Abstract
During development, the lung mesoderm generates a variety of cell lineages, including airway and vascular smooth muscle. Epigenetic changes in adult lung mesodermal lineages are thought to contribute towards diseases such as idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease, although the factors that regulate early lung mesoderm development are unknown. We show in mouse that the PRC2 component Ezh2 is required to restrict smooth muscle differentiation in the developing lung mesothelium. Mesodermal loss of Ezh2 leads to the formation of ectopic smooth muscle in the submesothelial region of the developing lung mesoderm. Loss of Ezh2 specifically in the developing mesothelium reveals a mesothelial cell-autonomous role for Ezh2 in repression of the smooth muscle differentiation program. Loss of Ezh2 derepresses expression of myocardin and Tbx18, which are important regulators of smooth muscle differentiation from the mesothelium and related cell lineages. Together, these findings uncover an Ezh2-dependent mechanism to restrict the smooth muscle gene expression program in the developing mesothelium and allow appropriate cell fate decisions to occur in this multipotent mesoderm lineage.
KEY WORDS: Ezh2, Polycomb repressive complex 2, Lung development, Smooth muscle, Mesoderm, Mesothelium
Summary: The PRC component Ezh2 acts cell-autonomously in the developing mouse lung mesothelium to repress smooth muscle differentiation and allow appropriate cell fate decisions to occur.
INTRODUCTION
Lung mesoderm-derived lineages provide a physical scaffold and inductive signaling for the conducting airway and gas-exchanging alveolar epithelium. Lung mesoderm develops into multiple tissue types, such as vascular smooth muscle, airway smooth muscle, and endothelium, in addition to poorly characterized parenchymal and interstitial mesenchymal cells. Lung mesodermal lineages are highly plastic in development and during injury repair, and destabilization of cell identity and quiescence are associated with diseases such as idiopathic pulmonary fibrosis (IPF) and chronic obstructive pulmonary disease (COPD) (Morrisey and Hogan, 2010; Herriges and Morrisey, 2014; Hogan et al., 2014).
The lung mesothelium is a multipotent mesoderm-derived cell lineage that forms a monolayer surrounding the lung and contributes to lung development through both direct contribution of mesodermally derived lineages as well as paracrine signaling. The lung mesothelium is contiguous with other pleural cell types, including the epicardium of the heart. Mesothelial cells migrate into the lung and contribute to the mesenchyme and generate a substantial proportion of vascular smooth muscle (Que et al., 2008; Dixit et al., 2013). Mesothelial cells may contribute to IPF, a disease characterized by inappropriate fibroblast differentiation and excessive proliferation (Mutsaers et al., 2015). Mesothelial cells differentiate into fibroblasts upon exposure to Tgfβ, a pro-fibrotic signaling molecule, which is upregulated in IPF lungs (Batra and Antony, 2015). Additionally, a mouse model of fibrosis by intratracheal Tgfβ1 instillation induces expression of the mesothelial-specific transcription factor Wilms tumor 1 (WT1) in the lung parenchyma (Karki et al., 2014). These data suggest that the mesothelium and mesothelial-derived cells might reactivate their developmental plasticity during lung injury and repair processes, and contribute to inappropriate fibrosis.
Ezh2 is the histone methyltransferase component of Polycomb repressive complex 2 (PRC2), which trimethylates lysine 27 of histone H3 (H3K27me3) and epigenetically represses transcription of developmentally regulated genes (Su et al., 2003; Boyer et al., 2006; Bracken et al., 2006; Lee et al., 2006). Ezh2 is highly expressed in the developing lung mesoderm, and its expression decreases as development proceeds (Galvis et al., 2015; Snitow et al., 2015). In addition to suppressing ectopic lineage differentiation during lung endoderm development (Galvis et al., 2015; Snitow et al., 2015), Ezh2 is required for normal development of many mesodermal tissues, such as the heart where it is essential for suppressing skeletal muscle genes in the myocardium (Delgado-Olguín et al., 2012). The crucial roles of Ezh2 in multiple mesodermal lineages led us to investigate its requirement in lung mesoderm development and lineage specification.
Here, we show that Ezh2 is required in the developing lung mesothelium to suppress smooth muscle differentiation. Ezh2 directly suppresses expression of the smooth muscle master transcription factor myocardin (Myocd) as well as the transcription factor Tbx18, which is known to promote smooth muscle development from the epicardium (Wu et al., 2013). Together, these data indicate that Ezh2/PRC2 is required to suppress ectopic smooth muscle development from the multipotent lung mesothelium by repressing the key transcriptional regulators Myocd and Tbx18.
RESULTS
Loss of Ezh2 in mesoderm inhibits lung growth and respiratory function
Ezh2 was previously shown to be highly expressed in the developing lung mesoderm (Galvis et al., 2015; Snitow et al., 2015). To assess the role of Ezh2 in lung mesoderm development, we generated a mesoderm-specific loss-of-function mutant by crossing Ezh2flox/flox mice with the pan-mesodermal Cre-expressing line Dermo1cre that has robust activity in the lung mesoderm by E10.5 (Su et al., 2003; Yu et al., 2003; Yin et al., 2008). We also crossed the R26RmTmG reporter line into these mutants to track Cre recombination activity (Muzumdar et al., 2007). Dermo1cre:R26RmTmG:Ezh2flox/flox mutant mice (hereafter referred to as Ezh2mesoderm-KO) die at P0 due to respiratory distress. They are cyanotic shortly after birth and attempt to breath, but are unable to inflate their lungs with air as assessed by buoyancy in PBS (Fig. 1A,B). Ezh2mesoderm-KO lungs are small (Fig. 1C,D) and have poorly developed alveoli with reduced mesenchymal development, as noted by reduced vimentin expression (Fig. S1A-C). Our laboratory recently showed that Tgfβ is crucial for sacculation and early alveologenesis in the lung (Wang et al., 2016). However, loss of Ezh2 did not alter the expression of Tgfβ pathway members in the lung mesoderm (Fig. S1D). Moreover, although the epithelium of Ezh2mesoderm-KO lungs is immature and exhibits a slight reduction in expression of Tgfβ pathway components (Fig. S1E-G), these effects are indirect and likely to result from reduced mesenchyme in Ezh2mesoderm-KO lungs.
