. We address “longevity matching” within LDKT, using a novel measure, kidney life years (KLYs), that is easier to conceptualize with respect to the added benefit 1 kidney may have over another. Kidney life years are defined as the number of years a graft has or is expected to function. For example, if graft “A” survives 7 years and graft “B” survives 9 years, then graft “B” has a +2 KLYs advantage over “A.” This information is important to patients with multiple direct donors and easy to match patient-donor pairs that are participating in kidney paired donation (KPD) (hereafter defined as “participants”) who may have more than 1 potential exchange donor to choose from.3,4 Therefore, we sought to identify characteristics that influence long-term LDKT graft survival and examine KLYs as it pertains to optimizing donor selection. Although KLYs is a novel concept, it is important because participants will understand this metric more easily compared with other models.
MATERIALS AND METHODS
We accessed the Scientific Registry for Transplant Research (SRTR) which includes data on all donors, waitlisted candidates, and transplant recipients in the United States.5 Records from the standard analysis file (March 31, 2015) were analyzed. Events were excluded after December 31, 2013, to maximize accuracy due to a lag in SRTR data updates via the United States Renal Data System and Social Security Master Death Files.
The focus was LDKTs occurring from 1987 to 2001 to allow sufficient time to assess long-term effects of independent variables on the dependent variable (KLYs), especially those that might be modified in donor selection such as would be available through KPD, or when more than 1 living donor is available. Several variables were excluded due to missing data: donor height, weight, body surface area, and kidney size, leaving 18 for analysis (Table 1). Type of immunosuppression and presence of donor-specific antibody (DSA) are absent in the SRTR data and could not be assessed.
TABLE 1.
Independent variables

Graft failure was defined as a patient who returned to permanent dialysis or waitlisting and those who received a repeat transplant. Patients dying with a functioning graft were censored. Dates of graft failure were used to calculate graft survival. We decided against reporting 5- or 10-year survival because patients are most interested in how long the transplanted kidney will last.
The first approach we took in answering the question, “Which LDKT kidney lasts the longest?” applied Cox regression to compute hazard ratios (HRs) for the independent variables affecting graft longevity (KLYs). A total of 40 371 transplants were performed between 1987 and 2001 and were followed through March 31, 2013. Of these, 15 821 were no longer functioning, and the exact KLYs could be computed, whereas 24 550 were “still-functioning” and exact KLYs could not be computed. Cox regression is designed to handle this issue of “still-functioning” grafts, using truncated KLYs (graft survival abridged at end of the study interval and recorded as such for matters of data inclusion) to compute HRs.
The second approach to answer the “which kidney lasts longest” question applied multiple regression to determine the expected difference in KLYs for alternative donors. For this analysis, the dependent variable, KLYs, was determined using 1 of 2 ways depending on whether the transplanted kidney had already failed or was “still functioning.” For failed kidneys, actual KLYs were used. For those cases with “still functioning” kidneys, the KLYs were calculated by adding the estimated T1/2 life to the known graft life, drawing from work done in other studies.6,7 For example, for kidneys that were still functioning after 20 years, 50% are expected to still be functioning 17 years later based on the estimated T1/2, thus the KLYs would be recorded as 37 years. Although we recognize that using the T1/2 estimates has the downside of using extrapolated values for the dependent variable, we believe that the resulting regression coefficients more accurately reflect the impact that the donor characteristics have on KLYs and is a superior approach as compared with not including the information associated with the nearly 26 000 transplant cases with “still-functioning” grafts. As will be shown, both the Cox and multiple regression models pointed to the same results internally validating this approach.
HLA Match Points were calculated using the formula used by the National Kidney Registry. A single “A” antigen match, 10 points; “B” match, 15 points; and “DR” match, 25 points. Matches on all 6 loci is equal to 100 points. The calculation is based on studies reporting HLA-matching affecting recipient graft outcome, with variable weighting assigned to the different HLA loci.8
Statistical Methods
Cox proportional hazards were calculated to demonstrate the degree to which each variable affected the chance for graft failure (event of interest). An HR greater than 1 denotes an increased likelihood of graft loss, whereas a value less than 1 denotes a reduced likelihood for graft loss. Multiple linear regression was used to estimate the impact each of the statistically significant independent variables had on KLYs. IBM SPSS Statistics Premium software was used. P value less than 0.05 was considered significant.
Disclaimer
The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the Organ Procurement and Transplant Network and SRTR contractors. The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. Note that, in lieu of a formal ethics committee, the principles of the Helsinki Declaration were followed.
RESULTS
Of 48681 transplants between October 1987 and December 2001, 40 371 cases met inclusion criteria after excluding patients who died with a functioning graft (8301) or missing key HLA data (9).
