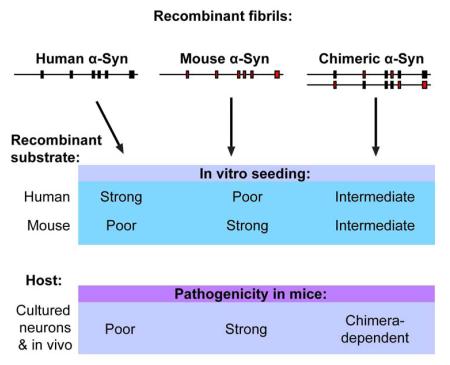
Summary
The accumulation and propagation of misfolded α-Synuclein (α-Syn) is a central feature of Parkinson’s disease (PD) and other synucleinopathies. Molecular compatibility between a fibrillar seed and its native protein state is a major determinant of amyloid self-replication. We show that cross-seeded aggregation of human (Hu) and mouse (Ms) α-Syn is bidirectionally restricted. Although fibrils formed by Hu-Ms-α-Syn chimeric mutants can overcome this inhibition in cell-free systems, sequence homology poorly predicts their efficiency in inducing α-Syn pathology in primary neurons or after intracerebral injection into wildtype mice. Chimeric α-Syn fibrils demonstrate enhanced or reduced pathogenicities compared to wildtype Hu- or Ms-α-Syn fibrils. Furthermore, α-Syn mutants induced to polymerize by fibrillar seeds inherit the functional properties of their template, suggesting transferable pathogenic and non-pathogenic states likely influence the initial engagement between exogenous α-Syn seeds with endogenous neuronal α-Syn. Thus, transmission of synucleinopathies is regulated by biological processes in addition to molecular compatibility.
Graphical abstract
Introduction
The propagation of misfolded proteins is an increasingly recognized disease mechanism in multiple neurodegenerative disorders (Jucker and Walker, 2013). Synucleinopathies, such as PD, are characterized by the intracellular accumulation of aggregated α-Syn (Lee and Trojanowski, 2006). Recent work indicates that fibrillar α-Syn seeds the pathological conversion of normal neuronal α-Syn when pathological α-Syn derived from diseased brains or recombinant α-Syn pre-formed fibrils (PFFs) enter neurons and induces the formation of fibrillary structures similar to Lewy bodies (LBs) and Lewy neurites (LNs) that typify PD (Luk et al., 2012a; Recasens et al., 2014; Volpicelli-Daley et al., 2011). Misfolded α-Syn propagation is further enhanced by the transfer of pathological α-Syn species from affected neurons to healthy ones (Desplats et al., 2009; Luk et al., 2012b) suggesting that the stereotypical spread of α-Syn pathology along neuroanatomical pathways observed in PD patients results from a templated-recruitment process reminiscent of that described for prion diseases (Braak et al., 2003; Dunning et al., 2013).
Despite its potentially central role in disease, little is known about the initial steps by which pathological α-Syn seeds corrupt native cellular α-Syn. Studies of mammalian and yeast amyloids have established that pathological seeding across species is generally inefficient. Resistance to cross-species templating likely arises at the molecular level, where sequence incompatibilities result in amyloid conformations or post-translational modifications that reduce the interface between the amyloid seed and substrate (Angers et al., 2010; Bett et al., 2012; Jones and Surewicz, 2005; Tessier and Lindquist, 2007). Even single mismatches in amino-acid sequence can greatly reduce the pathogenicity of amyloids or the susceptibility of a species as host for propagation (Santoso et al., 2000; Scott et al., 1993; Tycko, 2015). Distinct conformational strains of fibrillar α-Syn which induce dissimilar histopathological phenotypes have also been identified (Bousset et al., 2013; Guo et al., 2013; Peelaerts et al., 2015; Woerman et al., 2015), suggesting that tertiary and quaternary structure may influence disease pathogenesis in synucleinopathies.
The assembly kinetics of α-Syn indicate a nucleation-dependent recruitment process (Biere et al., 2000; Rochet and Lansbury, 2000; Wood et al., 1999). Substitution of the human sequence at either position 53 or 87 with the native mouse residue accelerates fibrillization rates substantially (Kang et al., 2011). Likewise, assembly rates for human-mouse chimeric α-Syn proteins fall between that of the two wildtype (wt) proteins and are proportional to their homology to wt mouse (Mswt α-Syn). Past studies have focused primarily on de novo α-Syn fibrillization in cell-free systems, although seeded fibrillization may be particularly relevant to the cell-to-cell spread likely occurring in PD. Recent studies showed that PFFs generated from Mswt α-Syn cause LBs/LNs within weeks after intracerebral injection in wt mice and are more potent than wt human (Huwt) α-Syn PFFs which induce pathology on the scale of months (Luk et al., 2012a; Masuda-Suzukake et al., 2013). We hypothesized that primary sequence differences between Mswt and Huwt α-Syn (Figure 1A; Larsen et al., 2009) account for this gap in seeding ability and designed a mutagenesis strategy targeting the 7 divergent positions between Mswt and Huwt α-Syn to identify key residues and domains that facilitate efficient templated conversion of α-Syn in vitro, in cultured neurons, and in wt mice.
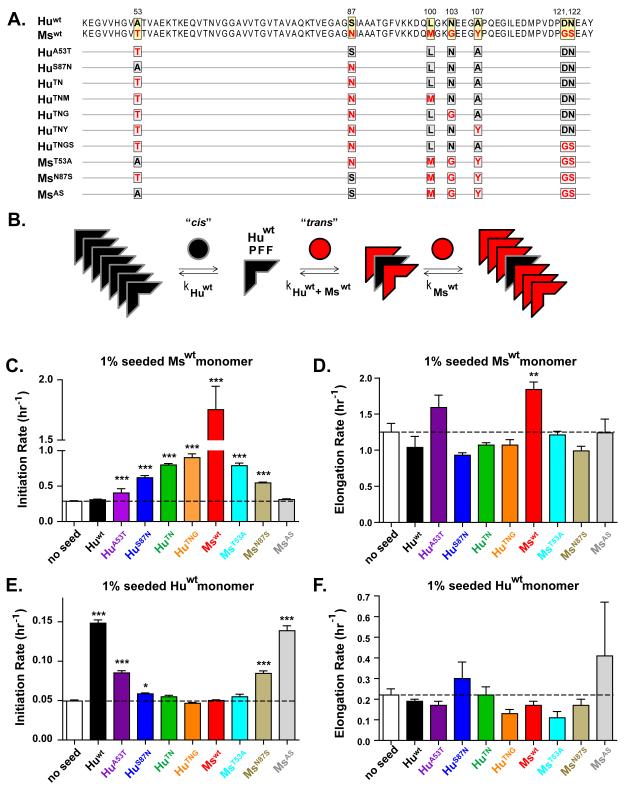
Figure 1. Sequence differences between Huwt and Mswt α-Syn reduce cross-seeded fibrillization rate in vitro.
(A), Amino-acid sequences of Huwt and Mswt α-Syn are denoted in red and black, respectively. Divergent residues are highlighted. Chimeric α-Syn proteins contain orthologous residues at the indicated positions. (B), Schematic of the proposed molecular mechanism for cross-seeded α-Syn aggregation. Huwt monomer (black circles) readily add to Huwt PFFs (black wedges) through cis-templating, but recruitment of Mswt monomer (red circles) is slower due to imperfect trans-templating. Upon overcoming this initial step, recruitment of Mswt monomer is facilitated by complete sequence homology to hybrid PFFs. (C,E), Normalized initiation rates for 1% PFF seeded Mswt (C) and Huwt (E) monomer. Data represent time to reach 50% aggregation. (D,F), Normalized elongation rates (slope from 10%-90% pelletable fibrils) for 1% PFF seeded Mswt (D) and Huwt (F). All data shown as mean ± SEM (N=4). Dashed lines denote rate for unseeded reactions. One-way ANOVA (Tukey post-hoc), *p<0.05, **p<0.01, *** p<0.001 vs Huwt PFFs. See also Figures S1-S3.
Surprisingly, while sequence homology correctly predicted inefficient cross-seeding between Huwt and Mswt α-Syn in both cultured neurons and in wt mice, we found that in vitro seeding efficiencies of chimeric α-Syn PFFs correlated poorly with their ability to induce LBs and LNs in vivo. Finally, mutant α-Syn PFFs can confer this enhanced or reduced propagation capacity to monomers of another sequence, providing evidence that the ectopic seeding efficiencies observed arise from unique properties encoded within α-Syn PFF preparations.
