Abstract
Background
Helicobacter pylori (H. pylori) infection is associated with a low platelet count in patients with immune thrombocytopenic purpura (ITP). While eradication of H. pylori is an established therapy for increasing the platelet count in ITP patients, it is unclear whether or not eradication will similarly affect the platelet counts in patients with chronic liver diseases (CLDs). We herein examined the effect of H. pylori eradication on the platelet counts in hepatitis C virus (HCV)-related CLD patients.
Methods
A total of 65 patients were enrolled, and the H. pylori-positive patients were treated to eradicate H. pylori. The eradication of H. pylori was assessed using a 13C-urea breath test 4 weeks after the completion of the therapy. In addition to the general laboratory variables of HCV-infected patients, including platelet counts, the prothrombin time (PT), and liver function markers (AST, ALT, total bilirubin, alkaline phosphatase, and albumin), we also investigated the presence of splenomegaly via ultrasonography. The platelet counts were measured at 1, 3, and 6 months after the final eradication therapy in order to assess the success of H. pylori eradication.
Results
Of the 65 patients with HCV-related CLD, 30 were found to be H. pylori-positive. The oral treatment regimen succeeded in eliminating H. pylori in 19 patients. These H. pylori-eradicated patients included eight males and 11 females, and 15 (78.9%) had liver cirrhosis. Regarding the patients who failed to achieve H. pylori eradication, their platelet counts did not markedly differ between pre- and post-treatment. Regarding the patients with H. pylori eradication, the platelet counts tended to increase 6 months after the treatment (9.2 ± 2.9 × 103/μL vs. 10.1 ± 3.7 × 103/μL, P = 0.085). We also found that the platelet count was significantly increased after the eradication in patients without splenomegaly (9.8 ± 2.8 ×103/μL vs. 11.0 ± 3.7 ×103/μL, P = 0.040). Regarding the seven patients whose platelet count increased by more than 20 × 103/μL after anti-H. pylori treatment, most (6/7, 85.7%) did not have splenomegaly.
Conclusion
H. pylori eradication may increase the platelet count in HCV-positive patients, particularly those without splenomegaly.
Keywords: Hepatitis C virus, Chronic liver disease, Thrombocytopenia, Helicobacter pylori, Splenomegaly
Introduction
Patients with chronic liver diseases (CLDs) are sometimes required to receive invasive treatments, such as endoscopic therapies for gastroesophageal varices, radiofrequency ablation for hepatocellular carcinoma (HCC), and interventional radiology for portal hypertension. However, thrombocytopenia, a commonly observed complication in patients with CLDs, can be a major problem in relation to such techniques associated with a risk for bleeding [1-3]. In CLD patients, splenomegaly and its associated hypersplenism are generally considered a major cause of thrombocytopenia. However, CLD patients with thrombocytopenia do not always have splenomegaly, and other causes may be involved in the decreased platelet count in such patients.
Helicobacter pylori (H. pylori) infection is associated with a low platelet count in patients with immune thrombocytopenic purpura (ITP) [4-8]. While eradication of H. pylori is an established therapy for increasing the platelet count in ITP patients, it is unclear whether or not eradication will similarly affect the platelet counts in CLD patients. Since hepatitis C virus (HCV) infection is the most frequent cause for CLD in Japan, we evaluated the effects of H. pylori eradication on the platelet counts in HCV-related CLD patients.
Patients and Methods
We prospectively evaluated the effect of H. pylori eradication on the platelet count in HCV-related CLD patients. HCV infection was confirmed based on positivity for HCV antibodies and HCV RNA, and patients with the following conditions were excluded from the study: receiving immunosuppressive therapy, co-infection with hepatitis B virus, and a history of eradication therapy for H. pylori.
We serologically determined the presence of H. pylori infection in a total of 65 HCV-infected patients who met the abovementioned criteria, as the measurement of the serum level of H. pylori-IgG antibody is considered the most reliable method of confirming H. pylori infection in treatment-naive patients in Japan [9, 10]. Then, the H. pylori-positive patients were treated with a standard triple therapy (20 mg b.i.d. omeprazole, 750 mg b.i.d. amoxicillin, and 400 mg b.i.d. clarithromycin) for 1 week. The successful eradication of H. pylori was assessed with a 13C-urea breath test 4 weeks after the completion of the therapy.