Mean overall KLYs was 24.4 years (range, 0-39 years). Median donor age was 38 years (range, 11-95 years [the 11-year-old donor was from December 24, 1997, and the two 95-year-old donors were from August 7, 1996, and December 20, 1996; these might represent data issues in the SRTR—author correspondence in progress]). Recipient median age was 36 years (range, < 1 year to 84 years). Living donors were, on average, 2.6 years older than recipients. Median HLA match points was 55 (range, 0-100). Overall, 57% of donors were women, whereas 43% of the recipients were women. Biological sibling donors/recipients accounted for 42% of the cases.
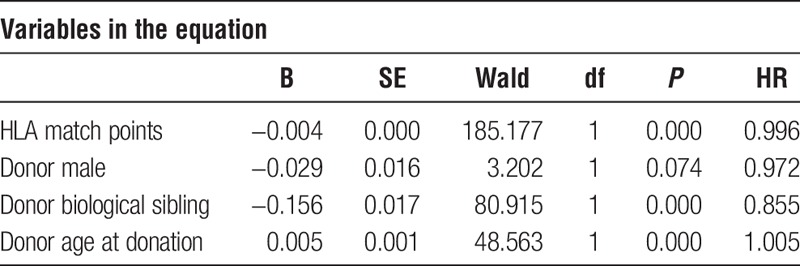
Cox regression was used to determine HRs for 4 statistically significant independent variables. Three donor characteristics (HLA match points, male donor, and biological sibling) have an HR less than 1, denoting a lower probability of graft failure at any point in time. Conversely, donor age is associated with an HR greater than 1 and thus an increased risk of graft loss (Table 2).
TABLE 2.
Cox regression results (1987-2001)
However, the Cox regression results are difficult to work with when trying to more precisely understand the actual advantage 1 donor may have over another. In an attempt to provide a tool to better describe differences among donors, multiple regression was used to explain variation in KLYs based on the independent variables. After accounting for multicollinearity effects and removing variables that became insignificant in the presence of others, the same 4 statistically significant variables that emerged during Cox regression were also significant using multiple regression (Tables 2 and 3).
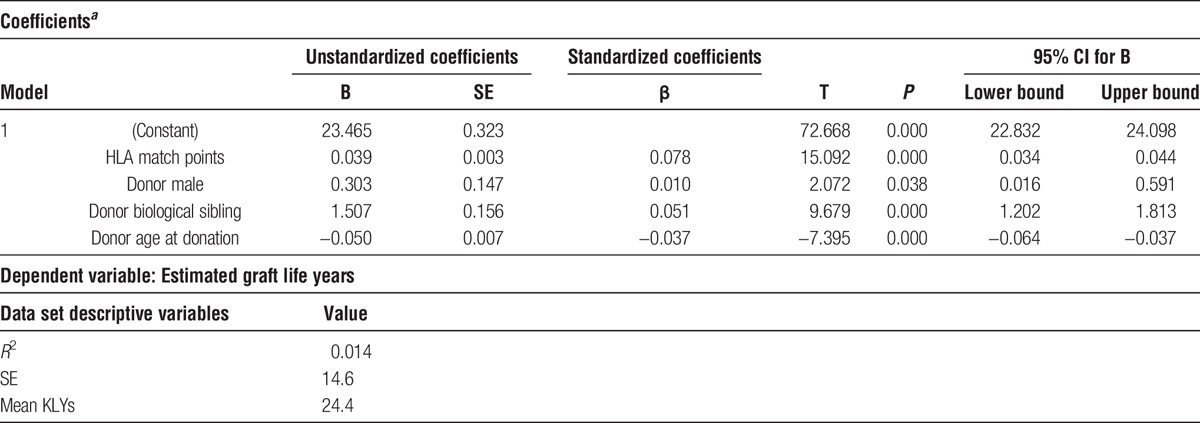
TABLE 3.
Multiple regression results (1987-2001)
From HRs in Table 2, each additional HLA match point is associated a 1-0.996 = 0.004 lower chance of graft failure. Therefore, a donor with 50 additional HLA match points would be associated with a 20% lower chance of graft failure at any point in time. A donor who is a biologic sibling is associated with a 14.5% reduced chance (1-0.855) of graft failure. On the other hand, for each year that a donor is older, the risk of graft loss is increased by 1-1.005 = 0.005 or 0.5%. Thus, comparing 2 donors, 1 of which is 20 years older than the other, the older donor is associated with a 10% higher probability of graft failure.
Referencing the multiple regression results in Table 3, the same 4 donor variables (HLA match points, male donor, biological sibling, and donor age) have both statistically and clinically significant impacts on KLYs. Based on the unstandardized coefficients (B), holding the other variables constant, each HLA match point added an average of 0.039 KLYs. A donor with 50 HLA match points more than another donor is associated with nearly 2 additional KLYs (50 × 0.039 = 1.95). A biological sibling donor is associated with 1.5 years additional KLYs, whereas a male donor is associated with 0.3 additional KLYs. Increased donor age negatively impacts KLYs. When comparing 2 donors, a donor who is twenty years older, holding all other factors constant, is, on average, associated with 1.0 less KLY.
