Abstract
Background: Footdrop is a significant problem in multiple sclerosis, reducing the safety and efficiency of walking. Functional electrical stimulation (FES) can produce dorsiflexion, correcting footdrop. The purpose of this retrospective analysis of clinical study data was to compare the effect of external and implanted FES devices for the correction of footdrop.
Methods: External FES was used for a minimum of 6 months before implantation. Walking performance was assessed using 10-m walking speed, 3-minute walking distance, the Physiological Cost Index, and health- and device-related quality of life and device-use questionnaires. Assessments were made before implantation and a mean (SD) of 128 (24) days after surgery, with additional walking speed measurements at 3 years.
Results: Twenty-three people with multiple sclerosis received the STIMuSTEP implant. Both devices enabled statistically significant increases in walking speed and walking distance, with a strong trend toward a reduced Physiological Cost Index, indicating that walking required less effort (P = .07). Both devices improved device-related quality of life. Walking speed gain with FES was maintained at 3 years. Three implants failed after falls, and there was one case of neuropraxia. The implant was used more days per week and was quicker to put on each day than the external FES device.
Conclusions: The STIMuSTEP implanted dropped foot stimulator is an effective long-term intervention for the correction of footdrop.
Multiple sclerosis (MS) affects approximately 127,000 people in the United Kingdom.1 Approximately 31% of the total MS population has walking difficulty, defined by an Expanded Disability Status Scale score of 4 to 7, with the most common walking problem being footdrop.2 Although traditionally an ankle-foot orthosis has been worn to correct footdrop, functional electrical stimulation (FES) is increasingly being used. In these systems, dorsiflexion is produced by stimulation of the common peroneal nerve, timed to the gait cycle using a footswitch placed in the shoe3 or a tilt sensor mounted on the lower leg.4 Skin surface electrodes are placed over the nerve as it passes over the head of the fibula or the popliteal fossa and over the tibialis anterior muscle. Studies have shown that FES can cause improved mobility, demonstrated by improved walking speed, reduced walking effort, and reduced incidence of falls, leading to improved quality of life in people with MS and other conditions.3–7 Functional electrical stimulation is a practical long-term intervention that has been demonstrated to be cost-effective.8 It may have some advantages over the use of an ankle-foot orthosis due to greater ground clearance and reduced walking effort.9,10
Although adherence to FES treatment is high,8,11 for a few patients, external FES systems can have disadvantages, which may limit their use. First, it is necessary to correctly place the electrodes each day. This may be mitigated to some extent by mounting electrodes on a cuff worn around the leg. However, clinical experience shows that not all FES users correctly place the cuff, misaligning the electrodes with the nerve. Placing electrodes correctly is also more challenging for people who have reduced hand function. A further problem can be skin irritation. The reported occurrence of skin irritation varies, with one study reporting that 48.5% of device users experienced irritation over a 42-week period,12 while another study reported a prevalence of 2.4% at all follow-up clinic appointments.11 Skin irritation can usually be managed by changing electrode type, adjusting stimulation parameters, or limiting the hours per day or days per week that FES is used. However, effective management is not always possible, and around 1% of FES users discontinue use due to persistent skin irritation.8 Finally, setting up external electrodes each day is a burden that some FES users may want to diminish. In a survey sent to 140 regular FES users, 66% reported that they would consider an implanted FES device.13
The STIMuSTEP implanted dropped foot stimulator was developed by the University of Twente (Enschede, the Netherlands) and Finetech Medical Ltd (Welwyn Garden City, UK).14–19 Two channels of stimulation are used to stimulate the two branches of the common peroneal nerve. The deep branch produces dorsiflexion and inversion, and the superficial branch produces eversion and plantarflexion of the foot. By adjusting the relative proportions of stimulation to each nerve, the movement of the foot can be controlled. The device consists of a passive receiver that receives stimulation pulses from an external controller strapped to the leg over the receiver, via close-coupled radio telemetry. The device uses epineural electrodes (a 9 × 2.75 × 0.8-mm assembly with a 1-mm diameter separated by 5 mm). The device is controlled using a pressure-sensitive footswitch placed in the shoe.
This article reports our experience in providing the STIMuSTEP implant as a clinical service in the United Kingdom's National Health Service.
Methods
This study is a retrospective analysis of clinical data from all people with MS referred to the FES service for the STIMuSTEP implant between 2006 and 2013. The selection criteria for STIMuSTEP use were the same as those for external FES use8: single or bilateral footdrop due to MS defined as a deficit of dorsiflexion and/or eversion of the ankle and the ability to stand from sitting and walk at least 10 m with appropriate aids but without assistance from another person. Contraindications to external FES were poorly controlled epilepsy, the presence of active implanted devices, pregnancy, and cancer in proximity to the area of the electrodes. An additional selection criterion for the STIMuSTEP was that external FES had been used for a minimum of 6 months. This was to ensure that FES provided useful assistance with walking. It also allowed those who had sufficient benefit from external FES to avoid an unnecessary surgical procedure. However, one study participant used external FES for only 3 months because although effective footdrop correction was demonstrated, poor hand function prevented the effective daily application of electrodes and, hence, the trial of external FES was stopped early. Further additional contraindications were diabetes, immunosuppressive drug use, and any standard medical factor that increased the risk of surgery under general anesthesia.
