Abstract
Background: There is evidence of the benefits of exercise training in multiple sclerosis (MS); however, few studies have been conducted in individuals with progressive MS and severe mobility impairment. A potential exercise rehabilitation approach is total-body recumbent stepper training (TBRST). We evaluated the safety and participant-reported experience of TBRST in people with progressive MS and compared the efficacy of TBRST with that of body weight–supported treadmill training (BWSTT) on outcomes of function, fatigue, and health-related quality of life (HRQOL).
Methods: Twelve participants with progressive MS (Expanded Disability Status Scale scores, 6.0–8.0) were randomized to receive TBRST or BWSTT. Participants completed three weekly sessions (30 minutes) of exercise training for 12 weeks. Primary outcomes included safety assessed as adverse events and patient-reported exercise experience assessed as postexercise response and evaluation of exercise equipment. Secondary outcomes included the Multiple Sclerosis Functional Composite, the Modified Fatigue Impact Scale, and the Multiple Sclerosis Quality of Life–54 questionnaire scores. Assessments were conducted at baseline and after 12 weeks.
Results: Safety was confirmed in both exercise groups. Participants reported enjoying both exercise modalities; however, TBRST was reviewed more favorably. Both interventions reduced fatigue and improved HRQOL (P ≤ .05); there were no changes in function.
Conclusions: Both TBRST and BWSTT seem to be safe, well tolerated, and enjoyable for participants with progressive MS with severe disability. Both interventions may also be efficacious for reducing fatigue and improving HRQOL. TBRST should be further explored as an exercise rehabilitation tool for patients with progressive MS.
There is evidence of the benefits of exercise training for people with multiple sclerosis (MS).1–3 These benefits, however, have primarily been established in populations with mild-to-moderate disease severity and a relapsing-remitting disease course.1–3 People with progressive MS with severe mobility impairment have limited opportunities to engage in exercise training because most traditional modalities are physically inaccessible. An alternative exercise modality that has been used for participants with mobility impairment is body weight–supported treadmill training (BWSTT),4 which allows an individual with reduced mobility to walk on a treadmill while being partially supported by an overhead pulley system. Supported treadmill walking has been examined in people with advanced MS and has been found to be safe and well tolerated, with preliminary results suggesting improvements in mobility, fatigue, and health-related quality of life (HRQOL).5–10 Although BWSTT may have benefits for participants with limited mobility, this type of intervention is rarely available in community settings, requires highly trained personnel to operate, and is costly to initiate and maintain.4 Furthermore, improvements gained from BWSTT might deteriorate once training is discontinued; therefore, developing long-term, feasible, and accessible exercise for people with advanced MS is critical.
An alternative adapted exercise modality to BWSTT is total-body recumbent stepper training (TBRST), a recumbent cross-trainer that provides participants with mobility impairment the opportunity to participate in full-body exercise in a safe and physically accessible manner.4 Using TBRST, participants can exercise with both the upper and lower extremities through the use of coupled arm levers and foot pedals. Despite the potential of TBRST as an exercise rehabilitation tool, few studies have evaluated TBRST. Compared with nonexercising controls, 12 weeks of TBRST improved aerobic capacity, upper- and lower-body muscular strength and endurance, and body composition in sedentary healthy adults.11 Walking speed, muscular strength, and blood pressure improved after 13 weeks of TBRST (≥9 min/wk) in a group of elderly individuals in an assisted-living community.12 An improvement in balance and impairment was observed after 8 weeks of TBRST in a study of participants more than 1 year post-stroke.13 To date, no studies have examined TBRST in people with MS.
In an attempt to address the limitations of previous research, the primary goal of this trial was to determine the safety and participant-reported experience of TBRST in people with progressive MS with severe mobility impairment (Expanded Disability Status Scale [EDSS] scores, 6.0–8.0). The secondary aim was to determine the efficacy of TBRST compared with that of BWSTT on outcomes of functional ability, fatigue, and HRQOL. If TBRST is safe, well tolerated, and beneficial for people with advanced MS, this represents a potential long-term rehabilitation solution.
Methods
Participants
Twelve participants with progressive MS were recruited through a local MS clinic and MS society chapter. The inclusion criteria were as follows: 1) clinically definite primary progressive or secondary progressive MS; 2) an EDSS score of 6.0 to 8.0; 3) age 18 to 60 years; 4) body weight less than 90 kg; 5) physician approval for exercise training; and 6) ability to visit study locations. The exclusion criteria were as follows: 1) pregnancy or plans to become pregnant during the study period; 2) current use or use within the previous 2 months of disease-modifying therapies, including interferon beta, glatiramer acetate, intravenous corticosteroids, mitoxantrone, azathioprine, and cylophosphamide; 3) acquired disability that could interfere with the evaluation of disability due to MS; 4) other serious medical conditions that might impair the ability to walk on a treadmill or participate in exercise training; and 5) previous experience with BWSTT or TBRST.
