Abstract
Several CD4+ T cell subtypes contribute to immune homeostasis in malaria, but the markers that define the main suppressive T cell subsets induced by this infection remain largely unknown. Here we provide a detailed phenotypic characterization of immunoregulatory CD4+ T cell populations in uncomplicated human malaria. We found an increased proportion of CD4+ T cells expressing CTLA-4, OX40, GITR, TNFRII, and CD69 in acute-phase single-species infections with Plasmodium vivax, P. falciparum, or both. Such an increase was not proportional to parasite density in P. vivax infections, but did not persist after parasite clearance. Significantly, less than 10% of CD4+ T cells expressing these regulatory molecules had the classical T regulatory (Treg) phenotype (CD4+CD25+CD127−FoxP3+). Two major Treg cell subpopulations, which together accounted for 19–23% of all Treg cells circulating in malaria patients, expressed surface receptors with opposing regulatory functions, either CTLA-4 or OX40. OX40+ Treg cells outnumbered their CTLA-4+ counterparts (1.8:1) during acute P. vivax infection, while a more balanced ratio (1.3:1) was observed following parasite clearance These data reveal new players in the complex CD4+ Treg cell network that maintains immune homeostasis in malaria and suggest potential targets for therapeutic interventions to improve parasite-specific effector immune responses.
Keywords: human malaria, immunoregulation, Treg cells, CTLA-4, OX-40
1. Introduction
Early inflammatory responses exert a major parasite-killing effect in malaria. However, downregulating inflammation once parasite multiplication is under control is crucial to prevent significant tissue damage and severe disease [1]. Interestingly, T cells from malaria patients typically fail to proliferate and secrete cytokines upon antigenic restimulation ex-vivo, while mitogen-induced responses are usually preserved. Although T-cell effector responses are partially restored after parasite clearance following chemotherapy [2–4], hyporesponsiveness may persist among subjects repeatedly exposed to new infections [5,6].
CD4+ regulatory T (Treg) cells have been implicated in the switch from predominantly inflammatory to anti-inflammatory responses and in the inhibition of effector T cell responses in human malaria [7–9], but the surface receptors that characterize highly suppressive Treg cell subsets induced by this infection remains largely unknown. Indeed, thymus-derived classical Treg cells, phenotypically defined as CD4+CD25+ cells that express the transcription factor FoxP3 but not the α chain of the IL-7 receptor (CD127), may display several additional regulatory molecules. Here we focus on seven surface receptors. These include the primary inhibitory receptor CTLA-4 (CD152), which competes with the stimulatory molecule CD28 for CD80 and CD86 binding on antigen-presenting cells [10,11]; the TNF receptor family costimulatory receptor OX40 (CD134) [12,13]; the glucocorticoid-inducible TNF receptor family-related protein (GITR/CD357) [14] and the TNF receptor II (TNFRII/CD120b), which can play either inhibitory or stimulatory roles [15]; CD69, an early activation marker with inhibitory properties [16]; CD39, an ectonuclease involved in the production of the immunosuppressive molecule adenosine [17]; and HLA-DR, an activation marker that identifies a Treg cell subset involved in contact-dependent immune suppression [18]. These receptors define functionally distinct regulatory cell phenotypes that are potential targets for immunomodulatory interventions [19].
Varying combinations of surface markers have been used to phenotype Treg cell populations in human malaria, limiting comparisons across studies [7–9]. Accordingly, increased proportions of CD4+CD25+CTLA-4+ [20], CD4+CD25hiCD69− [21], CD4+CD127lowFoxP3+ [22], CD4+CD127low/−FoxP3+ [23], and CD4+CD25+CD127lowFoxP3+ cells [24] have been found in Plasmodium falciparum-infected subjects in Africa and Indonesia. More recently, the proportion of CD4+CD25+CD127dimFoxP3+ cells in P. falciparum-infected African children was shown to correlate negatively with cumulative exposure to malaria; heavily exposed children actually had decreased absolute numbers and proportions of Treg cells [25]. However, similar proportions of CD4+CD25+FoxP3+ [26] and CD4+CD25+CD127lowFoxP3+ cells [27] were observed in the peripheral blood of falciparum malaria patients and uninfected controls exposed to less intense malaria transmission in Brazil and Peru. Likewise, increased proportions of CD4+CD25+FoxP3+ cells were described in human P. vivax infections in Thailand [28] and Brazil [29], but in another study from Brazil only the CTLA-4+ subset of CD4+CD25+FoxP3+ cells was found to be increased in frequency in response to this parasite [26]. The most recent report, from China, showed comparable proportions of CD4+CD25hiFoxP3+ Treg cells in uninfected malaria-exposed subjects, vivax malaria patients, and unexposed controls [30]. Here we sought to characterize phenotypically CD4+ T cell populations expressing a range of regulatory molecules in the peripheral blood of uncomplicated malaria patients from Brazil, compared to uninfected controls.
2. Materials and methods
2.1. Ethical statement
This study was approved by the Institutional Review Board of the Institute of Biomedical Sciences, University of São Paulo, Brazil (954/CEP). Written informed consent was obtained from all study participants or their parents or guardians if participants were minors.
2.2. Study subjects
We analyzed peripheral blood mononuclear cells (PBMC) from 38 acute-phase, symptomatic but uncomplicated P. vivax and 17 P. falciparum malaria patients and from 15 apparently healthy subjects living in the same area, who served as malaria-exposed but noninfected controls [26]. Malaria patients (regardless of the infecting species) had significantly lower counts of white blood cells, lymphocytes, T lymphocytes, and T CD4+ lymphocytes, when compared to uninfected controls (Table 1). To test whether changes in T cell populations expressing regulatory markers persisted after parasite clearance, we additionally examined paired PBMC samples, collected 28 ± 2 days after starting antimalarial chemotherapy, from 15 vivax malaria patients who had been enrolled during the acute phase of infection [26]. Plasmodium vivax malaria was treated according to the current therapy guidelines of the Ministry of Health of Brazil, with 25 mg/kg of chloroquine base over three days (maximum adult dose, 1.5 g over three days) and 0.5 mg/kg/day of primaquine base for seven days (maximum adult dose, 30 mg/day). After-treatment blood samples had significantly higher counts of lymphocytes, T lymphocytes, and T CD4+ lymphocytes, compared to paired acute-phase samples (Table 2), although still slightly lower than those found in uninfected controls (Table 1). No post-treatment samples were obtained from falciparum malaria patients.
Table 1.
