Abstract
Chondroitin sulfate (CS)/chondroitin (Chn) chains are indispensable for embryonic cell division and cytokinesis in the early developmental stages in Caenorhabditis elegans and mice, whereas heparan sulfate (HS) is essential for axon guidance during nervous system development. These data indicate that the fundamental functions of CS and HS are conserved from worms to mammals and that the function of CS/Chn differs from that of HS. Although previous studies have shown that C. elegans produces HS and non-sulfated Chn, whether the organism produces CS remains unclear. Here, we demonstrate that C. elegans produces a small amount of 4-O-sulfated Chn and report the identification of C41C4.1, an orthologue of the human chondroitin 4-O-sulfotransferase gene. Loss of C41C4.1 in C. elegans resulted in a decline in 4-O-sulfation of CS and an increase in the number of sulfated units in HS. C41C4.1 deletion mutants exhibited reduced survival rates after synchronization with sodium hypochlorite. Collectively, these results show for the first time that CS glycans are present in C. elegans and that the Chn 4-O-sulfotransferase responsible for the sulfation plays an important role in protecting nematodes from oxidative stress.
Keywords: Caenorhabditis elegans (C. elegans), chondroitin sulfate, oxidative stress, proteoglycan, sulfotransferase
Introduction
Chondroitin sulfate (CS),4 a linear polysaccharide that is covalently linked to specific core proteins to form CS proteoglycans (PG), is distributed on the surface of cells and within the extracellular matrix. Because CS moieties vary considerably with respect to the size and number of CS chains per core protein and in the position and degree of sulfation, they store a massive amount of functional information and thus exhibit a variety of biological functions (1). To date, CS has been postulated as playing roles not only in physiologic processes, such as cytokinesis, morphogenesis, and neuronal plasticity, but also in pathologic processes, including skeletal disorders, glial scar formation after brain injury, and viral and bacterial infections (1–4). Notably, we revealed that CS/chondroitin (Chn) chains are indispensable for embryonic cell division and cytokinesis during the early developmental stages in Caenorhabditis elegans and mice (5–8). These observations suggest that the function of CS/Chn is evolutionarily conserved from worms to mammals.
The CS/Chn biosynthetic mechanism in humans is quite similar to that in C. elegans (5, 6). The biosynthesis of CS chains occurs with N-acetylgalactosamine (GalNAc) and glucuronic acid (GlcUA) transferred alternately by Chn co-polymerases (1). CS chains are then modified by sulfotransferases (4, 9, 10). In humans, polymerization of CS disaccharide units is catalyzed by the following six homologous glycosyltransferases: Chn synthase-1 (ChSy-1) (11), ChSy-2 (12), ChSy-3 (13, 14), Chn polymerizing factor (ChPF) (15), Chn GalNAc transferase-1 (16, 17), and Chn GalNAc transferase-2 (18). In addition, to date, seven sulfotransferases involved in the sulfation of CS have been cloned (9). Four sulfotransferases that catalyze the sulfation of position 4 of GalNAc residues have been cloned. Chn 4-O-sulfotransferases-1, -2, and -3 (C4ST-1, -2, and -3) are responsible for the sulfation of position 4 of GalNAc residues in CS, whereas dermatan 4-O-sulfotransferase-1 catalyzes the transfer of a sulfate group to GalNAc residues adjacent to iduronic acid residues in dermatan sulfate (19–22). Chn 6-O-sulfotransferase-1 (C6ST-1) transfers a sulfate group to position 6 of GalNAc residues (23, 24). Uronyl 2-O-sulfotransferase catalyzes the 2-O-sulfation of GlcUA and iduronic acid (25). GalNAc 4-sulfate 6-O-sulfotransferase (GalNAc4S-6ST) transfers a sulfate group to position 6 of GalNAc(4-O-sulfate) formed by C4ST (26). In C. elegans, only two genes, sqv-5 (cChSy) and mig-22 (PAR2.4, cChPF, or pfc-1), are required for Chn biosynthesis (5, 6). However, the enzyme responsible for the sulfation of CS remains unknown (27), despite the identification of orthologues of genes encoding 3′-phosphoadenosine 5′-phosphosulfate (PAPS) synthase and the PAPS transporters (PAPST1 and PAPST2), which are required for the sulfation of CS and HS, a representative sulfated glycosaminoglycan (GAG) (28–30).
We recently showed that in mice, CS chains sulfated in specific patterns play important regulatory roles during the critical cortical plasticity period, which is induced by the maturation of parvalbumin-expressing interneurons through the incorporation of Otx2 (31). The specific sulfation of CS chains also plays an important role during mouse embryonic stem cell differentiation (32). In addition, we demonstrated that the neuritogenic effects of CSPG are mediated by interactions between CS chains exhibiting a specific sulfation pattern and contactin-1, a cell surface receptor that recognizes the specific sulfation pattern (33). Similarly, previous studies have revealed that HS is essential for embryogenesis during the later stages of development in C. elegans (34). It also has been shown that modification of HS plays an important role in nervous system development, particularly in axon guidance (35, 36). These data indicate that the sulfation patterns of CS and HS chains are important in determining specific functions. Although previous studies have shown that C. elegans produces HS and non-sulfated Chn, none have demonstrated the production of CS (37–39). Speculating that the fundamental mechanism underlying the biosynthesis and function of CS/Chn in C. elegans might be similar to that in humans, we hypothesized that C. elegans expresses a sulfotransferase orthologue. Previous screening of a C. elegans protein sequence database using the amino acid sequence of human C4ST-1 identified a candidate protein, C41C4.1 (27). Here, we demonstrated that C. elegans produces 4-O-sulfated CS and expresses an orthologue of C4ST, C41C4.1. In addition, we show that loss of the C41C4.1 gene in C. elegans results in a reduction in 4-O-sulfation of CS and an increase in the number of sulfated units of HS. We also report that C41C4.1 deletion mutants exhibit significantly lower survival rates under oxidative stress. Thus, this study shows a novel function of 4-O-sulfated CS in nematodes.
Results
Molecular Cloning of C41C4.1
Screening the WormBase using the deduced amino acid sequence of human C4ST-1 resulted in the identification of a few homologues in C. elegans (27). One homologue, C41C4.1, contained a 5′-untranslated region of 24 bp, a single open reading frame of 990 bp that codes for a protein of 329 amino acids, and a 3′-untranslated region of 125 bp. The predicted translation initiation site conformed to the Kozak initiation consensus sequence. A Kyte-Doolittle hydropathy plot revealed one prominent hydrophobic segment of 21 amino acid residues that begins at the amino terminus. In addition, the hydrophobic segment differs from a membrane anchor in that it contains two cationic residues and is not flanked by cationic residues. Moreover, an analysis using the SOSUIsignal software system (40) for the prediction of signal peptides and membrane proteins showed that the protein has a signal peptide. These results suggest that C41C4.1 is a soluble protein. Database searches revealed 21.3 and 20.6% amino acid sequence identity with human and mouse C4ST-1, respectively (Fig. 1). Thus, the features of the identified protein suggest that it is involved in the sulfation of Chn in C. elegans.
FIGURE 1.
Comparison of the predicted amino acid sequences of C. elegans C41C4.1, human C4ST-1, and mouse C4ST-1. The predicted amino acid sequences were aligned using the program GENETYX-MAC (version 10). The closed and shaded boxes indicate that the predicted amino acid in the alignment is identical in all three or any two sequences, respectively. Gaps introduced for maximal alignment are indicated by dashes. Putative membrane-spanning domains are boxed. The putative PAPS-binding domains, 5′-PSB and 3′-PB, are underlined.
