Abstract
IMPORTANCE
Zika virus (ZIKV) is an emerging arthropod-borne virus (arbovirus) in the genus Flavivirus that has caused a widespread outbreak of febrile illness, is associated with neurological disease, and has spread across the Pacific to the Americas in a short period.
OBSERVATIONS
In this review, we discuss what is currently known about ZIKV, neuroimmunologic complications, and the impact on global human health. Zika virus spread across Africa and Asia in part owing to unique genomic evolutionary conditions and pressures resulting in specific human disease manifestations, complications, and pathogenesis. Recent data suggest that acute ZIKV infection in pregnant women may result in acute infection of fetal tissue and brain tissue, causing microcephaly and potentially severe debilitation of the infant or even death of the fetus. Cases of acute ZIKV are also associated with Guillain-Barré syndrome. With the increased number of cases, new complications such as ocular involvement and sexual transmission have been reported.
CONCLUSIONS AND RELEVANCE
Zika virus is an emerging viral pathogen with significant consequences on human health throughout the world. Ongoing research into this pathogen is urgently needed to produce viable vaccine and therapeutic options.
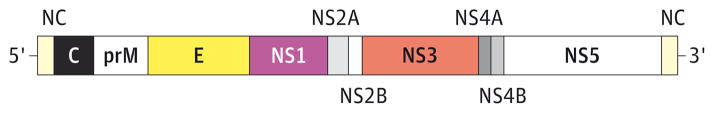
Zika virus (ZIKV) is in the family Flaviviridae, a group of positive-sense, single-stranded, enveloped RNA viruses. Members of the Flavivirus genus within this family cause widespread human diseases, including yellow fever, dengue, Japanese encephalitis, West Nile virus disease, and ZIKV infections. Flaviviruses have a characteristic RNA genomic organization with a capped, 5′ RNA followed by a short noncoding region, a single open-reading frame that codes for a polyprotein, and a 3′ noncoding region (Figure 1). The genomic RNA for flaviviruses is typically 10 to 11 kilobases in length, is structured to function in a similar fashion to host messenger RNAs within the host cell, and can quickly undergo translation by host factors.2 This allows flaviviruses to form replication complexes and create new RNA genomes and viral proteins.
Figure 1. Organization of the Flavivirus Genome.
Flaviviruses have capped RNA genomes with 5′ and 3′ noncoding (NC) regions. Following the 5′ NC region, flavivirus genes are translated as a single polyprotein and cleaved into structural proteins (capsid [C], premembrane [prM], and envelope [E]) and nonstructural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). Adapted from Petersen and Roehrig.1
Discussion
Timeline of the ZIKV Outbreak
Flaviviruses are spread by arthropod vectors, and ZIKV is transmitted by mosquito vectors in the Aedes genus. The first isolation of ZIKV occurred in 1947 from the blood of a febrile sentinel rhesus monkey and Aedes africanus mosquitoes at the edge of the Zika forest of Uganda as part of a project to collect yellow fever virus isolates.3,4 Because it was unclear whether ZIKV could cause human disease, in 1956 a human volunteer who was previously vaccinated against yellow fever virus was inoculated with ZIKV-infected mouse brain suspension; the volunteer subsequently developed a 1-week clinical syndrome of headache, fever, and malaise.5 The first reported natural case of ZIKV infection in a human occurred in 1964, in a European man working in Uganda who developed a 5-day syndrome of frontal headache; a maculopapular rash on the face, neck, trunk, and upper arms; fever; and myalgias.6 The patient was diagnosed as having ZIKV using acute and convalescent serum samples showing development of neutralizing ZIKV antibodies.6 Subsequent serologic data indicated that ZIKV infections occurred in West Africa (Nigeria, Sierra Leone, Gabon, and Senegal) as well as East Africa (Uganda).7–10 Zika virus infections were also found in parts of Asia, including Pakistan, Indonesia, and Malaysia.11–13 Based on nonstructural gene 5 (NS5) gene sequences, 3 lineages of ZIKV were found to represent infection in East Africa, West Africa, and Asia.14
In 2007, an outbreak of ZIKV disease in the Yap State of the Federated States of Micronesia highlighted the epidemic potential of this virus.15 During that outbreak, 59 probable cases and 49 confirmed cases of ZIKV infection were reported.15 Symptoms of acute ZIKV infection included fever, myalgias, rash, and conjunctivitis. An estimated 73% of residents on Yap were infected with ZIKV during the outbreak, with an estimated 900 cases of illness. Surveys and serologic studies indicated that approximately 19% of patients with seroconversion developed clinical illness.15 Despite the large number of cases, no complications were noted in the study: no hospitalizations, hemorrhagic manifestations, or deaths.15 Interestingly, no neurologic complications or fetal complications were noted during this outbreak.