Demographic, hematologic, and clinical characteristics of study participants.
| Value for groupa |
||||
|---|---|---|---|---|
| Characteristic | Uninfected controls (Co) |
P. vivax Infections (Pv) |
P. falciparum Infections (Pf) |
P value |
| No. of subjects | 15 | 38 | 17 | |
| Age (yr) | 33.5 (29–42) | 28.5 (21–43) | 43 (35–49) | 0.012b |
| Gender (% male) | 53.3% (8/15) | 68.4% (26/43) | 70.5% (12/17) | 0.694 |
| Duration (yr) of malaria exposure | 28.5 (22.0–34.7) | 21.0 (13.9–30.1) | 30.0 (21.2–37.7) | 0.036c |
| No. of WBCs (109/liter) | 7.3 (5.6–8.5) | 5.6 (4.3–6.7) | 5.1 (4.5–6.4) | 0.012d |
| No. of lymphocytes (109/liter) | 2.1 (1.5 3.3) | 1.2 (0.8–1.6) | 1.3 (0.9–1.7) | 0.001e |
| No. of T lymphocytes (109/liter) | 1.5 (1.2–2.4) | 0.8 (0.5–1.2) | 1.0 (0.6–1.1) | 0.001f |
| No. of CD4+ T lymphocytes (109/liter) | 1.0 (0.7–1.4) | 0.5 (0.3–0.7) | 0.5 (0.4–0.8) | 0.001g |
| Parasitemia (no. of parasites/ml) | NAh | 2385 (662–8088) | 515 (31–3638) | 0.038i |
| Duration of symptoms (days) | NAh | 3 (2–5) | 5 (4–7) | 0.035i |
Data are presented as medians (interquartile ranges) unless otherwise stated and are compared across two or three groups with Kruskal-Wallis or Mann-Whitney tests (continuous variables) or χ2 tests (proportions). We report uncorrected P values.
Pv < Pf (P = 0.006), Mann-Whitney test.
Pv < Co (P = 0.045) and Pv < Pf (P = 0.034), Mann-Whitney test.
Pv < Co (P = 0.017) and Pf < Co (P = 0.004), Mann-Whitney test.
Pv < Co (P < 0.0001) and Pf < Co (P = 0.002), Mann-Whitney test.
Pv < Co (P < 0.0001) and Pf < Co (P = 0.001), Mann-Whitney test.
Pv < Co (P = 0.001) and Pf < Co (P = 0.002), Mann-Whitney test.
NA = not applicable.
Pv < Pf, Mann-Whitney test.
Table 2.
Hematologic characteristics of vivax malaria patients (n = 14) during acute-phase infection and convalescence.
| Characteristic | Valuea |
P value | |
|---|---|---|---|
| Acute phase | Convalescence | ||
| No. of WBCs (109/liter) | 5.4 (4.0–6.8) | 5.8 (4.8–7.4) | 0.073 |
| No. of lymphocytes (109/liter) | 1.0 (0.6–1.4) | 1.8 (1.4–2.0) | 0.002 |
| No. of T lymphocytes (109/liter) | 0.7 (0.5–0.9) | 1.2 (0.8–1.4) | 0.011 |
| No. of CD4+ T lymphocytes (109/liter) | 0.4 (0.3–0.7) | 0.7 (0.4–0.9) | 0.013 |
Data are presented as medians (interquartile ranges) and compared with Wilcoxon tests.
All study subjects lived in the farming settlement of Remansinho, Western Amazon Basin of Brazil, characterized by year-round hypoendemic transmission of both P. vivax (which accounts for >90% of the local malaria burden) and P. falciparum [31]. Because many adults living in this frontier settlement are migrants from non-endemic sites, their ages do not necessarily equal the length of malaria exposure; we thus used the number of years living in a malaria-endemic setting as a proxy of cumulative exposure to malaria. On-site malaria diagnosis, based on thick blood smear microscopy, was confirmed by nested PCR with species-specific primers that target the 18S rRNA genes of P. falciparum and P. vivax; parasite density (number of parasites/µl of blood) was estimated by quantitative real-time PCR [26]. The detection threshold of this technique is approximately 2 parasites µl of blood. Healthy controls and convalescent subjects were negative for malaria parasites, by both microscopy and nested PCR, at the time of blood sampling.
2.3. PBMC
PBMC were separated onsite by gradient centrifugation with Ficoll-Paque Plus (GE Healthcare, United Kingdom) and cryopreserved in liquid nitrogen as described [26]. PBMC were thawed in a 37 °C water bath on the day o f flow cytometry analysis, evaluated for viability using a Countess automated cell counter (Invitrogen, USA), and resuspended in 10 ml of RPMI 1640 medium (Gibco, Scotland) supplemented with 10% (vol/vol) inactivated fetal bovine serum (FBS) (Gibco), 10 mM HEPES (Gibco), 2 mM L-glutamine (Gibco), 1 mM sodium pyruvate (Sigma-Aldrich, USA), 55 µM 2 mercaptoethanol (Sigma-Aldrich), and a 1% (vol/vol) solution containing 100 U/ml of penicillin, 10 µg/ml of streptomycin, and 25 µl/ml of amphotericin B (Gibco). PBMC were again evaluated for viability and only samples with >90% viability were used for flow cytometry analysis.
2.4. Monoclonal antibodies
The following labelled murine anti-human monoclonal antibodies were used to delineate lymphocyte populations by flow cytometry analysis: anti-CD3 (clone UCHT1l) labeled with phycoerythrin-Texas Red-X (ECD) (Beckman Coulter, USA), anti-CD4 (clone RPA-T4) labeled with V500 (BD Horizon, USA), anti-CD25 (clone BC96) labeled with allophycocyanin with cyanin-7 (APCcy7) (Biolegend, USA), anti-CD127 (clone A19D5) labeled with brilliant violet 421 (BV421) (Biolegend), anti- FoxP3 (clone 236a) labeled with peridinin chlorophyll protein complex with cyanin-5.5 (PerCP-Cy5.5) (BD Pharmigen), anti-CD39 (clone eBioA1) labeled with phycoerythrin (PE) (eBioscience, USA), anti-CTLA-4 (clone L3D10) labeled with allophycocyanin (APC) (Biolegend), anti-CD69 (clone FN50) labeled with phycoerythrin-carbocyanin (PE-Cy7) (Biolegend), anti-HLA-DR (clone L243) labeled with Alexa 700 (BD Pharmigen), anti-TNFRII (clone 22235) labeled with fluorescein isothiocyanate (FITC) (R&D Systems, USA), anti-OX40 (clone ACT35) labeled with PE-Cy7 (Biolegend), anti-GITR (110416) labeled with FITC (R&D Systems), anti-CD45RO (clone UCHL1) labeled with APC (Biolegend), and anti-CD45RA labeled with Alexa 700 (Biolegend). Antibody panels used in this study are presented in Supplementary Table 1. We titrated each antibody and selected its final dilution for an optimal specific staining associated with a low background.
