Abstract
Background
Although humans experience orgasms with a degree of statistical regularity, they remain among the most enigmatic of sexual responses; difficult to define and even more difficult to study empirically. The question of whether animals experience orgasms is hampered by similar lack of definition and the additional necessity of making inferences from behavioral responses.
Method
Here we define three behavioral criteria, based on dimensions of the subjective experience of human orgasms described by Mah and Binik, to infer orgasm-like responses (OLRs) in other species: 1) physiological criteria that include pelvic floor and anal muscle contractions that stimulate seminal emission and/or ejaculation in the male, or that stimulate uterine and cervical contractions in the female; 2) short-term behavioral changes that reflect immediate awareness of a pleasurable hedonic reward state during copulation; and 3) long-term behavioral changes that depend on the reward state induced by the OLR, including sexual satiety, the strengthening of patterns of sexual arousal and desire in subsequent copulations, and the generation of conditioned place and partner preferences for contextual and partner-related cues associated with the reward state. We then examine whether physiological and behavioral data from observations of male and female rats during copulation, and in sexually-conditioned place- and partner-preference paradigms, are consistent with these criteria.
Results
Both male and female rats display behavioral patterns consistent with OLRs.
Conclusions
The ability to infer OLRs in rats offers new possibilities to study the phenomenon in neurobiological and molecular detail, and to provide both comparative and translational perspectives that would be useful for both basic and clinical research.
Keywords: sex, pleasure, animals, male, female, genitosensory, ultrasonic vocalizations, conditioned preference, opioids, dopamine
Well you tried it just for once found it all right for kicks But now you found out that it's a habit that sticks ‘Orgasm Addict’ by the Buzzcocks (1977)
Orgasms are among the most wonderous and pleasurable events we experience as human beings. The term comes from the Greek term orgasmos meaning ‘excitement’ or ‘swelling’ and is synonymous with sexual ‘climax’ or the autonomic apex of copulation or masturbation (Coolen, Allard, Truitt, & McKenna, 2004; McKenna, 1999a). The Roman poet Ovid, in Ars Amatoria (2CE/1855), describes orgasm as a phase which ‘relieves’ or ‘completes’ the lovemaking of both partners (Vol. 2, p. 663). Albert Moll (1908/1912), one of the early German sexologists, described four phases of human sexual response resulting from a ‘tumescence and detumescence drive’ (referring to blood flowing into and out of erectile tissues), positioning orgasms as a ‘voluptuous acme’ or high point in tumescence that yields to a ‘sudden cessation of the voluptuous sensation’ and detumescence (pp. 22–23). Wilhelm Reich (1927/1945) described orgasms as a ‘bioelectric discharge’ of purely sexual tension, breaking away from Freud's (1920/1922) notion of orgasm as a death instinct or ‘Thanatos’ that the life instinct ‘Eros’ always moved its ‘libido’ toward. Kinsey, Pomeroy, and Martin (1948) and Kinsey, Pomeroy, Martin, and Gebhard (1953) noted that orgasms come largely from penile stimulation in men and clitoral stimulation (CLS) in women, although stimulation of other erogenous zones on the body could also sum up to, or in some cases generate, orgasms. Masters and Johnson (1966) reiterated Moll's four stages of human sexual response and identified orgasm at its own phase, the ecstatic period when some or all of the built-up sexual tension is released, followed by a longer-term ‘resolution’ or refractory phase where pelvic muscles and blood flow are relaxed. Despite a plethora of poetry and songs devoted to orgasms and their repercussions, the phenomenology of orgasm in humans is fraught with a multitude of definitions and interpretations, different subjective experiences across individuals, and even different experiences in the same individual across the lifespan. Another problem is that orgasms have occupied the ‘highest’ place in the human sexual response cycle. This drives many people to ‘achieve’ them or somehow ‘give’ them to others, which can diminish the value of sexual sensations experienced during other phases of the response cycle. That said, people's experiences with orgasms, especially first experiences, can be profound and life-changing.
Genitosensory, motor, and autonomic control of orgasm
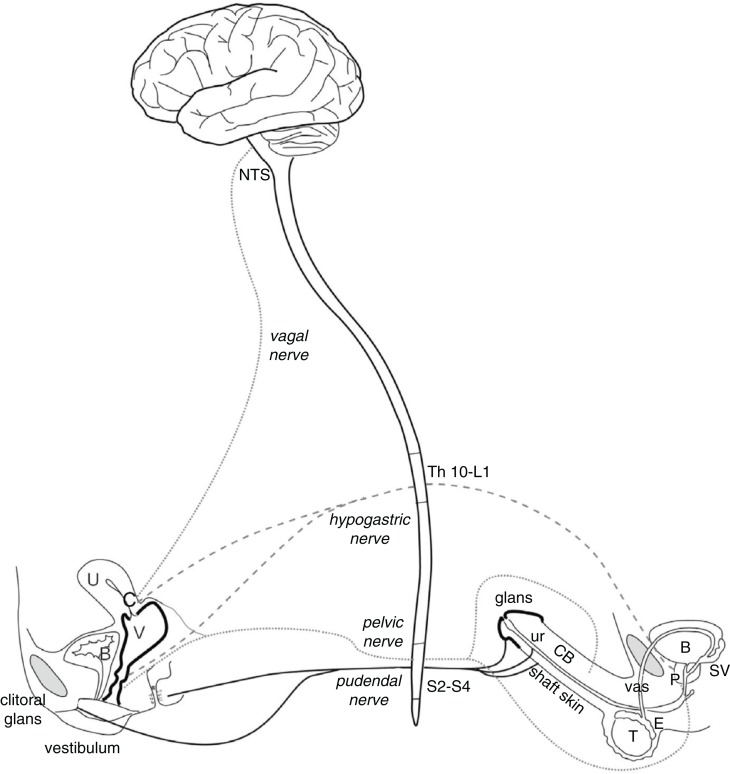
Orgasms are controlled by the autonomic nervous system (ANS) and spinal cord, and ultimately processed in the brain (Komisaruk, Beyer-Flores, & Whipple, 2006). They can be defined and observed objectively as a spinal reflex that results in rhythmic muscle contractions of the pelvic floor and anus, and a urethrogenital reflex often coincident with seminal emission (controlled by the hypogastric nerve) and ejaculation (controlled by the pudendal nerve) in men (Giuliano, 2011; McKenna, 1999b; McKenna, Chung, & McVary, 1991) and contractions of the uterus and cervix in women (Meston, Levin, Sipski, Hull, & Heiman, 2004). Stimulation of the glans and shaft of the erect penis also give rise to pelvic floor muscle contractions as orgasm nears. Stimulation of the vagina and erect clitoris induces pelvic floor muscle contractions (Shafik, 1995; Shafik, El-Sibai, Mostafa, Shafik, & Ahmed, 2005) that appear to increase in duration and intensity as orgasm approaches. The glans penis and external clitoris are homologous structures, and tactile stimulation activates similar sensory nerves (e.g. dorsal clitoral and penile nerves) that course into the pudendal nerve, which enters the spinal cord at the sacral divisions S2–S4 (Fig. 1). The pudendal nerve is a mixed sensory and motor nerve that serves the entire sacral plexus. In addition to the clitoris and penis, the pudendal nerve receives sensory inputs from labia, scrotum, and anus. Its motor division controls contractions of the external urethral and anal sphincters along with the rest of the pelvic floor muscles. The pelvic nerve controls contractions of the internal urethral and anal sphincters and is responsible for erection of the clitoris and penis. Stimulation of the hypogastric nerve activates the sympathetic outflow responsible for orgasm. In turn, the pelvic nerve carries sensory information from the cervix and pelvic floor muscle contractions during orgasm. Input from the cervix is likely also carried by the vagus nerve, which bypasses the spinal cord and sends sensory input directly to the medulla in the brainstem (Komisaruk, Beyer-Flores, & Whipple, 2006; Komisaruk & Whipple, 2005). These nerves thus subserve the genitosensory input to the spinal cord and brain that are processed ultimately as genital swelling, sexual stimulation, and orgasm (Fig. 2). In male rats, a set of spinothalamic neurons in the lower lumbar spinal cord (LSt neurons) has been identified that act as an ejaculation generator, coordinating inhibitory and excitatory outflow from brainstem and hypothalamic nuclei (notably nucleus paragigantocellularis, paraventricular (PVN) nucleus, and medial preoptic area (mPOA)) to control sympathetic, parasympathetic, and motor actions (Coolen et al., 2004; Truitt & Coolen, 2002). It is not known to what extent these neurons play a role in orgasm relative to the control of ejaculation, as ejaculation can occur without corresponding orgasm in men (McKenna, 1999b).
Fig. 1.
Nerves (pudendal, pelvic, hypogastric, and vagus) that subserve sexual arousal and orgasm in women and men. B: bladder. C: cervix. CB: corpora spongiosum of the bulbocavernosus. E: epididymis. NTS: nucleus of the solitary tract (brainstem). P: prostate. SV: seminal vesicle. T: testis. U: uterus. ur: urethra. V: vagina. vas: vas deferens. Adapted from Georgiadis, et al. (2012).
