Abstract
Background
Cortico-striatal network dysfunction in attention-deficit/hyperactivity disorder (ADHD) is generally investigated by comparing functional connectivity of the main striatal sub-regions (i.e., putamen, caudate, and nucleus accumbens) between an ADHD and a control group. However, dimensional analyses based on continuous symptom measures might help to parse the high phenotypic heterogeneity in ADHD. Here, we focus on functional segregation of regions in the striatum and investigate cortico-striatal networks using both categorical and dimensional measures of ADHD.
Methods
We computed whole-brain functional connectivity for six striatal sub-regions that resulted from a novel functional parcellation technique. We compared functional connectivity maps between adolescents with ADHD (N=169) and healthy controls (N=122), and investigated dimensional ADHD-related measures by relating striatal connectivity to ADHD symptom scores (N=444). Finally, we examined whether altered connectivity of striatal sub-regions related to motor and cognitive performance.
Results
We observed no case-control differences in functional connectivity patterns of the six striatal networks. In contrast, inattention and hyperactivity/impulsivity symptom scores were associated with increases in functional connectivity in the networks of posterior putamen and ventral caudate. Increased connectivity of posterior putamen with motor cortex and cerebellum was associated with decreased motor performance.
Conclusions
Our findings support hypotheses of cortico-striatal network dysfunction in ADHD by demonstrating that dimensional symptom measures are associated with changes in functional connectivity. These changes were not detected by categorical ADHD versus control group analyses, highlighting the important contribution of dimensional analyses to investigating the neurobiology of ADHD.
Keywords: ADHD, resting state fMRI, functional parcellation, cortico-striatal networks, putamen, dimensional analysis
Introduction
The striatum includes three main nuclei: the nucleus accumbens (NAcc), caudate, and putamen. These nuclei can be delineated based on histology, anatomical connectivity, and functional relevance (1-3). Specifically, the NAcc forms a network with anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC), and is associated with motivational control and reward processing (4). The caudate facilitates cognitive control through its connectivity with dorsolateral prefrontal cortex (DLPFC; 1), and the putamen primarily regulates motor function via connections with motor areas (5), but is also involved in cognitive tasks (6, 7).
Dysfunction in these cortico-striatal networks is thought to contribute to attention-deficit/hyperactivity disorder (ADHD) as the defining behavioral characteristics of ADHD coincide with the behaviors controlled by these networks. This idea is supported by structural meta-analyses reporting abnormalities of striatal nuclei in ADHD (8, 9) and by functional magnetic resonance imaging (fMRI) studies in ADHD (for reviews see 10-12). For example, patients with ADHD show decreased performance on, and altered neural responses during, response inhibition (13), working memory (14), reward processing (15), and motor function (16). Resting-state fMRI (rs-fMRI) studies further support the notion of aberrant integration of cortico-striatal networks in ADHD. Aberrant functional connectivity of NAcc, caudate, putamen, ACC, and frontal cortex (including DLPFC and inferior frontal gyrus) has been reported in ADHD (for reviews see 17, 18), and atypical functional connectivity of NAcc, putamen, and OFC has been associated with ADHD severity (19-21). However, existing literature is heterogeneous regarding the specific striatal and cortical regions implicated in ADHD and the direction of the effect. Some studies found decreased functional connectivity of putamen (19), NAcc (22), and caudate (23), others reported increased functional connectivity of NAcc (20, 21) and caudate (24) in cortico-striatal networks. Furthermore, in a previous study we investigated functional connectivity of structural definitions of putamen, caudate and NAcc as seeds in a large ADHD cohort (N=321). However, we did not observe differences in any of the whole brain cortico-striatal networks between participants with ADHD and controls (25).
To aid in clarifying the divergence in findings we propose to go beyond typical analytical approaches through incorporating two recent analytical developments. First, most studies have compared an ADHD group to a control group, i.e., used a categorical approach to investigate ADHD-related differences. This approach is based on clinical practice where patients are given an ADHD diagnosis when symptoms exceed a certain threshold, presuming underlying categorical mechanisms (26). However, high phenotypic heterogeneity and symptom overlap with other disorders have led to a shift toward dimensional characterization of ADHD (27). This approach corroborates the hypothesis that heterogeneous neurobiological mechanisms, comprising categorical and dimensional constructs underlie ADHD (27-29). Accordingly, dimensional analyses based on ADHD symptom scores might reveal ADHD-related changes not detected by a traditional ADHD versus control group comparison.
Second, striatal connectivity is typically investigated using putamen, caudate, and NAcc as homogeneous regions. However, recent evidence suggests that putamen and caudate may be functionally segregated into smaller sub-regions (1, 30). The putamen is considered to consist of an anterior region, connected to pre-supplementary motor area and ACC, and a posterior region, connected to primary and secondary motor areas (2, 31, 32). Anterior putamen has been associated with higher-order cognitive aspects of motor control such as learning new movements (31), while posterior putamen has been related to execution of skilled movements (32). A functional segregation of the caudate into a dorsal and ventral region has also been suggested (1, 33). Ventral caudate has been hypothesized to exert cognitive control over emotional circuits via connections with PFC, ACC and amygdala, whereas dorsal caudate has been associated with cognitive control over action-based networks via connections with PFC, motor cortex, and parietal cortex (33, 34). Investigating functional connectivity of the striatum in ADHD while taking these potential subdivisions of putamen and caudate into account might enable detection of ADHD-related effects localized within these sub-regions, that may not be revealed when considering caudate and putamen as homogeneous regions.
Here, we examined cortico-striatal network connectivity in ADHD while taking subdivisions within the striatum into account. We used functionally defined sub-regions of the striatum obtained by applying a novel parcellation strategy. We investigated resting-state functional connectivity of these fine-scale striatal sub-regions in a large ADHD cohort (N=444) using both categorical diagnosis and dimensional ADHD symptom measures. Finally, we explored whether altered connectivity of striatal sub-regions was related to motor and cognitive performance. As such, this study builds on previous reports, including our own work (25), by using fine-scale functionally-defined instead of structurally-defined striatal subregions and by conducting dimensional analyses in addition to categorical analyses.