2.5. CD4+ T cell phenotyping by flow cytometry
Thawed PBMC samples were centrifuged at 500 × g for 10 min, and 106 viable cells/ml were transferred to V-bottomed 96-well microplates (Nunc, USA) in 100 µl of staining buffer (PBS with 2% fetal bovine serum [FBS, Gibco] and 2 mm EDTA) for surface staining with directly conjugated antibodies. Cells were incubated at 4 °C in darkness for 30 min and washed twice with staining buffer. For FoxP3 expression analysis, cells were fixed and permeabilized using the Human FoxP3 buffer set (BD Pharmingen), stained with PerCP-Cy5.5-labelled anti-FoxP3 antibodies, and resuspended in 100 µl of fixation buffer (1% paraformaldehyde in PBS). Samples were acquired on an LSRFortessa flow cytometer (BD Biosciences) using the FACSDiva software (BD Biosciences). Anti-mouse IgG-coated beads (BD Biosciences) were stained with each fluorochrome separately and used for software-based compensation. We used the Live/Dead Fixable Blue Dead Cell Stain for UV excitation (Invitrogen) to determine cell viability and collected ≥ 300,000 events in each live gate. Fluorescence minus one (FMO) controls were used to define the gates for FoxP3, CTLA-4, OX40, GITR, and TNFRII in order to control for spectral overlap (Supplementary Fig. 1). Boolean analysis was applied to evaluate coexpression of different molecules by the same cells. Data analysis was carried out using FlowJo software version 8.8.6 (Tree Star, USA).
2.6. Statistical analysis
Because most continuous variables had an overdispersed distribution, results were summarized as medians and interquartile ranges (IQR). Comparisons across groups of subjects were done with nonparametric Kruskal-Wallis or Mann-Whitney tests (for continuous variables) or χ2 tests (for proportions). When individual Kruskal-Wallis tests indicated a significant difference (P < 0.05) among groups, Mann-Whitney tests were carried out to determine where the differences lay. Paired data were compared with Wilcoxon signed rank tests for continuous variables. Nonparametric correlation coefficients (r) were estimated using Spearman rank correlation tests. All analyses were performed using SPSS 17.0 software (SPSS, Chicago, IL). Because our analysis of T cell populations involved multiple comparisons, we used the Bonferroni correction to control the familywise error rate. The critical value for an individual test was defined by dividing the overall error rate (α = 0.05) by the number of familywise comparisons. For this purpose, we defined that each combined analysis of individual surface receptor expression in each cell compartment (either CD4+ T cells or classical Treg cells) from each patient population (either vivax or falciparum malaria patients) defined a "family" of statistical tests.
3. Results
3.1. Increased surface expression of regulatory receptors in the CD4+ T cell compartment of malaria patients
Using the gating strategy shown in Supplementary Fig. S2, we observed significantly higher proportions of CD4+ T cells expressing CTLA-4, TNFRII, GITR, and CD69 in vivax malaria patients, compared to uninfected controls (Table 3). The increase in the relative frequency of CD4+ T cells expressing OX40 was of borderline statistical significance (P = 0.010, above the Bonferroni-corrected critical P value of 0.0071) in P. vivax infections. A similar analysis of falciparum malaria patients revealed significantly increased proportions of CD4+ T cells expressing CTLA-4, OX40 and CD69, compared to uninfected controls; the P value for TNFRII (P = 0.014) did not reach statistical significance after Bonferroni correction (Table 3). We thus found an increased proportion of circulating CD4+ T subpopulations expressing several types of regulatory molecules during uncomplicated acute malaria.
Table 3.
Proportions of CD4+ T cells expressing selected receptors in malaria patients and uninfected controls.
| Value for groupa |
||||
|---|---|---|---|---|
| CD4+ T cell phenotype | Uninfected controls (Co) |
P. vivax Infections (Pv) |
P. falciparum Infections (Pf) |
P value |
| No. of subjectsb | 15 | 38 | 17 | |
| CTLA-4+ | 0.57 (0.41–0.74) | 1.29 (0.91–1.65) | 0.89 (0.67–1.89) | <0.0001c |
| TNFRII+ | 1.26 (0.83–1.39) | 1.89 (1.44–2.44) | 1.69 (1.25–2.26) | 0.001d |
| GITR+ | 0.74 (0.60–0.82) | 1.04 (0.77–1.50) | 0.76 (0.62–1.14) | 0.007e |
| OX40+ | 8.06 (6.56–11.00) | 12.55 (7.97–15.84) | 12.31 (8.23–15.60) | 0.015f |
| CD39+ | 6.45 (4.12–8.25) | 7.10 (4.15–11.21) | 7.58 (6.12–10.20) | 0.432 |
| CD69+ | 3.66 (1.67–4.20) | 8.37 (6.02–12.21) | 7.92 (3.93–12.70) | 0.001g |
| HLA-DR+ | 4.95 (3.61–6.47) | 5.66 (3.88–7.61) | 6.47 (4.33–9.62) | 0.169 |
| CD25+CD127−FoxP3+ (Treg) | 0.73 (0.66–0.94) | 0.77 (0.54–1.00) | 1.03 (0.66–1.46) | 0.214 |
Data are presented as medians (interquartile ranges) and compared across three groups with Kruskal-Wallis tests. Pairwise comparisons were carried out with Mann-Whitney tests. We report all uncorrected P values < 0.05 obtained in pairwise comparisons, but consider as significant, in pairwise comparisons, P values (underlined) below the critical Bonferroni-corrected value of 0.0071 (corresponding to 7 familywise comparisons).
Because of the limited availability of cells, the numbers of subjects with PBMC tested for TNFRII, CD69, HLA-DR staining were: Co, n = 12; Pv, n = 32; Pf, n = 15.
Pv > Co (P < 0.0001) and Pf > Co (P = 0.001), Mann-Whitney test.
Pv > Co (P < 0.0001) and Pf ≥ Co (P = 0.014), Mann-Whitney test.
Pv > Co (P = 0.002), Mann-Whitney test.
Pv ≥ Co (P = 0.010) and Pf > Co (P = 0.006), Mann-Whitney test.
Pv > Co (P < 0.0001) and Pf > Co (P = 0.005), Mann-Whitney test.
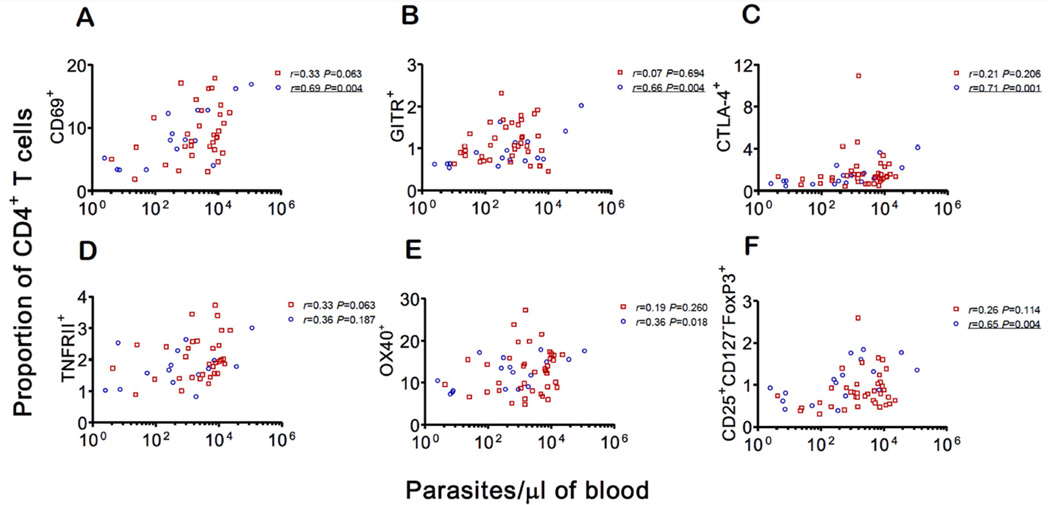
We next examined whether the relative frequency of circulating CD4+ T cells expressing regulatory markers that were found to be altered during acute infection (either in the P. vivax or P. falciparum group, or both) correlated with patients' parasitemias. Accordingly, the proportions of CD4+ T cells bearing CD69, GITR, and CTLA-4, but not TNFRII, correlated with P. falciparum parasite loads measured by quantitative real-time PCR (Fig. 1A–D). The correlation between the proportion of OX40+ cells and P. falciparum parasitemia (Fig. 1E) was of borderline statistical significance (P = 0.018; Bonferroni-corrected critical value, P < 0.0071). In contrast, we found no evidence for a parasite density-dependent increase in the proportion of CD4+ T cells expressing any of these receptors in vivax malaria patients (Fig. 1A–E). Interestingly, median parasite densities were slightly higher in P. vivax infections, a difference of borderline statistical significance (Table 1).
