Abstract
IMPORTANCE
Patients with chronic kidney disease (CKD) are at an increased risk of cardiovascular disease (CVD) compared with the general population. Prior studies have produced contradictory results on the association of dietary sodium intake with risk of CVD, and this relationship has not been investigated in patients with CKD.
OBJECTIVE
To evaluate the association between urinary sodium excretion and clinical CVD events among patients with CKD.
DESIGN, SETTING, AND PARTICIPANTS
A prospective cohort study of patients with CKD from 7 locations in the United States enrolled in the Chronic Renal Insufficiency Cohort Study and followed up from May 2003 to March 2013.
EXPOSURES
The cumulative mean of urinary sodium excretion from three 24-hour urinary measurements and calibrated to sex-specific mean 24-hour urinary creatinine excretion.
MAIN OUTCOMES AND MEASURES
A composite of CVD events defined as congestive heart failure, stroke, ormyocardial infarction. Events were reported every 6 months and confirmed by medical record adjudication.
RESULTS
Among 3757 participants (mean age, 58 years; 45% women), 804 composite CVD events (575 heart failure, 305 myocardial infarction, and 148 stroke) occurred during a median 6.8 years of follow-up. From lowest (<2894 mg/24 hours) to highest (≥4548 mg/24 hours) quartile of calibrated sodium excretion, 174, 159, 198, and 273 composite CVD events occurred, and the cumulative incidence was 18.4%, 16.5%, 20.6%, and 29.8% at median follow-up. In addition, the cumulative incidence of CVD events in the highest quartile of calibrated sodium excretion compared with the lowest was 23.2% vs 13.3% for heart failure, 10.9% vs 7.8% for myocardial infarction, and 6.4% vs 2.7% for stroke at median follow-up. Hazard ratios of the highest quartile compared with the lowest quartile were 1.36 (95% CI, 1.09–1.70; P = .007) for composite CVD events, 1.34 (95% CI, 1.03–1.74; P = .03) for heart failure, and 1.81 (95% CI, 1.08–3.02; P = .02) for stroke after multivariable adjustment. Restricted cubic spline analyses of the association between sodium excretion and composite CVD provided no evidence of a nonlinear association (P = .11) and indicated a significant linear association (P < .001).
CONCLUSIONS AND RELEVANCE
Among patients with CKD, higher urinary sodium excretion was associated with increased risk of CVD.
Chronic kidney disease (CKD) affects approximately 11% of the US general population1 and is associated with increased risk of end-stage renal disease, cardiovascular disease (CVD), and all-cause mortality.2,3 Greater than 1 in 3 US adults has CVD, and it is the leading cause of death in the United States.4 Those with CKD are at increased risk of CVD compared with those with normal kidney function, and risk increases as CKD progresses.2,3
A positive association between sodium intake and blood pressure is well established.5 However, the association between sodium intake and clinical CVD remains less clear.6 While some studies reported a J- or U-shaped association between dietary sodium and CVD,7,8 others found a positive monotonic association between sodium intake and risk of CVD, coronary heart disease, congestive heart failure (CHF), and stroke.9–11 Methodologic limitations, including inconsistencies in dietary sodium measurement methods, could contribute to these conflicting findings.6
Blood pressure of patients with CKD is more sensitive to high sodium intake than persons with normal kidney function due to a diminished capacity to excrete sodium.12 Despite this, there is limited prior research on the association between dietary sodium intake and CVD among those with impaired kidney function,13,14 and to our knowledge, no previous studies have examined the association between sodium intake and incident CVD among patients with CKD. In addition, few studies examining the association between dietary sodium and CVD have used the mean of multiple 24-hour urine samples to quantify urinary sodium excretion, which is considered the best method for estimating usual sodium intake.6,15 The objective of this study was to determine the prospective relationship between urinary sodium (and potassium) excretion, estimated from the mean of 3 repeated 24-hour urine samples, and risk of clinical CVD among patients with CKD enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study.
Methods
Study Participants
The CRIC Study is an ongoing, multicenter, prospective cohort study of adults aged 21 to 74 years with mild to moderate CKD designed to identify and examine risk factors for CKD progression and development of CVD in those with CKD. Details of the CRIC Study design and methods have been published previously.16 Briefly, a total of 3939 racially and ethnically diverse participants, approximately half of whom had diabetes, were recruited from 7 clinical centers in the United States from 2003 to 2008. Participants were eligible for the study if they met age-specific estimated glomerular filtration rate (eGFR) criteria of 20 to 70 mL/min/1.73 m2. Those with a history of kidney transplant, dialysis for at least 1 month, glomerulonephritis requiring immunosuppression, advanced heart failure, cirrhosis, or polycystic kidney disease were ineligible.
Institutional review boards at all participating institutions approved the study protocol, and the study adhered to the Declaration of Helsinki. All participants provided written informed consent.
Measurements
Self-reported sociodemographic and lifestyle characteristics, medical history, current medication use, and responses to a food frequency questionnaire,17 which was used to estimate total calorie intake, were obtained at the baseline visit. Race was self-reported in response to questions with fixed categories and was used to calculate eGFR to determine eligibility. Blood pressure, height, weight, and waist circumference were measured using standard protocols, and fat-free mass was obtained by bioelectrical impedance analysis.16,18 An overnight fasting blood sample was collected to measure serum creatinine, lipids, and plasma glucose. Serum creatinine measurements were calibrated to isotope dilution mass spectrometry traceable values. Hypertension was defined as mean blood pressure of 140/90 mm Hg or greater or self-reported use of antihypertensive medication, and diabetes was defined as a fasting plasma glucose of 126 mg/dL or greater, a nonfasting plasma glucose 200 mg/dL or greater, or self-reported use of antidiabetes medication. Glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation, which includes serum creatinine, age, sex, and race.1
Study participants were requested to collect 24-hour urine specimens at baseline and the first 2 annual follow-up visits. If either the total urine volume was less than 500 mL at the end of 24 hours or the duration of collection was not between 22 and 24 hours, participants were instructed to recollect the urine sample. Urinary sodium and potassium levels were measured by flame emission spectrophotometry (Instrumentation Laboratory Flame Photometer model 943). Urinary creatinine was measured on a BioTek Plate Reader ELx808 using a Jaffe reaction with a colorimetric end point and reagents from Sigma-Aldrich. All laboratory analyses were conducted at the CRIC Study central laboratory at the University of Pennsylvania with stringent quality control.
