ABSTRACT
Inflammatory response of blood–brain barrier (BBB) endothelial cells plays an important role in pathogenesis of many central nervous system inflammatory diseases, including multiple sclerosis; however, the molecular mechanism mediating BBB endothelial cell inflammatory response remains unclear. In this study, we first observed that knockdown of neuropilin-1 (NRP1), a co-receptor of several structurally diverse ligands, suppressed interferon-γ (IFNγ)-induced C-X-C motif chemokine 10 expression and activation of STAT1 in brain microvascular endothelial cells in a Rac1-dependent manner. Moreover, endothelial-specific NRP1-knockout mice, VECadherin-Cre-ERT2/NRP1flox/flox mice, showed attenuated disease progression during experimental autoimmune encephalomyelitis, a mouse neuroinflammatory disease model. Detailed analysis utilizing histological staining, quantitative PCR, flow cytometry and magnetic resonance imaging demonstrated that deletion of endothelial NRP1 suppressed neuron demyelination, altered lymphocyte infiltration, preserved BBB function and decreased activation of the STAT1–CXCL10 pathway. Furthermore, increased expression of NRP1 was observed in endothelial cells of acute multiple sclerosis lesions. Our data identify a new molecular mechanism of brain microvascular endothelial inflammatory response through NRP1–IFNγ crosstalk that could be a potential target for intervention of endothelial cell dysfunction in neuroinflammatory diseases.
KEY WORDS: Neuropilin-1, Interferon-γ, Inflammatory response, Brain microvascular endothelial cells, Neuroinflammatory diseases
Summary: This is the first study to define the role of neuropilin-1, a co-receptor of several structurally distinct cytokines, in the IFNγ-induced inflammatory response of endothelial cells in neuroinflammatory diseases.
INTRODUCTION
Blood–brain barrier (BBB) dysfunction is an early feature of many central nervous system (CNS) inflammatory diseases, including multiple sclerosis, and is correlated with negative clinical outcomes (Minagar and Alexander, 2003; Zivadinov and Leist, 2005). As an important component of BBB, endothelial cells have been observed to express chemokines and adhesion molecules that facilitate infiltration of circulating leukocytes into CNS parenchyma, which significantly contributes to increased BBB permeability, inflammatory demyelination, metabolic imbalance and other consequences, to aggravate the progression of neuroinflammatory diseases (Brück et al., 1997; Larochelle et al., 2011). Increased levels of inflammatory mediators in peripheral blood mononuclear cells and serum, such as interferon-γ (IFNγ), as well as infiltrating leukocytes and activated resident CNS cells, including astrocytes and microglia, have been shown to cause endothelial dysfunction (Eng et al., 1996; Larochelle et al., 2011; Rubio and Capa, 1993; Sørensen et al., 1999a). Despite the importance of endothelial cells for the maintenance of BBB integrity, little is known about the molecular mechanisms mediating the inflammatory response of endothelial cells during the pathogenesis of neuroinflammatory diseases.
Multiple sclerosis is the most common cause of nontraumatic disability in young adults in developed countries (Goodin, 2014). Multiple sclerosis is a chronic neuroinflammatory disease and is pathologically characterized by myelin-specific T cells, B cells and antibody-mediated demyelination, variable inflammation, axonal damage and gliosis in the CNS (Gerhard et al., 1985; Ryberg, 1978; Traugott et al., 1979). Relapsing-remitting multiple sclerosis is the most common clinical presentation of multiple sclerosis and is characterized by an acute or subacute onset of clinical dysfunction, which is followed by remission and then secondary, progressive disease. Relapse is considered to be the clinical expression of acute focal inflammatory demyelination (Giesser, 2011; Lucchinetti et al., 2000). Therapies to restrict the severity of multiple sclerosis relapse are limited (Weiner, 2009), and current clinical anti-inflammatory therapies for acute multiple sclerosis, such as interferon-β, are only effective in some cases, whereas other therapies, such as natalizumab, have a stronger anti-inflammatory effect but are associated an increased risk of fatal progressive multifocal leukoencephalopathy (Lindå et al., 2009). Therefore, there is an urgent need to understand the pathophysiology of acute multiple sclerosis and to identify new therapeutic targets.
Neuropilin-1 (NRP1) is a single-pass transmembrane protein that has been studied mostly with regard to its role in guiding the growth of axons during development as a receptor of semaphoring 3A and in the induction of angiogenesis as a receptor of vascular endothelial growth factor A (VEGF-A) in endothelial cells (Chen et al., 1997; Kolodkin et al., 1997). The essential role of NRP1 in the developing CNS has been highlighted by the embryonic lethality, perturbed growth of cranial nerves and severely-impaired embryonic CNS vascularization of NRP1-deficient mice (Gu et al., 2003a; Kawasaki et al., 1999; Kitsukawa et al., 1997). Further studies support its role in differentiation, development of cranial neural crest cell projections and in the patterning of the sympathetic nervous system (Kawasaki et al., 2002; Schwarz et al., 2009, 2008). Although NRP1 is highly expressed in specific subsets of developing neurons (Fujisawa et al., 1989; Takagi et al., 1991, 1987), it is expressed at relatively low levels in the normal CNS given that the expression of NRP1 is lost soon after neuronal circuits have been established (Fujisawa et al., 1995; Kawakami et al., 1996; Takagi et al., 1995). The potential involvement of NRP1 in the pathogenesis of neurological diseases had been indicated by evidence showing that NRP1 protein levels are induced in the animal models of both ischemic and mechanical injury to neurons (Skold et al., 2000; Zhang et al., 2001). Recent studies have shown that inhibition of NRP1 with a ATWLPPR peptide yields beneficial effects in a mouse model of CD8+-T-cell-initiated BBB disruption (Suidan et al., 2012). Although the role of NRP1 in immune cells has been studied in the murine multiple sclerosis model (Solomon et al., 2011), the importance of endothelial-cell-specific NRP1 in the pathogenesis of neuroinflammatory diseases, especially endothelial cell inflammatory response, is still unclear.
In this study, we report for the first time that knockdown of NRP1 suppresses IFNγ-induced expression of chemokines, including that of CXCL10, in human brain microvascular endothelial cells (HBMVECs) in a Rac1–STAT1-dependent manner. The importance of endothelial-specific NRP1 in inflammatory cell infiltration, BBB disruption and neuron demyelination was authenticated in a murine neuroinflammatory disease model with VECad-Cre-ERT2/NRP1flox/flox (VECad/NRP1−/−) mice. Furthermore, increased NRP1 levels were observed in microvascular endothelial cells in human active multiple sclerosis lesions, indicating the involvement of endothelial NRP1 during the pathogenesis of human neuroinflammatory diseases. Our results identify, for the first time, that NRP1 mediates the pro-inflammatory effect of cerebral endothelial cells in neuroinflammatory diseases.
RESULTS
Knockdown of NRP1 inhibits IFNγ-stimulated activation of STAT1 and expression of CXCL10 in a Rac1-dependent manner
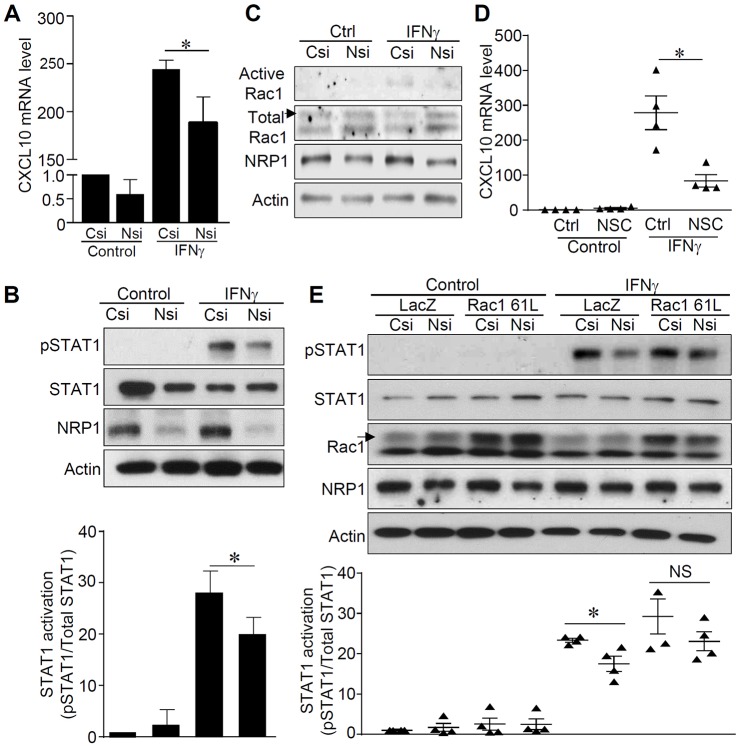
First, to examine the effect of NRP1 on the inflammatory response of BBB endothelial cells, human brain microvascular endothelial cells (HBMVECs) were cultured and stimulated with IFNγ, one of the pro-inflammatory cytokines elevated in neuroinflammatory diseases (Hohnoki et al., 1998; Minagar and Alexander, 2003). Our results showed that knockdown of NRP1 significantly attenuated IFNγ-induced chemokines, including CXCL10 and CXCL11, in HBMVECs (Fig. 1A; Fig. S1A,B). The inhibitory effects of siRNA against NRP1 (NRP1 siRNA) on CXCL10 levels was also observed in human dermal microvascular endothelial cells (HMVECs) (Fig. S1C). Notably, knockdown of NRP1 also significantly attenuated tumor necrosis factor α (TNFα)-induced CXCL10 in HBMVECs (Fig. S1D–F). Additionally, knockdown of NRP1 suppressed IFNγ-stimulated phosphorylation of STAT1 and STAT3 in HBMVECs (Fig. 1B; Fig. S2A). A different siRNA against NRP1 (siRNA_8#) showed the same inhibitive effects on the expression of CXCL10 and on the activation of STAT1 in HBMVECs (Figs S1G–I and S2B). These results support that NRP1 specifically controls both IFNγ- and TNFα-stimulated inflammatory responses in HBMVECs.