Results
Sequence homology predicts efficiency of α-Syn cross-seeding in vitro
Sequence homology between a misfolded protein (i.e., a fibrillar or oligomeric seed) and its native soluble monomer (substrate) strongly influences the efficiency with which amyloids initiate templated conversion (Cobb and Surewicz, 2009). From a molecular perspective, the current hypothesis supports the concept that the rate of homologous or “self” recruitment (cis templating; Figure 1B, left path) occurs faster than that of cross-seeding, mainly due to delayed elongation caused by the reduced trans-seeding efficacy between the non-homologous seed and substrate. Once levels of converted substrate reach a critical threshold, cis-seeding of this sequence becomes the dominant kinetic factor, resulting in efficient elongation (Figure 1B, right path).
To determine whether seeding by α-Syn PFFs also follows such a relationship, we first confirmed that full-length Huwt, Mswt, and α-Syn chimeras (in which divergent residues were substituted with the orthologous amino-acids) are assembly-competent (Figures 1A, S1). Following incubation with agitation, all wt and chimeric α-Syn constructs formed fibrils averaging 12-15 nm in diameter as observed by electron microscopy (Figure S1B). Furthermore, PFFs showed comparable (>85%) levels of insoluble amyloid α-Syn after sedimentation and Thioflavin T (ThT) binding, although ThT fluorescence intensity was not proportional to fibril quantity (Figure S1A, C).
We evaluated how α-Syn sequence affects the ability of fibrils to act as pathological seeds by comparing the relative rates of Mswt α-Syn monomer aggregation in the presence of PFFs made from the different α-Syn constructs. Self-seeding with Mswt PFFs at 1% (w/w) of the monomer concentration dramatically accelerated the assembly of Mswt α-Syn compared to unseeded reactions (Figures 1C and S2). By contrast, initiation rates of cross-seeding reactions containing Huwt PFFs were similar to unseeded reactions.
Chimeric PFFs seeded Mswt α-Syn assembly with efficiencies between that of Huwt and Mswt PFFs. This correlated closely to the degree of homology between substrate and seeds, but did not achieve the cis-templating efficiency of Mswt PFFs (Figures 1C and S2). Interspecies substitutions of Mswt with the Huwt residue at Thr53 to Ala (MsT53A) and Asn87 to Ser (MsN87S) respectively reduced efficiencies by ~65% and 75% compared to Mswt PFFs. Fibrils combining these two substitutions (MsAS PFFs) seeded with ~80% lower efficiency than Mswt α-Syn PFFs.
Conversely, interspecies substitutions of Huwt with Mswt residues increased their seeding capabilities with conversion of Ser87 to Asn (HuS87N) having a greater effect than the A53T substitution (Figure 1C and S2). Simultaneous substitution at both positions (HuTN) conferred an initiation rate approaching 50% that of Mswt PFFs. Additional C-terminal substitutions, e.g. Asn103 to Gly (HuTNG), had relatively minor effects on the seeding efficiency of chimeric PFFs, consistent with previous studies showing that regions immediately flanking the hydrophobic NAC domain work in concert to influence overall in vitro fibrillization propensity of α-Syn (Bertoncini et al., 2005). Although seeding initiation rates varied greatly between chimeric α-Syn PFFs as seeds, elongation rates were similar between these reactions (Figures 1D, S2E). Thus, sequence discrepancies between the monomer substrate (Mswt) and seeding PFFs primarily affect the initial trans-templating event (i.e., initiation rate).
We reasoned that if differences at specific residues lead to inefficient templating between Huwt and Mswt α-Syn, then seeding initiation rates for wt and chimeric α-Syn PFFs should follow the same rank order when Huwt α-Syn monomer is used as the substrate instead of Mswt. As expected, cis-templating of Huwt α-Syn monomers by Huwt PFFs was significantly more efficient compared to PFFs comprised of other α-Syn constructs (Figures 1E and S3). Trans-templating of Huwt monomers by Mswt, HuTN and HuTNG PFFs proceeded inefficiently with initiation rates similar to unseeded reactions. However, trans-templating with MsAS PFFs, which contain human residues at 53 and 87, was nearly as efficient as Huwt PFFs, in contrast to the effect of converting Huwt at residues 53 and 87 to the Mswt sequence which effectively nullified seeding activity. These observations support the view that compatibility at these two residues greatly facilitates seed/substrate interaction. Therefore, similar to other amyloids, the propagation of α-Syn amyloid in vitro appears to be driven largely by individual residues rather than unique motifs.
As observed with Mswt α-Syn monomer as the substrate, overall elongation rates of Huwt α-Syn monomer in PFF-seeded reactions were comparable regardless of the α-Syn sequence encoded by the seed (Figure 1F, S3E), further supporting the hypothesis that once a critical level of cross-seeding is achieved, templating switches to a self-seeding mode that is less dependent on the composition of the seed. Taken together, while both wt and chimeric α-Syn PFFs are capable of seeding fibril formation in vitro, the initiation rates of templating are strongly determined by sequence homology between seeds and substrate.
Reduced efficiency of human-mouse α-Syn cross-seeding in neurons
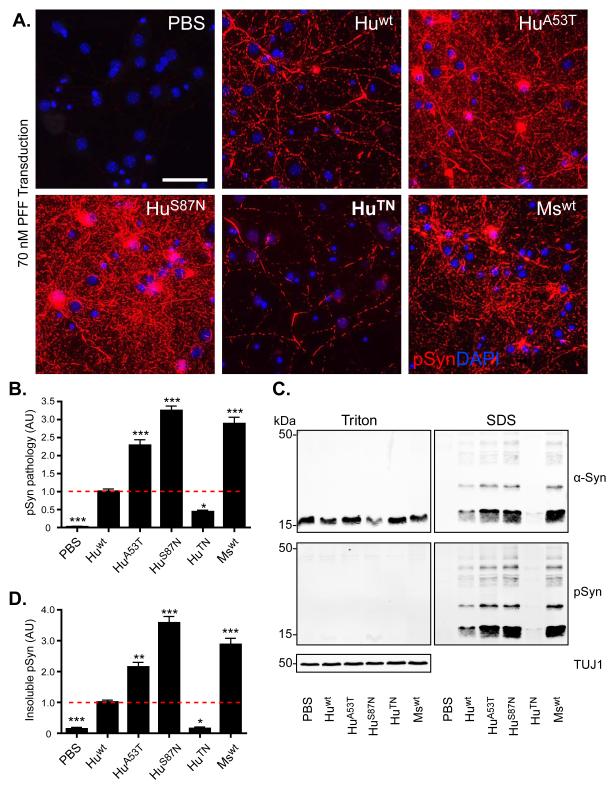
To determine if the cross-seeding properties identified in vitro also apply to seeding in cells, we next compared the ability of wt and chimeric α-Syn PFFs to induce LN- and LB-like inclusions in cultured neurons. Consistent with our previous studies (Volpicelli-Daley et al., 2011; Volpicelli-Daley et al., 2014), hippocampal neurons from non-transgenic (CD-1) mice treated with 70 nM Mswt PFFs for 7d showed a dramatic increase in pSer129 α-Syn (pSyn; Figure 2A, B), a highly specific marker of LBs and LNs in human PD brains (Fujiwara et al., 2002). As predicted by in vitro seeding data, and in agreement with cross-species seeding being inefficient, addition of Huwt PFFs at the same concentration elicited lower levels of pSyn inclusions. HuA53T α-Syn PFFs exhibited intermediate levels of LB and LN pathology that mirrored the in vitro seeding potency of these fibrils with Mswt monomer. Biochemical analysis of Triton-X100 soluble and insoluble α-Syn 7d after seeding yielded results consistent with the immunocytochemistry (Figure 2C, D). Additionally, while differences in seeding efficacy persisted, all PFFs were able to elicit detectable levels of pathology when added to neurons at a higher concentration (140 nM; Figure S4), indicating that although sequence mismatches between PFF and substrate delay the onset of pathology, insoluble inclusions develop once seeding has been initiated.
Figure 2. Sequence-dependent and -independent impediments to α-Syn seeding in neurons.