The patients who did not achieve H. pylori eradication with the first therapy were subjected to a second therapy (20 mg b.i.d. omeprazole, 750 mg b.i.d. amoxicillin, and 250 mg b.i.d. metronidazole), provided they agreed. The general laboratory variables of HCV-infected patients, including platelet counts, the prothrombin time (PT), and liver function markers (AST, ALT, total bilirubin, alkaline phosphatase, and albumin), were routinely measured. The platelet counts were measured at 1, 3, and 6 months after the final eradication therapy. We also investigated the presence of splenomegaly, via a previously reported method [11].
The numerical variables were expressed as the mean ± standard deviation (SD). The pre- and post-treatment platelet counts were compared using a Wilcoxon t-test. A P-value < 0.05 was considered statistically significant.
This study was approved by the Hyogo College of Medicine ethics committee (Approval No. 650) and was conducted according to the Declaration of Helsinki. The current study protocol was registered as a clinical trial (UMIN000002563, https://upload.umin.ac.jp/), and written informed consent was obtained from all of the patients.
Results
Baseline characteristics
A total of 65 patients with HCV-related CLD were enrolled in the present study, and 30 were found to be H. pylori-positive. The basic characteristics of the H. pylori-positive patients are shown in Table 1. The population included 16 male patients and 14 female patients, and the ages of the patients ranged from 56 to 75 years (median 69.5 years). All of the patients had HCV-related CLD, and 24 (80.0%) had liver cirrhosis. In these 24 cirrhotic patients, the Child-Pugh classification was grade A in 20 patients and grade B in four patients. No patients were classified as Child-Pugh C grade.
Table 1. Characteristics of the H. pylori-Positive Patients (N = 30).
| Age (years) | 68.8 ± 5.0 |
| Gender (male/female) | 16/14 |
| AST (IU/L) | 60.5 ± 33.1 |
| ALT (IU/L) | 57.8 ± 42.5 |
| γ-GTP (IU/L) | 47.0 ± 31.3 |
| ALP (IU/L) | 281.5 ± 87.6 |
| Total bilirubin (mg/dL) | 0.9 ± 0.3 |
| Albumin (g/dL) | 3.8 ± 0.5 |
| Hemoglobin (g/dL) | 12.3 ± 1.8 |
| Platelets (× 103/μL) | 98.2 ± 40.9 |
| Prothrombin time (%) | 80.9 ± 12.0 |
| HCV genotype (group 1/2) | 22/8 |
| HCV RNA (Log copies/mL) | 5.6 ± 0.8 |
| Chronic hepatitis/Child-Pugh A cirrhosis/Child-Pugh B cirrhosis/Child-Pugh C cirrhosis | 6/20/4/0 |
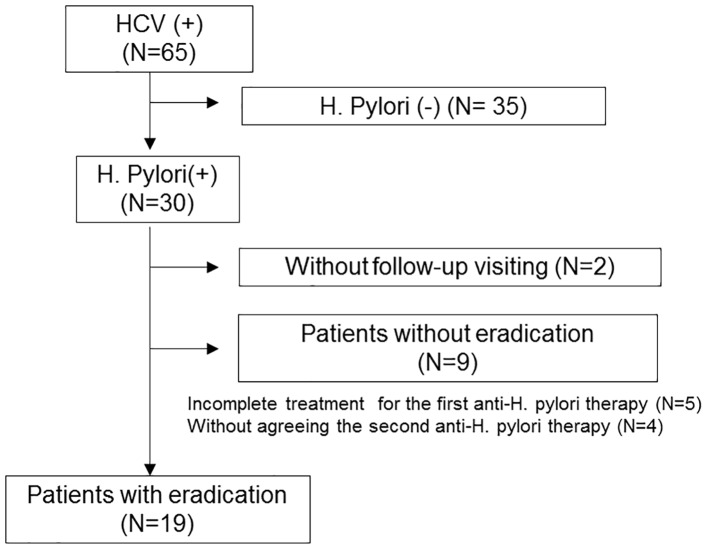
The oral treatment regimen succeeded in eliminating H. pylori in 19 patients. Of the 11 patients in whom H. pylori eradication could not be confirmed, nine actually failed to eradicate H. pylori, and two simply missed their follow-up visit (Fig. 1). The basic characteristics of the H. pylori-eradicated patients are shown in Table 2. This population included eight male patients and 11 female patients, and 15 (78.9%) had liver cirrhosis. In these 15 cirrhotic patients, the Child-Pugh classification was grade A in 13 patients and grade B in two patients.