The cumulative mean of 24-hour urinary sodium excretion obtained from the baseline visit and the first 2 annual follow-up visits prior to developing a study event was calculated as the exposure variable. This approach was selected to take full advantage of all available exposure and outcome data.19 Twenty-four-hour urinary sodium excretion was calibrated according to the sex-specific mean of 24-hour urinary creatinine excretion in the study population (1569 mg/24 hours in men and 1130 mg/24 hours in women) in order to reduce measurement error due to potential incompleteness of 24-hour urine collection. Calibration was done by multiplying each sample’s urinary sodium-to-creatinine ratio by sex-specific mean creatinine level in the study population. Uncalibrated mean 24-hour urinary sodium excretion was also used as an exposure variable in sensitivity analyses.
Outcome Assessment
Study participants attended annual clinic visits and received telephone calls every 6 months between in-person visits. Cardiovascular clinical outcomes were assessed using a standard medical event questionnaire at all follow-up contacts. Medical records were requested for event verification, and 2 physicians adjudicated each cardiovascular event and classified them as either probable or definite.16
The primary outcome of interest was composite CVD, defined as the first of CHF, myocardial infarction (MI), and stroke that occurred during follow-up. In addition, CHF, MI, and stroke were analyzed individually. All included CVD events were nonfatal. Congestive heart failure was identified by hospital admission for new or worsening CHF signs and symptoms, in addition to diminished cardiac output. Myocardial infarction was defined by characteristic changes in troponin and creatine kinase–MB levels, symptoms of myocardial ischemia, electrocardiogram changes, or new fixed profusion abnormalities. Stroke was defined as rapid onset of neurologic deficit, headache, or other nonvascular cause and clinically relevant lesion on brain imaging for longer than 24 hours or death within 24 hours. All events classified as probable or definite during adjudication were included in these analyses. Follow-up was censored at the first of either death, loss to follow-up, withdrawal, or March 2013.
Statistical Analysis
Baseline characteristics of study participants were compared across quartiles of calibrated urinary sodium excretion using χ2 tests for categorical variables and analysis of variance and Kruskal-Wallis tests for normally and nonnormally distributed continuous variables, respectively.
Cumulative incidence of composite CVD, CHF, MI, and stroke were calculated by quartile of urinary sodium excretion using the Kaplan-Meier method and compared using the log-rank test with the null hypothesis that the cumulative incidence was the same among quartiles of sodium excretion.20 Hazards ratios for the associations of urinary sodium excretion with composite CVD, CHF, MI, and stroke were estimated using Cox proportional hazards models with follow-up time used as the time scale.21 The assumption of proportionality was tested using Schoenfeld residuals and interaction terms with time for each exposure variable and covariate. No substantial deviations from proportionality were observed. Complete case analysis was used for the main findings. Multiple imputation for missing covariate values was performed in a sensitivity analysis, and no substantial differences were observed. Urinary sodium excretion was analyzed in quartiles and as a continuous variable.
Analyses of subgroups defined by sex, age, race, diabetes status, and history of CVD were performed, and effect modification was assessed by including interaction terms with calibrated sodium excretion in the models. Important covariates for CVD were selected based on prior knowledge and were adjusted in multivariable analyses. First, unmodifiable demographic risk factors, ie, age, sex, race, and CRIC clinic site, were adjusted in the multivariable models (model 1). Next, established modifiable CVD risk factors were added to the multivariable models (model 2): education, waist circumference, lean body mass index, body mass index, cigarette smoking, alcohol drinking, physical activity, low-density lipoprotein cholesterol level, glucose level, history of CVD, use of antidiabetic and lipid-lowering medications, use of diuretics, use of renin-angiotensin system blocking agents and other antihypertensive medications, and urinary creatinine. Finally, eGFR at baseline was included in the multivariable models (model 3). Systolic blood pressure was not adjusted in the main analyses because blood pressure might be on the causal pathway between urinary sodium excretion and CVD events.6 However, systolic blood pressure was adjusted in sensitivity analyses. In addition, because 24% of participants were missing data on total caloric intake, total caloric intake was not adjusted in the primary analyses but was adjusted in sensitivity analyses.
Possible nonlinear relationships between urinary sodium excretion and composite CVD, CHF, MI, and stroke were examined with restricted cubic splines.22 Analyses were multivariable-adjusted and used 3 knots, and the 2.5% highest and lowest sodium excretion observations were trimmed. Knots were located at the 5%, 50%, and 95% percentiles corresponding to values of calibrated urinary sodium excretion of 2175, 3636, and 6017 mg/d, respectively. Sensitivity analyses were conducted using alternate middle knot locations of 3800 and 4000 mg/d based on biologic plausibility, as well as using 4 or 5 knots instead of 3 knots. Tests for nonlinearity comparing a model with only the linear term to a model with the linear and restricted cubic spline terms were conducted using likelihood ratio tests. If a test for nonlinearity was not significant, a test for linearity was conducted comparing a model with the linear term to a model with only the covariates of interest.
All analyses were also conducted with urinary potassium excretion as the exposure of interest using the same methodologic approach described above. All analyses were conducted using SAS version 9.2, and all hypothesis testing was 2-tailed with P < .05 set as statistically significant.
Results
A total of 3757 participants (mean age, 58 years; 45% women) were included in this analysis. Participants with incomplete or missing 24-hour urine collection were excluded (n = 182): 54 participants who did not successfully collect any 24-hour urine specimens, 126 participants with incomplete 24-hour urine collection (3 with total urine volume <500 mL, 10 with collection duration <20 hours, and 113 with total creatinine excretion <7 mg/kg of body weight), and 2 participant with mean urinary sodium excretion less than 20 mmol/24 hours. A total of 2167 participants had 3 sodium measurements, 982 had 2 measurements, and 608 had only 1 measurement. There was no significant difference in mean urinary sodium excretion between those with all 3 measurements and those with fewer (mean [SD], 3725 [1328] mg/24 hours vs 3669 [1586] mg/24 hours; P for difference = .24).
Overall, mean (SD) 24-hour sodium excretion was 3701 (1443) mg. Participants with higher calibrated 24-hour urinary sodium excretion were more likely to be male, white, and current smokers; report a history of hypertension, diabetes, hypercholesterolemia, and CVD; and take antihypertensive medications (Table 1). Those with the highest sodium excretion also had the highest systolic and diastolic blood pressure; waist circumference; lean body mass index; daily calorie intake; and triglycerides, glucose, and glycated hemoglobin levels and the lowest low-density and high-density lipoprotein cholesterol values. Urinary potassium and protein excretion levels were highest, while eGFR was lowest, in participants with the highest calibrated sodium excretion.