DISCUSSION
With success of KPD,3,9 easy to match pairs can choose between multiple donors. They should understand matching characteristics that correlate with longer-functioning grafts. Though evidence exists for deceased donor graft longevity, less has been published for living donors.2,10,11 Brennan et al12 looked at over 8500 LDKTs calculating a suboptimal graft function score equating worse outcome with recipient serum creatinine 1.5 mg/dL or greater at 1 year. Aside from this being a crude measure of graft longevity, it does not provide concrete information how many added years of function 1 donor provides over another. More recently, a LDKT risk index was published where it was concluded that donor age, body mass index, race, cigarette use, and HLA B and DR mismatches were associated with risk of graft loss.2 We identified some similar factors using KLYs.
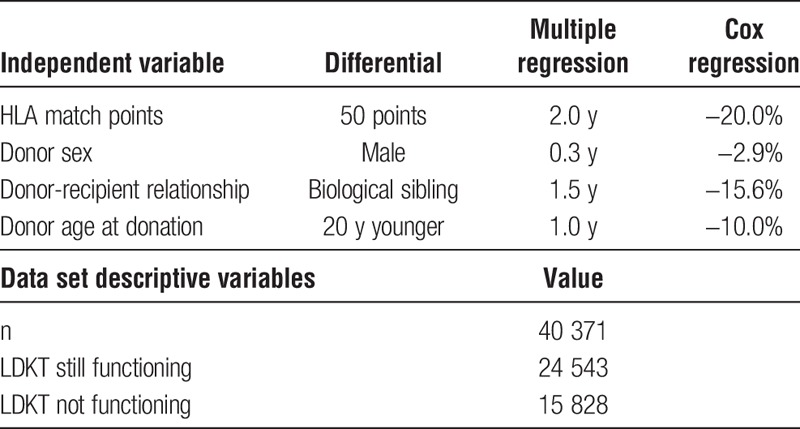
Four donor variables—HLA match points, donor sex, biological sibling, and donor age—significantly impact KLYs. Both the Cox and multiple regression models identified the same 4 variables internally validating their importance. Additionally, shown in Table 4, the impact of the independent variables are proportionally similar across both models.
TABLE 4.
Comparison of independent variables
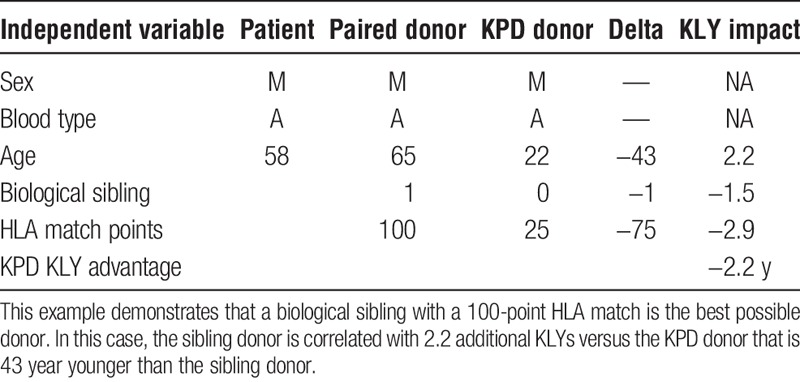
The regression coefficients are additive. This becomes important when there are 2 or more donors available, such as through KPD. Table 5 illustrates an example of how the results can be applied to determine which donor is best with respect to maximizing expected KLYs. In this example, a 49-year-old recipient is immunologically compatible with his 30-year-old male donor, but is a zero antigen match (HLA match points = 0). A 61-year-old male KPD donor with 75 HLA match points is also compatible and available via KPD. This scenario shows that even an older donor can be associated with higher expected KLYs (1.3 years) when there is a better HLA match. The negative impact of donor age is outweighed by the higher HLA match points. In another example, a younger KPD donor with a better HLA match would be associated far with more KLYs (4.1 years) than a poorer HLA-matched compatible husband-wife pair (Table 6). In still another example, a 6 antigen-matched compatible older brother of a potential recipient is a better match than a KPD donor with a poor HLA match who is 43 years younger than the brother (Table 7). The expected disadvantage of using the KPD donor in this scenario would be −2.2 KLYs, making the sibling donor the preferred option in terms of maximizing expected KLYs.
TABLE 5.