The procedure was performed in day surgery under general anesthesia by a plastic surgeon (J Hobby, MSK, or DEMS-S). Surgery took 45 to 90 minutes and was performed using loupe magnification. The common peroneal nerve was exposed via a 6-cm incision at the head of the fibula. An intraoperative stimulation probe was used to identify the branches of the nerve by observing the movement of the foot. Multiple locations around the nerve were tested until sites that produced dorsiflexion without excessive inversion/toe extension and eversion without excessive plantarflexion were found. Microscissors were used to make an incision in the epineurium, and a subepineural pocket was created. The electrode was slid into the pocket and held in place by polypropylene 6.0 sutures to the epineurium at the neck of the electrode and tags, 10 mm from the electrode. A subcutaneous pocket was created posterior and slightly inferior to the fibula head, into which the implant receiver was sutured in place. Before closure, the response was tested using the STIMuSTEP controller. The relative proportion of stimulation to the two nerve branches was adjusted until dorsiflexion with moderate eversion was produced. The response was tested with the knee in both flexion and extension to ensure that a consistent response was obtained.
After wound closure, the site was covered using a surgical dressing, and the leg was bandaged from the foot to above the knee to give compression and support. The dressing was changed to a lightweight dressing after 1 week. Three weeks were allowed for healing, after which the external controller was set up for walking by a clinical scientist (PNT) or a physiotherapist (IAWH). Follow-up was provided 4 weeks after setup, 3 months after that (20 weeks after surgery), 6 months later, and then yearly for as long as the device was used.
Walking performance was assessed using 10-m walking speed and the Physiological Cost Index (PCI; an estimate of walking effort derived by dividing the increase in heart rate measured at the end of a walk relative to the resting heart rate before walking by walking speed) and 3-minute walking distance and PCI.3,20,21 Ten-meter walking speed was measured over a 12-m straight course, with 1 m at each end for acceleration and deceleration. Two walks without FES were followed by a walk with FES. The first walk was used as a warm-up walk, and the second and third walks were used for data analysis. A single instruction to “walk briskly but safely” was given. For the PCI measurement, heart rate was recorded at the end of each walk using a Polar heart rate monitor (Polar Electro Inc, Lake Success, NY).7,11 The 3-minute walking distance was measured by walking repeated lengths of a room 14.2 m long. Heart rate was recorded at the end of the walk. No instruction was given except that participants could stop before the end of the 3 minutes if they became tired.
Health-related quality of life was measured using the 36-item Short Form Health Status Survey questionnaire,22 and device-related quality of life was recorded using the Psychosocial Impact of Assisted Devices Scale (PIADS).7,23 Participant experience using the devices was assessed using custom-designed questionnaires (Supplementary Appendix 1 (150.9KB, pdf) , published in the online version of this article at ijmsc.org).24,25 All the assessments were made at the preoperative assessment and again 20 weeks postoperatively. Ten-meter walking speed was also recorded at subsequent follow-up assessments.
The hospital's charge throughout the study period for delivery of the STIMuSTEP was £6442 in the first year and £351 per year thereafter for annual follow-up and device maintenance. The hospital's charge for the external device was £1640 in the first year and £300 per year thereafter.8 This work was performed in accordance with the Declaration of Helsinki.
Analysis
The following comparisons were made for 10-m walking speed, 10-m PCI, walking distance in 3 minutes, and PCI in 3 minutes3: orthotic effect (the difference in a parameter between walking with and without FES assistance on any same occasion), training effect (the difference in a parameter between walking without FES before implant and without FES at a later date), and total orthotic effect (the difference in a parameter between walking without FES before implant and with FES at a later date). The difference between orthotic effects before and after surgery was used to demonstrate equivalency of the two devices.