(A) Immunostaining of pSyn (red) in primary hippocampal neurons treated at DIV10 for 7d with 70 nM of indicated α-Syn PFFs. Nuclei are stained with DAPI (blue). Scale bar = 50 μm. (B) Quantification of pSyn immunoreactivity in cultures [(area occupied × density)/DAPI count]. Results shown as mean ± SEM (N=4) relative to Huwt levels of pSyn (dashed line). (C) Primary hippocampal neurons treated with PFFs were sequentially extracted with 1% Triton X-100 and 2% SDS. Lysates were immunoblotted for total α-Syn or, pSyn, with βIII tubulin (TUJ1) as a loading control. (D) Densitometric quantification of insoluble α-Syn fractions in experiment in C. Results shown as mean ± SEM (N=3). *p < 0.05; **p < 0.01, *** p<0.001, one-way ANOVA vs Huwt PFF treatment (Tukey post-hoc test). See also Figure S4.
Intriguingly, HuS87N α-Syn gave rise to PFFs whose seeding in neurons exceeded that of Mswt PFFs (Figure 2A-D), even though these PFFs show a significantly slower initiation rate in Mswt monomer than Mswt PFFs in the in vitro seeded-aggregation assay (Figure 1B). Unexpectedly, HuTN PFFs elicited 94% and 85% less pSyn than Mswt PFFs at 70 nM and 140 nM, respectively, and were even less potent than Huwt PFFs despite sharing more homology with endogenous Mswt α-Syn (Figure 2C, D) and being 2.5-fold more efficient than Huwt PFFs in seeding Mswt monomer assembly in vitro (Figures 1C and S2). Thus, the combination of Thr and Asn at positions 53 and 87 appears to restrict the ability to initiate pSyn inclusions in neurons. Altogether, these observations suggest that, in addition to sequence homology, there are biological processes that further regulate seeding at the cellular level.
C-terminal modifications further influence α-Syn seeding in neurons
While only modest changes in in vitro seeding efficiency were observed for chimeras containing C-terminal substitutions beyond residue 87, the relative inactivity of HuTN PFFs in neurons indicates that these remaining residues contribute significantly to the seeding of Mswt PFFs in neurons. Besides positions 53 and 87, the primary sequences between Huwt and Mswt α-Syn differ at 5 additional residues (100, 103, 107, 121, and 122; Figure 1A). We therefore expected that one or more additional substitutions to HuTN at these positions would have a restorative effect on seeding when the resulting PFFs were added to neurons. Indeed, seeding activity of HuTN PFFs could be restored by several of the substitutions, most prominently by reintroduction of the native mouse residue at 103 (i.e., HuTNG; Figure S4). Although HuTNG and HuTNY PFFs show significant rescue of seeding efficacy in neuronal culture, re-introduction of mutations both closer (L100M) and further (D121G and N122S) from the hydrophobic core of α-Syn elicited only partial recovery (Figure S4C, E).
To further test the role of the C-terminus in neuronal seeding and its interplay with the residues at positions 53 and 87, we generated PFFs containing only residues 1-99 of Huwt or Mswt α-Syn (1-99Huwt and 1-99Mswt). Treatment of neurons with these PFFs showed significantly reduced levels of pSyn-immunoreactive inclusions compared to full length α-Syn (compare Huwt and Mswt with 1-99Huwt and 1-99Mswt in Figure S4C-E) indicating that the C-terminus facilitates efficient seeding in neuronal cultures.
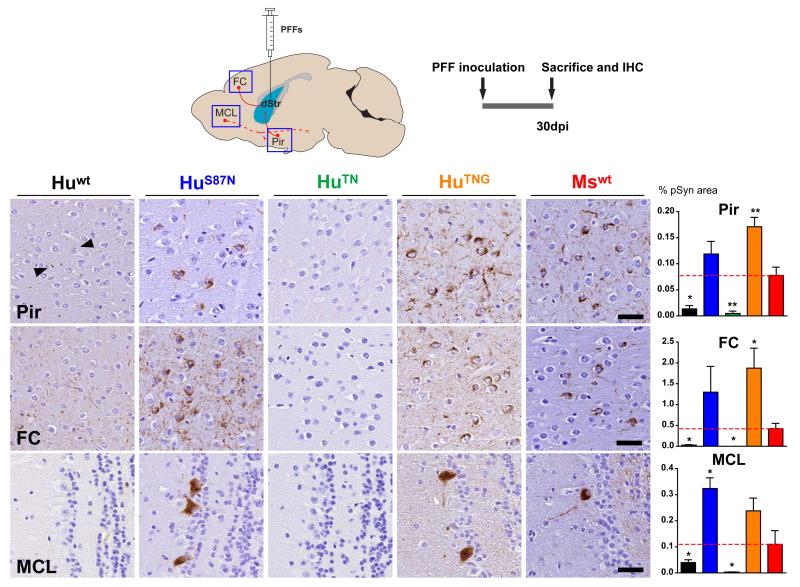
Pathological seeding properties of PFFs are preserved in vivo
The divergence in pathological seeding activities between chimeric α-Syn PFFs in neurons versus cell-free assays prompted us to evaluate whether PFFs have similar cross-seeding properties in vivo. C57Bl6/C3H mice were injected with either Mswt or Huwt PFFs by means of a single unilateral injection into the dorsal striatum, which induces Lewy-like pathology in multiple CNS regions connected to the injection site, including piriform cortex (Pir), frontal agranular cortex (FC), amygdala (Amyg), and dopamine (DA) neurons of the substantia nigra pars compacta (SNpc) (Luk et al., 2012a; Masuda-Suzukake et al., 2013). As expected, Mswt PFFs induced a higher burden of α-Syn pathology compared to Huwt PFFs when examined by immunohistochemistry (IHC) 30 days post injection (dpi) in the Pir and FC, two regions that rapidly develop α-Syn inclusions after intrastriatal PFF inoculation. Whereas LBs were present in Pir and FC of Mswt PFF treated animals at 30 dpi, the same regions in Huwt PFF injected mice showed only traces of LN-like accumulations, reflective of the early stages of seeded pathology (Osterberg et al., 2015), and consistent with inefficient cross-seeding between Huwt and Mswt α-Syn.
We then asked whether the variations in seeding observed among chimeric PFFs in cultured neurons are preserved in vivo by examining the distribution of α-Syn pathology in mice injected with PFFs generated from HuS87N, HuTN or HuTNG monomer. By 30 dpi, striatal injections of HuS87N PFFs induced abundant α-Syn positive LNs and LBs in Pir and ipsilateral FC (Figure 3, second column). In contrast, mice injected with HuTN PFFs were devoid of α-Syn pathology in all areas examined by IHC for pSyn (Figure 3) and misfolded α-Syn (Syn506, data not shown). The inability of HuTN PFFs to induce α-Syn pathology was reversed by the reintroduction of the native mouse residue at 103 since HuTNG PFFs elicited comparable levels of pathology to HuS87N PFFs in these same regions. Thus, the induction of Lewy-like inclusions with chimeric α-Syn PFFs in mice is more congruent with data from our neuronal culture than from in vitro seeding studies and further suggests that these differences are underpinned by similar cellular processes.
Figure 3. Cross-seeding efficiencyof Huwt and Mswt α-Syn PFFs in vivo.
Wt mice were injected with either wt (Hu or Ms) or chimeric (HuS87N, HuTN, or HuTNG) α-Syn PFFs via a single unilateral injection into the dorsal striatum. IHC for pSyn was used to visualize LB- and LN-like inclusions. Representative images are shown for piriform cortex (Pir), frontal agranular cortex (FC) or olfactory mitral cell layer (MCL) ipsilateral to the injection site at 30 dpi. Inclusions are visible by 30 dpi in all three regions following treatment with Mswt, HuS87N, or HuTNG PFFs whereas Huwt induced mild α-Syn accumulation in processes (arrowheads). No pathology was detectable in animals that received HuTN PFFs. Scale bars = 30 μm. *p < 0.05; **p < 0.01, one-way ANOVA vs Mswt PFF treatment (Tukey post-hoc test).
Notably, the mitral cell layer (MCL) of the ipsilateral olfactory bulb, a discrete neuron population lacking direct projections to the injection site, also showed numerous pSyn inclusions after striatal injection of Mswt, HuS87N and HuTNG PFFs (Figure 3, bottom row). The comparable overall distribution of α-Syn pathology induced by these PFFs also point to shared neuroanatomical routes of spread consistent with trans-cellular spread of misfolded α-Syn. The MCL of mice injected with Huwt or HuTN PFFs lacked any α-Syn pathology, suggesting that α-Syn pathology spreads at a slower rate than with Mswt, HuS87N or HuTNG PFFs, consistent with a delayed initiation of aggregation in these mice.