Outcome Measures
Disability
Neurologic status was assessed through a clinically administered EDSS14 performed by a neurologist (CD). Scores on the EDSS were used to characterize the disability level of the sample and to monitor stability of neurologic function over the 12-week period.
Safety
Participants were asked to report on any adverse events (eg, muscle and joint pain, physical discomfort, and excessive fatigue) experienced during or after each training session.
Participant Experience of Exercise Training
Participants completed a questionnaire evaluating the physical and psychological postexercise response, enjoyment of equipment, equipment accessibility and safety, perceived benefit to daily functioning, and recommendation and use of the exercise equipment. Affective response to exercise was assessed using the single-item Feeling Scale,15 whereby participants indicated how they felt immediately after exercise on an 11-point scale ranging from −5 (very bad) to 5 (very good). Feeling states after exercise were evaluated using the 12-item Exercise-Induced Feeling Inventory,16 which is composed of four subscales (revitalization, tranquility, positive engagement, and physical exhaustion), each assessed by three items. Participants specified the extent to which each item described how they felt after exercise on a scale from 0 (do not feel) to 4 (feel very strongly). A mean score was generated for each subscale. Pain was evaluated using a three-item scale developed from the Brief Pain Inventory.17 Participants responded to, “When using the exercise equipment, how much ______ did you experience,” inserting each of the following three items: shoulder pain, bodily pain, and physical discomfort. Items were scored on a 7-point scale ranging from 1 (not at all) to 7 (a lot), and a mean score for the three items was generated. The Feeling Scale, Exercise-Induced Feeling Inventory, and Brief Pain Inventory have demonstrated good sensitivity and sound psychometric properties.18,19
Participant enjoyment of the training modality was assessed using a single item rating how much participants liked using the equipment on a scale from 1 (not at all) to 7 (a lot). The accessibility and safety of the equipment were each evaluated by single items assessing how confident the participant was in using the exercise equipment without assistance (accessibility) and without causing injury (safety). Both items were rated on a 7-point scale from 1 (not at all confident) to 7 (completely confident). Perceived benefit of the equipment to improve daily functioning was assessed using a single item rating how useful participants believed the equipment was in improving fitness to help perform activities of daily living. Perceived benefit to daily functioning was rated on a scale from 1 (not at all) to 7 (very much). Recommendation and use of equipment was assessed with three single-item scales. Participants indicated whether they would recommend that fitness facilities purchase the exercise equipment on a scale from 1 (definitely not) to 7 (definitely yes). Participants indicated their anticipated use of the equipment if it were made available at an exercise facility they attended on a scale from 1 (never) to 7 (always). Finally, participants specified the amount of time (<5, 5, 10, 15, 20, 25, or 30 minutes or other) they imagined themselves using the exercise equipment (TBRST or BWSTT), assuming high motivation and fitness, in one exercise session without stopping.
Functional Ability
Functional ability was assessed using the Multiple Sclerosis Functional Composite (MSFC).20 The MSFC assesses lower-extremity, upper-extremity, and cognitive functioning using three scales: the Timed 25-Foot Walk test, the Nine-Hole Peg Test, and the Paced Auditory Serial Addition Test, respectively. Pre-baseline administration of the MSFC was conducted to minimize practice effects.21 The MSFC was scored according to standardized instruction.20 Z scores were calculated for each MSFC item by standardizing or comparing the test values with a reference population using mean baseline values from the overall study sample as the reference population. A composite Z score was then calculated as a mean Z score for the three individual scales. Z scores are expressed in units of standard deviation.
Fatigue
Fatigue was assessed using the 21-item Modified Fatigue Impact Scale,22,23 which assesses overall fatigue as well as physical, cognitive, and psychosocial fatigue. Each item assesses the impact of fatigue on daily activities and functioning and is scored from 0 (never) to 4 (always). The total score is computed by summing the responses on all 21 items and ranges from 0 to 84. Physical subscale scores range from 0 to 36, cognitive subscale scores range from 0 to 40, and psychosocial subscale scores range from 0 to 8. Higher total and subscale scores indicate a greater impact of fatigue. The reliability and validity of the Modified Fatigue Impact Scale have been established.22
Health-Related Quality of Life
HRQOL was assessed using the Multiple Sclerosis Quality of Life–54 questionnaire, which consists of 12 multi-item scales, 2 single-item scales, and 2 composite scores (physical and mental health).24 The individual scales and composite scores range from 0 to 100, where higher scores indicate better quality of life. This questionnaire has shown good reliability and validity.25
Interventions
TBRST
Participants completed the TBRST protocol using the NuStep T4/TRS 4000 (NuStep Inc, Ann Arbor, MI), which is a recumbent cross-trainer that allows for upper- and lower-body exercise in a seated position. A photograph and detailed description of the recumbent stepper setup is published elsewhere.4 The recumbent stepper allows participants to achieve a natural stepping motion against graded loads created by a magnetic resistance system. Arm levers and foot pedals are coupled and move in a bilateral reciprocal manner. Foot straps and leg stabilizers were used for added control and proper leg alignment when necessary. One trainer assisted participants with transferring and equipment setup as required.