Structural Analysis of C. elegans CS
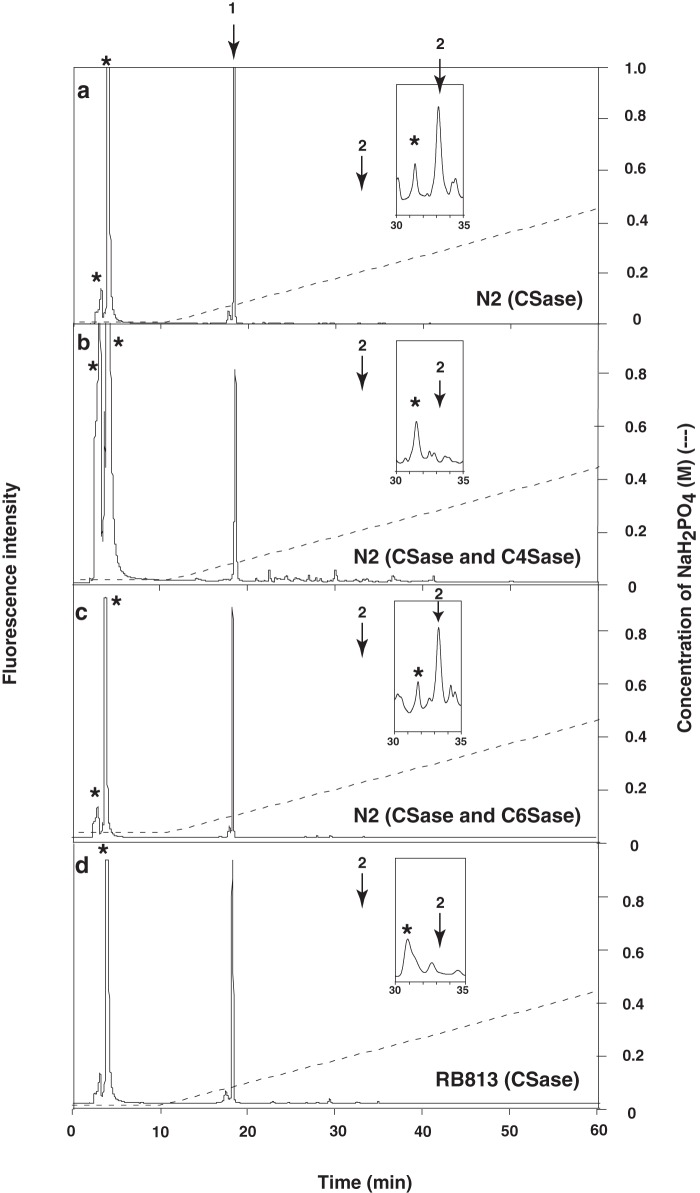
Previous studies have shown that C. elegans produces HS and non-sulfated Chn, but production of CS has not been demonstrated (37). It should be noted that in these previous studies, the amount of HS in C. elegans was so low that shark cartilage Chn 6-O-sulfate (Seikagaku Corp.) containing a negligible proportion of non-sulfated disaccharides was added as a carrier before the purification steps (5, 6, 37). Hence, we examined whether N2 worms contain sulfated Chn without adding the carrier. As shown in Fig. 2a and Table 1, peaks were evident following complete chondroitinase ABC (CSaseABC) digestion of the purified GAG fraction from C. elegans. A single major peak was detected at the elution position corresponding to the authentic non-sulfated Chn disaccharide ΔHexUAα1–3GalNAc (ΔDi-0S), and a small peak was detected at the elution position corresponding to the authentic Chn 4-O-sulfate disaccharide, ΔHexUAα1–3GalNAc(4-O-sulfate) (ΔDi-4S) (Fig. 2a). As shown in Fig. 2, b and c, the CSaseABC digestion yielded a small peak at the position of ΔDi-4S (Fig. 2a, inset). The peak disappeared following subsequent digestion with chondro-4-O-sulfatase (Fig. 2b) but not following digestion with chondro-6-O-sulfatase (Fig. 2c). In wild-type worms, HS disaccharide analysis revealed the expected profile (Table 2). Also, the amount of sulfated disaccharide in CS was similar to that in HS in wild-type worms (Tables 1 and 2). These results indicate that C. elegans produces 4-O-sulfated Chn.
FIGURE 2.
HPLC analysis of 2-aminobenzamide-labeled disaccharide composition by enzymatic digestion of N2 (wild-type) and RB813 (C41C4.1 mutant) nematodes. HPLC analysis of chondroitinase ABC (CSase) digest of N2 (a), chondroitinase ABC and chondro-4-O-sulfatase (C4Sase) digest of N2 (b), chondroitinase ABC and chondro-6-O-sulfatase (C6Sase) digest of N2 (c), and chondroitinase ABC digest of RB813 (d). a–d, the enzyme digest of the C. elegans GAG fraction was derivatized with 2-aminobenzamide and analyzed by HPLC using an amine-bound silica column (37). The peak eluting at ∼5 min and marked by an asterisk in the inset represents an acetate ion derived from the incubation buffer and an unknown substance derived from the chondroitinase ABC preparation, respectively. The insets show the magnified chromatogram between 30 and 35 min. The elution positions of authentic unsaturated disaccharides labeled with 2-aminobenzamide are indicated by numbered arrows: 1, ΔHexUAα1–3GalNAc; 2, ΔHexUAα1–3GalNAc(4-O-sulfate).
TABLE 1.
Disaccharide composition of C. elegans CS
Values are expressed as pmol of disaccharide/mg of dried worm homogenate. Means ± S.E. of three determinations are shown. ND, not detected (<0.01 pmol/mg).
| Composition | N2 | RB813 |
|---|---|---|
| pmol/mg (%) | ||
| ΔHexUAα1–3GalNAc | 1645 ± 114 (99.5) | 1894 ± 157 (100) |
| ΔHexUAα1–3GalNAc(4-O-sulfate) | 7.4 ± 0.2 (0.5) | ND |
| Total disaccharide | 1652.4 ± 105 | 1894 ± 157 |
TABLE 2.
Disaccharide composition of C. elegans HS
Values are expressed as pmol of disaccharide/mg of dried worm homogenate. The means ± S.E. of three determinations is shown. The degree of sulfation is expressed as the average number of sulfate groups/disaccharide unit. ND, not detected (<0.01 pmol/mg).
| Composition | N2 | RB813 |
|---|---|---|
| pmol/mg (%) | ||
| ΔHexUAα1–4GlcNAc | 3.4 ± 0.41 (60) | 4.2 ± 0.37 (55) |
| ΔHexUAα1–4GlcNAc(6-O-sulfate) | 0.6 ± 0.01 (11) | 0.9 ± 0.09 (12) |
| ΔHexUAα1–4GlcN(2-N-sulfate) | 0.7 ± 0.01 (13) | 1.1 ± 0.24 (14) |
| ΔHexUAα1–4GlcN(2-N-,6-O-disulfate) | ND | ND |
| ΔHexUA(2-O-sulfate) α1–4GlcN(2-N-sulfate) | 0.8 ± 0.01 (14) | 1.3 ± 0.08 (17) |
| ΔHexUA(2-O-sulfate) α1–4GlcN(2-N-,6-O-disulfate) | 0.1 ± 0.01 (2) | 0.1 ± 0.01 (2) |
| Total disaccharide | 5.6 ± 0.35 | 7.6 ± 0.46 |
| Degree of sulfation | 0.57 | 0.64 |
Expression of a Soluble Form of the Novel Sulfotransferase and Its Characterization as C. elegans C4ST
To facilitate the functional analysis of the putative sulfotransferase, a soluble form of the protein was generated by replacing the first 34 amino acids with a cleavable insulin signal sequence and a protein A-IgG-binding domain, as described under “Experimental Procedures.” The soluble putative sulfotransferase was then expressed in COS-1 cells as a recombinant enzyme fused with the protein A-IgG-binding domain. The fused enzyme expressed in the medium was adsorbed onto IgG-Sepharose beads to remove endogenous sulfotransferases, and the beads to which the enzyme was bound were used as the enzyme source. The bound fusion protein was assayed for sulfotransferase activity using Chn polymer as an acceptor substrate. As expected, sulfotransferase activity was detected with Chn polymer (0.52 ± 0.03 nmol/mg/h).
To identify the sulfotransferase reaction products, Chn polymer was labeled with [35S]sulfate by incubation with [35S]PAPS as a sulfate donor and the beads to which the enzyme was bound as the enzyme source. The reaction products were isolated by gel filtration and then digested with CSaseABC. The digest was analyzed by anion exchange HPLC using an amine-bound silica column, as described under “Experimental Procedures.” As shown in Fig. 3, a quantitative yield of a single 35S-labeled peak at the position of ΔHexUAα1–3GalNAc(4-O-sulfate) was obtained (Fig. 3a). This peak shifted to the position of inorganic sulfate upon subsequent digestion with chondro-4-O-sulfatase (Fig. 3b) but not upon digestion with chondro-6-O-sulfatase (Fig. 3c). These findings indicate that sulfate was incorporated exclusively at the GalNAc C-4 position in the non-sulfated disaccharide unit, GlcUAβ1–3GalNAc. In contrast, the recombinant enzyme exhibited no sulfotransferase activity toward N-acetylheparosan oligosaccharides or α-thrombomodulin containing the linkage region tetrasaccharide. Thus, the enzyme was identified as a homologue of human Chn 4-O-sulfotransferase.
FIGURE 3.