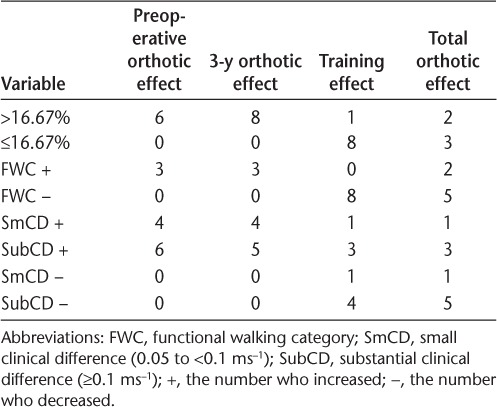
The number of participants achieving a clinically significant change in walking speed was calculated using three methods. This was to enable comparison with published studies for external FES and also with the Miller et al.26 model for the prediction of decline in walking speed in MS. Pereira et al.27 determined that the minimum change that was clinically meaningful was 0.05 ms−1, and a substantial change was 0.1 ms−1 or greater. Perry et al.28 related walking speed to functional independence, defining people with a walking speed of less than 0.4 ms−1 as household walkers, 0.4 to 0.58 ms−1 as the most restricted community walkers, 0.58 to 0.8 ms−1 as the least limited community walkers, and more than 0.8 ms−1 as nonlimited community walkers. Finally, Kaufman et al.29 determined that a meaningful change in 25-foot walk time for MS was 20%, which equates to a change in walking speed of 16.67%.
The data were explored for normal distribution, and it was found that some data sets were nonnormally distributed. Hence, except for demographic data, nonparametric analysis is used. Results are presented as median with 95% confidence interval. Change was calculated as the median of the differences and was tested using the Wilcoxon signed rank test using Minitab 14 statistical software (Minitab Inc, State College, PA). A significance level of P = .05 was used.
Results
Twenty-three people with MS received the STIMuSTEP implant (Table 1). All the participants achieved effective correction of footdrop using the implant, demonstrated by the production of dorsiflexion and eversion when the device was used, enabling a heel strike and safe walking in the clinic and at home. The mean (SD) time from preoperative assessment to surgery was 142 (104) days. The follow-up assessment was a mean (SD) of 128 (24) days after surgery, giving a total mean (SD) time between assessments of 270 (107) days.
Table 1.
Demographics, assistive device use, reason for receiving the implant, and reasons for discontinuing use (n = 23)
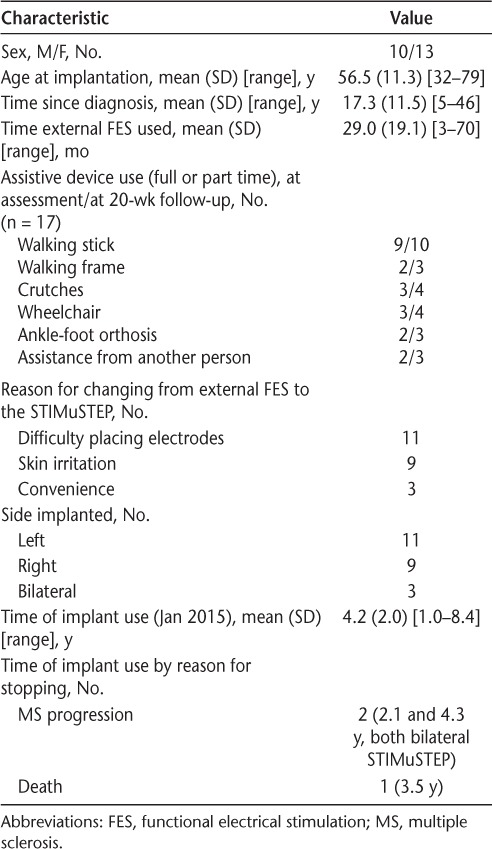
For 10-m walking speed, 20 complete sets of data were available for analysis. One preoperative assessment record was lost, one postoperative assessment was delayed by a relapse, and a third postoperative assessment was delayed by a fall (see the Adverse Events subsection later herein). In addition, two sets of 3-minute walking data were missing at baseline. Further PCI data were missing because of recording device failure or the use of β-blockers, preventing the reliable measurement of heart rate change in response to exercise. In addition, some questionnaire data were not recorded.
Comparisons After 18 Weeks of STIMuSTEP Use
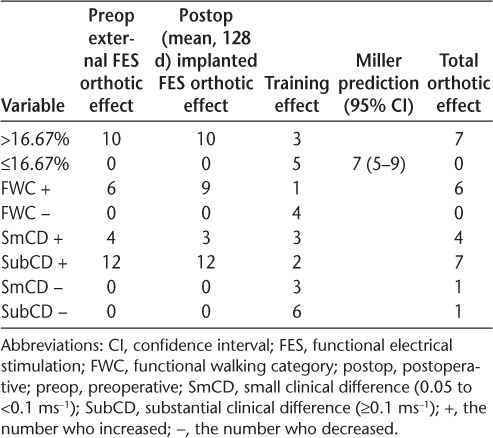
Both external and implanted devices gave a substantial clinically meaningful increase in walking speed (external FES: 0.125 ms−1, P < .001; implanted FES: 0.130 ms−1, P < .001) with no significant difference between the devices (0.01 ms−1, P = .627) (Table 2). Although there was no significant change in walking speed over 270 days when not assisted by FES (training effect: −0.037 ms−1, P = .235), five participants did experience a reduction of more than 16.67%. This is in line with the Miller et al.26 model prediction for 270 days of seven people with MS (37%) (95% confidence interval, 5–9), suggesting that receiving the implant did not adversely affect the progression of MS. However, three participants did achieve a training effect, increasing their walking speed by more than 16.67%, and ten walked faster than 16.67% when either device was used. Using Pereira et al.'s27 definition of clinically meaningful change in walking speed, 16 participants achieved either a small or a substantial clinical change using external FES and 15 using the implant. Six participants walked fast enough with external FES to improve their functional walking category, and nine achieved this using the implant. A summary of clinically meaningful changes is given in Table 3.