BWSTT
Participants underwent BWSTT using the Woodway Loko system (Woodway USA Inc, Wakesha, WI), which consists of a treadmill with an overhead pulley system connected to a supportive harness. A photograph and description of BWSTT has previously been published.4 The BWSTT allows participants with limited mobility to walk upright on a treadmill with a portion of their body weight counterbalanced. The BWSTT protocol was based on previous trials conducted by our research group in people with spinal cord injury and is appropriate for use in advanced MS.26,27 Following these parameters, the percentage of body weight supported was prescribed on an individual basis. Treadmill training was therapist assisted, with one trainer positioned at either lower limb to promote proper gait mechanics. When necessary, an additional trainer stood behind the participant to assist with weight shifting and trunk stability.
Procedures
All the procedures were approved by a research ethics board, and participants provided written informed consent. After enrollment, participants underwent a neurologic examination and completed functional, fatigue, and HRQOL measures. Due to the heterogeneity of the population and small sample, we did not attempt a matched design but rather randomized participants who met the inclusion criteria to receive one of two exercise interventions: TBRST or BWSTT. Randomization was conducted after baseline assessment and was determined by a computer-generated randomization program. After group assignment, participants completed three weekly sessions of BWSTT or TBRST for 12 weeks. Participants completed a pre-training familiarization session. The duration of each session was gradually increased according to participant ability and comfort up to a maximum of 30 minutes. To ensure that the intensity of exercise was similar between groups, rating of perceived exertion (RPE) was used as an indicator of effort using the Borg CR10 scale.28 Throughout the training program, participants were instructed to exercise at a perceived exertion of 3 to 5, which corresponded to a rating of moderate to strong on the 10-point scale. Exercise training heart rate was recorded (Polar Electro Oy, Kempele, Finland) at each training session. Safety (ie, adverse events) was evaluated at each training session. Exercise progression in both groups was based on perceived exertion, comfort, and participant progress. After completion of the training program, participants completed all of the same measures as at baseline and the experience of exercise training questionnaire.
Data Analysis
Demographic and clinical characteristics of participants were summarized using descriptive statistics. Values are presented in the text as mean (SD), unless otherwise noted. Baseline characteristics were compared between groups using independent-samples t tests and χ2 tests. Differences between groups in exercise adherence were determined using independent-samples t tests and Mann-Whitney tests. Adverse events were summarized for each training group. Participant evaluations of exercise training data were compared between groups using independent-samples t tests and Mann-Whitney tests. Effect sizes (ESs), expressed as Cohen's d, were used to compare differences in the evaluation of exercise training between groups. Between-group comparisons of exercise training parameters, functional ability, fatigue, and HRQOL were analyzed using a series of mixed-model analyses of variance, with time (baseline vs. 12 weeks) as the within-subjects factor and group (TBRST vs. BWSTT) as the between-subjects factor. Data that were not normally distributed were square root transformed. The ESs were used to evaluate the magnitude of change in outcome measures in each exercise intervention. The ESs have been used in previous physical activity and exercise training interventions in people with MS to express the magnitude of differences within and between groups after the interventions.5,29–32 The ESs were interpreted as small, moderate, and large based on criteria of 0.2, 0.5, and 0.8, respectively.33 Statistical significance was set at P ≤ .05.
Results
Participants
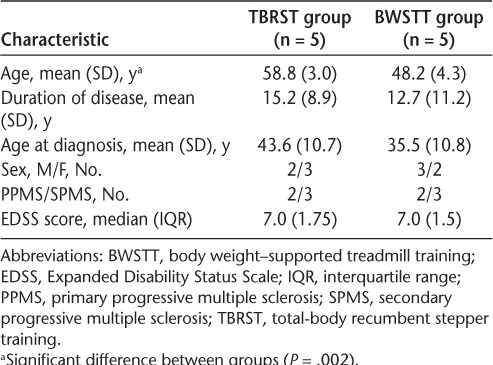
Twelve participants were enrolled in this study: six in the TBRST group (median [interquartile range] EDSS score, 7.0 [1.625]) and six in the BWSTT group (median [interquartile range] EDSS score, 6.75 [1.625]). Each group included three men and three women, two with primary progressive MS and four with secondary progressive MS. One participant dropped out of the BWSTT group after 17 training sessions. One participant in the TBRST group acquired an injury unrelated to exercise training after 34 sessions. Five participants in each group completed the intervention and follow-up (ie, 12-week) testing. Characteristics of the participants who completed the intervention are presented in Table 1. There were no differences between groups in baseline characteristics (all P > .05) except for age. Participants in the TBRST group were significantly older than those in the BWSTT group (P = .002); accordingly, age was used as a covariate in comparisons of intervention effects between groups. Overall, EDSS scores remained stable in both groups throughout the intervention (P < .05); only one individual in the TBRST group had a change in EDSS score, which increased by 0.5 at 12 weeks.