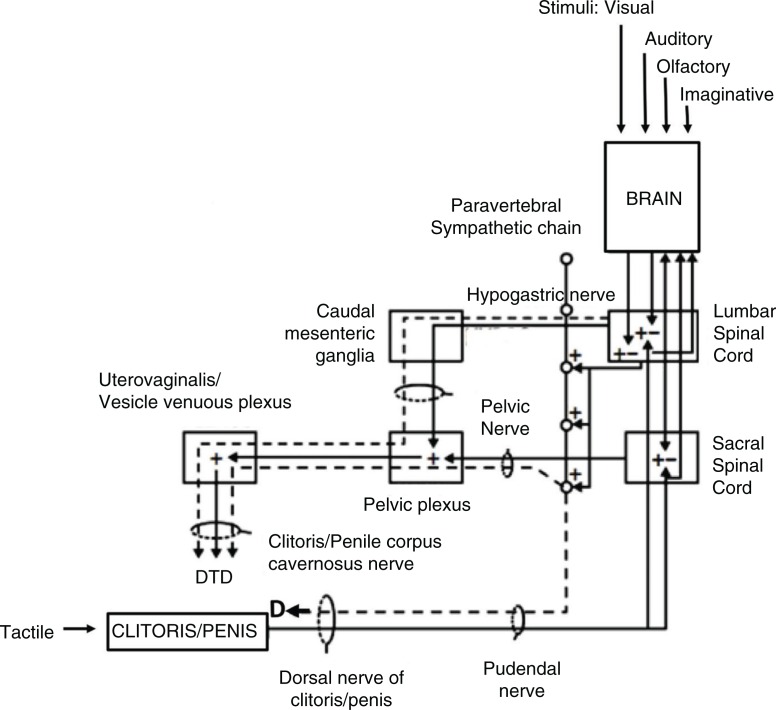
Fig. 2.
Schematic diagram of the spinal and supraspinal regulation of tumescence (T) and detumescence (D) of the clitoris and penis. Dashed lines are excitatory and solid lines inhibitory for genital blood flow. After De Groat and Steers (1998). LSt neurons that comprise the ejaculation generator in males are located in the lower lumbar cord (see Coolen et al., 2004).
Neuroanatomical and neurochemical correlates of orgasm
Orgasms produce a similar signature of brain activation in women and men undergoing functional magnetic resonance imaging (fMRI) scans, including deactivations of left ventromedial and orbitofrontal cortices, and activations of anterior cingulate, insula, parietal lobe, hippocampus, amygdala, basal ganglia (especially the putamen), nucleus accumbens (NAc), bed nucleus of the stria terminalis-preoptic area, hypothalamic PVN nucleus, cerebellum (including the anterior lobe of the cerebellar vermis and deep cerebellar nuclei), and lower brainstem (central gray, mesencephalic reticular formation, and nucleus of the solitary tract), with a slight bias for more activation of the central gray in men (Bianchi-Demicheli & Ortigue, 2007; Georgiadis, Kringelbach, & Pfaus, 2012; Georgiadis, Reinders, Paans, Renken, & Kortekaas, 2009; Komisaruk & Whipple, 2005; Stoléru, Fonteille, Cornélis, Joyal, & Moulier, 2012). Using positron emission tomography to analyze regional cerebral blood flow, Huynh, Willemsen, Holstege et al. (2013) found a greater activation of the pituitary following masturbation to orgasm in women relative to men. In another study, similar blood flow to the right ventrolateral pedunculopontine nucleus of the brainstem accompanied ejaculation in men and CLS -induced orgasm in women, with activation patterns corrected for head and facial movements made during ‘faked’ orgasm (Huynh, Willemsen, Lovick, & Holstege, 2013).
Multiple neurotransmitter systems, including endogenous opioids, serotonin, and endocannabinoids, are involved in the subjective feelings of pleasure, satiety, and sedation common to orgasms and their aftermath (Pfaus, 2009). In addition, plasma oxytocin and prolactin levels rise in both men and women during and following orgasm (Krüger et al., 2003, 2006; Murphy, Checkley, Seckl, & Lightman, 1990). The euphoric pleasure of orgasm is likely induced by opioids acting at µ-opioid receptors in different limbic and hypothalamic structures (Ågmo & Paredes, 1988; Pfaus & Gorzalka, 1987; Parada, Sparks, Censi, & Pfaus, 2014). Indeed, heroin addicts describe the rush of euphoria they experience upon injecting heroin in sexual terms, often equating it with orgasm (Chessick, 1960; Pfaus & Gorzalka, 1987). Accordingly, administration of the opioid receptor antagonist naloxone to men blunts the pleasure of orgasm and eliminates the oxytocin release associated with it (Murphy et al., 1990). In addition to the opioid contribution, orgasm depends critically on sympathetic activation, and there appear to be individually defined optimality curves for orgasm across increasing levels of arousal. For example, plasma levels of adrenaline and noradrenaline peak at orgasm and then fall precipitously after, perhaps giving rise to the subjective experience of a ‘release’ of tension and arousal that built up during sexual interaction. Furthermore, drugs or situations that reduce sympathetic tone can delay or abolish orgasm, whereas drugs or situations that increase sympathetic tone can either increase the magnitude or number of orgasms, although such increases must be specific to, or associated with, sexual interaction (Pfaus et al., 2010). However, if stressors are of sufficient magnitude, then the likelihood of becoming sexually aroused is reduced, making sexual behavior and orgasm unlikely to impossible (Barlow, 1986; Both, Everaerd, & Laan, 2003; Magariños & Pfaff, 2016).
Are orgasms unique to humans?
The study of sexual behavior in animals, mostly rodents and non-human primates, has provided analogies and homologies of human sexual behavior and revealed much about underlying physiological processes that subserve sexual arousal and desire, and to a lesser extent sexual reward and inhibition, that simply could not be obtained from humans (Ågmo, Turi, Ellingsen, & Kaspersen, 2004; Aragona & Wang, 2004; Frohmader, Pitchers, Balfour, & Coolen, 2010; Pfaus, Kippin, & Coria-Avila, 2003; Pfaus et al., 2010, 2012; Wallen, 1995). Accordingly, these models are used widely in sexual and reproductive medicine (Chianese et al., 2011; Giuliano et al., 2010; McMurray, Casey, & Naylor, 2006; Olivier et al., 2006; Pattij, Olivier, & Waldinger, 2005). Mechanisms of erection, ejaculation, and the post-ejaculatory refractory period have been studied in detail in rodents and other species (e.g. Chen, Chan, & Chang, 1999; Giuliano & Rampin, 2004; Levin, 2009; Newman, Reiss, & Northup, 1982). Ejaculation in a variety of species has been viewed as synonymous with sexual ‘climax’ (Fox & Fox, 1971), and indeed the autonomic and neurochemical mechanisms that control erection, seminal emission, and ejaculation are virtually identical in males of a variety of mammalian species, including humans. This begs a question: Can we assume that a male rat or rhesus macaque experiences the pleasure of ejaculation as we experience an orgasm? It may be more accurate to refer to this as an ‘orgasm-like response’ (OLR). Likewise, during copulation, female macaques can display intense tonic/clonic uterine contractions and sudden increases in heart rate coincident with a male's ejaculation (Goldfoot, Westerborg-van Loon, Groeneveld, & Slob, 1980; Slob, Groeneveld, & van der Werff ten Bosch, 1986; Troisi & Carosi, 1998; Zumpe & Michael, 1968), although this response is not always observed. During this time, some female macaques also open their mouths and tense their facial muscles in what Slob et al. referred to as ‘climax face’. Do female macaques or females of other species experience OLRs in response to sufficient copulatory stimulation? And if so, how could we know?
One advantage of studying orgasms in humans is that people can provide a subjective verbal assessment of their feelings that can be contrasted with physiological correlates. However, this comes with a caution: at best, humans do not have a common way to scale orgasm quality or intensity (despite available validated rating scales, like that of Mah and Binik (2002), which researchers doing objective assessments have not utilized); at worst, they lie. Moreover, it is virtually impossible to study human orgasms in any kind of natural environment without necessarily altering the context. And of course, we cannot study first experiences of orgasm except as Kinsey and colleagues did in the subjective retrospective of their participants.
Inferring subjective states from objective behaviors
Obviously, we do not know what it feels like to be another animal (Nagel, 1974). In fact, our empathy and intuition take us only so far in inferring the feelings of other people. For example, to someone who is sexually active but has never experienced an orgasm, the tales of those that have multiple orgasms must seem outlandish if not also depressing. However, consistent with Dennett (1991), if an orgasm could be distilled into observable (and testable) components, it would be easier to make quantitative determinations of differences in expression that might reflect lawful differences in qualitative experience. What might those components be? A hint is given from the work of Mah and Binik (2002).