Figure 1.
Algorithm for the classification of HCV-positive patients.
Table 2. Pre-Treatment Characteristics of Patients With Helicobacter pylori Eradication (N = 19).
| Age (years) | 68.1 ± 5.3 |
| Gender (male/female) | 8/11 |
| AST (IU/L) | 54.6 ± 30.8 |
| ALT (IU/L) | 50.8 ± 42.6 |
| γ-GTP (IU/L) | 41.4 ± 30.3 |
| ALP (IU/L) | 280.9 ± 103.4 |
| Total bilirubin (mg/dL) | 1.0 ± 0.3 |
| Albumin (g/dL) | 3.8 ± 0.5 |
| Hemoglobin (g/dL) | 12.1 ± 1.7 |
| Platelets (× 103/μL) | 91.6 ± 30.2 |
| Prothrombin time (%) | 80.8 ± 12.8 |
| HCV genotype (group 1/2) | 14/5 |
| HCV RNA (Log copies/mL) | 5.7 ± 0.8 |
| Chronic hepatitis/Child-Pugh A cirrhosis/Child-Pugh B cirrhosis/Child-Pugh C cirrhosis | 4/13/2/0 |
Effects of H. pylori eradication on the platelet counts in patients with HCV-related CLD
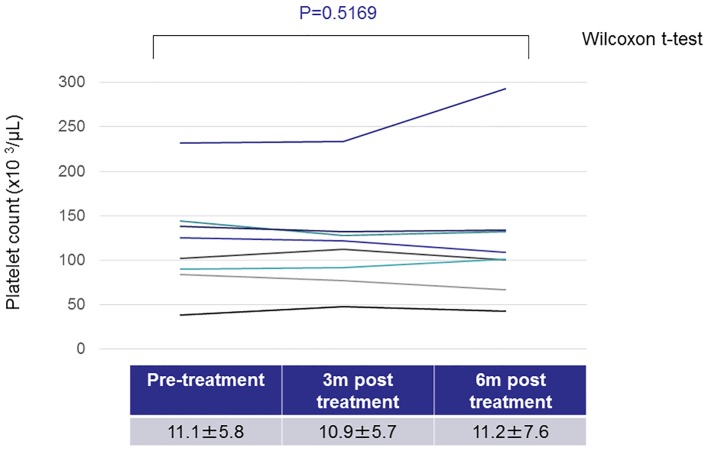
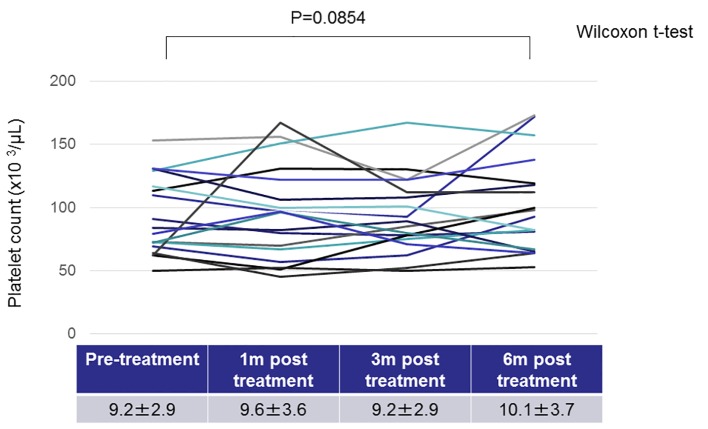
On investigation of the nine patients who did not achieve H. pylori eradication, the platelet counts did not change markedly between pre- and post-treatment (Fig. 2). Regarding the 19 patients with H. pylori eradication, the platelet counts tended to increase 6 months after the treatment, although the difference before and after treatment did not reach statistical significance (Fig. 3). We found that the platelet counts were significantly increased after the eradication in patients without splenomegaly (Table 3). In addition, regarding the seven patients whose platelet count increased by more than 20 × 103/μL after anti-H. pylori treatment, most (6/7, 85.7%) did not have splenomegaly.
Figure 2.
Changes in the platelet counts in patients without Helicobacter pylori eradication.
Figure 3.
Changes in the platelet counts in patients with Helicobacter pylori eradication.