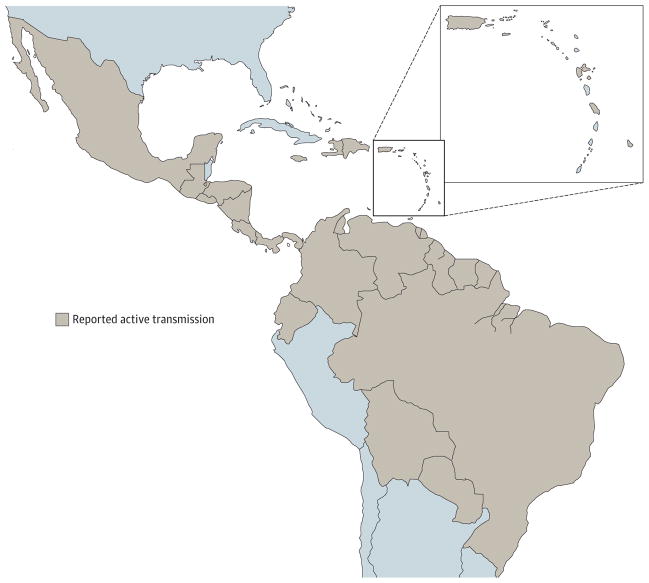
Zikavirus continued to spread east across the Pacific Ocean, with reported outbreaks in French Polynesia in 2013 followed by outbreaks in the Cook Islands, Easter Island, Vanuatu, and the Solomon Islands.16 In 2015, ZIKV appeared in Brazil and phylogenetic studies of gene sequences traced the virus to Asian isolates that had appeared in the outbreaks in the Pacific Islands.17,18 Since the outbreak in Brazil, ZIKV has rapidly spread through South America, Central America, Mexico, and many of the islands in the Caribbean (Figure 2). The outbreak of ZIKV in Brazil was closely associated with the distribution of Aedes mosquitoes, consistent with the known biology of the virus. In early 2015 in Brazil, many of the clinical symptoms remained unchanged from previously reported symptoms associated with acute ZIKV infection; however, by September 2015, reports of an increase in the number of infants born with microcephaly in ZIKV-affected areas began to emerge.20
Figure 2. Map of Current Regions in the Americas Experiencing Active Zika Virus Infections.
Adapted from the Centers for Disease Control and Prevention.19
ZIKV Complications
Prior to the Brazilian outbreak, ZIKV caused illness in about 20% of patients infected with the virus and was associated with an acute, mild, febrile illness with rare reported complications. By 2014, an increased number of Guillain-Barré syndrome (GBS) cases possibly associated with ZIKV infection were reported in French Polynesia and later in South America.21–24 During the outbreak in French Polynesia in November 2013, a Polynesian woman developed GBS 7 days after an acute febrile illness attributed to ZIKV based on serology and the ongoing epidemic on the island.21 The patient presented with bilateral paresthesias and ascending muscle weakness. By day 3 of admission, she developed tetraparesis, diffuse myalgia, and bilateral facial, asymmetric peripheral facial palsy. Deep tendon reflexes were absent, and cerebrospinal fluid examination revealed a white blood cell count of 7/mL with albuminocytological dissociation of 1.66 g/L protein. Results of testing for common causes of GBS were negative, but results for ZIKV-specific IgM were positive at 8 and 28 days after the original acute febrile episode. Zika virus seroconversion was confirmed with plaque reduction neutralization tests against ZIKV. Thus, the patient developed GBS associated with acute ZIKV infection.