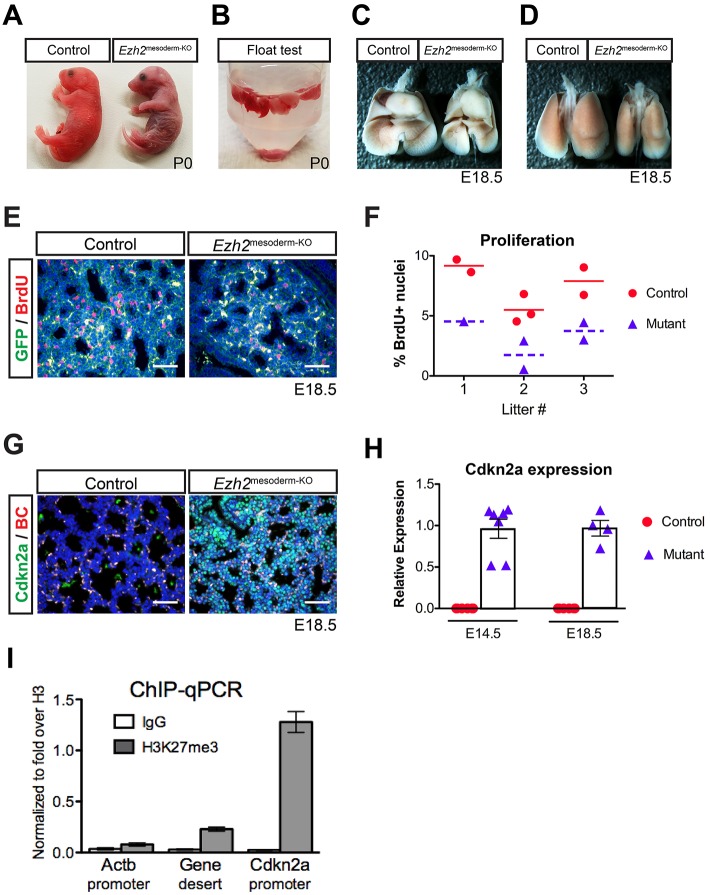
Fig. 1.
Ezh2 is required in mesoderm for lung development. (A) Ezh2mesoderm-KO mutant mice are cyanotic shortly after birth. (B) Lungs from Ezh2mesoderm-KO mice at P0 are not buoyant in PBS (n=3), whereas control lungs float (n=4). (C,D) Ventral (C) and dorsal (D) views of Ezh2mesoderm-KO show reduced lung size at E18.5. (E) IHC for BrdU at E18.5 reveals decreased BrdU incorporation in the GFP+ mesoderm of mutants. (F) BrdU incorporation quantified by litter, showing an average 50% reduction in Ezh2mesoderm-KO mutants relative to their siblings (P=0.0018). (G) IHC for Cdkn2a (green) reveals increased expression in mutants at E18.5; the red channel distinguishes autofluorescent blood cells (BC) from specific signal. (H) qPCR for Cdkn2a expression shows ectopic expression in the mutants at E14.5 and E18.5, whereas signal was not detected in controls. (I) ChIP-qPCR for H3K27me3 in isolated lung mesoderm at E18.5 (n=4). The Cdkn2a promoter is enriched relative to non-repressed control loci. Error bars indicate s.e.m. Scale bars: 50 µm.
Ezh2mesoderm-KO mutant lung mesenchyme is less proliferative than that of sibling controls, with a 50% reduction in BrdU incorporation (Fig. 1E,F). Cell cycle inhibitors such as Cdkn2a (also known as p16) are common targets of Ezh2-mediated epigenetic repression (Bracken et al., 2007), and Cdkn2a expression is readily observed at E14.5 and E18.5 in the Ezh2mesoderm-KO lung mesenchyme, but not in controls (Fig. 1G,H). To examine the Cdkn2a promoter for occupancy by the Ezh2/PRC2 histone mark H3K27me3, we performed ChIP-qPCR in isolated lung mesoderm. We found that the Cdkn2a promoter is enriched for the H3K27me3 mark, in contrast to a nearby gene desert and the constitutively active gene β-actin (Actb) (Fig. 1I). Thus, Ezh2/PRC2 is responsible for repressing the cell cycle inhibitor Cdkn2a in order to allow proliferation and appropriate growth of the developing lung mesenchyme.
Loss of Ezh2 leads to ectopic smooth muscle formation in the lung
Examination of Ezh2mesoderm-KO lungs revealed co-expression of SM22α (also known as Tagln) and smooth muscle actin (SMA) in regions along the periphery of the lung adjacent to the mesothelium (Fig. 2A-D). The co-expression of both SM22α and SMA suggests that these ectopic structures were composed of smooth muscle cells. 3D imaging by optical projection tomography (OPT) of whole-mount immunohistochemistry (IHC) for SM22α shows the ectopic smooth muscle forming disorganized branch-like structures and sheets, as well as smaller distal nodes (Fig. 2E,F, Movies 1,2). The phenotype is 100% penetrant, although the amount of surface area covered by ectopic smooth muscle varies between lobes of the lung (Fig. S2A,B). To determine when this phenotype arises, we performed IHC for SM22α and SMA on lungs from E12.5, E14.5 and E16.5 (Fig. 2G-L). There is no evidence for ectopic smooth muscle at E12.5 (Fig. 2J), but by E14.5 the Ezh2mesoderm-KO lungs have sporadic regions of ectopic smooth muscle in the very proximal portions of the lobes (Fig. 2K). By E16.5, the phenotype has expanded and extends distally through the lung, where it becomes more noticeable by E18.5 (Fig. S2C). These data show that the phenotype appears to develop in a proximal-to-distal manner over time. We did not observe other significant changes in lung mesoderm lineages, including airway or vascular smooth muscle (Fig. S2D). Thus, Ezh2 is required in lung mesoderm to prevent ectopic smooth muscle in the submesothelial mesenchyme.
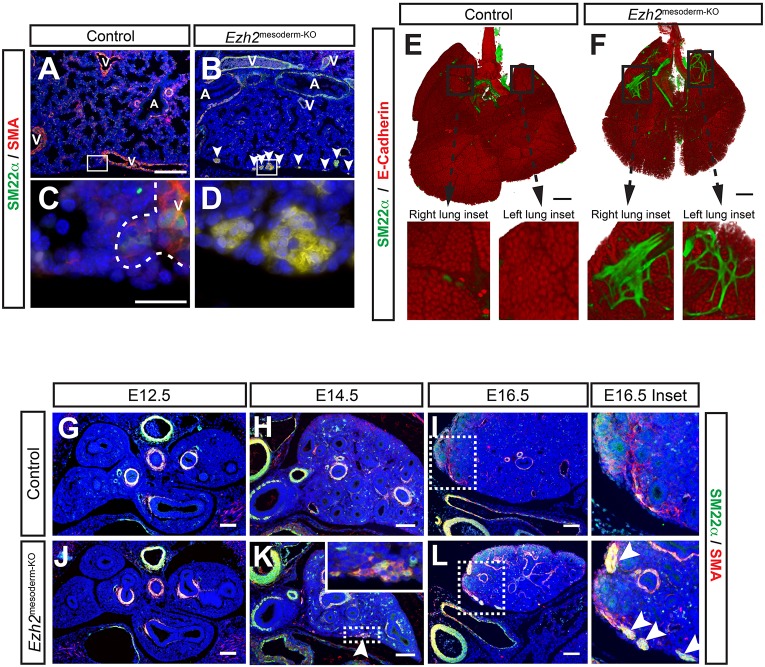
Fig. 2.