Table 3. Changes in the Platelet Count Between Pre- and Post-Helicobacter pylori Eradication.
| Factors | Classification | Platelet count |
P-value | |
|---|---|---|---|---|
| Pre-eradication | Post-eradication | |||
| Age | ≥ 70 years (N = 8) | 10.3 ± 2.9 | 11.1 ± 4.4 | 0.273 |
| < 70 years (N = 11) | 8.2 ± 2.7 | 9.2 ± 2.6 | 0.116 | |
| Sex | Male (N = 10) | 9.6 ± 3.2 | 11.3 ± 4.4 | 0.125 |
| Female (N = 9) | 8.9 ± 2.8 | 9.3 ± 2.9 | 0.253 | |
| Platelet count before treatment | ≥ 800 × 103/µL (N = 10) | 8.2 ± 2.7 | 9.2 ± 2.6 | 0.116 |
| < 800 × 103/µL (N = 9) | 7.2 ± 1.2 | 8.0 ± 1.8 | 0.188 | |
| Splenomegaly | Present (N = 6) | 7.9 ± 2.8 | 8.5 ± 2.7 | 0.593 |
| Absent (N = 13) | 9.8 ± 2.8 | 11.0 ± 3.7 | 0.040 | |
| Previous interferon treatment | Present (N = 6) | 9.3 ± 2.4 | 9.5 ± 2.8 | 0.343 |
| Absent (N = 13) | 9.2 ± 3.2 | 10.4 ± 4.0 | 0.081 | |
| Hepatocellular carcinoma | Present (N = 11) | 8.8 ± 3.3 | 10.0 ± 4.1 | 0.137 |
| Absent (N = 8) | 9.7 ± 2.4 | 10.3 ± 3.1 | 0.230 | |
Discussion
HCV infection is the most frequent cause of CLD in Japan, and a low platelet count in HCV-infected patients can be a major concern when performing certain clinical practices [1-3, 12, 13]. H. pylori infection has been reported to decrease the platelet count in ITP patients [4-8]. However, while H. pylori infection is highly prevalent in Japan, along with HCV infection, the association of these two infectious diseases has rarely been studied. We herein examined the effects of H. pylori elimination on the platelet count in patients with HCV-related CLD.
The pre- and post-eradication platelet counts in fully H. pylori-eradicated patients did not differ significantly. However, in those patients without splenomegaly, the platelet counts significantly increased in response to H. pylori eradication (Table 3). These findings suggested that H. pylori infection may be related to the decreased platelet count in HCV-related CLD, particularly in patents without splenomegaly.
Recently, oral thrombopoietin receptor agonists such as eltrombopag [14-16] and lusutrombopag [17] have been developed as agents to increase the platelet count. These new drugs reduce the bleeding risk without platelet transfusion under the invasive treatment and have also been reported to be clinically useful. However, eltrombopag administration may be associated with an increased risk of thromboembolic events in cirrhotic patients, although the precise mechanisms of this event have not been clarified. Therefore, thrombopoietin receptor agonists should be administered only following a careful evaluation of the risks and benefits [14-16]. Since H. pylori infection significantly increases the risk of gastric cancer, the Japanese guidelines recommend that all H. pylori-infected patients receive anti-H. pylori therapy [9]. In the present study, we observed no thromboembolic events; as such, the eradication of H. pylori may be a way for HCV-related CLD patients to increase their platelet counts before receiving thrombopoietin agonists.
Several limitations associated with the present study warrant mention. First, we found that the platelet counts were significantly increased after H. pylori eradication in patients without splenomegaly. Although these results suggest that H. pylori infection may be related to the decreased platelet counts in HCV-related CLD patients, we did not clarify the associated mechanism. Second, the effects of H. pylori eradication were evaluated in a small number of patients (N = 19), despite the fact that a total of 65 patients were enrolled. Although we were unable to determine the reason for the low rate of H. pylori infection, many Japanese patients with HCV-related CLD are elderly and may have severely atrophic gastric mucosa where H. pylori cannot thrive. Another study with a larger number of patients will be needed to confirm our results.
In summary, the present findings suggest that H. pylori eradication may increase the low platelet count in HCV-positive patients, particularly in those without splenomegaly.
Acknowledgments
We are grateful to R. Nakatani, N. Tawara, K. Wasai, N. Kanazawa, Y. Matsushita, S. Fujii, H. Kido, and K. Minemoto (Hyogo College of Medicine) for their technical and secretarial assistance.
