Abstract
The discovery that oxidized vitamin C, dehydroascorbate (DHA), can induce oxidative stress and cell death in cancer cells has rekindled interest in the use of high dose vitamin C (VC) as a cancer therapy. However, high dose VC has shown limited efficacy in clinical trials, possibly due to the decreased bioavailability of oral VC. Because human erythrocytes express high levels of Glut1, take up DHA, and reduce it to VC, we tested how erythrocytes might impact high dose VC therapies. Cancer cells are protected from VC-mediated cell death when co-cultured with physiologically relevant numbers of erythrocytes. Pharmacological doses of VC induce oxidative stress, GSH depletion, and increased glucose flux through the oxidative pentose phosphate pathway (PPP) in erythrocytes. Incubation of erythrocytes with VC induced hemolysis, which was exacerbated in erythrocytes from glucose-6-phosphate dehydrogenase (G6PD) patients and rescued by antioxidants. Thus, erythrocytes protect cancer cells from VC-induced oxidative stress and undergo hemolysis in vitro, despite activation of the PPP. These results have implications on the use of high dose VC in ongoing clinical trials and highlight the importance of the PPP in the response to oxidative stress.
Keywords: cancer, erythrocyte, glucose metabolism, glucose-6-phosphate dehydrogenase (G6PD or G6PDH), glycolysis, hydrogen peroxide, metabolomics, oxidative stress, pentose phosphate pathway (PPP), vitamin C, cancer, hydrogen peroxide
Introduction
Consistent with its function as a potent reducing agent essential for numerous biological reactions, VC2 can be readily oxidized to dehydroascorbate (DHA) in aqueous solution. DHA is transported intracellularly by Glut1 (solute carrier family 2, facilitated glucose transporter member 1) (1), after which it is recycled to reduced VC at the expense of cellular antioxidants, such as GSH (2, 3). This paradoxical role for VC as a source of oxidative stress has been found to be selectively toxic to some cancer cells, particularly to those overexpressing Glut1 (4, 5). Although the benefits of VC supplementation as a cancer therapy have been inconsistent (6), higher doses of intravenous VC have shown more promise, and several clinical trials are ongoing (7).
Like many cancer cells, human erythrocytes (RBCs) express very high levels of Glut1. The abundance of the Glut1 transporter in erythrocyte membrane allows RBCs to mediate the transport of glucose and DHA at rates that far exceed their capacity to utilize it (3). There is substantial evidence that erythrocytes participate in VC recycling in vivo (8, 9). In addition, erythrocytes have also been found to protect both tissues and cells from H2O2-mediated damage through their high capacity redox systems (10). We hypothesized that erythrocytes might protect cancer cells from VC-mediated toxicity through similar mechanisms. Indeed, we find that physiologically relevant numbers of erythrocytes protected HCT116, A375, and SK-MEL-28 cells from VC-mediated toxicity. Erythrocytes incubated with VC showed higher levels of oxidative stress and shifted glucose metabolism toward the oxidative pentose phosphate pathway (PPP), which produces NADPH for ROS mitigation. Finally, we find that VC induced hemolysis of erythrocytes, which was exacerbated by chemical or genetic inhibition of glucose-6-phosphate dehydrogenase (G6PD) and rescued by incubation with β-mercaptoethanol. This study broadens our current understanding of VC metabolism by erythrocytes and has implications for the use and monitoring of VC in the clinical setting.