Table 2.
10-m walking speed, 10-m Physiological Cost Index (PCI), 3-minute walk distance, and 3-minute walk PCI
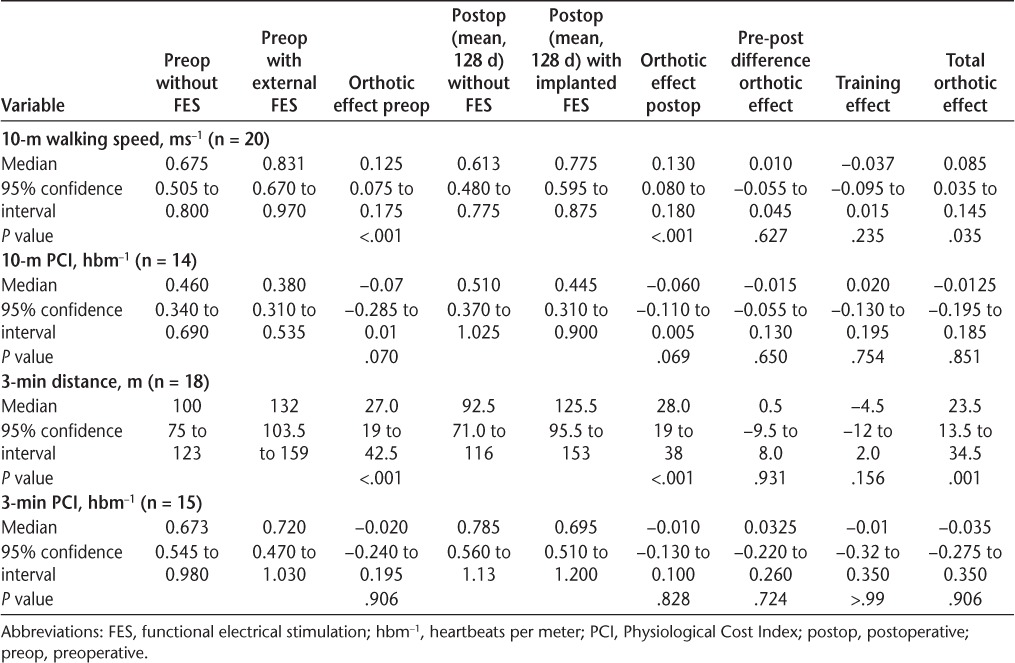
Table 3.
Number of participants who changed their walking speed when walking with and without FES sufficiently to be considered a clinically meaningful change (N = 20)
Both devices enabled a statistically significant increase in walking distance in 3 minutes relative to no device (external FES: 27 m, P < .001; implanted FES: 28 m, P < .001) (Table 2). The PCI showed a strong trend toward a reduction in walking effort before (P = .070) and after (P = .069) surgery when measured over 10 m. There was no reduction in the PCI when measured over 3 minutes (Table 2).
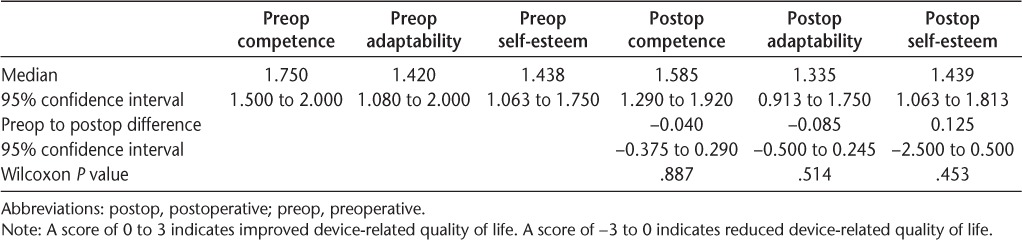
Overall, there were no differences in 36-item Short Form Health Status Survey scores between devices (Table 4), and both devices improved device-related quality of life (PIADS) (Table 5).
Table 4.