Older donor with better HLA
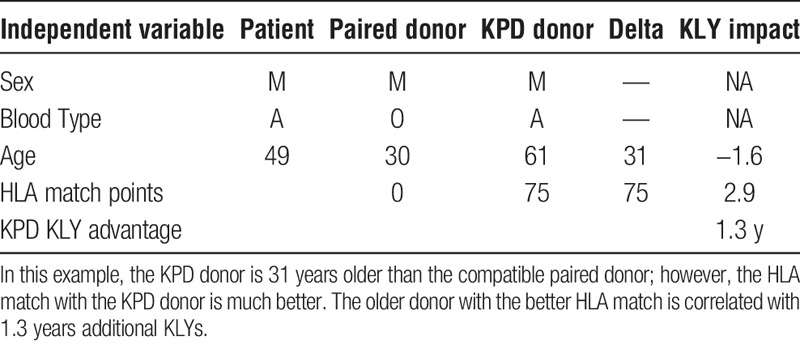
TABLE 6.
Younger donor with better HLA
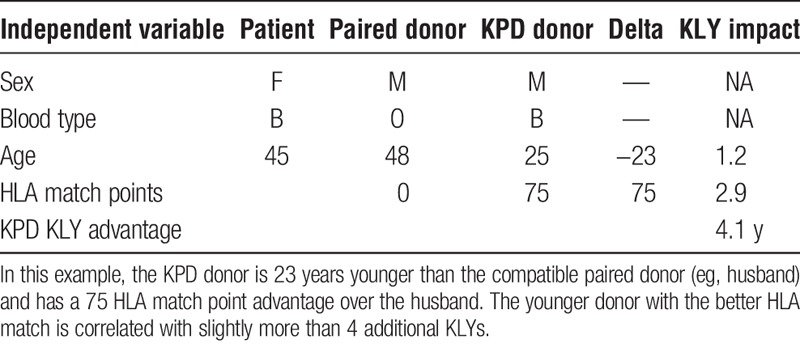
TABLE 7.
Six antigen-matched sibling versus younger donor
Although older donors are associated with lower expected KLYs, the impact is smaller than we anticipated. Looking further into the issue of living donor age (“older is worse”), the results show that when comparing 2 potential donors who are otherwise equivalent (same HLA match points, sex, and sibling relationship), a younger donor is preferred. However, there is bias among some participants that “older” donors are undesirable—that is, that donor quality diminishes as age increases, and that after a certain age, a living donor should be deemed unacceptable in the absence of considering other donor selection criteria. However, our results demonstrate that HLA match points have a more profound effect on KLYs versus donor age. Therefore, living donor age should not be considered in isolation of other variables.
Examining donor age in more detail, a scatterplot of donor age against KLYs did not indicate a discernable curvilinear relationship (or a linear relationship for that matter). The correlation between the 2 variables, although statistically significant, is weak. Second, we created dummy (0-1) variables to define age cutoffs (50, 55, 60, and 65 years). These variables were added to the other variables (donor HLA match points, donor sex, biological sibling, and donor age) and included in multiple regression. In the presence of the donor age variable, none of these dummy age variables added to the explanation of the variation in KLYs (results not shown).
We also examined the SRTR data to see what the actual transplant outcomes had been for the 40 371 transplants that occurred between 1987 and 2001. If “older” donors are to be considered unacceptable, we would expect to see a high percentage of “older” donor transplants failing early. To establish a baseline, the data show that 72% of all grafts lasted 10 or more years. Table 8 shows the percentages of grafts that survived 10 or more years with donors of different age groups. These results are consistent with our Cox and multiple regression findings in that they show the percentage of 10+ year graft survival drops as donor age increases. However, what is important is that even without considering any other factors, such as HLA, the 10+ year graft survival remains high regardless of the donor age category. Table 9 shows that for transplants performed when HLA match points were in the range 50 to 100, there was almost no drop in the percentage of grafts that survive 10 or more years when older donors are used. Figure 1 illustrates this point showing KLYs as they relate to donor age stratified by match points of 50 to 100. The mean KLYs remain fairly constant for donors between ages 10 and 39 years (around 25 years), declining slightly for donors aged 40 to 49 years (24.3 years), 40 to 40 years (23.1 years), 50 to 59 years (23.1 years), and 60 to 69 years (20.7 years). The uptick for donors aged 70 to 79 years (22.8 years) may be an aberration due to the small number of donors (51) in this group. Similar trends are seen with HLA match points less than 50 though the mean KLYs are generally lower as might be expected (Figure 2). Similar results occur when “still functioning” grafts are excluded (Figures 3 and 4).
TABLE 8.