Figure 1. Correlations between parasitemia (the number of parasites per microliter of blood, estimated by quantitative real-time PCR) and the proportion (%) of CD4+ T cells expressing selected surface markers.
A. CD69; B. GITR; C. CTLA-4; D. TNFRII; and E. OX40. F. Correlation between parasitemia and the proportion of CD4+ T cells with the classical Treg phenotype (CD25+CD127−FoxP3+). Red squares represent P. vivax-infected subjects (n = 32 for TNFRII and CD69 and n = 38 for all other markers); blue circles represent P. falciparum-infected subjects (n = 15 for TNFRII and CD69 and n = 17 for all other markers). Spearman correlations coefficients (r) are shown separately for each species. Because we made 6 familywise comparisons, we consider a Bonferroni-corrected P value < 0.0083 to indicate a significant correlation. P values below this critical value are underlined.
Classical Treg cells accounted for ≤ 1% of the circulating CD4+ T cell compartment in malaria patients and uninfected controls (Table 3; gating strategy shown in Supplementary Fig. S3). Because CD4+ T cell counts were substantially decreased in malaria patients, compared to uninfected controls (Table 1), absolute number of circulating CD4+ classical Treg cells decreased during malaria. Interestingly, the proportion of CD4+ cells that were classical Treg cells correlated positively with parasitemias in falciparum malaria patients (Fig. 1F). These results suggest that Treg cell frequencies are significantly elevated in a minority of P. falciparum-infected patients, the ones with the highest parasite densities, although no causal relationship can be inferred from this finding.
3.2. Increased frequency of CTLA-4+ and OX40+ Treg cell subsets in malaria patients
We next examined the surface expression of regulatory and activation markers in the circulating pool of classical Treg cells (Table 4). The vast majority (93–96%) of these Treg cells, from both from malaria patients and uninfected controls, were CD45RO+ and CD45RA− (data not shown) Overall, 75–80% of them were CD39+ in both infected and uninfected subjects. Surface expression of CTLA-4 was observed in 9–10% of the Treg cells from malaria patients, but in only 5% of those from uninfected controls; this difference was statistically significant for both vivax and falciparum malaria patients, compared to controls (Table 4). Only 0.3–0.5% of CTLA-4+ Treg cells coexpressed GITR, another well-defined suppressive receptor (Supplementary Table 2).
Table 4.
Proportions of classical Treg cells (CD4+CD25+CD127−FoxP3+) expressing selected receptors in malaria patients and uninfected controls.
| Value for groupa |
||||
|---|---|---|---|---|
| CD4+ Treg cell phenotype | Uninfected Controls (Co) |
P. vivax Infections (Pv) |
P. falciparum Infections (Pf) |
P value |
| No. of subjectsb | 15 | 38 | 17 | |
| CTLA-4+ | 4.85 (3.51–5.95) | 10.01 (6.83–13.85) | 8.85 (6.87–13.8) | <0.0001c |
| TNFRII+ | 6.10 (5.16–6.99) | 6.21 (4.84–7.81) | 7.34 (4.95–9.88) | 0.584 |
| GITR+ | 3.44 (2.22–4.02) | 3.60 (2.57–4.63) | 3.53 (2.99–4.18) | 0.759 |
| OX40+ | 11.30 (9.69–13.10) | 14.81 (11.52–19.74) | 12.02 (10.81–21.22) | 0.018d |
| CD39+ | 77.41 (39.62–88.02) | 74.66 (61.35–86.09) | 80.29 (68.62–84.84) | 0.749 |
| CD69+ | 9.01 (5.79–11.0) | 8.61 (6.86–11.62) | 7.76 (5.76–11.13) | 0.655 |
| HLA-DR+ | 38.70 (30.51–48.00) | 37.1 (25.66–40.35) | 42.65 (36.34–43.93) | 0.060 |
Data are presented as medians (interquartile ranges) and compared across three groups with Kruskal-Wallis tests. Pairwise comparisons were carried out with Mann-Whitney tests. We report all P values < 0.05 obtained in pairwise comparisons, but consider as significant, in pairwise comparisons, the P values (underlined) that are below the critical Bonferroni-corrected value of 0.0071 (corresponding to 7 familywise comparisons).
Because of the limited availability of cells, the numbers of subjects with PBMC tested for TNFRII, CD69, HLA-DR staining were: Co, n = 12; Pv, n = 32; Pf, n = 15.
Pv > Co (P < 0.0001) and Pf > Co (P = 0.001), Mann-Whitney test
Pv > Co (P = 0.003), Mann-Whitney test.