Results
Erythrocytes Protect Cancer Cells from VC- and H2O2-induced Cell Death
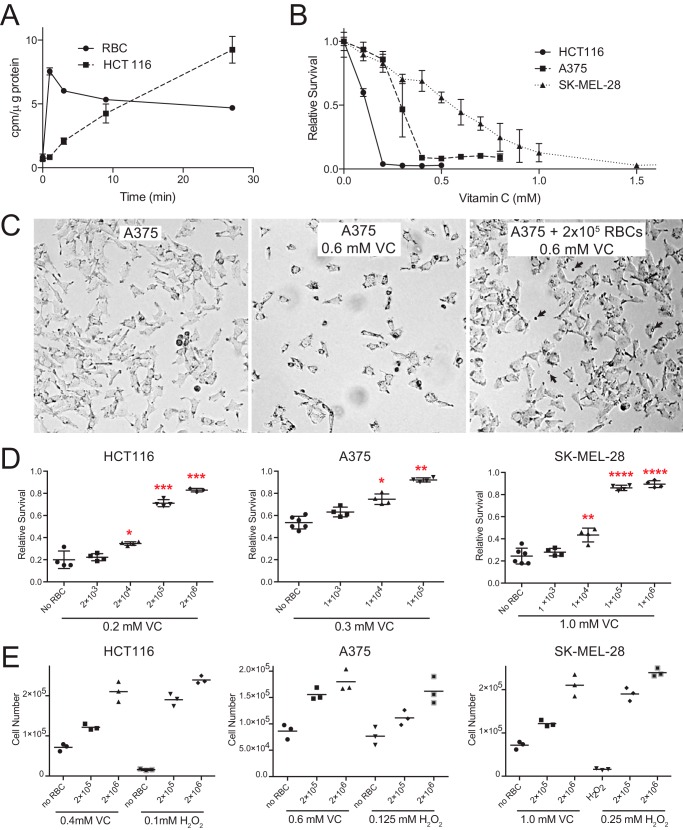
The selective toxicity of VC to KRAS (proto-oncogene v-Raf murine sarcoma viral oncogene homolog B) and BRAF (proto-oncogene v-Raf murine sarcoma viral oncogene homolog B) mutant colorectal cancer cells is due, in part, to the high expression levels of Glut1 and preferential uptake of oxidized DHA by the facilitative glucose transporter (5). Consistent with their very high expression of Glut1, erythrocytes have also been shown to transport DHA efficiently (11). We compared the kinetics of DHA uptake by erythrocytes and HCT116 at physiological concentrations of glucose and found that erythrocytes accumulated intracellular VC much more rapidly than HCT116 cells (Fig. 1A). Consistent with previous studies on the accumulation of ascorbate in erythrocytes (8), we found that accumulation of VC by RBCs peaked rapidly (∼1 min) and decreased slightly over time consistent with the slow release of recycled ascorbate back into the medium. In contrast, the kinetics of DHA uptake by HCT116 cells was delayed and increased slowly over 30 min. The level of rapid uptake and reduction of DHA by erythrocytes is consistent with the undetectable levels of DHA in healthy human serum (12).
FIGURE 1.
Erythrocytes protect cancer cells from VC and H2O2 toxicity. A, [14C]DHA uptake assay demonstrates that RBCs accumulate DHA more rapidly than HCT116 cells. B, HCT116 and BRAF mutant melanoma cells (A375 and SK-MEL-28) show a dose-dependent toxicity to VC-mediated toxicity as assessed by MTT. C, photomicrographs of A375 cells reveal that VC treatment (0.6 mm for 24 h) caused cytotoxicity. Co-culture with RBCs (indicated by arrows) rescues cell viability. D, the co-culture of RBCs with HCT116, A375, and SK-MEL-28 cancer cells protects them from VC-induced toxicity as assessed by the MTT assay. An equal number of cells cultured without VC or RBCs was used as the reference (100%) to calculate relative survival (n = 3, error bars = S.D.; analysis of variance with Dunnett's correction; *, p ≤ 0.05, **, p ≤ 0.01, ***, p ≤ 0.001, ****, p ≤ 0.0001). E, the co-culture of RBCs with HCT116, A375, and SK-MEL-28 protects them from VC-induced toxicity as assessed by cell number.
Given the rapid kinetics of DHA uptake by erythrocytes, we hypothesized that RBCs might protect cancer cells from VC-mediated toxicity. First, we determined the sensitivities of HCT116 and two BRAFV600E mutant melanoma cell lines to VC. Although HCT116 cells were the most sensitive to VC, both BRAF mutant melanoma cell lines were also sensitive to VC-induced cell death at physiological glucose concentrations (Fig. 1B). The intravenous administration of high dose ascorbate has the ability to increase serum ascorbate to >25 mm (7). Thus, all three mutant cells showed sensitivity to VC at pharmacologically relevant doses. Next, these cancer cells were co-cultured with RBCs and then treated with VC. The addition of as few as 10,000 RBCs significantly protected HCT116, A375, and SK-MEL-28 cells from VC-induced toxicity (Fig. 1, C–E). Higher numbers of erythrocytes offered near complete protection of all three cancer cell lines from VC-mediated toxicity. These striking in vitro findings suggest that circulating erythrocytes might exert modifying effects on VC toxicity in the in vivo setting.
The protective effects of RBCs against oxidative stress were not limited to VC. Consistent with reports that RBCs can protect tissues from hydrogen peroxide (H2O2)-induced tissue damage (10), we found that RBCs could protect cancer cells from H2O2-mediated cell death. After first determining the sensitivity of each of the cell lines to H2O2, we also found that as few as 10,000 RBCs could also protect cancer cells from H2O2 toxicity (data not shown). Moreover, the addition of both VC and H2O2 resulted in a synergistic toxicity to the cancer cell lines. Co-culture with erythrocytes provided protection against this synergistic oxidative stress (data not shown).