Fig. 1.
Knockdown of NRP1 inhibited IFNγ-stimulated expression of CXCL10 and activation of STAT1. See also Figs S1 and S2. (A) HBMVECs were transfected with control siRNA (Csi) or siRNA against NRP1 (Nsi) and then exposed to recombinant IFNγ (10 ng ml−1) for 24 h. Expression of CXCL10 was quantified by using qPCR and expressed as a relative fold of that in the control group, which was normalized to 1. n=5 for each group. (B) HBMVECs were transfected with control siRNA (Csi) or NRP1 siRNA (Nsi) for 24 h and then stimulated with IFNγ (10 ng ml−1) for 15 min. Total protein lysates were collected and subjected to western blotting with the indicated antibodies. Results are representative of five independent experiments. The bar graph shows the activation of STAT1 expressed as ratios of phosphorylated (p)STAT1 to total STAT1 and compared with that of the control group, which was normalized to 1. (C) The activity of Rac1 was examined in the lysates from the HBMVECs that had been transfected with control siRNA (Csi) or NRP1 siRNA (Nsi) for 24 h and stimulated with IFNγ (10 ng ml−1) for 15 min. Results are representative of three independent experiments. Western blots were scanned, analyzed and are presented in Fig. S2. (D) HBMVECs were pretreated with Rac1 inhibitor NSC 23766 (NSC) for 1 h and then stimulated with IFNγ (10 ng ml−1) for 24 h. Total RNA was extracted and subjected to qPCR analysis with primers against CXCL10 and β-actin genes. The expressed levels of CXCL10 are reported as a relative fold of the control group, which was normalized to 1. n=4 for each group. (E) HBMVECs were infected with a lentivirus expressing Rac1 61L or LacZ as control for 24 h and then transfected with control siRNA (Csi) or NRP1 siRNA (Nsi) for another 24 h. Total protein lysates were collected after stimulation with IFNγ (10 ng ml−1) for 15 min and subjected to western blotting for the indicated proteins. The bar graph shows the activation of STAT1 expressed as the ratios of pSTAT1 to total STAT1, and values were compared with those of the control group, which were normalized to 1. n=4 for each group. *P<0.05; NS, no significance. A nonparametric Mann-Whitney test was used to compare differences between control siRNA and NRP1 siRNA groups. Data are expressed as mean±s.e.m.
To further investigate how NRP1 regulates activation of STAT1, activity of Rac1 was examined because it has been reported to mediate the maximum activation of STAT1 (Park et al., 2004). As shown in Fig. 1C and Fig. S2C, knockdown of NRP1 attenuated IFNγ-induced activation of Rac1 in HBMVECs. Moreover, the Rac1 inhibitor NSC 23766 significantly suppressed IFNγ-induced expression of CXCL10 in HBMVECs, suggesting that Rac1 has an essential role in IFNγ-induced pro-inflammatory effects (Fig. 1D). To further define the role of Rac1 in NRP1-siRNA-mediated suppression of STAT1 phosphorylation, a constitutively-activated form of Rac1, Rac1 61L, was overexpressed in the control and NRP1-knockdown HBMVECs. As shown in Fig. 1E, in control HBMVECs (LacZ-overexpressing cells), the knockdown of NRP1 significantly inhibited IFNγ-stimulated phosphorylation of STAT1; however, this inhibitive effect on the phosphorylation of STAT1 was attenuated in Rac1-61L-overexpressing HBMVECs. Expression levels of Rac1 61L were similar between control and NRP1-knockdown HBMVECs (Fig. S2D). Taken together, these results show that NRP1 controls IFNγ-induced CXCL10 expression and STAT1 activation in a Rac1-dependent manner in HBMVECs.
Knockout of endothelial NRP1 modulates inflammatory cell infiltration and preserves BBB function in a mouse experimental autoimmune encephalomyelitis model
Endothelial-cell-specific tamoxifen-inducible NRP1-knockout mice, designated as VECad/NRP1−/−, were generated by crossing a mouse line expressing tamoxifen-inducible Cre-recombinase (Cre-ERT2) under the regulation of the vascular endothelial cadherin (VECad) promoter with NRP1flox/flox mice. Unlike TEK receptor tyrosine kinase (Tie2)/NRP1−/− mice, which are generated by crossing Tie2-Cre mice with NRP1flox/flox mice and show embryonic lethality (Gu et al., 2003a), the VECad/NRP1−/− mice showed normal viability after administration of tamoxifen. We also crossed VECad-Cre-ERT2 mice with ROSA26R reporter mice to examine the specificity and efficiency of Cre activity after tamoxifen administration. As shown in Fig. S3A, administration of tamoxifen induced efficient recombination in the endothelium of adult tissues, including the brain and spinal cord.
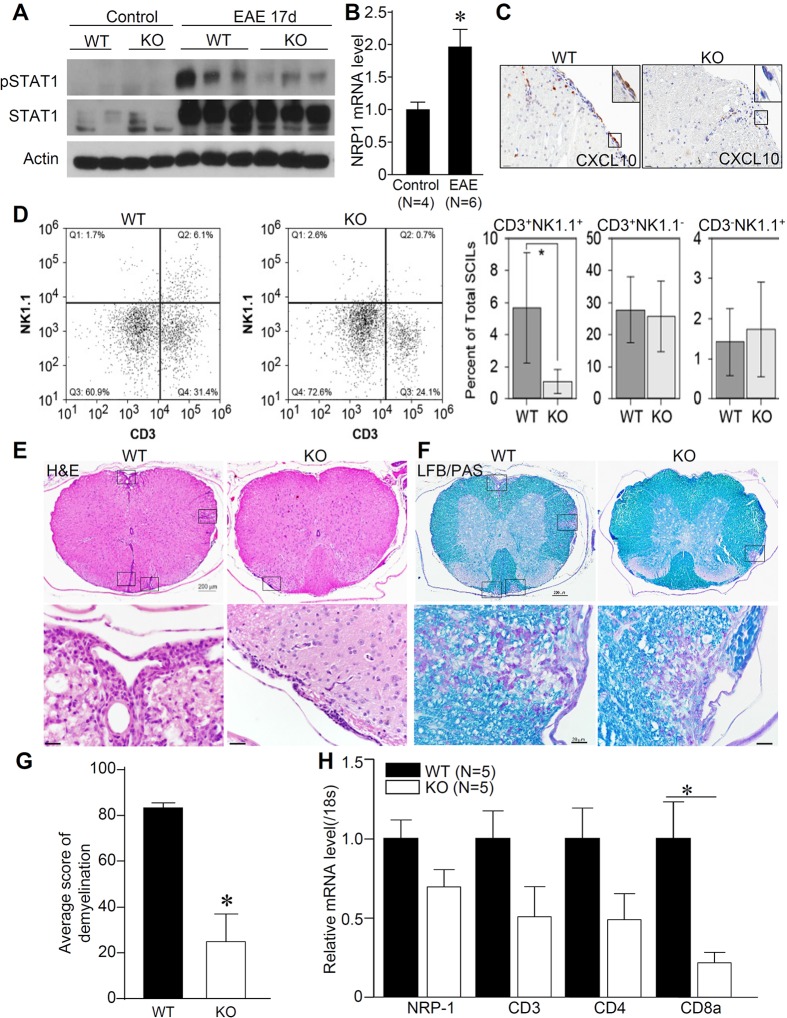
To investigate the role of endothelial NRP1 in neuroinflammatory diseases, an experimental autoimmune encephalomyelitis (EAE) model was then developed in VECad/NRP1−/− mice. Because previous studies have shown the potential role of tamoxifen in a mouse EAE model (Elloso et al., 2005), both VECad-Cre−/NRP1flox/flox (VECad/NRP1+/+) mice, designated as the wild-type (WT) group, and VECad/NRP1−/− mice (knockout group) were administered tamoxifen and then immunized with myelin oligodendrocyte glycoprotein peptide fragment 35–55 (MOG35–55) to induce EAE at 10 weeks of age. We first examined the activation of STAT1 and expression of CXCL10 in spinal cords after administration of MOG35–55. As shown in Fig. 2A and Fig. S3B, increased phosphorylation of STAT1 and STAT3 was observed in spinal cords of WT mice but not VECad/NRP1−/− mice after induction of EAE. Coincidentally, the expression of CXCL10 was extremely low in normal spinal cords; however, its expression was induced in spinal cords, including spinal cord endothelial cells, after induction of EAE (Fig. S3C), suggesting that endothelial-cell-derived chemokines contribute to inflammatory cell infiltration and EAE disease progression. Induction of NRP1 in spinal cords was also observed after MOG35–55 administration (Fig. 2B). Deletion of endothelial NRP1 led to a trend of suppressed expression of CXCL10 in the endothelial cells in spinal cords after induction of EAE (Fig. 2C; Fig. S3D). These results suggest that deletion of endothelial NRP1 suppressed the STAT1–CXCL10 pathway in spinal cords after administration of MOG35–55.
Fig. 2.