Materials and Methods
Participants
Participants in our study were part of the NeuroIMAGE cohort (35), the Dutch follow-up of the large-scale International Multicenter ADHD Genetics (IMAGE) study (36), consisting of families with one or more children ‘officially’ diagnosed with ADHD by a psychiatrist and control families without an ADHD diagnosis. ADHD (and comorbid oppositional defiant disorder (ODD), conduct disorder (CD), anxiety disorders and depression) were reassessed by a trained psychologist during the NeuroIMAGE assessment. To this end, the Schedule for Affective Disorders and Schizophrenia for School-Age Children - Present and Lifetime Version (K-SADS; 37) was used, complemented with Conners’ ADHD questionnaires (38, 39). For an ADHD diagnosis, six or more DSM-5 ADHD symptoms on at least one domain (inattention or hyperactivity/impulsivity) were required (five or more for participants >18 years). Participants from control families and unaffected siblings of ADHD participants were allowed to have a maximum of two ADHD symptoms per domain. The remaining participants were classified as subthreshold ADHD. Next to this categorical classification, we used inattention and hyperactivity/impulsivity symptom scores derived from the Conners’ Parent Rating Scale (CPRS-RL; 38) for our dimensional analyses. The CPRS-RL is an ADHD rating scale from which standardized T-scores ranging from 40-90 can be obtained. For a full description of the NeuroIMAGE cohort, including inclusion criteria and diagnostic assessment, see (35). Local ethical committees of the participating centers approved our study and we obtained written informed consent from all participants (for participants >12 years) and their legal guardians (for participants <18 years).
For the current analyses we selected participants who completed an anatomical and an 8-minute rs-fMRI scan. Detailed scan parameters and preprocessing procedures are provided in the Supplementary material. Participants with high head-motion (N=47) as determined by the root mean squared of the frame-wise displacement (RMS-FD>0.5; 40) and participants with insufficient brain coverage (N=16) were excluded. This procedure led to the inclusion of 444 participants, consisting of ADHD participants (N=169), healthy controls (N=122), unaffected siblings of ADHD participants (N=89), and subthreshold ADHD cases (N=64). The characteristics of these participants are specified in Table 1. In the ADHD group 130 participants were currently on stimulant medication, however, all participants discontinued stimulant medication use 48 hours before assessment.
Table 1.
Participant characteristics.
| Controls (C) N=122 | ADHD (A) N=169 | Test Statistic | Subthreshold N=64 | Siblings N=89 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographic (Mean, SD) | ||||||||||
| Age, years | 17.04 | 2.98 | 17.89 | 3.07 | t(289)=2.36* | A>C | 18.37 | 3.32 | 17.63 | 4.12 |
| IQ a | 106.6 | 13.9 | 96.08 | 15.1 | t(289)=−6.08** | A<C | 100.7 | 12.9 | 102.1 | 14.9 |
| Medication use, years | - | - | 2.41 | 3.12 | - | - | 1.95 | 3.13 | 0.002 | 0.01 |
| Motion b | 0.125 | 0.09 | 0.140 | 0.10 | t(289)=1.35 | - | 0.129 | 0.09 | 0.113 | 0.09 |
| Demographic (Number, %) | ||||||||||
| Sex, male | 54 | 44.3 | 123 | 72.8 | X2(1)=24.18** | A>C | 38 | 59.4 | 35 | 39.3 |
| Scan location, Nijmegen | 48 | 39.3 | 92 | 54.4 | X2(1)=6.65* | A>C | 36 | 56.3 | 46 | 51.7 |
| ODD c | - | - | 46 | 27.2 | - | - | 8 | 12.5 | 2 | 2.25 |
| CD d | - | - | 6 | 3.80 | - | - | - | - | - | - |
| Clinical (Mean, SD) | ||||||||||
| Hyperactive/impulsive symptoms e | 46.45 | 5.24 | 69.59 | 14.2 | t(289)=17.19** | A>C | 54.70 | 11.70 | 47.71 | 6.46 |
| Inattentive symptoms e | 46.12 | 5.72 | 66.09 | 10.9 | t(289)=18.49** | A>C | 54.07 | 8.57 | 46.81 | 6.16 |
Estimated IQ based on Wechsler Intelligence Scale for Children or Wechsler Adult Intelligence Scale–III Vocabulary and block design (52, 53).
Motion as measured by frame-wise displacement (40)
Oppositional Defiant Disorder (ODD)
Conduct Disorder (CD)
Conners Parent Rating Scale questionnaire, standardized T-score (38). Range min. 40 to max. 90 (≥63 is clinical threshold). The test statistics only compare the ADHD and control group given that only these groups were included in the categorical analysis. The dimensional analyses included all participants listed in this table independent of diagnostic label
p<.05
p < .001.
Functional segmentation of the striatum
We obtained functionally defined sub-regions within the striatum using a novel top-down parcellation strategy called Instantaneous Correlation Parcellation (ICP; Van Oort et al., in preparation). ICP delineates sub-regions within a larger predefined region of interest based on subtle differences in the timeseries of the regions’ voxels. To this end ICP applies group-level independent component analysis to temporally unfolded voxel-wise timeseries, which augments those subtle differences. The optimal number of sub-regions is determined by assessing reproducibility of the parcellation across random splits of the data sample. Details about the ICP implementation are provided in the Supplementary material.
Here, we generated an independent, high-resolution parcellation of the striatum using rs-fMRI data publically available in the Human Connectome Project (HCP; 41). The HCP dataset provides one hour of rs-fMRI data acquired using a multiband echo-planar imaging sequence with a spatial resolution of 2mm isotropic and a TR of 720ms. This results in 4800 available time points for every participant. This high temporal resolution is of great benefit as the ICP strategy specifically focuses on subtle differences in timeseries to delineate sub-regions. We used 50 females and 50 males from the S500 release (for subject IDs see Supplementary Table S1). Specifically, we used their ICA-FIX denoised data (41) and additionally applied a high-pass filter of 0.01Hz and spatial smoothing using a 3mm FWHM Gaussian kernel.