Table 1.
Characteristics of 3757 Patients With Chronic Kidney Disease According to Quartile of Calibrated 24-Hour Urinary Sodium Excretion: Chronic Renal Insufficiency Cohort Study
| Variable | Calibrated Urinary Sodium Excretion, mg/24 ha | P Value | |||
|---|---|---|---|---|---|
| <2894 (n = 939) | 2894–3649 (n = 940) | 3650–4547 (n = 939) | ≥4548 (n = 939) | ||
| Age, mean (SD), y | 57.2 (10.9) | 57.7 (11.3) | 58.2 (10.8) | 58.0 (10.6) | .24 |
| Men, No. (%) | 329 (35.0) | 469 (49.9) | 576 (61.3) | 714 (76.0) | <.001 |
| Race/ethnicity, No. (%) | |||||
| White | 362 (38.6) | 429 (45.6) | 475 (50.6) | 510 (54.3) | <.001 |
| Black | 483 (51.4) | 414 (44.0) | 351 (37.4) | 309 (32.9) | |
| Other | 94 (10.0) | 97 (10.3) | 113 (12.0) | 120 (12.8) | |
| High school graduate, No. (%) | 762 (81.2) | 751 (79.9) | 760 (80.9) | 737 (78.5) | .44 |
| Current smoking, No. (%) | 120 (12.8) | 95 (10.1) | 110 (11.7) | 155 (16.5) | <.001 |
| Weekly alcohol drinking, No. (%) | 220 (23.4) | 233 (24.8) | 261 (27.8) | 254 (27.1) | .11 |
| Physical activity, mean (SD), METs/wk | 190.5 (130.3) | 201.7 (144.4) | 206.5 (155.3) | 197.9 (146.9) | .11 |
| Hypertension, No. (%) | 753 (80.2) | 813 (86.5) | 814 (86.7) | 853 (90.8) | <.001 |
| Diabetes, No. (%) | 354 (37.7) | 412 (43.8) | 463 (49.3) | 566 (60.3) | <.001 |
| History of CVD, No. (%) | 256 (27.3) | 282 (30.0) | 328 (34.9) | 373 (39.7) | <.001 |
| Antihypertensive medication, No. (%) | 807 (86.5) | 856 (92.0) | 873 (93.6) | 890 (95.4) | <.001 |
| Diuretics | 528 (56.6) | 537 (57.7) | 531 (56.9) | 608 (65.2) | <.001 |
| RAS blocking agents | 564 (60.5) | 677 (72.8) | 664 (71.2) | 662 (71.0) | <.001 |
| Other antihypertensive medications | 611 (65.5) | 628 (67.5) | 677 (72.6) | 711 (76.2) | <.001 |
| Lipid-lowering medication, No. (%) | 491 (52.6) | 536 (57.6) | 601 (64.4) | 609 (65.3) | <.001 |
| Antidiabetic medication, No. (%) | 310 (33.2) | 372 (40.0) | 422 (45.2) | 508 (54.4) | <.001 |
| Systolic BP, mean (SD), mm Hg | 125.7 (21.7) | 126.3 (20.9) | 128.1 (21.7) | 132.3 (22.4) | <.001 |
| Diastolic BP, mean (SD), mm Hg | 70.7 (12.7) | 71.0 (12.8) | 71.4 (12.3) | 72.7 (13.0) | .006 |
| Waist circumference, mean (SD), cm | 104.4 (18.5) | 105.1 (16.8) | 106.0 (17.1) | 107.2 (17.2) | .004 |
| Body mass index, mean (SD)b | 31.7 (8.0) | 32.1 (7.5) | 31.9 (7.3) | 31.8 (7.5) | .70 |
| Lean body mass index, mean (SD)c | 20.0 (4.1) | 20.7 (3.8) | 21.3 (4.3) | 22.0 (4.2) | <.001 |
| Daily total calorie intake, mean (SD), kcal | 1745 (744) | 1792 (790) | 1853 (824) | 1948 (905) | <.001 |
| LDL cholesterol, mean (SD), mg/dL | 105.3 (36.5) | 103.9 (34.2) | 101.2 (35.5) | 99.0 (35.3) | <.001 |
| HDL cholesterol, mean (SD), mg/dL | 49.7 (16.5) | 48.3 (16.0) | 46.7 (14.5) | 45.3 (14.5) | <.001 |
| Triglycerides, mean (SD), mg/dL | 147.0 (119.4) | 156.0 (123.5) | 158.9 (113.7) | 164.1 (107.5) | .01 |
| Glucose, mean (SD), mg/dL | 109.2 (46.9) | 111.4 (49.5) | 113.6 (46.5) | 124.0 (55.8) | <.001 |
| HbA1c, mean (SD), % | 6.4 (1.4) | 6.6 (1.6) | 6.6 (1.4) | 6.9 (1.7) | <.001 |
| Urinary creatinine, mean (SD), mg/24 h | 1384 (490) | 1404 (472) | 1410 (474) | 1298 (417) | <.001 |
| Urinary sodium, mean (SD), mg/24 h | 2491 (870) | 3364 (925) | 4008 (1096) | 4941 (1518) | <.001 |
| Urinary potassium, mean (SD), mg/24 h | 2003 (854) | 2065 (820) | 2223 (1052) | 2352 (984) | <.001 |
| Calibrated sodium excretion, mean (SD), mg/24 ha | 2345 (436) | 3278 (216) | 4065 (249) | 5776 (1361) | <.001 |
| Calibrated potassium excretion, mean (SD), mg/24 ha | 1960 (862) | 2061 (777) | 2288 (909) | 2745 (1038) | <.001 |
| Urinary protein, median (IQR), g/24 h | 0.11 (0.06–0.53) | 0.12 (0.07–0.57) | 0.20 (0.08–0.85) | 0.49 (0.11–1.94) | <.001 |
| eGFR, mean (SD), mL/min/1.73 m2 | 45.4 (15.9) | 45.5 (14.5) | 44.0 (14.9) | 42.9 (14.1) | <.001 |
Abbreviations: BP, blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; METs, metabolic equivalents; RAS, renin-angiotensin system.
SI conversion factors: To convert LDL and HDL cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113; and glucose to mmol/L, multiply by 0.0555.
Calibrated to mean urinary creatinine excretion of 1569 mg/24 hours in men and 1130 mg/24 hours in women.
Calculated as weight in kilograms divided by height in meters squared.
Calculated as fat-free mass in kilograms divided by height in meters squared.
