Abstract
Purpose of review
Cancer cachexia is common and reduces function, treatment tolerability and quality of life. Given its multifaceted pathophysiology a multimodal approach to cachexia management is advocated for, but can be difficult to realise in practice. We use a case-based approach to highlight practical approaches to the multimodal management of cachexia for patients across the cancer trajectory.
Recent findings
Four cases with lung cancer spanning surgical resection, radical chemoradiotherapy, palliative chemotherapy and no anticancer treatment are presented. We propose multimodal care approaches that incorporate nutritional support, exercise, and anti-inflammatory agents, on a background of personalized oncology care and family-centred education. Collectively, the cases reveal that multimodal care is part of everyone's remit, often focuses on supported self-management, and demands buy-in from the patient and their family. Once operationalized, multimodal care approaches can be tested pragmatically, including alongside emerging pharmacological cachexia treatments.
Summary
We demonstrate that multimodal care for cancer cachexia can be achieved using simple treatments and without a dedicated team of specialists. The sharing of advice between health professionals can help build collective confidence and expertise, moving towards a position in which every team member feels they can contribute towards multimodal care.
Keywords: cancer cachexia, clinical education, multimodal care, supportive care
INTRODUCTION
Cancer cachexia is a ‘multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass, with or without a loss of fat mass, that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment’ [1]. It is common, especially among patients with advanced solid cancers [1,2], and impacts adversely on function [3,4], treatment tolerability [5], health service use [6], quality of life [7], and survival [8]. The pathophysiology of cancer cachexia is complex but can be characterized by a negative protein and energy balance, driven by a variable combination of reduced food intake and abnormal metabolism [9]. Persistent inflammatory and stress responses, coupled with tumour-derived factors, led to reduced food intake [9], increased resting energy expenditure [10], and net loss of lean tissue [11,12]. The degree of cachexia and the predominant phenotype depends on the tumour and patient characteristics [1,8,13], which can be uncovered with a comprehensive standardized assessment [14].
Given the multifaceted pathophysiology and heterogeneous presentation of cancer cachexia [15], a multimodal approach to its management has been proposed [16] and advocated for by experts in the field [9,11,14,17–20]. Core treatment goals of the approach include ensuring sufficient energy and protein intake, maintaining physical activity and muscle mass, and reducing systemic inflammation where present [14]. Core component interventions would therefore include nutritional support, physical activity and exercise, and anti-inflammatory agents, for example, n-3 fatty acids or NSAIDs. These should be offered on a background of personalized oncological and nursing management, and family-centred education [21].
A multimodal approach has a strong theoretical backing but can be difficult to realise in clinical practice [22]. With only a handful of specialized cachexia clinics and limited allied health professionals (AHPs), responsibility falls to those providing routine care, usually the oncologist and/or cancer nurse [23▪]. There are time and resource restraints, few guidelines providing recommendations for cachexia management, and there can be the perception that cachexia management demands specialist expertise [23▪]. In this article, we use a case-based approach to highlight practical approaches to the multimodal management of cachexia for patients across the cancer trajectory. The cases are based on patients attending a lung clinic in a tertiary cancer centre. The suggested treatments are informed and guided by recent evidence, but have been selected with the generalist in mind and do not require specialist equipment and expertise. The intention is to demonstrate that multimodal care can be implemented into routine clinical practice.
Box 1.

no caption available
CASE #1: LISTED FOR SURGICAL RESECTION
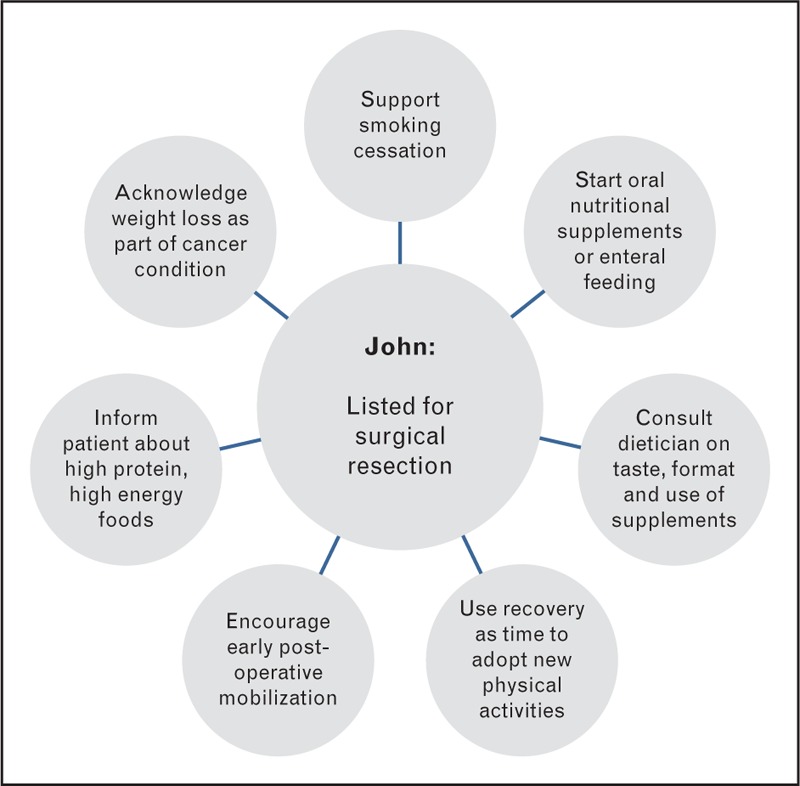
John, aged 75, has been listed for surgical resection of a T1aNoMo adenocarcinoma of the lung (Fig. 1). His current WHO performance status is 1. He has concurrent COPD, suffers from severe anxiety, and continues to smoke half-an-ounce of rolling tobacco daily. He reports losing about half a stone and his current BMI is 17.8 kg/m2, CRP is 119 mg/L, and appetite is lower than usual. Depending on Johns’ pathological staging, he may need further adjuvant chemotherapy. His oncologist has prescribed postoperative pabrinex and vitamin B12 and is supporting John to stop smoking.