In developing their adjective-based rating scale, Mah and Binik (2002) examined factor loadings and structure among a large set of words used by women and men to describe their subjective experience of orgasm from masturbation and copulation. These terms were then rated by several hundred university-age women and men, and then organized by principal components analysis into 12 factors. Further refinement was done by collapsing factors that reflected essentially the same phenomena into a two-factor model that encompassed sensory and cognitive/affective dimensions of the experience of orgasm. This model accounted for 73–84% of the intersubject variance across all measures. The sensory dimension consisted of terms that described sensations of building, flooding, flushing, shooting, throbbing, and muscle spasms. The cognitive–affective dimension described ecstasy, pleasurable and satisfying sensations, relaxation, and emotional intimacy. These dimensions appear to correspond to three components: (1) physical sensations generated by muscle contractions and sympathetic activation, (2) a cognitive/affective sense of ecstasy and pleasure, and (3) the relaxation/intimacy that corresponds to a longer-term refractory period. If we distill these components further into what might be observed in humans or other animals, we can derive at least three phenomena that could characterize an OLR:
Physiological changes, including pelvic floor and anal muscle contractions that stimulate seminal emission and/or ejaculation in the male, or that stimulate uterine and cervical contractions in the female. These contractions should occur in temporal synchrony with sympathetic activation and/or bloodflow from erectile tissues;
Short-term behavioral changes that reflect immediate awareness of a pleasurable hedonic reward state. These include measurable vocalization patterns, rejection of further genitosensory stimulation, and/or behavioral relaxation or quiescence, during and/or immediately after the OLR; and
Long-term behavioral changes that depend on the reward state induced by the OLR. These include an identifiable state of sexual satiety, the strengthening of patterns of sexual arousal and desire in subsequent copulations, and the generation of conditioned place and partner preferences for contextual and partner-related cues associated with the reward state.
How well these criteria might be applied to rats?
Copulatory responses in rats
To understand the potential expression of OLRs in a rat model, it is necessary to consider the form and patterning of rat copulatory behavior. Copulation in rats is initiated and controlled by females, who enforce or ‘pace’ the multiple mounts, vaginal intromissions, and ejaculations they receive from males (Madlafousek & Hliňák 1978; McClintock, 1984; Pearce & Nuttall, 1961; Pfaus, Jones, Flanagan-Cato, & Blaustein, 2015). Sexually receptive females actively solicit sexual interaction with males by making a headwise orientation to the male followed by an abrupt runaway. This often entices the male to chase them, after which the female holds a pre-lordosis crouch. Males mount with flank palpation, further increasing the lordosis posture of the female, allowing vaginal penetration as the male intromits with brief pelvic thrusts that typically last less than a second. If vaginal penetration has occurred, the male dismounts abruptly and grooms his erect penis into detumescence. The female typically runs away a short distance, and either hops and darts around the male or makes another full solicitation. Hopping and darting typically entices the male to mount again and is used as a proximal solicitation, especially if the male is not chasing or if the female does not have enough room in a testing environment to make a full solicitation with a longer runaway. Finally, stimulation of the cervix results in an inhibitory state. Indeed, in pacing chambers, females typically begin to fight with males after they receive approximately four ejaculatory series and display decreased sexual solicitations and hops and darts (Coopersmith, Candurra, & Erskine, 1996; Pfaus, Smith, & Coopersmith, 1999). This fighting behavior enforces longer and longer intervals between intromissions. Similarly, in large open fields with attached burrow systems, females typically take themselves out of the copulations after a smaller number of ejaculations compared with small chambers (McClintock, 1984). Collectively, these patterns of behavior reflect an ‘estrous termination’ that is dependent on the amount of vaginocervical stimulation (VCS) received by the female (Pfaus, Smith, Byrne, & Stephens, 2000) and corresponding activation of the pelvic nerve (Lodder & Zeilmaker, 1976; Pfaus, Manitt, & Coopersmith, 2006), which in turn activates a glutamate system in the ventromedial hypothalamus (VMH) that inhibits appetitive sexual behavior (Georgescu, Cyr, & Pfaus, 2012; Georgescu, Sabongui, Del Corpo, Marsan, & Pfaus, 2009). Ejaculation by males results in a post-ejaculatory refractory period, after which males resume copulating with the female toward another ejaculation. This can repeat through approximately 6–10 successive ejaculatory series until the male is ‘sexually exhausted’ (Beach & Jordan, 1956) and cannot engage in full copulatory responses for approximately 3–4 days (Rodriguez-Manzo, 1999; Rodríguez-Manzo & Fernández-Guasti, 1994, 1995).
Male responses to copulatory stimulation
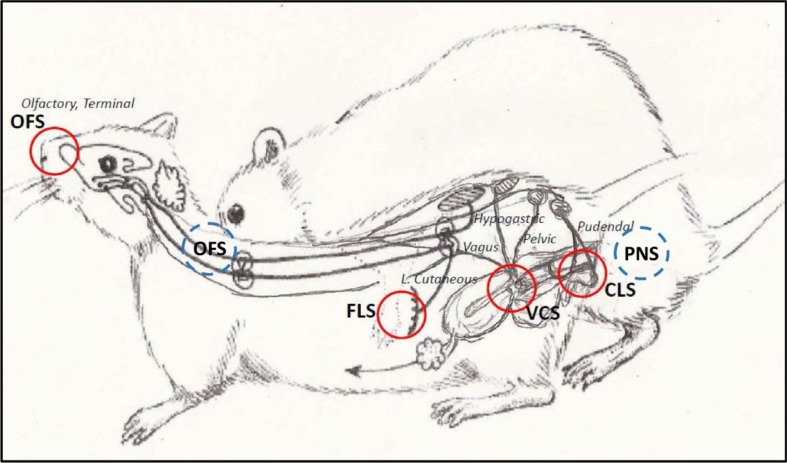
In response to female solicitations, male rats mount, intromit, and ejaculate, and males have relatively stable individual patterns of these behaviors (Pattij et al., 2005). In terms of sensory stimulation, males receive both olfactory stimulation from the odors/pheromones of the female along with penile stimulation from intromissions and ejaculations (Fig. 3). Deprivation of sensory stimulation from the penis by topical anesthesia or deafferentation results in males that mount at a higher rate than males given a control treatment but cannot intromit or ejaculate (Gray, Davis, & Dewsbury, 1976; Lodder, 1976). Deprivation of olfactory information, either from destruction of the olfactory epithelium by zinc sulfate, or bulbectomy, abolishes copulatory behavior in males (Carr, Loeb, & Dissinger, 1965; Thor & Flannelly, 1977). However, these disruptions of copulatory behavior do not occur if male rats have sufficient sexual experience (Pfaus et al., 2012), suggesting that sexual experience crystallizes and automates copulatory patterns in males. Males are also aware of their sexual state during copulation. For example, prior to each mount with intromission, males emit a short ultrasonic call in the 50 kHz range (McIntosh, Barfield, & Thomas, 1984; White, Cagiano, Moises, & Barfield, 1990). These precontact vocalizations increase in frequency as males learn to associate the ejaculatory reward state with their pre-ejaculatory copulatory behavior (Bialy, Rydz, & Kaczmarek, 2000; McGinnis & Vakulenko, 2003). These vocalizations are eliminated after castration and restored with exogenous androgen (Harding & Velotta, 2011). Immediately after ejaculation, males emit a long 22 kHz vocalization which corresponds to their period of behavioral quiescence (Burgdorf et al., 2008; McIntosh, Barfield, & Thomas, 1984; White et al., 1990).
Fig. 3.
Sensory stimulation of female (red) and male (blue) rats during sexual interaction. OFS: olfactory stimulation. FLS: flank (tactile) stimulation. VCS: vaginocervical stimulation. CLS: clitoral stimulation. PNS: penile stimulation.
Female responses to copulatory stimulation
Pacing is facilitated for females in large open fields, bilevel chambers (where she can run from level to level), or in unilevel chambers bisected by a Plexiglas partition with holes at the bottom that allow the female – but not the male – to cross from side to side. Females show regular patterns of pacing in these three testing environments. For example, in unilevel chambers with a partition, females display different latencies to return to the male (called ‘contact return latencies’) depending on the preceding copulatory stimulation (short if mounted, longer if intromitted, and longest if she received an ejaculation; Erskine, 1985). These observations bolstered the notion that females enforce an optimal timing between intromissions that leads to optimal timing for the induction of a progestational state (Adler, 1978). If females are tested in small chambers that do not allow efficient pacing, they are more likely to display defensive responses such as rearing or boxing to enforce intervals between mounts, intromissions, and ejaculations. They are also less likely to get pregnant (Frye & Erskine, 1990), suggesting that female rats have a similar ‘vaginal code’ to that first observed in female mice by Diamond (1970).