Metabolomic Flux Analysis of RBCs Treated with VC
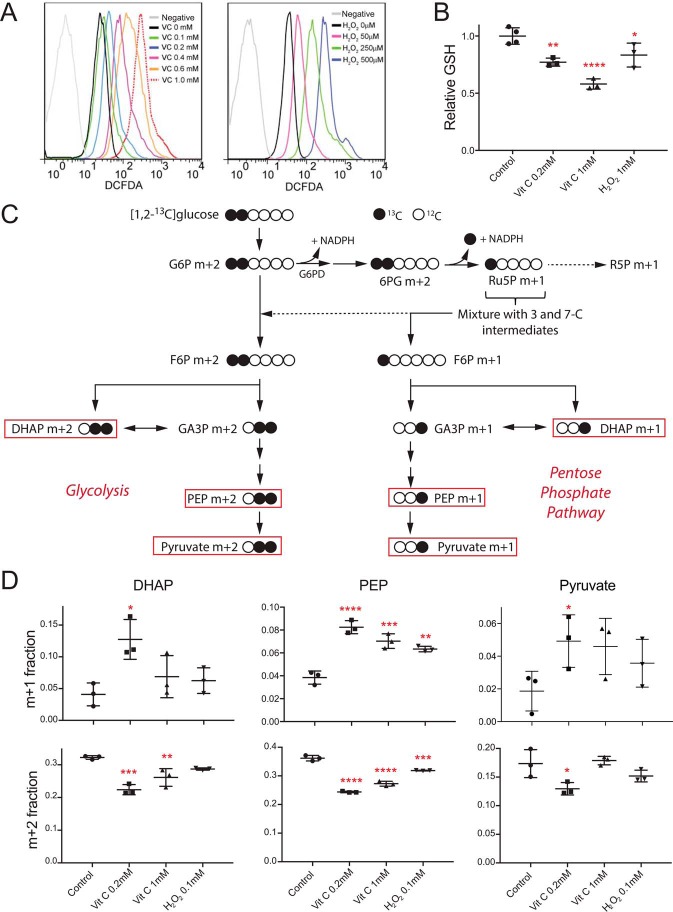
Despite the high redox capacity of erythrocytes, the uptake and recycling of large amounts of DHA might present an oxidative burden to erythrocytes. To test this, erythrocytes were incubated with the ROS-sensitive dye, 2′,7′-dichlorofluorescein diacetate (DCFDA). We found that VC increased the intracellular ROS of erythrocytes in a dose-dependent manner. The amount of oxidative stress induced by VC was comparable with similar concentrations of H2O2 (Fig. 2A). Consistent with the function of GSH as a the principal cellular antioxidant responsible for the recycling of DHA by erythrocytes (8), we found that erythrocyte GSH levels decreased significantly upon incubation with VC (Fig. 2B).
FIGURE 2.
Erythrocytes show increased oxidative stress, decreased GSH, and increased glucose flux through the oxidative PPP after treatment with VC. A, FACS analysis of erythrocytes treated with increasing amounts of VC (0, 0.1, 0.2, 0.4, 0.6, and 1 mm) or H2O2 (0, 0.05, 0.25, and 1 mm) shows increased levels of ROS as indicated by DCFDA fluorescence. The gray curve represents a control without DCFDA. B, intracellular GSH decreases after incubation with VC. C, schematic of [1,2-13C]glucose metabolic tracing experiments. Some of the glucose proceeding through the PPP results in the generation of m+1-labeled products. However, some products of the oxidative PPP will not be detected as m+1-labeled products (dashed arrows). Red boxes highlight the subset of monitored products. G6P, glucose 6-phosphate; 6PG, 6-phosphogluconic acid; Ru5P, ribulose 5-phosphate; R5P, ribose 5-phosphate; F6P, fructose 6-phosphate; GA3P, glyceraldehyde 3-phosphate; DHAP, dihydroxyacetone phosphate; PEP, phosphoenolpyruvate; D, quantitation of labeled products in metabolic tracing experiments. Incubation of erythrocytes with VC or H2O2 results in significant increases of m+1-labeled products, indicating increased flux through the PPP (top row), and significant decreases of m+2-labeled products, indicating decreased flux through glycolysis (bottom row) (n = 3, error bars = S.D.; analysis of variance with Dunnett's correction; *, p ≤ 0.05, **, p ≤ 0.01, ***, p ≤ 0.001, ****, p ≤ 0.0001 when compared with control).