FIGURE 1.
John, listed for surgical resection.
John's BMI is below normal and he is severely malnourished which could impact negatively on the outcome of his surgery in terms of complications and length of stay. His weight loss and associated problems should be acknowledged [24], with an explanation that surgery can cure lung cancer and, with adequate nutrition, the weight can be regained. A nutritional care plan over the next 7–10 days could include dietary intake and oral nutritional supplements or alternatively enteral tube feeding [25]. The latter is not often offered to patients but may be useful given John's anxiety if he is unable to meet his nutritional requirements orally. Food fortification advice would include information about the use of high energy and high protein foods, for example, the use of full cream milk, additional cheese or cream in soup or mashed potato, the use of flavoured oils (olive, nut or vegetable) on foods such as soup, rice or pasta. Use of high energy fluids can add to his energy intake, for example milky coffee, hot chocolate, malted milk drinks, milkshakes or fruit smoothies. High energy snacks between meals are also useful to increase energy intake including nuts, dried fruit, cheese or nut butter on crackers or toast, cake and biscuits, but dietary counselling must discuss the foods that the patient would be able to eat in addition to usual meals. Talking with and his wife together can facilitate dialogue about sensitive topics, conflicts, and strengths they have developed to manage his COPD that might be helpful now [26▪]. Oral nutritional supplements may improve overall dietary intake in some patients [27]. Adherence to taking these may be improved with the added expertise of a dietician to discuss taste preference, formats of supplements available and suitable usage in addition to food [28–31]. When introducing them talking about ways other couples have coped in similar situations could be used as an indirect way of normalizing experience, supporting problem solving and reinforcing existing coping strategies [26▪,32].
Without the need for neoadjuvant chemotherapy there is only a short window of opportunity to improve John's pulmonary function and fitness prior to surgery [33,34], so the focus should shift towards enhanced recovery after surgery. Early mobilization is an important aspect of postoperative care, to be encouraged by all the team. There is limited evidence on which protocol to adopt [35▪], but the patient who can mobilize quickly becomes an active participant in their care [36]. This might again reduce postoperative complications and risk of prolonged stay or readmission. John is a good candidate for pulmonary rehabilitation, which can reduce symptom burden and enhance exercise capacity following lung resection [37,38]. From the first contact this plan can be introduced, along with counselling to find new activities in the recovery phase. Patients often adopt sedentary behaviours despite successful primary treatment of disease, but something as simple as a step counter/pedometer may help them to self-manage this [39,40].
CASE #2: COMMENCED RADICAL CHEMORADIOTHERAPY
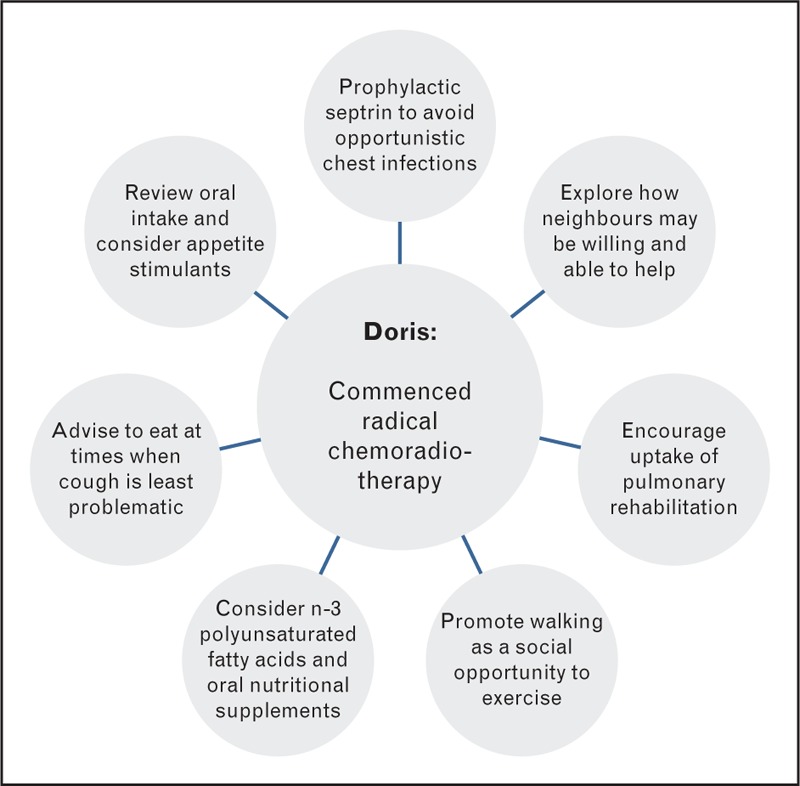
Doris, aged 65, has COPD and presented with cough and weight loss (Fig. 2). She was diagnosed with a radically treatable T1aN2Mo squamous cell carcinoma of the lung. She commenced radical chemo-radiation therapy but following cycle 1 of chemotherapy was admitted with acute sepsis. Her weight at diagnosis had dropped 13 kg since documented 2 years previously, and has since dropped a further 6 kg despite enteral support. Doris had a current BMI of 14.7 kg/m2 and performance status of 2. Her appetite remains poor and she has a high CRP of 280 mg/L. She is a retired landlady, is widowed with no children, and currently lives alone but has supportive neighbours. Her admission might prompt a change in medical management including stopping her chemotherapy and altering the dose per fraction of radiotherapy. Prophylactic septrin could help avoid opportunistic chest infections while her radiotherapy continues and nutritional support with pabrinex and vitamin B12 could be offered.