Chimeric α-Syn sequences can enhance or suppress pathological seeding in vivo
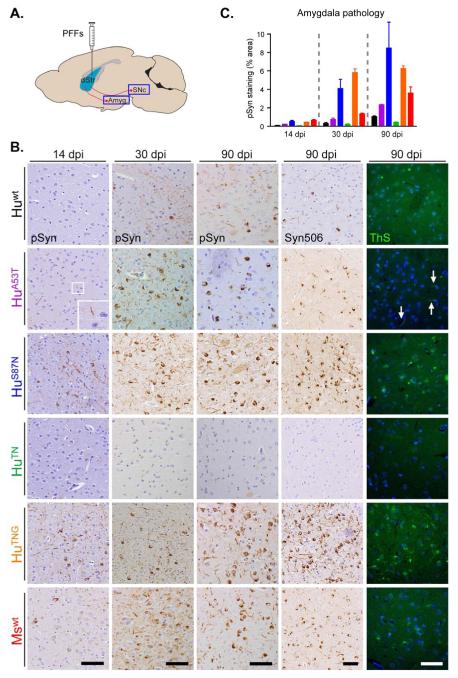
To more precisely determine differences in the relative seeding by PFFs in vivo, we compared the extent of α-Syn pathology they induced over time in the amygdala, one of the first areas to develop LB- and LN-like pathology after intrastriatal PFF injections (Luk et al., 2012a). At 14 dpi, neurons containing pSyn were virtually undetectable in animals inoculated with Huwt PFFs (Figure 4A-C). By this time, however, Mswt PFF-treated animals had accumulated considerable pSyn-positive inclusions, primarily in neurites of amygdaloid nuclei. Mice injected with HuS87N or HuTNG PFFs exhibited even more abundant LNs as well as mature LB-like inclusions. Injection of HuA53T α-Syn PFFs (which, like HuS87N PFFs, contain only one murine residue) resulted in mainly LN pathology at 14 dpi, indicating they are less potent than either HuS87N or HuTNG PFFs in eliciting α-Syn pathology. The rank-ordered efficiencies with which PFFs induced pathology in vivo is therefore consistent with the seeding efficiency observed in culture.
Figure 4. Relative pathological seeding efficacy of wt and chimeric α-Syn PFFs is preserved in vivo.
(A) Wt mice were sacrificed at 14, 30, or 90 d after intrastriatal injection of wt or chimeric α-Syn PFFs. (B) Inclusion pathology in the ipsilateral amygdala revealed by anti-pSyn, Syn506, or thioflavin S (ThS). Injected α-Syn PFFs are arranged with increasingly sequence homology to the host Mswt α-Syn from top to bottom. Pathology is detectable in mice injected with Mswt, HuS87N, and HuTNG PFFs at 14 dpi. HuA53T PFF injected mice developed sparse pSyn immunoreactive processes (inset) but no amygdala pathology was detectable in the Huwt cohort until 30 dpi. Scale bars = 50 μm. (C) Quantification of α-Syn pathology at each time point. Data expressed as mean % area occupied by pSyn inclusions ± SD (N ≥4 per group).
Examination of PFF-injected brains at 30, 90, and 180 dpi revealed that α-Syn pathology continued to evolve with time in all mice treated with pathogenic PFFs (Figure 4B, C). By 30 dpi, LB-like inclusions were prominent in the amygdala of mice exposed to HuA53T, HuS87N, HuTNG or Mswt PFFs. However, only LN-like inclusions could be detected in Huwt PFF-injected mice, consistent with delayed initiation of seeding in these animals. The appearance of cytoplasmic inclusions occurred at later incubation periods (90 dpi), matching the previously described evolution for seeded α-Syn pathology in vivo (Luk et al., 2012a; Osterberg et al., 2015). Neuronal inclusions were also labeled with an antibody selective for misfolded α-Syn (Syn506; Waxman et al., 2008) and thioflavin S, confirming pathological α-Syn as a major constituent. However, we failed to observe any pathology in mice injected with HuTN PFFs through all time points up to 180 dpi, suggesting a complete lack of pathogenicity of these PFFs in vivo that was not observed with neuronal cultures.
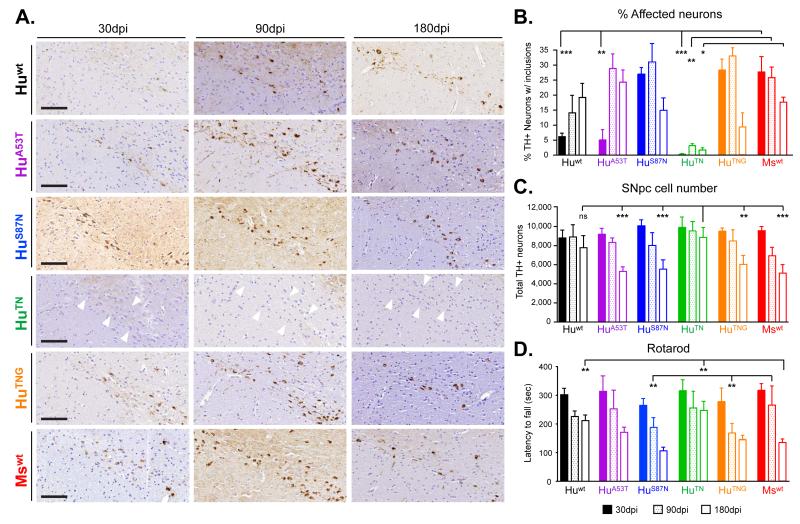
Pathogenicity of α-Syn PFFs correlates with rate of neurodegeneration in vivo
In agreement with our previous studies, pSyn immunoreactive inclusions could be found in ipsilateral ventral SNpc neurons 30 dpi with Mswt PFFs (Figure 5A). Comparable levels of pSyn positive pathology were also seen in the SNpc following injections of either HuS87N or HuTNG PFFs, whereas HuA53T PFFs resulted in primarily LN accumulations characteristic of early stages of seeded pathology. Inclusions were barely detectable in DA neurons after Huwt PFFs treatment and mice inoculated with HuTN PFFs remained free of α-Syn inclusions in all areas examined.
Figure 5. α-Syn pathology in midbrain DA neurons following injections of different α-Syn PFFs.
(A) Representative images of pSyn immunoreactive inclusions accumulating in SNpc DA neurons at 30 or 90d after inoculation with α-Syn PFFs. PFFs are listed in order of increasing homology to Mswt α-Syn. Inclusions were detectable in all cohorts except HuTN PFFs which remained clear of pSyn accumulation up to 90 dpi (white arrowheads). Scale bars = 100 μm. (B) Quantification of the proportion of SNpc DA neurons containing pSyn-positive inclusions. Initiation of α-Syn pathology is significantly delayed in mice treated with Huwt PFFs compared to Ms and chimeric PFFs. (C) DA neuron number in the SNpc after treatment with indicated wt or chimeric PFFs at 30, 90, and 180 dpi. All data shown are for the hemisphere ipsilateral to the injection site and shown as mean ± SEM (N = 5-8 per group). No inclusions were detected in the contralateral SNpc and neuron numbers there were unchanged for all groups. (D) Rotarod performance of α-Syn PFF-injected mice at 30, 90, and 180 dpi with indicated PFFs. *p < 0.05, **p <0.01, ***p < 0.001, two-way ANOVA (Tukey’s HSD). See also Figure S5.
Quantification of tyrosine-hydroxylase (TH)-positive SNpc neurons harboring pSyn inclusions confirmed that by 30 dpi following injections of Mswt PFFs, pathological α-Syn deposits were already present in ~28% of SNpc DA neurons (Figure 5B). A comparable percentage of DA neurons were affected in mice treated with HuS87N or HuTNG PFFs (26 and 29%, respectively) indicating that these PFFs likely initiate α-Syn pathology in similar DA subpopulations. Consistent with their delayed seeding capacities, this proportion was not reached in HuA53T PFF-treated mice until 90 dpi, whereas less than 20% of DA neurons in Huwt PFF injected animals displayed pSyn pathology at 180 dpi. Thus, with the exception of HuTN, DA cells can ultimately be seeded by each type of PFF. Interestingly, the proportion of SNpc neurons bearing α-Syn inclusions did not exceed 38% after injections with PFFs of any α-Syn construct at any time-point up to 180 dpi, suggesting that templated α-Syn pathology follows a similar trajectory of spread for pathogenic PFFs.