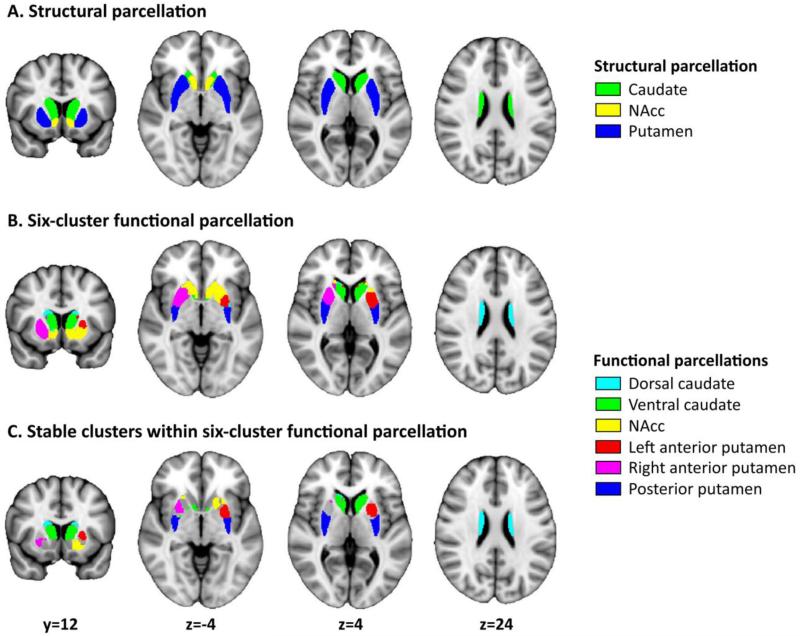
We applied ICP to the selected HCP rs-fMRI data, using a mask of bilateral striatum created by combining NAcc, caudate, and putamen as extracted from the Harvard-Oxford atlas (Figure 1A). As a first solution, ICP yielded highest split-half reproducibility for a parcellation including two sub-regions. This parcellation segregated the striatum into a cluster corresponding to putamen and a cluster corresponding to NAcc and caudate. The parcellation showing the second highest reproducibility (84% overlap between split-half analyses; Figure S3) yielded six sub-regions, which closely corresponded to the hypothesized sub-regions within the striatum (Figure 1B). Accordingly, we used this parcellation for further analyses.
Figure 1. Functional parcellation of striatum generated using Instantaneous Correlation Parcellation.
A) Structural parcellation of the striatum in NAcc (yellow), caudate (green), and putamen (blue) as provided in the Harvard-Oxford Atlas. B) Functional parcellation of the striatum into six sub-regions using the ICP strategy. C) The six final, stable sub-regions used for further analyses including: dorsal caudate (turquoise), ventral caudate (green), NAcc (yellow), left anterior putamen (red), right anterior putamen (violet), and posterior putamen (blue). We created the stable regions by excluding those voxels that were inconsistently associated with sub-regions across lower-level parcellations (e.g., a voxel that was associated with anterior putamen in a 4-region solution, and posterior putamen in the current 6-region solution would be excluded; see also Supplementary Figure S4).
Figure 1B illustrates the six sub-regions, including the hypothesized anterior-posterior division within putamen and dorsal-ventral division within caudate. All resulting sub-regions were bilateral, except for anterior putamen, which was split in separate left and right parts. To enhance the homogeneity of the obtained sub-regions we identified voxels within each sub-region that were part of the same sub-region across the lower-scale parcellations (scale 2-5; Supplementary Figure S4). This procedure excluded voxels that exhibited discordant associations when increasing the number of sub-regions. Especially voxels at the interface between two regions might be subject to altered associations depending on the number of requested sub-regions. The resulting coherent, stable sub-regions were used as regions of interest in subsequent functional connectivity analyses. These six sub-regions are displayed in Figure 1C and consisted of dorsal caudate (turquoise), ventral caudate (green), NAcc (yellow), left anterior putamen (red), right anterior putamen (violet), and posterior putamen (blue). Of note, we present a comparison with an alternative functional parcellation of striatum (30) in Supplementary Figure S5.
Seed-based functional connectivity analyses
We used the six stable sub-regions obtained from the ICP parcellation as masks to extract rs-fMRI timeseries for the NeuroIMAGE participants. Timeseries were extracted from each participant's rs-fMRI data after transformation to MNI152 2mm standard space (see Supplementary material). First we extracted timeseries for all voxels within each mask and applied a singular value decomposition. We then selected the first eigenvariate and used the associated timeseries as the timeseries that most accurately represented the seed mask. Next, we obtained native-space participant-level whole-brain voxel-wise functional connectivity maps for each seed by entering all seed timeseries simultaneously in a multiple regression. This approach resulted in unique whole-brain functional connectivity maps for each striatal sub-region that were not confounded by contributions of the other seeds. The whole-brain functional connectivity maps were transformed to MNI152 2mm standard space and were used in categorical and dimensional analyses that assessed effects of ADHD. In the categorical analysis we compared the functional connectivity maps of the six striatal regions between the ADHD (N=169) and control group (N=122). In the dimensional analyses we examined the relationship between functional connectivity of the six striatal regions and CPRS inattention and hyperactivity/impulsivity scores across all participants (N=444) in two separate linear models.
Both the categorical and dimensional models included covariates for age, age2, sex, scan location, and comorbid ODD/CD. The effects of both categorical and dimensional analyses were tested using permutation testing as implemented in FSL randomize (5000 permutations; 42). We applied threshold-free cluster enhancement and family-wise error correction. Results were considered statistically significant if they passed a threshold of p<0.0083 (i.e., p=0.05/6 investigated networks). To rule out that our findings were driven by scan location, sex, age, IQ, medication history, ODD/CD comorbidity or motion, we conducted post-hoc sensitivity analyses, as described in the Supplementary material (Tables S2-S6). Finally, to provide a comparison with current literature, we repeated our analyses using putamen, caudate, and NAcc as homogeneous (anatomically-defined) seed regions (see Supplementary material).
Relationships with motor performance and cognition
For regions showing significant ADHD-related effects in the categorical and/or dimensional analyses, we explored post-hoc whether these effects were related to motor or cognitive performance. We calculated mean connectivity for regions identified in the categorical and/or dimensional analyses for every participant and correlated these connectivity values with measures of motor or cognitive performance, while correcting for effects of age, age2, sex, scan location, and ODD/CD comorbidity. To index motor performance we used total motor performance scores obtained from the Developmental Coordination Disorder questionnaire (DCD-Q; 43), performance on a motor timing task (44, 45), and performance on motor speed, motor pursuit, and motor tracking obtained from the Amsterdam Neuropsychological Task battery (46, 47). To index cognitive functioning we used performance on the following tasks: visuospatial working memory (accuracy and reaction time; 48-50), response inhibition (stop signal reaction time and number of errors; 6, 51), and WISC/WAIS intellectual functioning (30, 47, 52, 53). Supplementary Table S8 lists participant characteristics for each measure.