Ectopic smooth muscle develops at the periphery of the lung. (A-D) IHC for smooth muscle markers SM22α and SMA indicates smooth muscle development around the periphery of the Ezh2mesoderm-KO lung at E18.5 (B, arrowheads). Inset shows morphological differences between control blood vessel (C, outlined by the dashed line) and Ezh2mesoderm-KO ectopic smooth muscle (D). A, airway; V, blood vessel. (E,F) OPT on whole-mount IHC for SM22α with E-cadherin counterstain to outline lung endoderm. Insets of right and left lung lobes reveal the patterning of ectopic smooth muscle in Ezh2mesoderm-KO lungs. (G-L) IHC for SM22α and SMA throughout lung development shows that ectopic smooth muscle development initiates by E14.5 (H,K) and expands between E14.5 and E16.5 (I,L, arrowheads in inset). Scale bars: 20 µm in A,C; 500 µm in E,F; 100 µm in G-L.
Since the lung mesoderm generates smooth muscle during development, we examined the expression of pathways known to regulate this process. These data did not reveal any significant dysregulation of these pathways in the Ezh2mesoderm-KO lungs (Fig. S3A-D) (Morrisey and Hogan, 2010). This is supported by normal vascular and airway smooth muscle development in Ezh2mesoderm-KO lungs (Fig. S2D). Thus, the ectopic smooth muscle in Ezh2mesoderm-KO lungs develops in close proximity to the mesothelium, but does not appear to be induced by alteration in paracrine signaling factors that promote smooth muscle development.
The smooth muscle master transcription factor Myocd is derepressed in ectopic smooth muscle
To explore the underlying cause of the ectopic smooth muscle in Ezh2mesoderm-KO lungs, we examined expression of Myocd, a crucial transcription factor of the smooth muscle lineage. Myocd is recruited to the promoters of SM22α and SMA, and directly induces their transcription at high levels (Du et al., 2003; Wang et al., 2003). Enforced Myocd expression can reprogram non-smooth muscle cells into smooth muscle, making it a potential candidate for promoting ectopic smooth muscle formation in Ezh2mesoderm-KO lungs (Du et al., 2003; Parmacek, 2007). In situ hybridization for Myocd shows expression in the forming ectopic smooth muscle nodules at E14.5 (Fig. 3A) and in the ectopic smooth muscle nodules at E18.5 (Fig. 3B).
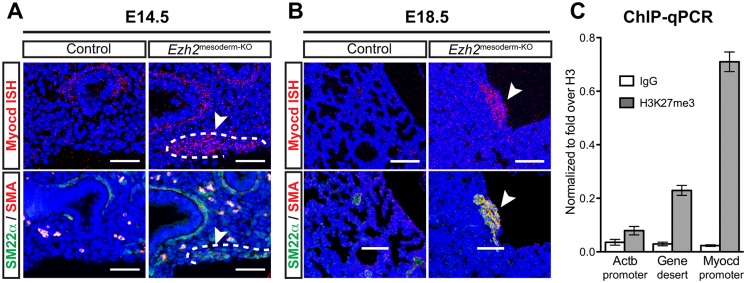
Fig. 3.
Myocardin is derepressed in ectopic smooth muscle. (A,B) In situ hybridization for myocardin (Myocd) reveals overlapping expression with SM22α and SMA in ectopic smooth muscle in Ezh2mesoderm-KO lungs (arrowheads, dashed lines) at E14.5 (A) and E18.5 (B). (C) ChIP-qPCR for H3K27me3 in isolated mesoderm at E18.5. The Myocd promoter is enriched relative to the non-repressed Actb locus and a nearby gene desert. Error bars indicate s.e.m. Scale bars: 50 µm in A; 100 µm in B.
Little is known about the epigenetic regulation of the Myocd gene. We found by ChIP-qPCR that the Myocd promoter is enriched for the H3K27me3 mark in isolated lung mesoderm (Fig. 3C). Thus, loss of Ezh2 leads to increased Myocd expression, possibly due to direct regulation by PRC2.
The ectopic smooth muscle structures in Ezh2mesoderm-KO lungs do not contain myocardium or vascular endothelium
One known regulator of Myocd expression is the myocardial transcription factor Nkx2.5, which promotes expression of a cardiac-specific isoform of Myocd (Ueyama et al., 2003). Nkx2.5 is expressed in cardiac myocardium and the pulmonary vein myocardium (Lien et al., 1999; Mommersteeg et al., 2007). Recent work has demonstrated the existence of a common cardiopulmonary progenitor that generates cardiac myocardium as well as pulmonary venous myocardium and pulmonary vascular smooth muscle in the developing lung (Peng et al., 2013). Therefore, one possible hypothesis to explain the Ezh2mesoderm-KO lung phenotype is that loss of Ezh2 in cardiopulmonary progenitors disrupts their normal migration into the lung and drives the ectopic expression of smooth muscle genes. We examined expression of the myocardial markers Nkx2.5 and myosin II heavy chain (MF20 antibody) in Ezh2mesoderm-KO lungs. These experiments revealed that the ectopic smooth muscle nodules in Ezh2mesoderm-KO lungs are not of myocardial origin (Fig. 4A,B). Additionally, despite having a branch-like structure, the nodules of ectopic smooth muscle do not contain vascular endothelium and they lack an obvious lumen (Fig. 4C). Thus, the ectopic smooth muscle in Ezh2mesoderm-KO lungs does not contain myocardium or a disorganized vasculature.
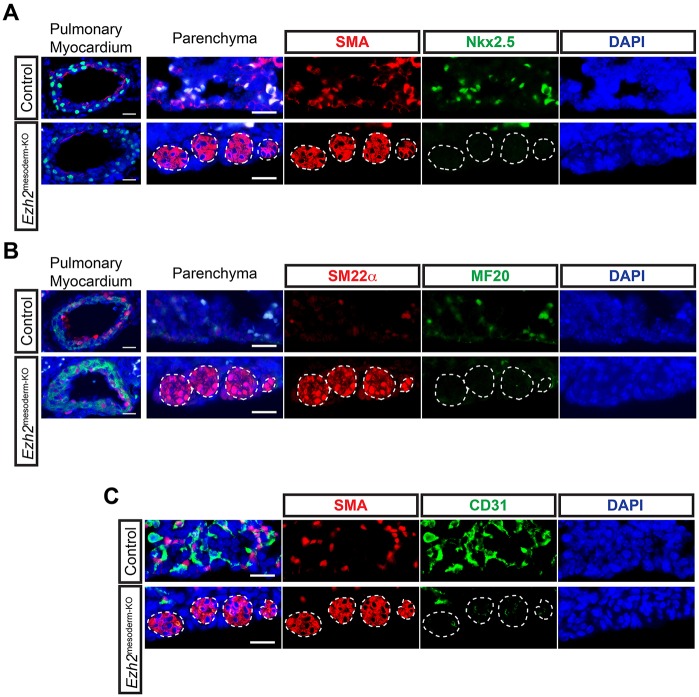
Fig. 4.
Ectopic smooth muscle is neither myocardium nor vasculature. (A) Co-IHC for SMA and Nkx2.5 and (B) SM22α and myosin II heavy chain (with MF20) in E18.5 lung parenchyma and pulmonary myocardium reveals that ectopic smooth muscle in Ezh2mesoderm-KO lungs does not express these myocardial markers, and that pulmonary myocardium forms normally in mutant lungs. (C) Co-IHC for SMA and CD31 shows that ectopic smooth muscle does not contain a vascular lumen with CD31+ endothelium. Ectopic smooth muscle is outlined. Scale bars: 20 µm.