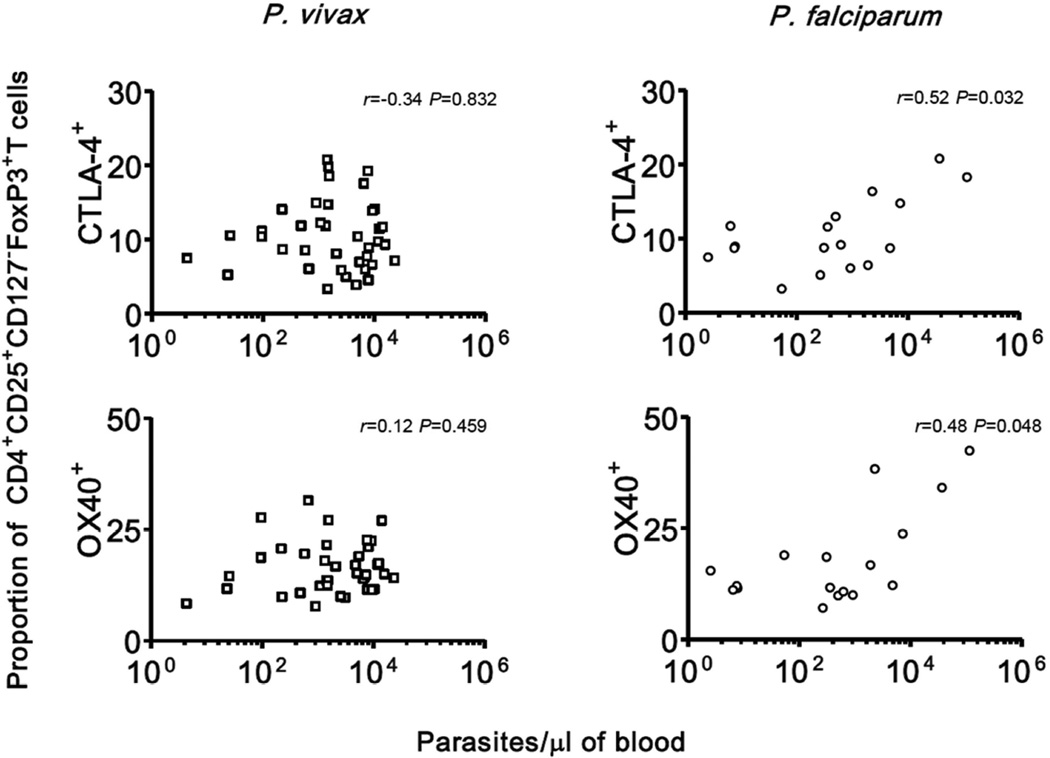
Treg cells expressing OX40 were also found in increased proportions during P. vivax (but not P. falciparum) infections, compared to uninfected controls (Table 4). The proportions of Treg cells expressing CTLA-4 and OX40 in vivax malaria were not correlated to each other (rs = 0.002, P = 0.991, n = 38); only 2% of the Treg cells coexpressed both markers (Supplementary Table 2). We thus characterized two distinct Treg cell subsets that express either CTLA-4 or OX40; together, these subsets accounted for nearly 23% of all circulating Tregs in vivax malaria patients and approximately 19% of those in P. falciparum infections. We next examined whether changes in the CLTA-4+ and OX40+ Treg cells were proportional to parasite densities. The proportion of CLTA-4+ and OX40+ Treg cells correlated weakly with P. falciparum parasitemias, but not with P. vivax parasitemias (Fig. 2). Malaria patients had no increased frequencies of Treg cells expressing other surface markers such as TNFRII, GITR, CD39, CD69, or HLA-DR (Table 2). Significantly, classical Treg cells represented minor fractions of the pool of circulating CD4+ T cells expressing CTLA-4 (5–9%), OX40 (around 1%), GITR (2–4%), TNFRII (2–4%) and CD39 (<1%) in malaria patients and uninfected controls (Supplementary Table 3). These findings indicate that the vast majority of CD4+ T cells expressing these regulatory receptors are actually conventional CD4+ T (Tconv) cells. Whether these cells might play a major immunoregulatory role during infection remains to be determined.
Figure 2. Correlations between parasitemia (the number of parasites per microliter of blood, estimated by quantitative real-time PCR) and the proportion (%) of CD4+CD25+CD127−FoxP3+ Treg cells expressing CTLA-4 and OX40.
Data and correlation coefficients are shown separately for P. vivax-infected subjects (n = 38) and P. falciparum-infected subjects (n = 17).
3.3. Changes in the circulating CD4+ T cell and Treg cell compartments after parasite clearance
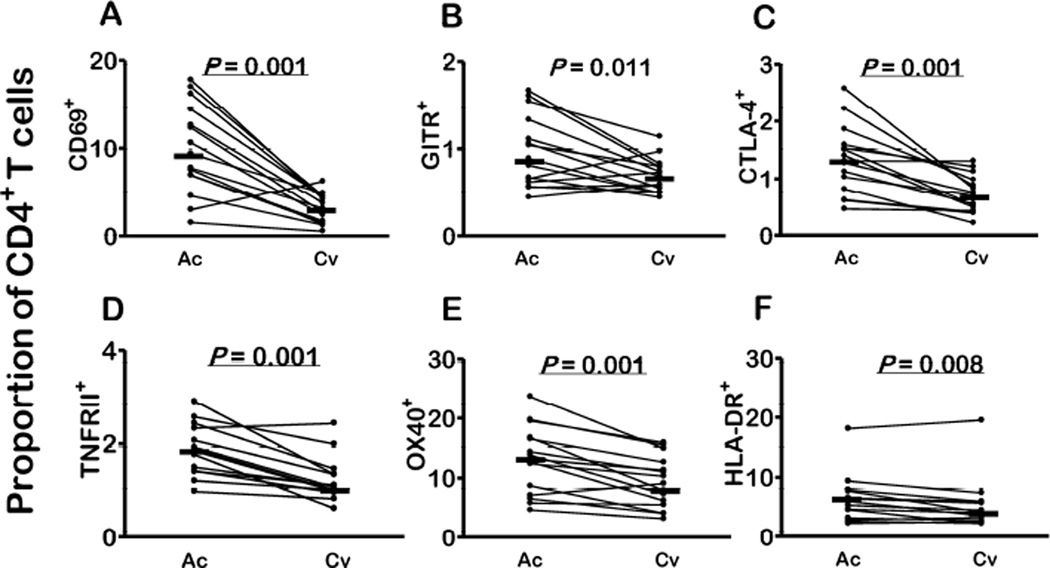
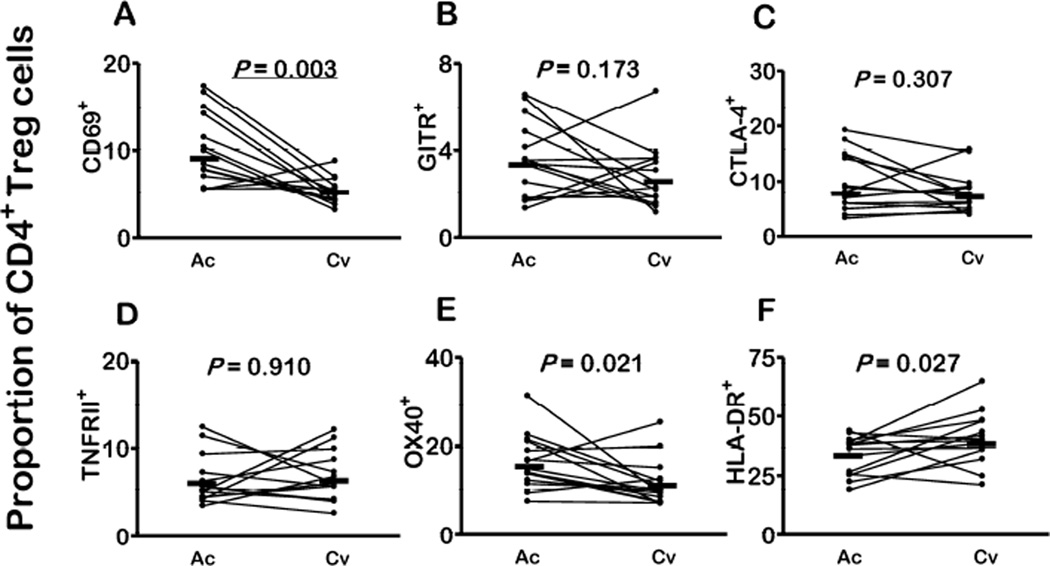
We next examined the expression of regulatory molecules by different CD4+ T cell types after P. vivax clearance following chemotherapy. The proportions of CD4+ T cells expressing CD69, CTLA-4, TNFRII, OX40, and HLA-DR significantly decreased after antimalarial chemotherapy; a decline of borderline significance was observed for CD4+ T cells expressing GITR (Fig. 3A–3F). These changes may be due to a downregulation in the surface expression of these markers in circulating cells or due to a return of marker-negative markers to the peripheral blood. Interestingly, the proportions of cells bearing these markers after P. vivax clearance were similar to those found in uninfected controls (Fig. 3A–3F; compare with data from Table 3; P values between 0.259 and 0.942 for comparisons between convalescent subjects and uninfected controls, Mann-Whitney tests). Similarly, the proportion of classical Treg cells expressing CD69 (but not CTLA-4, TNFRII, or GITR) significantly decreased after treatment; a decline of borderline significance was observed for the proportion of Treg cells expressing OX40 (Fig. 4A–E). The proportion of CTLA-4+ Treg cells remained elevated in convalescent patients, being significantly greater than in controls (P = 0.028, Mann-Whitney test). The ratio between OX40+ and CTLA-4+ Treg cells circulating in vivax malaria patients dropped from 1.8:1 in the acute phase to 1.3:1 after treatment. In contrast, increased proportions of HLA-DR+ Treg cells were found in convalescence, compared to acute infection (Fig. 4F).