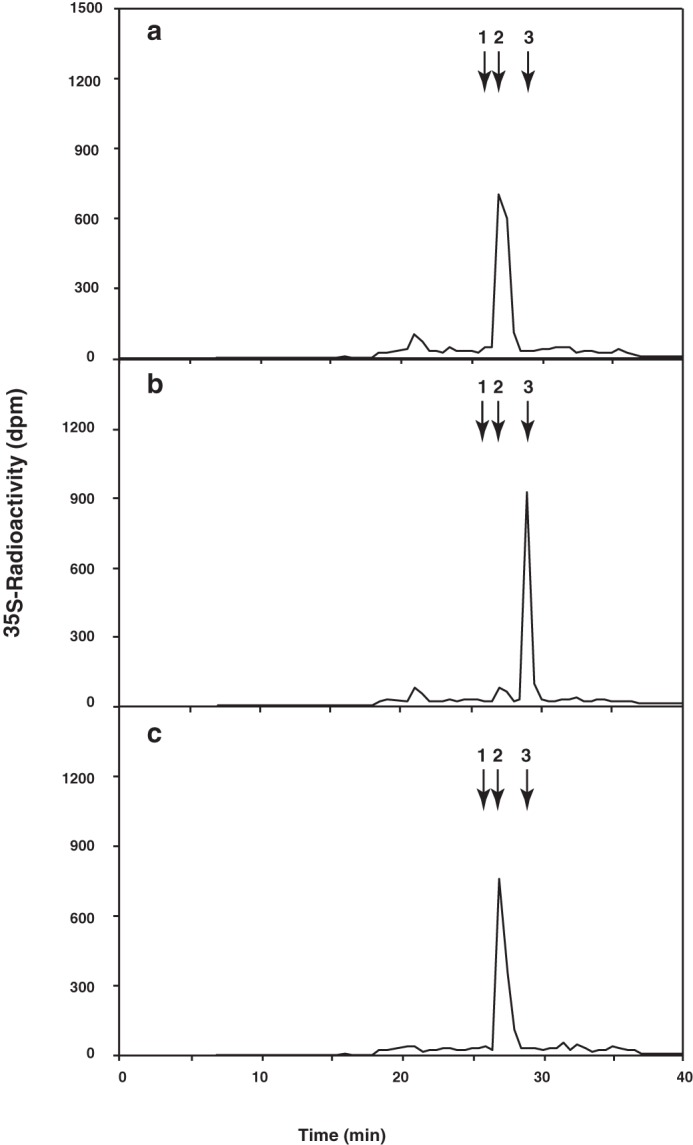
Identification of novel sulfotransferase reaction products. Enzymatic reactions were carried out using Chn polymer as an acceptor substrate under the incubation conditions described under “Experimental Procedures.” Reaction products were isolated by gel filtration using a syringe column packed with Sephadex G-25 and then digested with chondroitinase ABC (a), chondroitinase ABC and chondro-4-O-sulfatase (b), or chondroitinase ABC and chondro-6-O-sulfatase (c). The digests were analyzed by anion exchange HPLC using an amine-bound silica PA03 column as described under “Experimental Procedures.” The eluate was collected after 14 min of injection at 30-s intervals for radioactivity measurement by liquid scintillation counting. The numbered arrows indicate the elution positions of the authentic unsaturated disaccharides or inorganic sulfate: 1, ΔHexUAα1–3GalNAc(6-O-sulfate); 2, ΔHexUAα1–3GalNAc(4-O-sulfate); 3, inorganic [35S]sulfate.
Expression of the C41C4.1 Gene in the Nematode C. elegans
To examine the expression pattern of the identified C4ST gene (C41C4.1), EGFP-tagged C41C4.1 (C41C4.1::egfp) was introduced into N2 worms by microinjection. Strong EGFP fluorescence was observed in the head (Fig. 4, b–e) and tail (Fig. 4, g and h) regions of adult hermaphrodites. Counterstaining of the neurons of adult worms with DiI incorporation (Fig. 4, a and c–e, amphid neurons; Fig. 4, f and h, phasmid neurons) strongly indicates that amphid sheath cells (Fig. 4, b–e) and phasmid sheath cells (Fig. 4, g and h) are brightly fluorescent with EGFP.
FIGURE 4.
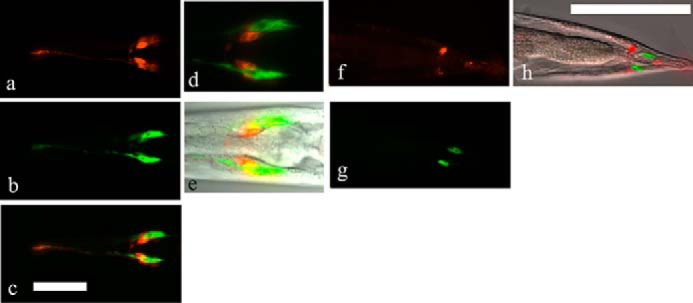
Expression of the C41C4.1 gene product in vivo. EGFP signals (green) were distributed in amphid sheath cells in the head region (b–e) and in phasmid sheath cells in the tail region (g and h) of transgenic worms. DiI (red) was taken in by neuronal cells (amphid/phasmid neurons) near the sheath cells. a, amphid neurons stained with DiI. b, amphid sheath cells in the head region expressing C41C4.1::egfp fluoresce brightly. c, merge, the distribution of the two signals was generally distinct. d, another merged photograph showing that the C41C4.1 gene is expressed in amphid sheath cells (green) adjacent to amphid neurons (red). e, image overlaid with the corresponding DIC image. f, in the tail region of transgenic worms, phasmid neurons (red) were stained with DiI. g, cells expressing C41C4.1::EGFP are found near the phasmid neurons in f. h, DIC image overlaid with f and g. Magnification: a–c, ×400; d–h, ×1000. Bar: 50 μm.
Immunohistologic Identification of 4-O-Sulfated Chn in Vivo
The antibody LY111, which recognizes 4-O-sulfated Chn, was used to determine the expression pattern of CS chains in C. elegans. In wild-type worms, strong staining in the cell-to-cell contact area of the spermatheca was observed (Fig. 5a), and the LY111 staining pattern (Fig. 5a) overlapped partially with the pattern observed in the spermatheca upon staining with the monoclonal antibody MH27, which recognizes the cell junction marker apical junction molecule-1 (AJM-1) (Fig. 5, d–f) (41). The uterus and eggshells of developing embryos were also stained strongly with the LY111 antibody (Fig. 5j). Strong LY111-associated fluorescence was observed in the head (Fig. 5m) and tail (Fig. 5o) regions. No similar staining patterns with the LY111 antibody were observed in RB813 worms lacking the C4ST gene (Fig. 5, b, h, k, n, and p). In the transgenic strain FX18330, which is a RB813 strain harboring the wild-type C4ST gene in extrachromosomal arrays, the positively stained cells for the LY111 antibody were mostly recovered (Fig. 5, c, i, and l). We also examined other types of antibodies that recognize CS in the wild-type and the RB813 animals (Fig. 6). A 1B5 antibody recognized non-sulfated Chn neoepitopes generated by predigestion with CSaseABC. As shown previously (5), immunohistochemical staining with the 1B5 antibody revealed strong expression of non-sulfated Chn in the eggshells (Fig. 6a). The staining patterns of the RB813 animals were comparable with those of wild-type animals (Fig. 6b). Although a 3B3 antibody is reactive with 6-O-sulfated unsaturated disaccharide neoepitope generated by predigestion with CSaseABC, it also recognizes non-sulfated unsaturated disaccharide neoepitope. Relatively weaker signals from eggshells stained with 3B3 were detected in both the wild-type (Fig. 6c) and the RB813 (Fig. 6d) animals. Because non-sulfated Chn is quite abundant in C. elegans and the staining patterns of the 3B3 antibody were similar to those of the 1B5, we assumed that the staining patterns of the 3B3 antibody reflect the expression of non-sulfated Chn. In contrast, after pretreatment with CSaseABC, a 2B6 antibody, which recognizes 4-O-sulfated unsaturated disaccharide neoepitope, did not show any reactivity to the eggshells of developing embryos (Fig. 6e) in wild-type animals, suggesting that 4-sulfation may not localize in the hexasaccharide linkage region. Similarly, the antibody CS56, which recognizes 6-O-sulfated Chn, exhibited no reactivity toward the eggshells of developing embryos (Fig. 6f) in wild-type animals, consistent with the absence of 6-O-sulfated Chn in C. elegans.
FIGURE 5.
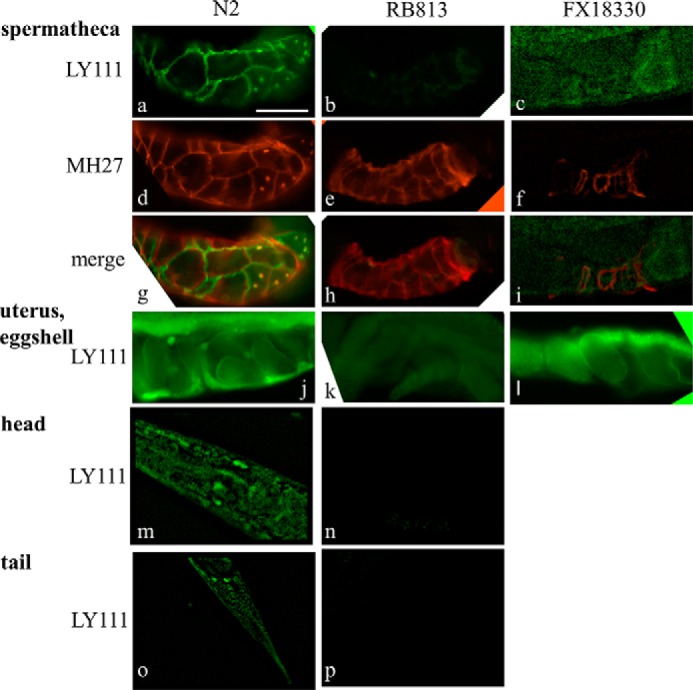
Immunohistologic identification of 4-O-sulfated Chn in vivo. a, in the germ line of wild-type worms, the spermatheca cells stained brightly with the anti-CS (LY111) antibody. Partial overlap of anti-CS staining (a) with anti-AJM-1 (MH27) staining (d) is indicated (g). In KO worms, no LY111 staining was observed (b), although anti-AJM-1 staining (e) was still positive. g–i, merged images of LY111 and MH27 staining. Eggshells, head region cells, and tail region cells (j, m, and o, respectively) were also stained with the LY111 antibody, but no signal was observed in RB813 worms (k, n, and p). LY111-positve cells were found in a transgenic strain carrying the wild-type C41C4.1 gene (FX18330) (c, i, and l). c, f, i, m, and o, images were sharpened using the 2D nearest-neighbor deconvolution algorithm. Bar: 20 μm.