During copulation, females receive olfactory stimulation from male odors and pheromones, somatosensory stimulation from the flanks (FLS) where males palpate, CLS from pelvic thrusts, and VCS from penile intromissions, which results in the seminal plug after ejaculation (Fig. 3), in addition to the cognitive–affective sense of control given the pacing conditions. Interestingly, VCS alone does not activate LSt neurons in the lower lumbar spinal cord that act in males as the spinal ejaculation generator (Truitt, Shipley, Veening, & Coolen, 2003). However, deprivation of sensory input from the clitoris and cervix through topical anesthesia resulted in shorter contact return latencies in unilevel pacing chambers following intromissions and ejaculations (Meerts, Strnad, & Schairer, 2015). This effect was also observed after transection of the pelvic nerve alone (Meerts & Clark, 2009). In contrast, chronic anesthesia of the external gland of the clitoris alone had the opposite effect in which females had a greater number of exits from and entrances to the male compartment but spent less time with the male (Parada et al., 2013). Females also emit ultrasonic vocalizations in the ‘appetitive’ 50 kHz range during copulation, especially as they approach males (White, Colona, & Barfield, 1991). These calls may be in response to male odor (Matochik, Barfield, & Nyby, 1992) or to the anticipation of rewarding copulatory contact. The calls are displayed reliably in females during proestrus/estrus, are eliminated by ovariectomy, and restored by hormone treatment that restores sexual receptivity (Matochik et al., 1992).
Paced copulation induces more vigorous mounts and intromissions by the male (Erskine, Kornberg, & Cherry, 1989), which result in stronger CLS and VCS from the male's pelvis and penis, respectively, during each mount with intromission and pelvic thrusting.
Together, these data suggest that genitosensory stimulation of the penis or clitoris induce a pleasurable state that males and females are aware of in terms of appetitive calls, and which they attempt to compensate for behaviorally if the stimulation is reduced by pharmacological agents or lesions to specific nerves.
Effects of repeated pleasurable copulatory stimulation
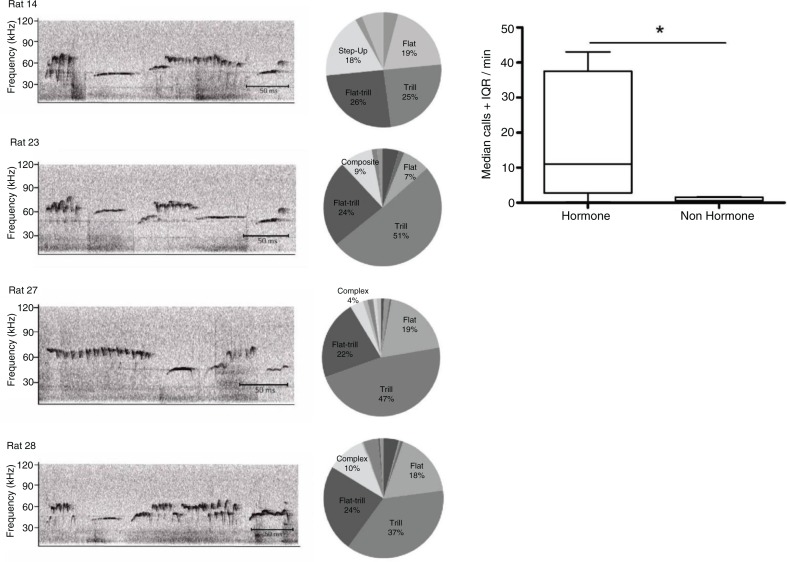
Copulation in both male and female rats also induces a reward state that can be associated by Pavlovian processes with contextual- or partner-related cues to form long-term conditioned place preferences (CPPs) or conditioned partner preferences. CPP is often demonstrated in an apparatus with two distinctive compartments that are connected to either side of a third neutral compartment. During training, the compartments are paired sequentially with different unconditional stimuli, (e.g. one side is paired with the reward state induced by copulation with a sex partner, eating food, or a drug with abuse potential, whereas the other side is paired with either nothing or a control manipulation). In the final test, the animal is placed into the neutral compartment between the two distinctive compartments, with the two doors on either side opened to allow free access to either compartment. CPP is said to have developed if the rat spends significantly more time in the reward-paired compartment than the other compartment. Sexual CPP is observed when male rats are placed into a distinctive compartment of the CPP box after ejaculation, in contrast to being placed into the other compartment after mounts or intromissions alone (Ågmo & Berenfeld, 1990; Everitt, 1990; Hughes, Everitt, & Herbert, 1990; Mehara & Baum, 1990). However, intromissions alone can maintain copulatory CPPs in males that have not yet experienced ejaculation (Hughes et al, 1990; Tenk, Wilson, Zhang, Pitchers, & Coolen, 2009). For females, sexual CPP is achieved by pairing either paced copulation or non-paced copulation with one of two distinct environments (Afonso, Woehrling, & Pfaus, 2006; Paredes & Alonso, 1997; Paredes & Vazquez, 1999). CPP can also be induced by CLS alone (Parada, Chamas, Censi, Coria-Avila, & Pfaus, 2010). We have mimicked the CLS females receive from males by applying it to the clitoris directly with a #4 camelhair paintbrush (Parada et al., 2010; Parada, Abdul-Ahad, Censi, Sparks, & Pfaus, 2011). CLS applied in intervals to mimic its distribution during paced copulation induces a strong CPP relative to continuous CLS or a control condition in which the female's tail is raised but no CLS is applied. Interestingly, the CPP induced by CLS is independent of hormone administration to ovariectomized females (Parada, Vargas, Kyres, Burnside, & Pfaus, 2012). CLS induces immediate 50 kHz vocalizations that follow individual trill-flat patterns (Fig. 4). These patterns are emitted in response to distributed but not continuous stimulation and are maximal in females that are fully sexually motivated and receptive. Thus, like male 50 kHz vocalizations, females appear to be aware of timed CLS and respond to it in a way consistent with the induction of a reward state.
Fig. 4.
Ultrasonic vocalizations made by female rats in response to distributed CLS. Left side depicts raw calls. Middle depicts the proportion of total calling during CLS taken by flats, trills, flat-trills, step-ups, and compound calls. Right side depicts median calls in females that receive full hormone priming with estradiol benzoate and progesterone versus no hormone (oil vehicle). *P<0.01.
In another group of studies, we have shown that placement of a neutral odor, such as almond or lemon, on a vigorous and receptive sex partner can be paired with the reward state induced by first experiences of ejaculation in males or paced copulation in females. Accordingly, these odors become discrete, partner-related, conditioned stimuli that direct a Pavlovian conditioned partner and mate preference in females (Coria-Avila, Ouimet, Pacheco, Manzo, & Pfaus, 2005; Coria-Avila & Pfaus, 2007) and conditioned ejaculatory preferences in males (Ismail, Gelez, Lachapelle, & Pfaus, 2009; Kippin, Talianakis, Schattmann, Bartholomew, & Pfaus, 1998; Kippin & Pfaus, 2001a, 2001b). Typically, these preferences are formed by paired copulation in bilevel or unilevel pacing chambers and then tested in a large open field with two potential sex partners, one scented and the other unscented. Unpaired controls who received repeated pairing with an unscented partner show a slightly weaker conditioned preference for the unscented partner on the final open-field test, and random-paired controls do not show any conditioning. Strain cues from pigmented versus non-pigmented partners also induce significant partner preferences in both females (Coria-Avila et al., 2006) and males (Ismail, Jones, Graham, Sylvester, & Pfaus, 2011). Interestingly, a female rat given her first paced copulatory experience with one particular male displays conditioned mate-guarding behavior in the presence of that male and a competitor female (Holley et al., 2015; Holley, Shalev, Bellevue, & Pfaus, 2014), a behavior that she does not display if the male is novel or there is no competition for him. These behaviors do not depend on an olfactory cue per se, instead are associated with more ‘pheromonal’ cues (e.g. major histocompatibility complexes) from the familiar male.
The reward state induced by ejaculation or paced copulation is dependent on the release of endogenous opioids in key limbic and hypothalamic brain regions (Paredes, 2014; Pfaus et al., 2012; van Furth, van Emst, & van Ree, 1995; van Furth, Wolterink, & van Ree, 1995). Repeated ejaculations increase whole-brain endorphin content in male rats (Szechtman, Hershkowitz, & Simantov, 1981). Indeed, opioid activation may also mediate the ability of orgasms in humans, and ejaculation in rats, to reduce anxiety and dysphoria (Fernández-Guasti, Roldán-Roldán, & Saldívar, 1989; McCarthy, 1977; Pfaus & Wilkins, 1995; Rodríguez-Manzo, López-Rubalcava, & Fernández-Guasti, 1999). Systemic treatment with the opioid antagonist naloxone, but not dopamine antagonists, during training abolishes the development of CPP and conditioned partner/ejaculatory preferences in both female and male rats (Ågmo & Berenfeld, 1990; Coria-Avila et al., 2008; Ismail, Girard-Bériault, Nakanishi, & Pfaus, 2009; Mehara & Baum, 1990; Paredes & Martinez, 2001). In fact, female rats given their first few sexual experiences under the influence of systemic naloxone lose their desire for sex, as indicated by the loss of solicitations and lordosis, and the induction of defensive responses when injected subsequently with saline (Pfaus et al., 2012). In males, the conditioned ejaculatory preference for the odor is shifted from the familiar scented female to unfamiliar unscented female if naloxone is infused to the mPOA during training, whereas it is abolished altogether if naloxone is infused to the ventral tegmental area (VTA) or NAc during conditioning (Quintana et al., in preparation). Similarly, infusions of naloxone into the mPOA abolish conditioning of CPP in both male (Ågmo & Gomez, 1993) and female (García-Horsman, Ågmo, & Paredes, 2008) rats. Naloxone was also effective in eliminating pacing-related CPP following infusions to the VMH or medial amygdala but not the NAc (García-Horsman et al., 2008). This suggests that the mPOA (and related hypothalamic structures such as the PVN and supraoptic (SON) nuclei) links the reward to particular partner-related cues, whereas the VTA and NAc are involved in the general attending to reward-related cues in males but not necessarily in females. Opioids are known to sensitize dopamine cell activity in the VTA, which results in sensitized release of dopamine in the NAc and elsewhere during reward-seeking behavior (Koob et al., 2014; Kornetsky, 2004; Spanagel, 1995). Opioid actions are also likely responsible for the sensitized activation of oxytocin and vasopressin neurons in the PVN and SON when conditioned rats are in the presence of partner-related cues (Holley et al., 2015).