Graft survival >10 y by donor age group
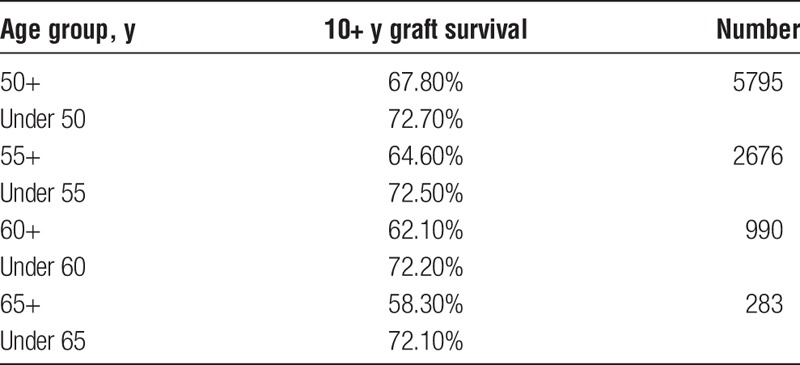
TABLE 9.
Graft survival >10 y by sonor age (HLA match points 50‐100)
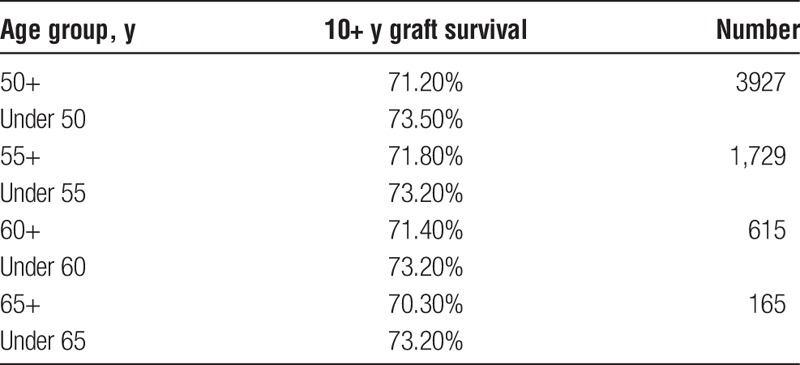
FIGURE 1.
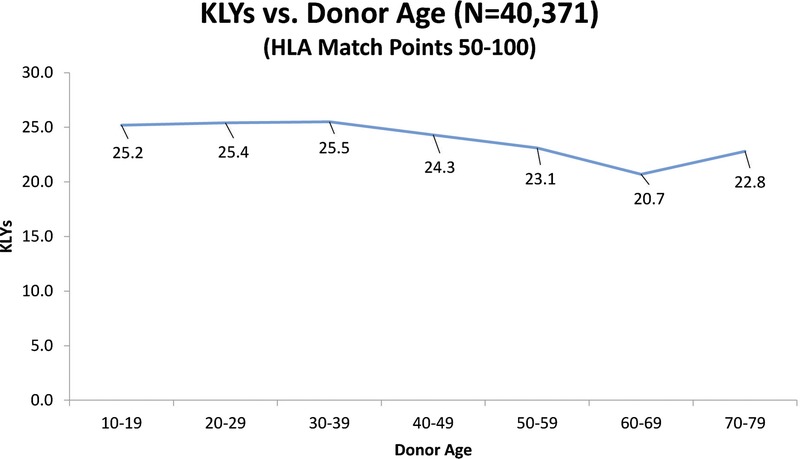
KLYs as they relate to donor age stratified by HLA match points (50-100).
FIGURE 2.
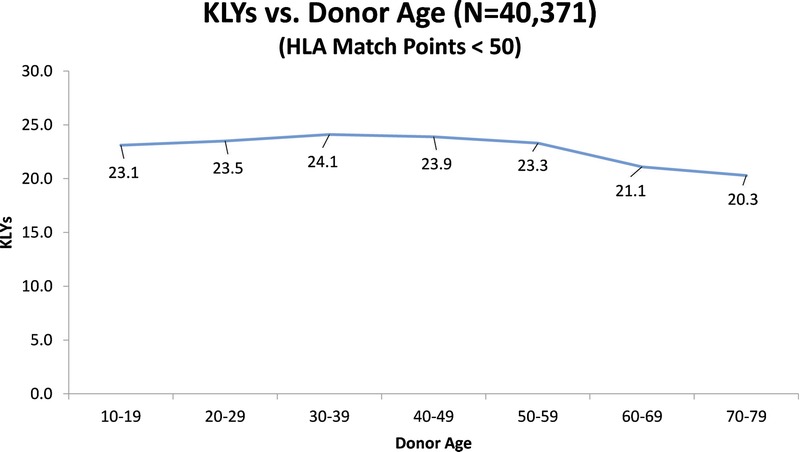
KLYs as they relate to donor age stratified by HLA match points (< 50).
FIGURE 3.