The regeneration of GSH from GSSG requires NADPH and GSH reductase. Keratinocytes, fibroblasts, and cancer cells can respond rapidly to oxidative stress by routing glucose flux through the oxidative PPP to generate NADPH (13, 14). Because erythrocytes lack organelles to mount a transcriptional response to oxidative stress, the reduction of NADP+ to NADPH might constitute an especially important facet of the response against RBC oxidative stress. DHA has previously been shown to stimulate oxidation of glucose through the pentose phosphate pathway (9). To quantitate the impact of oxidative stress on glucose flux in RBCs, we used [1,2-13C]glucose to monitor the labeling of glucose-derived metabolites. In this labeling scheme, m+1-labeled metabolites from the lower half of glycolysis (i.e. dihydroxyacetone phosphate, phosphoenolpyruvate, and pyruvate) arise from flux through the oxidative PPP- and m+2-labeled metabolites, from glycolysis (Fig. 2C). Importantly, these measurements underestimate total flux through the oxidative PPP as they report only carbons flowing back into the pools of glycolytic intermediates as opposed to supplying ribose-phosphate pools for nucleotide biosynthesis and other branch pathways. In unperturbed erythrocytes, ∼4–8% of lower glycolytic intermediates were labeled as m+1. After treatment with VC, the fractional abundance of m+1-labeled products increased significantly, as much as 3-fold for some metabolites (Fig. 2D, top row; supplemental data). Similar changes were observed in H2O2-treated cells. Analysis of other labeled isotopologues revealed significant decreases in the levels of m+2-labeled isotopologues consistent with decreases of up to 20% in the glycolytic pathway after VC treatment (Fig. 2D, bottom row; supplemental data). Interestingly, changes in glucose flux were actually less pronounced in RBCs treated with 1.0 mm VC or 0.1 mm H2O2 than those treated with 0.2 mm VC. High levels of oxidative stress can overwhelm the antioxidant systems of RBCs and compromise RBC membrane integrity and metabolism (15). Thus, we speculate that high levels of oxidant stress may irreparably damage some RBCs and render them metabolically inactive.
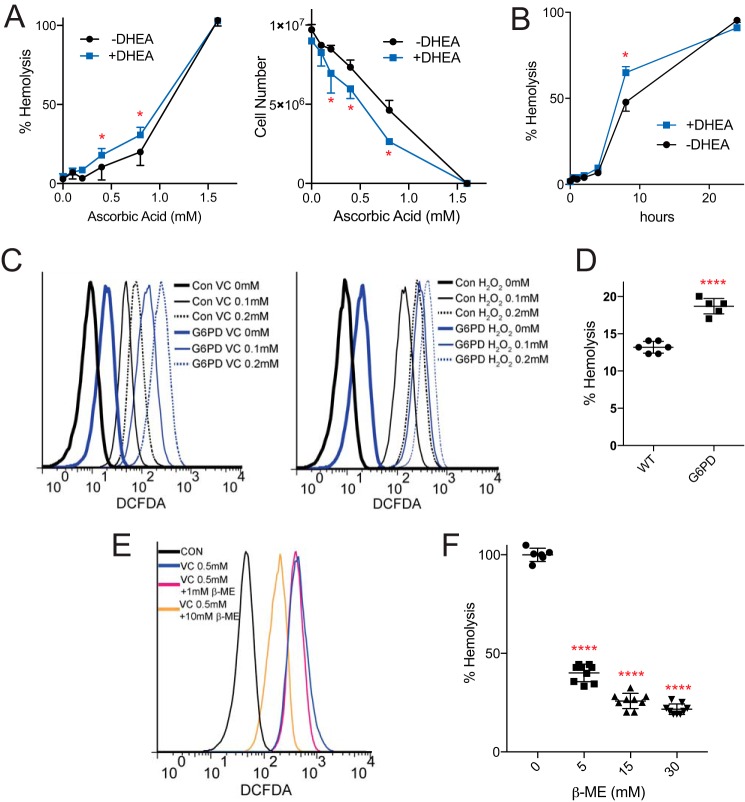
Hemolysis in Erythrocytes Treated with VC
Incubation of RBCs with VC results in an induction of oxidative stress, depletion of GSH, and increased flux through the PPP. These findings are consistent with the key role of PPP in producing NADPH for RBCs, and suggest that inhibition of the PPP could damage VC-treated RBCs. Although several cases of VC-induced hemolysis have been reported in the literature (16–18), the mechanism of this hemolysis has not been experimentally verified. Importantly, all patients reported to have significant VC-induced hemolysis were found to possess a genetic deficiency of G6PD, a key upstream enzyme of the PPP. First, we tested whether VC might induce hemolysis in normal erythrocytes in vitro. Indeed, we found that pharmacological concentrations of VC induced significant hemolysis as measured by both hemoglobin release and decreased RBC number, with complete hemolysis noted at 1.6 mm VC (Fig. 3A). At lower concentrations of VC, hemolysis was more striking at later time points, likely reflecting the depletion of glucose from the incubation medium (Fig. 3B). Consistent with an important role for the PPP in maintaining RBC redox, dehydroepiandrosterone (DHEA), a PPP inhibitor, significantly exacerbated VC-induced RBC hemolysis under all conditions (Fig. 3, A and B). Moreover, erythrocytes from a G6PD patient showed increased oxidative stress both at baseline and also in response to VC and H2O2 treatments (Fig. 3C). G6PD erythrocytes were also significantly more susceptible to VC-induced hemolysis (Fig. 3D). The incubation of normal RBCs with β-mercaptoethanol (β-ME), an antioxidant, partially rescued RBCs from both VC-induced oxidative stress (Fig. 3E) and hemolysis (Fig. 3F).