Knockout of endothelial NRP1 modulates inflammatory responses in EAE. See also Figs S2 and S3. (A) Protein lysates were obtained from the spinal cords of WT and VECad/NRP1−/− (KO) mice 17 days after EAE induction and were subjected to western blotting for the indicated proteins. (B) Spinal cords were collected from normal mice and mice at 17 days after EAE induction, and then subjected to qPCR. n=4 for normal mice and n=6 for EAE group. (C) Immunohistochemistry was performed to examine the expression of CXCL10 in the mouse spinal cords of WT and VECad/NRP1−/− (KO) mice 17 days after EAE induction. Results are representative from five samples from five animals in each group. Scale bars: 20 µm. (D) Spinal-cord-infiltrating leukocytes were collected at 13 days post EAE induction and analyzed by using flow cytometry. The three right bar graphs in D represent different cell populations. Data are expressed as mean±95% confidence intervals in the graphs on the right. Representative flow cytometry plots are shown on the left. n=5 for each group. (E,F) Spinal cords were harvested 17 days after EAE induction and cut into 10–12 slabs/per spinal cord, fixed, embedded in paraffin blocks, and subjected to Luxol-Fast-Blue and periodic-acid–Schiff (LFB/PAS) staining (F) and hematoxylin and eosin (H&E) staining (E). Representative images of five mice from each group are shown in E and F. Scale bars: 200 µm (top panels); 20 µm (bottom panels). LFB/PAS-stained images were blindly scored, quantified and compared (G). n=5 for each group. Rectangles in E and F indicate the inflammatory cell infiltration and demyelination lesions, and shown as enlarged images on the bottom. (H) The spinal cords of WT mice and VECad/NRP1−/− (KO) mice were harvested at 17 days after EAE induction and subjected to qPCR analysis. Data are expressed as relative folds (mean±s.e.m.) of control group values and normalized to expression of 18S ribosomal RNA (18s). n=5 for each group.*P<0.05, two-tailed unpaired Student's t-test were used for analysis in Fig. 2B,G,H.
Flow cytometry analysis was then performed to analyze immune cell infiltration in the spinal cord 13 days after induction of EAE, when mice showed signs of disease progression. When compared to age-matched littermate controls that had been treated at the same time, VECad/NRP1−/− mice showed a significant reduction in the number of NK1.1+CD3+ natural killer T (NKT) or NKT-like cells within the population of CD45+ spinal-cord-infiltrating leukocytes (SCILs; Fig. 2D). Unexpectedly, at this timepoint, we observed no difference in the percentage of CD4+CD8−, CD4+CD8+ or CD4−CD8+ T cells in the spinal cord infiltrate (Fig. S3E). Inflammatory responses were further evaluated in spinal cord tissues 17 days after administration of MOG35–55, when mice reached the peak of disease presentation. The results in Fig. 2E,F show extensive infiltration of inflammatory cells that is accompanied by local tissue destruction in WT mice, whereas limited infiltration of inflammatory cells was observed in the spinal cords of the VECad/NRP1−/− mice. Focal demyelination, presented as regions with a loss of myelin staining (blue), was observed in WT mice but was attenuated in the VECad/NRP1−/− mice. The results were further confirmed by scoring parenchymal demyelination and meningeal inflammatory cell infiltration (Fig. 2G; Fig. S3F). Semi-quantitative real-time (q)PCR analysis of T-cell markers in the spinal cords suggests that the accumulation of T cells, especially CD8+ T cells, was reduced in the spinal cords of VECad/NRP1−/− mice 17 days after EAE induction (Fig. 2H).
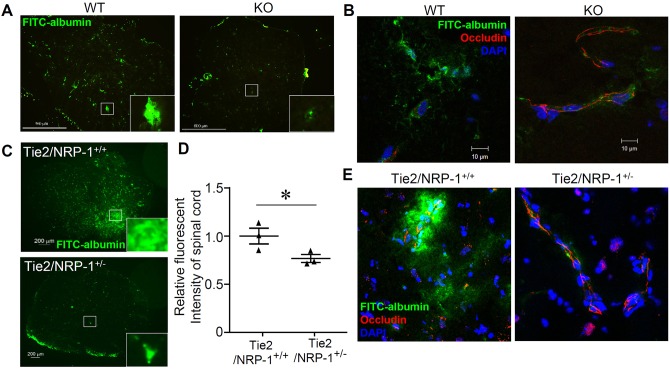
To evaluate BBB integrity, accumulation of FITC-labeled albumin, which is unable to migrate across the BBB under the normal conditions, was examined in the both VECad/NRP1−/− mice and Tie2/NRP1+/− mice after EAE induction. As shown in Fig. 3A, representative images of spinal cords from WT mice showed diffused distribution of albumin, whereas spinal cords of VECad/NRP1−/− mice exhibited a more restricted accumulation of albumin in the vasculature. Disruption of tight junction structures (staining for occludin), accompanied by perivascular leakage of FITC-labeled albumin, was present in spinal cords of the WT mice; however, in the VECad/NRP1−/− mice, tight junction structures were better preserved and were accompanied by much less leakage of FITC–albumin (Fig. 3B). The presence of BBB leakage in the cerebellums of WT mice was also visualized by using gadolinium-enhanced T1-weighted molecular resonance imaging (MRI) in both axial and coronal MRI images (Fig. S4). Furthermore, Tie2/NRP1+/− mice (heterozygous deletion of NRP1 in endothelial cells) also showed preserved BBB integrity after induction of EAE, which was shown by a smaller accumulation of FITC-labeled albumin (Fig. 3C), decreased total fluorescence intensity (Fig. 3D) and reduced disruption of tight junction structures (Fig. 3E) in the spinal cords. These results indicate that deletion of endothelial NRP1 results in modulated inflammatory cell infiltration and maintenance of BBB integrity in EAE.
Fig. 3.
Deletion of endothelial NRP1 preserves BBB function during EAE. See also Fig. S4. (A,B) FITC–albumin dissolved in PBS was administered to WT (VECad/NRP1+/+) mice and KO (VECad/NRP1−/−) littermates through tail vein injection 2 h before killing at 14 days after EAE induction. Spinal cords were harvested and subjected to frozen sectioning (A) and immunofluorescent staining with antibodies against occludin (red) (B). n=4 for WT group and n=5 for KO group. Scale bars: 500 µm (A); 10 µm (B). (C–E) FITC–albumin was administered to Tie2-Cre−/NRP1flox/wt (Tie2/NRP1+/+) mice and Tie2-Cre+/NRP1f/wt (Tie2/NRP1+/−) mice through tail vein injection 2 h before killing 21 days after EAE induction. Half of each spinal cord was subjected to frozen sectioning. Scale bars: 200 µm (C). The other half of each spinal cord was harvested, homogenized and analyzed for the fluorescence intensity of FITC–albumin, which was normalized to the fluorescence intensity of that in the serum and expressed as a relative level (mean±s.e.m.) of the control (D, Tie2/NRP-1+/+). Immunofluorescence staining of frozen sections was performed with an antibody against occludin (red). Nuclei were counterstained with DAPI (E, blue). n=3 for each group (C–E). Panel D shows the analysis using a two-tailed unpaired Student's t-test; *P<0.05.
Knockout of endothelial NRP1 attenuates disease progression in the mouse EAE model
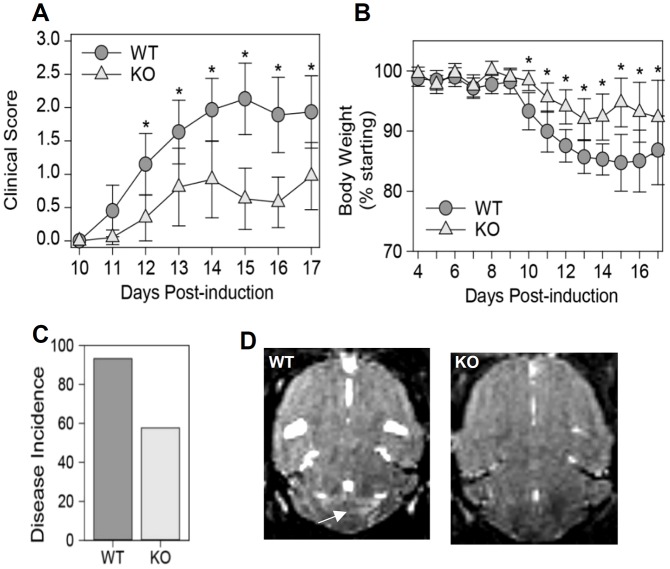
To define the role of NRP1 in disease progression after EAE induction, clinical scores and body weights of mice were monitored. As shown in Fig. 4A–C, EAE induction led to progressive disease in control mice, which was characterized by weight loss and ascending paralysis (limp tail followed by hindlimb weakness and finally complete hindlimb paralysis). However, VECad/NRP1−/− mice exhibited lower disease incidence (Fig. 4C) (risk ratio=0.67, P=0.007 by Fisher's exact test), preservation of body weight (Fig. 4B) (P<0.001 by two-way ANOVA), delayed disease onset and reduced clinical scores (P<0.001 by two-way ANOVA between WT and knockout) (Fig. 4A).
Fig. 4.
Deletion of endothelial NRP1 inhibits disease progression in EAE. See also Fig. S4. (A–C) Four-week-old WT (VECad/NRP1+/+) mice and KO (VECad/NRP1−/−) littermates were administered tamoxifen through gavage feeding for five consecutive days. Then, these mice were immunized with the MOG35–55 peptide followed by intraperitoneal injection of pertussis toxin (PTX) at 10 weeks of age. Behavioral scores (A) and body weights (B) were monitored. Data are expressed as mean±95% confidence intervals. Disease incidence (C) is presented as the percentage of diseased mice within the total population. n=42 for WT group, n=36 for VECad/NRP1−/− group (D). T2*-weighted MRI images of the brains of WT mice and VECad/NRP1−/− mice at 14 days after EAE induction. Arrow in D indicates a focal hyper-intense lesion in the cerebellum of a WT mouse. Results are representative of three animals in each group. In A,B, P<0.01 is denoted by * owing to limited space, and two tailed two-way ANOVA was used for analysis.