Since SNpc neurons that harbor α-Syn inclusions progressively degenerate in experimental studies (Luk et al., 2012a; Paumier et al., 2015; Recasens et al., 2014), we quantified the remaining number of DA neurons in this region at each time point to determine if the rate of inclusion formation correlated with cell loss (Figures 5C and S6). The most significant reduction in DA neurons was observed in cohorts injected with the most pathogenic PFFs (i.e., HuS87N, HuTNG or Mswt), where SNpc neurons were reduced by 9-22% at 90 dpi. This decrease approached ~40% by 180 dpi, corresponding to the drop in the number and proportion of pSyn-bearing neurons at this time point (Figure 5B, C). Consistent with this DA neuron loss, mice injected with HuS87N, HuTNG or Mswt PFFs also showed progressive deterioration in rotarod performance that was significant by 180 dpi (Figure 5D). Importantly, neither Huwt nor HuTN PFFs caused any significant reduction in DA neuron number up to 180 dpi.
Wildtype and chimeric α-Syn fibrils are potent seeds in non-neuronal cells
The notable deficiency in seeding activity exhibited by HuTN PFFs in neuron cultures and in vivo argues that its initial engagement with cellular α-Syn may be the rate-limiting step. To investigate this possibility, we measured the seeding activity of wt and chimeric PFFs in a previously established cell-model of seeded synucleinopathy where PFFs are delivered directly by cationic liposomes to the cytoplasm of HEK293 cells transfected with either Mswt or Huwt α-Syn (Luk et al., 2009). All PFF preparations, including HuTN PFFs, induced a similar amount of insoluble pSyn inclusions in cells (Figure S6). Thus, when differences in PFF internalization/delivery are eliminated, HuTN PFFs are equally potent to other PFFs in recruiting and converting endogenous α-Syn into pathological aggregates, consistent with observations from our in vitro seeding assays.
Non-pathogenic chimeric α-Syn PFFs shows normal entry into neurons
Given that HuTN PFFs are highly efficient in inducing pSyn inclusions when delivered into cells, yet seed poorly when passively administered to neurons or mice, we asked if the internalization of HuTN PFFs by neurons is compromised. We previously showed that α-Syn PFFs are detectable in >80% of neurons after 6 h of exposure to Huwt PFFs (Tran et al., 2014). Hippocampal neurons derived from Snca−/− mice were used to compare the internalization efficacy of Huwt, HuTN and Mswt PFFs at 6 and 24 h post transduction to eliminate confounds of detecting endogenous α-Syn. All PFF preparations were readily internalized as indicated by detectable α-Syn positive puncta juxtaposed to markers of early endosomes (EEA-1) and lysosomes (LAMP1) (Figure S6 D, E). The proportion of neurons containing internalized α-Syn PFFs was similar across these three constructs, suggesting that the lack of neuronal seeding seen with HuTN PFFs is not due to an overall reduction in PFF entry into neurons. Similarly, the proportion of neurons containing α-Syn PFF in LAMP1-positive structures were indistinguishable across these fibril types, suggesting that, at least at this early stage, they share a common route of internalization and subcellular processing (Figure S6 F, G). No differences were observed in the proportion of PFF-bearing neurons at 24 h post-treatment, further indicating that internalization is not responsible for the inability of HuTN PFFs to induce α-Syn inclusions in neurons (data not shown).
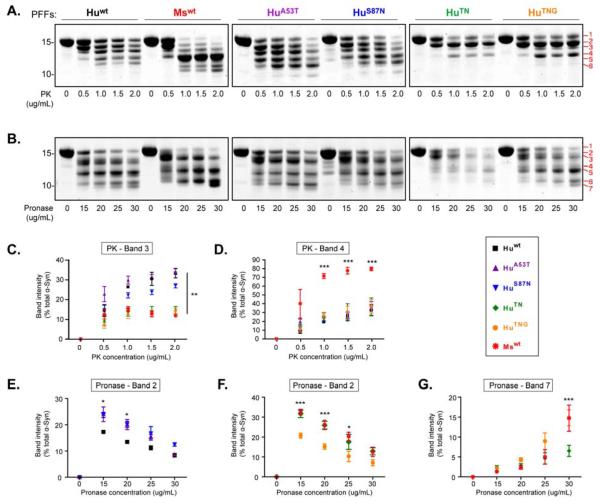
HuTN and chimeric PFFs display distinct protease sensitivities
Since HuTN differs in sequence from pathogenic chimeras such as HuA53T and HuS87N by a single amino acid, we tested whether wt and chimeric PFFs assembled de novo may encode distinct conformations. Partial digestion with various proteases revealed several unique fragmentation patterns among PFFs. Proteinase K (PK) treatment clearly differentiated Huwt, HuA53T, HuS87N, and Mswt PFFs (Figure 6A). We and others have previously shown that the five major bands following PK digestion of Huwt PFFs consist of 5 polypeptides: 1) aa1-140, 2) ~aa19-140, 3) ~aa32-140, 4) ~aa19-120, and 5) ~32-120 (Miake et al., 2002). Several trends emerge from the digestion patterns of PFFs as the α-Syn sequence is modified from Huwt to Mswt. Firstly, increased N-terminal cleavage occurs with perturbation of the Huwt sequence simultaneously at positions 53 and 87, resulting in a relative decline of full-length α-Syn (band 1) compared to bands 2-5, (compare band 1 for HuTN, HuTNG, and MsWT with Huwt, Hu A53T, and HuS87N in Figure 6A). Second, the shift from relatively equivalent amounts of bands 2-4 in Huwt, Hu A53T, and HuS87N PFF digests to a near total loss of band 3 in the HuTN, HuTNG chimeras indicates that these residue changes prevent further N-terminal truncation to generate band 3, while facilitating cleavage at the C-terminus to form band 4 (Figure 6A, B). Finally, PK digestion of Mswt PFFs shows a dramatically different pattern with rapid digestion to band 4, indicating facile degradation at both N- and C-termini (Figure 6A-D). When comparing the digestion pattern of HuTN and Mswt PFFs, in which only the C-termini vary, reduction in the stability of band 2 from Hu to Ms implies that the switch in C-terminal residues has a collective effect in exposing the N-terminus of Mswt PFFs for further degradation.
Figure 6. Protease treatment of wt and chimeric PFFs reveal distinct digestion patterns.
Wt and chimeric α-Syn PFFs were digested with Proteinase K (A) or Pronase E (B) at the indicated concentrations. Digestion products were resolved on a 12% Bis-tris gel and visualized with Coomassie Brilliant Blue. Major bands generated by each enzyme are indicated in red. (C-G) Quantification of specific PFF digestion fragments are shown. Results represent mean band intensity relative to undigested PFFs. Data shown as mean ± SEM from ≥3 independent digestion experiments. *p < 0.05, **p <0.01, ***p < 0.001, two-way ANOVA (Bonferroni test) for all groups vs. HuTN, HuTNG, and Mswt (C); Mswt vs. all groups (D); Huwt vs. all groups (E); HuTNG vs. all groups (F); HuTN vs. all groups (G). See also Figures S5-S7.
Since PK treatment did not differentiate all of the PFFs examined, particularly proseeding HuTNG PFFs from non-pathogenic HuTN PFFs, additional proteases were used. While trypsin and chymotrypsin yielded additional differences between Huwt, HuA53T, and HuS87N PFFs, HuTN and HuTNG PFFs continued to lack clear differences in digestion patterns despite their starkly different potency in vivo (Figure S7). However, partial digestion with Pronase E revealed distinct patterns of protein fragments for HuTN and HuTNG PFFs (Figure 6B, E-G). HuTN PFFs appeared to be more resistant than HuTNG PFFs at low concentrations (Figure 6E, F), but failed to build up the stable amounts of smaller fragments prominent in digestions of all seeding competent PFFs, including HuTNG (e.g., bands 5 and 7 in Figure 6B, G). Collectively, these results indicate the differences between Huwt and Mswt PFFs are largely driven by residues at 53 and 87, although terminal regions of α-Syn also play an important role in the overall structure of PFFs.
