Abstract
The embryonic tectum displays an anteroposterior gradient in development and produces the superior colliculus and inferior colliculus. Studies suggest that partition of the tectum is controlled by different strengths and durations of FGF signals originated from the so-called isthmic organizer at the mid/hindbrain junction; however, the underlying mechanism is unclear. We show that deleting Ptpn11, which links FGF with the ERK pathway, prevents inferior colliculus formation by depleting a previously uncharacterized stem cell zone. The stem-zone loss is attributed to shortening of S phase and acceleration of cell cycle exit and neurogenesis. Expression of a constitutively active Mek1 (Mek1DD), the known ERK activator, restores the tectal stem zone and the inferior colliculus without Ptpn11. By contrast, Mek1DD expression fails to rescue the tectal stem zone and the inferior colliculus in the absence of Fgf8 and the isthmic organizer, indicating that FGF and Mek1DD initiate qualitatively and/or quantitatively distinctive signaling. Together, our data show that the formation of the inferior colliculus relies on the provision of new cells from the tectal stem zone. Furthermore, distinctive ERK signaling mediates Fgf8 in the control of cell survival, tissue polarity and cytogenetic gradient during the development of the tectum.
KEY WORDS: ERK signaling pathway, Shp2, Ptpn11, FGF, Neural progenitor, Stem cells, Organizer, Mouse
Summary: FGF/ERK signaling controls the formation of the mouse inferior colliculus by maintaining a neural progenitor zone and by regulating cell survival, polarity and specification.
INTRODUCTION
During embryogenesis, the mammalian midbrain greatly enlarges. The anterior and posterior parts of the dorsal midbrain (tectum) differentially form the superior colliculus (SC; known as the optic tectum in other vertebrates) and inferior colliculus (IC), which are involved in visual and auditory processing, respectively. The developing tectum expands in an anterior-to-posterior direction and displays a developmental gradient – cells in the anterior tectum cease dividing and undergo neurogenesis whereas cells in the posterior continue proliferation (Altman and Bayer, 1981a; Cowan et al., 1968). Furthermore, cells across the tectum display gradients of molecular properties that control ordered ingrowth of retinotectal axons (Cang and Feldheim, 2013). Therefore, the developing tectum provides an excellent experimental paradigm to study molecular mechanisms that coordinate tissue specification, polarity, and growth.
A signaling center at the junction between the mesencephalon (mes) and rhombomere 1 (r1), called the isthmic organizer, controls pattern formation and subsequent development of the midbrain and cerebellum (Joyner et al., 2000; Wurst and Bally-Cuif, 2001). Fibroblast growth factor 8 (Fgf8) is the key signaling molecule of the isthmic organizer (Chi et al., 2003; Crossley and Martin, 1995; Martinez et al., 1999; Shamim et al., 1999). In addition, Fgf8 induces expression of two other FGF genes, Fgf17 and Fgf18, in a broader domain at the isthmus (Liu et al., 2003; Xu et al., 2000). Isthmus-derived FGF proteins form a smooth posteriorhigh-to-anteriorlow gradient in the mes, instructing the graded expression of En2 and neural-mapping labels, such as ephrin ligands and Eph receptors, in the tectum of chick embryos (Chen et al., 2009b). Distinct levels of FGF signaling may also specify SC and IC fates, as mutations that moderately reduce FGF activities cause a similar disruption of the IC in mice (Basson et al., 2008; Chi et al., 2003; Sgaier et al., 2007; Trokovic et al., 2003; Xu et al., 2000; Yang et al., 2013a). Furthermore, deleting Fgf8 at different embryonic stages results in variable truncation of the posterior tectum (Sato and Joyner, 2009). These findings suggest that both the strength and duration of FGF signaling are crucial for development of the tectum, particularly the IC. However, the reported FGF mutations all cause abnormal mes-r1 patterning, adding confounding variables to interpretation of the tectal phenotype at the late stages. It remains largely unknown how different strengths and durations of FGF signaling establish both a smooth gradient in gene expression and discrete SC and IC cell fates.
FGF controls diverse cellular processes, including survival, proliferation, specification and differentiation, during midbrain development (Chi et al., 2003; Lahti et al., 2011; Lee et al., 1997; Liu et al., 1999; Saarimäki-Vire et al., 2007). Although multiple intracellular signaling cascades have been implicated in FGF signaling, the extracellular signal-regulated kinase 1/2 [ERK1 (MAPK3) and ERK2 (MAPK1)] pathway appears to play a dominant role downstream of FGF receptors in brain development (Guillemot and Zimmer, 2011). Indeed, experiments in chick embryos suggested that high and low levels of FGF/ERK signaling differentially control the r1 fate and mes cell proliferation, respectively (Sato and Nakamura, 2004). It remains to be determined whether the ERK pathway mediates other FGF functions in the developing midbrain. Furthermore, how an intracellular signaling cascade, like the ERK pathway, transforms the graded FGF signals that are originated from the isthmus into a smooth developmental gradient and gene expression in the tectum, but discrete outputs in specifying SC and IC cell fates is still mystery.
We recently reported that specific deletion of Ptpn11, which codes for a protein phosphatase also known as Shp2 that links FGF with the ERK pathway (Hadari et al., 1998; Li et al., 2014a), from the early neural plate results in truncation of the tectum and malformation of the cerebellar cortex (Li et al., 2014b). Differing from other FGF mutations, the Ptpn11 conditional knockout (Ptpn11-cKO) appeared to have little effect on the pattern formation of the neural tube before embryonic day (E) 10.5 (Li et al., 2014b). We reasoned that Ptpn11-cKO mutants could provide a unique opportunity to study how FGF signaling controls tectal development at the later embryonic stages. In this study, we characterized the tectal phenotype in Ptpn11-cKO mice. By expressing a constitutive active MEK (MAP2K) to activate ERK in the absence of Ptpn11 or Fgf8, we investigated how the ERK signaling cascade mediates FGF function during tectal development. We have identified a unified model that explains how the localized sources of FGF control tissue specification, polarity, and growth during tectal development mostly through the ERK signaling pathway.
RESULTS
Deletion of Ptpn11 leads to specific loss of the IC
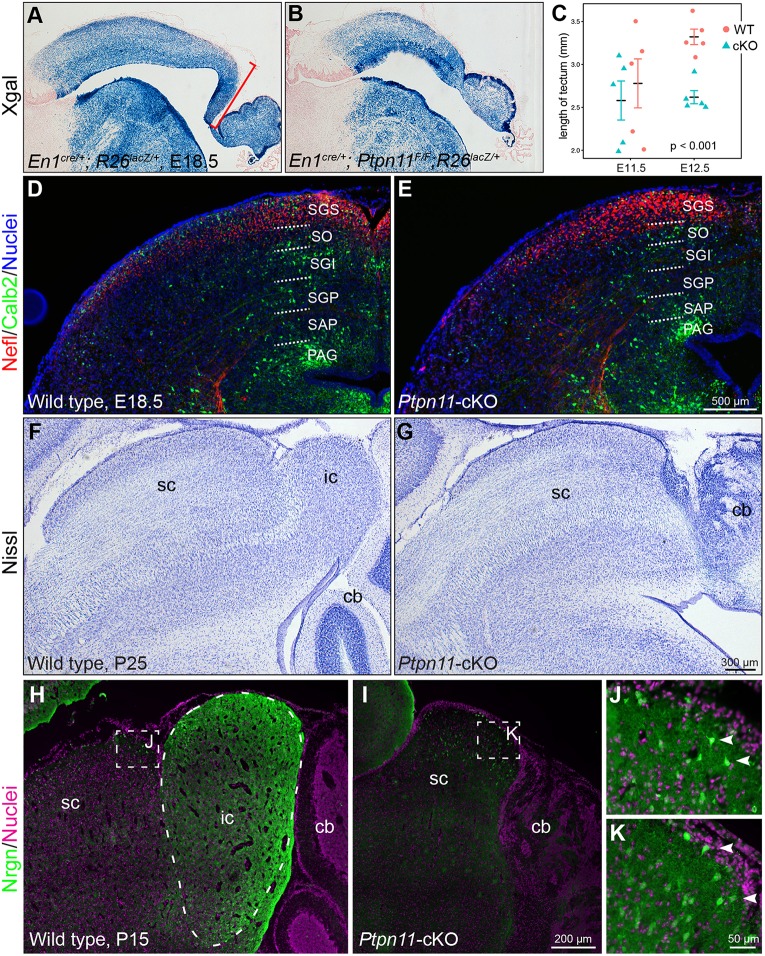
By combining an En1cre knock-in (Kimmel et al., 2000) and Ptpn11floxed (Ptpn11F) (Yang et al., 2013b) alleles, we previously reported that deleting Ptpn11 from the mes-r1 neural plate causes truncation of the tectum (Li et al., 2014b). To define the extent of tectal tissue loss, we generated En1cre/+; Ptpn11F/F; R26RlacZ/+ embryos, in which Cre-mediated recombination simultaneously removed Ptpn11 and induced permanent lacZ expression from the R26RlacZ locus (Soriano, 1999). X-gal histochemistry revealed that the midbrain and cerebellum were smaller in En1+/cre; Ptpn11F/F; R26RlacZ/+ embryos compared with En1+/cre; Ptpn11F/+; R26RlacZ/+ (control) at E18.5, with the most significant reduction in the tectum (Fig. 1A,B). By measuring the length of the tectum, we detected significant shortening of the tectum in Ptpn11-cKO embryos at E12.5 but not at E11.5 (Fig. 1C), indicating that Ptpn11-cKO impedes tectal expansion after E11.5.
Fig. 1.
Deletion of Ptpn11 causes truncation of the mesencephalon at E12.5 and loss of the inferior colliculus at birth. (A,B) X-gal histochemistry on sagittal mouse brain sections. The bracket demarcates the tectal region that is lost in Ptpn1-cKO embryos. (C) Quantification of the length of the dorsal mesencephalon (mean±s.e.m.). Two-tailed unpaired t-test with Welch's correction. (D,E) Immunofluorescence for neurofilament (Nefl) and calbindin (Calb2). Dotted lines delimit cell and fiber stratification patterns. Nomenclature based on Edwards et al. (1986). (F,G) Nissl staining of sagittal sections of brains of the indicated genotypes at P25. (H-K) Neurogranin (Nrgn) staining of the inferior colliculus (ic) (outlined by dashed line) on sagittal sections of brains at P15. Nuclei are stained with Hoechst 33342. Boxed areas in H and I are enlarged in J and K, respectively; arrowheads indicate isolated Nrgn+ cells in the superior colliculus (sc). cb, cerebellum; PAG, periaqueductal gray; SAP, stratum album profundum; SGI, stratum griseum intermedium; SGP, stratum griseum profundum; SGS, stratum griseum superficiale; SO, stratum opticum.