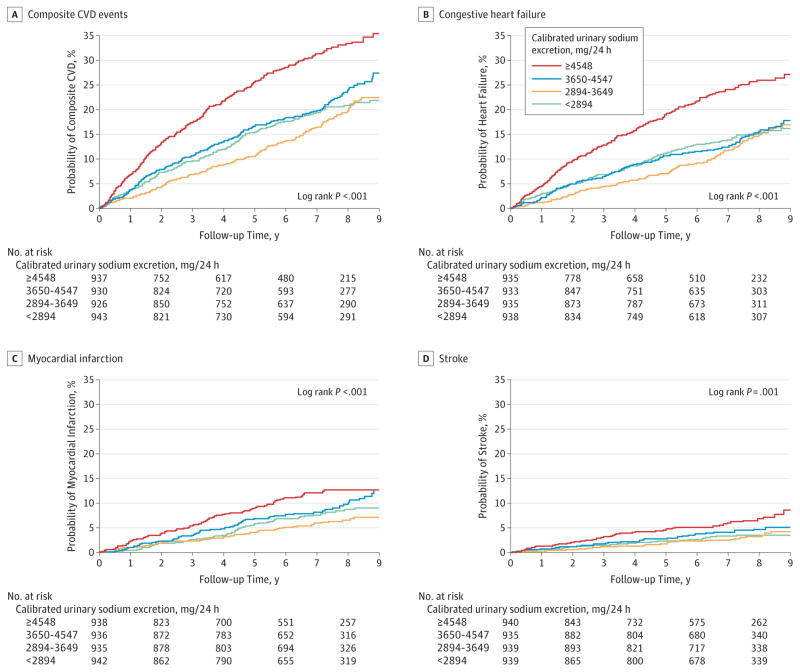
Over a median 6.8 years of follow-up, 804 composite first CVD events, 575 CHF events, 305 MI events, and 148 stroke events occurred, corresponding to incidence rates of 35.7 CVD events per 1000 person-years, 24.6 CHF events per 1000 person-years, 12.6 MI events per 1000 person-years, and 6.0 stroke events per 1000 person-years. From lowest (<2894 mg/24 hours) to highest (≥4548 mg/24 hours) quartile of calibrated sodium excretion, the cumulative incidence of composite CVD at median follow-up was 18.4%, 16.5%, 20.6%, and 29.8% (log-rank P < .001); the cumulative incidence of CHF was 13.3%, 12.0%, 13.3%, and 23.2% (log-rank P < .001); the cumulative incidence of MI was 7.8%, 5.8%,8.6%, and 10.9% (log-rank P < .001); and the cumulative incidence of stroke was 2.7%, 2.8%, 4.1%, and 6.4%(log-rank P = .001), respectively (Figure 1 and Table 2).
Figure 1.
Cumulative Kaplan-Meier Estimates of Cardiovascular Diseases According to Quartile of Calibrated 24-Hour Urinary Sodium Excretion
Because participants who only had urinary sodium excretion measurements after an outcome occurred were excluded from the analysis for that outcome, there are slightly fewer people at time = 0 years for each individual outcome compared with the 3757 participants described in Table 1, who contributed to at least 1 outcome but not necessarily all of them.
Table 2.
Composite Cardiovascular Disease, Congestive Heart Failure, Myocardial Infarction, and Stroke According to Quartile of Calibrated 24-Hour Urinary Sodium Excretion
| Variable | No. of Participants | Calibrated Urinary Sodium Excretion, mg/24 ha | ||||
|---|---|---|---|---|---|---|
| <2894 | 2894–3649 | 3650–4547 | ≥4548 | P Value for Trend | ||
| No. of participants | 939 | 940 | 939 | 939 | ||
| Composite CVDb | ||||||
| Events | 174 | 159 | 198 | 273 | ||
| Person-years | 5804 | 5972 | 5739 | 5012 | ||
| Cumulative incidence at median 6.8 y | 18.4 (15.8–20.9) | 16.5 (14.1–18.8) | 20.6 (18.0–23.1) | 29.8 (26.7–32.7) | <.001 | |
| follow-up, % (95% CI)c | ||||||
| Model 1, HR (95% CI)d | 3736 | 1 [Reference] | 0.88 (0.71–1.10) | 1.14 (0.93–1.41) | 1.79 (1.46–2.19) | <.001 |
| P value | .27 | .22 | <.001 | |||
| Model 2, HR (95% CI) | 3528 | 1 [Reference] | 0.85 (0.68–1.07) | 0.99 (0.80–1.24) | 1.31 (1.05–1.63) | .002 |
| P value | .17 | .96 | .02 | |||
| Model 3, HR (95% CI) | 3528 | 1 [Reference] | 0.87 (0.69–1.10) | 1.01 (0.81–1.26) | 1.36 (1.09–1.70) | <.001 |
| P value | .24 | .96 | .007 | |||
| Congestive Heart Failure | ||||||
| Events | 125 | 117 | 127 | 206 | ||
| Person-years | 5938 | 6216 | 5998 | 5235 | ||
| Cumulative incidence at median 6.8 y follow-up, % (95% CI)c | 13.3 (11.0–15.5) | 12.0 (9.9–14.0) | 13.3 (11.1–15.4) | 23.2 (20.3–26.0) | <.001 | |
| Model 1, HR (95% CI)d | 3741 | 1 [Reference] | 0.90 (0.70–1.16) | 1.01 (0.78–1.30) | 1.90 (1.50–2.41) | <.001 |
| P value | .41 | .96 | <.001 | |||
| Model 2, HR (95% CI) | 3533 | 1 [Reference] | 0.86 (0.66–1.13) | 0.83 (0.63–1.08) | 1.27 (0.98–1.65) | .02 |
| P value | .27 | .16 | .08 | |||
| Model 3, HR (95% CI) | 3533 | 1 [Reference] | 0.89 (0.68–1.17) | 0.84 (0.65–1.11) | 1.34 (1.03–1.74) | .008 |
| P value | .40 | .22 | .03 | |||
| Myocardial Infarction | ||||||
| Events | 69 | 54 | 83 | 99 | ||
| Person-years | 6195 | 6336 | 6175 | 5569 | ||
| Cumulative incidence at median 6.8 y follow-up, % (95% CI)c | 7.8 (6.0–9.7) | 5.8 (4.3–7.3) | 8.6 (6.8–10.3) | 10.9 (8.7–12.9) | .002 | |
| Model 1, HR (95% CI)d | 3751 | 1 [Reference] | 0.73 (0.51–1.04) | 1.10 (0.79–1.53) | 1.42 (1.02–1.98) | .002 |
| P value | .08 | .57 | .04 | |||
| Model 2, HR (95% CI) | 3540 | 1 [Reference] | 0.66 (0.44–0.97) | 1.00 (0.70–1.43) | 1.12 (0.77–1.62) | .14 |
| P value | .03 | .99 | .56 | |||
| Model 3, HR (95% CI) | 3540 | 1 [Reference] | 0.66 (0.45–0.97) | 1.00 (0.70–1.43) | 1.15 (0.79–1.66) | .11 |
| P value | .04 | .99 | .46 | |||
| Stroke | ||||||
| Events | 28 | 28 | 39 | 53 | ||
| Person-years | 6293 | 6479 | 6337 | 5719 | ||
| Cumulative incidence at median 6.8 y follow-up, % (95% CI)c | 2.7 (1.6–3.7) | 2.8 (1.7–3.8) | 4.1 (2.8–5.3) | 6.4 (4.6–8.1) | <.001 | |
| Model 1, HR (95% CI)d | 3753 | 1 [Reference] | 1.04 (0.61–1.77) | 1.55 (0.94–2.54) | 2.51 (1.55–4.07) | <.001 |
| P value | .88 | .09 | <.001 | |||
| Model 2, HR (95% CI) | 3542 | 1 [Reference] | 0.92 (0.53–1.59) | 1.38 (0.83–2.29) | 1.80 (1.08–3.00) | .007 |
| P value | .76 | .21 | .02 | |||
| Model 3, HR (95% CI) | 3542 | 1 [Reference] | 0.93 (0.54–1.61) | 1.38 (0.83–2.29) | 1.81 (1.08–3.02) | .006 |
| P value | .79 | .21 | .02 | |||
Abbreviations: CVD, cardiovascular disease; HR, hazard ratio.