36-item Short Form Health Status Survey quality of life (n = 18)
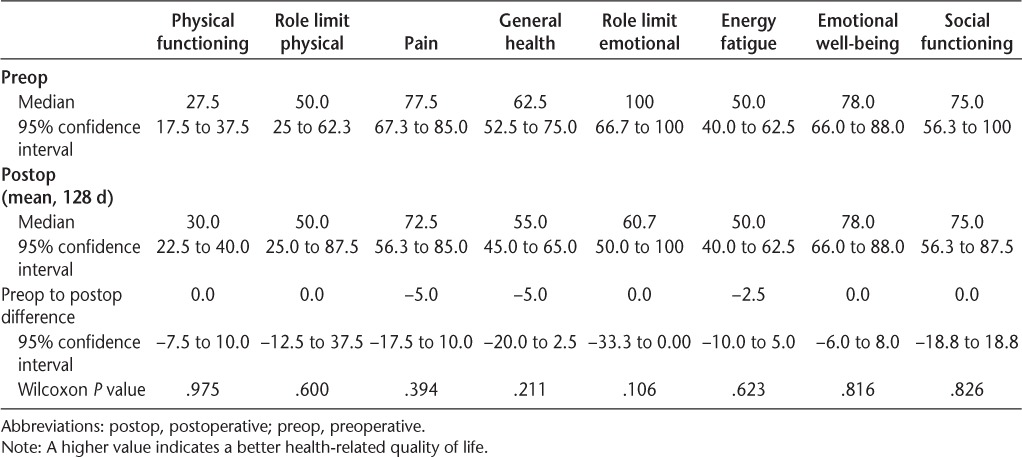
Table 5.
Psychosocial Impact of Assistive Devices Scale scores (n = 17)
Complete device use experience questionnaire data were available for 17 participants. There was an increase in the mean (SD) number of days per week that FES was used from 4.3 (2.9) with the external FES device to 6.5 (0.9) with the STIMuSTEP (P = .006). Both devices were used in similar activities of daily living except for work, where the STIMuSTEP was used by eight participants, three more people than external FES. Participants were asked to identify from a list of 13 possible responses their reasons for using FES. The reasons identified were the same for both devices (n = external/implanted), with the most commonly cited being reduced the effort of walking (17/17), reduced trips or falls (17/16), increased confidence while walking (17/15), walked further (14/15), walked faster (14/15), and greater independence (14/14). Participants were asked to rate the sensation they experienced from each device on a visual analogue scale. There was no significant difference in the mean (SD) score for each device: external FES, 4.8 (1.6) and the STIMuSTEP, 4.3 (1.6) (P = .41), where 10 is a very strong sensation and 1 is no sensation. When asked if they agreed with the statement that the sensation from each device was comfortable, 11 external FES and 14 STIMuSTEP users agreed and two external FES and one STIMuSTEP user disagreed with the statement. All the participants agreed or strongly agreed with the statement, “I am glad I have the external FES (or STIMuSTEP),” with four more strongly agreeing with the statement relating to the STIMuSTEP. Participants were asked to estimate the time it took them to put on each device. The mean (SD) estimate for the external FES device was 15.0 (11.3) minutes and for the STIMuSTEP was 2.6 (1.8) minutes (P < .001).
10-m Walking Test at 40 Months
Ten-meter walking speed data were also available for 12 of the 16 participants who had had the implant longer than 3 years (mean [SD], 1191 [99] days) (Tables 6 and 7). An overall clinically meaningful increase in walking speed was achieved when either device was used (external FES before implantation: 0.11 ms−1, P = .003; implanted FES at 40 months: 0.10 ms−1, P = .004), and again there was no significant difference between devices (0.005 ms−1, P = .937). However, there was a significant decline in unassisted walking speed over this period, in line with the progression of MS (−0.232 ms−1, P = .014).
Table 6.
10-m walking speed preoperatively and at 3 years (mean, 1191 days) postoperatively (n = 12)
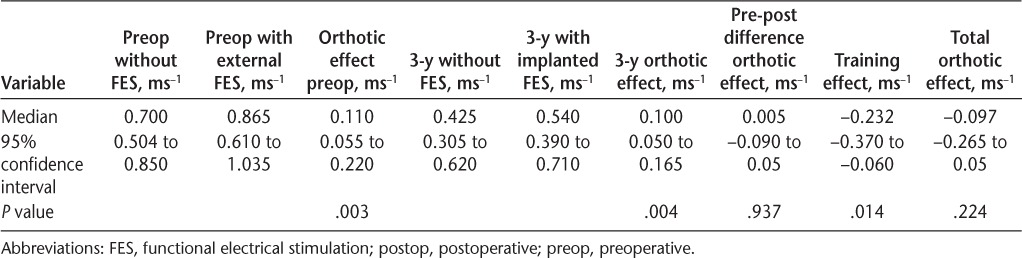
Table 7.
Clinically meaningful change in 10-m walking speed at 3 years (mean, 1191 days) postoperatively (n = 12)
Adverse Events
Four implant failures occurred, three of which happened after a fall. The cause of the other failure is unknown. All four devices were successfully replaced. However, one participant had a further fall after the second surgery and fractured her ulna. This prevented use of a walker and, consequently, delayed recommencement of walking with the STIMuSTEP. Walking is now achieved, but it has not yet been possible to repeat the assessments. Two cases of pain in response to stimulation with the implant have been recorded. One was likely due to neuropraxia and resolved after 6 months. The second case was found to be due to external pressure on the nerve from the external controller and leg strap, which was resolved after modification of the external components.