Table 1.
Participant characteristics
Exercise Training
Training Adherence
Participants in the TBRST group completed 33.2 (3.8) of a possible 36 training sessions with adherence of 89.1% (6.6%). Participants in the BWSTT group completed 35.6 (0.9) training sessions with adherence of 89.2% (10.4%). Adherence was defined as the percentage of available sessions attended. There was no significant difference between groups in program adherence.
Training Parameters
Participants in the TBRST group significantly increased mean training workload over the duration of the intervention from level 2.4 (1.2) to 4.9 (1.4) (P < .001) (Figure 1A). There was a nonsignificant increase in total steps per training session from 1753.1 (121.6) at baseline to 2043.4 (268.5) at 12 weeks (P = .13). Stepping cadence was consistent across the training program and ranged from 62.8 (4.8) to 68.2 (9.8) steps/min (P = .20). Participants in the BWSTT group significantly reduced the amount of body weight support required to walk on the treadmill from 72.2% (19.6%) at baseline to 43.1% (18.1%) at 12 weeks (P < .001) (Figure 1B). Treadmill walking distance increased significantly from 0.4 (0.2) km at baseline to 0.8 (0.3) km at 12 weeks (P < .001). Treadmill walking speed also increased significantly over the 12 weeks, from 0.9 (0.3) to 1.6 (0.6) km/hour (P = .001).
Figure 1.
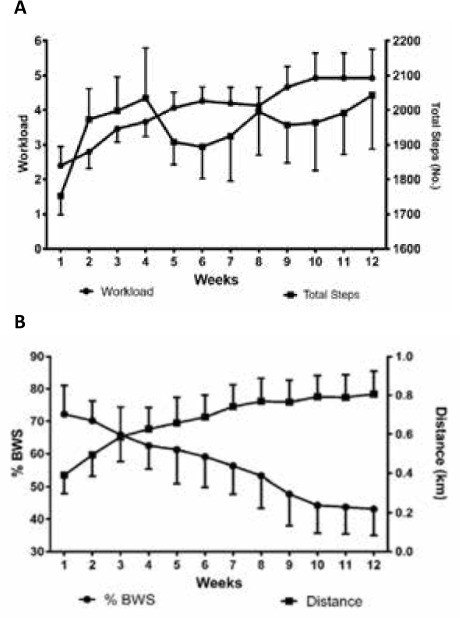
Changes in training parameters over 12 weeks of total-body recumbent stepper training (A) and body weight–supported (BWS) treadmill training (B)
Values are given as mean (SEM).
Exercise Intensity
Overall, mean weekly RPE ranged from 3.8 to 4.6 in the TBRST group and from 2.8 to 4.5 in the BWSTT group across the 12-week intervention. There was no significant difference in perceived exertion between groups (P = .63), and RPE did not change during the 12 weeks (P = .12) (Figure 2A). The difference in average training heart rate (HR) between the exercise modalities was compared relatively as a percentage of age-predicted maximum HR (ie, 220 – age). Average training HR expressed as a percentage of age-predicted maximum was stable across the interventions over time (P = .31); however, significantly higher training HRs were recorded in the TBRST group compared with the BWSTT group (P = .01) (Figure 2B).
Figure 2.
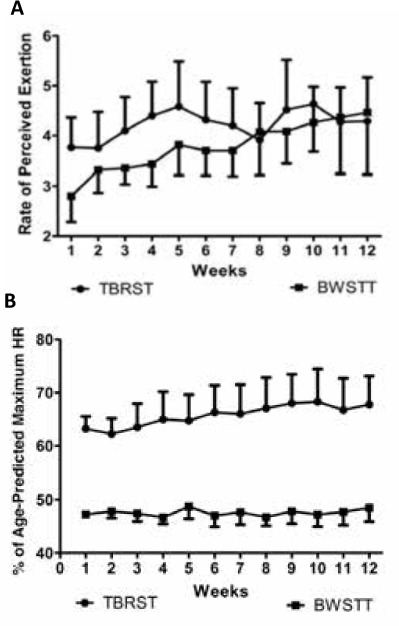
Changes in rate of perceived exertion (A) and average training heart rate (HR) as a percentage of age-predicted maximum HR (B) over 12 weeks of total-body recumbent stepper training (TBRST) and body weight–supported treadmill training (BWSTT)
Values are given as mean (SEM).