Results
Whole-brain functional connectivity of the six striatal regions
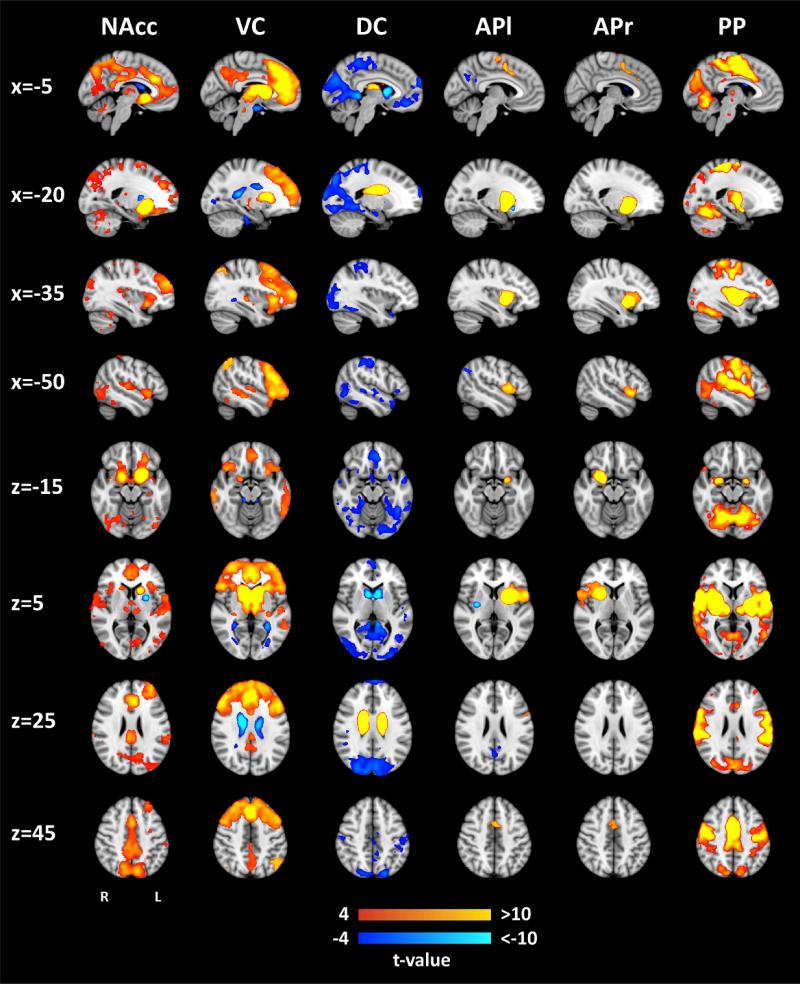
Figure 2 shows the whole-brain functional connectivity networks of the six striatal sub-regions in the control group. The observed networks largely correspond with striatal networks as reported in existing literature (1, 2). The network of NAcc comprised OFC, ACC, and precuneus. The ventral caudate network (VC) included medial and lateral PFC, lateral OFC and ACC, whereas the network of dorsal caudate (DC) was restricted to striatal regions. The left (APl) and right (APr) anterior putamen networks were unilateral and smaller compared to the other networks, including dorsal ACC and pre-supplementary motor area. Finally, the posterior putamen (PP) was extensively connected with areas of the motor system including primary motor cortex and cerebellum. Supplementary Figure S6 compares the current networks to functional networks of the striatum defined in an earlier study (1).
Figure 2. Whole-brain functional connectivity networks for each of the six striatal sub-regions.
Whole-brain networks shown here were derived through multiple regression using data of healthy controls only (N=122). X- and z-values represent x- and z-MNI152 coordinates. Abbreviations: NAcc=nucleus accumbens, VC=ventral caudate, DC=dorsal caudate, APl=anterior putamen left, APr=anterior putamen right, PP=posterior putamen. The network for the right anterior putamen seed (APr) is displayed for the corresponding x-values in the right hemisphere (x=5, x=20, x=35, and x=50). To aid visualization, the threshold for the network of posterior putamen seed (PP) was set at |t|≥ 7.
Categorical effects of ADHD
We did not observe significant differences between the ADHD and control group in the functional connectivity patterns of any of the six striatal regions. Results obtained using a non-Bonferroni corrected threshold of p<0.05 are presented in Supplementary Figure S9. Further, to allow comparison with earlier studies we conducted univariate (instead of multivariate) categorical analyses investigating connectivity for each striatal seed region in a separate model. These analyses did also not reveal significant differences between the ADHD and control group.
Effects of dimensional ADHD-related measures
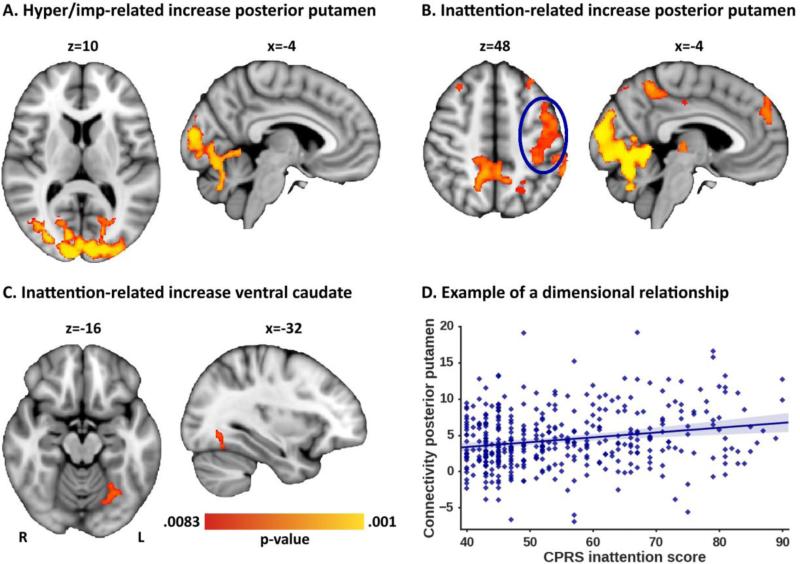
Dimensional analyses using participant-level ADHD symptom ratings of all 444 participants, revealed that increased hyperactivity/impulsivity (Figure 3A) and increased inattention scores (Figure 3B) were associated with increased functional connectivity of posterior putamen with occipital cortex and cerebellum. Higher inattention scores were furthermore associated with increased functional connectivity of posterior putamen with prefrontal cortex, left motor cortex, and precuneus (Figure 3B). Increased inattention scores also correlated with increased functional connectivity between ventral caudate and occipital fusiform gyrus (Figure 3C). Peak coordinates of the significant clusters are listed in Table 2; Figure S7 illustrates the relationship between symptom scores and functional connectivity for each cluster. Of note, although effects were observed across the whole sample, relationships between functional connectivity and symptom scores were strongest in ADHD participants and their siblings; See Supplementary Table S4). No significant dimensional relationships were observed for functional connectivity patterns of the other striatal regions (NAcc, dorsal caudate, left and right anterior putamen). Post-hoc sensitivity analyses showed that the observed dimensional effects were not related to scan location, sex, age, IQ, medication history, or ODD/CD comorbidity (Supplementary Tables S2 and S3).