The case for OLRs in rats
Both male and female rats display physiological and behavioral changes consistent with the induction of OLRs during sexual interaction. They are clearly aware of the sexual stimuli they receive and behave in ways to maximize the reward received by them. The urethrogenital ejaculatory reflexes of male rats, and the cervicouterine reflexes of female rats, depend on pelvic floor muscle contractions that are experienced at discrete intervals during sex. Responses made to those physiological events include both the induction of short- and long-term inhibitory states for further sexual stimulation, such as refractory periods in males, pacing behaviors by females, along with sexual exhaustion and estrous termination. Longer-term sensitization of contextual- and partner-related cues also occurs that is associated with the induction of a hedonically positive, opioid-induced sexual reward state. This reward state augments the activation of excitatory neurochemical systems, such as dopamine, noradrenaline, oxytocin, and vasopressin (Pfaus, 2009), that drive sexual arousal, CPP, and partner/mate preference in the appropriate circumstances. Thus, female and male rats meet the criteria set forth for OLRs, and they do so with a rich repertoire of behaviors to study beyond copulatory responses alone. Although we can never know if rats experience OLRs the same way we experience orgasms, it is notable that these cognitively mediated behaviors reflect both evaluation and expectation of sexual pleasure or reward. This places them squarely into the realm of responses studied by human sex researchers as a function of sexual pleasure (Georgiadis et al., 2012). In turn, the ability to define OLRs opens new possibilities to study the effects of sexual reward and non-reward, and the manner in which arousal, sexual disinhibition, and other developmental, situational, or partner-related variables might enhance the experience of sexual pleasure from a comparative and translational perspective.
Acknowledgements
Research from our laboratory was supported by operating grants from the Canadian Institutes for Health Research (MOP-111254), Natural Sciences and Engineering Research Council of Canada (OGP-0138878), and an infrastructure grant from Fonds de la Recherche en Santé du Québec (FRSQ Groupe de Recherche) to the Center for Studies in Behavioral Neurobiology at Concordia University. JGP is indebted to Drs. William J. Jacobs, Barry R. Komisaruk, Beverly Whipple, Janniko R. Georgiadis, Frederick Toates, Frédérique Courtois, Tilman Krüger, Barry Everitt, Ben Sachs, Stan Althof, Ken Mah, Yitzchak Binik, Marcel Waldinger, William Fisher, Michael Sand, the late Sandra Leiblum, Ron Barfield, and Carlos Beyer, for many inspiring and fruitful discussions.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- Adler N. T. On the mechanisms of sexual behavior and their evolutionary constraints. In: Hutchison J. B, editor. Biological determinants of sexual behavior. Chichester: Wiley; 1978. pp. 657–695. [Google Scholar]
- Afonso V. M, Woehrling A, Pfaus J. G. Sensory mediation of female–male mounting in the rat: I. Role of olfactory cues. Physiology and Behavior. 2006;87:857–862. doi: 10.1016/j.physbeh.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Ågmo A, Berenfeld R. Reinforcing properties of ejaculation in the male rat: The role of opioids and dopamine. Behavioral Neuroscience. 1990;104:177–182. doi: 10.1037//0735-7044.104.1.177. [DOI] [PubMed] [Google Scholar]
- Ågmo A, Gomez M. Sexual reinforcement is blocked by infusion of naloxone into the medial preoptic area. Behavioral Neuroscience. 1993;107:812–818. doi: 10.1037//0735-7044.107.5.812. [DOI] [PubMed] [Google Scholar]
- Ågmo A, Paredes R. G. Opioids and sexual behavior in the male rat. Pharmacology, Biochemistry, and Behavior. 1988;30:1021–1034. doi: 10.1016/0091-3057(88)90135-9. [DOI] [PubMed] [Google Scholar]
- Ågmo A, Turi A. L, Ellingsen E, Kaspersen H. Preclinical models of sexual desire: Conceptual and behavioral analyses. Pharmacology, Biochemistry, and Behavior. 2004;78:379–404. doi: 10.1016/j.pbb.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Aragona B. J, Wang Z. The prairie vole (Microtus ochrogaster): An animal model for behavioral neuroendocrine research on pair bonding. ILAR Journal. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- Barlow D. H. Causes of sexual dysfunction: The role of anxiety and cognitive interference. Journal of Consulting and Clinical Psychology. 1986;54:140–148. doi: 10.1037//0022-006x.54.2.140. [DOI] [PubMed] [Google Scholar]
- Beach F. A, Jordan L. Sexual exhaustion and recovery in the male rat. Quarterly Journal of Experimental Psychology. 1956;8:121–133. [Google Scholar]
- Bialy M, Rydz M, Kaczmarek L. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behavioral Neuroscience. 2000;114:983–990. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- Bianchi-Demicheli F, Ortigue S. Toward an understanding of the cerebral substrates of women's orgasm. Neuropsychologia. 2007;45:2645–2659. doi: 10.1016/j.neuropsychologia.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Both S, Everaerd W, Laan E. Modulation of spinal reflexes by aversive and sexually appetitive stimuli. Psychophysiology. 2003;40:469–476. doi: 10.1111/1469-8986.00019. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes R. A, Moskal J. R, Pfaus J. G, Brudzynski S. M, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. Journal of Comparative Psychology. 2008;122:357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- Carr W. J, Loeb L. S, Dissinger M. L. Responses of rats to sex odors. Journal of Comparative and Physiological Psychology. 1965;59:370–377. doi: 10.1037/h0022036. [DOI] [PubMed] [Google Scholar]
- Chen K. K, Chan J. Y, Chang L. S. Dopaminergic neurotransmission at the paraventricular nucleus of hypothalamus in central regulation of penile erection in the rat. Journal of Urology. 1999;162:237–242. doi: 10.1097/00005392-199907000-00074. [DOI] [PubMed] [Google Scholar]
- Chessick R. D. The ‘pharmacogenic orgasm’ in the drug addict. Archives of General Psychiatry. 1960;3:545–556. doi: 10.1001/archpsyc.1960.01710050095010. [DOI] [PubMed] [Google Scholar]
- Chianese R, Chioccarelli T, Cacciola G, Ciaramella V, Fasano S, Pierantoni R, et al. The contribution of lower vertebrate animal models in human reproduction research. General and Comparative Endocrinology. 2011;171:17–27. doi: 10.1016/j.ygcen.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Coolen L. M, Allard J, Truitt W. A, McKenna K. E. Central regulation of ejaculation. Physiology & Behavior. 2004;83:203–215. doi: 10.1016/j.physbeh.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Coopersmith C, Candurra C, Erskine M. S. Effects of paced mating and intromissive stimulation on feminine sexual behavior and estrus termination in the cycling rat. Journal of Comparative Psychology. 1996;110:176–186. doi: 10.1037/0735-7036.110.2.176. [DOI] [PubMed] [Google Scholar]
- Coria-Avila G. A, Jones S. L, Solomon C. E, Gavrila A. M, Jordon G. L, Pfaus J. G. Conditioned partner preference in female rats for strain of male. Physiology and Behavior. 2006;88:529–537. doi: 10.1016/j.physbeh.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Coria-Avila G. A, Ouimet A. J, Pacheco P, Manzo J, Pfaus J. G. Olfactory conditioned partner preference in the female rat. Behavioral Neuroscience. 2005;119:716–725. doi: 10.1037/0735-7044.119.3.716. [DOI] [PubMed] [Google Scholar]
- Coria-Avila G. A, Pfaus J. G. Neuronal activation by stimuli that predict sexual reward in female rats. Neuroscience. 2007;148:623–632. doi: 10.1016/j.neuroscience.2007.05.052. [DOI] [PubMed] [Google Scholar]
- Coria-Avila G. A, Solomon C. E, Barbosa Vargas E, Lemme I, Ryan R, Ménard S, et al. Neurochemical basis of conditioned partner preference in the female rat: I. Disruption by naloxone. Behavioral Neuroscience. 2008;122:385–395. doi: 10.1037/0735-7044.122.2.385. [DOI] [PubMed] [Google Scholar]
- De Groat W. C, Steers W. D. Neuroanatomy and neurophysiology of penile erection. In: Tanagho E. A, Lue T. F, McLure R. D, editors. Contemporary management of impotence and infertility. Baltimore, MD: Williams and Wilkins; 1998. pp. 3–27. [Google Scholar]