With the outbreak in Brazil, reports began to surface of possible other complications associated with ZIKV infection. In 2015, the Brazil Ministry of Health established a task force to investigate the possible association of ZIKV with microcephaly during pregnancy.20 Microcephaly is defined as a decrease in head circumference greater than 2 SDs below the mean for sex and gestational age at birth. During a reported outbreak of ZIKV in northeast Brazil in early 2015,17 the Brazil Ministry of Health confirmed an increase in birth prevalence of microcephaly in the same region compared with previously reported rates of 0.5/10 000 live births.20 Despite likely underreporting of microcephaly prior to the outbreak, the Brazil Ministry of Health established a microcephaly registry and reported an increase in microcephaly cases in areas of the ZIKV outbreak.20 By December 2015, ZIKV RNA was detected in the amniotic fluid of 2 pregnant women whose fetuses were found to have microcephaly and in the brain and tissues of an infant with microcephaly who died immediately in the neonatal period.20,25 Additionally, ZIKV RNA was found in amniotic fluid from 2 women pregnant with children with microcephaly.25
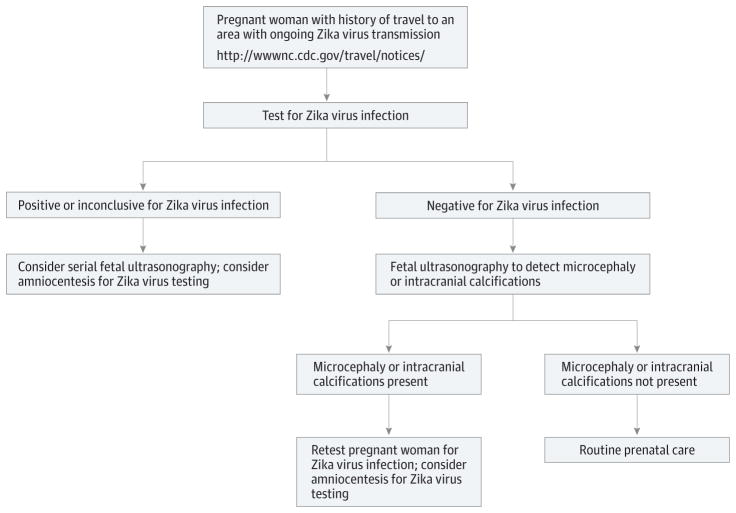
Out of an abundance of precaution, the Centers for Disease Control and Prevention (CDC) issued a travel advisory for ZIKV-affected regions and on February 1, 2016, the World Health Organization declared the ZIKV outbreak a Public Health Emergency of International Concern.26 The CDC also published guidelines for caring for women with possible ZIKV exposure who are or may become pregnant.26 If a pregnant woman has a history of travel to an area with ongoing ZIKV transmission, then the CDC recommends a ZIKV testing algorithm to evaluate for microcephaly (Figure 3).26
Figure 3. Centers for Disease Control and Prevention Algorithm for Evaluation of Pregnant Women With a Possible Exposure to Zika Virus.
Adapted from Oduyebo et al.26
Subsequently, a case report provided additional evidence of microcephaly associated with ZIKV infection.27 A 25-year-old European woman who lived in Brazil became ill during the 13th week of pregnancy with likely ZIKV infection; fetal ultrasonography results at 14 and 20 weeks of gestation were normal.27 By 32 weeks of gestation, ultrasonography of the fetus revealed severe microcephaly. Autopsy of the fetus revealed ZIKV RNA in the fetal brain tissue with 99.7% sequence identity to a ZIKV isolate from the French Polynesia outbreak in 2013. Microscopic evaluation of the fetal brain tissue revealed extensive inflammation, astrocyte and microglial activation, and injury in the cortex and the lateral corticospinal tracts; electron microscopy analysis revealed evidence of flavivirus-like virions in the fetal brain tissue. The complete genome of ZIKV was recovered from the fetal brain tissue and exhibited a high degree of homology to Asian strains and more distant relation to the African strains of the virus.
As the outbreak has continued, other reports of complications and new modes of potential transmission of ZIKV have surfaced. In a small case series of 29 infants with microcephaly born to 29 mothers, 23 of the mothers reported suspected ZIKV symptoms during pregnancy and ocular abnormalities were found in 10 of the 29 children (34.5%) with microcephaly.28 Of these 10 affected children, 7 had bilateral disease; the most common findings were focal pigment mottling of the retina and chorioretinal atrophy in 11 of the 17 eyes with abnormalities (64.7%), followed by optic nerve abnormalities in 8 of the 17 eyes (47.1%). These findings suggest that ZIKV infection in neonates can also cause significant eye disease, and infants born to symptomatic mothers should be screened for eye disease. Additional studies have recently found evidence of human-to-human transmission of ZIKV through sexual transmission.29,30 In support of these observations, ZIKV RNA has now been found in saliva,31 semen, and urine of infected patients.29,32 The duration of infectivity of ZIKV in the semen and urine is not currently known, but in these cases infectious particles of ZIKV were recovered from semen in the absence of viremia,29 implying that infectivity in semen can occur after symptoms and viremia resolve.