Furthermore, T2-weighted MRI scans were used to evaluate lesions in brains 14 days post EAE induction. As shown in Fig. 4D, hyper-intense regions, which correspond to inflammation, demyelination and axonal loss, were observed in the cerebellum of WT mice but were absent in the VECad/NRP1−/− mice.
Endothelial NRP1 is upregulated in human multiple sclerosis lesions
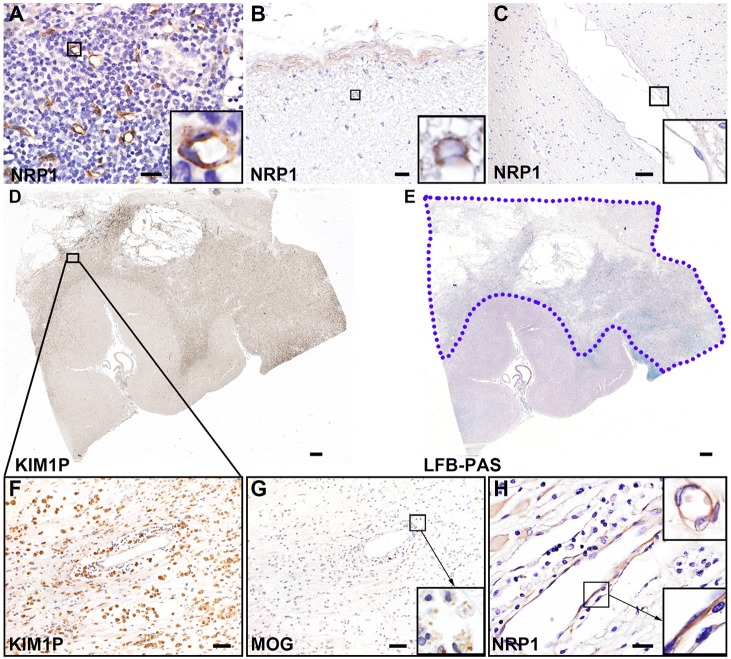
To further confirm the role of NRP1 in human neuroinflammatory diseases, immunohistochemistry was performed in normal and multiple-sclerosis-affected CNS tissues to identify the expression pattern of NRP1. Optimization of the staining protocol for NRP1 was first performed in human normal tonsil tissues to confirm the specificity of the NRP1 antibody (Sigma, catalog number HPA030278) (Fig. 5A). Normal human brain and spinal cords had limited NRP1 staining in glial cells in subpial regions (Fig. 5B). No obvious NRP1 immunoreactivity was detected in the normal CNS endothelium (Fig. 5C). However, in multiple sclerosis early active demyelinating lesions – which showed massive infiltration of macrophages (identified using the pan-macrophage marker KIM1P; Fig. 5D,F), the presence of destructive demyelinating lesions that were characterized by the loss of myelin staining (Fig. 5E) and the engulfment of myelin oligodendrocyte glycoprotein by macrophages (Fig. 5G) – NRP1 immunoreactivity was increased in both microvascular endothelial cells and active astrocytes, as revealed by the presence of membrane cytoplasmic staining (Fig. 5H). These results support that endothelial NRP1 plays a role in the pathogenesis of human multiple sclerosis.
Fig. 5.
Endothelial NRP1 is induced in human acute multiple sclerosis lesions. (A) Immunohistochemistry analysis of NRP1 in normal human tonsil tissues reveals strong NRP1 expression in the endothelium. Images are representative of three technical replicates. (B) Normal human spinal cord samples showed limited NRP1 immunoreactivity in subpial regions. Enlarged view (inset) shows NRP1+ glial cells. (C) Normal human brain white matter shows no NRP1 signal. The enlarged view indicates NRP1− endothelial cells. (D) The low-magnification image of KIM1P immunohistochemical staining indicates extensive macrophage infiltration in white matter of a multiple sclerosis brain. (E) Luxol-Fast-Blue and periodic-acid–Schiff (LFB-PAS) staining on the multiple sclerosis tissue section reveals a destructive demyelinating lesion in the white matter (shown by the blue-dotted line). (F) The enlarged view of panel D shows a region of interest with numerous macrophage infiltrations (KIM1P staining). (G) Staining of myelin oligodendrocyte glycoprotein (MOG) in the same region as that shown in F indicates early active demyelinating activity, characterized by myelin debris with macrophages (enlarged view in the inset). (H) Increased NRP1 immunoreactivity in the reactive astrocytes and endothelial cells in the lesion shown in D–G (NRP1 staining). Results are representative of three samples in both normal human brains and multiple sclerosis lesions (B–H). Enlarged views in H show the positive staining of NRP1 in endothelial cells. Scale bars: 20 µm (A,B,H), 50 µm (C,F,G), 1 mm (D,E).
DISCUSSION
In this study, we identified that NRP1 acts as a previously unidentified mediator of the inflammatory response of BBB endothelial cells in neuroinflammatory diseases by obtaining evidence from human multiple sclerosis lesions, a mouse EAE model and cultured human cerebral endothelial cells. Our findings provide evidence that deletion of NRP1 inhibits the effect of the pro-inflammatory cytokines IFNγ and TNFα in HBMVECs. Additionally, our results reveal the beneficial roles of endothelial NRP1 deletion in a mouse EAE model and the induction of endothelial NRP1 in human acute multiple sclerosis lesions. Collectively, our results suggest that targeting endothelial NRP1 signaling could be a potential avenue to suppress inflammatory responses and maintain BBB function, which might help to diminish the progression of neuroinflammatory diseases.
Proinflammatory cytokines are elevated in both the CNS parenchyma and serum of multiple sclerosis individuals and are correlated with disease progression (Larochelle et al., 2011). Our results show, for the first time, the pro-inflammatory effects of NRP1 in cerebral endothelial cells, which are evidenced by attenuated chemokine expression and modulated lymphocyte infiltration in endothelial-NRP1-deletion mice after administration of MOG35–55. The roles of NRP1 in the human immune system have been extensively reported (Kumanogoh and Kikutani, 2013); however, the role and mechanism of NRP1 in pro-inflammatory-cytokine-stimulated effects in endothelial cells remain unclear. NRP1 is known as a specific marker of plasmacytoid dendritic cells (Dzionek et al., 2000), and mediates dendritic-cell-stimulated T-cell activation (Tordjman et al., 2002) and mononuclear phagocyte recruitment (Dejda et al., 2014). Neutralizing antibodies against NRP1 attenuate virus-induced IFN-α production of plasmacytoid dendritic cells (Grage-Griebenow et al., 2007). Our results show, for the first time, that NRP1 mediates IFNγ-stimulated expression of chemokines in cerebral endothelial cells through a Rac1–STAT1-dependent pathway. Furthermore, we have identified that Rac1 acts as a downstream mediator of NRP1 to activate STAT1 in HBMVECs, which is consistent with previous reports that overexpression of NRP1 potentiates activation of Rac1 in hepatic stellate cells (Cao et al., 2010). However, our current results do not rule out a possibility that NRP1-independent Rac1 activation is involved in IFNγ-stimulated inflammatory responses.
The T-cell response to myelin antigen is considered one of the biggest causes of demyelination in multiple sclerosis (Fife et al., 2001). NRP1 has been shown in previous studies to be expressed in certain subsets of T-regulatory (T-reg) cells and to interact with semaphorin-4a to maintain stability and the immune-suppressive functions of these cells (Lalor and Segal, 2013; Stiles et al., 2009). Additionally, in mouse EAE models, deletion of NRP1 in CD4+ T cells causes preferential T helper (Th)-17 lineage commitment, decreased T-reg cell functionality and enhanced disease severity. Conversely, mice receiving CD4+ T cells that overexpress NRP1 are highly resistant to disease progression through a mechanism that includes TGFβ (Argaw et al., 2012). Our study identifies that NRP1 acts as a central mediator, engaging the signaling of VEGF-A, IFNγ and others in endothelial cells to facilitate lymphocyte infiltration and disease progression in mouse EAE models. These studies suggest that NRP1 signaling could have different effects in distinct cell types that are associated with neuroinflammatory diseases.