Ezh2 is required to repress smooth muscle lineage differentiation in the lung mesothelium
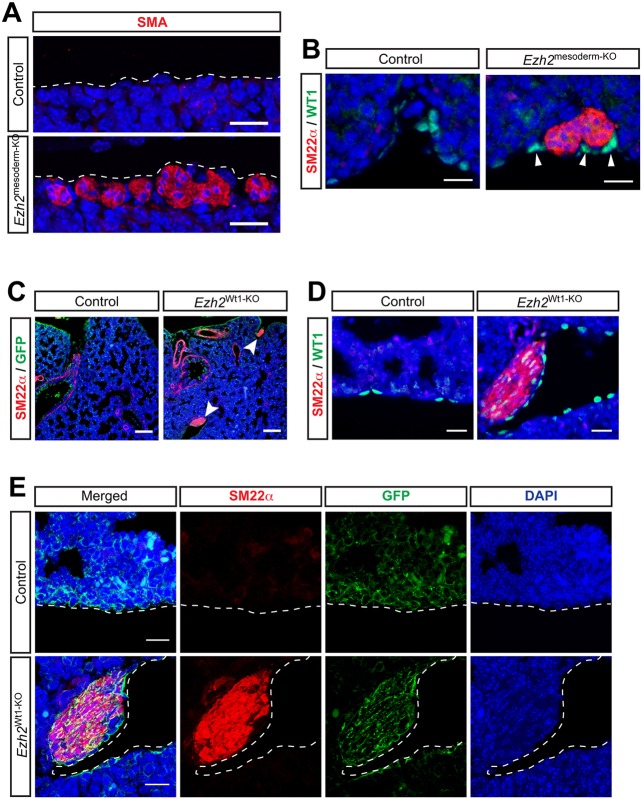
The mesothelium is a mesodermally derived epithelial monolayer that envelopes the lung and reduces friction against the chest cavity and other organs. During development, a population of mesothelial cells delaminate and migrate into the lung, contributing to vascular smooth muscle (Que et al., 2008; Dixit et al., 2013). To determine whether the mesothelium in Ezh2mesoderm-KO lungs contributes to ectopic smooth muscle, we investigated the association of these tissues. Confocal microscopy on sectioned tissue revealed that the SMA+ ectopic smooth muscle is immediately adjacent to, or forms part of, the exterior cell layer of the lung, and is thus intimately associated with the mesothelium (Fig. 5A). Co-IHC for the mesothelial marker WT1 with SM22α revealed that the SM22α+ ectopic smooth muscle is in close contact with WT1+ mesothelial cells (Fig. 5B).
Fig. 5.
Loss of Ezh2 specifically in the lung mesothelium leads to the formation of ectopic smooth muscle nodules. (A) IHC for SMA shows that ectopic smooth muscle forms along the exterior cell layer of the lung at E18.5. (B) Co-IHC for SM22α and WT1 shows that ectopic smooth muscle forms next to the WT1+ mesothelium at E18.5. (C) IHC for SM22α and GFP lineage tracing reveals ectopic smooth muscle (arrowheads) forming in Ezh2Wt1-KO lungs along the GFP+ mesothelium at E18.5. (D) IHC for WT1 and SM22α confirms the close association of ectopic smooth muscle with mesothelium at E18.5. (E) IHC for SM22α and GFP lineage tracing showing colocalization of these markers by confocal microscopy confirms that ectopic smooth muscle arises from the WT1+ lineage in Ezh2Wt1-KO mutants. Scale bars: 20 µm in A,B,D,E; 100 µm in C.
The localization of the ectopic smooth muscle nodules suggests that the mesothelium could provide either a niche for other mesodermal lineages to develop into smooth muscle or that it directly generates the ectopic smooth muscle (Fig. 5A,B). To address whether loss of Ezh2 specifically in the mesothelium would lead to ectopic smooth muscle formation, we crossed Ezh2flox/flox mice with Wt1cre mice and RosamTmG mice to delete Ezh2 specifically in the mesothelium and lineage trace the targeted cells (Muzumdar et al., 2007; Zhou et al., 2008). The Wt1cre:Ezh2flox/flox:RosamTmG mutant embryos (hereafter Ezh2Wt1-KO) exhibit multiple heart and lung phenotypes (data not shown). Importantly, they develop ectopic smooth muscle that expresses SM22α (Fig. 5C). The ectopic smooth muscle is closely associated with WT1+ cells at the periphery of the lung, similar to the Ezh2mesoderm-KO lungs (Fig. 5D, Fig. S4), and is lineage traced with the Wt1cre line (Fig. 5E). Other organs, including the heart, intestine and kidney, did not exhibit formation of ectopic smooth muscle nodules (data not shown). Thus, Ezh2 is required autonomously in the pulmonary mesothelium to suppress differentiation into ectopic smooth muscle.
Aberrant expression of Tbx15/18 in Ezh2mesoderm-KO lungs
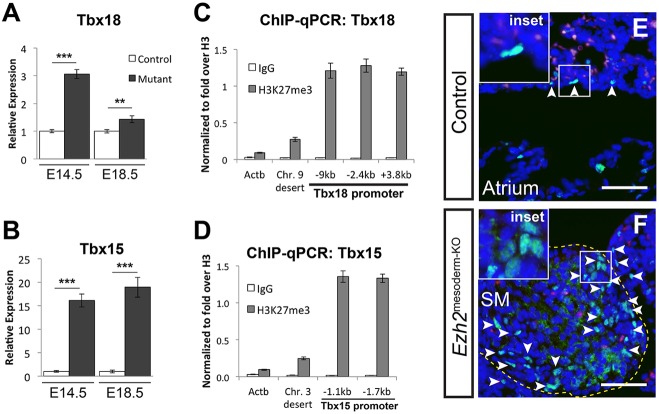
The formation of ectopic smooth muscle at the mesothelial layer of the lung superficially resembles coronary vessel smooth muscle that develops at the surface of the heart from the epicardium. Since the lung mesothelium and epicardium are both generated from WT1+ cells during development, we examined Ezh2mesoderm-KO lungs for expression of the key epicardial transcription factor Tbx18 (Kraus et al., 2001; Wu et al., 2013). Tbx18 null embryos exhibit reduced development of coronary smooth muscle in the heart (Wu et al., 2013). We found that Tbx18 expression is increased in Ezh2mesoderm-KO lungs at E14.5 and E18.5 (Fig. 6A). Moreover, expression of the highly related Tbx15 gene is also increased in Ezh2mesoderm-KO lungs at E14.5 and E18.5 (Fig. 6B). Consistent with previous observations that Tbx genes are regulated by PRC2 (Boyer et al., 2006), we found that the Tbx18 and Tbx15 promoters are decorated by H3K27me3 in isolated lung mesoderm (Fig. 6C,D). Immunostaining using an antibody that recognizes both Tbx15 and Tbx18 shows Tbx15/18 expression in rare mesothelial and submesothelial cells in control E18.5 lungs, whereas Tbx15/18+ cells are found expanded and intermixed among ectopic smooth muscle cells in Ezh2mesoderm-KO lungs (Fig. 6E,F). Thus, Ezh2 is required to repress the transcription factors Tbx15 and Tbx18. The derepression of these two factors along with Myocd is likely to drive ectopic smooth muscle differentiation in Ezh2mesoderm-KO and Ezh2Wt1-KO lungs (Fig. 7).