There are limited data on the best diagnostic approaches for acute ZIKV infection in humans.33 Unlike acute West Nile virus infection, patients presenting with acute ZIKV symptoms are viremic for a mean of 3 days following initial symptoms such that the virus can be detected in clinical samples using reverse transcription–polymerase chain reaction assay.33 The CDC ZIKV assay uses two 1-step real-time reverse transcription–polymerase chain reactions that target the ZIKV premembrane and envelope genes.33,34 Other ZIKV gene targets have been used for other polymerase chain reaction assays as well. Clinical samples that have tested positive for ZIKV RNA by polymerase chain reaction include serum or plasma, saliva, urine, semen, and amniotic fluid. Serologic diagnosis using assays measuring ZIKV IgM and IgG production with paired acute and convalescent serum is another important diagnostic method.33 However, other common flaviviruses such as dengue virus or Japanese encephalitis virus can exhibit a high degree of serologic cross-reactivity to ZIKV serology even with the use of capture enzyme-linked immunosorbent assay. At times more specific tests such as the plaque reduction neutralization tests can differentiate between flaviviruses, but they still can exhibit low-level cross-neutralization with other flaviviruses.33 Thus, the diagnosis of acute ZIKV infection based on serology alone must be evaluated with caution in regions with other endemic flavivirus infections.
ZIKV Genomic Evolution and Pathogenesis
Owing to the rapidity of sequencing technology, several ZIKV isolates from this outbreak are already reported and available for analysis and comparison with past isolates.14,35–37 A recent analysis of the molecular evolution of ZIKV isolates throughout the emergence of the virus in the 20th century revealed several insights.36 Based on phylogenetic analysis, ZIKV likely first emerged in Uganda around 1920 followed by 2 independent ZIKV introductions into West and Central Africa from the eastern portion of the continent and then a third introduction event into Malaysia around 1945.36 During its emergence, ZIKV underwent 13 recombination events, which is an unusual feature for a flavivirus since these viruses are often genetically restricted by the need to replicate in evolutionarily disparate invertebrate (mosquitoes) and vertebrate hosts.36 The unusual evolutionary plasticity of the ZIKV genome is likely due in part to vertebrate host preferences for the virus,38 which may contribute to ZIKV genomic adaptation to specific vector–vertebrate host environments. This likely resulted in an increased frequency of ZIKV activity every 1 to 2 years when compared with other arboviruses, which exhibit an activity frequency of 5 to 8 years for dengue virus and yellow fever virus.36 The unique genomic features of ZIKV likely contributed to the rapid emergence of the virus over a very large geographic area.
Very little is currently known about the biology and pathogenesis of ZIKV. The envelope protein for flaviviruses is largely responsible for host range due to receptor binding and immune responses. The ZIKV envelope gene underwent several selective changes mainly associated with negative selection, implying an important role for this gene in vertebrate host selectivity and emergence.36 Acute ZIKV infection in 6 human cases was characterized by robust, acute polyclonal T-cell activation and acute increases in serum interleukin (IL) responses (IL-1β, IL-2, IL-4, IL-6, IL-9, IL-13, and IL-17).39 Also, acute infection in these patients was associated with increases in RANTES (regulated on activation, normal T cell expressed and secreted), macrophage inflammatory protein 1α, and vascular endothelial growth factor.39 These data provide some initial information to characterize the acute inflammatory response in these patients.
Cell culture and mouse models of ZIKV are not well established at this stage. Recent data have shown that human dermal fibroblasts, epidermal keratinocytes, human neurospheres,40 and immature dendritic cells are permissive to recent ZIKV isolates and that several entry or adhesion factors (DC-SIGN, AXL, Tyro3, and TIM-1) permitted ZIKV entry, implying that the virus can use a range of host receptors to gain entry into different cell types.41 During acute infection in cell culture, ZIKV induced transcription of Toll-like receptor 3 (TLR3), retinoic acid-inducible gene 1 (RIG-I), and melanoma differentiation-associated protein 5 (MDA5) as well as several common interferon-stimulated genes, implying that the innate immune response found to be important for control of other flaviviruses also plays an important role in the detection and control of ZIKV.41 Zika virus was also sensitive to type I and II interferons in these cell culture systems.41 These data are consistent with what is known about other flaviviruses and help to characterize the role of the immune response for this virus. A better understanding of the mechanisms of immune control of ZIKV will enable targeted vaccination or therapeutic treatments in the future.