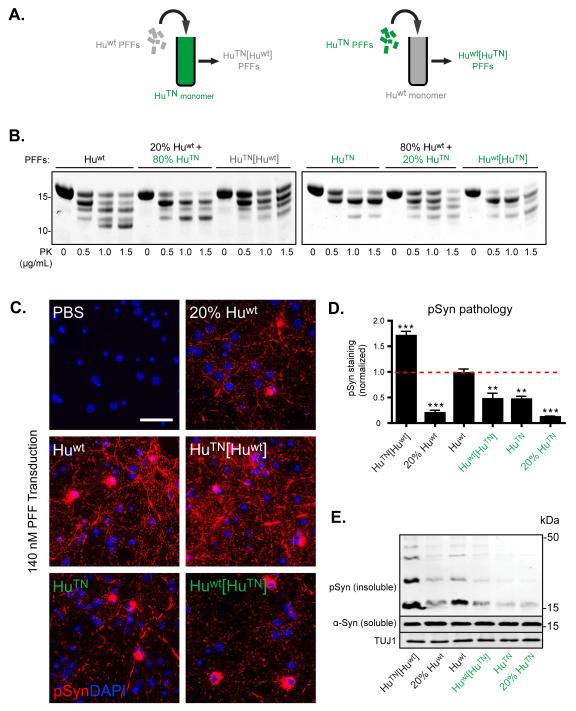
Pathological seeding properties of α-Syn PFFs are transferrable
Since our biochemical data thus far established that HuTN and Huwt PFFs have distinct tertiary structures and that HuTN PFFs formed de novo are non-seeding in neurons, we determined if these two properties are interdependent. To test whether the nature of α-Syn seeds dictate PFF tertiary structure and/or neuronal seeding ability, we seeded assembly of HuTN monomer with 20% Huwt PFFs to generate HuTN[Huwt] PFFs (Figure 7A). In the same manner, Huwt[HuTN] PFFs were made by seeding monomeric Huwt with de novo HuTN fibrils. Upon PK treatment, HuTN[Huwt] PFFs produced a banding pattern nearly identical to that of Huwt PFFs, despite being comprised primarily of HuTN monomer (Figure 7B). Importantly, none of the bands examined represented truncation events beyond positions ~32 or ~110, terminal areas in which these constructs share identical sequences, thus eliminating the possibility that the substitutions within the HuTN sequence were responsible for the observed differences. Moreover, this pattern was clearly distinct from PK digestion of de novo Huwt and HuTN PFFs mixed together just before PK treatment. Conversely, the digestion pattern of Huwt[HuTN] PFFs mirrored that of HuTN PFFs (Figure 7B ). Thus, under these seeding conditions, Huwt and HuTN α-Syn monomers are capable of adopting structural properties of the templating PFFs.
Figure 7. Pathogenic and non-pathogenic properties of PFFs are transferable.
(A) Schematic illustrating generation of hybrid fibrils through seeding with pathogenic and non-pathogenic PFFs. (B) Wt and chimeric α-Syn PFFs assembled de novo were digested with PK at the indicated concentrations. Digestion products in Bis-tris gels were stained with Coomassie Brilliant Blue. (C) Immunostaining for pSyn in primary hippocampal neurons treated with Huwt PFFs (140 nM or 28 nM to test the effect of the quantity used for seeding), HuTN[Huwt] PFFs (20% HuWT seeded HuTN PFFs), HuTN PFFs, and Huwt[HuTN] PFFs (20%HuTN seeded Huwt PFFs) at 140 nM. Primary hippocampal neurons (DIV10) were treated with α-Syn PFFs and 7d later cells were fixed and stained. Nuclei are stained with DAPI (blue). Scale bar = 50 μM. (D) Quantification of pSyn levels in PFF-treated neurons [(area occupied × average intensity)/DAPI]. Results shown as mean ± SEM (N=3; *p < 0.05; **p < 0.01, one-way ANOVA vs Huwt PFF treatment with Tukey post-hoc test). (E) DIV10 primary hippocampal neurons were treated for 7d with 140 nM PFFs and then sequentially extracted with 1% Triton X-100 followed by 2% SDS. Lysates from Triton and SDS fractions were immunoblotted with Syn9027 (total α-Syn), MJF-R13 (pSyn), and TUJ1 loading control. See also Figure S7.
To determine if HuTN[Huwt] and Huwt[HuTN] PFFs generated by cross-seeding also inherit the pathogenicity of the templating PFFs, we compared their ability to induce α-Syn inclusions in hippocampal neurons (Figure 7D-F). As predicted by its digestion pattern, HuTN[Huwt] PFFs were potent in eliciting LBs and LNs in cultured neurons (Figure 7C). Remarkably, HuTN[Huwt] PFFs treated cultures developed approximately 4-fold more insoluble pSyn pathology as measured by IF and immunoblot compared to de novo aggregated HuTN PFFs, indicating that the pathogenicity of Huwt PFFs had been successfully conferred onto HuTN α-Syn (Figure 7D, E). Surprisingly, the level of pSyn pathology induced by HuTN[Huwt] PFFs was nearly twice the amount in cultures treated with Huwt PFFs, likely due to the additional homology between HuTN and Ms α-Syn. Conversely, neurons exposed to Huwt[HuTN] PFFs showed a marked reduction in α-Syn inclusions compared to cultures that received de novo aggregated Huwt PFFs, indicating that Huwt had adopted the non-pathogenic state of the HuTN seeds used to generate this material, demonstrating that both a pathogenic and a non-pathogenic state are transferable between different α-Syn sequences.
Discussion
Recent studies have shown that misfolded α-Syn species template the conversion of native α-Syn into pathological PD-like inclusions, a process that likely underlies the spread of cellular α-Syn pathologies observed in prototypical synucleinopathies such as PD. Although fibril-seeded LN and LB formation has been observed in both neuron cultures and in vivo models, how pathological α-Syn potentiates the conversion of the endogenous normal α-Syn pool is unclear. Our data here show that, similar to other amyloids, PFF-mediated cross-seeding between homologous α-Syn sequences is significantly more efficient than that of Hu and Ms α-Syn. Using a series of Hu-Ms chimeric α-Syn PFFs, we find that at the molecular level seeding efficiency is determined by sequence homology between the α-Syn PFF seeds and soluble α-Syn monomers.
However, our findings reveal a more complex picture at the cellular level as the in vitro seeding potencies for several PFFs did not directly correlate with their ability to induce pathology. While Hu-Ms cross-seeding is markedly less effective when induced by PFFs in neurons and in vivo, we also identified chimeric α-Syn proteins that give rise to PFFs that either significantly enhanced (HuS87N and HuTNG) or suppressed (HuTN) induction of α-Syn pathologies compared to Mswt α-Syn PFFs. Among the residues examined, the two residues closest to the core hydrophobic (NAC) domain of α-Syn (53 and 87) show the strongest influence on PFF cross-seeding in vivo, as highlighted by the fact that single substitutions towards resembling either Huwt or Mswt immediately restore neuronal seeding activity to PFFs, although pathogenicity is also influenced by interactions between these two residues and the C-terminal region (particularly around aa 103-107).
Our in vivo data also showed that these altered seeding characteristics apply across multiple brain regions and neuronal subtypes. Most strikingly, HuTN PFFs have a lower seeding capacity than even Huwt PFFs in both neuron culture and in vivo despite its greater homology with Ms α-Syn and higher seeding potency in cell-free systems. Thus, in addition to molecular compatibility between seed and substrate, processes operating at the cellular level also determine the efficiency of α-Syn pathology transmission.
When cross-seeded with Huwt PFFs, HuTN monomers form pathogenic PFFs. Similarly, Huwt monomer assembled in the presence of non-pathogenic HuTN seeds display reduced potency in neurons, making it unlikely that co-purifying contaminants are responsible for the different activities of seeding or non-seeding α-Syn constructs. These data also suggest that pathogenicity can be transferred between these two sequences through in vitro templating, consistent with the notion that α-Syn fibrils can assume multiple conformations (Bousset et al., 2013; Guo et al., 2013). It is possible that specific fibril conformations have distinct biological activities, although further studies are needed to elucidate the relationship between structure and pathogenicity in neurons. Indeed, our PFF preparations may potentially contain multiple misfolded species with differing pathogenicities, and it is conceivable that alteration of the α-Syn primary sequence could shift this equilibrium.