FIGURE 6.
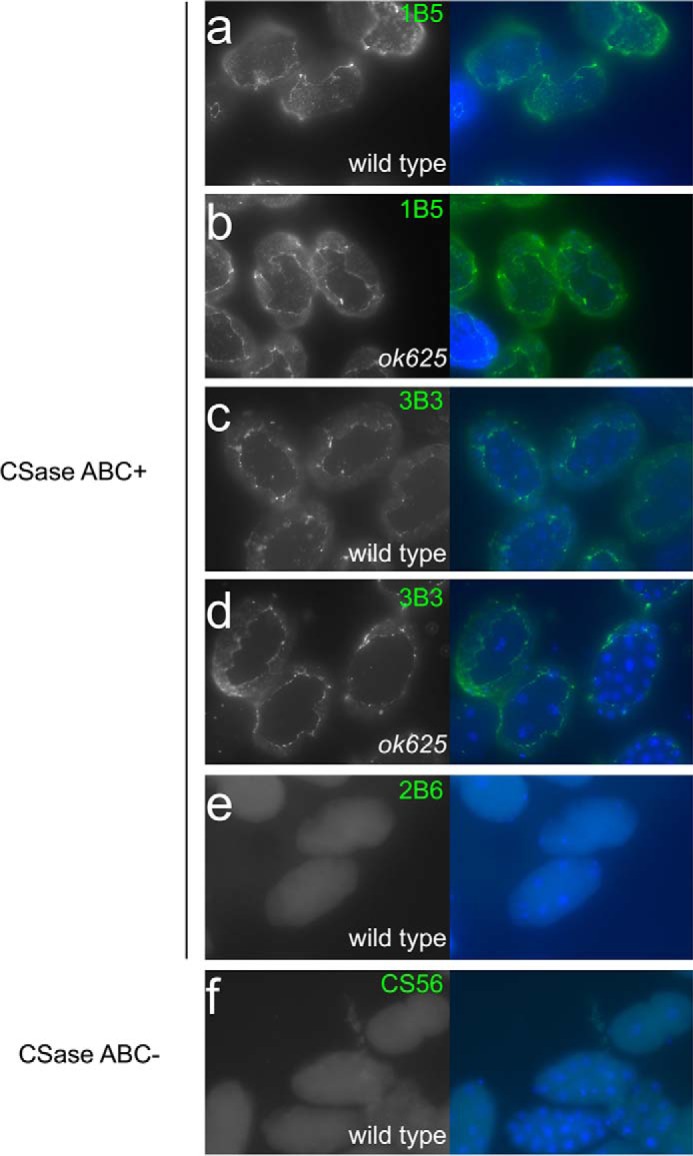
Immunohistologic analysis using 1B5, 3B3, 2B6, and CS56 anti-CS antibodies. In the wild-type and RB813 (ok625) mutant animals, the eggshells of the developing embryos were reactive with 1B5 (a and b) and 3B3 (c and d) antibodies. No signal was observed in the wild-type animals for 2B6 or CS56 antibodies (e and f). The specimens were pretreated with (a–e) or without (f) CSaseABC.
RNAi of C41C4.1 Gene and Deletion Mutant Strains
The C41C4.1 RNAi construct from the Ahringer library exhibited no apparent abnormalities (42, 43). Thus, the deletion alleles ok625 and tm576 from the Caenorhabditis Genetics Center (CGC, Minneapolis, MN) and the National BioResource Project, Japan, respectively, were analyzed. The tm576 allele, which leads to sterility and is lethal, was maintained in a heterozygous state. Although the deletion region of the gene of ok625 is wider than that of the tm576 allele, the RB813 strain harboring the ok625 allele is neither lethal nor sterile. This is suitable for biochemical analysis, and thus we selected the ok625 allele for further analysis. We backcrossed this allele more than seven times and used it in the study. The original RB813 strain from the CGC showed a significant life span extension phenotype (data not shown), but after backcrossing five times, the life span extension phenotype disappeared, suggesting that other affected sequences in the genome are responsible for the phenotype.
Determination of the Chn-4-O-sulfate Structure in C41C4.1 Deletion Mutants
To test whether worms harboring the ok625 allele lack 4-O-sulfated Chn, we examined the disaccharide composition of knock-out (KO) worms. The CS and HS sulfation patterns in C4ST mutants (RB813) were determined by CSaseABC or heparin lyase digestion followed by HPLC analysis, as described under “Experimental Procedures.” In the RB813 mutants, the level of non-sulfated CS units increased, whereas no 4-O-sulfated CS units were detected (Fig. 2d and Table 1). In addition, the level of non-sulfated HS units decreased in RB813 mutants, whereas the level of disulfated disaccharide units (ΔHexUA(2-O-sulfate)α1–4GlcNS) and the degree of sulfation increased slightly in RB813 mutants (Table 2). These results suggest that C41C4.1 is indispensable for the 4-O-sulfation of CS in vivo and that the sulfation patterns of CS and HS depend on C41C4.1 activity.
Low Survival Rate of KO Worms
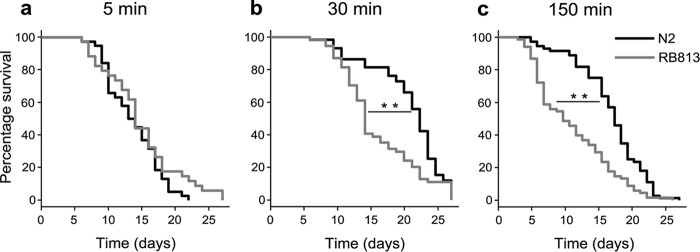
No apparent morphologic disorders were observed in either KO (RB813) or RNAi-treated C41C4.1. The germ line abnormalities and abnormal cell division during early embryogenesis observed in sqv-5/mig-22-depleted (ChSy-/ChPF-deficient) worms were not observed in either KO (RB813) or RNAi-treated C41C4.1 worms, indicating that sulfated Chn PG play a minimal role during early embryonic division. The RNA-Seq study carried out by Kimble and colleagues (44) revealed that the C41C4.1 gene is highly expressed in the germ line in a gender-neutral manner, suggesting that this maternally expressed gene does play a role in the germ line lineage. Thus we examined germ line phenotypes of the deletion allele. Because we detected no apparent morphologic abnormalities in the gonads of KO worms, we next examined brood size. Alkaline bleach (sodium hypochlorite (NaOCl)) treatment for 5 min is widely used to obtain developing nematode eggs (45). Experimental animals were synchronized with the standard bleach treatment to collect synchronized eggs from gravid worms in order to determine the brood size. No reduction in the brood size was observed after the standard bleach treatment. Because the original RB813 worms (before outcrossing) exhibited a longer life span, the difference in the life span of N2 worms and the backcrossed RB813 worms was also determined after bleach treatment. Bleach-treated RB813 worms (30- and 150-min bleach treatments, Fig. 7, b and c, respectively) exhibited a lower survival rate than control N2 worms, although the total life span was not affected. After 30 min of bleach treatment, the wild-type worms exhibited a higher survival rate than the untreated wild-type worms, whereas a significant decrease in survival rate (p = 0.001) was observed with the RB813 worms. Longer treatment (150 min) amplified the difference, and the survival rate of the KO worms decreased drastically, although the life span of the wild-type and KO worms did not change (p = 0.003). We detected no significant extension in the life span of the N2 and RB813 worms after the bleach treatment. No differences in life span or survival rate were observed between N2 and RB813 worms not subjected to bleach treatment (data not shown).
FIGURE 7.
Increased mortality in C41C4.1 KO worms. Kaplan-Meier plots of bleach-treated (5, 30, and 150 min, a–c, respectively) N2 and RB813 worms. Bleach-treated RB813 worms exhibited a higher mortality rate than bleach-treated N2 worms. **, p < 0.005.
Tolerance to Oxidative Stress
Longer bleach treatment (150 min) drastically reduced the number of hatching eggs in N2 and RB813 worms. Tolerance to the treatment was markedly lower in RB813 worms. To determine whether the effect of bleach treatment was the result of oxidative stress, we next examined the effect of hydrogen peroxide, another oxidizing agent, on survival. After the standard bleach treatment for two generations, the young adult animals were treated with hydrogen peroxide (60 mm) for 2 h. The treated animals showed a lower survival rate for RB813 animals compared with similarly treated N2 animals (Table 3). The phenotype to the oxidative stress was rescued in the RB813 animals harboring the wild-type C41C4.1 transgene (FX18330 strain) (Table 3). These results indicate that these oxidizing agents produce similar toxic effects that are detrimental to embryo survival, suggesting that sulfated Chn played a role in protecting the worms from oxidative stress.