The SC exhibits six cellular and fibrous layers, whereas the IC lacks obvious layered organization (Fig. 1D,F) (Altman and Bayer, 1981a; Edwards et al., 1986). In Ptpn11-cKO embryos at E18.5 and thereafter, the remaining tectum displayed the characteristic laminated structure for the SC, but lacked a discernible IC (Fig. 1E-G). To confirm the loss of the IC, we performed immunostaining for neurogranin (Nrgn) on a series of sagittal sections of wild-type and Ptpn11-cKO brains at postnatal day (P) 15. Nrgn immunoreactivity labeled the entire IC in wild-type mice (Sato and Joyner, 2009), but the Nrgn-positive (Nrgn+) cell cluster was missing in the remaining tectum in Ptpn11-cKO mice (Fig. 1H,I; data not shown). By contrast, scattered Nrgn+ cells were detected in the SC of Ptpn11-cKO mice as found in controls (Fig. 1J,K), indicating the specific loss of Nrgn+ IC neurons in the absence of Ptpn11. Because most Ptpn11-cKO mice die or become severely retarded by P7, when the SC and IC can be clearly demarcated, we did not attempt to measure the SC size. We cannot conclude whether the SC is similar in size between mutants and controls. Nevertheless, our data show that Ptpn11 deletion prevents growth of the tectum after E11.5 and formation of the IC.
Deletion of Ptpn11 has no obvious effect on FGF/ERK signaling in the mes-r1 at E10.5
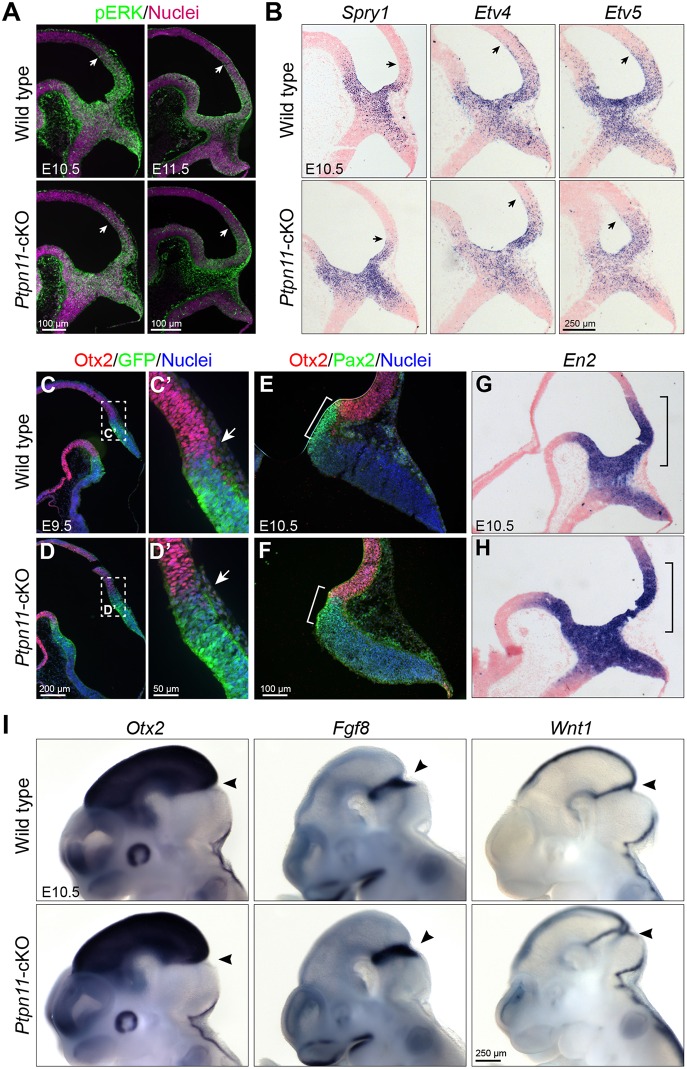
Ptpn11 proteins are greatly reduced from mes-r1 neuroepithelium in Ptpn11-cKO embryos at E8.5 (Li et al., 2014b). Interestingly, analysis of phosphorylated ERK (pERK), which indicates ERK activation, showed normal pERK immunoreactivity in the posterior mes of Ptpn11-cKO embryos before E11.5 (Fig. 2A). Furthermore, the expression of Spry1, Etv4 and Etv5, which are readout genes of FGF signaling, was unchanged in Ptpn11-cKO embryos at E10.5 (Fig. 2B). Therefore, FGF/ERK signaling is mostly normal in the mes-r1 region of Ptpn11-cKO embryos at E10.5.
Fig. 2.
Pattern formation of the mes-r1 area is normal in Ptpn11-cKO embryos. (A) Immunofluorescence for pERK on sagittal sections. (B) In situ hybridization for Spry1, Etv4 and Etv5. Arrows indicate the anterior limit of detected signals in A,B. (C-F) Immunofluorescence on sections of E9.5 embryos carrying the Gbx2creER allele (C-D′) and E10.5 embryos (E,F). The boxed areas are enlarged in C′ and D′; arrows indicate the border between Otx2+ and GFP+ cells. The brackets demarcate the Pax2 expression domain. (G-I) In situ hybridization on sections (G,H) and whole mount (I) of E10.5 embryos. Arrowheads indicate the isthmus; brackets demarcate the En2 expression domain in the posterior mesencephalon.
We performed detailed analyses to examine the anterior-posterior patterning of the mes-r1 region. Interactions between the homeobox genes Otx2 and Gbx2 position the mes-r1 boundary (Broccoli et al., 1999; Millet et al., 1999), and define the expression domain of Fgf8, Wnt1, Pax2 and En1/2 (Li and Joyner, 2001). To monitor Gbx2 expression, we crossed a Gbx2creER allele, which contains a creER-ires-EGFP cassette in the Gbx2 locus (Chen et al., 2009a), into the Ptpn11-cKO background. Immunostaining for Otx2 and GFP showed normal segregation between Otx2+ and GFP+ cells at the mes-r1 border in Ptpn11-cKO embryos at E9.5 (Fig. 2C-D′). Pax2, which is essential for the induction of Fgf8 in the isthmus (Ye et al., 2001), was expressed in the anterior r1 in Ptpn11-cKO embryos at E10.5 as found in controls (Fig. 2E,F). The expression of En2, Otx2, Fgf8 and Wnt1 was indistinguishable between wild-type and Ptpn11-cKO embryos at E10.5 (Fig. 2G-I). Collectively, our data demonstrate that Ptpn11-cKO does not overtly alter the FGF/ERK function and the early patterning in the mes-r1 region at E10.5.
Deletion of Ptpn11 transiently increased apoptosis at the diencephalic-mesencephalic border
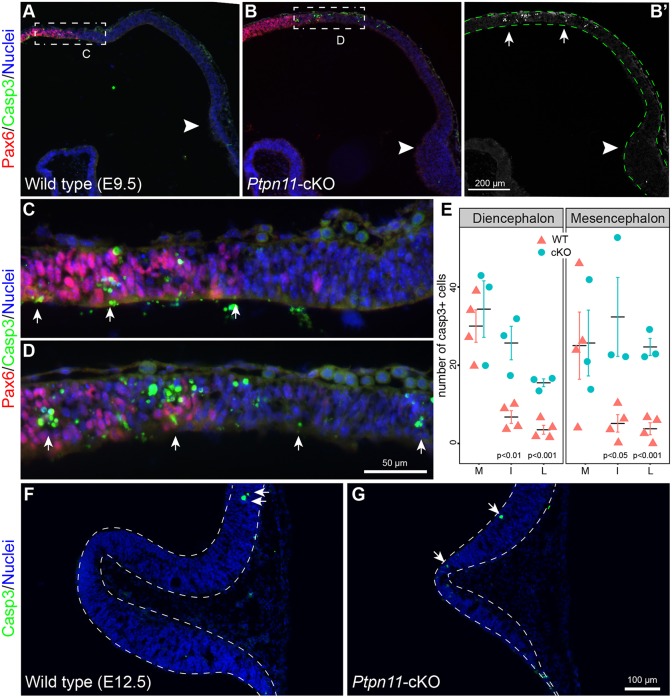
We next sought to examine whether abnormal cell death causes IC deletion in Ptpn11-cKO mice by performing immunofluorescence for activated caspase 3 (Casp3) and Pax6, which respectively label apoptotic and diencephalic cells. We detected numerous Casp3+ cells near the diencephalic-mesencephalic boundary (DMB), particularly along the dorsal midline, in wild-type embryos at E9.5 (Fig. 3A,C). Ptpn11-cKO exacerbated cell death in both diencephalon and mes within 250 µm of the DMB, with significant elevations in regions lateral to the dorsal midline compared with controls (Fig. 3A-E). Remarkably, Casp3+ cells were hardly detected in the posterior part of the mes in Ptpn11-cKO embryos between E9.5 and E11.5 (Fig. 3B,B′,G). Furthermore, few Casp3+ cells were present at the DMB after E9.5 (data not shown). Therefore, deletion of Ptpn11 briefly increases apoptosis near the DMB at E9.5. However, because the tectal truncation occurs after E11.5 (Fig. 1C), other mechanisms, rather than cell death, probably play bigger roles in the absence of the IC in Ptpn11-cKO embryos.
Fig. 3.