Conflicts of Interest
The authors declare no conflicts of interest in association with this study.
Abbreviations
- CLD
chronic liver disease
- HCC
hepatocellular carcinoma
- H. pylori
Helicobacter pylori
- ITP
immune thrombocytopenic purpura
- HCV
hepatitis C virus
- PT
prothrombin time
- SD
standard deviation
References
- 1.Shah SH, Hayes PC, Allan PL, Nicoll J, Finlayson ND. Measurement of spleen size and its relation to hypersplenism and portal hemodynamics in portal hypertension due to hepatic cirrhosis. Am J Gastroenterol. 1996;91(12):2580–2583. [PubMed] [Google Scholar]
- 2.Boyer TD, Habib S. Big spleens and hypersplenism: fix it or forget it? Liver Int. 2015;35(5):1492–1498. doi: 10.1111/liv.12702. [DOI] [PubMed] [Google Scholar]
- 3.Gangireddy VG, Kanneganti PC, Sridhar S, Talla S, Coleman T. Management of thrombocytopenia in advanced liver disease. Can J Gastroenterol Hepatol. 2014;28(10):558–564. doi: 10.1155/2014/532191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352(9131):878. doi: 10.1016/S0140-6736(05)60004-9. [DOI] [PubMed] [Google Scholar]
- 5.Stasi R, Sarpatwari A, Segal JB, Osborn J, Evangelista ML, Cooper N, Provan D. et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood. 2009;113(6):1231–1240. doi: 10.1182/blood-2008-07-167155. [DOI] [PubMed] [Google Scholar]
- 6.Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 7.Kuwana M. Helicobacter pylori-associated immune thrombocytopenia: clinical features and pathogenic mechanisms. World J Gastroenterol. 2014;20(3):714–723. doi: 10.3748/wjg.v20.i3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang JJ, Lee DH, Yoon H, Shin CM, Park YS, Kim N. The Effects of Helicobacter pylori Eradication Therapy for Chronic Idiopathic Thrombocytopenic Purpura. Gut and Liver. 2016;10(3):356–361. doi: 10.5009/gnl14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer. 2013;132(6):1272–1276. doi: 10.1002/ijc.27965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SY. Current progress toward eradicating Helicobacter pylori in East Asian countries: differences in the 2013 revised guidelines between China, Japan, and South Korea. World J Gastroenterol. 2014;20(6):1493–1502. doi: 10.3748/wjg.v20.i6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oshibuchi M, Nishi F, Sato M, Ohtake H, Okuda K. Frequency of abnormalities detected by abdominal ultrasound among Japanese adults. J Gastroenterol Hepatol. 1991;6(2):165–168. doi: 10.1111/j.1440-1746.1991.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 12.Aizawa N, Enomoto H, Takashima T, Sakai Y, Iwata K, Ikeda N, Tanaka H. et al. Thrombocytopenia in pegylated interferon and ribavirin combination therapy for chronic hepatitis C. J Gastroenterol. 2014;49(8):1253–1263. doi: 10.1007/s00535-013-0884-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda N, Imanishi H, Aizawa N, Tanaka H, Iwata Y, Enomoto H, Saito M. et al. Nationwide survey in Japan regarding splenectomy/partial splenic embolization for interferon treatment targeting hepatitis C virus-related chronic liver disease in patients with low platelet count. Hepatol Res. 2014;44(8):829–836. doi: 10.1111/hepr.12184. [DOI] [PubMed] [Google Scholar]
- 14.Mihaila RG, Cipaian RC. Eltrombopag in chronic hepatitis C. World J Gastroenterol. 2014;20(35):12517–12521. doi: 10.3748/wjg.v20.i35.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danish FI, Yasmin S. The role of eltrombopag in the management of hepatitis C virus-related thrombocytopenia. Hepat Med. 2013;5:17–30. doi: 10.2147/HMER.S27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tillmann HL, McHutchison JG. Use of thrombopoietic agents for the thrombocytopenia of liver disease. Semin Hematol. 2010;47(3):266–273. doi: 10.1053/j.seminhematol.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Kim ES. Lusutrombopag: First Global Approval. Drugs. 2016;76(1):155–158. doi: 10.1007/s40265-015-0525-4. [DOI] [PubMed] [Google Scholar]