Figure 3. Changes in the circulating CD4+ T cell compartment in malaria patients after P. vivax clearance following chemotherapy.
The proportions (%) of CD4+ T cells expressing A. CD69, B. GITR, C. CTLA-4, D. TNFRII, E. OX40, and F. HLA-DR during the acute phase of infection (Ac) and 26–30 days after starting chemotherapy (convalescence, Cv). Each comparison included 15 paired samples; 26 horizontal bars indicate median values and P values were calculated with Wilcoxon tests. Because we made 6 familywise comparisons, we consider a Bonferroni-corrected P value < 0.0083, to indicate a significant change. P values below this critical value are underlined.
Figure 4. Changes in the circulating CD4+CD25+CD127−FoxP3+ Treg cell compartment in malaria patients after P. vivax clearance following chemotherapy.
The proportions (%) of classical Treg cells expressing A. CD69, B. GITR, C. CTLA-4, D. TNFRII, E. OX40, and F. HLA-DR in paired samples collected during the acute infection (Ac) and 26–30 days after treatment (convalescence, Cv). Each comparison included 15 paired samples; horizontal bars indicate median values and P values were calculated with Wilcoxon tests. Because we made 6 familywise comparisons, we consider a Bonferroni-corrected P value < 0.0083 to indicate a significant change. P values below this critical value are underlined.
4. Discussion
Here we show that acute malaria is associated with an increased proportion of peripheral CD4+ T cells expressing several regulatory receptors, with some species-specific differences. Of note, surface expression of CTLA-4, the primary suppressive receptor of Treg cells, was detected in 1.3% of all CD4+ T cells from acute vivax malaria patients, but in only 0.7% of those sampled after parasite clearance. Only 5% of these CTLA-4-bearing CD4+ T cells were phenotypically defined as classical Treg cells. Similar figures have been recently found in HIV-infected subjects and uninfected controls, respectively [32]. CTLA-4 upregulation on CD4+ T cells has been interpreted as a mere correlate of extensive T cell activation during infections such as malaria and HIV [33–36]. However, CTLA-4+ T cells that are not classical Treg cells can exert a cell-extrinsic negative regulation on CTLA-4− effector T cells in mice [37,38]. Accordingly, blocking CTLA-4 on the surface of Tconv cells, using monoclonal antibodies, enhances antitumor immunity in mice, further suggesting that CTLA-4+ Tconv cells are highly suppressive [35]. Although these findings suggest that all circulating CTLA-4+ T cells, and not only classical CTLA-4+ Treg cells, can suppress effector responses and thus limit excessive inflammation in mice, this effect had not been directly observed so far in experimental or human malaria.
Whether the population of CD4+ Tconv cells expressing CD69, which was found in increased frequency during acute-phase infection but not after parasite clearance, can also exert a major inhibitory function in malaria patients remains to be determined. CD4+CD25−CD69+FoxP3− T cells suppress effector T cell responses in mice through membrane-bound transforming growth factor (TGF)-β [39] and a similarly strong immunosuppressive function in vitro has been documented for human CD4+CD69+TGF-β+ cells [40].
In addition to inhibitory molecules, stimulatory receptors such as OX40 (in falciparum malaria patients), TNFRII and GITR (in vivax malaria patients) were more frequently expressed by peripheral CD4+ T cells in infected, compared to uninfected subjects. Although we cannot offer a clear-cut explanation for the differences in relative frequency of CD4+ T cell subpopulations circulating in vivax and falciparum malaria patients, we note that, on average, P. vivax-infected subjects in our study were younger and had less years of exposure to malaria than their P. falciparum-infected counterparts (Table 1). It remains unclear whether these differences between vivax malaria patients and other study subjects could lead to increased expression of selected T cell surface markers.
OX40 is transiently expressed on recently activated Tconv cells, peaking at 48 hours and disappearing after 72 to 96 hours after T cell receptor (TCR) triggering, and strongly modulate their function. OX40 signaling expands effector T cells and improves their function and survival [13]. Recent studies showed increased OX40 expression on Tconv cells not only in human malaria, but also in experimental infections in mice [36,41]. Similarly, TNFRII and GITR expressed on Tconv cells promote clonal expansion and survival of effector T cells and enhance their function [42], but a malaria-associated upregulation of these molecules on Tconv cells had not been previously reported. We hypothesize that Tconv cells expressing OX40, TNFRII and GITR can play a major role, which remains largely unexplored, in enhancing effector T cell responses during acute malaria.
We next examined the classical Treg cell compartment and found no increase in the proportion of CD4+ T cells that were phenotypically defined as classical Tregs in malaria patients, compared to uninfected controls. However, the proportion of Treg cells correlated positively with P. falciparum parasite density, suggesting that patients with the highest parasitemias are more likely to have increased proportions of these cells. Interestingly, we found no malaria-associated expansion of CD39+CD4+ Treg cells, which constitute a major immunoregulatory T cell population induced by tumors [43] and chronic infections with viruses such as HIV [32] and HTLV-1 [44]. However, two particular subsets, together accounting for almost one fourth of all CD4+ Treg cells, were found in increased proportions in the peripheral blood of vivax malaria patients. Importantly, surface molecules with potentially antagonistic properties -- either inhibitory (CTLA-4) or stimulatory (OX40) -- characterize these Treg subsets. While the proportion of CTLA-4+ Treg cells has been previously found to be increased in P. vivax infections [26,29], we note that this is the first report of increased frequency of OX40+ Treg cells in human malaria.