Deletion of Ptpn11 causes cell death at the diencephalic-mesencephalic junction but not in the posterior part of the mesencephalon. (A-D) Immunofluorescence for Pax6 and activated Casp3 on sagittal sections of E9.5 embryos. B′ is Casp3-channel image of B with dashed lines outlining the neural tube; arrowheads indicate the isthmus; boxed areas are enlarged in C and D. (E) Quantification of Casp3+ cells at the diencephalic-mesencephalic junction on medial (M), intermediate (I) and lateral (L) sections of the neural tube (mean±s.e.m.) of wild-type (WT) and Ptpn11-cKO embryos. Two-tailed unpaired t-test with Welch's correction, n=4 embryos per group. (F,G) Immunofluorescence for Casp3 on sagittal sections. The arrows in B′-G indicate Casp3+ cells.
Ptpn11 is essential to maintain a stem zone in the posterior tectum
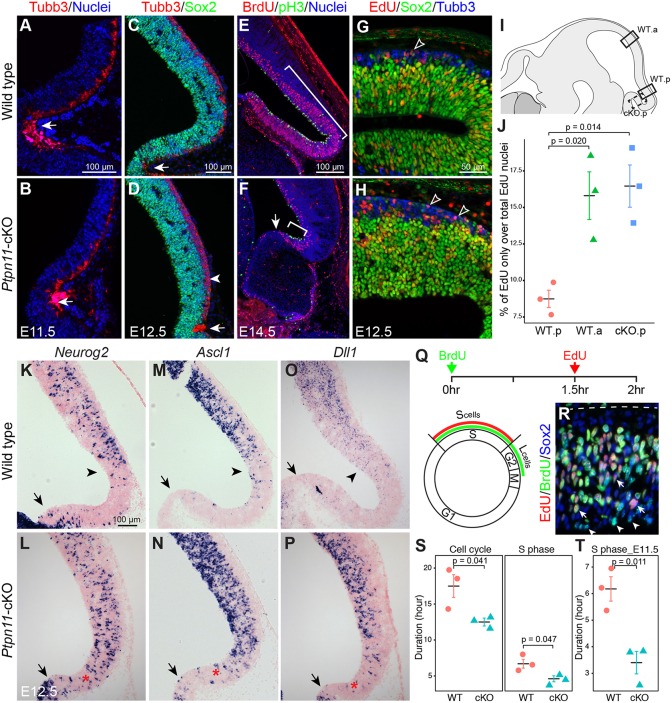
We investigated whether an imbalance between cell proliferation and differentiation causes the IC loss in Ptpn11-cKO mice. Immunofluorescence for neural stem cell markers, such as Ki67 (Mki67) and Sox2, or the postmitotic neuronal precursor cell marker Tubb3 did not reveal any obvious abnormality in the tectum of Ptpn11-cKO embryos from E9.5 to E11.5 (Fig. 4A,B; data not shown). From E12.5 and thereafter, a notable increase of postmitotic neural precursors was detected in the posterior tectum of Ptpn11-cKO embryos compared with controls (Fig. 4C,D; Fig. 5F′,G′). In wild-type embryos at E14.5, the posterior tectum was mostly composed of proliferating cells with a few postmitotic cells (Fig. 4E), and this zone of active proliferation persisted until E16.5 (data not shown). By contrast, this proliferative domain was missing in the Ptpn11-cKO tectum by E14.5 (Fig. 4F), indicating that Ptpn11 is required to maintain a pool of neural progenitors in the posterior tectum. We refer to this progenitor cell zone that is mostly devoid of postmitotic neurons as the tectal stem zone (TSZ).
Fig. 4.
Depletion of neural progenitors in the posterior tectum in the absence of Ptpn11. (A-H) Immunofluorescence with the indicated antibodies on sagittal sections. The arrows point to the isthmus; the arrowhead in D indicates the thickened Tubb3+ cell layer; brackets in E and F demarcate the proliferative zone; unfilled arrowheads in G and H denote Tubb3+/EdU+/Sox2− cells that have exited the cell cycle. (I) Schematic of the anterior (WT.a) and posterior (WT.b) areas (boxed) that were analyzed for cell cycle re-entry and cell cycle length. The dashed box indicates the region closer to the isthmus that was examined in Ptpn11-cKO embryos (cKO.p). (J) Quantification of percentage of nuclei expressing EdU only out of total EdU+ nuclei (mean±s.e.m.). Two-tailed unpaired Student's t-test. (K-P) In situ hybridization on sagittal sections of E12.5 midbrain. Arrows indicate the isthmus, arrowheads show the posterior limit of gene expression in wild-type embryos; asterisks denote the abnormal expression in the posterior end of the midbrain. (Q) Schematic illustrating the estimation of cell cycle parameters by double labeling with BrdU and EdU. (R) Immunofluorescence on sagittal sections of the posterior tectum at E12.5. Arrows and arrowheads indicate BrdU+/EdU+ nuclei and nuclei expressing BrdU only, respectively; the dashed line shows the pial surface. (S,T) Quantification of duration of the total cell cycle and S phase at E12.5 (S) and S phase at E11.5 (T) in wild-type (WT) and Ptpn11-cKO embryos. Two-tailed unpaired Student's t-test.
Fig. 5.
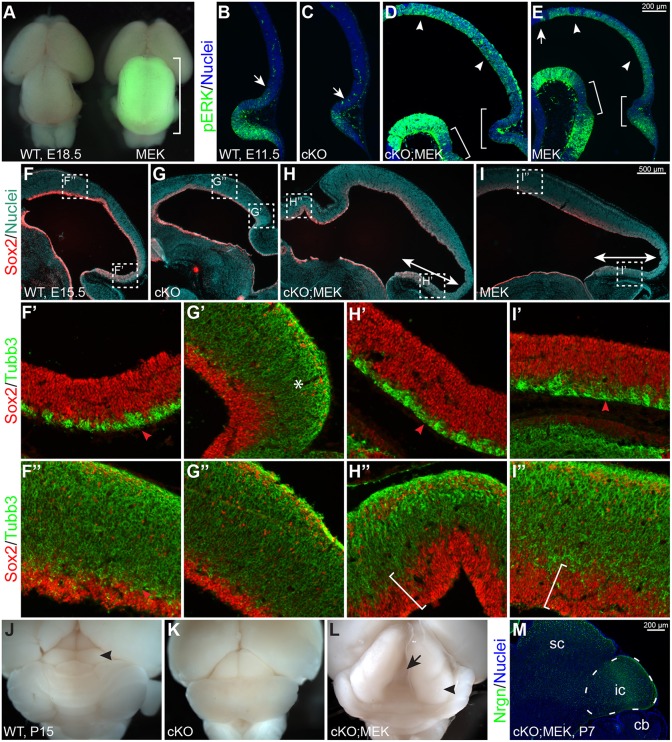
Expressing Mek1DD rescues the tectal stem zone and inferior colliculus in Ptpn11-cKO mutants. (A) Dorsal view of wild-type (WT) and En1cre/+;R26Mek1DD/+ (MEK) brains at E18.5. The bracket shows the enlarged tectum with GFP fluorescence, which marks Mek1DD expression. (B-E) Immunofluorescence for phosphorylated ERK (pERK) on sagittal sections of WT, Ptpn11-cKO (cKO), Ptpn11-cKO;MEK (cKO;MEK) and MEK embryos at E11.5. Arrows demarcate the anterior limit of pERK immunoreactivity; arrowheads show the pERK immunoreactivity throughout the mes; brackets show reduced intensity of pERK near the isthmus. (F-I″) Immunofluorescence on sagittal sections of E15.5 brains. The boxed areas in F-I are enlarged in F′-I″. The double arrows show the enlarged tectal stem zone (TSZ) with Mek1DD expression; the asterisk indicates increased accumulation of Tubb3+ cells; the arrowheads show the thin layer of Tubb3+ cells in the presumptive TSZ; the brackets denote the thickened ventricular zone. (J-L) Dorsal view of P15 brains. The arrowheads indicate the inferior colliculus (ic); the arrow shows the splitting of the tectum, a phenotype observed in mice with Mek1DD expression after birth. (M) Immunofluorescence on sagittal sections of P7 Ptpn11-cKO;MEK embryos. The IC is demarcated with a dashed line. cb, cerebellum; sc, superior colliculus.
Deletion of Ptpn11 results in abnormal cell cycle and neurogenesis in the TSZ
Cell cycle progression, including interkinetic nuclear migration (INM), cell cycle re-entry and cell cycle duration, is important in maintaining neural progenitor cells (Sun and Hevner, 2014). To study INM, we labeled cells that underwent DNA synthesis with a pulse of 5-ethynyl-2′-deoxyuridine (EdU) or (5-bromo-2′-deoxyuridine) BrdU, and determined the position of S-phase labeled nuclei at 30, 60, 90, 120 and 240 min. Most labeled nuclei were initially present at the basal side at 30 min and they had reached the ventricular surface by 90 min; after reaching the ventricular surface, they entered mitosis as judged by phosphorylated histone 3 (pH3) immunoreactivity (Fig. S1A). We found no difference in the distribution of EdU/BrdU+ and pH3+ nuclei in the posterior tectum between wild-type and Ptpn11-cKO embryos at E11.5 (Fig. S1D,E), suggesting that Ptpn11 deletion does not affect INM in the TSZ at this stage.
To examine cell cycle exit, we labeled proliferating cells with a pulse of EdU at E11.5, harvested the brain 24 h later, and immunostained for Sox2. Thus, EdU+/Sox2− cells represent those that had exited the cell cycle over the 24 h period, whereas EdU+/Sox2+ cells represent those that had divided and re-entered cell cycle. In agreement with the developmental gradient in the tectum, we found that fewer cells exited the cell cycle in the posterior than in the anterior tectum (Fig. 4J). Remarkably, 88% more progenitors exited the cell cycle in the posterior Ptpn11-cKO tectum compared with the control (Fig. 4J), indicating that Ptpn11 deletion enhances cell cycle exit in the TSZ between E11.5 and E12.5.
The early steps of cell cycle exit and neurogenesis of neural progenitors are regulated by proneural genes (Bertrand et al., 2002). In situ hybridization for the proneural genes Neurog2, Ascl1 and their target Dll1 revealed the presence of their transcripts in a ‘salt-and-pepper’ pattern in the anterior part, but absence from the posterior part, of the tectum at E12.5 (Fig. 4K,M,O). By contrast, their transcripts were detected throughout the Ptpn11-cKO tectum at E12.5 (Fig. 4L,N,P), indicating ectopic neurogenesis in the presumptive TSZ in Ptpn11-cKO embryos. Together, the increase in cell cycle exit and neurogenesis counts for the premature exhaustion of the TSZ in Ptpn11-cKO embryos.