Our study shows that selective deletion of NRP1 in endothelial cells attenuates EAE and alters endothelial cell production of CXCL10 in response to IFNγ stimulation. CXCL10 was initially reported to be elevated in human cerebrospinal fluid (CSF) during acute demyelinating events and to be accompanied by expression of its receptor C-X-C motif receptor 3 in perivascular infiltrating inflammatory cells in active multiple sclerosis lesions (Sørensen et al., 1999b). A later study reports that endothelial cells are primary sources of CXCL10 in sural nerve biopsies from individuals with classical Guillain–Barré syndrome (acute inflammatory demyelinating polyradiculoneuropathy) (Kieseier et al., 2002). In this study, we observed that the knockdown of NRP1 attenuated CXCL10 expression in HBMVECs and contributed to the suppressed expression of CXCL10 and the inflammatory response in mouse spinal cords after EAE induction (Fig. 2). Because HBMVECs are difficult to transfect, we only achieved ∼30% Nrp1 knockdown (Fig. S1B,F,I). Even with this level of knockdown, IFNγ-stimulated CXCL10 expression was shown to be reduced by 30–50% (Fig. 1A; Fig. S1A,D,G), indicating that NRP1 is an important regulator of CXCL10 expression. However, our current results do not rule out the possibility that NRP1-independent regulation of CXCL10 is involved. Notably, we also observed the positive staining of CXCL10 in inflammatory cells after EAE induction in WT mice and in mice in which endothelial cell NRP1 had been knocked out (Fig. 2C), indicating that non-endothelial-cell-derived CXCL10 is also involved in the progression of neuroinflammatory diseases. Our study is consistent with previous studies showing that treatment with anti-CXCL10 antibody reduces clinical and histological disease severity and prevents recruitment of activated CD4+ T cells to the CNS parenchyma in the myelin proteolipid protein (PLP) EAE model (Fife et al., 2001). Likewise, although we did not measure a difference in CD4+ or CD8+ T cell burden at 13 days post induction, we did observe a significant reduction in T-cell markers in the spinal cord at 17 days post induction, consistent with a role for CXCL10 in the retention of T cells in the CNS (Lalor and Segal, 2013; Stiles et al., 2009). Furthermore, our findings mirror the observed decrease in clinical severity during MOG-induced EAE in mice with astrocyte-specific deletion of CXCL10 (Mills Ko et al., 2014). Indeed, the apparent role of CXCL10 in the accumulation of CD4+ T cells in spinal cord perivascular spaces but not in the recruitment of T cells to these spaces, coupled to the observation that CXCR3 controls the parenchymal distribution of T cells (Muller et al., 2007), indicates that many of the CD4+ and CD8+ T cells measured in the SCILs at 13 days post induction could have been trapped in the perivascular space rather than in the parenchyma, thereby limiting tissue damage and demyelination. In this context, it is also notable that we observed a decrease in NK1.1+ CD3+ NKT or NKT-like cells at 13 days post induction. Given that this population of cells produces IFNγ, it is possible that an early reduction in spinal cord NKT cell number attenuates ongoing endothelial responses that retain pathogenic T cells, reducing overall disease severity. In the future, it might be reasonable to identify the T-cell receptor restriction of these NKT cells to determine whether they are a direct cause of pathogenesis or are immunomodulatory. It will also be useful to carefully assess the relative perivascular and parenchymal distributions of effector and regulatory T cells at earlier timepoints in the disease course, and to determine whether CXCL10 is the key chemokine mediating the effect of NRP1 deficiency in endothelial cells. Additionally, the induction of C-X-C chemokines, such as CXCL10, might directly contribute to the BBB endothelial cell dysfunction in EAE, which has been reported to inhibit proliferation and induce the apoptosis of endothelial cells in several studies (Luster et al., 1995; Wilson et al., 2013). Collectively, our current findings support a new role for NRP1-dependent signaling in CNS endothelial cells that could be tied to CXCL10-mediated control of lymphocyte trafficking, perivascular retention and parenchymal infiltration.
Previous studies have shown that NRP1 acts as a co-receptor for VEGF-A and that it is required for VEGF-A-induced permeability in endothelial cells (Becker et al., 2005; Soker et al., 1998). The increased expression of VEGF-A in neurons (Suidan et al., 2010) and astrocytes (Argaw et al., 2012) has been previously reported in different mouse neuroinflammatory disease models, and importantly, the administration of a peptide, which selectively inhibits the binding of VEGF-A to NRP1 (Starzec et al., 2007), has shown therapeutic effects in a mouse model of CD8+-T-cell-initiated BBB disruption (Suidan et al., 2012). Consistent with these studies, our results showed that deletion of endothelial NRP1 attenuates leaking of FITC–albumin from the CNS parenchyma and preserves tight junction structures. Furthermore, infiltrated inflammatory cells, especially CD8+ lymphocytes, were shown to stimulate resident CNS cells, including astrocytes, microglia and neurons, to release factors to increase BBB permeability; moreover, these cells secrete several cytokines, such as TNFα, as well as reactive oxygen species and matrix metalloproteinases, that act on BBB endothelial cells directly to disrupt BBB integrity (Larochelle et al., 2011). As we also observed less lymphocyte infiltration in VECad/NRP1−/− mice, our results suggest that decreased expression and accumulation of BBB permeability mediators might also be present in the CNS of VECad/NRP1−/− mice after EAE induction.
Another important contribution of this study is that we identified the expression pattern of NRP1 in early multiple sclerosis lesions and the normal CNS in humans. Our results show that the basal level of NRP1 is relatively low in the normal CNS but that it is induced in early multiple sclerosis lesions, which are pathologically characterized by the presence of active demyelination and cortical and meningeal inflammation (Lucchinetti et al., 2011). Interestingly, induced expression of NRP1 was also observed in active astrocytes in early multiple sclerosis lesions, which play dual roles in both immune responses and neuron demyelination (Nair et al., 2008). A recent study has also reported that NRP1 is expressed in microglia and macrophages in human multiple sclerosis lesions (Costa et al., 2015), suggesting that NRP1 is involved in astrocyte, microglia and macrophages activation in neuroinflammatory diseases.
Taken together, the results of our study identify a dual pro-inflammatory role of NRP1 in cerebral endothelial cells and provide evidence that the deletion of endothelial NRP1 in mice maintains BBB function and attenuates disease progression after induction of EAE. Moreover, the staining of human multiple sclerosis samples showed the clinical relevance of NRP1 expression in early multiple sclerosis lesions in humans. Our results raise the possibility that NRP1 acts as a central mediator of BBB endothelial dysfunction during neuroinflammatory diseases and could be a potential therapeutic target.
MATERIALS AND METHODS
Mice
Tamoxifen-inducible endothelial-cell-specific NRP1-knockout mice were generated by breeding VECad-Cre-ERT2 (C57BL/6 background; a kind gift from Ralf H. Adams, University of Münster, Münster, Germany) (Sörensen et al., 2009) with NRP1flox/flox mice (C57BL/6 background; a kind gift from Professor Alex L. Kolodkin, Johns Hopkins University, Baltimore, MD) (Gu et al., 2003b). VECad-Cre-ERT2 mice were crossed with ROSA 26R mice (C57BL/6 background) to generate VECad-ROSA mice. To induce deletion of endothelial NRP1, tamoxifen (Sigma Chemicals) was dissolved in corn oil (15 mg ml−1) and administered to 4-week-old mice through gavage feeding (100 µl) for five consecutive days, which was performed at the animal facilities at Mayo Clinic. The investigator who performed the oral gavage was blind to the genotypes of the mice. Tie2-Cre mice (C57BL/6 background) were crossed with NRP1flox/flox mice to generate the Tie2/NRP1+/− mice. Animal sample size was determined on the basis of Institutional Animal Care and Use Committee (IACUC) limitations and previous experience of EAE experiments. None of the mice were excluded from this study. Animals were allocated to experimental groups owing to their predetermined type, and no randomization was used. Animal care and experimental procedures were performed under protocols approved by the IACUC of Mayo Clinic.
Immunohistochemistry staining of NRP1 in human brain tissues
Neuropathological studies on human tissue were performed on archival formalin-fixed paraffin-embedded CNS tissue from normal CNS controls and from autopsied individuals who had been clinically and pathologically diagnosed as having multiple sclerosis. Written or oral consent from all the subjects of this surgical procedure was obtained. The study was approved by the Institutional Review Board of Mayo Clinic, Rochester, MN. Hematoxylin and eosin, Luxol-Fast-Blue and periodic-acid–Schiff staining, as well as Bielschowsky silver impregnation, were used to stain 5-µm-thick sections for routine pathological evaluation. Immunohistochemistry was performed by using an avidin–biotin technique with primary antibodies against MOG (1:1000, Abcam, cat no. ab109746), KIM1P (1:5000; gift from Dr Wolfgang Brück, University of Göttingen, Göttingen, Germany) and NRP1 (1:200, Sigma, HPA030278). Demyelinating activity was described as active demyelination, inactive demyelination, remyelination and periplaque white matter and/or grey matter, as previously described (Popescu and Lucchinetti, 2012).
EAE
The mice were housed in a conventional facility at Mayo Clinic with a reverse 12-h-light and 12-h-dark cycle and ad libitum access to food and water. All behavior experiments were performed during daylight hours. The investigators who performed the EAE induction, behavioral scoring and measurements of body weight were blinded to the genotypes of the mice. For disease induction, 10- to 12-week-old male mice were immunized subcutaneously in both flanks with 100 mg of MOG35-55 peptide that was emulsified in complete Freund's adjuvant containing Mycobacterium tuberculosis H37Ra (400 mg/mice). Pertussis toxin (Sigma Chemicals; 200 ng) was then injected intraperitoneally at day 0 and day 2 post immunization. Clinical disease was monitored daily in a blinded fashion by measuring disease severity according to the standard EAE grading system: 0, normal; 1, loss of tail tone; 2, hindlimb weakness; 3, hindlimb paralysis; 4, hindlimb paralysis and forelimb paralysis or weakness; 5, moribund or dead.
Histology
Mice killed after EAE induction were perfused with PBS and then 4% paraformaldehyde. Spinal cords were cut into 10-to-12 transverse slabs and paraffin-embedded by using standard methods (Tsunoda and Fujinami, 1996). Sections were stained with hematoxylin and eosin, and Luxol-Fast-Blue and periodic-acid–Schiff.
Semi-quantification of inflammatory cell infiltration and demyelination in the spinal cord was performed as previously described (Miller et al., 1997). Meningeal inflammation and parenchymal demyelination was assessed in a blinded manner. The investigator who performed the semi-quantification was blind to the group information. Briefly, each quadrant from each transverse section from the same mouse was examined for the presence or absence of a pathological abnormality. The maximum score of 100 indicates that there was a pathological abnormality present in all four quadrants of the spinal cord sections. A score of 0 indicates that no quadrant showed an abnormality.