Fig. 6.
Repression of the T-box factors Tbx18 and Tbx15 by Ezh2 in the lung mesoderm. (A,B) qPCR for the epicardial transcription factor Tbx18 (A) and its close paralog Tbx15 (B) shows ectopic expression of these transcription factors in Ezh2mesoderm-KO lungs at E14.5 and E18.5. **P<0.01, ***P<0.001. (C,D) ChIP-qPCR for H3K27me3 in isolated mesoderm at E18.5. The Tbx18 (C) and Tbx15 (D) promoters are enriched relative to non-repressed control loci. Error bars indicate s.e.m. (E,F) IHC for Tbx15/18 reveals their expression (arrowheads) in the developing lung mesothelium (E), and that expression is localized in the ectopic smooth muscle (SM) nodules in Ezh2mesoderm-KO lungs at E18.5 (F). Scale bars: 50 µm.
Fig. 7.
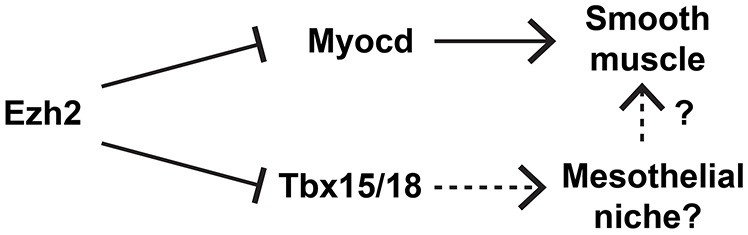
Model of Ezh2 repression of the smooth muscle lineage during lung development. Ezh2 represses Myocd, the master transcription factor of the smooth muscle lineage. Ezh2 also represses Tbx18, which might contribute to a specialized epicardial-like niche that promotes smooth muscle development.
DISCUSSION
Previous studies have demonstrated key roles for epigenetic regulation of patterning and lineage decisions in the lung endoderm (Zacharek et al., 2011; Wang et al., 2013; Galvis et al., 2015; Snitow et al., 2015). Studies on human lung diseases have demonstrated loss of epigenetic regulation in adult lung mesodermal cell lineages (Ito et al., 2002, 2005; Huang et al., 2013). However, little is known about epigenetic regulation of the developing mesoderm of the lung. In this study, we have shown an essential role for Ezh2/PRC2 in restricting the differentiation of the smooth muscle lineage from the mesothelium of the lung.
Ezh2 is known to regulate the development of a variety of mesodermal tissues, including B cell development (Su et al., 2003), skeletal muscle satellite stem cells (Juan et al., 2011; Woodhouse et al., 2013), limb bud development (Wyngaarden et al., 2011), heart development (Chen et al., 2012; Delgado-Olguín et al., 2012; He et al., 2012), fetal hematopoiesis (Mochizuki-Kashio et al., 2011) and endothelial development (Delgado-Olguín et al., 2014). In many of these tissues, and as found in our study presented here, there is a requirement for Ezh2-mediated suppression of the cell cycle inhibitor Cdkn2a, to allow progenitor cell proliferation. More importantly, our studies have revealed a previously unappreciated role for Ezh2 in suppressing ectopic expression of the smooth muscle transcription factor Myocd in the developing lung mesothelium. The direct regulation of Myocd by Ezh2 is supported by our finding that the Myocd promoter region is decorated by the Ezh2 repressive mark H3K27me3.
The mesothelium is a multipotent mesodermally derived epithelial layer that surrounds the lung, and migrates into the lung to generate multiple lung mesoderm lineages including lung alveolar mesenchyme and vascular smooth muscle (Que et al., 2008; Dixit et al., 2013). Mesothelia surrounding the gut (serosal mesothelium) and heart (epicardium) similarly generate vascular smooth muscle in these organs (Mikawa and Gourdie, 1996; Wilm et al., 2005; Wu et al., 2013). The origins of smooth muscle lineages in the lung remain poorly understood. Recent evidence demonstrates that a multipotent cardiopulmonary progenitor (CPP) generates both vascular and airway smooth muscle in the lung (Peng et al., 2013). Despite these findings and those presented in the present report, the overall quantitative contribution of mesothelial, CPP, or other sources of smooth muscle in the lung remains unclear.
The restricted pattern of the ectopic smooth muscle nodules in our Ezh2 lung mesoderm mutants suggests that lack of Ezh2 creates a permissive rather than an instructive environment for Myocd expression. Such instructive cues could come from niche effects exerted by the WT1+ mutant mesothelium, which closely associates with the ectopic smooth muscle. However, we did not observe any significant gene expression changes in signaling pathways known to regulate smooth muscle development from the mesothelium (White et al., 2006; Dixit et al., 2013). We did observe derepression of both Tbx15 and Tbx18, which are transcription factors implicated in regulating muscle development (Agulnik et al., 1998; Kraus et al., 2001; Singh et al., 2005; Airik et al., 2006). In particular, Tbx18 plays a crucial role in the development of epicardial smooth muscle (Wu et al., 2013). Tbx18 may function to create a niche for smooth muscle development through the regulation of signaling factors such as such as Tgfβ or Notch that promote smooth muscle differentiation in the epicardium, in lung vascular smooth muscle, and in mesothelial delamination and migration into the developing lung (Farin et al., 2007; Morimoto et al., 2010; Greulich et al., 2012). Although we have not observed alterations in these pathways in our studies, they could be disrupted in small subsets of cells that are difficult to detect and whose altered differentiation leads to the observed phenotype. Since our lineage tracing analysis shows that Ezh2Wt1-KO mice also develop ectopic smooth muscle nodules, these data support the concept that Ezh2 is required in mesothelial cells to directly suppress smooth muscle lineage differentiation through the inhibition of multiple transcriptional regulators of the smooth muscle lineage, including Myocd and Tbx18.
Lung mesothelium has been implicated in the pathogenesis of multiple diseases including IPF, where fibrotic lesions often initiate in the submesothelial mesenchyme and expand internally into the lung. Expression of the lung mesothelial marker WT1 has been observed in lung fibrotic lesions, suggesting that mesothelial cells or their differentiated progeny may contribute to lesion formation (Mubarak et al., 2012; Karki et al., 2014). Mesothelial contribution to fibrosis has also been demonstrated in the liver, where the lineage-traced mesothelium invades into the submesothelial region and differentiates into fibroblasts following chemical injury (Asahina et al., 2011; Li et al., 2013). The etiology of IPF is unknown, and our results suggest that Polycomb-mediated transcriptional repression might have a role in regulating mesothelial fate in the adult to prevent this disease. Our findings, along with the studies mentioned above, suggest that mesothelial cells may contribute significantly towards fibrotic lung disease through their predisposition to differentiate into smooth muscle or myofibroblast cells. Future studies to assess whether pathological smooth muscle remodeling in pulmonary hypertension, COPD, bronchiolitis obliterans and asthma involves Polycomb-mediated regulation might provide important information regarding the etiology of these diseases.