We next measured the cell cycle parameters by labeling dividing cells first with BrdU and then EdU with an interval of 1.5 h (Fig. 4Q) as described previously (Martynoga et al., 2005). Because neural progenitors are not synchronized in vivo, cells in the initial BrdU-labeled cohort will leave S phase at a constant rate during the 1.5-h window, and this leaving fraction (Lcells) will be labeled by BrdU but not EdU (Fig. 4Q,R). We detected a significant increase in the ratio of BrdU-only cells (Lcells) to BrdU+/EdU+ cells (Scells) in the posterior tectum of Ptpn11-cKO compared with controls at E12.5 (0.33±0.03 versus 0.23±0.02; Student's t-test, P=0.048), suggesting shortening of S phase due to Ptpn11-cKO. Indeed, the estimated lengths of S phase (Ts) and total cell cycle (Tc) were significantly reduced in the posterior Ptpn11-cKO tectum compared with controls (Fig. 4S). We detected similar S-phase shortening in the posterior Ptpn11-cKO tectum at E11.5 (Fig. 4T). The latter finding is important because it suggests that abnormal cell cycle is likely to be a cause, rather than a result, of tissue loss, which is apparent until E12.5 in Ptpn11-cKO embryos (Fig. 1C). Recent studies demonstrated that S-phase duration is the key factor in maintenance of proliferative division of neural stem/progenitor cells; neural stem/progenitor cells shorten S phase on commitment to neuron production (Arai et al., 2011; Turrero García et al., 2016). Altogether, our data suggest that Ptpn11 deletion causes premature commitment of neural progenitors to neurogenic division, leading to the loss of the TSZ.
The Ptpn11-regulated ERK pathway controls formation of the IC by maintaining the TSZ
Although Ptpn11 has been implicated in several signaling pathways (Qu, 2000), our previous studies demonstrated that Ptpn11 plays a crucial role in the FGF/ERK signaling pathway in the development of the mes-r1, especially the cerebellum (Li et al., 2014b). To determine whether Ptpn11 regulates ERK in the maintenance of TSZ, we generated En1cre/+; Ptpn11F/F; R26Mek1DD/+ mice, in which cre-mediated recombination simultaneously removes Ptpn11 and expresses a constitutively active Mek1, Mek1DD (Cowley et al., 1994). Expressing Mek1DD throughout mes-r1 induced enlargement of the midbrain (Fig. 5A). In En1cre/+; Ptpn11F/F; R26Mek1DD/+ embryos at E10.5, Mek1DD expression not only restored pERK immunoreactivity near the isthmus but also induced robust pERK in the anterior mes (Fig. 5B-D). Interestingly, the levels of pERK appeared to be reduced near the isthmus in En1cre/+; R26Mek1DD/+ and En1cre/+; Ptpn11F/F; R26Mek1DD/+ embryos (Fig. 5D,E), suggesting that the isthmus-derived signals cause a feedback inhibition of ERK.
To examine the TSZ, we performed immunofluorescence for Sox2 and Tubb3. As expected, the presumptive TSZ, which was defined as a Sox2+ region with few Tubb3+ cells, was missing in Ptpn11-cKO embryos (Fig. 5F,F′,G,G′). By contrast, the TSZ occupied a much broader area in the posterior tectum in En1cre/+; R26Mek1DD/+ and En1cre/+; Ptpn11F/F; R26Mek1DD/+ embryos compared with controls (Fig. 5F-I′). The ventricular zone marked by Sox2 was notably thicker throughout the tectum in En1cre/+; R26Mek1DD/+ and En1cre/+; Ptpn11F/F; R26Mek1DD/+ embryos than in wild-type and Ptpn11-cKO embryos at E13.5 (Fig. 5F-I″). We detected few cells exiting the cell cycle in the posterior tectum of En1cre/+; Ptpn11F/F; R26Mek1DD/+ embryos from E11.5 to E12.5 (Fig. S2A). After a single EdU administration at E11.5, the EdU labeling index was significantly lower than that of controls in the posterior tectum of En1cre/+; Ptpn11F/F; R26Mek1DD/+ embryos at E12.5 (Fig. S2B), indicating reduced cell cycle activity caused by Mek1DD expression. Therefore, Mek1DD expression reverses the phenotype caused by Ptpn11 deletion and rescues the TSZ by reducing cell cycle exit and/or cell cycle activity.
At P7, morphologically and histologically discernible SC and IC were found in En1cre/+; Ptpn11F/F; R26Mek1DD/+ mice (Fig. 5L,M; Fig. S2C,D). Collectively, our data demonstrate that the MEK/ERK signaling pathway mediates Ptpn11 to maintain the TSZ, which is essential for IC formation.
The Ptpn11-regulated ERK signaling pathway mediates isthmic organizer activity to maintain the TSZ
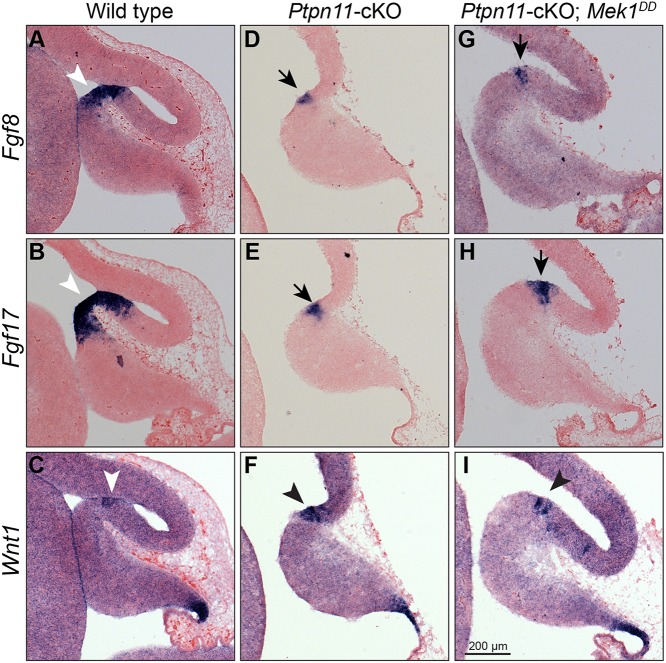
In En1cre/+; Ptpn11F/F; R26Mek1DD/+ embryos, although ERK hyperphosphorylation is present throughout the mes, the TSZ is enlarged but limited to the posterior end of the developing tectum (Fig. 5D,H-I′). This suggests that Mek1DD rescues the TSZ by potentiating remaining isthmic organizer function in Ptpn11-cKO embryos. To test this hypothesis, we analyzed how Ptpn11 deletion and Mek1DD expression affected transcription of isthmic organizer genes. Three members of the Fgf8 subfamily, Fgf8, Fgf17 and Fgf18, are expressed in the isthmus; Fgf8 interacts with Fgf17 to promote the growth of the tectum and cerebellum (Xu et al., 2000). In addition, Wnt1, which codes for a secreted signaling protein, is expressed in a transverse band immediately anterior to the isthmus (Wurst and Bally-Cuif, 2001). In situ hybridization revealed that Ptpn11 deletion narrowed the expression domain of Fgf8 and Fgf17, but had little effect on Wnt1 expression, at the isthmus of E12.5 embryos (Fig. 6A-F). Remarkably, the expression patterns of Fgf8, Fgf17 and Wnt1 at the isthmus were indistinguishable between En1cre/+; Ptpn11F/F and En1cre/+; Ptpn11F/F; R26Mek1DD/+ embryos at E12.5 (Fig. 6G-I). These findings suggest that Ptpn11 and Mek1DD regulate the TSZ primarily through modulation of intracellular signaling of isthmic organizer rather than transcription of organizer genes.
Fig. 6.
Mek1DD expression rescues the tectal stem zone and inferior colliculus independently of the transcriptional regulation of the isthmic organizer genes in Ptpn11-cKO mutants. (A-I) In situ hybridization on sagittal sections of E12.5 brains of the indicated genotypes. Arrowheads indicate normal expression at the isthmus; arrows denote the restricted expression domains.
The graded expression of axon-mapping labels is controlled by ERK signaling
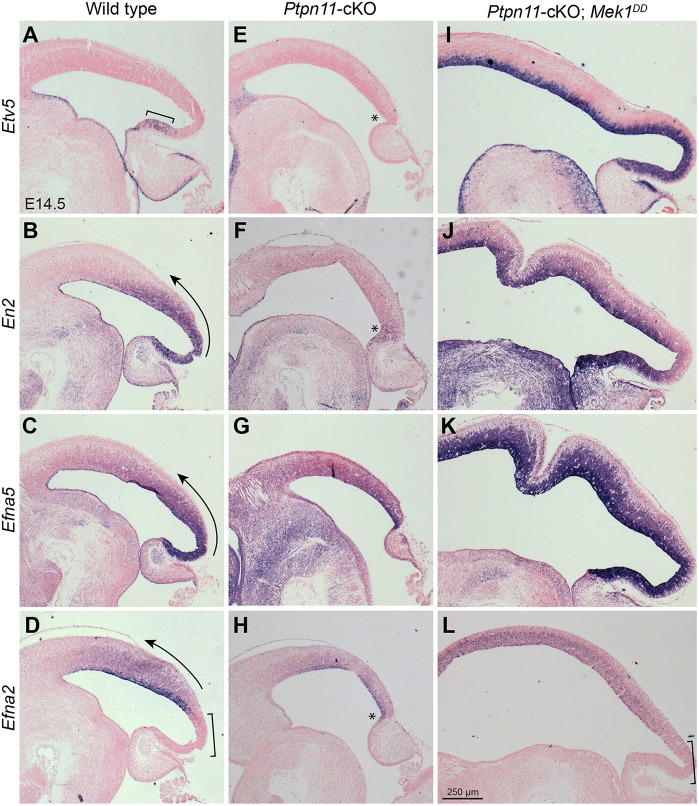
Graded distribution of FGF ligands establishes the smooth expression gradient of En2 and ephrin A (Efna5 and Efna2) in the tectum of chick embryos (Chen et al., 2009b). However, the intracellular signaling pathway that mediates FGFs to generate this molecular polarity in the tectum had not been determined. In agreement with previous findings in chick embryos (Sato and Nakamura, 2004; Suzuki-Hirano et al., 2009), we observed a gradient of pERK immunoreactivity in the mouse mes (Fig. 2A; Fig. 5B), suggesting that the ERK pathway controls molecular polarity in the tectum. To test this notion, we examined the expression of Etv5 (a known transcriptional target and effector of FGF/ERK signaling; Mao et al., 2009; Zhang et al., 2009; Znosko et al., 2010), En2 and its targets Efna5 and Efna2 in the tectum of wild-type, Ptpn11-cKO and Ptpn11-cKO;MEK embryos at E14.5. As described previously (Sato and Nakamura, 2004; Suzuki-Hirano et al., 2009), Etv5, En2, Efna5 and Efna2 are expressed in a posteriorhigh-anteriorlow gradient (Fig. 7A-D). Different from the other genes, the Efna2 transcripts are absent from the TSZ (Fig. 7D). In the Ptpn11-cKO tectum, the transcripts of Etv5, En2 and Efna5 were depleted or greatly reduced (Fig. 7E-H). In line with the TSZ deletion, the negative domain of Efna2 was absent from the posterior tectum in Ptpn11-cKO embryos at E14.5 (Fig. 7H). In Ptpn11-cKO;MEK embryos, Etv5, En2 and Efna5 were robustly induced throughout the tectum, whereas Efna2 was broadly expressed in the presumptive SC (Fig. 7I-L). Our data show that the ERK pathway mediates graded FGF activities to establish a smooth gradient of axon-mapping labels in the tectum.