FIGURE 2.
Doris, commenced radical chemoradiotherapy.
This patient has severe malnutrition despite enteral support. The persistent inflammatory response, possibly fuelled by recurrent infection, has contributed to her ongoing weight loss and treatment may have further reduced appetite and food intake. A full review of symptoms is warranted. Ideally this would have occurred prior to her starting treatment to improve tolerance to it. The use of n-3 polyunsaturated fatty acids in addition to protein and energy dense nutritional supplements may have helped support performance status and quality of life [41]. A self-management approach to eating can help retain a sense of control [42,43]. Doris could be advised to eat and drink at times when her cough is least problematic and use nutrition dense snacks and fluids, for example, milk drinks of fortified soups, to increase intake in spite of symptoms. Appetite stimulants may have a role. On completion of radiotherapy proactive monitoring and management of oesophagitis is required to ensure this does not further exacerbate the poor intake. Enteral tube feeding may be required in circumstances if oral intake is particularly compromised and nutritional status deteriorating. Treatment-related anaemia, pneumonitis and physical inactivity might have reduced Doris’ exercise tolerance, particularly during her recent hospital admission [3,44]. Studies support exercise alongside chemoradiotherapy therapy [45▪▪]. Although no study has purposefully selected cachectic patients [46], exercise has an anti-inflammatory effect [47] and can help maintain function. If local rehabilitation services are available a referral should be discussed. With her concurrent COPD diagnosis Doris would be an excellent candidate for pulmonary rehabilitation [48,49] despite her current frailty [50▪,51]. Doris should be encouraged to stay as active as she feels able [52,53], perhaps to start walking with friends or neighbours on a few days of each week as a social activity [54].
CASE #3: RECEIVING PALLIATIVE CHEMOTHERAPY
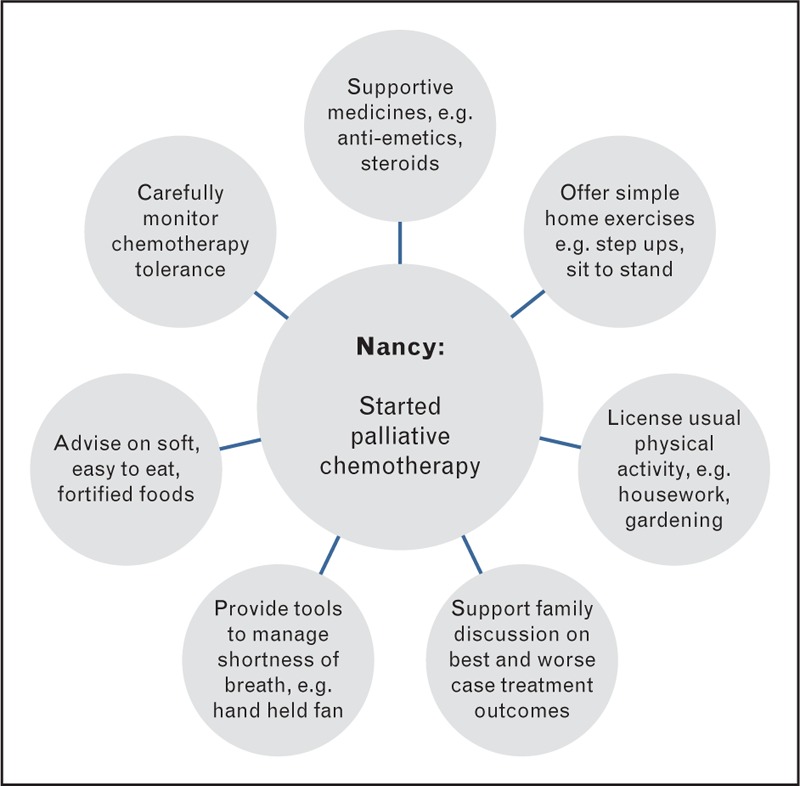
Nancy, aged 71, was recently diagnosed with stage IV squamous cell lung carcinoma having presented to her GP with breathlessness (Fig. 3). She had a pleural effusion which was drained, and has a medical history of a cardiac pacemaker, small hiatus hernia, and hypercholesteraemia. She lives with her husband and has four grown children nearby. Her performance status is 1 and she mobilizes with a walking stick, mainly for confidence. Nancy's BMI at diagnosis was 28.5 kg/m2. She had lost 5 kg but this did not cause her particular concern as she had been keen to lose weight. Her appetite remains low and her breathlessness persists. She was prescribed palliative chemotherapy with gemcitabine/carboplatin and baseline bloods revealed an ESR of 116 mm/h and CRP of 58 mg/L. Her oncological management included prescribing postchemotherapy supportive medications (antiemetics, proton pump inhibitors and steroids), treating constipation, and close monitoring of tolerance to chemotherapy.