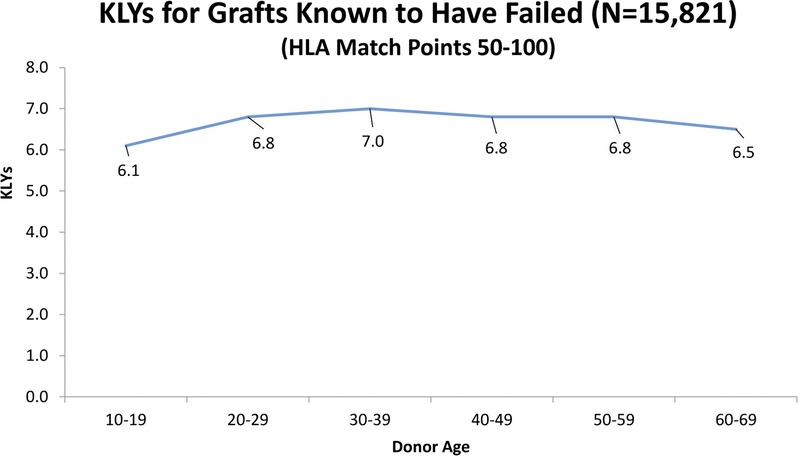
KLYs as they relate to donor age stratified by HLA match points (50-100) strictly for grafts known to have failed.
FIGURE 4.
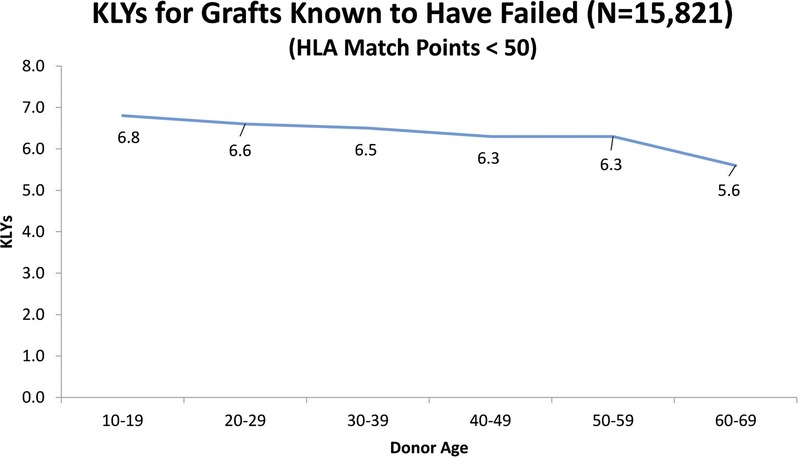
KLYs as they relate to donor age stratified by HLA match points (< 50) strictly for grafts known to have failed.
Although we can control for other factors besides donor age, the important finding is that although younger donors are preferred, viable “older” donors should not be automatically rejected. There does not appear to be a clinically significant age where the mean KLYs fall precipitously. Addressing the concept of age-matching, Chang et al6 examined data from the United States Renal Data System and found that aside from recipients aged 18 to 39 years who achieved optimal longevity with living donor kidneys also aged 18 to 39 years, there were near equivalent outcomes with any of the other age pairings. However, they did not control for HLA which we demonstrated to be the strongest predictor of graft longevity. HLA match points are therefore both statistically and clinically more significant regarding impact on KLYs (Tables 1 to 9, Figures 1 to 4). As a result, when choosing between 2 donors, one should not be surprised by Table 7 showing that even a donor who is 43 years older, but a better HLA match, can be associated with a better expected long-term outcome. Participants should therefore take the time to seek the donor kidney that is expected to last the longest, but also to avoid making the mistake of excluding donors who would otherwise be suitable (or better) options in their clinical situation. This is especially important for highly sensitized incompatible pairs participating in KPD who lack matching power and who may not have the leverage to maximize KLYs unlike easy to match pairs.
In practice, many KPD participants routinely turn down a better HLA matched donor in favor of a younger donor even though a better HLA-matched donor would be associated with higher KLYs. It is uncommon in KPD to have 2 potential living donors with a 30-year age discrepancy (1.5 KLY impact), whereas it is common to see 2 potential living donors with a 50 HLA match point discrepancy (2.0 KLY impact). Others refuse to accept any living donor over the age of 55 to 65 years, fearing a very short expected KLYs result which is not supported by our findings. In doing so, they accept the risk of declining health of the recipient on the waitlist, the morbidity, and mortality of dialysis, plus reduced graft survival in delaying transplant. At the time of this writing, only 14 (5%) of 277 active National Kidney Registry participants awaiting a match set a restriction on the minimum HLA match points they will accept (even though HLA is the strongest predictor of graft longevity), but 130 (47%) set a restriction on maximum donor age preference (ie, donor age must be below the 30-65 years age range [variable by center]). Adjusting practice patterns described above would not only reduce wait time to transplant for participants in KPD, but also increase the number of transplants that occur while at the same time improving KLYs for the recipients involved.3,20
Improved long-term outcomes also appeared to result from male donors (+0.3 KLYs). This may be related to the fact that they tend to be larger than female donors (more nephron mass), or it may be a downstream consequence of the Y-chromosome. In the North American Pediatric Renal Trials and Collaborative Studies review (NAPRTCS, Transplantation, 2006), there was a correlation between creatinine clearance and graft survival factoring donor size. Hugen et al13 reported that size of the donor kidney does matter in live donor transplantation.