Fig. 7.
ERK signaling controls the graded expression of neural mapping labels in the tectum. (A-L) In situ hybridization on sagittal sections of E14.5 brains of the indicated genotypes. The arrows indicate the expression gradient; the brackets show Etv5 expression in the posterior end of the tectum in A and the lack of Efna2 expression in D and L; the asterisks indicate the loss of Etv5 (E), restricted En2 (F) and ectopic Efna2 expression (H).
Mek1DD expression rescues cell death, but not the TSZ or IC, in the mes without Fgf8
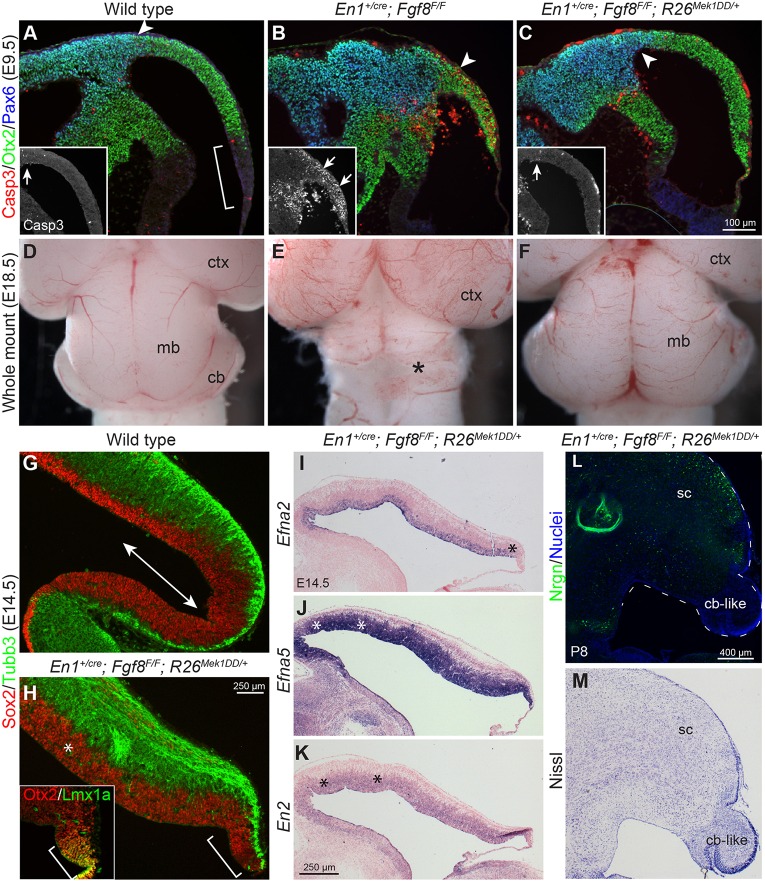
In En1cre/+; R26Mek1DD/+ and En1cre/+; Ptpn11F/F; R26Mek1DD/+ mice, although the widespread ERK hyperphosphorylation abolishes molecular polarity in the tectum (Fig. 7), the tectum is partitioned into the SC and IC (Fig. 5L,M; Fig. S2C,D), suggesting that isthmus-derived signaling, but not Mek1DD-induced ERK, controls the discrete cell fates of the SC and IC. Because the isthmic organizer, especially Fgf8, is essential for cell survival throughout the mes-r1 neural plate (Chi et al., 2003; Saarimäki-Vire et al., 2007), it has been impossible to evaluate how the tectum develops without the isthmic organizer. We investigated whether Mek1DD expression could rescue cell death without Fgf8 by generating En1+/cre; Fgf8F/F; R26Mek1DD/+ embryos, in which Fgf8 deletion and Mek1DD expression simultaneously occur in the mes-r1 neural plate. In contrast to the widespread cell death in the En1+/cre; Fgf8F/F mes at E9.5, few Casp3+ cells were detected in the mes, which was defined by Otx2+/Pax6– cells, in En1+/cre; Fgf8F/F; R26Mek1DD/+ embryos (Fig. 8A-C). Terminal deoxynucleotidyl transferase dUTP nick end labeling corroborated the rescue of cell death in En1+/cre; Fgf8F/F; R26Mek1DD/+ embryos (data not shown). At E18.5, the tectum was discernible in En1+/cre; Fgf8F/F; R26Mek1DD/+ embryos (Fig. 8D-F). Our data demonstrate that Mek1DD expression prevents cell death in the mes without Fgf8.
Fig. 8.
Mek1DD expression rescues the cell survival but not the tectal stem zone and inferior colliculus in the absence of Fgf8. (A-C) Immunofluorescence on sagittal sections of E9.5 embryos of the indicated genotypes. Insets show single channel of Casp3 staining; the arrows indicate Casp3+ apoptotic cells; the bracket demarcates the Otx2– region corresponding to the isthmus and r1; arrowheads mark the posterior limit of Pax6 expression. (D-F) Dorsal view of E18.5 brains. The asterisk shows the loss of midbrain (mb) and cerebellum (cb). ctx, neocortex. (G,H) Immunostaining on sagittal sections. The inset shows Otx2/Lmx1a staining on a section adjacent to that shown in H; the brackets delimit the rhombic lip marked by Lmx1a; the asterisk indicates the thickened ventricular zone; double arrow indicates the tectal stem zone. (I-K) In situ hybridization on sagittal sections of E14.5 En1cre/+;Fgf8F/F;R26Mek1DD/+ tectum. Asterisks indicate ectopic expression compared with controls (see Fig. 7B-D). (L,M) Nrgn (L) and Nissl (M) staining on sagittal sections of P8 En1cre/+;Fgf8F/F;R26Mek1DD/+ brains. sc, superior colliculus; cb-like, cerebellum-like tissue.
In both En1+/cre; Fgf8F/F and En1+/cre; Fgf8F/F; R26Mek1DD/+ embryos at E9.5, the Otx2– domain posterior to the mes was missing (Fig. 8A-C), suggesting the absence of the isthmus and r1. Lmx1a is expressed at the rhombic lip, the junction between the neural tube and the roof plate of the fourth ventricle (Chizhikov et al., 2006). We detected Lmx1a+/Otx2+ cells at the posterior end of the mes that juxtaposed the roof of the fourth ventricle in En1cre/+; Fgf8F/F; R26Mek1DD/+ embryos at E12.5 and E14.5 (Fig. 8H, inset; data not shown), indicating the induction of the rhombic lip at the junction between the mes and roof plate of the fourth ventricle in these mutants. Collectively, our data show that Mek1DD expression fails to rescue the specification of the isthmus and r1 in the absence of Fgf8.
We then examined whether Mek1DD expression rescued the TSZ and the IC without the isthmus. As found in the tectum with Mek1DD expression (Fig. 5H′,I′,H″,I″), the ventricular zone marked by Sox2 was thickened in the En1+/cre; Fgf8F/F; R26Mek1DD/+ tectum at E13.5 (Fig. 8G,H). However, the posterior part of the En1+/cre; Fgf8F/F; R26Mek1DD/+ tectum was remarkably similar to that found in Ptpn11-cKO mutants, containing abundant postmitotic neuronal precursor cells marked by Tubb3 and lacking the Efna2– domain (Fig. 5G′; Fig. 7H; Fig. 8H,I), indicating the loss of the TSZ in En1+/cre; Fgf8F/F; R26Mek1DD/+ embryos. In agreement with the broad Mek1DD expression, Efna2, Efna5 and En2 were evenly expressed in the remaining tectum of En1+/cre; Fgf8F/F; R26Mek1DD/+ embryos at E14.5 (Fig. 8I-K). Nrgn and Nissl staining revealed the absence of the IC in En1+/cre; Fgf8F/F; R26Mek1DD/+ mice at P8 (Fig. 8L,M), demonstrating that Mek1DD expression fails to rescue the IC without Fgf8. Therefore, Mek1DD expression promotes self-renewal of neural progenitor cells in the TSZ depending on isthmus-derived FGF. Our findings further support the notion that the maintenance of the TSZ is essential for the formation of the IC.
DISCUSSION
By characterizing the tectal phenotype in Ptpn11-cKO mutants with or without Mek1DD, we demonstrated that FGF/ERK signaling controls the anterior-to-posterior expansion of the tectum and formation of the IC by maintaining a newly discovered stem zone, the TSZ, in the posterior tectum. By expressing Mek1DD in the mes-r1 without Fgf8, we have defined the crucial role of the ERK signaling pathway in mediating diverse FGF function, including regionalization, cell survival, tissue polarity and tissue specification, during tectal development.