Our findings present a number of possible, non-exclusive, scenarios as to how different α-Syn seeds affect pathological α-Syn propagation. Firstly, PFFs may differ in their ability to enter into neurons. In this regard, we did not detect differences in the internalization or subcellular compartmentalization of HuTN PFFs vs. other pathogenic fibrils although more sensitive methods are necessary to interrogate specific differences in subcellular localization or if various PFFs differ in their susceptibility to intracellular processing or degradation. Secondly, neuronal α-Syn is preferentially localized to presynaptic vesicles and is associated with membranes under physiological conditions where it maintains a different conformation than its unfolded state in cell-free systems (Dettmer et al., 2015). Therefore, monomeric α-Syn could be highly accessible to pathological α-Syn species of various configurations (hence sequence compatibility governs cell-free seeding rate) whereas membrane-bound α-Syn might interface with only a subset of configurations such as those found within pathogenic PFF preparations (Sidhu et al., 2016). This remains to be confirmed, although in agreement with this, HuTN PFFs efficiently induce α-Syn inclusions when transduced into α-Syn overexpressing non-neuronal cells, suggesting a reduced ability to interact with endogenous neuronal α-Syn. Thirdly, pathogenic and non-pathogenic PFFs may differ in their interaction with as yet unknown cofactors that could facilitate pathological α-Syn seeding in neurons (Supattapone, 2014).
It is interesting that while the species barrier for the infectious conformer of human prion protein (PrPsc), which in its normal conformation is a surface glycoprotein, is particularly sensitive to changes in the amino acid sequence, the phenomenon described here for α-Syn, an intracellular protein, shows a dramatic dependence on features beyond primary structure (Cobb and Surewicz, 2009; Collinge and Clarke, 2007). This suggests that where a seed and the endogenous protein meet is a key consideration for understanding how the various proteins that aggregate in neurodegenerative disease propagate. Further understanding of the fate of internalized seeding-incompetent PFFs comprised of HuTN α-Syn should help elucidate the nature of this initial interaction and thus provide important mechanistic insights into the process of templated α-Syn seeding in neurons.
Lastly, our data showing that similar neuroanatomical regions develop α-Syn pathology regardless of the cell-free seeding efficiency of the injected α-Syn PFFs suggests that the sequences described here alter their ability to trigger the initial misfolding of cellular α-Syn, and is in line with a model whereby α-Syn pathology is induced through seeding in trans but that subsequent propagation proceeds through cycles of seeding in cis involving primarily endogenous α-Syn. Consistent with this, we observed that DA neuron degeneration was most rapid following injection of Mswt and chimeric α-Syn PFFs with greater neuronal seeding rates and, consequently, more quickly developing α-Syn pathology. This observation suggests a direct relationship between the onset of α-Syn pathology burden and neuronal toxicity. In summary, our investigation of cross-seeding between Hu and Ms α-Syn has revealed important insights into the pathogenicity of a protein central to neurodegeneration.
Experimental Procedures
Animals
Wt (C57BL6/C3H) mice were obtained from the Jackson Laboratories (Bar Harbor, ME). Primary neuronal cultures were prepared from timed-pregnant (CD-1) mice (Charles River Laboratories; Wilmington, MA) and Snca−/− mice (Abeliovich et al., 2000) that were maintained on a C57BL6 background. All housing, breeding, and procedures were performed according to the NIH Guide for the Care and Use of Experimental Animals and approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC).
Recombinant α-Syn Purification and In Vitro Fibrillization
Constructs for chimeric α-Syn proteins were generated through PCR site-directed mutagenesis with the PRK172/Huwt α-Syn or PRK172/Mswt α-Syn plasmid as template. Wt and chimeric α-Syn constructs were expressed in E.coli BL21 (DE3) RIL cells and purified as previously described (Giasson et al., 2001; Luk et al., 2012a; Luk et al., 2009; Volpicelli-Daley et al., 2014). Fibrillization was conducted by diluting recombinant α-Syn to 360 μM (5 mg/mL) in sterile Dulbecco’s PBS (pH 7.0; Mediatech Inc.) with constant agitation (1,000 rpm, at 37°C) for 7 days in an Eppendorf Thermomixer C™ with ThermoTop™. Successful fibrillization was verified by sedimentation, TEM analysis, and ThT-binding. Further details are described in Supplemental Experimental Procedures.
Kinetic analysis of seeded α-Syn in vitro fibrillization
Efficacy of seeding for a particular construct was assayed by adding 1% PFFs (25 μg/mL) in to a fibrillization reaction containing 99% freshly thawed and spun Mswt or Huwt monomer (2.5 mg/mL in the final reaction). All seeded fibrillization reactions were performed at a final concentration of 2.5 mg/mL (180 μM) and final volume of 750 μL in order to facilitate differentiation of aggregation rates. Sedimentation was performed at the time points indicated. For each α-Syn construct, >2 independently prepared monomer batches were tested for each fibrillization paradigm, and >2 series of seeded fibrillization reactions were run for each monomer batch. Relative quantities of protein in each fraction were assessed by densitometery using a Licor Odyssey scanner at 680 nm. Graphing was performed with Graphpad Prism 4 and the curve-fitting feature of the software was used to generate the elongation rate (Hill slope) and initiation rate (EC50) data for Figures 1, S2 & S3.
Primary Neuron Cultures and Fibril Transduction
Primary neuron cultures were prepared from CD1 or Snca−/− mice on embryonic day (E) 15–E17 as previously described (Volpicelli-Daley et al., 2014). Dissociated hippocampal neurons were plated onto poly-D-lysine coated coverslips in 24-well plates (100,000 cells/coverslip). Transduction was performed at 10DIV using α-Syn PFFs diluted to 2.4 μM in PBS (without Mg2+/Ca2+) and sonicated with a Biorupter (Diagenode; Denville, NJ) at high power for 10 cycles (30s on, 30s off at 10°C ). At 4 d post-transduction, 30% of the media was gently aspirated from each well and replaced with 200 μL of fresh media. For biochemical studies, PFFs (140 nM) were added to neurons in a 6-well plate containing 1 million cells per well. Transduced neurons were harvested for immunocytochemistry or sequential extraction at 7 days post transduction (17 DIV).
Proteolytic digestion of PFFs
Proteinase K (P-2308), trypsin (T-9201), chymotrypsin (C-4129), and Pronase E (P-5147) were obtained from Sigma and stock solutions prepared in water. Chymotrypsin was dissolved in 1 mM HCl. Ten micrograms of PFFs generated from de novo assembly reactions were digested with the indicated concentrations of protease in Dulbecco’s PBS. Digestions were performed in a final volume of 20 μL and incubated at 37°C for either 30 min (Proteinase K, trypsin) or 40 min (Pronase E, chymotrypsin). Digestions were stopped with 1 mM PMSF or 100 μM aprotinin. Reaction samples were then boiled with SDS-sample buffer for 5 min and resolved on NuPAGE Novex 12% Bis-Tris gels (Invitrogen) for Proteinase K, chymotrypsin, and Pronase E reactions or Novex 16% Tricine gels for trypsin digestions. All experiments were preformed 3-5 times with at least 2 independently prepared batches of PFFs. Representative images from Coomassie stained gels are shown in Figure 6 and Figure S7.
Stereotaxic injection of PFFs
Prior to intrastriatal injection, PFFs were first diluted in sterile PBS and sonicated as described above. Mice (2-3 months old) were anesthetized with ketamine hydrochloride (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). A single injection (5 μg PFFs) was stereotaxically injected into the striatum (co-ordinates: +0.2 mm relative to Bregma, +2.0 mm from midline, +2.6 mm beneath the dura). Injections were made with 10 μL syringes (Hamilton, NV) at a rate of 0.1 μL per min (2.5 μL total) with the needle in place for ≥ 5 min at each target. Animals were monitored regularly following recovery from surgery, and sacrificed at various pre-determined time points (14, 30, 90, or 180 dpi) by overdose with ketamine/xylazine. For histological studies the brain and spinal cord were removed after transcardial perfusion with PBS and underwent overnight postfixation in either neutral buffered formalin (Fisher Scientific) or 70% ethanol (in 150 mM NaCl, pH 7.4), before being processed and embedded in paraffin.
Statistical analysis
Data from in vitro cross-seeding reactions, neuronal culture experiments and immunoblot quantification were compared with GraphPad Prism software (v.4), using one-way analysis of variance (ANOVA). Post-hoc testing was performed using the Tukey method. One-way ANOVA was also used to compare in vivo pSyn pathology within select regions of interest at 30dpi. Comparison of histopathological and behavioral data in the timed-cohort series was performed using STATA software (v.11). Means from each group were analyzed by two-way ANOVA with α-Syn PFF treatment and incubation time as factors. Post-hoc pairwise comparisons were performed using Tukey’s HSD method. Results from protease digestion experiments were analyzed by two-way ANOVA (α-Syn PFF and protease concentration as factors). Bonferonni correction was applied to pairwise comparisons.