The fact that HLA matching had the most important impact on KLYs should not be surprising. Terasaki and Cai14 argued that chronic rejection is a function of DSAs targeting mismatched antigens, and directly correlates with graft longevity. Similarly, analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing Renal Transplant Registry showed that graft half-lives were longer with better HLA matching.15 Another possible reason for the effect on KLYs is that HLA mismatches correlate with risk of patient death because of the requirement for higher immunosuppression, and more antirejection therapy.16-18 Though we were not able to study the influence of DSA on KLYs because of missing data, we may learn in the future that epitope matching will have an even greater impact on KLYs.19 There are no data to support that newer immunosuppression protocols will affect the independent variables influence on KLYs, but we anticipate that better immunosuppression and optimal donor selection will extend KLYs working in tandem because of the ceiling forced by chronic rejection.14,19
Looking at applications for KLYs, both immunologically compatible and incompatible participants will be interested in the results highlighted by this article, especially in the era of national multicenter KPD where there is a community of biologically diverse donors available for exchange.4 All participants should consider options that may translate into greater graft longevity or KLYs. There are at least 8 reasons why a participant (either compatible or incompatible) may choose to enroll in KPD with the goal of “trading-up” for a better outcome through donor optimization (measured in KLYs) among other reasons, listed as follows: (1) transplants may last longer (ie, higher expected KLYs) with better donor selection; (2) recipients may require less immunosuppression with better matching, and therefore experience less side effects from medication; (3) recipients may experience less morbidity/mortality by seeking a better match, especially in cases where aggressive desensitization can be avoided entirely16-18; (4) recipients will be easier to transplant in the future (either with a living, or deceased donor) should they require it, because they will be sensitized to fewer antigens (DSA)20; (5) participants help others while at the same time helping the intended recipient who faces the possibility of a repeat transplant if a graft fails—this not only by offloading competition on the waitlist by enabling more transplants per unit time, but also by facilitating more difficult to accomplish transplants in sensitized cohorts of recipients of which the intended recipient may become a member (by developing DSA) should they require a repeat transplant in the future, these concepts being supported by the mathematics driving KPD3,4,20,21 (6) donor-recipient anatomic, and other physiologic considerations (eg, CMV, EBV status); (7) registration in an advanced donation program,22 and; (8) altruistic motives, “helping the greater good,” by creating more transplants via KPD. Confidence in KPD is growing—allowing for mainstream KLYs optimization to take place—and is reinforced by early results showing favorable outcomes with shipped living donor kidneys.3,4,23 In considering KLYs optimization, the ethics of both incompatible and compatible exchange have been described and are more recently supported in a publication by Cuffy calling for multicenter trials using compatible pairs in KPD.24-26
Recipients with 3 to 6 antigen-matched biological sibling donors are typically the best matches that can rarely be improved upon, and are just 1 example that can be determined informing participants on “Which LDKT kidney lasts the longest?” if calculations are done using the methods described in this paper.
CONCLUSIONS
We present the first long-term analysis of KLYs in LDKTs. HLA matching had the most impact on KLYs and should be emphasized in the donor selection process, among other variables. These results confirm the critical importance of HLA matching in long-term living donor graft survival and should be incorporated into the living donor selection process supported by the largest body of national multicenter data available (SRTR).
ACKNOWLEDGMENT
The authors thank J. Michael Cecka, Professor, Pathology and Laboratory Medicine, University of California, Los Angeles (UCLA), Los Angeles, CA.
Footnotes
Published online 6 June 2016.
The authors declare no funding or conflicts of interest.
J.M. participated in research design, writing of the article, performance of the research, and data analysis. M.L.M. participated in research design, writing of the article, performance of the research, and data analysis. B.L. participated in research design, writing of the article, and data analysis. J.V. participated in research design, writing of the article, and data analysis. M.R. participated in performance of the research and data analysis. T.D.A. participated in performance of the research and data analysis. G.H. participated in research design, writing of the article, performance of the research, and data analysis. P.C.F. participated in research design, writing of the article, performance of the research, and data analysis. P.W.S. participated in research design, writing of the article, performance of the research, and data analysis.
REFERENCES
- 1.Israni A, Salkowski N, Gustafson S, et al. New national allocation policy for deceased donor kidneys in the united states and possible effect on patient outcomes. J Am Soc Nephrol. 2014;25:1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massie AB, Leanza J, Fahmy LM, et al. A risk index for living donor kidney transplantation. Am J Transplant. doi: 10.1111/ajt.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melcher ML, Leeser DB, Gritsch HA, et al. Chain transplantation: initial experience of a large multicenter program. Am J Transplant. 2012;12:2429. [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Registry. National Kidney Registry Home Page: National Kidney Registry, http://www.kidneyregistry.org/. Updated 2016. Accessed 9/1/2015.