Figure 3. Significant dimensional effects for posterior putamen and ventral caudate connectivity.
A) Increased hyperactivity/impulsivity scores were associated with increased functional connectivity of posterior putamen with regions in occipital cortex and cerebellum. B) Increased inattention scores were associated with increased functional connectivity of posterior putamen with the various highlighted regions. C) Increased inattention scores were furthermore related to increased functional connectivity between ventral caudate and occipital fusiform gyrus. D) Representation of the relationship between inattention and functional connectivity between posterior putamen and motor cortex (as circled in panel B).
Table 2.
Peak coordinates of significant clusters observed in the dimensional analyses.
| Inattention-related increase in posterior putamen connectivity | Peak coordinates (MNI) | ||
|---|---|---|---|
| Occipital cortex | x=−12 | y=−94 | z=8 |
| Cerebellum | x=−6 | y=−68 | z=−22 |
| Precuneus | x=10 | y=−46 | z=46 |
| Motor cortex | x=−46 | y=2 | z=50 |
| extending to supramarginal gyrus | x=−58 | y=−40 | z=38 |
| Prefrontal cortex | x=−22 | y=44 | z=30 |
| Inattention-related increase in ventral caudate connectivity | |||
| Occipital cortex | x=−24 | y=−68 | z=−18 |
| Hyperactivity/Impulsivity-related increase in posterior putamen connectivity | |||
| Posterior putamen – Occipital cortex | x=24 | y=−86 | z=16 |
| Posterior putamen – Cerebellum | x=14 | y=−74 | z=−18 |
Relationships with motor performance and cognition
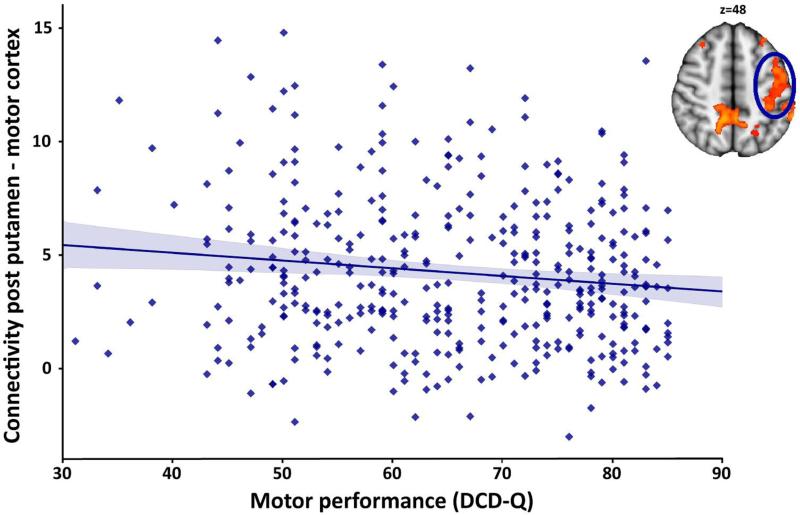
Given the role of the posterior putamen in motor function, we conducted post-hoc analyses exploring whether functional connectivity between posterior putamen and significant clusters identified in the dimensional analyses was related to motor performance (using a Bonferroni corrected p-value of 0.00625, i.e., 0.05/8 examined clusters). These analyses revealed that DCD-Q total motor performance decreased as functional connectivity between posterior putamen and motor cortex increased (r=−0.14, p=0.006; see Figure 4). Similarly, DCD-Q total motor performance decreased as posterior putamen-cerebellum connectivity increased (r=−0.20, p<0.001). These effects are however not unexpected given the presence of motor problems in ADHD. Correlations between connectivity of posterior putamen and the other measures of motor performance were not significant.
Figure 4.
Increased functional connectivity between posterior putamen and motor cortex is associated with decreased motor performance, r=−0.14, p=0.006. Motor performance was based on the Developmental Coordination Disorder Questionnaire (DCD-Q; 43); higher scores indicate better performance. The correlation between posterior putamen-cerebellum connectivity and motor performance (r=−0.20, p<0.001) yielded a similar effect.
Finally, we explored whether functional connectivity between ventral caudate and occipital fusiform gyrus, which was related to symptoms of inattention, was also related to impaired cognitive functioning, as the caudate has been implicated in cognitive control (54). We did not observe associations between ventral caudate-occipital fusiform gyrus connectivity and our cognitive performance measures.
Discussion
In this report we investigated categorical and dimensional ADHD-related alterations in whole-brain functional connectivity of six striatal sub-regions obtained using a novel functional parcellation technique. We did not replicate case-control differences in cortico-striatal network connectivity observed in previous studies, yet we did observe that functional connectivity in the networks of posterior putamen and ventral caudate increased significantly with increasing inattention and hyperactivity/impulsivity symptom scores. In addition, we showed that increased connectivity of posterior putamen with motor cortex and cerebellum correlated with decreased motor performance. Our findings support hypotheses of cortico-striatal dysfunction in ADHD and highlight the dimensional aspects of the disorder.
Increased inattention scores were associated with increased functional connectivity of posterior putamen with several regions including motor cortex, cerebellum, precuneus, and PFC. The observed increase in functional connectivity of posterior putamen with motor cortex and cerebellum could be interpreted in light of impairments in motor execution, which are typical for many children with ADHD (16, 55). This idea is supported by our observation that increased connectivity of posterior putamen with cerebellum and motor cortex was associated with subjective ratings of decreased motor performance, and also corresponds with previous studies relating increased white matter tract strength between posterior putamen and primary motor cortex to decreased motor performance in healthy adults (7). Our findings furthermore corroborate reports of increasing motor tract anisotropy with increasing ADHD symptom scores (56), and task-based fMRI studies that relate aberrant activity of the motor system to impaired motor function in ADHD (57, 58). We note that the direction of this dimensional effect, i.e., increased functional connectivity, contradicts task-based fMRI and rs-fMRI studies that reported reduced activation or connectivity in cortico-striatal networks in ADHD (for reviews see 12, 17, 18). Regarding the motor network, both increased (59) and decreased (19) functional connectivity have been reported in ADHD. This dichotomy might relate to potential opposite effects of ADHD characteristics in clinical ADHD versus the healthy population (15), yet other studies do not report such opposite effects (60).