TABLE 3.
C41C4.1 KO worms exhibit hypersensitivity to hydrogen peroxide
The number of living animals after 2 h of hydrogen peroxide treatment was determined. KO (RB813 strain) animals exhibited lower tolerance to hydrogen peroxide than N2 (wild-type) animals (N2 vs. RB813, p = 0.00017, Holm's test). KO worms harboring the wild-type C41C4.1 gene (FX18330 strain) showed a tolerance phenotype comparable with the N2 animals (N2 vs. FX18330, p = 0.876; FX18330 vs. RB813, p = 0.00112, Holm's test).
| H2O2 treatment | N2 (n = 131) | RB813 (n = 124) | FX18330 (n = 63) | p value |
|---|---|---|---|---|
| % survival rate | ||||
| 2 h | 60.3 | 34.7 | 61.9 | 3.04 × 10−5 |
Discussion
In this study, we showed for the first time that C. elegans produces 4-O-sulfated Chn and that the C41C4.1 gene is the C. elegans orthologue of human C4ST. In addition, we showed that loss of C41C4.1 in C. elegans results in a reduction of 4-O-sulfation of CS and an increase in the number of sulfated units of HS. This increased degree of HS sulfation was similar to that reported for cChSy- and cChPF-RNAi F1 worms (5, 6). Moreover, the C41C4.1 exhibited no sulfotransferase activity toward N-acetylheparosan oligosaccharides or α-thrombomodulin containing the linkage region tetrasaccharide. These results demonstrate that the levels of CS and HS in vivo are interdependent and that C41C4.1 is directly involved in the synthesis of 4-O-sulfated CS disaccharides in C. elegans. Furthermore, loss of the C41C4.1 gene in C. elegans increases the death rate of worms treated with oxidizing agents. This increased susceptibility to bleach/hydrogen peroxide indicates that sulfated Chn in the eggshells plays an important role in protecting the embryo from oxidizing agents.
The major CS found in mammalian tissues contains a sulfate group at position 4 or 6 of GalNAc residues. The ratio of 4-sulfation to 6-sulfation reportedly changes during development in chicken and human epiphyseal cartilage and rat skin (46–48). Characteristically, the proportion of 6-O-sulfation gradually decreases, whereas that of 4-O-sulfation progressively increases, resulting in an incremental change in the 4S to 6S ratio during development (48). Sulfotransferase expression plays a significant role in regulating the sulfation profile of CS structures (48). In contrast to the CS found in mammalian tissues, C. elegans and Drosophila contain only 4-O-sulfated CS structures and considerably more non-sulfated structures than 4-O-sulfated structures (this study and Ref. 38).
Previously, we demonstrated that blocking Chn synthesis results in defects of cytokinesis during early embryogenesis in nematodes (5, 6). However, in this report, the embryos of C41C4.1-null mutants did not exhibit any cytokinesis defects, suggesting that 4-O-sulfation of CS is dispensable for cytokinesis in C. elegans. Consistent with those results, in RB813 mutant worms, the number of non-sulfated units in CS increased, whereas no 4-O-sulfated units were detected. These data suggest that the amount of Chn is important for cytokinesis in C. elegans.
The loss of C4ST activity resulted in hypersensitivity to bleach and hydrogen peroxide, possibly due to inhibition of the formation of intact eggshells. The presence of 4-O-sulfated Chn in the eggshells of N2 worms and its absence in the eggshells of mutant worms (Fig. 5, j and k) supports this possibility. If eggshell permeability is affected by the loss of 4-O-sulfated Chn, abnormal cytokinesis resulting from osmotic stress might be expected (49). However, RB813 mutant embryos did not exhibit an abnormal osmosensitive cytokinesis defect phenotype. Thus, the possibility that sulfated Chn plays a role in osmolarity regulation seems less likely. Nematode eggshells consist of a vitelline layer, a chitin layer with an underlying Chn PG layer, a perivitelline layer, a permeability layer, and a periembryonic space above the embryonic cell membrane (50). In the standard bleach treatment (3–5 min), the outer eggshell layer of C. elegans embryos is removed, whereas the chitinous and lipid layers remain intact (50, 51). To examine the effect of long-term bleach treatment on eggshell permeability, we stained mutant eggshells (of nematodes subjected to 150-min bleach treatment) with FM4-64 dye (51) to determine whether the permeability barrier of the embryos remained intact or was affected by oxidation. We found that the permeability barrier remained intact or was least affected in the bleach-treated embryos (data not shown). These results suggest that 4-O-sulfated Chn PG in the eggshell play an anti-oxidant role but do not play a major role in maintaining eggshell permeability.
Previously reported DNA microarray results (using significance analysis of microarrays (SAM)) (52) indicated that inhibition of the cyc-1 gene, which encodes a component of complex III (cytochrome c reductase), significantly reduces the expression of the C41C4.1 gene. The cyc-1 gene is essential in the respiratory chain, as it is involved in regulating H2O2 levels in C. elegans. Inhibition of the cyc-1 gene by RNAi extends the life span and reduces the fluctuations in H2O2 (53). Fu et al. (53) also showed that externally added H2O2 increases the internal H2O2 level as well as the degree of fluctuation in H2O2 levels in C. elegans. Thus, the decrease in survival rate observed in our study after bleach treatment could have been the result of increased internal H2O2 levels.
Expression of the C41C4.1 gene was strong in both amphid sheath and phasmid sheath cells, both of which are glial cells in the C. elegans neuronal system. Our previous study indicated that 3′-phosphoadenosine 5′-phosphate synthase (PAP) and PAPS transporter genes, which are indispensable for sulfation, are expressed in amphid sheath cells (28). A previous DNA microarray study reported that C41C4.1 mRNA is enriched (18.3-fold) in amphid sheath cells (54). These results strongly suggest that C4ST plays a role in the nematode glial system. Laser ablation of amphid sheath cells showed that glia-ablated worms exhibit defective amphid wing C cell-mediated odortaxis toward 1% isoamyl alcohol and 0.5% benzahldehyde (54). It would be intriguing to examine whether ablation of the C41C4.1 sulfotransferase or PAPS-related genes (pps-1 and pst-1) also affects nematode odortaxis behavior.
CS PG are major components of the central nervous system extracellular matrix in mammals. It has been well established that up-regulation of glial-derived CS PG expression within glial scars and perineuronal nets (PNN) creates a barrier to axonal regrowth and sprouting. Recent studies have shown that CS chains in PNN have a critical role in neuroprotection against oxidative stress, which can be a risk factor of many neurodegenerative disorders (55). It is well known that protein aggregates are not uniformly distributed but occur in selective regions of brains with Alzheimer's disease. Notably, it has been shown that regions rich in PNN tend to be protected from the deposition of protein aggregates as compared with regions with sparse PNN (56). CS chains in PNN may exert a neuroprotective effect by acting as antioxidants, because depositions of lipofuscin pigment, which are generated by iron-catalyzed oxidative processes, are rarely observed in neurons with PNN (57). Thus, the polyanionic nature of CS chains possibly reduces the local oxidative potential by scavenging reactive oxygen species, in view of the fact that CS chains in the PNN consist mainly of 4-O-sulfation (31, 58) and loss of 4-O-sulfated Chn in C. elegans increases the death rate of worms treated with oxidizing agents, as shown in the present study. In a few in vitro experiments, Cu2+/Fe2+-mediated human LDL oxidation is inhibited by 4-O-sulfated CS. The inhibitory effect was unique to 4-O-sulfated CS, and 6-O-sulfated CS was ineffective (59, 60). Thus, 4-O-sulfation in CS chains plays an important role in protecting animals from oxidative stress, and the function of 4-O-sulfation in CS chains seems to be evolutionarily conserved from worms to mammals.
Experimental Procedures
Nematode Strains
Most of the C. elegans strains used in this study were obtained from the CGC. The deletion strain RB813 (ok625) was isolated by the C. elegans Gene Knock-out Consortium and was obtained from the CGC. A C. elegans KO allele, tm576, which was obtained from the National BioResource Project Japan C. elegans, was also examined.
Materials
35S-Labeled PAPS (1.69 mCi/mmol) was purchased from PerkinElmer Life Sciences. Chn (a chemically desulfated derivative of whale cartilage chondroitin sulfate A), CSaseABC (EC 4.2.2.4), chondro-4-O-sulfatase (EC 3.1.6.9), chondro-6-O-sulfatase (EC 3.1.6.10), heparinase (EC 4.2.2.7), and heparitinase (EC 4.2.2.8) were purchased from Seikagaku Corp. (Tokyo).
Transgenic Strains
We made six transgenic strains for the rescue experiment. However, five of them showed a slow growth phenotype for some reason, so that we excluded them from use in the oxidative stress assay. The remaining healthy strain, FX18330 (ok625, tmEx4398), was used for the rescue experiments. An extrachromosomal array of tmEx4398 was made by injecting 50 ng/μl pFX_C41C4.1::EGFP, 10 ng/μl Pmyo-2::mCherry, and 140 ng/μl DNA ladder into RB813 (ok625) animals.