Mutations reducing FGF function at the isthmus were known to cause abnormal apoptosis in the anterior mes (Basson et al., 2008; Sato and Joyner, 2009). Our study has extended the previous findings by showing that elevated apoptosis is normally present in both diencephalon and mes areas immediately next to the DMB at E9.5, and that Ptpn11 deletion enhances the phenotype (Fig. 3A-E). How Ptpn11-cKO intensifies apoptosis around the DMB, including the diencephalon (a non-cell-autonomous effect), is unclear. It is possible that cell death is caused by abnormal DMB formation, a process that is dependent on isthmus-originated Fgf8 (Scholpp et al., 2003), in Ptpn11-cKO embryos. It has been suggested that cell death in the anterior mes is accompanied by transformation of the remaining IC progenitors into an SC fate, which in turn causes IC deletion (Basson et al., 2008; Sato and Joyner, 2009). However, in Ptpn11-cKO embryos the anterior-posterior patterning of the mes-r1 is mostly normal at E10.5 (Fig. 2), and shortening of the tectum does not occur until E12.5 (Fig. 1C), suggesting that cell death and/or misspecification are unlikely to account for the IC deletion. As we are unaware of any marker that specifically defines SC or IC progenitors, we cannot rule out the possibility that Ptpn11-cKO causes an SC-to-IC transformation after E10.5. We link the failure of IC formation to the loss of the TSZ in Ptpn11-cKO and En1+/cre; Fgf8F/F; R26Mek1DD/+ embryos. Conversely, rescuing the TSZ by Mek1DD restores the IC without Ptpn11. Our results strongly suggest that the isthmus-originated FGF function is essential for the maintenance of the TSZ and thereby the formation of the IC.
Genetic fate-mapping experiments revealed that cells in the posterior mes are mostly destined for the IC (Sgaier et al., 2007; Zervas et al., 2004), and that the SC and IC are almost exclusively formed by neuronal precursor cells that undergo neurogenesis around E12.5 and E15.5, respectively (Kim et al., 2008). In rat, IC neurons are produced between E14 and the perinatal period in an orderly sequence: the earliest born neurons are situated anteriorly, and latest generated cells reside posteriorly (Altman and Bayer, 1981a). In contrast to the IC, SC neurons are arranged in the inside-out and outside-in sequence in the deep and superficial layers, respectively, in rodents (Altman and Bayer, 1981b). Taken together, we propose the following model for tectal development in mice. Cells that are distal to the isthmus receive low levels of FGF/ERK signaling, undergo neurogenesis before E12.5, and the generated neurons are arranged in laminar manner to form the SC. Neural progenitor cells that are proximal to the isthmus receive high levels of FGF/ERK activity and thereby undergo proliferative division to constitute the TSZ. As the stem zone expands due to cell proliferation, cells in the anterior-most region of the TSZ receive less FGF/ERK activity, switch from proliferative to neurogenic division around E15.5, and produce neurons to the IC in an anterior-to-posterior order.
According to our model, graded FGF/ERK activities dictate the temporal and spatial order of neurogenesis of progenitors within the TSZ. It explains why both the strength and duration of FGF are important for IC formation. Failures in maintaining the TSZ probably account for the IC deletion in mouse mutants in which FGF signaling is reduced in the mes-r1 (Basson et al., 2008; Chi et al., 2003; Sgaier et al., 2007; Trokovic et al., 2003; Xu et al., 2000; Yang et al., 2013a), or in which Fgf8 is deleted after E9.5 (Sato and Joyner, 2009). It is worth noting that deleting Fgf8 before E9.5 (Sato and Joyner, 2009) or severely reducing FGF function (Chi et al., 2003; Trokovic et al., 2003) causes partial loss of the SC in addition to complete absence of the IC, suggesting that the TSZ might provide SC precursor cells at the early stage.
It is well-known that self-renewal of neural stem/progenitor cells (NSPCs) is inversely correlated with cell cycle length, especially G1 duration (Dehay and Kennedy, 2007; Salomoni and Calegari, 2010). Lengthening or shortening of G1 can accelerate or delay, respectively, neurogenesis in the embryonic forebrain (Calegari and Huttner, 2003; Lange et al., 2009; Pilaz et al., 2009). However, emerging evidence suggests that S-phase length is linked to NSPC maintenance, and S-phase shortening signifies the transition of NSPCs from proliferative to neurogenic division (Arai et al., 2011; Cabochette et al., 2015; Turrero García et al., 2016). Remarkably, Ptpn11 deletion shortens the S phase and total cell cycle in the TSZ. Our estimated cell cycle parameters (Tc=17.5, Ts=6.7 h at E12.5; Ts=6.2 at E11.5) are comparable to those (Tc=19.1, Ts=5.0 h) determined by cumulative EdU labeling for apical progenitors in E14.5 mouse cortex (Arai et al., 2011). We found that almost all mitotic nuclei became EdU+ in the 2 h after EdU administration in both wild-type and Ptpn11-cKO embryos at E11.5 (Fig. S1A), indicating an average G2 length of <2 h, similar to that reported previously (Arai et al., 2011; Tapias et al., 2014). Given the relatively short and constant M phase of neural progenitors (0.5-1.0 h) (Arai et al., 2011; Tapias et al., 2014), we deduce that Ptpn11 deletion does not significantly change G1 duration but mainly affects the S phase. Although we did not measure cell cycle parameters, we showed that Mek1DD expression inhibited cell cycle exit and reduced cell cycle activity in the posterior tectum (Fig. S2S,B), and consequently it not only rescued but also expanded the TSZ with or without Ptpn11 (Fig. 5H′,I′; Fig. 7L). Our data thus suggest that the MEK/ERK signaling pathway regulates cell cycle progression, especially the S phase, to control the timely transition from proliferative to neurogenic division in the TSZ.
By expressing Mek1DD in the mes-r1 lacking Ptpn11 or Fgf8, we have gained new insight into the intracellular signaling pathway responsible for the isthmus-derived FGF function. We demonstrated that the MEK/ERK cascade mediates Fgf8 to control cell survival (Fig. 8A-C). Moreover, the MEK/ERK signaling pathway mediates graded FGF activities to establish the expression gradient of neural mapping labels in the tectum (Fig. 7; Fig. 8I-K). We also found that Fgf8 and Mek1DD initiate qualitatively and/or quantitatively distinctive signaling because Mek1DD expression failed to rescue regionalization of the mes-r1 neural plate or to maintain the TSZ in the absence of Fgf8. Interestingly, pERK immunoreactivity was reduced near the isthmus in En1+/cre; Ptpn11F/F; R26Mek1DD/+ and En1+/cre; R26Mek1DD/+ embryos (Fig. 5D,E), suggesting that endogenous FGFs initiate feedback signaling whereas Mek1DD cannot. Previous studies showed that sustained or transient activation of ERK differentially causes differentiation or proliferation, respectively, in PC12 cells (Marshall, 1995). Moreover, feedback inhibition of ERK signaling differs from sustained ERK activation in the control of mes-r1 regionalization (Suzuki-Hirano et al., 2009). Therefore, different timing, amplitude and durations of ERK signaling may dictate distinct cellular responses in the tectum. Alternatively, Fgf8 might activate other signaling cascades upstream of MEK/ERK.
Although Mek1DD expression caused thickening of the ventricular zone (marked by Sox2) throughout the tectum (Fig. 6F-I″; Fig. 8G,H), it was insufficient to block neurogenesis to induce or maintain the TSZ without isthmus-derived FGFs (Fig. 8H). Interestingly, the basic helix-loop-helix protein Hes1, a potent inhibitor of neurogenesis, is specifically expressed in the posterior tectum (Hirata et al., 2001; data not shown). The spatially restricted Hes1 expression could explain why Mek1DD itself cannot induce/maintain the TSZ in the anterior tectum. Moreover, cyclical Hes1 expression is important for NSPC maintenance (Imayoshi et al., 2013; Shimojo et al., 2008); FGF and its downstream signaling cascade, the JAK/STAT pathway, have been implicated in the control of Hes1 oscillation (Kamakura et al., 2004; Nakayama et al., 2008; Yoshiura et al., 2007). Therefore, isthmus-derived FGF signaling may regulate Hes1 oscillation to induce/maintain the TSZ in the posterior tectum. Remarkably, a previous report showed that Hes1 expression primarily oscillates during the S phase in vivo (Shimojo et al., 2008). As Hes1 expression oscillates with a period of 2-3 h because of intrinsic feedback inhibition, S-phase duration could dictate the number of Hes1 oscillation cycles and thereby step-wise accumulation of Hes1 downstream genes, which may in turn control the timing of neurogenesis and/or cell fate decisions in the TSZ. Further studies will be needed to determine whether ERK-dependent and ERK-independent FGF signaling act in concert by differentially regulating S phase and Hes1 expression oscillation in the control of TSZ maintenance and IC development.
In summary, we found that the Ptpn11-regulated Fgf8/ERK signaling pathway is instrumental in maintaining the TSZ, which is in turn responsible for the developmental gradient of developing tectum and, ultimately, formation of the IC. These insights are important for understanding how tissue expansion is related to patterning, differentiation and specification of mature tissue structures in other areas of the developing embryo.
MATERIALS AND METHODS
Mouse and tissue preparation
All animal procedures described herein were approved by the Animal Care Committee at the University of Connecticut Health. All mouse strains were maintained on an ICR outbred genetic background. Noon of the day on which a vaginal plug was detected was designated as E0.5 in staging of embryos. Generation and characterization of En1cre (En1tm2(cre)Wrst/J; #007916) (Li et al., 2002), Ptpn11floxed (Yang et al., 2013b), Gbx2creER (Gbx2tm1.1(cre/ERT2)Jyhl/J; #022135) (Chen et al., 2009a), Fgf8floxed (Meyers et al., 1998), R26RlacZ (Soriano, 1999) and R26Mek1DD (Gt(ROSA)26Sortm8(Map2k1*,EGFP)Rsky/J; #012352) alleles have been described previously.
Embryonic mouse brains were dissected in cold PBS and fixed in 4% paraformaldehyde for 40 min or overnight. Brains were cryoprotected, frozen in OCT freezing medium (Sakura Finetek), and sectioned at 10-16 μm thickness using a cryomicrotome (CM3050, Leica).