Further, we observed that increased inattention scores were associated with increased connectivity between posterior putamen and precuneus. The precuneus is a multifaceted region and is also part of the default mode network (DMN). The DMN, as opposed to task-positive networks, is associated with self-referential cognitive processes that are typically inhibited during externally oriented, attention-demanding tasks (61). Our findings correspond with previous studies reporting decreased anti-correlation between precuneus (as part of the DMN) and task-positive networks, or in other words diminished DMN suppression, during sustained attention in ADHD (62). Diminished DMN suppression is thought to disrupt ongoing cognition and behavior, leading to periodic lapses in task-performance, a hallmark of ADHD (18, 63).
Finally, we observed increased functional connectivity of both posterior putamen and ventral caudate with occipital cortex at higher ADHD symptom scores. Deficits in occipital cortex, which is involved in visual processing, are not typically considered in the etiology of ADHD. Yet, there is ample evidence for structural (64, 65) and functional occipital cortex abnormalities in ADHD (for review see 63). Furthermore, local functional connectivity within occipital cortex was significantly increased in ADHD patients (23, 66). Increased activation and connectivity of occipital cortex has been hypothesized to reflect mechanisms compensating for inattention in ADHD (14, 63). Accordingly, our finding of increased functional connectivity between ventral caudate and occipital cortex as a function of increased inattention could be explained in the context of interactions between occipital cortex, caudate, and attention networks that are aimed at maintaining attention to relevant stimuli while suppressing attention to irrelevant stimuli (67).
In contrast to the dimensional ADHD-related effects we did not find significant case-control differences on cortico-striatal network connectivity when using categorical definitions of the disorder. A recent rs-fMRI study characterized both categorical and dimensional variations in four large-scale brain networks (default mode, dorsal attention, executive control, and salience network; 28). Distinct categorical and dimensional effects were reported, but overlap between some of the neural correlates of both measures was also demonstrated, confirming the hypothesis that both categorical and dimensional mechanisms contribute to ADHD. We did not find significant categorical differences, which in inconsistent with this hypothesis and previous reports investigating functional connectivity in corticostriatal networks in ADHD (for reviews see 17, 18). However, when lowering the threshold for significance we observed increased connectivity between posterior putamen and occipital cortex in the ADHD group compared to controls, which overlapped with our significant dimensional findings (see Supplementary Figure S9). These observations seem to suggest that dimensional analyses using inattention and hyperactivity/impulsivity measures might be more sensitive than a categorical analysis to reveal the neuroimaging correlates of ADHD. Yet, our findings do not rule out that different mechanisms underlie the ADHD-related effects observed in categorical and dimensional analyses of cortico-striatal network connectivity (28). Of note, we are preparing a manuscript focusing on statistical techniques to disentangle effects of hyperactivity/impulsivity, inattention, and general ADHD-related effects (Pruim et al., in preparation). Further, although not yielding significance, the categorical analyses revealed similar effects sizes as the dimensional analyses. The average effect size of the dimensional effects is r=0.237 versus r=0.225 of the subthreshold categorical effect depicted in Supplementary Figure S9. This suggests that the difference in results might at least be partly related to increased statistical power in the dimensional analyses through the inclusion of more participants.
A factor that might have contributed to the absence of significant categorical differences in our study is the potential heterogeneity in our large sample (N=444). We explicitly aimed to include an ADHD sample sufficiently broad to provide a valid representation of the general ADHD population: we selected participants independent of ADHD subtype, sex, and stimulant treatment within a large age range (8-25 years). This approach however, might have concealed categorical differences detected in previous studies. That is, previous studies have mostly been conducted in smaller, potentially more homogeneous samples consisting of participants with the combined (and most severe) ADHD subtype, while all ADHD subtypes were included in our study. Dimensional measures, in contrast, capture such developmental, sex, and treatment differences by allowing individual variation.
A limitation of our study is that medication use differed among ADHD participants, with 130 out of 169 participants being on stimulant medication. Importantly, stimulant medication has been shown to alter activation and/or functional connectivity of prefrontal and striatal regions in controls (68) and participants with ADHD (for meta-analyses see 69-71). However, sensitivity analyses described in the Supplementary material indicate that medication status (i.e., medicated versus medication-naïve; Table S5 and Figure S8) and the duration of medication use (Table S2) did not significantly impact our findings. We could not distinguish medicated from medication-naïve participants.
In this report we investigated cortico-striatal networks using functionally defined sub-regions of the striatum obtained via a novel functional parcellation strategy (i.e., ICP). Using this approach we avoided imposing structural borders while taking functional subdivisions of the striatum into account. We clearly demonstrated the potency of ICP to segregate the striatum into functional sub-regions: the parcellation of the striatum not only confirmed the traditional subdivision into NAcc, putamen, and caudate, but also the hypothesized anterior-posterior division of putamen and ventral-dorsal division of caudate were retrieved. We furthermore demonstrated the benefit of our fine-scale functional subdivision over a traditional anatomical subdivision of striatum: the analyses using putamen, caudate, and NAcc as homogeneous seed regions only revealed a significant inattention-related increase in functional connectivity in the network of putamen, which was of smaller spatial extent than the inattention-related increases observed in the network of the functionally defined posterior putamen region (Supplementary Figure S10).
To conclude, we demonstrated the effectiveness of ICP to functionally segregate the striatum into biologically valid sub-regions. Using these regions we confirmed cortico-striatal network dysfunction in ADHD by revealing symptom-related increases in functional connectivity of posterior putamen and ventral caudate. Our results highlight the potential of data-driven functional connectivity studies for explaining variance in the behavioral heterogeneity of ADHD beyond a categorical definition of the disorder.