Construction of a Soluble Form of C41C4.1
A cDNA fragment encoding a truncated form of C41C4.1 (lacking the first 34 N-terminal amino acids, including the putative cytoplasmic and transmembrane domains) was amplified by reverse transcription-PCR with adult C. elegans total RNA as the template using a forward primer (5′-CGGGATCCCCATACTTATTCTCGATTTTAT-3′) containing a BamHI restriction site and a reverse primer (5′-CGGGATCCTCAAACTTTTATTTCATACCAA-3′) containing a BamHI restriction site and a stop codon. PCR was carried out with KOD polymerase (Toyobo, Osaka, Japan) in 5% (v/v) dimethyl sulfoxide for 32 cycles of 94 °C for 30 s, 60 °C for 30 s, and 68 °C for 180 s. The resulting PCR fragment was subcloned into the BamHI site of pGIR201protA (61) resulting in fusion of the insulin signal sequence and the protein A sequence present in the vector as described previously (5, 6).
Expression of the Soluble Form of C41C4.1 and Enzyme Assays
The expression plasmid (6.0 μg) was transfected into COS-1 cells using FuGENETM 6 (Roche Applied Science) according to the manufacturer's instructions, and the cells were cultured in 100-mm plates. After 2 days of culture at 37 °C, 6 ml of the culture medium was collected and incubated with 10 μl of IgG-Sepharose (Amersham Biosciences) for 6 h at 4 °C. The beads were recovered by centrifugation, washed with the assay buffer, and then resuspended in the assay buffer and tested for sulfotransferase activity. Assays of sulfotransferase activity were carried out as described previously (22) with slight modifications. Briefly, the standard reaction mixture (30 μl) contained 10 μl of resuspended beads, 50 mm imidazole-HCl (pH 6.8), 2 mm dithiothreitol, 10 μm [35S]PAPS (3 × 105 dpm), and 100 μg of Chn, N-acetylheparosan oligosaccharides (34), or α-thrombomodulin containing the linkage region tetrasaccharide (16) as an acceptor substrate. The reaction mixture was incubated at 37 °C overnight and then gel-filtered using a syringe column packed with Sephadex G-25 (super-fine) (62).
Identification of Transferase Reaction Products
Radioactive fractions containing the enzyme reaction products were pooled and desiccated. The dried products were digested with 20 mIU of CSaseABC, and the digest was then analyzed by anion exchange HPLC using an amine-bound silica PA03 column as described previously (63). To confirm the disaccharide structure, chondro-4-O-sulfatase or 6-O-sulfatase digestion of the CSaseABC digest was conducted with the remainder of the 35S-labeled isolated product as described previously (63).
Analysis of GAG
The GAG fraction was analyzed as described elsewhere (5, 6, 37). Freshly cultured nematodes were sonicated using a GE-70 ultrasonic processor (Branson Ultrasonics) and then freeze-dried. The dried samples (1250 mg of wild-type worms and 990 mg of RB813 worms) were extracted with acetone and then treated with 6 ml of 1.0 m NaBH4/0.05 m NaOH at 4 °C for 20 h. The purified GAG fraction was digested with CSaseABC or a mixture of heparin lyases I and II, and then the digests were derivatized with 2-aminobenzamide and analyzed by HPLC to determine the disaccharide composition. To confirm the disaccharide structure, chondro-4-O-sulfatase or 6-O-sulfatase digestion of the CSaseABC digest was conducted with the remainder of the purified GAG fraction.
Expression of C41C4.1::EGFP in the Nematode C. elegans
The promoter and coding region of the C41C4.1 cDNA was fused with an EGFP coding sequence as described previously (28, 30), and the resulting product was microinjected into N2 worms to generate the transgenic strain. The transgenic worms were observed under a fluorescence microscope as described previously (5).
Immunohistologic Identification of Sulfated Glycans
A monoclonal antibody against 4-O-sulfated Chn (LY111, Seikagaku Corp.) or 6-O-sulfated Chn (CS56, Sigma) was used to detect CS in nematodes. Monoclonal antibodies 1B5, 2B6, and 3B3 (Cosmo Bio, Tokyo) were used at a 1:20 ratio. CSaseABC (50 mIU) treatment was performed as described (5) before the primary antibodies were added.
Worms were fixed in 100% methanol (−20 °C) for 10 min. The fixed worms were freeze-cracked and then stained with the LY111 antibody (1:100 dilution in washing buffer:PBS with 3% bovine serum albumin (protease-free, Intergen)) as described previously (5). The MH27 antibody was obtained from the Developmental Studies Hybridoma Bank of the University of Iowa and used (at a 1:15 dilution in the washing buffer) to detect AJM-1 (41). For the secondary antibody, Alexa Fluor 488-conjugated goat anti-mouse IgM (μ-chain specific, Invitrogen) (×1000) and Alexa Fluor 594-conjugated ant-mouse IgG (γ-chain specific, Invitrogen) (×1000) were used, respectively. Nematodes were fed DiI (Wako Chemicals) to stain amphid neurons and phasmid neurons as described elsewhere (64). Differential interference contrast (DIC) and fluorescence images were obtained using a Leica DMRXA fully automatic microscope (Leica, Wetzlar, Germany) or Olympus BX51 fluorescence/DIC microscope (Tokyo) as described previously (5). The acquired images were processed using MetaMorph software (version 6.1r5, Molecular Devices Corp.). Some images were sharpened using the MetaMorph 2D nearest-neighbor deconvolution tool.
Determination of the Lethality of Bleach and Hydrogen Peroxide and Their Effect on Brood Size
Worms were treated with 150 mm NaOCl (45). Gravid worms were treated with the bleach solution for various incubation times (5–150 min). Immediately following the treatment, the number of eggs just after the treatment was counted, and the number of hatched worms after 24 and 48 h was counted for an accurate count of the birth rate.
After the standard bleach treatment for two generations, the young adult animals (3 days after the bleach treatment) were collected into the 3-well plates and incubated with H2O2 solution (50 mm in M9) for 60 min. After the incubation, the worms were collected with a Pasteur pipette and plated onto a region of an agar plate without the Escherichia coli OP50 lawn. After 10 min, a fresh M9 solution was pipetted onto the worms, and the remnant of H2O2 was diluted with the M9 solution. After 10 min, the worms were transferred to a new plate with E. coli OP50 with a worm picker. After a 2-h incubation at 20 °C, the number of surviving animals was determined by gently touching the worms with a worm picker under a stereomicroscope (Olympus SZX16, Tokyo) with high magnification (×115 to ×230).
Statistical Analysis
Statistical analyses, including Fisher's exact test and one-way analysis of variance followed by Holm's multiple comparison test, were performed using the R statistical package (R, version 2.11.0). Survival was assessed using the Kaplan-Meier method and log rank test with R-Commander and its plugin package, EZR (65).
Author Contributions
T. I. performed the experiments, analyzed the results shown in Figs. 1–3 and Tables 1 and 2, and helped write the paper. K. D. carried out transgenic analyses, constructed rescued strains, carried out the immunostaining shown in Fig. 6, and collected samples for biochemical analyses. Y. W. performed the experiments and analyzed the data shown in Tables 1 and 2. K. H. N. analyzed the transgenic, KO, and rescued strains, carried out immunohistologic staining in Figs. 4 and 5, and helped write the paper. D. M. carried out transgenic analyses and sample preparation for biochemical analyses. N. K. isolated the backcrossed KO strain, carried out brood size and life span analyses, and helped write the paper. M. R. carried out immunostaining of the rescued strains and carried out phenotype analyses under oxidative stress of all the strains with M. T. E. Y. and A. K. carried out phenotype analyses under oxidative stress conditions on wild-type and KO strain. S. M. isolated one of the KO alleles (tm576) and analyzed the results obtained by using the KO animals. K. N. designed the C. elegans experiments, carried out statistical analyses, and helped write the paper. H. K. conceived and coordinated the study, designed the experiments, and helped write the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgment
We thank Masanori Fukuoka for technical assistance.
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT)-supported Program for the Strategic Research Foundation at Private Universities, 2012–2016 (to H. K.) and by Grants-in-aid for Scientific Research on Innovative Areas (23110003 to H. K.), Scientific Research B (25293014 and 16H05088 to H. K.), and Scientific Research C (16K07298 to K. N.). This work was also supported by grants from the Japan Society for the Promotion of Science (JSPS) and the Core Research for Evolutional Science and Technology (CREST) Program of the Japan Science and Technology Corp. (to K. N.) and by Grant 120140 from the Mizutani Foundation (to K. N.). The authors declare that they have no conflicts of interest with respect to the contents of this article.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) AB862094
- CS
- chondroitin sulfate
- Chn
- chondroitin
- ChSy
- chondroitin synthase
- ChPF
- chondroitin polymerizing factor
- CSaseABC
- chondroitinase ABC
- DiI
- 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- HS
- heparan sulfate
- PG
- proteoglycan
- GlcUA
- d-glucuronic acid
- C4ST
- chondroitin 4-O-sulfotransferase
- PAPS
- 3′-phosphoadenosine 5′-phosphosulfate
- PNN
- perineuronal net
- GAG
- glycosaminoglycan
- CGC
- Caenorhabditis Genetics Center
- AJM-1
- apical junction molecule-1
- DIC
- differential interference contrast.