FIGURE 3.
VC-induced hemolysis is exacerbated by G6PD inhibition. A, dose-dependent hemolysis of RBCs by VC as assessed by hemoglobin absorbance (left) and cell counts (right) after 1.5 h of incubation. DHEA significantly exacerbated hemolysis as indicated by red asterisks (n = 3, error bars = S.D.; Student's t test with Holm-Šídák correction, α = 0.05). Data represent replicate measurements that are representative of more than three independent experiments. B, incubation of RBCs with 0.6 mm VC resulted in extensive hemolysis at 8 h. DHEA significantly exacerbated hemolysis at 8 h (n = 3, error bars = S.D.; Student's t test with Holm-Šídák correction, α = 0.05). C, RBCs from a G6PD patient showed higher levels of ROS at baseline, when treated with VC (0.1 and 0.2 mm), and when treated with H2O2 (0.1, 0.2 mm) when compared with control RBCs as indicated by DCFDA fluorescence. Con, control. D, RBCs from a G6PD patient showed significantly higher levels of hemolysis in response to incubation with VC (1.0 mm, 1.5 h) when compared with control RBCs (n = 3, Student's t test, p ≤ 0.0001). E, incubation of RBCs with β-mercaptoethanol rescued VC-induced oxidative stress. F, incubation of RBCs with β-ME resulted in a dose-dependent rescue of hemolysis (right) (n = 3, Student's t test, p ≤ 0.0001).
Discussion
Our findings that erythrocytes can protect cancer cells from VC-induced oxidative stress extend previous studies, suggesting an antioxidant role for erythrocytes. Although most clinical trials involving high dose VC include G6PD deficiency as an exclusion criterion, we suggest that all patients at risk of hemolysis be excluded from such trials. Consistent with this suggestion, a recent randomized, double-blind, placebo-controlled trial found that oral supplementation of VC significantly worsened hemolytic markers in sickle cell anemia and β-thalassemia patients, contrary to their expectations (19). Although the presence of sufficient serum glucose might mitigate hemolysis in ongoing high dose VC trials, low levels of hemolysis might be detectable even in otherwise healthy patients. To test this possibility, clinical monitoring of hemolytic parameters could be considered in future or ongoing clinical trials. Moreover, our studies suggest that VC alone may have limited utility in blood storage, where it has been tested extensively (20, 21). Rather, preservation of erythrocytes in solutions with glucose and alternative anti-oxidants might better prevent RBC oxidative stress and hemolysis and promote longer term erythrocyte stability during storage.
Our results also highlight the importance of glucose flux through the oxidative PPP as a critical component in the response to oxidative stress. Although many studies have focused on glucose's roles as an energy source and biosynthetic intermediate, more recent studies have begun to probe the role of glucose flux through the PPP in the response to oxidative stress (13, 22). We speculate that the dependence of RBCs on the PPP in the response to oxidative stress might help explain why they have evolved to express extraordinarily high levels of the Glut1 transporter. It will be interesting to test whether other cell types that express high levels of glucose transporters show a similar dependence on glucose in the response to oxidative stress.
Experimental Procedures
Cell Lines, Cell Culture, RBC Co-culture, and Treatments
A375 and SK-MEL-28 cell lines were cultured in DMEM (Gibco 11965) supplemented with 10% FBS and 1% penicillin-streptomycin. HCT116 cells were cultured in the same medium with 5% FBS. For VC and/or H2O2 treatment, cells were seeded at a density of 5000 cells/well in a 96-well plate in 100 μl of DMEM (Gibco A14430) supplemented with 5 mm glucose and 10% heat-inactivated FBS. After 36 h, 100 μl of fresh medium containing VC and/or H2O2 was added to the existing culture, and cells were cultured for an additional 24 h. For co-culture experiments, human RBCs were added to the culture just prior to supplementation with VC and/or H2O2. After 24 h, the RBCs were gently washed out before measuring cell viability. Cell viability was determined through the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Thermo Fisher Scientific) according to the manufacturer's instructions. Cancer cells cultured in the absence of RBCs and treatments were used as the reference to calculate the relative survival of the different experimental conditions. Cell viability was also separately determined by counting cells that were seeded at a density of 1 × 105 cells/well in a 12-well plate in 1 ml of medium. Cells were trypsinized and counted in the presence of trypan blue. For β-ME rescue experiments, 0.5 mm VC was added first to the RBCs, followed quickly by the indicated amount of β-ME. Because the toxicity of VC varied between 96- and 12-well plates, the concentrations of VC used in MTT and cell counting experiments were independently determined.