FIGURE 3.
Nancy, receiving palliative chemotherapy.
Breathlessness and poor appetite here will have had a significant impact on the dietary intake for this patient leading to malnutrition. Malnourished patients have a poorer outcome compared with those who are not malnourished at diagnosis [55] so symptom control is a priority. Given Nancy's age and comorbidities, polypharmacy may be contributing to her symptoms and a geriatric assessment is worthwhile. Achieving sufficient dietary intake can be difficult in the older breathless patient, who often reduce the frequency of eating and have dietary profiles with little variety and unusually high proportions of liquids [56]. Consideration should be given to foods and fluids that maximize dietary intake but are easy to eat and drink, for example, foods that are soft and can be fortified. Oral nutritional supplements may be an option if found suitably palatable. The home support from her husband and grown children is important. Families can support or challenge the patient's approach to progressing disease and changing eating habits [57,58] and should be closely involved in management where possible. It can be important to support the patient's plans to tell family about best and worst case scenario from treatment and negotiate with their family. There is also a role in preparing the family in the case of rapidly progressing disease where they may engage in futile attempts to feed that cause distress [43]. Acknowledging the importance of meals for maintaining relationships can be useful, as can suggesting ways Nancy can continue to be part of daily family routines and events without feeling pressure to eat, such as offering ‘help yourself’ meals.
Nancy's favourable performance status should assist her getting through her palliative chemotherapy, indeed performance status, functional capacity and activity behaviours are all predictors of overall survival [59]. Physical activity level should be seen as a ‘vital sign’ reflecting current wellbeing. Whilst when Nancy feels in need of rest this should happen, she should be permitted and encouraged to continue usual activity behaviours such as the housework, gardening and hobbies. With her symptoms, advice should be offered on activity planning and pacing ‘balancing activity and rest’ and ‘listening to your body’, along with simple way to manage breathlessness, such as using a handheld fan or cooled water spray. Reassuring Nancy and her family about normal exertional responses to activity can also be helpful [60]. They could be directed towards web-based information and guidance to support active living, for example, from Macmillan Cancer Care. Although formal exercise services are not often available, many patients report a readiness to take up light self-managed exercise during chemotherapy [61,62]. Simple home exercises that do not require equipment can be taught, for example step-ups and sit-to-stands with a focus on a slow and controlled movement. Furthermore palliative care services, including hospices, may provide rehabilitation services that the patient may be willing to explore [63–65].
CASE #4: NO PLANNED ANTI-CANCER THERAPY
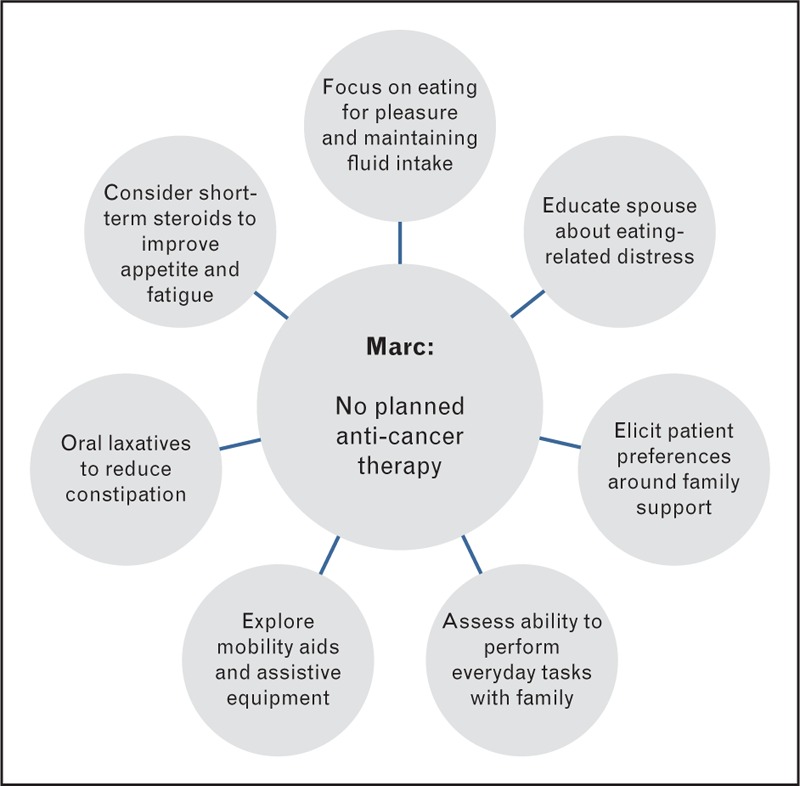
Marc, aged 63, was diagnosed with a Stage IV mixed large cell/neuroendocrine lung tumour following flu-like symptoms that restricted his work as a carpenter (Fig. 4). His performance status had deteriorated rapidly in the last month, from WHO 1 to 3, and he reported significant weight loss. Additional symptoms included chronic fatigue, cough, constipation, peripheral oedema. Marc's BMI was 19.3 kg/m2 and blood tests revealed a high CRP of 232 mg/L. His planned management was for best supportive care. The oncologist assessed Marc for palliative radiotherapy to improve his cough, prescribed oral laxatives for his constipation as a nutritional impact symptom, diuretics to improve peripheral oedema, and a short-course of corticosteroids to stimulate appetite and alleviate fatigue. Timely involvement of palliative care was also prioritized.