- Dennett D. C. Consciousness explained. Boston, MA: Little, Brown; 1991. [Google Scholar]
- Diamond M. Intromission pattern and species vaginal code in relation to induction of pseudopregnancy. Science. 1970;169:995–997. doi: 10.1126/science.169.3949.995. [DOI] [PubMed] [Google Scholar]
- Erskine M. S. Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized-adrenalectomized hormone-primed rats. Behavioral Neuroscience. 1985;99:151–161. doi: 10.1037//0735-7044.99.1.151. [DOI] [PubMed] [Google Scholar]
- Erskine M. S, Kornberg E, Cherry J. A. Paced copulation in rats: Effects of intromission frequency and duration on luteal activation and estrus length. Physiology & Behavior. 1989;45:33–39. doi: 10.1016/0031-9384(89)90163-7. [DOI] [PubMed] [Google Scholar]
- Everitt B. J. Sexual motivation: A neural and behavioral analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neuroscience and Biobehavioral Reviews. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Fernández-Guasti A, Roldán-Roldán G, Saldívar A. Reduction in anxiety after ejaculation in the rat. Behavioural Brain Research. 1989;32:23–29. doi: 10.1016/s0166-4328(89)80068-3. [DOI] [PubMed] [Google Scholar]
- Fox C. A, Fox B. A comparative study of coital physiology, with special reference to the sexual climax. Journal of Reproduction and Fertility. 1971;24:319–336. doi: 10.1530/jrf.0.0240319. [DOI] [PubMed] [Google Scholar]
- Freud S. Beyond the pleasure principle. Vienna: International Psycho-Analytic Press; 1920/1922. (C. J. M. Hubback, Trans.) [Google Scholar]
- Frohmader K. S, Pitchers K. K, Balfour M. E, Coolen L. M. Mixing pleasures: Review of the effects of drugs on sex behavior in humans and animal models. Hormones and Behavior. 2010;58:149–162. doi: 10.1016/j.yhbeh.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Frye C. A, Erskine M. S. Influence of time of mating and paced copulation on induction of pseudopregnancy in cyclic female rats. Journal of Reproduction and Fertility. 1990;90:375–385. doi: 10.1530/jrf.0.0900375. [DOI] [PubMed] [Google Scholar]
- García-Horsman S. P, Ågmo A, Paredes R. G. Infusions of naloxone into the medial preoptic area, ventromedial nucleus of the hypothalamus, and amygdala block conditioned place preference induced by paced mating behavior. Hormones and Behavior. 2008;54:709–716. doi: 10.1016/j.yhbeh.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Georgescu M, Cyr D, Pfaus J. G. AMPA/kainate receptors in the ventromedial hypothalamus mediate the effects of glutamate on estrus termination in the rat. Pharmacology, Biochemistry, and Behavior. 2012;102:146–150. doi: 10.1016/j.pbb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Georgescu M, Sabongui C, Del Corpo A, Marsan L, Pfaus J. G. Vaginocervical stimulation induces Fos in glutamate neurons in the ventromedial hypothalamus: Attenuation by estrogen and progesterone. Hormones and Behavior. 2009;56:450–456. doi: 10.1016/j.yhbeh.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Georgiadis J, Kringelbach M. L, Pfaus J. G. Sex for fun: A synthesis of human and animal neurobiology. Nature Reviews Urology. 2012;9:486–498. doi: 10.1038/nrurol.2012.151. [DOI] [PubMed] [Google Scholar]
- Georgiadis J. R, Reinders A. A, Paans A. M, Renken R, Kortekaas R. Men versus women on sexual brain function: Prominent differences during genital tactile stimulation, but not during orgasm. Human Brain Mapping. 2009;30:3089–3101. doi: 10.1002/hbm.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano F. Neurophysiology of erection and ejaculation. Journal of Sexual Medicine. 2011;8(Suppl 4):310–315. doi: 10.1111/j.1743-6109.2011.02450.x. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Pfaus J, Srilatha B, Hedlund P, Hisasue S, Marson L, et al. Experimental models for the study of female and male sexual function. Journal of Sexual Medicine. 2010;7:2970–2995. doi: 10.1111/j.1743-6109.2010.01960.x. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Rampin O. Neural control of erection. Physiology & Behavior. 2004;83:189–201. doi: 10.1016/j.physbeh.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Goldfoot D. A, Westerborg-van Loon H, Groeneveld W, Slob A. K. Behavioral and physiological evidence of sexual climax in the female stump-tailed macaque (Macaca arctoides) Science. 1980;208:1477–1479. doi: 10.1126/science.7384791. [DOI] [PubMed] [Google Scholar]
- Gray G. D, Davis H. N, Dewsbury D. A. Masculine sexual behavior in male and female rats following perinatal manipulation of androgen: Effects of genital anesthetization and sexual experience. Hormones and Behavior. 1976;7:317–329. doi: 10.1016/0018-506x(76)90037-4. [DOI] [PubMed] [Google Scholar]
- Harding S. M, Velotta J. P. Comparing the relative amount of testosterone required to restore sexual arousal, motivation, and performance in male rats. Hormones and Behavior. 2011;59:666–673. doi: 10.1016/j.yhbeh.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Holley A, Bellevue S, Vosberg D, Wenzel K, Roorda S, Jr, Pfaus J. G. The role of oxytocin and vasopressin in conditioned mate guarding behavior in the female rat. Physiology & Behavior. 2015;144:7–14. doi: 10.1016/j.physbeh.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Holley A, Shalev S, Bellevue S, Pfaus J. G. Conditioned mate-guarding behavior in the female rat. Physiology & Behavior. 2014;131:136–141. doi: 10.1016/j.physbeh.2014.04.034. [DOI] [PubMed] [Google Scholar]
- Hughes A. M, Everitt B. J, Herbert J. Comparative effects of preoptic area infusions of opioid peptides, lesions and castration on sexual behaviour in male rats: studies of instrumental behaviour, conditioned place preference and partner preference. Psychopharmacology (Berl) 1990;102:243–256. doi: 10.1007/BF02245929. [DOI] [PubMed] [Google Scholar]
- Huynh H. K, Willemsen A. T, Holstege G. Female orgasm, but not male ejaculation, activates the pituitary: A PET-neuroimaging study. Neuroimage. 2013;76:178–182. doi: 10.1016/j.neuroimage.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Huynh H. K, Willemsen A. T, Lovick T. A, Holstege G. Pontine control of ejaculation and female orgasm. Journal of Sexual Medicine. 2013;10:3038–3048. doi: 10.1111/jsm.12300. [DOI] [PubMed] [Google Scholar]
- Ismail N, Gelez H, Lachapelle I, Pfaus J. G. Pacing conditions contribute to the conditioned ejaculatory preference for a familiar female in the male rat. Physiology and Behavior. 2009;96:201–208. doi: 10.1016/j.physbeh.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Ismail N, Girard-Bériault F, Nakanishi S, Pfaus J. G. Naloxone, but not flupenthixol, disrupts the development of conditioned ejaculatory preference in the male rat. Behavioral Neuroscience. 2009;123:992–999. doi: 10.1037/a0017096. [DOI] [PubMed] [Google Scholar]
- Ismail N, Jones S. L, Graham M. D, Sylvester S, Pfaus J. G. Partner preference for strain of female in Long-Evans male rats. Physiology and Behavior. 2011;102:285–290. doi: 10.1016/j.physbeh.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Kinsey A. C, Pomeroy W. B, Martin C. E. Sexual behavior in the human male. Philadelphia, PA: W. B. Saunders; 1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey A. C, Pomeroy W. B, Martin C. E, Gebhard P. H. Sexual behavior in the human femal. Philadelphia, PA: W. B. Saunders; 1953. [Google Scholar]
- Kippin T. E, Pfaus J. G. The development of olfactory conditioned ejaculatory preferences in the male rat: I. Nature of the unconditioned stimulus. Physiology & Behavior. 2001a;73:457–469. doi: 10.1016/s0031-9384(01)00484-x. [DOI] [PubMed] [Google Scholar]
- Kippin T. E, Pfaus J. G. The nature of the conditioned response mediating olfactory conditioned ejaculatory preference in the male rat. Behavioural Brain Research. 2001b;122:11–24. doi: 10.1016/s0166-4328(01)00162-0. [DOI] [PubMed] [Google Scholar]
- Kippin T. E, Talianakis S, Schattmann L, Bartholomew S, Pfaus J. G. Olfactory conditioning of sexual behavior in the male rat (Rattus norvegicus) Journal of Comparative Psychology. 1998;112:389–399. [Google Scholar]
- Komisaruk B. R, Beyer-Flores C, Whipple B. The science of orgasm. Baltimore, MD: The Johns Hopkins Press; 2006. [Google Scholar]