The role of OX40 signaling in CD4+ Treg cell function is currently under debate. The engagement of OX40 expressed by classical Treg cells with agonist antibodies abrogates their suppressive capacity in mice [45]. However, the effects of OX40 signaling on Treg cell expansion and survival are less clear-cut and vary according to whether inflammation is present at the time of receptor engagement [12,46]. It is tempting to speculate that the ratio between CTLA-4+ and OX40+ Treg cells may be a key factor to determine inflammatory homeostasis and effector T cell function in malaria. Interestingly, OX40+ Treg cells clearly outnumbered their CTLA-4+ counterparts (1.8:1) during acute P. vivax infection, while a more balanced ratio (1.3:1) was observed after treatment, consistent with less inflammatory immune responses following parasite clearance [1].
The combined administration of blocking anti-CTLA-4 antibodies and agonist anti-OX40 antibodies enhances antitumor immunity in experimental cancer models [19,35,47]. Whether a similar approach might be useful to circumvent the T-cell hyporesponsiveness in malaria remains to be determined. Our preliminary data show that CTLA-4 blockade alone did not enhance lymphocyte proliferation upon antigenic restimulation ex-vivo with P. vivax-infected red blood cells (data not shown). Similarly, preclinical data showed a limited efficacy of CTLA-4 targeting alone against some tumors [35]. In contrast, improved T cell responses to malaria parasites both ex-vivo and in vivo can be induced by the simultaneous blockade of CTLA-4 and other inhibitory receptors, e.g. lymphocyte activation gene-3 (LAG-3), programmed death-1 (PD-1) and T cell immunoglobulin mucin-3 (TIM-3) [48–50]. Moreover, enhancing OX40 signaling with agonist antibodies improved effector T cell responses, antibody production, and parasite clearance in experimental murine malaria [36]. These data suggest that the combined targeting of multiple regulatory receptors with either agonist or blocking antibodies, some of which have already been approved for clinical use in cancer patients [19], might be worth exploring as a strategy to improve parasite-specific effector immune responses in malaria.
In conclusion, we showed an increased expression of several stimulatory and inhibitory molecules on CD4+ T cells during human malaria. Two of them (CTLA-4 and OX40), with opposing effects, are specifically upregulated in Treg cells during vivax malaria. These data reveal new players in the complex regulatory network that maintains immune homeostasis in malaria and provide potential targets for therapeutic interventions.
Supplementary Material
Acknowledgments
This research was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant 2010/52146-0 to MUF and doctoral scholarships to RMG-L and NFL); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grant 470315/2012-1 to MUF and senior research scholarships to EGK and MUF); and the National Institutes of Health (grant U19 AI089681 to Joseph M. Vinetz, University of California, San Diego).
We thank the population of Remansinho for their enthusiastic participation in this study and Maria José Menezes, Danielle S. Menchaca Vega, and Melissa da Silva Bastos for their superb laboratory support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that no competing interests exist.
References
- 1.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 2.Troye-Blomberg M, Romero P, Patarroyo ME, Björkman A, Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria III. Proliferative response to antigen in vitro and subset composition of T cells from patients with acute infection or from immune donors. Clin Exp Immunol. 1984;58:380–387. [PMC free article] [PubMed] [Google Scholar]
- 3.Ho M, Webster HK, Looareesuwan S, Supanaranond W, Phillips RE, Chanthavanich P, et al. Antigen-specific immunosuppression in human malaria due to Plasmodium falciparum . J Infect Dis. 1986;153:763–771. doi: 10.1093/infdis/153.4.763. [DOI] [PubMed] [Google Scholar]
- 4.Riley EM, Andersson G, Otoo LN, Jepsen S, Greenwood BM. Cellular immune responses to Plasmodium falciparum antigens in Gambian children during and after an acute attack of falciparum malaria. Clin Exp Immunol. 1988;73:17–22. [PMC free article] [PubMed] [Google Scholar]
- 5.Goonewardene R, Carter R, Gamage CP, Del Giudice G, David PH, Howie S, et al. Human T cell proliferative responses to Plasmodium vivax antigens: evidence of immunosuppression following prolonged exposure to endemic malaria. Eur J Immunol. 1990;20:1387–1391. doi: 10.1002/eji.1830200626. [DOI] [PubMed] [Google Scholar]
- 6.Braga EM, Carvalho LH, Fontes CJ, Krettli AU. Low cellular response in vitro among subjects with long-term exposure to malaria transmission in Brazilian endemic areas. Am J Trop Med Hyg. 2002;66:299–303. doi: 10.4269/ajtmh.2002.66.299. [DOI] [PubMed] [Google Scholar]
- 7.Hansen DS, Schofield L. Natural regulatory T cells in malaria: host or parasite allies? PLoS Pathog. 2010;6:e1000771. doi: 10.1371/journal.ppat.1000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scholzen A, Minigo G, Plebanski M. Heroes or villains? T regulatory cells in malaria infection. Trends Parasitol. 2010;26:16–25. doi: 10.1016/j.pt.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Finney OC, Riley EM, Walther M. Regulatory T cells in malaria--friend or foe? Trends Immunol. 2010;31:63–70. doi: 10.1016/j.it.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 11.Walker LS, Sansom DM. Confusing signals: recent progress in CTLA-4 biology. Trends Immunol. 2015;36:63–70. doi: 10.1016/j.it.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruby CE, Yates MA, Hirschhorn-Cymerman D, Chlebeck P, Wolchok JD, Houghton AN, et al. Cutting Edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J Immunol. 2009;183:4853–4857. doi: 10.4049/jimmunol.0901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nocentini G, Riccardi C. GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. Eur J Immunol. 2005;35:1016–1022. doi: 10.1002/eji.200425818. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Oppenheim JJ. The phenotypic and functional consequences of tumour necrosis factor receptor type 2 expression on CD4+ FoxP3+ regulatory T cells. Immunology. 2011;133:426–433. doi: 10.1111/j.1365-2567.2011.03460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González-Amaro R, Cortés JR, Sánchez-Madrid F, Martín P. Is CD69 an effective brake to control inflammatory diseases? Trends Mol Med. 2013;19:625–632. doi: 10.1016/j.molmed.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 18.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 19.Karimi S, Chattopadhyay S, Chakraborty NG. Manipulation of regulatory T cells and antigen-specific cytotoxic T lymphocyte-based tumour immunotherapy. Immunology. 2015;144:186–196. doi: 10.1111/imm.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brustoski K, Moller U, Kramer M, Hartgers FC, Kremsner PG, Krzych U, et al. Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J Infect Dis. 2006;193:146–154. doi: 10.1086/498578. [DOI] [PubMed] [Google Scholar]