Supplementary Material
Acknowledgements
Maarten Mennes is supported by a Marie Curie International Incoming Fellowship under the European Union's Seventh Framework Programme (FP7/2007-2013), grant agreement n° 327340 (Brain Fingerprint). Jan K. Buitelaar is supported by NWO Large Investment grant 1750102007010, EU FP7 grant TACTICS (grant no. 278948), ZonMW Addiction: Risk Behaviour and Dependency Grant 60-60600-97-193, NWO Brain & Cognition: an Integrative Approach grant 433-09-242, and NWO National Initiative Brain & Cognition 056-13-015. Christian F. Beckmann is supported by the Netherlands Organisation for Scientific Research (NWO-Vidi 864-12-003) and received funding from the Wellcome Trust UK Strategic Award [098369/Z/12/Z]. Barbara Franke's research is supported by grants from the Netherlands Organization for Scientific Research (NWO), i.e., the NWO Brain & Cognition Excellence Program (grant 433-09-229) and a Vici grant (grant 016-130-669). Her research also received funding from the European Community's Seventh Framework Programme (FP7/2007 – 2013) under grant agreements n° 602805 (Aggressotype) and n° 602450 (IMAGEMEND), and from the European Community's Horizon 2020 Programme (H2020/2014 – 2020) under grant agreement n° 643051 (MiND). In addition, her work is supported by a grant for the ENIGMA Consortium (grant number U54 EB020403) from the BD2K Initiative of a cross-NIH partnership. This work was further supported by National Institutes of Health grant R01MH62873 (Dr. Faraone) and grants from Radboudumc, University Medical Center Groningen, Accare, and VU University Amsterdam. The data described in this paper has been orally presented at both the ADHD World Congress 2015 in Glasgow, Scotland, and at the Human Brain Mapping conference 2015 in Honolulu, HI, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Jan K. Buitelaar has been a consultant to/member of advisory boards of and/or a speaker for Janssen-Cilag BV, Eli Lilly, Bristol-Myers Squibb, Schering Plough, UCB, Shire, Novartis, Roche, and Servier. He is not an employee of any of these companies, and not a stock shareholder of any of these companies. Barbara Franke has received a speaker fee from Merz. Pieter J. Hoekstra has received an unrestricted research grant from Shire and has been member of the advisory boards of Shire and Eli Lilly. Jaap Oosterlaan has received an unrestricted investigator initiated research grant from Shire pharmaceuticals. All other authors reported no biomedical financial interests or conflicts of interest.
References
- 1.Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 2.Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson's disease. Cereb Cortex. 2010;20:1175–1186. doi: 10.1093/cercor/bhp178. [DOI] [PubMed] [Google Scholar]
- 3.Alexander GE, Delong MR, Strick PL. Parallel Organization of Functionally Segregated Circuits Linking Basal Ganglia and Cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 4.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A. 2005;102:12566–12571. doi: 10.1073/pnas.0502762102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rooij D, Hoekstra PJ, Mennes M, von Rhein D, Thissen AJ, Heslenfeld D, et al. Distinguishing Adolescents With ADHD From Their Unaffected Siblings and Healthy Comparison Subjects by Neural Activation Patterns During Response Inhibition. Am J Psychiatry. 2015;172:674–683. doi: 10.1176/appi.ajp.2014.13121635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Wit S, Watson P, Harsay HA, Cohen MX, van de Vijver I, Ridderinkhof KR. Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. J Neurosci. 2012;32:12066–12075. doi: 10.1523/JNEUROSCI.1088-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry. 2011;168:1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- 9.Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand. 2012;125:114–126. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- 10.Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 12.Cubillo A, Halari R, Smith A, Taylor E, Rubia K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex. 2012;48:194–215. doi: 10.1016/j.cortex.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Lei D, Du M, Wu M, Chen T, Huang X, Du X, et al. Functional MRI reveals different response inhibition between adults and children with ADHD. Neuropsychology. 2015;29:874–881. doi: 10.1037/neu0000200. [DOI] [PubMed] [Google Scholar]
- 14.Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plichta MM, Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev. 2014;38:125–134. doi: 10.1016/j.neubiorev.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stray LL, Kristensen O, Lomeland M, Skorstad M, Stray T, Tonnessen FE. Motor regulation problems and pain in adults diagnosed with ADHD. Behav Brain Funct. 2013;9:18. doi: 10.1186/1744-9081-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oldehinkel M, Francx W, Beckmann CF, Buitelaar JK, Mennes M. Resting state FMRI research in child psychiatric disorders. Eur Child Adoles Psy. 2013;22:757–770. doi: 10.1007/s00787-013-0480-0. [DOI] [PubMed] [Google Scholar]
- 18.Posner J, Park C, Wang Z. Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol Rev. 2014;24:3–15. doi: 10.1007/s11065-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao X, Cao Q, Long X, Sun L, Sui M, Zhu C, et al. Abnormal resting-state functional connectivity patterns of the putamen in medication-naive children with attention deficit hyperactivity disorder. Brain Res. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 20.Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, et al. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2012;23:33–45. doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomasi D, Volkow ND. Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71:443–450. doi: 10.1016/j.biopsych.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Posner J, Rauh V, Gruber A, Gat I, Wang Z, Peterson BS. Dissociable attentional and affective circuits in medication-naive children with attention-deficit/hyperactivity disorder. Psychiatry Res. 2013;213:24–30. doi: 10.1016/j.pscychresns.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Q, Zang Y, Sun L, Sui M, Long X, Zou Q, et al. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport. 2006;17:1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- 24.Mennes M, Vega Potler N, Kelly C, Di Martino A, Castellanos FX, Milham MP. Resting state functional connectivity correlates of inhibitory control in children with Attention-Deficit/Hyperactivity Disorder. Frontiers in Psychiatry. 2012;2 doi: 10.3389/fpsyt.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Rhein D, Oldehinkel M, Beckmann CF, Oosterlaan J, Heslenfeld D, Hartman CA, et al. Aberrant local striatal functional connectivity in attention-deficit/hyperactivity disorder. J Child Psychol Psyc. 2016 doi: 10.1111/jcpp.12529. [DOI] [PubMed] [Google Scholar]
- 26.Sonuga-Barke EJ. Categorical models of childhood disorder: a conceptual and empirical analysis. J Child Psychol Psychiatry. 1998;39:115–133. [PubMed] [Google Scholar]