MATERIALS AND METHODS
Animals
Dermo1cre, Wt1cre, Ezh2flox/flox and RosamTmG mice and their genotyping have been described previously (Su et al., 2003; Yu et al., 2003; Muzumdar et al., 2007; Zhou et al., 2008). BrdU was administered intraperitoneally (60 mg/kg body weight) 90 min prior to dissection. All animal procedures were performed in accordance with the Institute for Animal Care and Use Committee at the University of Pennsylvania.
Float test
Lungs were collected from seven neonatal pups shortly after birth. Lungs from three cyanotic pups and four of their non-cyanotic siblings were sequentially placed in PBS to determine buoyancy. Pups were subsequently genotyped and matched with lung sample data.
Quantitative RT-PCR
Quantitative PCR (qPCR) was performed as previously described (Snitow et al., 2015) using the primers listed in Table S1. Statistical significance was calculated with GraphPad Prism 5 software using a two-tailed unpaired t-test.
ChIP-qPCR
The epithelial cell depletion protocol was modified from Wang et al. (2016). Lung mesoderm was isolated by digesting E18.5 lungs in 1× dispase (BD Biosciences), 480 U/ml collagenase type I (Life Technologies) and 0.33 U/ml DNase I (Roche) for 20 min at 37°C, pipetting briefly every 5 min. 5 mM EDTA was then added to prevent epithelial cells from clumping. Digested lung cells were gently pelleted, and washed twice with PBS containing 5 mM EDTA and 1% BSA. Epithelial cells were removed by incubation with 5 µg rat anti-EpCAM/CD326 (eBioscience, 14-5791-85) in 1% BSA in PBS containing 5 mM EDTA for 30 min at 4°C, washed three times with 1% BSA in PBS containing 5 mM EDTA, then incubated with 50 µl sheep anti-rat IgG Dynabeads (4.5 µm in diameter; Life Technologies) for 30 min at 4°C. Dynabeads linked to epithelial cells were removed by magnetic pull-down, and the mesoderm-containing supernatant was washed three times in PBS prior to cross-linking.
Lung mesoderm was cross-linked with 1% formaldehyde for 10 min and sonicated to an average length of 150-500 bp using a Diagenode Bioruptor on high amplitude for 10 cycles of 30 s on/off. Immunoprecipitation was performed as previously described (Snitow et al., 2015), and analyzed by real-time qPCR using the primers listed in Table S1.
Histology
Tissues were fixed overnight in fresh 2% or 4% paraformaldehyde, dehydrated, and embedded in paraffin wax and sectioned at a thickness of 6-8 μm. Hematoxylin and Eosin (H&E) staining was performed using standard procedures. The Myocd in situ hybridization probe was described previously (Du et al., 2003). In situ hybridization and IHC were performed as described (Wang and Morrisey, 2010; Tian et al., 2011; Li et al., 2012; Wang et al., 2013). IHC used the following antibodies: p16/Cdkn2a (Santa Cruz, sc-1661; 1:100), GFP (Aves, GFP-1020; 1:1000), BrdU (Abcam, ab6326; 1:100), SM22α (Abcam, ab10135; 1:100), SMA (Sigma, A5228; 1:200), Nkx2.5 (Santa Cruz, sc-8697; 1:50), MF20 (Developmental Studies Hybridoma Bank; 1:20), PECAM (CD31; R&D Systems, MAB3628; 1:500), WT1 (Santa Cruz, sc-192; 1:50), Tbx15/18 (ThermoFisher, PA5-38563; 1:50), SP-C (Chemicon, AB3786; 1:500) and Pdpn (Developmental Studies Hybridoma Bank, T1 alpha 8.1.1; 1:50).
Slides were mounted with Vectashield mounting medium containing DAPI (Vector Laboratories). BrdU+ nuclei and DAPI-stained nuclei were counted manually, assisted by the multipoint counting tool in Fiji software (Schindelin et al., 2012); epithelial cells were excluded from this analysis. Statistical analysis was performed with GraphPad Prism software using a one-tailed paired t-test. P<0.05 was considered significant.
Whole-mount immunohistochemistry
The whole-mount IHC protocol was modified from Metzger et al. (2008). Lungs were dissected and fixed overnight in a 1:4 ratio of dimethyl sulfoxide:methanol, bleached for 5-10 h in a 1:1:4 ratio of 30% H2O2:DMSO:methanol, washed and stored in 100% methanol. Lungs were rehydrated in a gradient series of methanol in PBST (PBS with 0.1% Tween 20): 75, 50, 25, 0%. Lungs were blocked overnight in blocking buffer (2.5% Triton X-100, 10% donkey serum and 0.05% sodium azide in PBS). Lungs were incubated for 48 h with primary antibodies SM22α (Abcam, ab10135; 1:200) and E-cadherin (Sigma, U3254; 1:200) diluted in blocking buffer, washed overnight in PBST, incubated for 48 h in secondary antibody, washed overnight in PBST, then washed for several hours in PBS. Lungs were then embedded in 1% low-melting agarose, dehydrated overnight in 100% methanol, then cleared overnight in a 1:2 benzoyl alcohol:benzoyl benzoate solution (BABB). OPT was imaged on a Bioptonics OPT Scanner 3001 M and reconstructed with the included software packages. Lungs imaged for surface area analysis were not optically cleared with BABB. Surface area was measured using Fiji software.
Acknowledgements
We gratefully acknowledge Yi Wang and David Frank for advice on lung digestion and epithelial cell depletion; Rachel Kadzik and Kurt Engleka for advice on whole-mount IHC; Jessica Grindheim and Shanru Li for helpful discussion and advice on chromatin immunoprecipitation; and the Histology Core at the University of Pennsylvania Cardiovascular Institute for histology services on sectioned tissue.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
M.S. and E.E.M. designed the experiments, analyzed the data and wrote the paper. M.S. performed experiments. M.M.L., L.C. and S.Z. performed histological sectioning, in situ hybridization and IHC of sectioned tissue.