- 21.Walther M, Tongren JE, Andrews L, Korbel D, King E, Fletcher H, et al. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23:287–296. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Walther M, Jeffries D, Finney OC, Njie M, Ebonyi A, Deininger S, et al. Distinct roles for FOXP3− and FOXP3+ CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog. 2009;5:e1000364. doi: 10.1371/journal.ppat.1000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finney OC, Nwakanma D, Conway DJ, Walther M, Riley EM. Homeostatic regulation of T effector to Treg ratios in an area of seasonal malaria transmission. Eur J Immunol. 2009;39:1288–1300. doi: 10.1002/eji.200839112. [DOI] [PubMed] [Google Scholar]
- 24.Minigo G, Woodberry T, Piera KA, Salwati E, Tjitra E, Kenangalem E, et al. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog. 2009;5:e1000402. doi: 10.1371/journal.ppat.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyle MJ, Jagannathan P, Farrington LA, Eccles-James I, Wamala S, McIntyre TI, et al. Decline of FoxP3+ regulatory CD4 T cells in peripheral blood of children heavily exposed to malaria. PLoS Pathog. 2015;11:e1005041. doi: 10.1371/journal.ppat.1005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonçalves RM, Salmazi KC, Santos BA, Bastos MS, Rocha SC, Boscardin SB, et al. CD4+ CD25+ Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in uncomplicated malaria: do different parasite species elicit similar host responses? Infect Immun. 2010;78:4763–4772. doi: 10.1128/IAI.00578-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres KJ, Villasis E, Bendezú J, Chauca J, Vinetz JM, Gamboa D. Relationship of regulatory T cells to Plasmodium falciparum malaria symptomatology in a hypoendemic region. Malar J. 2014;13:108. doi: 10.1186/1475-2875-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jangpatarapongsa K, Chootong P, Sattabongkot J, Chotivanich K, Sirichaisinthop J, Tungpradabkul S, et al. Plasmodium vivax parasites alter the balance of myeloid and plasmacytoid dendritic cells and the induction of regulatory T cells. Eur J Immunol. 2008;38:2697–2705. doi: 10.1002/eji.200838186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bueno LL, Morais CG, Araújo FF, Gomes JA, Corrêa-Oliveira R, Soares IS, et al. Plasmodium vivax : induction of CD4+CD25+FoxP3+ regulatory T cells during infection are directly associated with level of circulating parasites. PLoS One. 2010;5:e9623. doi: 10.1371/journal.pone.0009623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jangpatarapongsa K, Xia H, Fang Q, Hu K, Yuan Y, Peng M, et al. Immunity to malaria in Plasmodium vivax infection: a study in central China. PLoS One. 2012;7:e45971. doi: 10.1371/journal.pone.0045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbosa S, Gozze AB, Lima NF, Batista CL, Bastos Mda S, Nicolete VC, et al. Epidemiology of disappearing Plasmodium vivax malaria: a case study in rural Amazonia. PLoS Negl Trop Dis. 2014;8:e3109. doi: 10.1371/journal.pntd.0003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulze Zur Wiesch J, Thomssen A, Hartjen P, Tóth I, Lehmann C, Meyer-Olson D, et al. Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J Virol. 2011;85:1287–1297. doi: 10.1128/JVI.01758-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steiner K, Waase I, Rau T, Dietrich M, Fleischer B, Bröker BM. Enhanced expression of CTLA-4 (CD152) on CD4+ T cells in HIV infection. Clin Exp Immunol. 1999;115:451–457. doi: 10.1046/j.1365-2249.1999.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlotmann T, Waase I, Jülch C, Klauenberg U, Müller-Myhsok B, Dietrich M, et al. CD4 αβ T lymphocytes express high levels of the T lymphocyte antigen CTLA-4 (CD152) in acute malaria. J Infect Dis. 2000;182:367–370. doi: 10.1086/315690. [DOI] [PubMed] [Google Scholar]
- 35.Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94:25–39. doi: 10.1189/jlb.1212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zander RA, Obeng-Adjei N, Guthmiller JJ, Kulu DI, Li J, Ongoiba A, et al. PD-1 Co-inhibitory and OX40 co-stimulatory crosstalk regulates helper T cell differentiation and anti-Plasmodium humoral immunity. Cell Host Microbe. 2015;17:628–641. doi: 10.1016/j.chom.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corse E, Allison JP. Cutting edge: CTLA-4 on effector T cells inhibits in trans. J. Immunol. 2012;189:1123–1127. doi: 10.4049/jimmunol.1200695. [DOI] [PubMed] [Google Scholar]
- 38.Wang CJ, Kenefeck R, Wardzinski L, Attridge K, Manzotti C, Schmidt EM, et al. Cutting edge: cell-extrinsic immune regulation by CTLA-4 expressed on conventional T cells. J Immunol. 2012;189:1118–1122. doi: 10.4049/jimmunol.1200972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han Y, Guo Q, Zhang M, Chen Z, Cao X. CD69+ CD4+ CD25− T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-β 1. J Immunol. 2009;182:111–120. doi: 10.4049/jimmunol.182.1.111. [DOI] [PubMed] [Google Scholar]
- 40.Gandhi R, Farez MF, Wang Y, Kozoriz D, Quintana FJ, Weiner HL. Cutting edge: human latency-associated peptide+ T cells: a novel regulatory T cell subset. J Immunol. 2010;184:4620–4624. doi: 10.4049/jimmunol.0903329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oakley MS, Majam V, Mahajan B, Gerald N, Anantharaman V, Ward JM, et al. Pathogenic roles of CD14, galectin-3, and OX40 during experimental cerebral malaria in mice. PLoS One. 2009;4:e6793. doi: 10.1371/journal.pone.0006793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Croft M. The TNF family in T cell differentiation and function--unanswered questions and future directions. Semin Immunol. 2014;26:183–190. doi: 10.1016/j.smim.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuler PJ, Schilling B, Harasymczuk M, Hoffmann TK, Johnson J, Lang S, et al. Phenotypic and functional characteristics of CD4+ CD39+ FOXP3+ and CD4+ CD39+ FOXP3− T-cell subsets in cancer patients. Eur J Immunol. 2012;42:1876–1885. doi: 10.1002/eji.201142347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leal FE, Ndhlovu LC, Hasenkrug AM, Bruno FR, Carvalho KI, Wynn-Williams H, et al. Expansion in CD39+ CD4+ immunoregulatory T cells and rarity of Th17 cells in HTLV-1 infected patients is associated with neurological complications. PLoS Negl Trop Dis. 2013;7:e2028. doi: 10.1371/journal.pntd.0002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4+ CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 46.Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, et al. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 47.Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol Res. 2014;2:142–153. doi: 10.1158/2326-6066.CIR-13-0031-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costa PA, Leoratti FM, Figueiredo MM, Tada MS, Pereira DB, Junqueira C, et al. Induction of inhibitory receptors on T cells during Plasmodium vivax malaria impairs cytokine production. J Infect Dis. 2015;212:1999–2010. doi: 10.1093/infdis/jiv306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2011;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hafalla JC, Claser C, Couper KN, Grau GE, Renia L, de Souza JB, et al. The CTLA-4 and PD-1/PD-L1 inhibitory pathways independently regulate host resistance to Plasmodium-induced acute immune pathology. PLoS Pathog. 2012;8:e1002504. doi: 10.1371/journal.ppat.1002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.