- 27.Chabernaud C, Mennes M, Kelly C, Nooner K, Di Martino A, Castellanos FX, et al. Dimensional brain-behavior relationships in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71:434–442. doi: 10.1016/j.biopsych.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elton A, Alcauter S, Gao W. Network connectivity abnormality profile supports a categorical-dimensional hybrid model of ADHD. Hum Brain Mapp. 2014;35:4531–4543. doi: 10.1002/hbm.22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asherson P, Trzaskowski M. Attention-deficit/hyperactivity disorder is the extreme and impairing tail of a continuum. J Am Acad Child Adolesc Psychiatry. 2015;54:249–250. doi: 10.1016/j.jaac.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aramaki Y, Haruno M, Osu R, Sadato N. Movement initiation-locked activity of the anterior putamen predicts future movement instability in periodic bimanual movement. J Neurosci. 2011;31:9819–9823. doi: 10.1523/JNEUROSCI.4473-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tricomi E, Balleine BW, O'Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci. 2009;29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, et al. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage. 2012;60:117–129. doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Draganski B, Kherif F, Kloeppel S, Cook PA, Alexander DC, Parker GJM, et al. Evidence for segregated and integrative connectivity patterns in the human basal ganglia. Journal of Neuroscience. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Rhein D, Mennes M, van Ewijk H, Groenman AP, Zwiers MP, Oosterlaan J, et al. The NeuroIMAGE study: a prospective phenotypic, cognitive, genetic and MRI study in children with attention-deficit/hyperactivity disorder. Design and descriptives. Eur Child Adoles Psy. 2014;24:265–281. doi: 10.1007/s00787-014-0573-4. [DOI] [PubMed] [Google Scholar]
- 36.Muller UC, Asherson P, Banaschewski T, Buitelaar JK, Ebstein RP, Eisenberg J, et al. The impact of study design and diagnostic approach in a large multi-centre ADHD study. Part 1: ADHD symptom patterns. BMC Psychiatry. 2011;11:54. doi: 10.1186/1471-244X-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 38.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 39.Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- 40.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 41.Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, et al. Resting-state fMRI in the Human Connectome Project. Neuroimage. 2013;80:144–168. doi: 10.1016/j.neuroimage.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson BN, Kaplan BJ, Crawford SG, Campbell A, Dewey D. Reliability and validity of a parent questionnaire on childhood motor skills. Am J Occup Ther. 2000;54:484–493. doi: 10.5014/ajot.54.5.484. [DOI] [PubMed] [Google Scholar]
- 44.Rommelse NN, Altink ME, de Sonneville LM, Buschgens CJ, Buitelaar J, Oosterlaan J, et al. Are motor inhibition and cognitive flexibility dead ends in ADHD? J Abnorm Child Psychol. 2007;35:957–967. doi: 10.1007/s10802-007-9146-z. [DOI] [PubMed] [Google Scholar]
- 45.Thissen AJ, Luman M, Hartman C, Hoekstra P, van Lieshout M, Franke B, et al. Attention-deficit/hyperactivity disorder (ADHD) and motor timing in adolescents and their parents: familial characteristics of reaction time variability vary with age. J Am Acad Child Adolesc Psychiatry. 2014;53:1010–1019. e1014. doi: 10.1016/j.jaac.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 46.De Sonneville L. Amsterdam Neuropsychological Tasks: A computer-aided assessment program. Computers in psychology. 1999;6:187–203. [Google Scholar]
- 47.Thissen AJ, Rommelse NN, Altink ME, Oosterlaan J, Buitelaar JK. Parent-of-origin effects in ADHD: distinct influences of paternal and maternal ADHD on neuropsychological functioning in offspring. J Atten Disord. 2014;18:521–531. doi: 10.1177/1087054712443159. [DOI] [PubMed] [Google Scholar]
- 48.Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- 49.McNab F, Leroux G, Strand F, Thorell L, Bergman S, Klingberg T. Common and unique components of inhibition and working memory: an fMRI, within-subjects investigation. Neuropsychologia. 2008;46:2668–2682. doi: 10.1016/j.neuropsychologia.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 50.van Ewijk H, Heslenfeld DJ, Luman M, Rommelse NN, Hartman CA, Hoekstra P, et al. Visuospatial working memory in ADHD patients, unaffected siblings, and healthy controls. J Atten Disord. 2014;18:369–378. doi: 10.1177/1087054713482582. [DOI] [PubMed] [Google Scholar]
- 51.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 52.Wechsler D. WISC-III Handleiding. The Psychological Corporation; London: 2002. [Google Scholar]
- 53.Wechsler D. WAIS-III Nederlandstalige bewerking. Technische handleiding. The Psychological Corporation; London: 2000. [Google Scholar]
- 54.Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Fliers E, Rommelse N, Vermeulen SH, Altink M, Buschgens CJ, Faraone SV, et al. Motor coordination problems in children and adolescents with ADHD rated by parents and teachers: effects of age and gender. J Neural Transm. 2008;115:211–220. doi: 10.1007/s00702-007-0827-0. [DOI] [PubMed] [Google Scholar]
- 56.van Ewijk H, Heslenfeld DJ, Zwiers MP, Faraone SV, Luman M, Hartman CA, et al. Different mechanisms of white matter abnormalities in attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2014;53:790–799. e793. doi: 10.1016/j.jaac.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Suskauer SJ, Simmonds DJ, Caffo BS, Denckla MB, Pekar JJ, Mostofsky SH. fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. J Am Acad Child Adolesc Psychiatry. 2008;47:1141–1150. doi: 10.1097/CHI.0b013e3181825b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valera EM, Spencer RM, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, et al. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:359–367. doi: 10.1016/j.biopsych.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.An L, Cao XH, Cao QJ, Sun L, Yang L, Zou QH, et al. Methylphenidate Normalizes Resting-State Brain Dysfunction in Boys With Attention Deficit Hyperactivity Disorder. Neuropsychopharmacology. 2013;38:1287–1295. doi: 10.1038/npp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoogman M, Rijpkema M, Janss L, Brunner H, Fernandez G, Buitelaar J, et al. Current self-reported symptoms of attention deficit/hyperactivity disorder are associated with total brain volume in healthy adults. PLoS One. 2012;7:e31273. doi: 10.1371/journal.pone.0031273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Christakou A, Murphy CM, Chantiluke K, Cubillo AI, Smith AB, Giampietro V, et al. Disorder-specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with autism. Mol Psychiatry. 2013;18:236–244. doi: 10.1038/mp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahrendts J, Rusch N, Wilke M, Philipsen A, Eickhoff SB, Glauche V, et al. Visual cortex abnormalities in adults with ADHD: a structural MRI study. World J Biol Psychiatry. 2011;12:260–270. doi: 10.3109/15622975.2010.518624. [DOI] [PubMed] [Google Scholar]
- 65.Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, et al. Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Arch Gen Psychiatry. 2011;68:1122–1134. doi: 10.1001/archgenpsychiatry.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X, Jiao Y, Tang T, Wang H, Lu Z. Altered regional homogeneity patterns in adults with attention-deficit hyperactivity disorder. Eur J Radiol. 2013;82:1552–1557. doi: 10.1016/j.ejrad.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 67.Shulman GL, Astafiev SV, Franke D, Pope DL, Snyder AZ, McAvoy MP, et al. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci. 2009;29:4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mueller S, Costa A, Keeser D, Pogarell O, Berman A, Coates U, et al. The effects of methylphenidate on whole brain intrinsic functional connectivity. Hum Brain Mapp. 2014;35:5379–5388. doi: 10.1002/hbm.22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 70.McCarthy H, Skokauskas N, Frodl T. Identifying a consistent pattern of neural function in attention deficit hyperactivity disorder: a meta-analysis. Psychol Med. 2014;44:869–880. doi: 10.1017/S0033291713001037. [DOI] [PubMed] [Google Scholar]
- 71.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.