References
- 1. Mikami T., and Kitagawa H. (2013) Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta 1830, 4719–4733 [DOI] [PubMed] [Google Scholar]
- 2. Sugahara K., and Kitagawa H. (2000) Recent advances in the study of the biosynthesis and functions of sulfated glycosaminoglycans. Curr. Opin. Struct. Biol. 10, 518–527 [DOI] [PubMed] [Google Scholar]
- 3. Sugahara K., Mikami T., Uyama T., Mizuguchi S., Nomura K., and Kitagawa H. (2003) Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 13, 612–620 [DOI] [PubMed] [Google Scholar]
- 4. Kitagawa H. (2014) Using sugar-remodeling to study chondroitin sulfate function. Biol. Pharm. Bull. 37, 1705–1712 [DOI] [PubMed] [Google Scholar]
- 5. Mizuguchi S., Uyama T., Kitagawa H., Nomura K. H., Dejima K., Gengyo-Ando K., Mitani S., Sugahara K., and Nomura K. (2003) Chondroitin proteoglycans are involved in cell division of Caenorhabditis elegans. Nature 423, 443–448 [DOI] [PubMed] [Google Scholar]
- 6. Izumikawa T., Kitagawa H., Mizuguchi S., Nomura K. H., Nomura K., Tamura J., Gengyo-Ando K., Mitani S., and Sugahara K. (2004) Nematode chondroitin polymerizing factor showing cell-/organ-specific expression is indispensable for chondroitin synthesis and embryonic cell division. J. Biol. Chem. 279, 53755–53761 [DOI] [PubMed] [Google Scholar]
- 7. Izumikawa T., Kanagawa N., Watamoto Y., Okada M., Saeki M., Sakano M., Sugahara K., Sugihara K., Asano M., and Kitagawa H. (2010) Impairment of embryonic cell division and glycosaminoglycan biosynthesis in glucuronyltransferase-I-deficient mice. J. Biol. Chem. 285, 12190–12196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hwang H. Y., Olson S. K., Esko J. D., and Horvitz H. R. (2003) Caenorhabditis elegans early embryogenesis and vulval morphogenesis require chondroitin biosynthesis. Nature 423, 439–443 [DOI] [PubMed] [Google Scholar]
- 9. Kusche-Gullberg M., and Kjellén L. (2003) Sulfotransferases in glycosaminoglycan biosynthesis. Curr. Opin. Struct. Biol. 13, 605–611 [DOI] [PubMed] [Google Scholar]
- 10. Miyata S., and Kitagawa H. (2015) Mechanisms for modulation of neural plasticity and axon regeneration by chondroitin sulfate. J. Biochem. 157, 13–22 [DOI] [PubMed] [Google Scholar]
- 11. Kitagawa H., Uyama T., and Sugahara K. (2001) Molecular cloning and expression of a human chondroitin synthase. J. Biol. Chem. 276, 38721–38726 [DOI] [PubMed] [Google Scholar]
- 12. Izumikawa T., Uyama T., Okuura Y., Sugahara K., and Kitagawa H. (2007) Involvement of chondroitin sulfate synthase-3 (chondroitin synthase-2) in chondroitin polymerization through its interaction with chondroitin synthase-1 or chondroitin-polymerizing factor. Biochem. J. 403, 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Izumikawa T., Koike T., Shiozawa S., Sugahara K., Tamura J., and Kitagawa H. (2008) Identification of chondroitin sulfate glucuronyltransferase as chondroitin synthase-3 involved in chondroitin polymerization: chondroitin polymerization is achieved by multiple enzyme complexes consisting of chondroitin synthase family members. J. Biol. Chem. 283, 11396–11406 [DOI] [PubMed] [Google Scholar]
- 14. Gotoh M., Yada T., Sato T., Akashima T., Iwasaki H., Mochizuki H., Inaba N., Togayachi A., Kudo T., Watanabe H., Kimata K., and Narimatsu H. (2002) Molecular cloning and characterization of a novel chondroitin sulfate glucuronyltransferase that transfers glucuronic acid to N-acetylgalactosamine. J. Biol. Chem. 277, 38179–38188 [DOI] [PubMed] [Google Scholar]
- 15. Kitagawa H., Izumikawa T., Uyama T., and Sugahara K. (2003) Molecular cloning of a chondroitin polymerizing factor that cooperates with chondroitin synthase for chondroitin polymerization. J. Biol. Chem. 278, 23666–23671 [DOI] [PubMed] [Google Scholar]
- 16. Uyama T., Kitagawa H., Tamura Ji J., and Sugahara K. (2002) Molecular cloning and expression of human chondroitin N-acetylgalactosaminyltransferase: the key enzyme for chain initiation and elongation of chondroitin/dermatan sulfate on the protein linkage region tetrasaccharide shared by heparin/heparan sulfate. J. Biol. Chem. 277, 8841–8846 [DOI] [PubMed] [Google Scholar]
- 17. Izumikawa T., Sato B., Mikami T., Tamura J., Igarashi M., and Kitagawa H. (2015) GlcUAβ1–3Galβ1–3Galβ1–4Xyl(2-O-phosphate) is the preferred substrate for chondroitin N-acetylgalactosaminyltransferase-1. J. Biol. Chem. 290, 5438–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uyama T., Kitagawa H., Tanaka J., Tamura J., Ogawa T., and Sugahara K. (2003) Molecular cloning and expression of a second chondroitin N-acetylgalactosaminyltransferase involved in the initiation and elongation of chondroitin/dermatan sulfate. J. Biol. Chem. 278, 3072–3078 [DOI] [PubMed] [Google Scholar]
- 19. Hiraoka N., Nakagawa H., Ong E., Akama T. O., Fukuda M. N., and Fukuda M. (2000) Molecular cloning and expression of two distinct human chondroitin 4-O-sulfotransferases that belong to the HNK-1 sulfotransferase gene family. J. Biol. Chem. 275, 20188–20196 [DOI] [PubMed] [Google Scholar]
- 20. Kang H. G., Evers M. R., Xia G., Baenziger J. U., and Schachner M. (2002) Molecular cloning and characterization of chondroitin-4-O-sulfotransferase-3: a novel member of the HNK-1 family of sulfotransferases. J. Biol. Chem. 277, 34766–34772 [DOI] [PubMed] [Google Scholar]
- 21. Evers M. R., Xia G., Kang H. G., Schachner M., and Baenziger J. U. (2001) Molecular cloning and characterization of a dermatan-specific N-acetylgalactosamine 4-O-sulfotransferase. J. Biol. Chem. 276, 36344–36353 [DOI] [PubMed] [Google Scholar]
- 22. Mikami T., Mizumoto S., Kago N., Kitagawa H., and Sugahara K. (2003) Specificities of three distinct human chondroitin/dermatan N-acetylgalactosamine 4-O-sulfotransferases demonstrated using partially desulfated dermatan sulfate as an acceptor: implication of differential roles in dermatan sulfate biosynthesis. J. Biol. Chem. 278, 36115–36127 [DOI] [PubMed] [Google Scholar]
- 23. Fukuta M., Uchimura K., Nakashima K., Kato M., Kimata K., Shinomura T., and Habuchi O. (1995) Molecular cloning and expression of chick chondrocyte chondroitin 6-sulfotransferase. J. Biol. Chem. 270, 18575–18580 [DOI] [PubMed] [Google Scholar]
- 24. Tsutsumi K., Shimakawa H., Kitagawa H., and Sugahara K. (1998) Functional expression and genomic structure of human chondroitin 6-sulfotransferase. FEBS Lett. 441, 235–241 [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi M., Sugumaran G., Liu J., Shworak N. W., Silbert J. E., and Rosenberg R. D. (1999) Molecular cloning and characterization of a human uronyl 2-sulfotransferase that sulfates iduronyl and glucuronyl residues in dermatan/chondroitin sulfate. J. Biol. Chem. 274, 10474–10480 [DOI] [PubMed] [Google Scholar]
- 26. Koike T., Mikami T., Shida M., Habuchi O., and Kitagawa H. (2015) Chondroitin sulfate-E mediates estrogen-induced osteoanabolism. Sci. Rep. 5, 8994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mizuguchi S., Dejima K., and Nomura K. (2009) Sulfation and related genes in Caenorhabditis elegans. Trends Glycosci. Glycotechnol. 21, 179–191 [Google Scholar]
- 28. Dejima K., Seko A., Yamashita K., Gengyo-Ando K., Mitani S., Izumikawa T., Kitagawa H., Sugahara K., Mizuguchi S., and Nomura K. (2006) Essential roles of 3′-phosphoadenosine 5′-phosphosulfate synthase in embryonic and larval development of the nematode Caenorhabditis elegans. J. Biol. Chem. 281, 11431–11440 [DOI] [PubMed] [Google Scholar]
- 29. Bhattacharya R., Townley R. A., Berry K. L., and Bülow H. E. (2009) The PAPS transporter PST-1 is required for heparan sulfation and is essential for viability and neural development in. C. elegans. J. Cell Sci. 122, 4492–4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dejima K., Murata D., Mizuguchi S., Nomura K. H., Izumikawa T., Kitagawa H., Gengyo-Ando K., Yoshina S., Ichimiya T., Nishihara S., Mitani S., and Nomura K. (2010) Two Golgi-resident 3′-phosphoadenosine 5′-phosphosulfate transporters play distinct roles in heparan sulfate modifications and embryonic and larval development in Caenorhabditis elegans. J. Biol. Chem. 285, 24717–24728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miyata S., Komatsu Y., Yoshimura Y., Taya C., and Kitagawa H. (2012) Persistent cortical plasticity by up regulation of chondroitin 6-sulfation. Nat. Neurosci. 15, 414–422 [DOI] [PubMed] [Google Scholar]