FIGURE 4.
Marc, no planned anticancer therapy.
Marc's nutritional markers (weight loss, poor appetite and increased CRP) suggest refractory cachexia [1] and with such rapid deterioration the provision of nutrition is unlikely to prevent further change in body composition. The deterioration in performance status and loss of body weight is likely to cause concern and distress to Marc and his family [21]. Therapeutic discussion should cover the causes of weight loss, main symptoms and what changing dietary intake can and cannot realistically achieve. Regarding nutrition, the focus might be to eat for pleasure whilst maintaining an adequate fluid intake – finding foods and fluids that provide best taste, are easy to eat, and taking small portion sizes. The link between constipation and drinking plenty of fluids could also be made. The use of oral nutritional supplements is unlikely to provide clinical benefit so these should only be considered if they are liked by the patient and requested. It can be helpful to include those preparing food in the discussion, often the family, to ensure their role is supportive. A clear explanation of what causes weight loss, for example, there may be a malnutrition component, but it is likely predominantly caused by disease and not anyone's fault, may help to alleviate the anxiety around appetite and eating, and help manage expectations [66]. The reduced performance status will also threaten Marc's physical independence and normal ‘role’, particularly with regard to his work [23▪,67]. An intense exercise programme targeting change in body composition is unlikely to be realistic or helpful. Instead the focus should be on maintaining independence in activities of daily living, involving the family who will be at home supporting Marc. The provision of a mobility aid and/or assistive equipment should be considered, to promote impendence in and around the home [55,68]. It is common for family members to be uncertain what to do, so a supportive approach that encourages the patient to talk about their preferences and how best to manage change can be useful [69], for example asking: ‘how would you prefer them to help?’. Family members sometimes default to doing everything for their loved one in an attempt to preserve their energy levels, including tasks they are capable of doing independently. A reminder that independence is often important to people may help family members to offer care that enables rather than limits daily activity.
IMPLEMENTING MULTIMODAL CARE IN PRACTICE
These cases highlight numerous component treatments to consider, and provide three key messages to support multimodal care for cancer cachexia in practice. First, every member of the clinical team can contribute towards multimodal care. Multimodal care includes addressing nutritional impact symptoms, for example, sore mouth, constipation, pain, which interfere with intake and absorption and compound cachexia. Assessing and managing these symptoms is an important part of multimodal care within reach of all clinicians. A holistic needs assessment can uncover additional patient and family needs relating to cachexia and prompt appropriate education and management. It is not always possible to refer to specialists, or there can be long wait lists, so simple practical advice should become part of each team member's repertoire. This would be supported by a commitment to inter-professional learning and working. Second, multimodal care for cancer cachexia should be person centred and focus on supported self-management. Treatments to improve or maintain physical, psychological and social functioning often require behaviour change and should be tailored to individual and the surrounding context. The patient's family and social support network are often important, and including them in the promotion and monitoring of health behaviours, for example, preparing meals or being physically active, can be invaluable. In line with this patient reported outcomes also have a role in recognizing successful care [70]. Third, multimodal approaches can ask a lot of the patient and their family. When introducing new treatments it is helpful to remind patients that they are buying-in to an approach to care, rather than signing up to a strict, often challenging treatment regimen. Adherence is key, so new requests or treatments should always be considered and introduced in line with the whole approach.
Once multimodal care is operationalized it can then be tested pragmatically, either alone or alongside pharmacological treatments. Some centres have developed cachexia clinics and offer working models to build an evidence base for multimodal care. Emerging trials also show promise for pharmacological agents, for example, ghrelin receptor agonists, myostatin, SARMs [19,71▪▪]. There may soon be new therapies to prescribe for cancer cachexia, but arguably these should be offered on a background of good multimodal care [23▪,71▪▪]. To test this argument trials may be needed to monitor the combined effects of exercise and nutrition, and anti-inflammatory medicines, with or without cachexia drug therapies [20]. Such designs have increasing importance given the regulatory perspective on functional trial endpoints [19,72].
CONCLUSION
This case-based paper highlights practical ways to provide multimodal care for cancer cachexia. Across a range of clinical scenarios, we demonstrate that this can be achieved with simple treatments and without a dedicated team of specialists. As multimodal care is part of everyone's remit, the sharing of advice and experiences between health professionals can help build confidence and expertise, moving towards a position where every team member feels they can contribute towards cachexia care.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by an unrestricted educational grant by Chugai Pharma UK Limited. M.M. is supported by Cicely Saunders International. A.R. is a Macmillan Lung Cancer Clinical Nurse Specialist.