Calibrated to mean urinary creatinine excretion of 1569 mg/24 hours in men and 1130 mg/24 hours in women.
Composite CVD is defined as congestive heart failure, stroke, and myocardial infarction.
Adjusted for age, sex, race, and clinic site.
Model 1: adjusted for age, sex, race, and clinic site. Model 2: model 1 plus education; waist circumference; lean body mass index; body mass index; cigarette smoking; alcohol drinking; physical activity; low-density lipoprotein cholesterol; glucose; history of CVD; use of antidiabetic medications, lipid-lowering medications, diuretics, renin-angiotensin system blocking agents, and other antihypertensive medications; and urinary creatinine excretion. Model 3: model 2 plus adjustment for baseline estimated glomerular filtration rate.
After adjustment for age, sex, race, and clinic site, those in the highest quartile of calibrated urinary sodium excretion were at an increased risk for composite CVD, CHF, MI, and stroke compared with the lowest quartile of calibrated urinary sodium excretion (Table 2). After additional adjustment for modifiable CVD risk factors, calibrated sodium excretion remained significantly associated with composite CVD, CHF, and stroke but was no longer significantly associated with MI. In fully adjusted models, including adjustment for baseline eGFR, the highest quartile of urinary sodium excretion was significantly associated with composite CVD (hazard ratio [HR], 1.36 [95% CI, 1.09–1.70]; P = .007), CHF (HR, 1.34 [95% CI, 1.03–1.74]; P = .03), and stroke (HR, 1.81 [95% CI, 1.08–3.02]; P = .02), but not with MI (HR, 1.15 [95% CI, 0.79–1.66]; P = .46). Results for urinary sodium excretion not calibrated with urinary creatinine excretion were similar to those using calibrated urinary sodium excretion (eTable 1 in the Supplement). For example, compared with the lowest quartile of urinary sodium excretion (<2686 mg/24 hours), those in the highest quartile of urinary sodium excretion (≥4474 mg/24 hours) were at an increased risk for composite CVD (HR, 1.35 [95% CI, 1.05–1.72]; P = .02), CHF (HR, 1.25 [95% CI, 0.94–1.67]; P = .12), MI (HR, 1.30 [95% CI, 0.87–1.95]; P = .20), and stroke (HR, 1.91 [95% CI, 1.08–3.33]; P = .03).
Multivariable-adjusted restricted cubic spline analyses suggested no evidence of a nonlinear association between calibrated urinary sodium excretion and composite CVD, MI, or stroke (Figure 2). They also indicated a significant linear association between calibrated urinary sodium excretion and composite CVD and stroke, but not with MI. There was no evidence of a nonlinear association between uncalibrated urinary sodium excretion and any CVD outcome using restricted cubic spline analyses (eFigure 1 in the Supplement). There was evidence of a significant linear relationship between uncalibrated urinary sodium excretion and composite CVD (P = .002), stroke (P = .02), and MI (P = .048).
Figure 2.
Multiple-Adjusted Hazard Ratios and 95% Confidence Intervals of Cardiovascular Diseases Associated With Calibrated 24-Hour Urinary Sodium Excretion
Hazard ratios were adjusted for age, sex, race, clinic site, education, waist circumference, lean body mass index, body mass index, cigarette smoking, alcohol drinking, physical activity, low-density lipoprotein cholesterol, glucose, history of cardiovascular disease, antidiabetic medications, lipid-lowering medications, diuretics, renin-angiotensin system blocking agents, other antihypertensive medications, urinary creatinine excretion, and baseline estimated glomerular filtration rate.
Hazard ratios for composite CVD, CHF, MI, and stroke associated with a 1000-mg/24-hour increase in calibrated urinary sodium excretion were 1.10 (95% CI, 1.05–1.16; P < .001), 1.09 (95% CI, 1.02–1.15; P = .005), 1.07 (95% CI, 0.98–1.16; P = .11), and 1.16 (95% CI, 1.05–1.28; P = .003), respectively (Table 3). The magnitude of these associations was consistent across predefined subgroups of sex, race, age, and history of diabetes and CVD, although some did not reach statistical significance. In addition, no significant P values for interaction were observed for any of the subgroups of interest with calibrated sodium excretion. Sensitivity analyses that additionally adjusted for total calorie intake and systolic blood pressure did not substantially change the magnitude of any of the associations between a 1000-mg/24-hour increase in calibrated urinary sodium and the CVD outcomes of interest (eTable 2 in the Supplement).
Table 3.