- 32. Izumikawa T., Sato B., and Kitagawa H. (2014) Chondroitin sulfate is indispensable for pluripotency and differentiation of mouse embryonic stem cells. Sci. Rep. 4, 3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mikami T., Yasunaga D., and Kitagawa H. (2009) Contactin-1 is a functional receptor for neuroregulatory chondroitin sulfate-E. J. Biol. Chem. 284, 4494–4499 [DOI] [PubMed] [Google Scholar]
- 34. Kitagawa H., Izumikawa T., Mizuguchi S., Dejima K., Nomura K. H., Egusa N., Taniguchi F., Tamura J., Gengyo-Ando K., Mitani S., Nomura K., and Sugahara K. (2007) Expression of rib-1, a Caenorhabditis elegans homolog of the human tumor suppressor EXT genes, is indispensable for heparan sulfate synthesis and embryonic morphogenesis. J. Biol. Chem. 282, 8533–8544 [DOI] [PubMed] [Google Scholar]
- 35. Bülow H. E, and Hobert O. (2004) Differential sulfations and epimerization define heparan sulfate specificity in nervous system development. Neuron 41, 723–736 [DOI] [PubMed] [Google Scholar]
- 36. Kinnunen T., Huang Z., Townsend J., Gatdula M. M., Brown J. R., Esko J. D., and Turnbull J. E. (2005) Heparan 2-O-sulfotransferase, hst-2, is essential for normal cell migration in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 102, 1507–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamada S., Van Die I., Van den Eijnden D. H., Yokota A., Kitagawa H., and Sugahara K. (1999) Demonstration of glycosaminoglycans in Caenorhabditis elegans. FEBS Lett. 459, 327–331 [DOI] [PubMed] [Google Scholar]
- 38. Toyoda H., Kinoshita-Toyoda A., and Selleck S. B. (2000) Structural analysis of glycosaminoglycans in Drosophila and Caenorhabditis elegans and demonstration that tout-velu, a Drosophila gene related to EXT tumor suppressors, affects heparan sulfate in vivo. J. Biol. Chem. 275, 2269–2275 [DOI] [PubMed] [Google Scholar]
- 39. Kiselova N., Dierker T., Spillmann D., and Ramström M. (2014) An automated mass spectrometry-based screening method for analysis of sulfated glycosaminoglycans. Biochem. Biophys. Res. Commun. 450, 598–603 [DOI] [PubMed] [Google Scholar]
- 40. Hirokawa T., Boon-Chieng S., and Mitaku S. (1998) SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 14, 378–379 [DOI] [PubMed] [Google Scholar]
- 41. Köppen M., Simske J. S., Sims P. A., Firestein B. L., Hall D. H., Radice A. D., Rongo C., and Hardin J. D. (2001) Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat. Cell Biol. 3, 983–991 [DOI] [PubMed] [Google Scholar]
- 42. Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., Gotta M., Kanapin A., Le Bot N., Moreno S., Sohrmann M., Welchman D. P., Zipperlen P., and Ahringer J. (2003) Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237 [DOI] [PubMed] [Google Scholar]
- 43. Ahringer J. (2006) Reverse genetics, in WormBook (The C. elegans Research Community, ed) doi/ 10.1895/wormbook.1.47.1 [DOI] [Google Scholar]
- 44. Ortiz M. A., Noble D., Sorokin E. P., and Kimble J. (2014) A new dataset of spermatogenic vs. oogenic transcriptomes in the nematode. Caenorhabditis elegans. G3 (Bethesda) 4, 1765–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shaham S. (2006) Methods in cell biology, in WormBook (The C. elegans Research Community, ed) doi/ 10.1895/wormbook.1.49.1 [DOI] [Google Scholar]
- 46. Mathews M. B., and Glagov S. (1966) Acid mucopolysaccharide patterns in aging human cartilage. J. Clin. Invest. 45, 1103–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Habuchi H., Kimata K., and Suzuki S. (1986) Changes in proteoglycan composition during development of rat skin: the occurrence in fetal skin of a chondroitin sulfate proteoglycan with high turnover rate. J. Biol. Chem. 261, 1031–1040 [PubMed] [Google Scholar]
- 48. Kitagawa H., Tsutsumi K., Tone Y., and Sugahara K. (1997) Developmental regulation of the sulfation profile of chondroitin sulfate chains in the chicken embryo brain. J. Biol. Chem. 272, 31377–31381 [DOI] [PubMed] [Google Scholar]
- 49. Johnston W. L., and Dennis J. W. (2012) The eggshell in the C. elegans oocyte-to-embryo transition. Genesis 50, 333–349 [DOI] [PubMed] [Google Scholar]
- 50. Olson S. K., Greenan G., Desai A., Mueller-Reichert T., and Oegema K. (2012) Hierarchical assembly of the eggshell and permeability barrier in C. elegans. J. Cell Biol. 198, 731–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rappleye C. A., Paredez A. R., Smith C. W., McDonald K. L., and Aroian R. V. (1999) The coronin-like protein POD-1 is required for anterior-posterior axis formation and cellular architecture in the nematode Caenorhabditis elegans. Genes Dev. 13, 2838–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cristina D., Cary M., Lunceford A., Clarke C., and Kenyon C. (2009) A regulated response to impaired respiration slows behavioral rates and increases lifespan in Caenorhabditis elegans. PLoS Genet. 5, e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fu X., Tang Y., Dickinson B. C., Chang C. J., and Chang Z. (2015) An oxidative fluctuation hypothesis of aging generated by imaging H2O2 levels in live Caenorhabditis elegans with altered lifespans. Biochem. Biophys. Res. Commun. 458, 896–900 [DOI] [PubMed] [Google Scholar]
- 54. Bacaj T., Tevlin M., Lu Y., and Shaham S. (2008) Glia are essential for sensory organ function in C. elegans. Science 322, 744–747, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Suttkus A., Morawski M., and Arendt T. (2016) Protective properties of neural extracellular matrix. Mol. Neurobiol. 53, 73–82 [DOI] [PubMed] [Google Scholar]
- 56. Brückner G., Hausen D., Härtig W., Drlicek M., Arendt T., and Brauer K. (1999) Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer's disease. Neuroscience 92, 791–805 [DOI] [PubMed] [Google Scholar]
- 57. Morawski M., Brückner M. K., Riederer P., Brückner G., and Arendt T. (2004) Perineuronal nets potentially protect against oxidative stress. Exp. Neurol. 188, 309–315 [DOI] [PubMed] [Google Scholar]
- 58. Yutsudo N., and Kitagawa H. (2015) Involvement of chondroitin 6-sulfation in temporal lobe epilepsy. Exp. Neurol., 274, 126–133 [DOI] [PubMed] [Google Scholar]
- 59. Albertini R., Ramos P., Giessauf A., Passi A., De Luca G., and Esterbauer H. (1997) Chondroitin 4-sulphate exhibits inhibitory effect during Cu2+-mediated LDL oxidation. FEBS Lett. 403, 154–158 [DOI] [PubMed] [Google Scholar]
- 60. Albertini R., De Luca G., Passi A., Moratti R., and Abuja P. M. (1999) Chondroitin-4-sulfate protects high-density lipoprotein against copper-dependent oxidation. Arch. Biochem. Biophys. 365, 143–149 [DOI] [PubMed] [Google Scholar]
- 61. Kitagawa H., and Paulson J. C. (1994) Cloning of a novel α 2,3-sialyltransferase that sialylates glycoprotein and glycolipid carbohydrate groups. J. Biol. Chem. 269, 1394–1401 [PubMed] [Google Scholar]
- 62. Kitagawa H., Tsutsumi K., Ujikawa M., Goto F., Tamura J., Neumann K. W., Ogawa T., and Sugahara K. (1997) Regulation of chondroitin sulfate biosynthesis by specific sulfation: acceptor specificity of serum β-GalNAc transferase revealed by structurally defined oligosaccharides. Glycobiology 7, 531–537 [DOI] [PubMed] [Google Scholar]
- 63. Kitagawa H., Fujita M., Ito N., and Sugahara K. (2000) Molecular cloning and expression of a novel chondroitin 6-O-sulfotransferase. J. Biol. Chem. 275, 21075–21080 [DOI] [PubMed] [Google Scholar]
- 64. Ohkura K., and Bürglin T. R. (2011) Dye-filling of the amphid sheath glia: implications for the functional relationship between sensory neurons and glia in. Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 406, 188–193 [DOI] [PubMed] [Google Scholar]
- 65. Kanda Y. (2013) Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 48, 452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]