Funding
This work was supported by funding from the National Institutes of Health [HL087825, HL110942 and HL100405]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.134932.supplemental
References
- Agulnik S. I., Papaioannou V. E. and Silver L. M. (1998). Cloning, mapping, and expression analysis of TBX15, a new member of the T-Box gene family. Genomics 51, 68-75. 10.1006/geno.1998.5278 [DOI] [PubMed] [Google Scholar]
- Airik R., Bussen M., Singh M. K., Petry M. and Kispert A. (2006). Tbx18 regulates the development of the ureteral mesenchyme. J. Clin. Invest. 116, 663-674. 10.1172/JCI26027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K., Zhou B., Pu W. T. and Tsukamoto H. (2011). Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology 53, 983-995. 10.1002/hep.24119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra H. and Antony V. B. (2015). Pleural mesothelial cells in pleural and lung diseases. J. Thorac. Dis. 7, 964-980. 10.3978/j.issn.2072-1439.2015.02.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer L. A., Plath K., Zeitlinger J., Brambrink T., Medeiros L. A., Lee T. I., Levine S. S., Wernig M., Tajonar A., Ray M. K. et al. (2006). Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441, 349-353. 10.1038/nature04733 [DOI] [PubMed] [Google Scholar]
- Bracken A. P., Dietrich N., Pasini D., Hansen K. H. and Helin K. (2006). Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 20, 1123-1136. 10.1101/gad.381706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken A. P., Kleine-Kohlbrecher D., Dietrich N., Pasini D., Gargiulo G., Beekman C., Theilgaard-Mönch K., Minucci S., Porse B. T., Marine J.-C. et al. (2007). The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 21, 525-530. 10.1101/gad.415507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Ma Y., Kim E. Y., Yu W., Schwartz R. J., Qian L. and Wang J. (2012). Conditional ablation of Ezh2 in murine hearts reveals its essential roles in endocardial cushion formation, cardiomyocyte proliferation and survival. PLoS ONE 7, e31005 10.1371/journal.pone.0031005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Olguín P., Huang Y., Li X., Christodoulou D., Seidman C. E., Seidman J. G., Tarakhovsky A. and Bruneau B. G. (2012). Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nat. Genet. 44, 343-347. 10.1038/ng.1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Olguín P., Dang L. T., He D., Thomas S., Chi L., Sukonnik T., Khyzha N., Dobenecker M.-W., Fish J. E. and Bruneau B. G. (2014). Ezh2-mediated repression of a transcriptional pathway upstream of Mmp9 maintains integrity of the developing vasculature. Development 141, 4610-4617. 10.1242/dev.112607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R., Ai X. and Fine A. (2013). Derivation of lung mesenchymal lineages from the fetal mesothelium requires hedgehog signaling for mesothelial cell entry. Development 140, 4398-4406. 10.1242/dev.098079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K. L., Ip H. S., Li J., Chen M., Dandre F., Yu W., Lu M. M., Owens G. K. and Parmacek M. S. (2003). Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol. Cell. Biol. 23, 2425-2437. 10.1128/MCB.23.7.2425-2437.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin H. F., Bussen M., Schmidt M. K., Singh M. K., Schuster-Gossler K. and Kispert A. (2007). Transcriptional repression by the T-box proteins Tbx18 and Tbx15 depends on Groucho corepressors. J. Biol. Chem. 282, 25748-25759. 10.1074/jbc.M703724200 [DOI] [PubMed] [Google Scholar]
- Galvis L. A., Holik A. Z., Short K. M., Pasquet J., Lun A. T. L., Blewitt M. E., Smyth I. M., Ritchie M. E. and Asselin-Labat M.-L. (2015). Repression of Igf1 expression by Ezh2 prevents basal cell differentiation in the developing lung. Development 142, 1458-1469. 10.1242/dev.122077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greulich F., Farin H. F., Schuster-Gossler K. and Kispert A. (2012). Tbx18 function in epicardial development. Cardiovasc. Res. 96, 476-483. 10.1093/cvr/cvs277 [DOI] [PubMed] [Google Scholar]
- He A., Ma Q., Cao J., von Gise A., Zhou P., Xie H., Zhang B., Hsing M., Christodoulou D. C., Cahan P. et al. (2012). Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ. Res. 110, 406-415. 10.1161/CIRCRESAHA.111.252205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges M. and Morrisey E. E. (2014). Lung development: orchestrating the generation and regeneration of a complex organ. Development 141, 502-513. 10.1242/dev.098186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. L. M., Barkauskas C. E., Chapman H. A., Epstein J. A., Jain R., Hsia C. C. W., Niklason L., Calle E., Le A., Randell S. H. et al. (2014). Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15, 123-138. 10.1016/j.stem.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. K., Scruggs A. M., Donaghy J., Horowitz J. C., Zaslona Z., Przybranowski S., White E. S. and Peters-Golden M. (2013). Histone modifications are responsible for decreased Fas expression and apoptosis resistance in fibrotic lung fibroblasts. Cell Death Dis. 4, e621 10.1038/cddis.2013.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Caramori G., Lim S., Oates T., Chung K. F., Barnes P. J. and Adcock I. M. (2002). Expression and activity of histone deacetylases in human asthmatic airways. Am. J. Respir. Crit. Care Med. 166, 392-396. 10.1164/rccm.2110060 [DOI] [PubMed] [Google Scholar]
- Ito K., Ito M., Elliott W. M., Cosio B., Caramori G., Kon O. M., Barczyk A., Hayashi S., Adcock I. M., Hogg J. C. et al. (2005). Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N. Engl. J. Med. 352, 1967-1976. 10.1056/NEJMoa041892 [DOI] [PubMed] [Google Scholar]
- Juan A. H., Derfoul A., Feng X., Ryall J. G., Dell'Orso S., Pasut A., Zare H., Simone J. M., Rudnicki M. A. and Sartorelli V. (2011). Polycomb EZH2 controls self-renewal and safeguards the transcriptional identity of skeletal muscle stem cells. Genes Dev. 25, 789-794. 10.1101/gad.2027911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S., Surolia R., Hock T. D., Guroji P., Zolak J. S., Duggal R., Ye T., Thannickal V. J. and Antony V. B. (2014). Wilms’ tumor 1 (Wt1) regulates pleural mesothelial cell plasticity and transition into myofibroblasts in idiopathic pulmonary fibrosis. FASEB J. 28, 1122-1131. 10.1096/fj.13-236828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus F., Haenig B. and Kispert A. (2001). Cloning and expression analysis of the mouse T-box gene Tbx18. Mech. Dev. 100, 83-86. 10.1016/S0925-4773(00)00494-9 [DOI] [PubMed] [Google Scholar]
- Lee T. I., Jenner R. G., Boyer L. A., Guenther M. G., Levine S. S., Kumar R. M., Chevalier B., Johnstone S. E., Cole M. F., Isono K.-i. et al. (2006). Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125, 301-313. 10.1016/j.cell.2006.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Wang Y., Zhang Y., Lu M. M., DeMayo F. J., Dekker J. D., Tucker P. W. and Morrisey E. E. (2012). Foxp1/4 control epithelial cell fate during lung development and regeneration through regulation of anterior gradient 2. Development 139, 2500-2509. 10.1242/dev.079699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang J. and Asahina K. (2013). Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc. Natl. Acad. Sci. USA 110, 2324-2329. 10.1073/pnas.1214136110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien C. L., Wu C., Mercer B., Webb R., Richardson J. A. and Olson E. N. (1999). Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development 126, 75-84. [DOI] [PubMed] [Google Scholar]