Composite Cardiovascular Disease, Congestive Heart Failure, Myocardial Infarction, and Stroke Associated With a 1000-mg Difference in Calibrated 24-Hour Urinary Sodium Excretiona
| Subgroups | Calibrated Urinary Sodium Excretion per 1000 mg/24 hb | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | HR (95% CI) | P Value | P Value for Interaction | No. | HR (95% CI) | P Value | P Value for Interaction | |
| Composite CVDc | Congestive Heart Failure | |||||||
| Overall | 3528 | 1.10 (1.05–1.16) | <.001 | 3533 | 1.09 (1.02–1.15) | .005 | ||
| Sex | .55 | .55 | ||||||
| Men | 1946 | 1.12 (1.06–1.18) | <.001 | 1949 | 1.10 (1.03–1.18) | .006 | ||
| Women | 1582 | 1.09 (0.99–1.20) | .07 | 1584 | 1.07 (0.96–1.20) | .20 | ||
| Race | .44 | .23 | ||||||
| Black | 1460 | 1.14 (1.06–1.23) | <.001 | 1461 | 1.15 (1.05–1.26) | .003 | ||
| Nonblack | 2068 | 1.08 (1.02–1.16) | .02 | 2072 | 1.05 (0.97–1.14) | .23 | ||
| Age, y | .14 | .13 | ||||||
| <60 | 1759 | 1.11 (1.04–1.19) | .001 | 1760 | 1.10 (1.02–1.19) | .02 | ||
| ≥60 | 1769 | 1.08 (1.00–1.16) | .04 | 1773 | 1.06 (0.97–1.15) | .20 | ||
| Diabetes status | .52 | .74 | ||||||
| Diabetes | 1674 | 1.11 (1.05–1.17) | <.001 | 1677 | 1.09 (1.02–1.16) | .01 | ||
| No diabetes | 1854 | 1.10 (1.00–1.21) | .06 | 1856 | 1.07 (0.94–1.21) | .33 | ||
| History of CVD | .50 | .29 | ||||||
| CVD history | 1135 | 1.12 (1.05–1.20) | <.001 | 1138 | 1.09 (1.01–1.18) | .02 | ||
| No CVD history | 2393 | 1.08 (1.00–1.17) | .05 | 2395 | 1.09 (0.99–1.19) | .08 | ||
| Myocardial Infarction | Stroke | |||||||
| Overall | 3540 | 1.07 (0.98–1.16) | .11 | 3542 | 1.16 (1.05–1.28) | .003 | ||
| Sex | .68 | .97 | ||||||
| Men | 1951 | 1.06 (0.96–1.17) | .23 | 1950 | 1.15 (1.02–1.30) | .02 | ||
| Women | 1589 | 1.13 (0.96–1.34) | .14 | 1592 | 1.20 (0.98–1.47) | .08 | ||
| Race | .30 | .69 | ||||||
| Black | 1468 | 0.99 (0.85–1.15) | .88 | 1472 | 1.22 (1.05–1.42) | .01 | ||
| Nonblack | 2072 | 1.10 (0.99–1.21) | .07 | 2070 | 1.10 (0.94–1.29) | .24 | ||
| Age, y | .66 | .79 | ||||||
| <60 | 1766 | 1.11 (0.98–1.25) | .10 | 1767 | 1.17 (1.02–1.34) | .03 | ||
| ≥60 | 1774 | 1.05 (0.93–1.18) | .42 | 1775 | 1.15 (0.98–1.34) | .09 | ||
| Diabetes status | .64 | .98 | ||||||
| Diabetes | 1682 | 1.08 (0.98–1.19) | .11 | 1684 | 1.17 (1.05–1.32) | .006 | ||
| No diabetes | 1858 | 1.03 (0.86–1.23) | .74 | 1858 | 1.13 (0.93–1.36) | .21 | ||
| History of CVD | .74 | .89 | ||||||
| CVD history | 1140 | 1.09 (0.98–1.21) | .10 | 1140 | 1.19 (1.05–1.35) | .005 | ||
| No CVD history | 2400 | 1.04 (0.90–1.21) | .59 | 2402 | 1.08 (0.91–1.29) | .38 | ||
Abbreviations: CVD, cardiovascular disease; HR, hazard ratio.
Adjusted for age, sex, race, clinic site, education, waist circumference, lean body mass index, body mass index, cigarette smoking, alcohol drinking, physical activity, low-density lipoprotein cholesterol, glucose, history of CVD, antidiabetic medications, lipid-lowering medications, diuretics, renin-angiotensin system blocking agents, other antihypertensive medications, urinary creatinine excretion, and baseline estimated glomerular filtration rate.
Calibrated to mean urinary creatinine excretion of 1569 mg/24 hours in men and 1130 mg/24 hours in women.
Composite CVD is defined as congestive heart failure, stroke, and myocardial infarction.
Baseline characteristics of CRIC participants by quartile of calibrated 24-hour urinary potassium excretion are presented in eTable 3 in the Supplement. In unadjusted analyses, higher calibrated urinary potassium excretion was significantly associated with cumulative incidence of composite CVD (cumulative incidence at median follow-up of 28.2% in highest quartile compared with 17.2% in lowest quartile; log-rank P < .001) and CHF (cumulative incidence of 21.1% in highest quartile compared with 11.9% in lowest quartile; log-rank P = .03), but not significantly associated with MI (cumulative incidence of 9.7% in highest quartile compared with 7.2% in lowest quartile; log-rank P = .08) or stroke (cumulative incidence of 5.7% in highest quartile compared with 3.1% in lowest quartile; log-rank P = .50) (eFigure 2 and eTable 4 in the Supplement). Higher calibrated potassium excretion was associated with composite CVD, CHF, and stroke after adjustment for age, sex, race, and clinic site (eTable 4), but after full multivariable adjustment, calibrated potassium excretion was no longer associated with any of the outcomes of interest. Spline analysis showed a significant linear association with composite CVD (P for linearity = .048) and CHF (P for linearity = .03), but no evidence of an association between urinary potassium excretion and MI or stroke (eFigure 3 in the Supplement).
In a sensitivity analysis of spline regression using alternate numbers of knots and middle knot locations, the associations between 24-hour urinary sodium excretion and cardiovascular disease were consistent (eTable 5 in the Supplement).
Discussion
To our knowledge, this study is the first to investigate the association between sodium excretion and CVD incidence in a population with CKD. These analyses documented a significantly increased risk of CVD in individuals with the highest urinary sodium excretion independent of several important CVD risk factors, including use of antihypertensive medications, baseline eGFR, and history of CVD. Findings were consistent across subgroups and independent of further adjustment for total caloric intake and systolic blood pressure. However, CVD risk was not significantly different among participants with a urinary sodium excretion of 4547 mg/24 hours or less. These findings, if confirmed by clinical trials, suggest that moderate sodium reduction among patients with CKD and high sodium intake may lower CVD risk.