Outcomes
Safety
Adverse events in either group occurred rarely and were mild in severity. Adverse events (physical discomfort, muscle pain, and excessive fatigue) were reported by one of the five participants (on two separate occasions) in the TBRST group. Adverse events (physical discomfort, minor bruising, joint pain, and excessive fatigue) were reported by four of the five participants (on five separate occasions) in the BWSTT group.
Participant Experience of Exercise Training
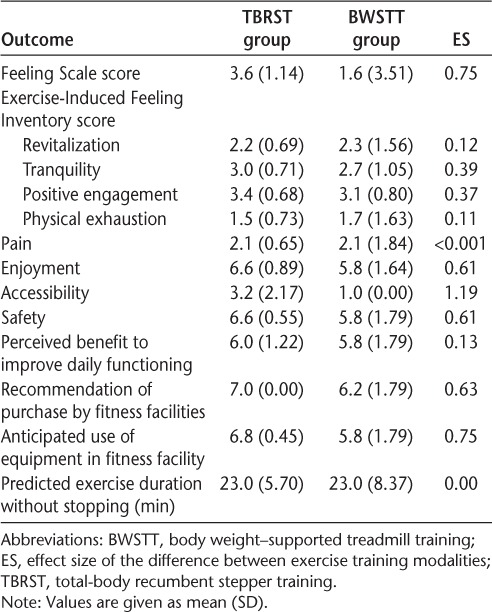
There were no significant differences between groups in participant experience of exercise training (all P > .05) (Table 2). Based on ESs, TBRST was more favorably rated by participants than was BWSTT. A large ES was determined for the difference between groups on the Feeling Scale; the TBRST group reported feeling better after exercise training than the BWSTT group. Moderate-to-large ESs were observed for equipment enjoyment, accessibility, safety, recommendation of equipment purchase by fitness facilities, and anticipated use of the equipment by participants in a fitness facility, all in favor of TBRST over BWSTT.
Table 2.
Participant experience of TBRST and BWSTT
Functional Ability
There were no significant effects on functional ability measures (all P > .05), and ESs further reflect a lack of change in MSFC scores (Table 3).
Table 3.
Functional, fatigue, and quality of life outcomes at baseline and after 12 weeks of TBRST and BWSTT
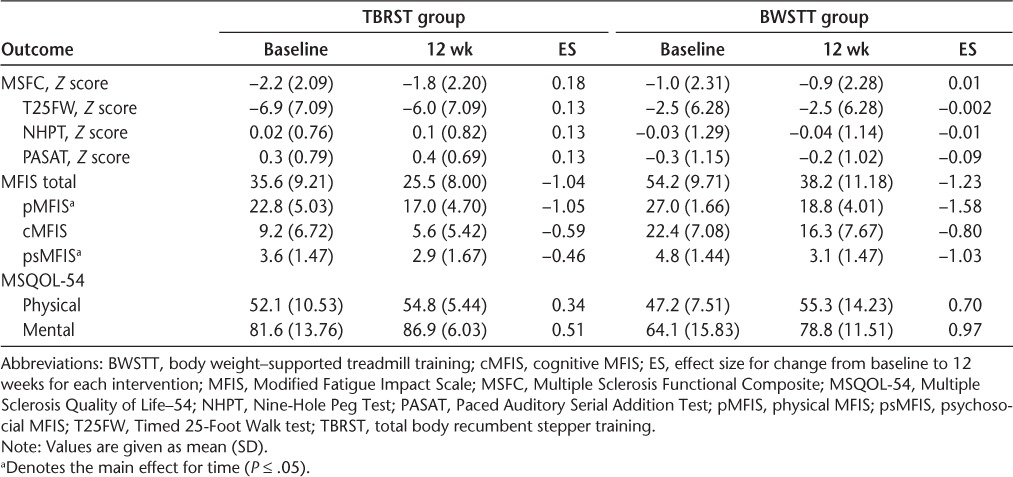
Fatigue
There was a significant effect of time on physical (P = .04) and psychosocial (P = .01) fatigue subscales; overall, participants perceived less fatigue after exercise training (Table 3). There were no significant effects of the intervention on the total or cognitive fatigue scores (all P > .05). There was a large effect of TBRST on total and physical fatigue, and a moderate effect on cognitive fatigue. With respect to BWSTT, there was a large effect for training on total and all fatigue subscales. This finding suggests that TBRST and BWSTT are efficacious for reducing fatigue in people with advanced MS.