Vascular permeability in vivo
To examine the BBB permeability after EAE induction, FITC–albumin (Sigma Chemicals) was dissolved in PBS (10 mg ml−1) and administered to mice through a 100-µl tail vein injection 2 h before killing. Spinal cords and sera were then collected. Half of the spinal cords were homogenized in RIPA buffer and centrifuged, and the supernatant collected. The fluorescence intensity of 100 µl of lysate was measured, normalized to the fluorescence intensity of the serum, and expressed as a relative fold of the control group. The other half of the spinal cords were snap frozen in optimal cutting temperature (OCT) compound embedding medium and subjected to confocal microscopy or fluorescent staining analysis with an antibody against occludin (1:100; N-19, Santa Cruz Biotechnology). Nuclei were counterstained with DAPI.
qPCR
Total mRNA was isolated from cells and tissues using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and was reverse-transcribed by using oligo (dT) priming using the iScript cDNA Synthesis kit (Bio-Rad). qPCR analyses were performed using the ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) and SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK). The results were normalized to 18S ribosomal RNA (mouse) or the β-actin gene (human). Primer sequences are shown in Table S1.
Reagents
Primary HBMVECs were purchased from Cell System (Kirkland, WA) and cultured by using Complete Classic Medium Kit With Serum and CultureBoost (Cell Systems, 4Z0-500). HMVECs (were purchased from Lonza (Allendale, NJ) and cultured in EBM-2 medium with the EGM-2 MV Bulletkit (Lonza). No cell line listed in the database of misidentified cell lines (maintained by International Cell Line Authentication Committee) was used. HBMVECs and HMVECs were authenticated by testing for the expression of von Williebrand Factor VIII and cytoplasmic uptake of acetylated low-density lipoprotein. All the cells were negative for mycoplasma. For siRNA-mediated knockdown of NRP1, NRP1 siRNAs (Hs_NRP1_7 FlexiTube siRNA, Hs_NRP1_8 FlexiTube siRNA) and control siRNA (AllStars Negative Control siRNA) were purchased from Qiagen. Oligofectamine (Life Technologies) was used as the transfection reagent. Recombinant IFNγ and TNFα, and antibodies against STAT1 (#9172; 1:1000), phosphorylated STAT1 (#9167; 1:1000), STAT3 (#4904; 1:1000) and phosphorylated STAT3 (#9145; 1:1000) were purchased from Cell Signaling Technology. Tamoxifen and the antibody against β-Actin (A2228; 1:5000) were purchased from Sigma-Aldrich. The antibody against NRP1 for western blotting (#3725; 1:1000) was purchased from Cell Signaling Technology, and the antibody against NRP1 (HPA030278; 1:200) for immunohistochemistry staining of human multiple sclerosis tissues was purchased from Sigma-Aldrich. CXCL10 antibody (orb10277; 1:100) was purchased from Biorbyt (San Francisco, CA). Quantification of Rac1 activity in HMBVECs was performed using an active Rac1-pull-down and -detection kit according to the manufacturer's protocol (Thermo Scientific, Waltham, MA). Rac1 inhibitor NSC 23766 was purchased from Tocris Bioscience (Bristol, UK).
X-gal staining
Fresh tissues were harvested, snap-frozen in OCT and sectioned at 5 µm. The staining was performed as previously described (Ji et al., 2008). Briefly, frozen sections were postfixed with 0.25% glutaraldehyde in PBS, and then washed with rinse solution (0.1 M phosphate buffer, pH 7.3, 0.1% deoxycholic acid, 0.2% NP-40, and 2 mM MgCl2). X-gal staining was performed by incubating samples in staining buffer (2.5 mg ml−1 X-gal, 5 mM potassium ferricyanide and 5 mM potassium ferrocyanide) overnight at 37°C, followed by counterstaining with Nuclear Fast Red.
Flow cytometry
For all experiments, flow cytometry buffer contained 1% bovine serum albumin and 0.02% sodium azide in PBS, as previously described (Howe et al., 2012). Briefly, blocking buffer contained flow cytometry buffer, supernatant from 2.4G2 hybridoma (Fc block; anti-CD16 and -CD32; American Type Culture Collection, no. HB-197) and fetal bovine serum at a ratio of 10:5:1. After isolation, cell suspensions were incubated in the blocking buffer at 4°C for 30 min. Antibodies were added to the blocked cells at 1:200 and incubated for 30 min at 4°C. Stained cells were then washed three times in flow cytometry buffer before flow cytometry analysis. Files were analyzed offline using FlowJo 7.5 (Windows version; Tree Star, Inc., Ashland, OR). CD45 was detected with antibody clone 30-F11 (BD Biosciences no. 557235). CD11B was detected with clone M1/70 (BD Biosciences no. 553312). Ly6C and Ly6G (lymphocyte antigens) were detected with clone Gr1 of antibody RB6-8C5 (BD Biosciences no. 553128). Ly6G was detected with clone 1A8 (BD Biosciences no. 560599). CD45.1 was detected with clone A20 (BD Biosciences no. 553776). Cocktail 1: FITC–anti-CD45, Phycoerythrin (PE)–anti-TCRγδ, peridinin chlorophyll (PerCP)–Cy5.5–anti-CD8, APC–anti-CD4 antibodies; cocktail 2: FITC–anti-CD45, PE–anti-TCRβ, PerCP–Cy5.5–anti-CD8, APC–anti-CD4 antibodies; cocktail 3: FITC–anti-CD3, PE–anti-CD1d, PerCP–Cy5.5–anti-CD11b, APC–anti-NK1.1 antibodies; cocktail 4, FITC–anti-CD3, PE–anti-TCRγδ, PerCP–Cy5.5–anti-CD11b, APC–anti-NK1.1 antibodies; cocktail 5: FITC–anti-CD69, PE–anti-CD62L, PerCP–Cy5.5–anti-CD8, APC–anti-CD4 antibodies.
MRI
The imaging system used for image acquisition was a Bruker Avance II 7 Tesla vertical bore small animal MRI system (Bruker Biospin). Mice were anesthetized through isoflurane inhalation and were maintained under 3–4% isoflurane. Each mouse's respiratory rate was monitored during MRI acquisition, and the monitoring system in use was a model 1030, SA Instruments, Inc. (Stony Brook, NY). Three-dimensional T2*-weighted images were acquired in the following manner [gradient echo fast imaging pulse sequence: repetition time (TR)=150 ms, echo time (TE)=10 ms, flip angle=15°, field of view=4×2.5×2.5 cm, matrix: 256×128×128, number of excitations=4] (Johnson et al., 2012). T2*-weighted images were analyzed using the Analyze function (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). Intraperitoneal injections of gadolinium using weight-based dosing of 100 mg kg−1 of body weight were given to each mouse and, after a standard delay of 15 min, a volume acquisition T1-weighted spin echo sequence was used (TR=150 ms, TE=8 ms, matrix: 256×256×18, number of averages=1) to obtain T1-weighted images. We experienced no animal death, and the animals recovered after anesthesia and image acquisition.
Statistics
All analyses were performed using GraphPad Prism 5 (GraphPad Software). Experiments were routinely repeated at least three times, and the number was increased according to effect size or sample variation. Values in Figs 2D, 4A,B and Fig. S3E are expressed as mean±95% confidence intervals, and all the other values are expressed as the mean±s.e.m. Nonparametric analysis was used for analysis in Fig. 1, Figs S1 and S2. Statistical differences were determined to be significant at P<0.05. An unpaired two-tailed Student's t-test was performed for Figs 2B,G,H and 3D. Disease incidence of Fig. 4C was analyzed by using Fisher's exact test. Clinical behavior and body weight changes after EAE induction in Fig. 4A,B were analyzed by using two-way ANOVA. For small sample sizes (n<5) in Figs 1D,E, 3D, Figs S1 and S2, scatter plots instead of bar graphs are used to present data.
Acknowledgements
We thank Dr Alex Kolodkin (Johns Hopkins University) and Dr Ralf H. Adams (Max Planck Institute) for the NRP1flox/flox mice and VECad-Cre-ERT2 mice, respectively. We thank Ms. Brandy H. Edenfield and Dr Laura Lewis-Tuffin of the Mayo Clinic Jacksonville Histopathology Facility for the kind help. We thank Dr Marcus Fruttiger (UCL Institute of Ophthalmology) for the tamoxifen induction protocols. We thank Ms. Kelly Viola for helpful suggestions for writing the article.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Y.W. performed in vitro experiments, BBB permeability assays, qPCR and western blotting analysis of animal tissues, and drafted the manuscript. Y.C. initiated this study and conducted the in vitro experiments. A.K.M. and B.D.C. performed EAE induction. Y.G. performed histological quantification and helped to optimize the staining protocol of human tissues. R.S.A. and B.D.C. conducted the flow cytometry analysis of spinal cords, and C.L.H. performed the analysis. J.D.G., P.A.A. and I.P. performed the mouse MRI analyses. Y.Z. conducted all the gavage feeding of tamoxifen. E.W. evaluated the EAE clinical scores and assisted Y.W. in animal breeding. R.S.A. assisted in the in vitro experiments. B.J. and K.D. helped with the analysis of reporter mice. A.K.M., C.L.H., B.J. and D.M. revised the manuscript. A.K.M., B.J., C.F.L., C.L.H. and D.M. helped to design the experiments and analyzed the results, and D.M. provided overall supervision of this project.