- Komisaruk B. R, Whipple B. Functional MRI of the brain during orgasm in women. Annual Review of Sex Research. 2005;16:62–86. [PubMed] [Google Scholar]
- Koob G. F, Buck C. L, Cohen A, Edwards S, Park P. E, Schlosburg J. E, et al. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt B):370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C. Brain-stimulation reward, morphine-induced oral stereotypy, and sensitization: Implications for abuse. Neuroscience and Biobehavioral Reviews. 2004;27:777–786. doi: 10.1016/j.neubiorev.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Krüger T. H, Haake P, Chereath D, Knapp W, Janssen O. E, Exton M. S, et al. Specificity of the neuroendocrine response to orgasm during sexual arousal in men. Journal of Endocrinology. 2003;177:57–64. doi: 10.1677/joe.0.1770057. [DOI] [PubMed] [Google Scholar]
- Krüger T. H, Schiffer B, Eikermann M, Haake P, Gizewski E, Schedlowski M. Serial neurochemical measurement of cerebrospinal fluid during the human sexual response cycle. European Journal of Neuroscience. 2006;24:3445–3452. doi: 10.1111/j.1460-9568.2006.05215.x. [DOI] [PubMed] [Google Scholar]
- Levin R. J. Revisiting post-ejaculation refractory time–what we know and what we do not know in males and in females. Journal of Sexual Medicine. 2009;6:2376–2389. doi: 10.1111/j.1743-6109.2009.01350.x. [DOI] [PubMed] [Google Scholar]
- Lodder J. Penile deafferentation and the effect of mating experience on sexual motivation in adult male rats. Physiology & Behavior. 1976;17:571–573. doi: 10.1016/0031-9384(76)90153-0. [DOI] [PubMed] [Google Scholar]
- Lodder J, Zeilmaker G. H. Role of pelvic nerves in the postcopulatory abbreviation of behavioral estrus in female rats. Journal of Comparative and Physiological Psychology. 1976;90:925–929. doi: 10.1037/h0077278. [DOI] [PubMed] [Google Scholar]
- Madlafousek J, Hliňák Z. Sexual behavior of the female laboratory rat: Inventory, patterning, and measurement. Behaviour. 1978;63:129–173. [Google Scholar]
- Magariños A. M, Pfaff D. W. Sexual motivation in the female and its opposition by stress. Current Topics in Behavioral Neuroscience. 2016;27:35–49. doi: 10.1007/7854_2015_392. [DOI] [PubMed] [Google Scholar]
- Mah K, Binik Y. M. Do all orgasms feel alike? Evaluating a two-dimensional model of the orgasm experience across gender and sexual context. Journal of Sex Research. 2002;39:104–113. doi: 10.1080/00224490209552129. [DOI] [PubMed] [Google Scholar]
- Masters W. H, Johnson V. E. Human sexual response. Boston, MA: Little, Brown; 1966. [Google Scholar]
- Matochik J. A, Barfield R. J, Nyby J. Regulation of sociosexual communication in female Long-Evans rats by ovarian hormones. Hormones and Behavior. 1992;26:545–555. doi: 10.1016/0018-506x(92)90021-m. [DOI] [PubMed] [Google Scholar]
- McCarthy B. W. Strategies and techniques for the reduction of sexual anxiety. Journal of Sex and Marital Therapy. 1977;3:243–248. doi: 10.1080/00926237708402991. [DOI] [PubMed] [Google Scholar]
- McClintock M. K. Group mating in the domestic rat as a context for sexual selection: Consequences for the analysis of sexual behavior and neuroendocrine responses. Advances in the Study of Behavior. 1984;14:1–50. [Google Scholar]
- McGinnis M. Y, Vakulenko M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiology & Behavior. 2003;80:81–88. doi: 10.1016/s0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]
- McIntosh T. K, Barfield R. J, Thomas D. Electrophysiological and ultrasonic correlates of reproductive behavior in the male rat. Behavioral Neuroscience. 1984;98:1100–1103. doi: 10.1037//0735-7044.98.6.1100. [DOI] [PubMed] [Google Scholar]
- McKenna K. E. Orgasm. In: Knobil E, Neill J. D, editors. Encyclopedia of reproduction. III. New York: Academic Press; 1999a. pp. 528–531. [Google Scholar]
- McKenna K. E. Ejaculation. In: Knobil E, Neill J. D, editors. Encyclopedia of reproduction. I. New York: Academic Press; 1999b. pp. 1002–1008. [Google Scholar]
- McKenna K. E, Chung S. K, McVary K. T. A model for the study of sexual function in anesthetized male and female rats. American Journal of Physiology. 1991;30:R1276–R1285. doi: 10.1152/ajpregu.1991.261.5.R1276. [DOI] [PubMed] [Google Scholar]
- McMurray G, Casey J. H, Naylor A. M. Animal models in urological disease and sexual dysfunction. British Journal of Pharmacology. 2006;147(Suppl 2):S62–S79. doi: 10.1038/sj.bjp.0706630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts S. H, Clark A. S. Conditioned place preference for mating is preserved in rats with pelvic nerve transection. Behavioral Neuroscience. 2009;123:539–546. doi: 10.1037/a0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts S. H, Strnad H. K, Schairer R. S. Paced mating behavior is affected by clitoral–vaginocervical lidocaine application in combination with sexual experience. Physiology & Behavior. 2015;140:222–229. doi: 10.1016/j.physbeh.2014.12.043. [DOI] [PubMed] [Google Scholar]
- Mehara B. J, Baum M. J. Naloxone disrupts the expression but not the acquisition by male rats of a conditioned place preference response for an oestrous female. Psychopharmacology (Berl) 1990;101:118–125. doi: 10.1007/BF02253728. [DOI] [PubMed] [Google Scholar]
- Meston C. M, Levin R. J, Sipski M. L, Hull E. M, Heiman J. R. Women's orgasm. Annual Review of Sex Research. 2004;15:153–257. [PubMed] [Google Scholar]
- Moll A. The sexual life of the child. New York: Macmillan; 1908. [Google Scholar]
- Murphy M. R, Checkley S. A, Seckl J. R, Lightman S. L. Naloxone inhibits oxytocin release at orgasm in man. Journal of Clinical Endocrinology and Metabolism. 1990;71:1056–1058. doi: 10.1210/jcem-71-4-1056. [DOI] [PubMed] [Google Scholar]
- Nagel T. What is it like to be a bat? Philosophical Review. 1974;83:435–450. [Google Scholar]
- Newman H. F, Reiss H, Northup J. D. Physical basis of emission, ejaculation, and orgasm in the male. Urology. 1982;19:341–350. doi: 10.1016/0090-4295(82)90186-8. [DOI] [PubMed] [Google Scholar]
- Olivier B, Chan J. S, Pattij T, de Jong T. R, Oosting R. S, Veening J. G, et al. Psychopharmacology of male rat sexual behavior: Modeling human sexual dysfunctions? International Journal of Impotence Research. 2006;18(Suppl 1):S14–S23. doi: 10.1038/sj.ijir.3901330. [DOI] [PubMed] [Google Scholar]
- Ovid (Publius Ovidius Naso, 2 CE/1855) Ars amatoria (Art of love) New York: Perseus Books; [Google Scholar]
- Parada M, Abdul-Ahad F, Censi S, Sparks L, Pfaus J. G. Context alters the ability of clitoral stimulation to induce a sexually-conditioned partner preference in the rat. Hormones and Behavior. 2011;59:520–527. doi: 10.1016/j.yhbeh.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Parada M, Chamas L, Censi S, Coria-Avila G, Pfaus J. G. Clitoral stimulation induces conditioned place preference and Fos activation in the rat. Hormones and Behavior. 2010;57:112–118. doi: 10.1016/j.yhbeh.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Parada M, Sparks L. M, Censi S, Pfaus J. G. Clitoral anesthesia disrupts paced copulation in the female rat. Physiology & Behavior. 2014;123:180–186. doi: 10.1016/j.physbeh.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Parada M, Vargas E. B, Kyres M, Burnside K, Pfaus J. G. The role of ovarian hormones in sexual reward states of the female rat. Hormones and Behavior. 2012;62:442–447. doi: 10.1016/j.yhbeh.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Parada M, Jafari N, Pfaus J. G. Sexual experience blocks the ability of clitoral stimulation to induce a conditioned place preference in the rat. Physiology & Behavior. 2013;119:97–102. doi: 10.1016/j.physbeh.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Paredes R. G. Opioids and sexual reward. Pharmacology, Biochemistry, and Behavior. 2014;121:124–131. doi: 10.1016/j.pbb.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Paredes R. G, Alonso A. Sexual behavior regulated (paced) by the female induces conditioned place preference. Behavioral Neuroscience. 1997;111:123–128. doi: 10.1037//0735-7044.111.1.123. [DOI] [PubMed] [Google Scholar]
- Paredes R. G, Martinez I. Naloxone blocks place preference conditioning after paced mating in female rats. Behavioral Neuroscience. 2001;115:1363–1367. [PubMed] [Google Scholar]