Discussion
Overall, both devices were equivalent in functional outcome in terms of benefit to walking and device-related quality of life. Both devices improved walking speed and distance. These effects were identified in the questionnaire as important reasons for using FES by the participants. The questionnaire also identified the reduction in effort while walking as an important factor, and a strong trend toward a reduced PCI was recorded for both devices over 10 m. However, there was no significant reduction in the PCI over 3 minutes. Stein et al.4 also found no orthotic benefit in terms of PCI from using external FES, although a training effect was observed over 3 months. Increased gait efficiency when using external FES has been reported in three studies that recorded oxygen consumption over a 5-minute walk, although Miller et al. found this only for slower walkers.10,30,31 The PCI measured over 10 m can be considered a non–steady-state measure of the PCI, reflecting the immediate demand on the cardiovascular system placed on walking, and the PCI recorded over 3 minutes may be considered a steady-state measure. The lack of a difference in the 3-minute PCI may indicate that participants were walking at their maximum capacity.
The overall change in walking speed (orthotic effect) when walking with either device was substantially significant using the criteria of Pereira et al.,27 with 60% of participants achieving this level. This is a slightly greater proportion than reported by Street et al.,11 who found that 49% of external FES users achieved this improvement. Despite the same number achieving a substantial change, three more participants increased the functional walking category when using the STIMuSTEP (45%) than external FES (30%). The latter is in line with Street et al.,11 who found that 32% of patients changed functional walking category. Similarly, the percentage of participants experiencing a decline in walking speed of 16.67% was at the low end of the confidence limit of the prediction made by the Miller et al.26 model in both studies. Of the three methods used to demonstrate clinical significance, two produced similar results, with nine people with MS increasing their functional walking category and ten increasing their walking speed by more than 16.67% when the implant was used. Twelve people with MS achieved Pereira et al.'s27 thresholds for a substantial clinical change and three people with MS achieved a small clinical change, suggesting that this method may have greater sensitivity to change. Although there are no established figures for clinically meaningful change for 3-minute walking distance, 50 m is accepted as meaningful change for the 6-minute walk.27 Because more than half of this value was achieved with either device, it is likely that the increase in walking distance in 3 minutes is also clinically meaningful.
At the time of writing, more than half of the participants had used the STIMuSTEP for more than 4 years and were able to achieve an orthotic effect that remained the same 3 years after implantation. This suggests that the device is an effective long-term mobility aid despite the progression of MS demonstrated by the reduction in walking speed both with and without FES.
The STIMuSTEP may have advantages in terms of participation: the number of days per week it was used and the number of people using it at work were greater than those for external FES. This may be due to greater convenience and ease of use, demonstrated by the significant difference in the perceived time to put the device on. It may also relate to the elimination of skin irritation, ending the need to ration the use of FES to preserve skin integrity.
Both devices improved device-related quality of life, as demonstrated by a positive PIADS score. Interestingly, the PIADS score is approximately double in all three domains compared with that reported for external FES by Barrett and Taylor.7 This may indicate that those who choose the implanted device may perceive the benefit from FES to be greater than does the general population of external FES users.
The cost of providing the implant compared with external FES is significantly higher in the first year but comparable in subsequent years. Case series data have demonstrated that the average time that external FES is used by each person with MS is approximately 5.5 years.32 The total cost of providing FES for this period was £3348, giving a cost per quality-adjusted life-year (QALY)33 of £14,492. Although the implant program has not been in existence long enough to demonstrate the mean period of use, our experience so far indicates that the mean time of use is likely to be comparable or greater. The projected cost for provision of the implant to 5.5 years is £7846. Assuming the same QALY gain, the cost per QALY over 5.5 years would be £34,794. The greater PIADS score found in this study compared with external FES suggests that the QALY gain may be greater for the STIMuSTEP, possibly resulting in a lower cost per QALY.
The present study has several limitations. It is of relatively small size, limiting its statistical power, and it has some missing data. Data were also not available from the start of external FES use; therefore, no comparison with pre-device walking was possible. However, the main purpose of this study was to compare the STIMuSTEP with the established external FES intervention, and this was achieved. The lack of a control group also prevents comparison with those continuing with external FES. However, most participants were receiving the implant to overcome difficulty using external FES, due to either skin irritation or difficulty placing electrodes, often due to reduced hand function, so do not represent the general population of external FES users, precluding a randomized controlled trial model.
Both external FES device and the implanted STIMuSTEP dropped foot stimulator improve walking function, demonstrated by an increase in walking speed and 3-minute walking distance when the devices are turned on (orthotic effect). This is associated with an improvement in device-related quality of life. Although there was no difference in gait performance between devices, setup time was shorter, and the number of days the device was used per week was greater for the STIMuSTEP. Although there is a risk of implant failure and surgical complication, the STIMuSTEP is a practical alternative to external FES for the correction of footdrop when skin irritation or difficulty placing electrodes prevents effective or consistent use of external FES.