Supplementary Material
Acknowledgements
We thank Drs. Kurt Brunden and Jing Guo for discussions and suggestions. This research was funded primarily by the Morris K. Udall Parkinson’s Disease Center of Excellence (NIH NS053488), NIH grants NS088322 and T32-AG000255, the Ofer Nimerovsky Family Fund and the Jeff and Anne Keefer Fund.
Footnotes
Author contributions
Conceptualization, KCL, DJC, JQT and VMYL; Methodology, KCL, DJC, BZ, and VMYL; Investigation, KCL, DJC, VK, BZ, IS, RMP, and SCD; Writing – Original Draft, KCL and DJC; Writing – Review and Editing, KCL, DJC, JQT, and VMYL; Funding Acquisition, KCL, JQT, and VMYL; Resources, KCL, JQT, and VMYL; Supervision, KCL and VMYL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Angers RC, Kang HE, Napier D, Browning S, Seward T, Mathiason C, Balachandran A, McKenzie D, Castilla J, Soto C, et al. Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science. 2010;328:1154–1158. doi: 10.1126/science.1187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1430–1435. doi: 10.1073/pnas.0407146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett C, Fernandez-Borges N, Kurt TD, Lucero M, Nilsson KP, Castilla J, Sigurdson CJ. Structure of the beta2-alpha2 loop and interspecies prion transmission. FASEB J. 2012;26:2868–2876. doi: 10.1096/fj.11-200923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biere AL, Wood SJ, Wypych J, Steavenson S, Jiang Y, Anafi D, Jacobsen FW, Jarosinski MA, Wu GM, Louis JC, et al. Parkinson's disease-associated alpha-synuclein is more fibrillogenic than beta- and gamma-synuclein and cannot cross-seed its homologs. The Journal of biological chemistry. 2000;275:34574–34579. doi: 10.1074/jbc.M005514200. [DOI] [PubMed] [Google Scholar]
- Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B, Madiona K, Olieric V, Bockmann A, Meier BH, Melki R. Structural and functional characterization of two alpha-synuclein strains. Nature communications. 2013;4:2575. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Cobb NJ, Surewicz WK. Prion diseases and their biochemical mechanisms. Biochemistry. 2009;48:2574–2585. doi: 10.1021/bi900108v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science (New York, NY) 2007;318:930–936. doi: 10.1126/science.1138718. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee H-J, Bae E-J, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee S-J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer U, Selkoe D, Bartels T. New insights into cellular α-synuclein homeostasis in health and disease. Current opinion in neurobiology. 2015;36:15–22. doi: 10.1016/j.conb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Dunning CJ, George S, Brundin P. What's to like about the prion-like hypothesis for the spreading of aggregated alpha-synuclein in Parkinson disease? Prion. 2013;7:92–97. doi: 10.4161/pri.23806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Murray IVJ, Trojanowski JQ, Lee VMY. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. Journal of Biological Chemistry. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, Zhang B, Riddle DM, Kwong LK, Xu Y, Trojanowski JQ, Lee VM. Distinct alpha-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013;154:103–117. doi: 10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EM, Surewicz WK. Fibril conformation as the basis of species- and strain-dependent seeding specificity of mammalian prion amyloids. Cell. 2005;121:63–72. doi: 10.1016/j.cell.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Wu K-P, Vendruscolo M, Baum J. The A53T mutation is key in defining the differences in the aggregation kinetics of human and mouse α-synuclein. Journal of the American Chemical Society. 2011;133:13465–13470. doi: 10.1021/ja203979j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen K, Hedegaard C, Bertelsen M, Bendixen C. Threonine 53 in alpha-synuclein is conserved in long-living non-primate animals. Biochemical and biophysical research communications. 2009;387:602–605. doi: 10.1016/j.bbrc.2009.07.070. [DOI] [PubMed] [Google Scholar]
- Lee VMY, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: New targets for drug discovery. Neuron. 2006;52:33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Luk K, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski J, Lee V. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012a;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk K, Kehm V, Zhang B, O'Brien P, Trojanowski J, Lee V. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med. 2012b;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk K, Song C, O'Brien P, Stieber A, Branch J, Brunden K, Trojanowski J, Lee V. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miake H, Mizusawa H, Iwatsubo T, Hasegawa M. Biochemical characterization of the core structure of alpha-synuclein filaments. The Journal of biological chemistry. 2002;277:19213–19219. doi: 10.1074/jbc.M110551200. [DOI] [PubMed] [Google Scholar]
- Osterberg VR, Spinelli KJ, Weston LJ, Luk KC, Woltjer RL, Unni VK. Progressive aggregation of alpha-synuclein and selective degeneration of lewy inclusion-bearing neurons in a mouse model of parkinsonism. Cell reports. 2015;10:1252–1260. doi: 10.1016/j.celrep.2015.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumier KL, Luk KC, Manfredsson FP, Kanaan NM, Lipton JW, Collier TJ, Steece-Collier K, Kemp CJ, Celano S, Schulz E, et al. Intrastriatal injection of pre-formed mouse alpha-synuclein fibrils into rats triggers alpha-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiology of disease. 2015;82:185–199. doi: 10.1016/j.nbd.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V. alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- Recasens A, Dehay B, Bove J, Carballo-Carbajal I, Dovero S, Perez-Villalba A, Fernagut PO, Blesa J, Parent A, Perier C, et al. Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol. 2014;75:351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- Rochet JC, Lansbury PT., Jr. Amyloid fibrillogenesis: themes and variations. Current opinion in structural biology. 2000;10:60–68. doi: 10.1016/s0959-440x(99)00049-4. [DOI] [PubMed] [Google Scholar]
- Santoso A, Chien P, Osherovich LZ, Weissman JS. Molecular basis of a yeast prion species barrier. Cell. 2000;100:277–288. doi: 10.1016/s0092-8674(00)81565-2. [DOI] [PubMed] [Google Scholar]
- Scott M, Groth D, Foster D, Torchia M, Yang SL, DeArmond SJ, Prusiner SB. Propagation of prions with artificial properties in transgenic mice expressing chimeric PrP genes. Cell. 1993;73:979–988. doi: 10.1016/0092-8674(93)90275-u. [DOI] [PubMed] [Google Scholar]
- Sidhu A, Segers-Nolten I, Subramaniam V. Conformational Compatibility Is Essential for Heterologous Aggregation of α-Synuclein. ACS chemical neuroscience. 2016 doi: 10.1021/acschemneuro.5b00322. [DOI] [PubMed] [Google Scholar]
- Supattapone S. Synthesis of high titer infectious prions with cofactor molecules. The Journal of biological chemistry. 2014;289:19850–19854. doi: 10.1074/jbc.R113.511329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier PM, Lindquist S. Prion recognition elements govern nucleation, strain specificity and species barriers. Nature. 2007;447:556–561. doi: 10.1038/nature05848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HT, Chung CH, Iba M, Zhang B, Trojanowski JQ, Luk KC, Lee VM. Alpha-synuclein immunotherapy blocks uptake and templated propagation of misfolded alpha-synuclein and neurodegeneration. Cell reports. 2014;7:2054–2065. doi: 10.1016/j.celrep.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycko R. Amyloid polymorphism: structural basis and neurobiological relevance. Neuron. 2015;86:632–645. doi: 10.1016/j.neuron.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley L, Luk K, Patel T, Tanik S, Riddle D, Stieber A, Meaney D, Trojanowski J, Lee V. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Lee VM. Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nature protocols. 2014;9:2135–2146. doi: 10.1038/nprot.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman E, Duda J, Giasson B. Characterization of antibodies that selectively detect alpha-synuclein in pathological inclusions. Acta neuropathologica. 2008;116:37–46. doi: 10.1007/s00401-008-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerman AL, Stohr J, Aoyagi A, Rampersaud R, Krejciova Z, Watts JC, Ohyama T, Patel S, Widjaja K, Oehler A, et al. Propagation of prions causing synucleinopathies in cultured cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E4949–4958. doi: 10.1073/pnas.1513426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Wypych J, Steavenson S, Louis JC, Citron M, Biere AL. alpha-synuclein fibrillogenesis is nucleation-dependent - Implications for the pathogenesis of Parkinson's disease. Journal of Biological Chemistry. 1999;274:19509–19512. doi: 10.1074/jbc.274.28.19509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.