- Paredes R. G, Vazquez B. What do female rats like about sex? Paced mating. Behavioural Brain Research. 1999;105:117–127. doi: 10.1016/s0166-4328(99)00087-x. [DOI] [PubMed] [Google Scholar]
- Pattij T, Olivier B, Waldinger M. D. Animal models of ejaculatory behavior. Current Pharmaceutical Design. 2005;11:4069–4077. doi: 10.2174/138161205774913363. [DOI] [PubMed] [Google Scholar]
- Peirce J. T, Nuttall R. L. Self-paced sexual behavior in the female rat. Journal of Comparative and Physiological Psychology. 1961;54:310–313. doi: 10.1037/h0040740. [DOI] [PubMed] [Google Scholar]
- Pfaus J. G, Manitt C, Coopersmith C. B. Effects of pelvic, pudendal, or hypogastric nerve cuts on Fos induction in the rat brain following vaginocervical stimulation. Physiology & Behavior. 2006;89:627–636. doi: 10.1016/j.physbeh.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Pfaus J. G. Pathways of sexual desire. Journal of Sexual Medicine. 2009;6:1506–1533. doi: 10.1111/j.1743-6109.2009.01309.x. [DOI] [PubMed] [Google Scholar]
- Pfaus J. G, Gorzalka B. B. Opioids and sexual behavior. Neuroscience and Biobehavioral Reviews. 1987;11:1–34. doi: 10.1016/s0149-7634(87)80002-7. [DOI] [PubMed] [Google Scholar]
- Pfaus J. G, Jones S. L, Flanagan-Cato L, Blaustein J. D. Chapter 50: Female sexual behavior. In: Plant A. M, Zeleznik A. J, editors. Knobil & Neill's Physiology of reproduction. fourth edition. New York: Elsevier; 2015. pp. 2287–2370. [Google Scholar]
- Pfaus J. G, Kippin T. E, Coria-Avila G. A. What can animal models tell us about human sexual response? Annual Review of Sex Research. 2003;14:1–63. [PubMed] [Google Scholar]
- Pfaus J. G, Kippin T. E, Coria-Avila G. A, Gelez H, Afonso V. M, Ismail N, et al. Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance. Archives of Sexual Behavior. 2012;41:31–62. doi: 10.1007/s10508-012-9935-5. [DOI] [PubMed] [Google Scholar]
- Pfaus J. G, Smith W. J, Byrne N, Stephens G. Appetitive and consummatory sexual behaviors of female rats in bilevel chambers. II. Patterns of estrus termination following vaginocervical stimulation. Hormones and Behavior. 2000;37:96–107. doi: 10.1006/hbeh.1999.1562. [DOI] [PubMed] [Google Scholar]
- Pfaus J. G, Smith W. J, Coopersmith C. B. Appetitive and consummatory sexual behaviors of female rats in bilevel chambers. I. A correlational and factor analysis and the effects of ovarian hormones. Hormones and Behavior. 1999;35:224–240. doi: 10.1006/hbeh.1999.1516. [DOI] [PubMed] [Google Scholar]
- Pfaus J. G, Wilkins M. F. A novel environment disrupts copulation in sexually naive but not experienced male rats: Reversal with naloxone. Physiology & Behavior. 1995;57:1045–1049. doi: 10.1016/0031-9384(94)00394-k. [DOI] [PubMed] [Google Scholar]
- Pfaus J. G, Wilkins M. F, Di Pietro N, Benibgui M, Toledano R, Rowe A, et al. Inhibitory and disinhibitory effects of psychomotor stimulants and depressants on the sexual behavior of male and female rats. Hormones and Behavior. 2010;58:163–176. doi: 10.1016/j.yhbeh.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Reich W. The function of the orgasm. New York: Farrar, Strauss, Giroux; 1927/1945. (Theodore P. Wolfe, Trans.) [Google Scholar]
- Rodriguez-Manzo G. Blockade of the establishment of the sexual inhibition resulting from sexual exhaustion by the Coolidge effect. Behavioural Brain Research. 1999;100:245–254. doi: 10.1016/s0166-4328(98)00137-5. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Manzo G, Fernández-Guasti A. Reversal of sexual exhaustion by serotonergic and noradrenergic agents. Behavioural Brain Research. 1994;62:127–134. doi: 10.1016/0166-4328(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Manzo G, Fernández-Guasti A. Opioid antagonists and the sexual satiation phenomenon. Psychopharmacology (Berl) 1995;122:131–136. doi: 10.1007/BF02246087. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Manzo G, López-Rubalcava C, Fernández-Guasti A. Anxiolytic-like effect of ejaculation under various sexual behavior conditions in the male rat. Physiology & Behavior. 1999;67:651–657. doi: 10.1016/s0031-9384(99)00119-5. [DOI] [PubMed] [Google Scholar]
- Shafik A. Vagino-levator reflex: Description of a reflex and its role in sexual performance. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 1995;60:161–164. doi: 10.1016/0028-2243(95)02095-a. [DOI] [PubMed] [Google Scholar]
- Shafik A, El-Sibai O, Mostafa R, Shafik A. A, Ahmed I. Response of the internal reproductive organs to clitoral stimulation: The clitorouterine reflex. International Journal of Impotence Research. 2005;17:121–126. doi: 10.1038/sj.ijir.3901278. [DOI] [PubMed] [Google Scholar]
- Slob A. K, Groeneveld W. H, van der Werff ten Bosch J. J. Physiological changes during copulation in male and female stumptail macaques (Macaca arctoides) Physiology & Behavior. 1986;38:891–895. doi: 10.1016/0031-9384(86)90059-4. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Modulation of drug-induced sensitization processes by endogenous opioid systems. Behavioural Brain Research. 1995;70:37–49. doi: 10.1016/0166-4328(94)00176-g. [DOI] [PubMed] [Google Scholar]
- Stoléru S, Fonteille V, Cornélis C, Joyal C, Moulier V. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: A review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2012;36:1481–1509. doi: 10.1016/j.neubiorev.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Szechtman H, Hershkowitz M, Simantov R. Sexual behavior decreases pain sensitivity and stimulated endogenous opioids in male rats. European Journal of Pharmacology. 1981;26:279–285. doi: 10.1016/0014-2999(81)90161-8. [DOI] [PubMed] [Google Scholar]
- Tenk C. M, Wilson H, Zhang Q, Pitchers K. K, Coolen L. M. Sexual reward in male rats: Effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Hormones and Behavior. 2009;55:93–97. doi: 10.1016/j.yhbeh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor D. H, Flannelly K. J. Social–olfactory experience and initiation of copulation in the virgin male rat. Physiology & Behavior. 1977;19:411–417. doi: 10.1016/0031-9384(77)90287-6. [DOI] [PubMed] [Google Scholar]
- Troisi A, Carosi M. Female orgasm rate increases with male dominance in Japanese macaques. Animal Behaviour. 1998;56:1261–1266. doi: 10.1006/anbe.1998.0898. [DOI] [PubMed] [Google Scholar]
- Truitt W. A, Coolen L. M. Identification of a potential ejaculation generator in the spinal cord. Science. 2002;297:1566–1569. doi: 10.1126/science.1073885. [DOI] [PubMed] [Google Scholar]
- Truitt W. A, Shipley M. T, Veening J. G, Coolen L. M. Activation of a subset of lumbar spinothalamic neurons after copulatory behavior in male but not female rats. Journal of Neuroscience. 2003;23:325–331. doi: 10.1523/JNEUROSCI.23-01-00325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth W. R, van Emst M. G, van Ree J. M. Opioids and sexual behavior of male rats: Involvement of the medial preoptic area. Behavioral Neuroscience. 1995;109:123–134. doi: 10.1037//0735-7044.109.1.123. [DOI] [PubMed] [Google Scholar]
- van Furth W. R, Wolterink G, van Ree J. M. Regulation of masculine sexual behavior: Involvement of brain opioids and dopamine. Brain Research Brain Research Reviews. 1995;21:162–184. doi: 10.1016/0165-0173(96)82985-7. [DOI] [PubMed] [Google Scholar]
- Wallen K. The evolution of female sexual desire. In: Abramson P. R, Pinkerton S. D, editors. Sexual nature sexual culture. Chicago, IL: University of Chicago Press; 1995. pp. 57–79. [Google Scholar]
- White N. R, Cagiano R, Moises A. U, Barfield R. J. Changes in mating vocalizations over the ejaculatory series in rats (Rattus norvegicus) Journal of Comparative Psychology. 1990;104:140–146. doi: 10.1037/0735-7036.104.3.255. [DOI] [PubMed] [Google Scholar]
- White N. R, Colona L. C, Barfield R. J. Sensory cues that elicit ultrasonic vocalizations in female rats (Rattus norvegicus) Behavioral and Neural Biology. 1991;55:154–165. doi: 10.1016/0163-1047(91)80136-3. [DOI] [PubMed] [Google Scholar]
- Zumpe D, Michael R. P. The clutching reaction and orgasm in the female rhesus monkey (Macaca mulatta) Journal of Endocrinology. 1968;40:117–123. doi: 10.1677/joe.0.0400117. [DOI] [PubMed] [Google Scholar]