Funding
This work was supported by National Institutes of Health [grant numbers HL70567 and CA78383 to D.M.], Clinical and Translational Science Awards (CTSA) [grant numbers UL1 TR000135 and NIH K12 CA90628 to B.J.], Florida Department of Health Cancer Research Chair's Fund Florida [grant number 3J-02 to D.M.], and the American Heart Association [grant number AHA-14POST20390029 to Y.W.]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.190702.supplemental
References
- Argaw A. T., Asp L., Zhang J., Navrazhina K., Pham T., Mariani J. N., Mahase S., Dutta D. J., Seto J., Kramer E. G.. et al. (2012). Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Invest. 122, 2454-2468. 10.1172/JCI60842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker P. M., Waltenberger J., Yachechko R., Mirzapoiazova T., Sham J. S. K., Lee C. G., Elias J. A. and Verin A. D. (2005). Neuropilin-1 regulates vascular endothelial growth factor-mediated endothelial permeability. Circ. Res. 96, 1257-1265. 10.1161/01.RES.0000171756.13554.49 [DOI] [PubMed] [Google Scholar]
- Brück W., Bitsch A., Kolenda H., Brück Y., Stiefel M. and Lassmann H. (1997). Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann. Neurol. 42, 783-793. 10.1002/ana.410420515 [DOI] [PubMed] [Google Scholar]
- Cao S., Yaqoob U., Das A., Shergill U., Jagavelu K., Huebert R. C., Routray C., Abdelmoneim S., Vasdev M., Leof E.. et al. (2010). Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-beta signaling in hepatic stellate cells. J. Clin. Invest. 120, 2379-2394. 10.1172/JCI41203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Chedotal A., He Z., Goodman C. S. and Tessier-Lavigne M. (1997). Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 19, 547-559. 10.1016/S0896-6273(00)80371-2 [DOI] [PubMed] [Google Scholar]
- Costa C., Martínez-Sáez E., Gutiérrez-Franco A., Eixarch H., Castro Z., Ortega-Aznar A., Ramón Y. C. S., Montalban X. and Espejo C. (2015). Expression of semaphorin 3A, semaphorin 7A and their receptors in multiple sclerosis lesions. Mult. Scler. 21, 1632-1643. 10.1177/1352458515599848 [DOI] [PubMed] [Google Scholar]
- Dejda A., Mawambo G., Cerani A., Miloudi K., Shao Z., Daudelin J.-F., Boulet S., Oubaha M., Beaudoin F., Akla N.. et al. (2014). Neuropilin-1 mediates myeloid cell chemoattraction and influences retinal neuroimmune crosstalk. J. Clin. Invest. 124, 4807-4822. 10.1172/JCI76492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzionek A., Fuchs A., Schmidt P., Cremer S., Zysk M., Miltenyi S., Buck D. W. and Schmitz J. (2000). BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 165, 6037-6046. 10.4049/jimmunol.165.11.6037 [DOI] [PubMed] [Google Scholar]
- Elloso M. M., Phiel K., Henderson R. A., Harris H. A. and Adelman S. J. (2005). Suppression of experimental autoimmune encephalomyelitis using estrogen receptor-selective ligands. J. Endocrinol. 185, 243-252. 10.1677/joe.1.06063 [DOI] [PubMed] [Google Scholar]
- Eng L. F., Ghirnikar R. S. and Lee Y. L. (1996). Inflammation in EAE: role of chemokine/cytokine expression by resident and infiltrating cells. Neurochem. Res. 21, 511-525. 10.1007/BF02527717 [DOI] [PubMed] [Google Scholar]
- Fife B. T., Kennedy K. J., Paniagua M. C., Lukacs N. W., Kunkel S. L., Luster A. D. and Karpus W. J. (2001). CXCL10 (IFN-gamma-inducible protein-10) control of encephalitogenic CD4+ T cell accumulation in the central nervous system during experimental autoimmune encephalomyelitis. J. Immunol. 166, 7617-7624. 10.4049/jimmunol.166.12.7617 [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Ohtsuki T., Takagi S. and Tsuji T. (1989). An aberrant retinal pathway and visual centers in Xenopus tadpoles share a common cell surface molecule, A5 antigen. Dev. Biol. 135, 231-240. 10.1016/0012-1606(89)90175-9 [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Takagi S. and Hirata T. (1995). Growth-associated expression of a membrane protein, neuropilin, in Xenopus optic nerve fibers. Dev. Neurosci. 17, 343-349. 10.1159/000111304 [DOI] [PubMed] [Google Scholar]
- Gerhard W., Taylor A., Sandberg-Wollheim M. and Koprowski H. (1985). Longitudinal analysis of three intrathecally produced immunoglobulin subpopulations in an MS patient. J. Immunol. 134, 1555-1560. [PubMed] [Google Scholar]
- Giesser B. S. (2011). Primer on Multiple Sclerosis. Oxford, New York: Oxford University Press. [Google Scholar]
- Goodin D. S. (2014). The epidemiology of multiple sclerosis: insights to disease pathogenesis. Handb. Clin. Neurol. 122, 231-266. 10.1016/B978-0-444-52001-2.00010-8 [DOI] [PubMed] [Google Scholar]
- Grage-Griebenow E., Löseke S., Kauth M., Gehlhar K., Zawatzky R. and Bufe A. (2007). Anti-BDCA-4 (neuropilin-1) antibody can suppress virus-induced IFN-alpha production of plasmacytoid dendritic cells. Immunol. Cell Biol. 85, 383-390. 10.1038/sj.icb.7100048 [DOI] [PubMed] [Google Scholar]
- Gu C., Rodriguez E. R., Reimert D. V., Shu T., Fritzsch B., Richards L. J., Kolodkin A. L. and Ginty D. D. (2003a). Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 5, 45-57. 10.1016/S1534-5807(03)00169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C., Rodriguez E. R., Reimert D. V., Shu T., Fritzsch B., Richards L. J., Kolodkin A. L. and Ginty D. D. (2003b). Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 5, 45-57. 10.1016/S1534-5807(03)00169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohnoki K., Inoue A. and Koh C.-S. (1998). Elevated serum levels of IFN-gamma, IL-4 and TNF-alpha/unelevated serum levels of IL-10 in patients with demyelinating diseases during the acute stage. J. Neuroimmunol. 87, 27-32. 10.1016/S0165-5728(98)00053-8 [DOI] [PubMed] [Google Scholar]
- Howe C. L., Lafrance-Corey R. G., Sundsbak R. S., Sauer B. M., Lafrance S. J., Buenz E. J. and Schmalstieg W. F. (2012). Hippocampal protection in mice with an attenuated inflammatory monocyte response to acute CNS picornavirus infection. Sci. Rep. 2, 545 10.1038/srep00545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B., Song J., Tsou L., Bi Y., Gaiser S., Mortensen R. and Logsdon C. (2008). Robust acinar cell transgene expression of CreErT via BAC recombineering. Genesis 46, 390-395. 10.1002/dvg.20411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. L., Chen Y., Suidan G. L., McDole J. R., Lohrey A. K., Hanson L. M., Jin F., Pirko I. and Johnson A. J. (2012). A hematopoietic contribution to microhemorrhage formation during antiviral CD8 T cell-initiated blood-brain barrier disruption. J. Neuroinflammation 9, 60 10.1186/1742-2094-9-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami A., Kitsukawa T., Takagi S. and Fujisawa H. (1996). Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. J. Neurobiol. 29, 1-17. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Kitsukawa T., Bekku Y., Matsuda Y., Sanbo M., Yagi T. and Fujisawa H. (1999). A requirement for neuropilin-1 in embryonic vessel formation. Development 126, 4895-4902. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Bekku Y., Suto F., Kitsukawa T., Taniguchi M., Nagatsu I., Nagatsu T., Itoh K., Yagi T. and Fujisawa H. (2002). Requirement of neuropilin 1-mediated Sema3A signals in patterning of the sympathetic nervous system. Development 129, 671-680. [DOI] [PubMed] [Google Scholar]
- Kieseier B. C., Tani M., Mahad D., Oka N., Ho T., Woodroofe N., Griffin J. W., Toyka K. V., Ransohoff R. M. and Hartung H.-P. (2002). Chemokines and chemokine receptors in inflammatory demyelinating neuropathies: a central role for IP-10. Brain 125, 823-834. 10.1093/brain/awf070 [DOI] [PubMed] [Google Scholar]
- Kitsukawa T., Shimizu M., Sanbo M., Hirata T., Taniguchi M., Bekku Y., Yagi T. and Fujisawa H. (1997). Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron 19, 995-1005. 10.1016/S0896-6273(00)80392-X [DOI] [PubMed] [Google Scholar]
- Kolodkin A. L., Levengood D. V., Rowe E. G., Tai Y.-T., Giger R. J. and Ginty D. D. (1997). Neuropilin is a semaphorin III receptor. Cell 90, 753-762. 10.1016/S0092-8674(00)80535-8 [DOI] [PubMed] [Google Scholar]
- Kumanogoh A. and Kikutani H. (2013). Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat. Rev. Immunol. 13, 802-814. 10.1038/nri3545 [DOI] [PubMed] [Google Scholar]
- Lalor S. J. and Segal B. M. (2013). Th1-mediated experimental autoimmune encephalomyelitis is CXCR3 independent. Eur. J. Immunol. 43, 2866-2874. 10.1002/eji.201343499 [DOI] [PubMed] [Google Scholar]
- Larochelle C., Alvarez J. I. and Prat A. (2011). How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 585, 3770-3780. 10.1016/j.febslet.2011.04.066 [DOI] [PubMed] [Google Scholar]
- Lindå H., von Heijne A., Major E. O., Ryschkewitsch C., Berg J., Olsson T. and Martin C. (2009). Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N. Engl. J. Med. 361, 1081-1087. 10.1056/NEJMoa0810316 [DOI] [PubMed] [Google Scholar]