Histochemistry, immunofluorescence and in situ hybridization
Standard protocols were used for X-gal histochemistry, immunofluorescence, and in situ hybridization, as described previously (Chen et al., 2009a). Detailed protocols are available on the Li Laboratory website (http://lilab.uchc.edu/protocols/index.html). Primary and secondary antibodies are listed in the Table S1.
For EdU and BrdU labeling, EdU and BrdU (30 mg/kg body weight, dissolved in PBS) were administered intraperitoneally to pregnant mice. EdU+ nuclei were visualized using the Click-iT EdU Alexa Fluor 647 Imaging Kit (Thermo Fisher Scientific).
Analysis of cell cycle progression, interkinetic nuclear migration, and length of the tectum
To analyze INM, we performed EdU and/or BrdU labeling in conjunction with Sox2 and/or pH3 staining. We chose Sox2 because it colocalized with Ki67 in the mes at E11.5, but produced better quality of signals than Ki67 allowing unbiased nucleus counting with ImageJ software. To determine the positions of EdU+ or BrdU+ nuclei 40, 60, 90, 120 and 240 min after EdU and/or EdU injection, we divided the posterior tectum into four bins parallel to the ventricular surface, and determined the number of EdU+, PH3+ and Sox2+ nuclei per bin using ImageJ. To measure the mesencephalic length, we used Adobe Illustrator to trace the mes on three parallel sections per embryo using the posterior limit of Pax6 expression and the isthmus as the anterior and posterior limits in five embryos of each genotype.
Quantification and statistical analysis
For analysis of cell cycle parameters, cell counting was performed manually with the examiner blind to relevant variables, such as genotypes and embryonic stages. At least two adjacent sections per embryo, and three or more embryos per group were analyzed. Cell-cycle (Tc) and S-phase (Ts) length were calculated as previously described (Martynoga et al., 2005): Ts=Ti/(Lcells/Scells); Ti=1.5 h (intervals between BrdU and EdU injections); Tc=Ts/(Scells/Sox2 cells). For cell death analysis, Casp3+ cells in both Pax6+ and Pax6− domains that were within 250 µm of the DMB were counted on five serial sections at 48 µm intervals from the midline in the lateral direction. For intermediate and lateral positions, numbers of Casp3+ cells on two adjacent sections were averaged.
Data processing, statistical analysis and plotting were performed in R version 3.1.2. An unpaired two-tailed t-test with Welch's correction or Student's t-test was used for analysis of experiments involving two groups. One-way ANOVA followed by the Turkey–Kramer multiple comparisons test was used for analysis of experiments involving more than two groups with one comparison. Bartlett's test was performed to verify the equal variance assumption before ANOVA and Student's t-test. Reproducible results were obtained from three or more samples, and quantitative data are expressed as mean± s.e.m.
Acknowledgements
We thank Dr N. Abimbola Sunmonu for her contribution to the initial characterization of Ptpn11-cKO mice, and Ms Carissa Sirois for her technical help in cell counting. We thank Drs Alexandra Joyner and Xin Zhang for providing plasmids to make RNA probes. The monoclonal anti-neurofilament antibody (2H3) and anti-Pax6 were developed by Drs T. M. Jessell, J. Dodd and A. Kawakami, and obtained through the Developmental Studies Hybridoma Bank under the auspices of the NICHD and maintained by The University of Iowa (Iowa City, IA).
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
A.D., K.L., X.H. and Q.G., performed experiments, analyzed data, discussed and commented on the written manuscript; A.D. helped write the manuscript; J.Y.H.L. conceived and directed the project, analyzed data and wrote the paper.
Funding
This work was supported by a grant from the National Institutes of Health [R01MH094914]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.138537.supplemental
References
- Altman J. and Bayer S. A. (1981a). Time of origin of neurons of the rat inferior colliculus and the relations between cytogenesis and tonotopic order in the auditory pathway. Exp. Brain. Res. 42, 411-423. 10.1007/bf00237506 [DOI] [PubMed] [Google Scholar]
- Altman J. and Bayer S. A. (1981b). Time of origin of neurons of the rat superior colliculus in relation to other components of the visual and visuomotor pathways. Exp. Brain. Res. 42, 424-434. 10.1007/bf00237506 [DOI] [PubMed] [Google Scholar]
- Arai Y., Pulvers J. N., Haffner C., Schilling B., Nüsslein I., Calegari F. and Huttner W. B. (2011). Neural stem and progenitor cells shorten S-phase on commitment to neuron production. Nat. Comms. 2, 154 10.1038/ncomms1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson M. A., Echevarria D., Ahn C. P., Sudarov A., Joyner A., Mason I., Martinez S. and Martin G. R. (2008). Specific regions within the embryonic midbrain and cerebellum require different levels of FGF signaling during development. Development 135, 889-898. 10.1242/dev.011569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N., Castro D. S. and Guillemot F. (2002). Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 3, 517-530. 10.1038/nrn874 [DOI] [PubMed] [Google Scholar]
- Broccoli V., Boncinelli E. and Wurst W. (1999). The caudal limit of Otx2 expression positions the isthmic organizer. Nature 401, 164-168. 10.1038/43670 [DOI] [PubMed] [Google Scholar]
- Cabochette P., Vega-Lopez G., Bitard J., Parain K., Chemouny R., Masson C., Borday C., Hedderich M., Henningfeld K. A., Locker M. et al. (2015). YAP controls retinal stem cell DNA replication timing and genomic stability. eLife 4, e08488 10.7554/eLife.08488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calegari F. and Huttner W. B. (2003). An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J. Cell Sci. 116, 4947-4955. 10.1242/jcs.00825 [DOI] [PubMed] [Google Scholar]
- Cang J. and Feldheim D. A. (2013). Developmental mechanisms of topographic map formation and alignment. Annu. Rev. Neurosci. 36, 51-77. 10.1146/annurev-neuro-062012-170341 [DOI] [PubMed] [Google Scholar]
- Chen L., Guo Q. and Li J. Y. H. (2009a). Transcription factor Gbx2 acts cell-nonautonomously to regulate the formation of lineage-restriction boundaries of the thalamus. Development 136, 1317-1326. 10.1242/dev.030510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Mohammadi M. and Flanagan J. G. (2009b). Graded levels of FGF protein span the midbrain and can instruct graded induction and repression of neural mapping labels. Neuron 62, 773-780. 10.1016/j.neuron.2009.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi C. L., Martinez S., Wurst W. and Martin G. R. (2003). The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development 130, 2633-2644. 10.1242/dev.00487 [DOI] [PubMed] [Google Scholar]
- Chizhikov V. V., Lindgren A. G., Currle D. S., Rose M. F., Monuki E. S. and Millen K. J. (2006). The roof plate regulates cerebellar cell-type specification and proliferation. Development 133, 2793-2804. 10.1242/dev.02441 [DOI] [PubMed] [Google Scholar]
- Cowan W. M., Martin A. H. and Wenger E. (1968). Mitotic patterns in the optic tectum of the chick during normal development and after early removal of the optic vesicle. J. Exp. Zool. 169, 71-92. 10.1002/jez.1401690110 [DOI] [PubMed] [Google Scholar]
- Cowley S., Paterson H., Kemp P. and Marshall C. J. (1994). Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell 77, 841-852. 10.1016/0092-8674(94)90133-3 [DOI] [PubMed] [Google Scholar]
- Crossley P. H. and Martin G. R. (1995). The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 121, 439-451. [DOI] [PubMed] [Google Scholar]
- Dehay C. and Kennedy H. (2007). Cell-cycle control and cortical development. Nat. Rev. Neurosci. 8, 438-450. 10.1038/nrn2097 [DOI] [PubMed] [Google Scholar]
- Edwards M. A., Caviness V. S. and Schneider G. E. (1986). Development of cell and fiber lamination in the mouse superior colliculus. J. Comp. Neurol. 248, 395-409. 10.1002/cne.902480308 [DOI] [PubMed] [Google Scholar]
- Guillemot F. and Zimmer C. (2011). From cradle to grave: the multiple roles of fibroblast growth factors in neural development. Neuron 71, 574-588. 10.1016/j.neuron.2011.08.002 [DOI] [PubMed] [Google Scholar]
- Hadari Y. R., Kouhara H., Lax I. and Schlessinger J. (1998). Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol. Cell. Biol. 18, 3966-3973. 10.1128/MCB.18.7.3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Tomita K., Bessho Y. and Kageyama R. (2001). Hes1 and Hes3 regulate maintenance of the isthmic organizer and development of the mid/hindbrain. EMBO J. 20, 4454-4466. 10.1093/emboj/20.16.4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I., Isomura A., Harima Y., Kawaguchi K., Kori H., Miyachi H., Fujiwara T., Ishidate F. and Kageyama R. (2013). Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science 342, 1203-1208. 10.1126/science.1242366 [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Liu A. and Millet S. (2000). Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr. Opin. Cell Biol. 12, 736-741. 10.1016/S0955-0674(00)00161-7 [DOI] [PubMed] [Google Scholar]
- Kamakura S., Oishi K., Yoshimatsu T., Nakafuku M., Masuyama N. and Gotoh Y. (2004). Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nat. Cell Biol. 6, 547-554. 10.1038/ncb1138 [DOI] [PubMed] [Google Scholar]
- Kim E. J., Battiste J., Nakagawa Y. and Johnson J. E. (2008). Ascl1 (Mash1) lineage cells contribute to discrete cell populations in CNS architecture. Mol. Cell. Neurosci. 38, 595-606. 10.1016/j.mcn.2008.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel R. A., Turnbull D. H., Blanquet V., Wurst W., Loomis C. A. and Joyner A. L. (2000). Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes Dev. 14, 1377-1389. [PMC free article] [PubMed] [Google Scholar]