Health-Related Quality of Life
There were no significant intervention effects on HRQOL outcomes (all P > .05) (Table 3). There was a moderate positive effect for TBRST on mental HRQOL, and a small positive effect of TBRST on physical HRQOL. There was a large positive effect of BWSTT on mental HRQOL and a moderate-to-large positive effect of BWSTT on physical HRQOL. Overall, both TBRST and BWSTT might be efficacious for improving physical and, to a greater extent, mental HRQOL in people with advanced MS. Considering the potentially positive impact of the interventions on HRQOL, we collapsed the effect of exercise training (ie, the TBRST and BWSTT groups combined) on HRQOL and calculated an overall effect for the physical (d = .56) and mental (d = .66) health composite scores. Using these values, we determined that a minimum overall sample of 22 (mental) to 28 (physical) participants would be necessary to detect a condition × time interaction (intraclass correlation coefficient = 0.50, α = .05, β = .20, ϵ = 1.0) of adapted exercise training on HRQOL in patients with severe progressive MS.
Discussion
To our knowledge, this is the first trial to evaluate the efficacy of recumbent stepper training in participants with MS and to compare recumbent stepper training with supported treadmill walking. Overall, we determined that TBRST is a safe and well-tolerated exercise training modality for people with progressive MS with severe mobility impairment. Second, TBRST and BWSTT are both beneficial for improving fatigue and HRQOL in this population. The results of this preliminary study suggest that TBRST is a feasible exercise training modality for people with MS with severe mobility impairment.
Importantly, TBRST was well tolerated, with few adverse events reported by participants. In both groups, most adverse events occurred early in the training program, when participants were likely adjusting to the increase in activity and the specialized exercise equipment. When adverse events were reported, participants were often unsure whether the symptoms were directly related to exercise participation or were due to other health issues. Adverse events were reported by more participants in the BWSTT group, likely due to the specialized equipment, particularly the supportive harness, causing minor discomfort and pain. With respect to participant experience of exercise training, there was no difference in participants' evaluation of the two training modalities. The ESs of the difference between groups reflected a more favorable experience of recumbent stepper training. A preference for recumbent stepper training over traditional aerobic exercise modalities was similarly reported by a group of elderly individuals.34 It is likely that participants felt that the accessibility and simplicity of the recumbent stepper, compared with the supported treadmill, made it more safe, easy, and enjoyable to use. This suggests that TBRST is a safe, well-tolerated, and enjoyable form of exercise training for people with advanced MS and represents an important first step in evaluating the potential of TBRST as an adapted rehabilitation tool in this population.
Interestingly, participants with progressive MS were able to achieve higher training HRs while engaging in recumbent stepping compared with BWSTT despite a similar perception of effort (ie, RPE). Other researchers have compared the cardiorespiratory responses of six women with MS with gait impairment (mean EDSS score, 4.6) using multiple exercise modalities (ie, treadmill walking, leg cycling, combined arm and leg cycling, and TBRST).35 Similar to the present findings, peak HR and peak oxygen consumption were higher with recumbent stepping compared with treadmill exercise. In the present study, recumbent stepping likely provided a greater cardiovascular challenge by engaging a larger muscle mass through the combined use of upper- and lower-body exercise. Furthermore, a 12-week TBRST intervention was found to significantly improve maximal oxygen consumption assessed by recumbent stepping and treadmill testing protocols in healthy sedentary adults compared with nonexercising controls.11 Future trials should examine the effect of TBRST and BWSTT on cardiorespiratory fitness to determine whether TBRST is superior to BWSTT in people with MS. This would have important implications for improving physical fitness and, consequently, daily functioning in people with advanced MS.
Regarding secondary outcomes, both TBRST and BWSTT improved fatigue and HRQOL. These results are consistent with previous studies that have reported improvements in fatigue and HRQOL in MS after participation in exercise training in general and BWSTT specifically.8,9,36 We further extend previous results by demonstrating improvements in these outcomes in response to TBRST. These initial findings are promising and should be replicated in a large-scale randomized controlled trial design. We did not observe an improvement in functional outcomes in this study. Similarly, previous work by our research group did not determine significant changes in functional outcomes after 12 weeks of BWSTT in participants with progressive MS with severe disability; however, there was a small positive effect on MSFC scores.9 Previous research also supports the beneficial effects of BWSTT for improving mobility in participants with MS.5–7 A factor that may have contributed to inconsistency in results is the high level of neurologic impairment of participants in the present study compared with previous trials. Of note, we did not observe a decline in function over 12 weeks in this study. We did not include a nonexercise condition and, therefore, cannot assume that a group receiving no treatment would not have deteriorated in function over the same period.