A recent mouse model of ZIKV brain infection was developed in interferon signaling–deficient mice.42 Work with classic ZIKV isolates in suckling mice revealed neurotropism and 100% mortality by day 10 following intracerebral injection.3,43–45 These studies revealed neuronal injury especially in the hippocampus characterized by astrocyte activation and new virions on ultrastructural examination in networks of the endoplasmic reticulum of neurons.43 Adult mice in these studies seemed to be more resistant to peripheral infection with ZIKV but did develop neurologic disease following intracerebral infection.44 Further development of animal models for modern ZIKV isolates is needed to understand the complex pathogenesis and immune responses required to prevent disease.
Conclusions
Zika virus has rapidly emerged throughout the 20th century and now is causing a large epidemic of disease in the Americas that has concerning links to possible intrauterine infection of the brains of developing fetuses. There is no current therapy or vaccine for this infection and the best approach to avoid complications from ZIKV is to avoid exposure to mosquitoes by using insect repellant, wearing long-sleeved shirts and pants, and using air conditioning and window screens to keep mosquitoes outside. For women traveling to regions undergoing a current outbreak of ZIKV, cautions must be undertaken in the case of pregnancy as outlined by the CDC.46 For men and women living in the endemic region of the ZIKV outbreak, we need urgent solutions and study of this virus to produce interventions that can prevent birth defects now linked with acute ZIKV infection in pregnant women.
Footnotes
Conflict of Interest Disclosures: None reported.
Author Contributions: Dr Beckham had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Beckham, Pastula, Tyler.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Beckham, Tyler.
Critical revision of the manuscript for important intellectual content: All authors.
Administrative, technical, or material support: Beckham, Pastula.
Study supervision: Beckham, Tyler.
References
- 1.Petersen LR, Roehrig JT. West Nile virus: a reemerging global pathogen. Emerg Infect Dis. 2001;7(4):611–614. doi: 10.3201/eid0704.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shives KD, Beatman EL, Chamanian M, O’Brien C, Hobson-Peters J, Beckham JD. West Nile virus-induced activation of mammalian target of rapamycin complex 1 supports viral growth and viral protein expression. J Virol. 2014;88(16):9458–9471. doi: 10.1128/JVI.01323-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dick GW. Zika virus, II: pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46(5):521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- 4.Dick GW, Kitchen SF, Haddow AJ. Zika virus, I: isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 5.Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg. 1956;50(5):442–448. [PubMed] [Google Scholar]
- 6.Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg. 1964;58:335–338. [PubMed] [Google Scholar]
- 7.Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979;83(2):213–219. doi: 10.1017/s0022172400025997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jan C, Languillat G, Renaudet J, Robin Y. A serological survey of arboviruses in Gabon [in French] Bull Soc Pathol Exot Filiales. 1978;71(2):140–146. [PubMed] [Google Scholar]
- 9.McCrae AW, Kirya BG. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans R Soc Trop Med Hyg. 1982;76(4):552–562. doi: 10.1016/0035-9203(82)90161-4. [DOI] [PubMed] [Google Scholar]
- 10.Monlun E, Zeller H, Le Guenno B, et al. Surveillance of the circulation of arbovirus of medical interest in the region of eastern Senegal [in French] Bull Soc Pathol Exot. 1993;86(1):21–28. [PubMed] [Google Scholar]
- 11.Darwish MA, Hoogstraal H, Roberts TJ, Ahmed IP, Omar F. A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Trans R Soc Trop Med Hyg. 1983;77(4):442–445. doi: 10.1016/0035-9203(83)90106-2. [DOI] [PubMed] [Google Scholar]
- 12.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18(3):411–415. doi: 10.4269/ajtmh.1969.18.411. [DOI] [PubMed] [Google Scholar]
- 13.Olson JG, Ksiazek TG, Suhandiman, Triwibowo Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg. 1981;75(3):389–393. doi: 10.1016/0035-9203(81)90100-0. [DOI] [PubMed] [Google Scholar]
- 14.Baronti C, Piorkowski G, Charrel RN, Boubis L, Leparc-Goffart I, de Lamballerie X. Complete coding sequence of Zika virus from a French Polynesia outbreak in 2013. Genome Announc. 2014;2(3):e00500–e00514. doi: 10.1128/genomeA.00500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 16.Musso D. Zika virus transmission from French Polynesia to Brazil. Emerg Infect Dis. 2015;21(10):1887. doi: 10.3201/eid2110.151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21(10):1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110(4):569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. [Accessed February 10, 2016];Areas with Zika. http://www.cdc.gov/zika/geo/index.html.