Very limited prior research on the association of dietary sodium with the risk of CVD in patients with impaired kidney function has been conducted.13,14 Dong et al13 reported an increase in risk of CVD mortality in those with the lowest mean sodium intake measured from a 3-day diet record among 305 Japanese peritoneal dialysis patients in a retrospective cohort study. Using data from 2 clinical trials investigating the efficacy of angiotensin receptor blockers (ARBs) on kidney outcomes in patients with type 2 diabetes and overt proteinuria, Lambers Heerspink et al14 conducted a post hoc analysis and found that among those with lower sodium excretion, ARBs were more effective at decreasing kidney and CVD risk. These findings from previous studies, conducted in either a retrospective cohort of peritoneal dialysis patients or in clinical trial participants with diabetes and overt proteinuria, are not directly applicable to patients with CKD in the general population. In contrast, the CRIC Study is a large cohort of diverse participants with CKD that was designed to investigate risk factors for CVD in those with CKD. Therefore, this study should provide an estimate of the association between dietary sodium intake and CVD incidence that is more applicable to those with CKD in the general population.
The results of this study provide evidence of a positive relationship between urinary sodium excretion and clinical CVD with individuals having the highest sodium excretion at the greatest risk of CVD events. Prior work has reported mixed results on the nature of the association between dietary sodium and CVD. Some prior studies have found a positive association between dietary sodium intake and risk of CVD, coronary heart disease, CHF, and stroke,9–11,23,24 while others have reported an inverse or J-shaped association between dietary sodium intake and CVD events. 7,8,25–27 The findings in this analysis as a whole support a positive association between urinary sodium excretion and CVD, which is particularly strong in individuals with the highest urinary sodium excretion.
Several methodologic limitations have been described that might account for the inconsistent findings in the sodium-CVD association, including measurement error in dietary sodium intake, confounding, and collinearity of nutritional explanatory variables.6,28 To address these limitations, this study used repeated 24-hour urine samples. If conducted properly, 24-hour urine collection for sodium excretion is considered the most accurate method of estimating dietary sodium intake at the individual level. It was estimated that approximately 90% of consumed sodium is absorbed and excreted in the urine, and the correlation between measured dietary sodium intake and urinary sodium excretion was more than 0.75.6,15 The CRIC Study also has well-quantified data on a large number of potential confounding factors that were adjusted in these analyses. In addition, the correlations of urinary sodium excretion with potential nutritional explanatory variables, including urinary potassium excretion (r = 0.36) and total calorie intake (r = 0.08), were not very strong in the study sample.
The findings reported here were independent of adjustment for systolic blood pressure, suggesting other mechanisms may play a role in the effect of dietary sodium on CVD in CKD patients with high proportions of treated blood pressure. This is consistent with previous findings of a positive association between dietary sodium and CVD independent of blood pressure.10,23,24 Some possible mechanisms for the direct effects of dietary sodium intake on CVD include endothelial dysfunction,29 increased oxidative stress leading to vascular damage,30 insulin resistance,31 and direct effects on renin-angiotensin-aldosterone system activity.32
This analysis found no significant association between urinary potassium excretion and CVD after multivariable adjustment. Prior work in the general population has found a protective effect of dietary potassium on CVD risk,33 but to our knowledge, the association between potassium intake or excretion and incident CVD has not been investigated in individuals with CKD. The nature of the associations of dietary potassium intake and excretion with CVD in patients with CKD is challenging to understand as a result of abnormal potassium homeostasis and the confounding effect of medications, such as renin-angiotensin-aldosterone blockers.34 There is also some uncertainty in the degree to which urinary potassium excretion represents dietary potassium intake, particularly in patients with impaired kidney function.35 In addition, dietary potassium restriction is often advised for those with impaired kidney function.36 Further research is needed to better understand the association between potassium intake, potassium excretion, and risk of CVD in those with CKD.
This study should be interpreted in light of several limitations. First, it was not possible to compare clinical outcomes among patients with urinary sodium excretion less than 2300 mg sodium/day or less than 1500 mg sodium/day, which are the recommended low sodium targets in the general population and in patients with CKD,6,37 because of very small sample sizes among these subgroups. Second, only three 24-hour urinary specimens were collected, which might not be enough to accurately reflect habitual intake and could underestimate the associations of interest due to regression dilution bias.38 Third, while many potential confounding factors were adjusted, significant differences across calibrated sodium quartiles were observed for many baseline variables, suggesting confounding by unmeasured or unadjusted factors could be present. Fourth, to our knowledge, the degree to which urinary sodium excretion approximates dietary sodium intake has not been assessed in patients with CKD, whose altered sodium handling by the kidney might affect the intake-excretion relationship. Fifth, total energy intake had substantial missing values (24%), so it was not adjusted in the primary analyses. However, in a sensitivity analysis, the results did not change significantly after adjustment for total energy intake, although residual confounding could be present due to the possibility of inaccurate total energy quantification based on a single food frequency questionnaire.39
Conclusions
Among patients with CKD, higher urinary sodium excretion was associated with increased risk of CVD.
Supplementary Material
Acknowledgments
Funding/Support: This study was funded by a research grant (R01DK074615) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Funding for the CRIC Study was obtained under a cooperative agreement from the NIDDK (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the University of Pennsylvania Clinical and Translational Science Award (NIH/NCATS UL1TR000003), Johns Hopkins University (UL1 TR-000424), University of Maryland (GCRC M01 RR-16500), Clinical and Translational Science Collaborative of Cleveland (UL1TR000439), Michigan Institute for Clinical and Health Research (UL1TR000433), University of Illinois at Chicago (CTSA UL1RR029879), Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases (P20 GM109036), and Kaiser (NIH/NCRR UCSF-CTSI UL1 RR-024131). Dr Mills is supported in part by the National Heart, Lung, and Blood Institute Cardiovascular Epidemiology training grant (T32HL007024, principal investigator: Coresh).
Role of the Funder/Sponsor: John Kusek, PhD, program director at the National Institute of Diabetes and Digestive and Kidney Diseases, contributed to the design and conduct of the study; analysis and interpretation of the data; and review and approval of the manuscript. Otherwise, the sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Mills and He had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chen, Appel, Kusek, Mohler, Ojo, Rahman, He.