Limitations
The results of this study are primarily limited by the small sample of participants, the heterogeneity of the sample, and the short duration of the training intervention. The use of RPE is limited as an indirect measure of exercise intensity, although this method has been validated in previous studies in patients with spinal cord injury.37 Given that there are so few studies of exercise interventions in people with MS with such high disability, the findings from this preliminary study are novel and promising.
Conclusion
This is the first trial to examine the effects of TBRST in participants with advanced MS. Findings from this preliminary investigation suggest that TBRST is safe and well tolerated by people with progressive MS with severe disability. The experience of TBRST was reviewed more favorably by participants than the experience of BWSTT. TBRST is similarly efficacious as BWSTT for improving the outcomes of fatigue and HRQOL. Interestingly, TBRST was a more challenging cardiovascular training modality than BWSTT. Overall, TBRST represents a viable, cost-effective alternative to BWSTT and should be considered as an exercise rehabilitation modality for participants with advanced MS.
PracticePoints
Little is known about the benefits of exercise training in patients with progressive MS.
We examined 12 weeks of recumbent stepper training in patients with progressive MS with severe disability.
In patients with progressive MS, recumbent stepper training was safe and enjoyable, reduced fatigue, and improved quality of life.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
References
- 1. Motl RW, Pilutti LA. The benefits of exercise training in multiple sclerosis. Nat Rev Neurol. 2012; 8: 487– 497. [DOI] [PubMed] [Google Scholar]
- 2. Latimer-Cheung AE, Pilutti LA, Hicks AL, et al. The effects of exercise training on fitness, mobility, fatigue, and health related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil. 2013; 94: 1800– 1828. [DOI] [PubMed] [Google Scholar]
- 3. Dalgas U, Ingemann-Hansen T, Stenager E. Physical exercise and MS recommendations. Int MS J MS Forum. 2009; 16: 5– 11. [PubMed] [Google Scholar]
- 4. Pilutti LA, Hicks AL. Rehabilitation of ambulatory limitations. Phys Med Rehabil Clin N Am. 2013; 24: 277– 290. [DOI] [PubMed] [Google Scholar]
- 5. Beer S, Aschbacher B, Manoglou D, Gamper E, Kool J, Kesselring J. Robot-assisted gait training in multiple sclerosis: a pilot randomized trial. Mult Scler. 2008; 14: 231– 236. [DOI] [PubMed] [Google Scholar]
- 6. Giesser B, Beres-Jones J, Budovitch A, Herlihy E, Harkema S. Locomotor training using body weight support on a treadmill improves mobility in persons with multiple sclerosis: a pilot study. Mult Scler. 2007; 13: 224– 231. [DOI] [PubMed] [Google Scholar]
- 7. Lo AC, Triche EW. Improving gait in multiple sclerosis using robot-assisted, body weight supported treadmill training. Neurorehabil Neural Repair. 2008; 22: 661– 671. [DOI] [PubMed] [Google Scholar]
- 8. Wier LM, Hatcher MS, Triche EW, Lo AC. Effect of robot-assisted versus conventional body-weight-supported treadmill training on quality of life for people with multiple sclerosis. J Rehabil Res Dev. 2011; 48: 483– 492. [DOI] [PubMed] [Google Scholar]
- 9. Pilutti LA, Lelli DA, Paulseth JE, et al. Effects of 12 weeks of supported treadmill training on functional ability and quality of life in progressive multiple sclerosis: a pilot study. Arch Phys Med Rehabil. 2011; 92: 31– 36. [DOI] [PubMed] [Google Scholar]
- 10. Campbell E, Coulter EH, Mattison PG, Miller L, McFadyen A, Paul L. Physiotherapy rehabilitation for people with progressive multiple sclerosis: a systematic review. Arch Phys Med Rehabil. 2016; 97: 141– 151.e3. [DOI] [PubMed] [Google Scholar]
- 11. Hass CJ, Garzarella L, de Hoyos DV, Connaughton DP, Pollock ML. Concurrent improvements in cardiorespiratory and muscle fitness in response to total body recumbent stepping in humans. Eur J Appl Physiol. 2001; 85: 157– 163. [DOI] [PubMed] [Google Scholar]
- 12. Johnson TR, McPhee SD, Dietrich MS. Effects of recumbent stepper exercise on blood pressure, strength and mobility in residents of assisted living communities: a pilot study. Phys Occup Ther Geriatr. 2002; 21: 27– 40. [Google Scholar]
- 13. Page SJ, Levine P, Teepen J, Hartman EC. Resistance-based, reciprocal upper and lower limb locomotor training in chronic stroke: a randomized, controlled crossover study. Clin Rehabil. 2008; 22: 610– 617. [DOI] [PubMed] [Google Scholar]