- Lucchinetti C., Brück W., Parisi J., Scheithauer B., Rodriguez M. and Lassmann H. (2000). Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 47, 707-717. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C. F., Popescu B. F. G., Bunyan R. F., Moll N. M., Roemer S. F., Lassmann H., Brück W., Parisi J. E., Scheithauer B. W., Giannini C.. et al. (2011). Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 365, 2188-2197. 10.1056/NEJMoa1100648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster A. D., Greenberg S. M. and Leder P. (1995). The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. J. Exp. Med. 182, 219-231. 10.1084/jem.182.1.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. J., Bright J. J., Sriram S. and Rodriguez M. (1997). Successful treatment of established relapsing experimental autoimmune encephalomyelitis in mice with a monoclonal natural autoantibody. J. Neuroimmunol. 75, 204-209. 10.1016/S0165-5728(97)00027-1 [DOI] [PubMed] [Google Scholar]
- Mills Ko E., Ma J. H., Guo F., Miers L., Lee E., Bannerman P., Burns T., Ko D., Sohn J., Soulika A. M.. et al. (2014). Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. J. Neuroinflammation 11, 105 10.1186/1742-2094-11-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagar A. and Alexander J. S. (2003). Blood-brain barrier disruption in multiple sclerosis. Mult. Scler. 9, 540-549. 10.1191/1352458503ms965oa [DOI] [PubMed] [Google Scholar]
- Muller M., Carter S. L., Hofer M. J., Manders P., Getts D. R., Getts M. T., Dreykluft A., Lu B., Gerard C., King N. J. C.. et al. (2007). CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J. Immunol. 179, 2774-2786. 10.4049/jimmunol.179.5.2774 [DOI] [PubMed] [Google Scholar]
- Nair A., Frederick T. J. and Miller S. D. (2008). Astrocytes in multiple sclerosis: a product of their environment. Cell. Mol. Life Sci. 65, 2702-2720. 10.1007/s00018-008-8059-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E. J., Ji K.-A., Jeon S.-B., Choi W.-H., Han I.-O., You H.-J., Kim J.-H., Jou I. and Joe E.-H. (2004). Rac1 contributes to maximal activation of STAT1 and STAT3 in IFN-gamma-stimulated rat astrocytes. J. Immunol. 173, 5697-5703. 10.4049/jimmunol.173.9.5697 [DOI] [PubMed] [Google Scholar]
- Popescu B. F. G. and Lucchinetti C. F. (2012). Meningeal and cortical grey matter pathology in multiple sclerosis. BMC Neurol. 12, 11 10.1186/1471-2377-12-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio N. and Capa L. (1993). Differential IL-1 synthesis by astrocytes from Theiler's murine encephalomyelitis virus-susceptible and -resistant strains of mice. Cell. Immunol. 149, 237-247. 10.1006/cimm.1993.1151 [DOI] [PubMed] [Google Scholar]
- Ryberg B. (1978). Multiple specificities of antibrain antibodies in multiple sclerosis and chronic myelopathy. J. Neurol. Sci. 38, 357-382. 10.1016/0022-510X(78)90142-9 [DOI] [PubMed] [Google Scholar]
- Schwarz Q., Vieira J. M., Howard B., Eickholt B. J. and Ruhrberg C. (2008). Neuropilin 1 and 2 control cranial gangliogenesis and axon guidance through neural crest cells. Development 135, 1605-1613. 10.1242/dev.015412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Q., Maden C. H., Vieira J. M. and Ruhrberg C. (2009). Neuropilin 1 signaling guides neural crest cells to coordinate pathway choice with cell specification. Proc. Natl. Acad. Sci. USA 106, 6164-6169. 10.1073/pnas.0811521106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld M., Cullheim S., Hammarberg H., Piehl F., Suneson A., Lake S., Sjögren A., Walum E. and Risling M. (2000). Induction of VEGF and VEGF receptors in the spinal cord after mechanical spinal injury and prostaglandin administration. Eur. J. Neurosci. 12, 3675-3686. 10.1046/j.1460-9568.2000.00263.x [DOI] [PubMed] [Google Scholar]
- Soker S., Takashima S., Miao H. Q., Neufeld G. and Klagsbrun M. (1998). Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92, 735-745. 10.1016/S0092-8674(00)81402-6 [DOI] [PubMed] [Google Scholar]
- Solomon B. D., Mueller C., Chae W.-J., Alabanza L. M. and Bynoe M. S. (2011). Neuropilin-1 attenuates autoreactivity in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 108, 2040-2045. 10.1073/pnas.1008721108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen T. L., Tani M., Jensen J., Pierce V., Lucchinetti C., Folcik V. A., Qin S., Rottman J., Sellebjerg F., Strieter R. M.. et al. (1999a). Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Invest. 103, 807-815. 10.1172/JCI5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen T. L., Tani M., Jensen J., Pierce V., Lucchinetti C., Folcik V. A., Qin S., Rottman J., Sellebjerg F., Strieter R. M.. et al. (1999b). Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Invest. 103, 807-815. 10.1172/JCI5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörensen I., Adams R. H. and Gossler A. (2009). DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood 113, 5680-5688. 10.1182/blood-2008-08-174508 [DOI] [PubMed] [Google Scholar]
- Starzec A., Ladam P., Vassy R., Badache S., Bouchemal N., Navaza A., du Penhoat C. H. and Perret G. Y. (2007). Structure-function analysis of the antiangiogenic ATWLPPR peptide inhibiting VEGF(165) binding to neuropilin-1 and molecular dynamics simulations of the ATWLPPR/neuropilin-1 complex. Peptides 28, 2397-2402. 10.1016/j.peptides.2007.09.013 [DOI] [PubMed] [Google Scholar]
- Stiles L. N., Liu M. T., Kane J. A. and Lane T. E. (2009). CXCL10 and trafficking of virus-specific T cells during coronavirus-induced demyelination. Autoimmunity 42, 484-491. 10.1080/08916930902810708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suidan G. L., Dickerson J. W., Chen Y., McDole J. R., Tripathi P., Pirko I., Seroogy K. B. and Johnson A. J. (2010). CD8 T cell-initiated vascular endothelial growth factor expression promotes central nervous system vascular permeability under neuroinflammatory conditions. J. Immunol. 184, 1031-1040. 10.4049/jimmunol.0902773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suidan G. L., Dickerson J. W., Johnson H. L., Chan T. W., Pavelko K. D., Pirko I., Seroogy K. B. and Johnson A. J. (2012). Preserved vascular integrity and enhanced survival following neuropilin-1 inhibition in a mouse model of CD8 T cell-initiated CNS vascular permeability. J. Neuroinflammation 9, 218 10.1186/1742-2094-9-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S., Tsuji T., Amagai T., Takamatsu T. and Fujisawa H. (1987). Specific cell surface labels in the visual centers of Xenopus laevis tadpole identified using monoclonal antibodies. Dev. Biol. 122, 90-100. 10.1016/0012-1606(87)90335-6 [DOI] [PubMed] [Google Scholar]
- Takagi S., Hirata T., Agata K., Mochii M., Eguchi G. and Fujisawa H. (1991). The A5 antigen, a candidate for the neuronal recognition molecule, has homologies to complement components and coagulation factors. Neuron 7, 295-307. 10.1016/0896-6273(91)90268-5 [DOI] [PubMed] [Google Scholar]
- Takagi S., Kasuya Y., Shimizu M., Matsuura T., Tsuboi M., Kawakami A. and Fujisawa H. (1995). Expression of a cell adhesion molecule, neuropilin, in the developing chick nervous system. Dev. Biol. 170, 207-222. 10.1006/dbio.1995.1208 [DOI] [PubMed] [Google Scholar]
- Tordjman R., Lepelletier Y., Lemarchandel V., Cambot M., Gaulard P., Hermine O. and Romeo P. H. (2002). A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 3, 477-482. 10.1038/ni789 [DOI] [PubMed] [Google Scholar]
- Traugott U., Scheinberg L. C. and Raine C. S. (1979). Multiple sclerosis: circulating antigen-reactive lymphocytes. Ann. Neurol. 6, 425-429. 10.1002/ana.410060509 [DOI] [PubMed] [Google Scholar]
- Tsunoda I. and Fujinami R. S. (1996). Two models for multiple sclerosis: experimental allergic encephalomyelitis and Theiler's murine encephalomyelitis virus. J. Neuropathol. Exp. Neurol. 55, 673-686. 10.1097/00005072-199606000-00001 [DOI] [PubMed] [Google Scholar]
- Weiner H. L. (2009). The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann. Neurol. 65, 239-248. 10.1002/ana.21640 [DOI] [PubMed] [Google Scholar]
- Wilson N. O., Solomon W., Anderson L., Patrickson J., Pitts S., Bond V., Liu M. and Stiles J. K. (2013). Pharmacologic inhibition of CXCL10 in combination with anti-malarial therapy eliminates mortality associated with murine model of cerebral malaria. PLoS ONE 8, e60898 10.1371/journal.pone.0060898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. G., Tsang W., Zhang L., Powers C. and Chopp M. (2001). Up-regulation of neuropilin-1 in neovasculature after focal cerebral ischemia in the adult rat. J. Cereb. Blood Flow Metab. 21, 541-549. 10.1097/00004647-200105000-00008 [DOI] [PubMed] [Google Scholar]
- Zivadinov R. and Leist T. P. (2005). Clinical-magnetic resonance imaging correlations in multiple sclerosis. J. Neuroimaging 15, 10S-21S. 10.1177/1051228405283291 [DOI] [PubMed] [Google Scholar]