Patient Enrollment and Preparation of RBCs
Erythrocyte analyses were approved by the institutional review board, and written informed consent was received from participants prior to inclusion in the study. The G6PD patient had a documented G6PD deficiency of 0.8 IU/g of Hb (reference values: 8.8–13.4 units/g of Hb). Venous blood was collected from volunteers in heparinized tubes; anticoagulated blood was centrifuged at 500 × g for 10 min at 4 °C, and the leukocytes were aspirated. The RBC pellet was then washed three times with Dulbecco's PBS under the same conditions. Washed cells were resuspended in PBS at ∼80% hematocrit and kept at 4 °C until use. RBCs were used on the day of collection.
Intracellular ROS and GSH Assay
2 × 107 RBCs were plated in 6-well plates in 2 ml of DMEM (Gibco A14430) supplemented with 5 mm glucose and 10% heat-inactivated FBS at 37 °C in at 5% CO2. ROS levels in RBCs were quantified by BD FACSCalibur flow cytometer using DCFDA staining. For H2O2 treatment, 5 μm DCFDA was first added to the cells, followed quickly by the addition of different doses of H2O2; samples were collected after 30 min. For VC treatment, VC was first added to the RBCs, and after a 2-h incubation, 5 μm DCFDA was added, and then cells were collected after 30 min. For collection, the samples were placed on ice, centrifuged at 1500 rpm for 4 min, gently washed once with FACS buffer (Dulbecco's PBS with 2% FBS and 0.2 mm EDTA), and resuspended in FACS buffer for analysis. For cancer cell ROS measurements, cells were seeded at a density of 1 × 105 cells/well in a 12-well plate in the same medium as described for VC and H2O2 treatment. After 24 h, 1 × 106 RBCs were added to the indicated wells. Next, H2O2 was added to the cells, followed quickly by the addition of DCFDA. Cancer cells were collected after 30 min for FACS analysis. For intracellular GSH measurement, RBCs were treated with VC or H2O2 for 120 or 30 min, respectively, quickly collected on ice, gently washed once with ice-cold culture medium, and then lysed in 200 μl of GSH assay buffer. GSH was measured using the GSH Colorimetric Assay Kit (BioVision) following the manufacturer's instructions.
[1,2-13C]Glucose Flux Analysis
2 × 107 RBCs were added to 5 ml of medium supplemented with 5 mm [1,2-13C]glucose or unlabeled glucose and 10% dialyzed FBS with or without vitamin C or H2O2. RBCs were carefully dispersed after addition to the medium. After 6 h, cells were collected by centrifugation at 1500 rpm for 10 min at 4 °C. The medium was aspirated, and the cells were frozen in liquid nitrogen, lysed with three freeze-thaw cycles in 1 ml of 50% methanol (MeOH), and then centrifuged to remove protein debris. The lysates were dried, methoximated, and derivatized with 10 mg/ml methoxyamine (Fisher PI-45950) at 70 °C for 10 min followed by 105 μl of MTBSTFA (Sigma 394882) at 70 °C for 1 h. After derivatization, 1 μl of the sample was injected onto an Agilent 6970 gas chromatograph networked to an Agilent 5973 mass selective detector. The abundance of the following ions was monitored: m/z 484–487 for dihydroxyacetone phosphate, m/z 261–264 for lactate, m/z 453–456 for phosphoenolpyruvate, and m/z 174–177 for pyruvate.