Supplementary Material
Acknowledgments
We thank John Hobby for early technique development and surgery; Laura Humphreys, Vicky Parry, and Kirstyne Kennaugh for data collection; and Yat Ting Kwan for data management. The STIMuSTEP devices were supplied by Finetech Medical Ltd. The external FES devices (ODFS III, ODFS Pace, and O2CHSII) were supplied by Odstock Medical Ltd.
PracticePoints
Functional electrical stimulation (FES) is an effective means of improving walking for people who have footdrop due to MS.
Implanted and external FES devices have equivalent effects on walking ability.
Implanted FES is an effective alternative to external FES when consistent use of external FES cannot be achieved because of difficulty in using skin electrodes.
Footnotes
Financial Disclosures: Clinical provision of the STIMuSTEP was by Odstock Medical Ltd (OML) at Salisbury District Hospital. OML is majority owned by Salisbury NHS Foundation Trust, the organization that runs Salisbury District Hospital. Drs. Taylor and Wilkinson Hart hold a small amount of stock in OML.
References
- 1. Mackenzie IS, Morant SV, Bloomfield GA, MacDonald TM, O'Riordan J. Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research. J Neurol Neurosurg Psychiatry. 2014; 85: 76– 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacobs LD, Wende KE, Brownscheidle CM, et al. A profile of multiple sclerosis: the New York State Multiple Sclerosis Consortium. Mult Scler. 1999; 5: 369– 376. [DOI] [PubMed] [Google Scholar]
- 3. Taylor PN, Burridge JH, Dunkerley AL, et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil. 1999; 80: 1577– 1583. [DOI] [PubMed] [Google Scholar]
- 4. Stein RB, Everaert DG, Thompson AK, et al. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair. 2010; 24: 152– 167. [DOI] [PubMed] [Google Scholar]
- 5. Esnouf J, Taylor P, Mann G, Barrett C. Impact on activities of daily living using a functional electrical stimulation device to improve dropped foot in people with multiple sclerosis, measured by the Canadian Occupational Performance Measure. Mult Scler. 2010; 16: 1141– 1147. [DOI] [PubMed] [Google Scholar]
- 6. Taylor P, Barrett C, Mann G, Wareham W, Swain IA. Feasibility study to investigate the effect of functional electrical stimulation and physiotherapy exercise on the quality of gait of people with multiple sclerosis: FES for dropped foot and hip stability in MS. Neuromodulation. 2014; 17: 75– 84. [DOI] [PubMed] [Google Scholar]
- 7. Barrett CL, Taylor PN. The effects of the Odstock Drop Foot Stimulator on perceived quality of life for people with stroke and multiple sclerosis. Neuromodulation. 2010; 13: 58– 64. [DOI] [PubMed] [Google Scholar]
- 8. Taylor P, Humphreys L, Swain I. The long-term cost-effectiveness of the use of functional electrical stimulation for the correction of dropped foot due to upper motor neuron lesion. J Rehabil Med. 2013; 45: 154– 160. [DOI] [PubMed] [Google Scholar]
- 9. van Swigchem R, Hanneke JR, van Duijnhoven HJR, den Boer J, Geurts AC, Weerdesteyn V. Effect of peroneal electrical stimulation versus an ankle-foot orthosis on obstacle avoidance ability in people with stroke-related foot drop. Phys Ther. 2012; 92: 398– 406. [DOI] [PubMed] [Google Scholar]
- 10. Khurana S, Ference T, Beranger A. Comparison of functional electric stimulation neuroprosthesis and ankle foot orthosis in persons with multiple sclerosis. Paper presented at: 26th European Committee for Treatment and Research in Multiple Sclerosis Congress and 18th Rehabilitation in Multiple Sclerosis Meeting; Copenhagen, Denmark; October 2–5, 2013. [Google Scholar]
- 11. Street TD, Taylor PN, Swain ID. The effectiveness of functional electrical stimulation on walking speed, functional walking category and clinically meaningful changes for people with multiple sclerosis. Arch Phys Med. 2015; 96: 667– 672. [DOI] [PubMed] [Google Scholar]
- 12. O'Dell MW, Dunning K, Kluding P, et al. Response and prediction of improvement in gait speed from functional electrical stimulation in persons with poststroke drop foot. J Am Acad Phys Med Rehabil. 2014; 6: 587– 601. [DOI] [PubMed] [Google Scholar]
- 13. Taylor PN, Mann GE, Wood DE, Hobby J. Pilot study to evaluate the safety and efficacy of an implanted dropped foot stimulator (IMPULSE). Paper presented at: 8th Annual Conference of the International FES Society; July 1–5, 2003; Maroochydore, Queensland, Australia. [Google Scholar]