- Metzger R. J., Klein O. D., Martin G. R. and Krasnow M. A. (2008). The branching programme of mouse lung development. Nature 453, 745-750. 10.1038/nature07005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T. and Gourdie R. G. (1996). Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev. Biol. 174, 221-232. 10.1006/dbio.1996.0068 [DOI] [PubMed] [Google Scholar]
- Mochizuki-Kashio M., Mishima Y., Miyagi S., Negishi M., Saraya A., Konuma T., Shinga J., Koseki H. and Iwama A. (2011). Dependency on the polycomb gene Ezh2 distinguishes fetal from adult hematopoietic stem cells. Blood 118, 6553-6561. 10.1182/blood-2011-03-340554 [DOI] [PubMed] [Google Scholar]
- Mommersteeg M. T., Brown N. A., Prall O. W. J., de Gier-de Vries C., Harvey R. P., Moorman A. F. M. and Christoffels V. M. (2007). Pitx2c and Nkx2-5 are required for the formation and identity of the pulmonary myocardium. Circ. Res. 101, 902-909. 10.1161/CIRCRESAHA.107.161182 [DOI] [PubMed] [Google Scholar]
- Morimoto M., Liu Z., Cheng H.-T., Winters N., Bader D. and Kopan R. (2010). Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J. Cell Sci. 123, 213-224. 10.1242/jcs.058669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey E. E. and Hogan B. L. M. (2010). Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell 18, 8-23. 10.1016/j.devcel.2009.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubarak K. K., Montes-Worboys A., Regev D., Nasreen N., Mohammed K. A., Faruqi I., Hensel E., Baz M. A., Akindipe O. A., Fernandez-Bussy S. et al. (2012). Parenchymal trafficking of pleural mesothelial cells in idiopathic pulmonary fibrosis. Eur. Respir. J. 39, 133-140. 10.1183/09031936.00141010 [DOI] [PubMed] [Google Scholar]
- Mutsaers S. E., Birnie K., Lansley S., Herrick S. E., Lim C.-B. and Prêle C. M. (2015). Mesothelial cells in tissue repair and fibrosis. Front. Pharmacol. 6, 113 10.3389/fphar.2015.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar M. D., Tasic B., Miyamichi K., Li L. and Luo L. (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593-605. 10.1002/dvg.20335 [DOI] [PubMed] [Google Scholar]
- Parmacek M. S. (2007). Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ. Res. 100, 633-644. 10.1161/01.RES.0000259563.61091.e8 [DOI] [PubMed] [Google Scholar]
- Peng T., Tian Y., Boogerd C. J., Lu M. M., Kadzik R. S., Stewart K. M., Evans S. M. and Morrisey E. E. (2013). Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature 500, 589-592. 10.1038/nature12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J., Wilm B., Hasegawa H., Wang F., Bader D. and Hogan B. L. M. (2008). Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc. Natl. Acad. Sci. USA 105, 16626-16630. 10.1073/pnas.0808649105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. K., Petry M., Haenig B., Lescher B., Leitges M. and Kispert A. (2005). The T-box transcription factor Tbx15 is required for skeletal development. Mech. Dev. 122, 131-144. 10.1016/j.mod.2004.10.011 [DOI] [PubMed] [Google Scholar]
- Snitow M. E., Li S., Morley M. P., Rathi K., Lu M. M., Kadzik R. S., Stewart K. M. and Morrisey E. E. (2015). Ezh2 represses the basal cell lineage during lung endoderm development. Development 142, 108-117. 10.1242/dev.116947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su I.-H., Basavaraj A., Krutchinsky A. N., Hobert O., Ullrich A., Chait B. T. and Tarakhovsky A. (2003). Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat. Immunol. 4, 124-131. 10.1038/ni876 [DOI] [PubMed] [Google Scholar]
- Tian Y., Zhang Y., Hurd L., Hannenhalli S., Liu F., Lu M. M. and Morrisey E. E. (2011). Regulation of lung endoderm progenitor cell behavior by miR302/367. Development 138, 1235-1245. 10.1242/dev.061762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama T., Kasahara H., Ishiwata T., Nie Q. and Izumo S. (2003). Myocardin expression is regulated by Nkx2.5, and its function is required for cardiomyogenesis. Mol. Cell. Biol. 23, 9222-9232. 10.1128/MCB.23.24.9222-9232.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. and Morrisey E. E. (2010). Regulation of cardiomyocyte proliferation by Foxp1. Cell Cycle 9, 4251-4252. 10.4161/cc.9.21.13924 [DOI] [PubMed] [Google Scholar]
- Wang Z., Wang D.-Z., Pipes G. C. T. and Olson E. N. (2003). Myocardin is a master regulator of smooth muscle gene expression. Proc. Natl. Acad. Sci. USA 100, 7129-7134. 10.1073/pnas.1232341100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Tian Y., Morley M. P., Lu M. M., Demayo F. J., Olson E. N. and Morrisey E. E. (2013). Development and regeneration of Sox2+ endoderm progenitors are regulated by a Hdac1/2-Bmp4/Rb1 regulatory pathway. Dev. Cell 24, 345-358. 10.1016/j.devcel.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Frank D. B., Morley M. P., Zhou S., Wang X., Lu M. M., Lazar M. A. and Morrisey E. E. (2016). HDAC3-dependent epigenetic pathway controls lung alveolar epithelial cell remodeling and spreading via miR-17-92 and TGF-beta signaling regulation. Dev. Cell 36, 303-315. 10.1016/j.devcel.2015.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. C., Xu J., Yin Y., Smith C., Schmid G. and Ornitz D. M. (2006). FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development 133, 1507-1517. 10.1242/dev.02313 [DOI] [PubMed] [Google Scholar]
- Wilm B., Ipenberg A., Hastie N. D., Burch J. B. E. and Bader D. M. (2005). The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 132, 5317-5328. 10.1242/dev.02141 [DOI] [PubMed] [Google Scholar]
- Woodhouse S., Pugazhendhi D., Brien P. and Pell J. M. (2013). Ezh2 maintains a key phase of muscle satellite cell expansion but does not regulate terminal differentiation. J. Cell Sci. 126, 565-579. 10.1242/jcs.114843 [DOI] [PubMed] [Google Scholar]
- Wu S.-P., Dong X.-R., Regan J. N., Su C. and Majesky M. W. (2013). Tbx18 regulates development of the epicardium and coronary vessels. Dev. Biol. 383, 307-320. 10.1016/j.ydbio.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyngaarden L. A., Delgado-Olguin P., Su I.-h., Bruneau B. G. and Hopyan S. (2011). Ezh2 regulates anteroposterior axis specification and proximodistal axis elongation in the developing limb. Development 138, 3759-3767. 10.1242/dev.063180 [DOI] [PubMed] [Google Scholar]
- Yin Y., White A. C., Huh S.-H., Hilton M. J., Kanazawa H., Long F. and Ornitz D. M. (2008). An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev. Biol. 319, 426-436. 10.1016/j.ydbio.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Xu J., Liu Z., Sosic D., Shao J., Olson E. N., Towler D. A. and Ornitz D. M. (2003). Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130, 3063-3074. 10.1242/dev.00491 [DOI] [PubMed] [Google Scholar]
- Zacharek S. J., Fillmore C. M., Lau A. N., Gludish D. W., Chou A., Ho J. W. K., Zamponi R., Gazit R., Bock C., Jäger N. et al. (2011). Lung stem cell self-renewal relies on BMI1-dependent control of expression at imprinted loci. Cell Stem Cell 9, 272-281. 10.1016/j.stem.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., Ma Q., Rajagopal S., Wu S. M., Domian I., Rivera-Feliciano J., Jiang D., von Gise A., Ikeda S., Chien K. R. et al. (2008). Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454, 109-113. 10.1038/nature07060 [DOI] [PMC free article] [PubMed] [Google Scholar]