DHA Uptake Assay
Transport measurements were performed as described previously with modifications (11, 23). Ascorbic acid, l-[1-14C] (ARC 1569) was purchased from American Radiolabeled Chemicals. Labeled ascorbic acid purchased from PerkinElmer did not perform as reported in control uptake assays. HCT116 cells were seeded in triplicate in 12-well plates (250,000 cells/well) for 18–24 h, washed twice with PBS, and incubated with serum-free DMEM containing 0.1% BSA for 2 h. Cells were then washed and incubated in Ringer's solution (126 mm NaCl, 5 mm KCl, 1 mm MgSO4, 32 mm HEPES, 2.5 mm glucose, 1 mm CaCl2, 0.1% BSA, pH 6.0). Ascorbate oxidase (10 units/ml) was used to convert [14C]AA to [14C]DHA, and transport was initiated by the addition of 0.5 μCi of [14C]AA and 100 μm cold AA to each well. Uptake assays were performed for 0, 1, 3, 9, and 27 min at room temperature. Transport was terminated by the removal of uptake solution followed by four washes with ice-cold stop solution containing 100 μm phloretin and 10 μm cytochalasin B in PBS. For DHA uptake in RBCs, 20 μl of erythrocyte suspension containing 2.5 × 106 cells in Ringer's solution was used for each reaction. Uptake was initiated by adding 100 μl of Ringer solution containing 0.5 μCi of 100 μm cold AA to each sample for the indicated time points. Transport was terminated by the addition of ice-cold stop solution. Cells were centrifuged at 16,000 × g for 1 min followed by three additional washes. Both HCT116 and pelleted erythrocytes were lysed in 0.5 ml of 0.2% SDS. 375 μl of the lysate was quantitated by liquid scintillation counting.
RBC Hemolysis Assay
Washed RBCs were resuspended in Ringer's solution at a cell density of 1 × 107 cells/ml. RBC suspensions were preincubated with 25 μm DHEA or DMSO control at 37 °C with shaking for 15 min prior to treatment with VC. For antioxidant rescue experiments, RBCs were preincubated with Ringer's solution supplemented with the indicated amounts of β-ME. Aliquots of 2 ml of RBC suspension containing 1 × 107 cells/ml were used in each replicate. RBC suspensions were incubated with various concentrations of freshly prepared VC at 37 °C for the indicated time points. For time course experiments, 0.6 mm VC was used for all time points. RBC hemolysis was determined by both absorbance at 540 nm and cell count from the same sample. Absorbance at 540 nm from hemoglobin released into RBC supernatants was normalized to the absorbance of RBCs completely hemolyzed with 3 mm VC (100% hemolysis).
Author Contributions
Z. Z. Z., E. E. L., R. J. D., and R. C. W. designed the experiments; Z. Z. Z., E. E. L., and J. S. performed experiments; Z. Z. Z., E. E. L., J. S., and Y. Y. acquired data; Z. Z. Z., E. E. L., J. S., R. J. D., and R. C. W. analyzed data; D. G. and A. Z. enrolled volunteers; Z. Z. Z. and R. C. W. wrote the manuscript with contributions from E. E. L., J. S., and R. J. D.
Supplementary Material
Acknowledgments
We thank the volunteers for their participation and Amanda Ziegeweid for help in recruiting patients.
This work was supported by National Institutes of Health Grant R01 CA157996 (to R. J. D.), K23 AR069728 (to D. G.), K23 HL132054 (to A. Z.), and K08 CA164047 (to R. C. W.). This work was also supported by Burroughs Wellcome Fund Career Award for Medical Scientists (CAMS) (Grant 1010978) and Disease Oriented Clinical Scholar Awards (to R. C. W.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.

This article contains supplemental data.
- VC
- vitamin C
- DCFDA
- 2′,7′-dichlorofluorescin diacetate
- DHA
- dehydroascorbate
- DHEA
- dehydroepiandrosterone
- PPP
- pentose phosphate pathway
- G6PD
- glucose-6-phosphate dehydrogenase
- ROS
- reactive oxygen species
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- β-ME
- β-mercaptoethanol
- MTBSTFA
- N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide
- AA
- ascorbic acid.