Conflicts of interest
M.M. and C.S. have received lecture fees from Fresenius Kabi. J.H. is a member of the Scientific Board for the Cachexia Hub, Helsinn Healthcare. KCHF has received lecture fees, consultancy fees, research support or attended advisory boards for Nutricia, Abbot, Fresenius Kabi, Lilly, Helsinn, Chugai, Ono, Novartis and Aveo Oncology. For the remaining authors none were declared.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011; 12:489–495. [DOI] [PubMed] [Google Scholar]
- 2.Tan BH, Fearon KC. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metabol Care 2008; 11:400–407. [DOI] [PubMed] [Google Scholar]
- 3.Maddocks M, Murton AJ, Wilcock A. Improving muscle mass and function in cachexia: nondrug approaches. Curr Opin Support Palliat Care 2011; 5:361–364. [DOI] [PubMed] [Google Scholar]
- 4.Maddocks M, Byrne A, Johnson CD, et al. Physical activity level as an outcome measure for use in cancer cachexia trials: a feasibility study. Support Care Cancer 2010; 18:1539–1544. [DOI] [PubMed] [Google Scholar]
- 5.Kazemi-Bajestani SM, Mazurak VC, Baracos V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin Cell Dev Biol 2016; 54:2–10. [DOI] [PubMed] [Google Scholar]
- 6.Tarricone R, Ricca G, Nyanzi-Wakholi B, Medina-Lara A. Impact of cancer anorexia-cachexia syndrome on health-related quality of life and resource utilisation: a systematic review. Crit Rev Oncol/Hematol 2016; 99:49–62. [DOI] [PubMed] [Google Scholar]
- 7.LeBlanc TW, Nipp RD, Rushing CN, et al. Correlation between the international consensus definition of the Cancer Anorexia-Cachexia Syndrome (CACS) and patient-centered outcomes in advanced nonsmall cell lung cancer. J Pain Symptom Manage 2015; 49:680–689. [DOI] [PubMed] [Google Scholar]
- 8.Martin L, Senesse P, Gioulbasanis I, et al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol 2015; 33:90–99. [DOI] [PubMed] [Google Scholar]
- 9.Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol 2013; 10:90–99. [DOI] [PubMed] [Google Scholar]
- 10.de Vos-Geelen J, Fearon KC, Schols AM. The energy balance in cancer cachexia revisited. Curr Opin Clin Nutr Metabol Care 2014; 17:509–514. [DOI] [PubMed] [Google Scholar]
- 11.Laviano A, Meguid MM, Inui A, et al. Therapy insight: cancer anorexia-cachexia syndrome – when all you can eat is yourself. Nat Clin Pract Oncol 2005; 2:158–165. [DOI] [PubMed] [Google Scholar]
- 12.Johns N, Stephens NA, Fearon KC. Muscle wasting in cancer. Int J Biochem Cell Biol 2013; 45:2215–2229. [DOI] [PubMed] [Google Scholar]
- 13.Johns N, Tan BH, MacMillan M, et al. Genetic basis of interindividual susceptibility to cancer cachexia: selection of potential candidate gene polymorphisms for association studies. J Genet 2014; 93:893–916. [DOI] [PubMed] [Google Scholar]
- 14.Aapro M, Arends J, Bozzetti F, et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann Oncol 2014; 25:1492–1499. [DOI] [PubMed] [Google Scholar]
- 15.Blum D, Omlin A, Baracos VE, et al. Cancer cachexia: a systematic literature review of items and domains associated with involuntary weight loss in cancer. Crit Rev Oncol/Hematol 2011; 80:114–144. [DOI] [PubMed] [Google Scholar]
- 16.Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer 2008; 44:1124–1132. [DOI] [PubMed] [Google Scholar]
- 17.Molfino A, Formiconi A, Rossi Fanelli F, Muscaritoli M. Cancer cachexia: towards integrated therapeutic interventions. Expert Opin Biol Therapy 2014; 14:1379–1381. [DOI] [PubMed] [Google Scholar]
- 18.Balstad TR, Kaasa S, Solheim TS. Multimodal nutrition/anabolic therapy for wasting conditions. Curr Opin Clin Nutr Metabol Care 2014; 17:226–235. [DOI] [PubMed] [Google Scholar]
- 19.Borg JJ, Anker SD, Rosano G, et al. Multimodal management as requirement for the clinical use of anticachexia drugs - a regulatory and a clinical perspective. Curr Opin Support Palliat Care 2015; 9:333–345. [DOI] [PubMed] [Google Scholar]
- 20.Fearon K. Cachexia: Treat wasting illness on multiple fronts. Nature 2016; 529:156. [DOI] [PubMed] [Google Scholar]
- 21.Hopkinson JB. The nursing contribution to nutritional care in cancer cachexia. Proc Nutr Soc 2015; 74:413–418. [DOI] [PubMed] [Google Scholar]
- 22.Solheim TS, Laird BJ. Evidence base for multimodal therapy in cachexia. Curr Opin Support Palliat Care 2012; 6:424–431. [DOI] [PubMed] [Google Scholar]
- 23▪.Del Fabbro E, Jatoi A, Davis M, et al. Health professionals’ attitudes toward the detection and management of cancer-related anorexia-cachexia syndrome, and a proposal for standardized assessment. J Community Support Oncol 2015; 13:181–187. [DOI] [PubMed] [Google Scholar]; Describes views and practice patterns of community oncologists and oncology nurses concerning the assessment and management of cancer cachexia.
- 24.Reid J, McKenna H, Fitzsimons D, McCance T. Fighting over food: patient and family understanding of cancer cachexia. Oncol Nurs Forum 2009; 36:439–445. [DOI] [PubMed] [Google Scholar]
- 25.Arends J, Bodoky G, Bozzetti F, et al. ESPEN guidelines on enteral nutrition: nonsurgical oncology. Clin Nutr 2006; 25:245–259. [DOI] [PubMed] [Google Scholar]
- 26▪.Hopkinson JB. Food connections: a qualitative exploratory study of weight- and eating-related distress in families affected by advanced cancer. Eur J Oncol Nurs 2016; 20:87–96. [DOI] [PubMed] [Google Scholar]; An in-depth qualitative study involving patient-family carer dyads flagging concerns of the individual patients and carers, but also with interactions between distressed family members affected by symptoms of cancer cachexia.