- Lahti L., Saarimäki-Vire J., Rita H. and Partanen J. (2011). FGF signaling gradient maintains symmetrical proliferative divisions of midbrain neuronal progenitors. Dev. Biol. 349, 270-282. 10.1016/j.ydbio.2010.11.008 [DOI] [PubMed] [Google Scholar]
- Lange C., Huttner W. B. and Calegari F. (2009). Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell 5, 320-331. 10.1016/j.stem.2009.05.026 [DOI] [PubMed] [Google Scholar]
- Lee S. M., Danielian P. S., Fritzsch B. and McMahon A. P. (1997). Evidence that FGF8 signalling from the midbrain-hindbrain junction regulates growth and polarity in the developing midbrain. Development 124, 959-969. [DOI] [PubMed] [Google Scholar]
- Li J. Y. and Joyner A. L. (2001). Otx2 and Gbx2 are required for refinement and not induction of mid-hindbrain gene expression. Development 128, 4979-4991. [DOI] [PubMed] [Google Scholar]
- Li J. Y. H., Lao Z. and Joyner A. L. (2002). Changing requirements for Gbx2 in development of the cerebellum and maintenance of the mid/hindbrain organizer. Neuron 36, 31-43. 10.1016/S0896-6273(02)00935-2 [DOI] [PubMed] [Google Scholar]
- Li H., Tao C., Cai Z., Hertzler-Schaefer K., Collins T. N., Wang F., Feng G.-S., Gotoh N. and Zhang X. (2014a). Frs2α and Shp2 signal independently of Gab to mediate FGF signaling in lens development. J. Cell Sci. 127, 571-582. 10.1242/jcs.134478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Leung A. W., Guo Q., Yang W. and Li J. Y. H. (2014b). Shp2-dependent ERK signaling is essential for induction of Bergmann glia and foliation of the cerebellum. J. Neurosci. 34, 922-931. 10.1523/JNEUROSCI.3476-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A., Losos K. and Joyner A. L. (1999). FGF8 can activate Gbx2 and transform regions of the rostral mouse brain into a hindbrain fate. Development 126, 4827-4838. [DOI] [PubMed] [Google Scholar]
- Liu A., Li J. Y. H., Bromleigh C., Lao Z., Niswander L. A. and Joyner A. L. (2003). FGF17b and FGF18 have different midbrain regulatory properties from FGF8b or activated FGF receptors. Development 130, 6175-6185. 10.1242/dev.00845 [DOI] [PubMed] [Google Scholar]
- Mao J., McGlinn E., Huang P., Tabin C. J. and McMahon A. P. (2009). Fgf-dependent Etv4/5 activity is required for posterior restriction of sonic hedgehog and promoting outgrowth of the vertebrate limb. Dev. Cell 16, 600-606. 10.1016/j.devcel.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J. (1995). Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179-185. 10.1016/0092-8674(95)90401-8 [DOI] [PubMed] [Google Scholar]
- Martinez S., Crossley P. H., Cobos I., Rubenstein J. L. and Martin G. R. (1999). FGF8 induces formation of an ectopic isthmic organizer and isthmocerebellar development via a repressive effect on Otx2 expression. Development 126, 1189-1200. [DOI] [PubMed] [Google Scholar]
- Martynoga B., Morrison H., Price D. J. and Mason J. O. (2005). Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev. Biol. 283, 113-127. 10.1016/j.ydbio.2005.04.005 [DOI] [PubMed] [Google Scholar]
- Meyers E. N., Lewandoski M. and Martin G. R. (1998). An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat. Genet. 18, 136-141. 10.1038/ng0298-136 [DOI] [PubMed] [Google Scholar]
- Millet S., Campbell K., Epstein D. J., Losos K., Harris E. and Joyner A. L. (1999). A role for Gbx2 in repression of Otx2 and positioning the mid/hindbrain organizer. Nature 401, 161-164. 10.1038/43664 [DOI] [PubMed] [Google Scholar]
- Nakayama K., Satoh T., Igari A., Kageyama R. and Nishida E. (2008). FGF induces oscillations of Hes1 expression and Ras/ERK activation. Curr. Biol. 18, R332-R334. 10.1016/j.cub.2008.03.013 [DOI] [PubMed] [Google Scholar]
- Pilaz L.-J., Patti D., Marcy G., Ollier E., Pfister S., Douglas R. J., Betizeau M., Gautier E., Cortay V., Doerflinger N. et al. (2009). Forced G1-phase reduction alters mode of division, neuron number, and laminar phenotype in the cerebral cortex. Proc. Natl. Acad. Sci. USA 106, 21924-21929. 10.1073/pnas.0909894106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C. K. (2000). The SHP-2 tyrosine phosphatase: signaling mechanisms and biological functions. Cell Res. 10, 279-288. 10.1038/sj.cr.7290055 [DOI] [PubMed] [Google Scholar]
- Saarimäki-Vire J., Peltopuro P., Lahti L., Naserke T., Blak A. A., Vogt Weisenhorn D. M., Yu K., Ornitz D. M., Wurst W. and Partanen J. (2007). Fibroblast growth factor receptors cooperate to regulate neural progenitor properties in the developing midbrain and hindbrain. J. Neurosci. 27, 8581-8592. 10.1523/JNEUROSCI.0192-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomoni P. and Calegari F. (2010). Cell cycle control of mammalian neural stem cells: putting a speed limit on G1. Trends Cell Biol. 20, 233-243. 10.1016/j.tcb.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Sato T. and Joyner A. L. (2009). The duration of Fgf8 isthmic organizer expression is key to patterning different tectal-isthmo-cerebellum structures. Development 136, 3617-3626. 10.1242/dev.041210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T. and Nakamura H. (2004). The Fgf8 signal causes cerebellar differentiation by activating the Ras-ERK signaling pathway. Development 131, 4275-4285. 10.1242/dev.01281 [DOI] [PubMed] [Google Scholar]
- Scholpp S., Lohs C. and Brand M. (2003). Engrailed and Fgf8 act synergistically to maintain the boundary between diencephalon and mesencephalon. Development 130, 4881-4893. 10.1242/dev.00683 [DOI] [PubMed] [Google Scholar]
- Sgaier S. K., Lao Z., Villanueva M. P., Berenshteyn F., Stephen D., Turnbull R. K. and Joyner A. L. (2007). Genetic subdivision of the tectum and cerebellum into functionally related regions based on differential sensitivity to engrailed proteins. Development 134, 2325-2335. 10.1242/dev.000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamim H., Mahmood R., Logan C., Doherty P., Lumsden A. and Mason I. (1999). Sequential roles for Fgf4, En1 and Fgf8 in specification and regionalisation of the midbrain. Development 126, 945-959. [DOI] [PubMed] [Google Scholar]
- Shimojo H., Ohtsuka T. and Kageyama R. (2008). Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron 58, 52-64. 10.1016/j.neuron.2008.02.014 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71. 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Sun T. and Hevner R. F. (2014). Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat. Rev. Neurosci. 15, 217-232. 10.1038/nrn3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki-Hirano A., Harada H., Sato T. and Nakamura H. (2009). Activation of Ras-ERK pathway by Fgf8 and its downregulation by Sprouty2 for the isthmus organizing activity. Dev. Biol. 337, 284-293. 10.1016/j.ydbio.2009.10.044 [DOI] [PubMed] [Google Scholar]
- Tapias A., Zhou Z.-W., Shi Y., Chong Z., Wang P., Groth M., Platzer M., Huttner W., Herceg Z., Yang Y.-G. et al. (2014). Trrap-dependent histone acetylation specifically regulates cell-cycle gene transcription to control neural progenitor fate decisions. Cell Stem Cell 14, 632-643. 10.1016/j.stem.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Trokovic R., Trokovic N., Hernesniemi S., Pirvola U., Vogt Weisenhorn D. M., Rossant J., McMahon A. P., Wurst W. and Partanen J. (2003). FGFR1 is independently required in both developing mid- and hindbrain for sustained response to isthmic signals. EMBO J. 22, 1811-1823. 10.1093/emboj/cdg169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrero García M., Chang Y., Arai Y. and Huttner W. B. (2016). S-phase duration is the main target of cell cycle regulation in neural progenitors of developing ferret neocortex. J. Comp. Neurol. 524, 456-470. 10.1002/cne.23801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst W. and Bally-Cuif L. (2001). Neural plate patterning: upstream and downstream of the isthmic organizer. Nat .Rev. Neurosci. 2, 99-108. 10.1038/35053516 [DOI] [PubMed] [Google Scholar]
- Xu J., Liu Z. and Ornitz D. M. (2000). Temporal and spatial gradients of Fgf8 and Fgf17 regulate proliferation and differentiation of midline cerebellar structures. Development 127, 1833-1843. [DOI] [PubMed] [Google Scholar]
- Yang J., Brown A., Ellisor D., Paul E., Hagan N. and Zervas M. (2013a). Dynamic temporal requirement of Wnt1 in midbrain dopamine neuron development. Development 140, 1342-1352. 10.1242/dev.080630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Wang J., Moore D. C., Liang H., Dooner M., Wu Q., Terek R., Chen Q., Ehrlich M. G., Quesenberry P. J. et al. (2013b). Ptpn11 deletion in a novel progenitor causes metachondromatosis by inducing hedgehog signalling. Nature 499, 491-495. 10.1038/nature12396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W., Bouchard M., Stone D., Liu X., Vella F., Lee J., Nakamura H., Ang S.-L., Busslinger M. and Rosenthal A. (2001). Distinct regulators control the expression of the mid-hindbrain organizer signal FGF8. Nat. Neurosci. 4, 1175-1181. 10.1038/nn761 [DOI] [PubMed] [Google Scholar]
- Yoshiura S., Ohtsuka T., Takenaka Y., Nagahara H., Yoshikawa K. and Kageyama R. (2007). Ultradian oscillations of Stat, Smad, and Hes1 expression in response to serum. Proc. Natl. Acad. Sci. USA 104, 11292-11297. 10.1073/pnas.0701837104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervas M., Millet S., Ahn S. and Joyner A. L. (2004). Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron 43, 345-357. 10.1016/j.neuron.2004.07.010 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Verheyden J. M., Hassell J. A. and Sun X. (2009). FGF-regulated Etv genes are essential for repressing Shh expression in mouse limb buds. Dev. Cell 16, 607-613. 10.1016/j.devcel.2009.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znosko W. A., Yu S., Thomas K., Molina G. A., Li C., Tsang W., Dawid I. B., Moon A. M. and Tsang M. (2010). Overlapping functions of Pea3 ETS transcription factors in FGF signaling during zebrafish development. Dev. Biol. 342, 11-25. 10.1016/j.ydbio.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]