Acquisition, analysis, or interpretation of data: Mills, Chen, Yang, Appel, Alper, Delafontaine, Keane, Rahman, Ricardo, Soliman, Steigerwalt, Townsend, He.
Drafting of the manuscript: Mills, He.
Critical revision of the manuscript for important intellectual content: Mills, Chen, Yang, Appel, Kusek, Alper, Delafontaine, Keane, Mohler, Ojo, Rahman, Ricardo, Soliman, Steigerwalt, Townsend, He.
Statistical analysis: Mills, Yang, Keane, He.
Obtained funding: Appel, Ojo, Rahman, Townsend, He.
Administrative, technical, or material support: Mills, Appel, Kusek, Alper, Keane, Ricardo, Steigerwalt, Townsend, He.
Study supervision: Appel, Alper, Delafontaine, Mohler, Ojo, Rahman, He.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Steigerwalt reported having received support from Medtronic as a principal investigator for 2 trials and travel expenses from ATCOR. Dr Townsend reported having received support from Medtronic, Janssen, Relypsa, and UpToDate. No other disclosures were reported.
References
- 1.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Matsushita K, van der Velde M, Astor BC, et al. Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 5.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- 6.IOM (Institute of Medicine) Sodium Intake in Populations: Assessment of Evidence. Washington, DC: National Academies Press; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donnell MJ, Yusuf S, Mente A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306(20):2229–2238. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 8.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, et al. European Project on Genes in Hypertension (EPOGH) Investigators. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305(17):1777–1785. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 9.Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129(9):981–989. doi: 10.1161/CIRCULATIONAHA.113.006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J, Ogden LG, Vupputuri S, Bazzano LA, Loria C, Whelton PK. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA. 1999;282(21):2027–2034. doi: 10.1001/jama.282.21.2027. [DOI] [PubMed] [Google Scholar]
- 11.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Dietary sodium intake and incidence of congestive heart failure in overweight US men and women: first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med. 2002;162(14):1619–1624. doi: 10.1001/archinte.162.14.1619. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodríguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346(12):913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 13.Dong J, Li Y, Yang Z, Luo J. Low dietary sodium intake increases the death risk in peritoneal dialysis. Clin J Am Soc Nephrol. 2010;5(2):240–247. doi: 10.2215/CJN.05410709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambers Heerspink HJ, Holtkamp FA, Parving HH, et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82(3):330–337. doi: 10.1038/ki.2012.74. [DOI] [PubMed] [Google Scholar]
- 15.Holbrook JT, Patterson KY, Bodner JE, et al. Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr. 1984;40(4):786–793. doi: 10.1093/ajcn/40.4.786. [DOI] [PubMed] [Google Scholar]
- 16.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study Group. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diet history questionnaire, version 1.0. National Institutes of Health, Applied Research Program, National Cancer Institute; 2007. [Google Scholar]
- 18.Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88(5 pt 1):2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 19.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481. [Google Scholar]
- 21.Cox DR. Regression models and life tables. J R Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 22.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 23.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) BMJ. 2007;334(7599):885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagata C, Takatsuka N, Shimizu N, Shimizu H. Sodium intake and risk of death from stroke in Japanese men and women. Stroke. 2004;35(7):1543–1547. doi: 10.1161/01.STR.0000130425.50441.b0. [DOI] [PubMed] [Google Scholar]
- 25.Alderman MH, Madhavan S, Cohen H, Sealey JE, Laragh JH. Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertension. 1995;25(6):1144–1152. doi: 10.1161/01.hyp.25.6.1144. [DOI] [PubMed] [Google Scholar]
- 26.Cohen HW, Hailpern SM, Fang J, Alderman MH. Sodium intake and mortality in the NHANES II follow-up study. Am J Med. 2006;119(3):275.e7–275.e14. doi: 10.1016/j.amjmed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 27.Ekinci EI, Clarke S, Thomas MC, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011;34(3):703–709. doi: 10.2337/dc10-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whelton PK, Appel LJ, Sacco RL, et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126(24):2880–2889. doi: 10.1161/CIR.0b013e318279acbf. [DOI] [PubMed] [Google Scholar]
- 29.Currie G, Delles C. Proteinuria and its relation to cardiovascular disease. Int J Nephrol Renovasc Dis. 2013;7:13–24. doi: 10.2147/IJNRD.S40522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobrian AD, Schriver SD, Lynch T, Prewitt RL. Effect of salt on hypertension and oxidative stress in a rat model of diet-induced obesity. Am J Physiol Renal Physiol. 2003;285(4):F619–F628. doi: 10.1152/ajprenal.00388.2002. [DOI] [PubMed] [Google Scholar]
- 31.Ogihara T, Asano T, Fujita T. Contribution of salt intake to insulin resistance associated with hypertension. Life Sci. 2003;73(5):509–523. doi: 10.1016/s0024-3205(03)00315-1. [DOI] [PubMed] [Google Scholar]
- 32.Frohlich ED. The salt conundrum: a hypothesis. Hypertension. 2007;50(1):161–166. doi: 10.1161/HYPERTENSIONAHA.107.088328. [DOI] [PubMed] [Google Scholar]
- 33.Yang Q, Liu T, Kuklina EV, et al. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011;171(13):1183–1191. doi: 10.1001/archinternmed.2011.257. [DOI] [PubMed] [Google Scholar]
- 34.Weir MR, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010;5(3):531–548. doi: 10.2215/CJN.07821109. [DOI] [PubMed] [Google Scholar]
- 35.Sandle GI, Gaiger E, Tapster S, Goodship THJ. Enhanced rectal potassium secretion in chronic renal insufficiency: evidence for large intestinal potassium adaptation in man. Clin Sci (Lond) 1986;71(4):393–401. doi: 10.1042/cs0710393. [DOI] [PubMed] [Google Scholar]
- 36.Musso CG. Potassium metabolism in patients with chronic kidney disease (CKD), part I: patients not on dialysis (stages 3–4) Int Urol Nephrol. 2004;36(3):465–468. doi: 10.1007/s11255-004-6193-z. [DOI] [PubMed] [Google Scholar]
- 37.KDOQI. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(2 suppl 2):S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Liu K, Cooper R, McKeever J, et al. Assessment of the association between habitual salt intake and high blood pressure: methodological problems. Am J Epidemiol. 1979;110(2):219–226. doi: 10.1093/oxfordjournals.aje.a112806. [DOI] [PubMed] [Google Scholar]
- 39.Subar AF, Kipnis V, Troiano RP, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158(1):1–13. doi: 10.1093/aje/kwg092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.