- 27.Baldwin C, Weekes CE. Dietary counselling with or without oral nutritional supplements in the management of malnourished patients: a systematic review and meta-analysis of randomised controlled trials. J Hum Nutr Diet 2012; 25:411–426. [DOI] [PubMed] [Google Scholar]
- 28.Ozcagli TG, Stelling J, Stanford J. A study in four European countries to examine the importance of sensory attributes of oral nutritional supplements on preference and likelihood of compliance. Turk J Gastroenterol 2013; 24:266–272. [PubMed] [Google Scholar]
- 29.Kaspar K, Herentrey K, Pradon E. Superior preference for a specific fruit-based oral nutritional supplement among older adults. Clin Nutr, Supplement 2012; 7:1744–2161. [Google Scholar]
- 30.Kennedy O, Law C, Methven L, et al. Investigating age-related changes in taste and affects on sensory perceptions of oral nutritional supplements. Age Ageing 2010; 39:733–738. [DOI] [PubMed] [Google Scholar]
- 31.Rahemtulla Z, Baldwin C, Spiro A, et al. The palatability of milk-based and nonmilk-based nutritional supplements in gastrointestinal cancer and the effect of chemotherapy. Clin Nutr 2005; 24:1029–1037. [DOI] [PubMed] [Google Scholar]
- 32.Hopkinson JB, Fenlon DR, Okamoto I, et al. The deliverability, acceptability, and perceived effect of the Macmillan approach to weight loss and eating difficulties: a phase II, cluster-randomized, exploratory trial of a psychosocial intervention for weight- and eating-related distress in people with advanced cancer. J Pain Symptom Manage 2010; 40:684–695. [DOI] [PubMed] [Google Scholar]
- 33.Granger CL. Physiotherapy management of lung cancer. J Physiother 2016; 62:60–67. [DOI] [PubMed] [Google Scholar]
- 34.Sebio Garcia R, Yanez Brage MI, Gimenez Moolhuyzen E, et al. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 2016; 23:486–497. [DOI] [PubMed] [Google Scholar]
- 35▪.Castelino T, Fiore JF, Jr, Niculiseanu P, et al. The effect of early mobilization protocols on postoperative outcomes following abdominal and thoracic surgery: a systematic review. Surgery 2016; 159:991–1003. [DOI] [PubMed] [Google Scholar]; A detailed review of early mobilization protocols following abdominal and thoracic surgery. Uncovers a need for further research to guide clinicians in effective early mobilization practice.
- 36.Slim K, Vignaud M. Enhanced recovery after surgery: the patient, the team, and the society. Anaesth Crit Care Pain Med 2015; 34:249–250. [DOI] [PubMed] [Google Scholar]
- 37.Rivas-Perez H, Nana-Sinkam P. Integrating pulmonary rehabilitation into the multidisciplinary management of lung cancer: a review. Respir Med 2015; 109:437–442. [DOI] [PubMed] [Google Scholar]
- 38.Cavalheri V, Tahirah F, Nonoyama M, et al. Exercise training for people following lung resection for nonsmall cell lung cancer - a Cochrane systematic review. Cancer Treat Rev 2014; 40:585–594. [DOI] [PubMed] [Google Scholar]
- 39.Cavalheri V, Jenkins S, Cecins N, et al. Patterns of sedentary behaviour and physical activity in people following curative intent treatment for nonsmall cell lung cancer. Chron Respir Dis 2016; 13:82–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Granger CL, McDonald CF, Irving L, et al. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer 2014; 83:292–299. [DOI] [PubMed] [Google Scholar]
- 41.Van BSM, Langius JAE, Smit EF, et al. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III nonsmall cell lung cancer during multimodality treatment. J Nutr 2010; 140:1774–1780. [DOI] [PubMed] [Google Scholar]
- 42.Hopkinson J, Corner J. Helping patients with advanced cancer live with concerns about eating: a challenge for palliative care professionals. J Pain Symptom Manage 2006; 31:293–305. [DOI] [PubMed] [Google Scholar]
- 43.Hopkinson JB. Psychosocial impact of cancer cachexia. J Cachexia Sarcopenia Muscle 2014; 5:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones LW, Eves ND, Haykowsky M, et al. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol 2009; 10:598–605. [DOI] [PubMed] [Google Scholar]
- 45▪▪.Xu YJ, Cheng JC, Lee JM, et al. A walk-and-eat intervention improves outcomes for patients with esophageal cancer undergoing neoadjuvant chemoradiotherapy. Oncologist 2015; 20:1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]; A nurse-led intervention using activity and nutritional advice to successfully preserve functional capacity and nutritional status in patients receiving chemoradiotherapy.