- 5.Organ Procurement and Transplantation Network. OPTN/SRTR 2012 Annual Report. http://srtr.transplant.hrsa.gov/annual_reports/2012/pdf/01_kidney_13.pdf. Published 2012. Accessed 9/1/2015.
- 6.Chang P, Gil J, Dong J, et al. Living donor age and kidney allograft half-life: implications for living donor paired exchange programs. Clin J Am Soc Nephrol. 2012;7:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hariharan S, Johnson CP, Bresnahan BA, et al. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:1837–1838. [DOI] [PubMed] [Google Scholar]
- 8.Roberts JP, Wolfe RA, Braagg-Gresham JL, et al. Effect of changing the priority for HLA matching on the rates and outcomes of kidney transplantation in minority groups. N Engl J Med. 2004;350:545–551. [DOI] [PubMed] [Google Scholar]
- 9.Gentry SE, Montgomery RA, Segev DL. Kidney paired donation: fundamentals, limitations, and expansions. Am J Kidney Dis. 2011;57:144–151. [DOI] [PubMed] [Google Scholar]
- 10.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231–236. [DOI] [PubMed] [Google Scholar]
- 11.Park KS, Shin JH, Jang HR, et al. Impact of donor kidney function and donor age on poor outcome of living unrelated kidney transplantation (KT) in comparison with living related kidney transplantation. Clin Transplant. 2014;28:953–960. [DOI] [PubMed] [Google Scholar]
- 12.Brennan TV, Bostrom A, Feng S. Optimizing living donor kidney graft function by donor-recipient pair selection. Transplantation. 2006;82:651–656. [DOI] [PubMed] [Google Scholar]
- 13.Hugen CM, Polcari AJ, Fitzgerald MP, et al. Size does matter: donor renal volume predicts recipient function following live donor renal transplantation. J Urol. 2010;185:605–609. [DOI] [PubMed] [Google Scholar]
- 14.Terasaki PI, Cai J. Human leukocyte antigen antibodies and chronic rejection: from association to causation. Transplantation. 2008;86:377. [DOI] [PubMed] [Google Scholar]
- 15.Cecka JM. The OPTN/UNOS Renal Transplant Registry. Clin Transpl. 2005:1–16. [PubMed] [Google Scholar]
- 16.Orandi BJ, Garonzik-Wang JM, Massie AB, et al. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant. 2014; 14: 1573–1580. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery JR, Berger JC, Warren DS, et al. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012;93:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opelz G, Dohler B. Association of HLA mismatch with death with a functioning graft after kidney transplantation: a collaborative transplant study report. Am J Transplant. 2012;12:3031–3038. [DOI] [PubMed] [Google Scholar]
- 19.Terasaki PI. A personal perspective: 100-year history of the humoral theory of transplantation. Transplantation. 2012;93:751–756. [DOI] [PubMed] [Google Scholar]
- 20.Meier-Kriesche HU, Scornik JC, Susskind B, et al. A lifetime versus a graft life approach redefines the importance of HLA matching in kidney transplant patients. Transplantation. 2009;88:23–29. [DOI] [PubMed] [Google Scholar]
- 21.Melcher ML, Veale JL, Javaid B, et al. Kidney transplant chains amplify benefit of nondirected donors. JAMA Surg. 2013;148:165. [DOI] [PubMed] [Google Scholar]
- 22.Flechner SM, Leeser D, Pelletier R, et al. The incorporation of an advanced donation program into kidney paired exchange: initial experience of the National Kidney Registry. Am J Transplant. 2015;10:2712–2717. [DOI] [PubMed] [Google Scholar]
- 23.Segev DL, Veale JL, Berger JC, et al. Transporting live donor kidneys for kidney paired donation: initial national results. Am J Transplant. 2011;11:356. [DOI] [PubMed] [Google Scholar]
- 24.Ratner LE, Rana A, Ratner ER, et al. The altruistic unbalanced paired kidney exchange: proof of concept and survey of potential donor and recipient attitudes. Transplantation. 2010;89:15–22. [DOI] [PubMed] [Google Scholar]
- 25.Ross LF, Rubin DT, Siegler M, et al. Ethics of a paired-kidney-exchange program. N Engl J Med. 1997;336:1752. [DOI] [PubMed] [Google Scholar]
- 26.Cuffy MC, Ratner LE, Siegler M, et al. Equipoise: ethical, scientific, and clinical trial design considerations for compatible pair participation in kidney exchange programs. Am J Transplant. 2015;15:1484–1489. [DOI] [PubMed] [Google Scholar]