- 14. Kenney L, Bultstra G, Buschman R, et al. An implantable two channel drop foot stimulator: initial clinical results. Artif Organs. 2002; 26: 267– 270. [DOI] [PubMed] [Google Scholar]
- 15. van der Aa HE, Bultstra G, Verloop AJ, et al. Application of a dual channel peroneal nerve stimulator in a patient with a central drop foot. Acta Neurochir Suppl. 2002; 79: 105– 107. [DOI] [PubMed] [Google Scholar]
- 16. Kottink AIR, Buschman HPJ, Kenney LPJ, et al. The sensitivity and selectivity of an implantable two-channel peroneal nerve stimulator system for restoration of dropped foot. Neuromodulation. 2004; 7: 277– 283. [DOI] [PubMed] [Google Scholar]
- 17. Kottink AI, Oostendorp LJ, Buurke JH, Nene AV, Hermens HJ, Ijzerman MJ. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: a systematic review. Artif Organs. 2004; 28: 577– 586. [DOI] [PubMed] [Google Scholar]
- 18. Kottink AI, Hermens HJ, Nene AV, et al. A randomized controlled trial of an implantable 2-channel peroneal nerve stimulator on walking speed and activity in poststroke hemiplegia. Arch Phys Med Rehabil. 2007; 88: 971– 978. [DOI] [PubMed] [Google Scholar]
- 19. Kottink AIR, Tenniglo MJB, de Vries WHK, Hermens HJ, Buurke JH. Effects of an implantable two-channel peroneal nerve stimulator versus conventional walking device on spatiotemporal parameters and kinematics of hemiparetic gait. J Rehabil Med. 2012; 44: 51– 57. [DOI] [PubMed] [Google Scholar]
- 20. Nene A. Physiological cost index of walking in able-bodied adolescents and adults. Clin Rehabil. 1993; 7: 319– 326. [Google Scholar]
- 21. Bailey M, Ratcliffe C. Reliability of physiological cost index in walking normal subjects using steady-state and non-steady-state and post-exercise. Physiotherapy. 1995; 81: 618– 623. [Google Scholar]
- 22. Anderson C, Laubscher S, Burns R. Validation of the Short Form 36 (SF-36) health survey questionnaire among stroke patients. Stroke. 1996; 27: 1812– 1816. [DOI] [PubMed] [Google Scholar]
- 23. Jutai J, Day H. Psychosocial impact of assistive devices scale (PIADS). Technol Disabil. 2002; 14: 107– 111. [Google Scholar]
- 24. Taylor PN, Burridge JH, Wood DE, et al. Patient perceptions of the Odstock Drop Foot Stimulator. Clin Rehabil. 1999; 13: 333– 340. [DOI] [PubMed] [Google Scholar]
- 25. Burridge JH, Haugland M, Larsen B, et al. Patients' perceptions of the benefits and problems of using the ActiGait implanted drop-foot stimulator. J Rehabil Med. 2008; 40: 873– 875. [DOI] [PubMed] [Google Scholar]
- 26. Miller DM, Thompson NR, Cohen JA, et al. Factors associated with clinically significant increased walking time in multiple sclerosis: results of a survival analysis of short-term follow-up data from a clinical database. Mult Scler. [published online August 11, 2014]. [DOI] [PubMed] [Google Scholar]
- 27. Pereira S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults: meaningful change and performance. J Am Geriatr Soc. 2006; 54: 743– 749. [DOI] [PubMed] [Google Scholar]
- 28. Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995; 26: 982– 989. [DOI] [PubMed] [Google Scholar]
- 29. Kaufman M, Moyer D, Norton J. The significant change for the Timed 25-foot Walk in the multiple sclerosis functional composite. Mult Scler. 2000; 6: 286– 290. [DOI] [PubMed] [Google Scholar]
- 30. Paul L, Rafferty D, Young S, Miller L, Mattison P, McFadyen A. The effect of functional electrical stimulation on the physiological cost of gait in people with multiple sclerosis. Mult Scler. 2008; 14: 954. [DOI] [PubMed] [Google Scholar]
- 31. Miller L, Rafferty D, Paul L, Mattison P. The impact of walking speed on the effects of functional electrical stimulation for foot drop in people with multiple sclerosis. Disabil Rehabil Assist Technol. [published online March 31, 2015]. [DOI] [PubMed] [Google Scholar]
- 32. Taylor P, Humphreys L, Swain I. A 15 year cost-effectiveness study of the use of FES for the correction of dropped foot in multiple sclerosis. Mult Scler J. 2014; 20: 1001– 1002. [Google Scholar]
- 33. Centre for Evidence-Based Purchasing Economic Report: Functional Electrical Stimulation for Dropped Foot of Central Neurological Origin: CEP10012. NHS Purchasing and Supply Agency; February 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