- 14. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983; 33: 1444– 1452. [DOI] [PubMed] [Google Scholar]
- 15. Hardy CJ, Rejeski WJ. Not what but how one feels: the measurement of affect during exercise. J Sport Exerc Psychol. 1989; 11: 304– 317. [Google Scholar]
- 16. Gauvin L, Rejeski WJ. The Exercise-Induced Feeling Inventory: development and initial validation. J Sport Exerc Psychol. 1993; 15: 403– 423. [Google Scholar]
- 17. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994; 23: 129– 138. [PubMed] [Google Scholar]
- 18. Gauvin L, Rejeski WJ, Reboussin BA. Contributions of acute bouts of vigorous physical activity to explaining diurnal variations in feeling states in active, middle-aged women. Health Psychol. 2000; 19: 365– 375. [DOI] [PubMed] [Google Scholar]
- 19. Martin Ginis KA, Latimer AE. The effects of single bouts of body-weight supported treadmill training on the feeling states of people with spinal cord injury. Spinal Cord. 2007; 45: 112– 115. [DOI] [PubMed] [Google Scholar]
- 20. Fisher JS, Jak AJ, Knicker JE, Rudick RA, Cutter G. Multiple Sclerosis Functional Composite (MSFC): Administration and Scoring Manual. New York, NY: National Multiple Sclerosis Society; 2001. [Google Scholar]
- 21. Solari A, Radice D, Manneschi L, Motti L, Montanari E. The Multiple Sclerosis Functional Composite: different practice effects in the three test components. J Neurol Sci. 2005; 228: 71– 74. [DOI] [PubMed] [Google Scholar]
- 22. Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the Fatigue Impact Scale. Clin Infect Dis. 1994; 18: S79– S83. [DOI] [PubMed] [Google Scholar]
- 23. Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci. 1994; 21: 9– 14. [PubMed] [Google Scholar]
- 24. Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995; 4: 187– 206. [DOI] [PubMed] [Google Scholar]
- 25. Miller A, Dishon S. Health-related quality of life in multiple sclerosis: psychometric analysis of inventories. Mult Scler. 2005; 11: 450– 458. [DOI] [PubMed] [Google Scholar]
- 26. Hicks AL, Adams MM, Martin Ginis K, et al. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord. 2005; 43: 291– 298. [DOI] [PubMed] [Google Scholar]
- 27. Giangregorio LM, Hicks AL, Webber CE, et al. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord. 2005; 43: 649– 657. [DOI] [PubMed] [Google Scholar]
- 28. Borg G. The Borg CR10 Scale® Folder: A Method for Measuring Intensity of Experience. Hasselby, Sweden: Borg Perception; 2004. [Google Scholar]
- 29. Pilutti L, Dlugonski D, Sandroff B, Klaren R, Motl R. Randomized controlled trial of a behavioral intervention targeting symptoms and physical activity in multiple sclerosis. Mult Scler J. 2014; 5: 594– 601. [DOI] [PubMed] [Google Scholar]
- 30. Romberg A, Virtanen A, Ruutiainen J. Long-term exercise improves functional impairment but not quality of life in multiple sclerosis. J Neurol. 2005; 252: 839– 845. [DOI] [PubMed] [Google Scholar]
- 31. Motl RW, Dlugonski D, Wójcicki TR, McAuley E, Mohr DC. Internet intervention for increasing physical activity in persons with multiple sclerosis. Mult Scler. 2011; 17: 116– 128. [DOI] [PubMed] [Google Scholar]
- 32. Rampello A, Franceschini M, Piepoli M, et al. Effect of aerobic training on walking capacity and maximal exercise tolerance in patients with multiple sclerosis: a randomized crossover controlled study. Phys Ther. 2007; 87: 545– 559. [DOI] [PubMed] [Google Scholar]
- 33. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 34. Looney MA, Rimmer JH. Aerobic exercise equipment preferences among older adults: a preliminary investigation. J Appl Meas. 2003; 4: 43– 58. [PubMed] [Google Scholar]
- 35. Gappmaier E, Estes H, Davis SL. Cardio-respiratory responses to maximal exercise of persons with MS using different modes of exercise. Med Sci Sports Exerc. 2001; 33: S130. [Google Scholar]
- 36. Rasova K, Havrdova E, Brandejsky P, Zalisova M, Foubikova B, Martinkova P. Comparison of the influence of different rehabilitation programmes on clinical, spirometric and spiroergometric parameters in patients with multiple sclerosis. Mult Scler. 2006; 12: 227– 234. [DOI] [PubMed] [Google Scholar]
- 37. Pelletier CA, Ditor DS, Latimer-Cheung AE, Warburton DE, Hicks AL. Exercise equipment preferences among adults with spinal cord injury. Spinal Cord. 2014; 52: 874– 879. [DOI] [PubMed] [Google Scholar]