- 20.Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al. Brazilian Medical Genetics Society–Zika Embryopathy Task Force. Possible association between Zika virus infection and microcephaly: Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62. doi: 10.15585/mmwr.mm6503e2. [DOI] [PubMed] [Google Scholar]
- 21.Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barré syndrome: case report, French Polynesia, December 2013. Euro Surveill. 2014;19(9):20720. doi: 10.2807/1560-7917.es2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- 22.Pan American Health Organization; World Health Organization. [Accessed February 10, 2016];Zika virus infection. http://www.paho.org/hq/index.php?option=com_topics&view=readall&cid=7880&Itemid=41484&lang=en.
- 23.Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. doi: 10.1016/S0140-6736(16)00562-6. [published online February 29, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watrin L, Ghawché F, Larre P, Neau JP, Mathis S, Fournier E. Guillain-Barré syndrome (42 cases) occurring during a Zika virus outbreak in French Polynesia. Medicine (Baltimore) 2016;95(14):e3257. doi: 10.1097/MD.0000000000003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martines RB, Bhatnagar J, Keating MK, et al. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses: Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(6):159–160. doi: 10.15585/mmwr.mm6506e1. [DOI] [PubMed] [Google Scholar]
- 26.Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure: United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(5):122–127. doi: 10.15585/mmwr.mm6505e2. [DOI] [PubMed] [Google Scholar]
- 27.Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 28.de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol. doi: 10.1001/jamaophthalmol.2016.0267. [published online February 9, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21(2):359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol. 2015;68:53–55. doi: 10.1016/j.jcv.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis. 2015;21(1):84–86. doi: 10.3201/eid2101.140894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waggoner JJ, Pinsky BA. Zika virus: diagnostics for an emerging pandemic threat. J Clin Microbiol. 2016;54(4):860–867. doi: 10.1128/JCM.00279-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enfissi A, Codrington J, Roosblad J, Kazanji M, Rousset D. Zika virus genome from the Americas. Lancet. 2016;387(10015):227–228. doi: 10.1016/S0140-6736(16)00003-9. [DOI] [PubMed] [Google Scholar]
- 36.Faye O, Freire CC, Iamarino A, et al. Molecular evolution of Zika virus during its emergence in the 20th century. PLoS Negl Trop Dis. 2014;8(1):e2636. doi: 10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuno G, Chang GJ. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol. 2007;152(4):687–696. doi: 10.1007/s00705-006-0903-z. [DOI] [PubMed] [Google Scholar]
- 38.Kuno G, Chang GJ. Biological transmission of arboviruses: reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin Microbiol Rev. 2005;18(4):608–637. doi: 10.1128/CMR.18.4.608-637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tappe D, Pérez-Girón JV, Zammarchi L, et al. Cytokine kinetics of Zika virus-infected patients from acute to reconvalescent phase. Med Microbiol Immunol. doi: 10.1007/s00430-015-0445-7. [published online December 24, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcez PP, Loiola EC, Madeiro da Costa R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. doi: 10.1126/science.aaf6116. [published online April 10, 2016] [DOI] [PubMed] [Google Scholar]
- 41.Hamel R, Dejarnac O, Wichit S, et al. Biology of Zika virus infection in human skin cells. J Virol. 2015;89(17):8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazear HM, Govero J, Smith AM, et al. A mouse model of Zika virus pathogenesis. Cell Host Microbe. doi: 10.1016/j.chom.2016.03.010. [published online April 5, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell TM, Field EJ, Narang HK. Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch. 1971;35(2):183–193. doi: 10.1007/BF01249709. [DOI] [PubMed] [Google Scholar]
- 44.Reagan RL, Brueckner AL. Comparison by electron microscopy of the Ntaya and Zika viruses. Tex Rep Biol Med. 1953;11(2):347–351. [PubMed] [Google Scholar]
- 45.Weinbren MP, Williams MC. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg. 1958;52(3):263–268. doi: 10.1016/0035-9203(58)90085-3. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. [Accessed April 22, 2016];Zika virus: for pregnant women. http://www.cdc.gov/zika/pregnancy/