- 46.Grande AJ, Silva V, Riera R, et al. Exercise for cancer cachexia in adults. Cochrane Database Syst Rev 2014; Cd010804. [DOI] [PubMed] [Google Scholar]
- 47.Maddocks M, Jones LW, Wilcock A. Immunological and hormonal effects of exercise: implications for cancer cachexia. Curr Opin Support Palliat Care 2013; 7:376–382. [DOI] [PubMed] [Google Scholar]
- 48.Holland AE, Wadell K, Spruit MA. How to adapt the pulmonary rehabilitation programme to patients with chronic respiratory disease other than COPD. Eur Respir Rev 2013; 22:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spruit MA, Singh SJ, Garvey C, et al. An Official American Thoracic Society/European Respiratory Society Statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188:e13–e64. [DOI] [PubMed] [Google Scholar]
- 50▪.Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax 2016; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; A large prospective cohort study examining the Fried frailty phenotype in people with respiratory disease referred for pulmonary rehabilitation. Similar studies are underway in populations with cancer.
- 51.Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015; 70:213–218. [DOI] [PubMed] [Google Scholar]
- 52.Ferriolli E, Skipworth RJ, Hendry P, et al. Physical activity monitoring: a responsive and meaningful patient-centered outcome for surgery, chemotherapy, or radiotherapy? J Pain Symptom Manage 2012; 43:1025–1035. [DOI] [PubMed] [Google Scholar]
- 53.Maddocks M, Wilcock A. Exploring physical activity level in patients with thoracic cancer: implications for use as an outcome measure. Support Care Cancer 2012; 20:1113–1116. [DOI] [PubMed] [Google Scholar]
- 54.Berra K, Rippe J, Manson JE. Making physical activity counseling a priority in clinical practice: the time for action is now. JAMA 2015; 314:2617–2618. [DOI] [PubMed] [Google Scholar]
- 55.Bentley R, Hussain A, Maddocks M, Wilcock A. Occupational therapy needs of patients with thoracic cancer at the time of diagnosis: findings of a dedicated rehabilitation service. Support Care Cancer 2013; 21:1519–1524. [DOI] [PubMed] [Google Scholar]
- 56.Hutton JL, Martin L, Field CJ, et al. Dietary patterns in patients with advanced cancer: implications for anorexia-cachexia therapy. Am J Clin Nutr 2006; 84:1163–1170. [DOI] [PubMed] [Google Scholar]
- 57.Hopkinson JB. Carers’ influence on diets of people with advanced cancer. Nurs Times 2008; 104:28–29. [Google Scholar]
- 58.McClement SE, Degner LF, Harlos MS. Family beliefs regarding the nutritional care of a terminally ill relative: a qualitative study. J Palliat Med 2003; 6:737–748. [DOI] [PubMed] [Google Scholar]
- 59.Jones LW, Hornsby WE, Goetzinger A, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic nonsmall cell lung cancer. Lung Cancer 2012; 76:248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maddocks M, Taylor V, Klezlova R, et al. When will I get my breath back? Recovery time of exercise-induced breathlessness in patients with thoracic cancer. Lung Cancer 2012; 76:128–129. [DOI] [PubMed] [Google Scholar]
- 61.Oechsle K, Jensen W, Schmidt T, et al. Physical activity, quality of life, and the interest in physical exercise programs in patients undergoing palliative chemotherapy. Support Care Cancer 2011; 19:613–619. [DOI] [PubMed] [Google Scholar]
- 62.Maddocks M, Armstrong S, Wilcock A. Exercise as a supportive therapy in incurable cancer: exploring patient preferences. Psycho-Oncology 2011; 20:173–178. [DOI] [PubMed] [Google Scholar]
- 63.Rice HT, Malcolm L, Norman K, et al. An evaluation of the St Christopher's Hospice rehabilitation gym circuits classes: patient uptake, outcomes, and feedback. Progr Palliat Care 2014; doi: 10.1179/1743291X14Y.0000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wosahlo P, Maddocks M. Benchmarking the provision of palliative rehabilitation within the hospice setting. Palliat Med 2015; 29:477–478. [DOI] [PubMed] [Google Scholar]
- 65.Oldervoll LM, Loge JH, Lydersen S, et al. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. The Oncologist 2011; 16:1649–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reid J, McKenna HP, Fitzsimons D, McCance TV. An exploration of the experience of cancer cachexia: what patients and their families want from healthcare professionals. Eur J Cancer Care (Engl) 2010; 19:682–689. [DOI] [PubMed] [Google Scholar]
- 67.Chochinov HM, Hassard T, McClement S, et al. The landscape of distress in the terminally ill. J Pain Symptom Manage 2009; 38:641–649. [DOI] [PubMed] [Google Scholar]
- 68.Eva G, Wee B. Rehabilitation in end-of-life management. Curr Opin Support Palliat Care 2010; 4:158–162. [DOI] [PubMed] [Google Scholar]
- 69.Helgeson VS. Social support and quality of life. Quality Life Res 2003; 12 Suppl 1:25–31. [DOI] [PubMed] [Google Scholar]
- 70.Wheelwright SJ, Johnson CD. Patient-reported outcomes in cancer cachexia clinical trials. Curr Opin Support Palliat Care 2015; 9:325–332. [DOI] [PubMed] [Google Scholar]
- 71▪▪.Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with nonsmall-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol 2016; 17:519–531. [DOI] [PubMed] [Google Scholar]; A publication reporting results on two phase III trials of a novel cachexia drug for people with lung cancer. One of the first pharmacological agents to move beyond phase II testing.
- 72.Fearon K, Argiles JM, Baracos VE, et al. Request for regulatory guidance for cancer cachexia intervention trials. J Cachexia Sarcopenia Muscle 2015; 6:272–274. [DOI] [PMC free article] [PubMed] [Google Scholar]