References
- 1. Rumsey S. C., Kwon O., Xu G. W., Burant C. F., Simpson I., and Levine M. (1997) Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 272, 18982–18989 [DOI] [PubMed] [Google Scholar]
- 2. May J. M., Qu Z. C., and Whitesell R. R. (1995) Ascorbic acid recycling enhances the antioxidant reserve of human erythrocytes. Biochemistry 34, 12721–12728 [DOI] [PubMed] [Google Scholar]
- 3. Cura A. J., and Carruthers A. (2012) Role of monosaccharide transport proteins in carbohydrate assimilation, distribution, metabolism, and homeostasis. Compr. Physiol. 2, 863–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bram S., Froussard P., Guichard M., Jasmin C., Augery Y., Sinoussi-Barre F., and Wray W. (1980) Vitamin C preferential toxicity for malignant melanoma cells. Nature 284, 629–631 [DOI] [PubMed] [Google Scholar]
- 5. Yun J., Mullarky E., Lu C., Bosch K. N., Kavalier A., Rivera K., Roper J., Chio I. I., Giannopoulou E. G., Rago C., Muley A., Asara J. M., Paik J., Elemento O., Chen Z., et al. (2015) Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350, 1391–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobs C., Hutton B., Ng T., Shorr R., and Clemons M. (2015) Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? A systematic review. Oncologist 20, 210–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duconge J., Miranda-Massari J. R., Gonzalez M. J., Jackson J. A., Warnock W., and Riordan N. H. (2008) Pharmacokinetics of vitamin C: insights into the oral and intravenous administration of ascorbate. P. R. Health Sci. J. 27, 7–19 [PubMed] [Google Scholar]
- 8. Mendiratta S., Qu Z. C., and May J. M. (1998) Erythrocyte ascorbate recycling: antioxidant effects in blood. Free Radic. Biol. Med. 24, 789–797 [DOI] [PubMed] [Google Scholar]
- 9. May J. M., Qu Z., and Morrow J. D. (2001) Mechanisms of ascorbic acid recycling in human erythrocytes. Biochim. Biophys. Acta 1528, 159–166 [DOI] [PubMed] [Google Scholar]
- 10. Toth K. M., Clifford D. P., Berger E. M., White C. W., and Repine J. E. (1984) Intact human erythrocytes prevent hydrogen peroxide-mediated damage to isolated perfused rat lungs and cultured bovine pulmonary artery endothelial cells. J. Clin. Invest. 74, 292–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sage J. M., and Carruthers A. (2014) Human erythrocytes transport dehydroascorbic acid and sugars using the same transporter complex. Am. J. Physiol. Cell Physiol. 306, C910–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dhariwal K. R., Hartzell W. O., and Levine M. (1991) Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am. J. Clin. Nutr. 54, 712–716 [DOI] [PubMed] [Google Scholar]
- 13. Kuehne A., Emmert H., Soehle J., Winnefeld M., Fischer F., Wenck H., Gallinat S., Terstegen L., Lucius R., Hildebrand J., and Zamboni N. (2015) Acute activation of oxidative pentose phosphate pathway as first-line response to oxidative stress in human skin cells. Mol. Cell 59, 359–371 [DOI] [PubMed] [Google Scholar]
- 14. Anastasiou D., Poulogiannis G., Asara J. M., Boxer M. B., Jiang J. K., Shen M., Bellinger G., Sasaki A. T., Locasale J. W., Auld D. S., Thomas C. J., Vander Heiden M. G., and Cantley L. C. (2011) Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lang F., Abed M., Lang E., and Föller M. (2014) Oxidative stress and suicidal erythrocyte death. Antioxid. Redox Signal. 21, 138–153 [DOI] [PubMed] [Google Scholar]
- 16. Campbell G. D. Jr, Steinberg M. H., and Bower J. D. (1975) Letter: Ascorbic acid-induced hemolysis in G-6-PD deficiency. Ann. Intern. Med. 82, 810. [DOI] [PubMed] [Google Scholar]
- 17. Mehta J. B., Singhal S. B., and Mehta B. C. (1990) Ascorbic-acid-induced haemolysis in G-6-PD deficiency. Lancet 336, 944. [DOI] [PubMed] [Google Scholar]
- 18. Rees D. C., Kelsey H., and Richards J. D. (1993) Acute haemolysis induced by high dose ascorbic acid in glucose-6-phosphate dehydrogenase deficiency. BMJ 306, 841–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arruda M. M., Mecabo G., Rodrigues C. A., Matsuda S. S., Rabelo I. B., and Figueiredo M. S. (2013) Antioxidant vitamins C and E supplementation increases markers of haemolysis in sickle cell anaemia patients: a randomized, double-blind, placebo-controlled trial. Br. J. Haematol 160, 688–700 [DOI] [PubMed] [Google Scholar]
- 20. Pallotta V., Gevi F., D'alessandro A., and Zolla L. (2014) Storing red blood cells with vitamin C and N-acetylcysteine prevents oxidative stress-related lesions: a metabolomics overview. Blood Transfus. 12, 376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vani R., Soumya R., Carl H., Chandni V. A., Neha K., Pankhuri B., Trishna S., and Vatsal D. P. (2015) Prospects of vitamin C as an additive in plasma of stored blood. Adv. Hematol. 2015, 961049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jeon S. M., Chandel N. S., and Hay N. (2012) AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485, 661–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee E. E., Ma J., Sacharidou A., Mi W., Salato V. K., Nguyen N., Jiang Y., Pascual J. M., North P. E., Shaul P. W., Mettlen M., and Wang R. C. (2015) A protein kinase C phosphorylation motif in GLUT1 affects glucose transport and is mutated in GLUT1 deficiency syndrome. Mol. Cell 58, 845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.