Abstract
Importance
Peripheral neuropathy is a prevalent condition that usually warrants a thorough history and examination, but limited diagnostic evaluation. Rare localizations of peripheral neuropathy, however, often require more extensive diagnostic testing and different treatments.
Objective
To describe rare localizations of peripheral neuropathy, including the appropriate diagnostic evaluation and available treatments.
Evidence Review
References were identified from PubMed searches with an emphasis on systematic reviews and randomized clinical trials. Articles were also identified through the use of the author's own files. Search terms included common rare neuropathy localizations and their causes, as well as epidemiology, pathophysiology, diagnosis, and treatment.
Findings
Diffuse, non-length dependent neuropathies, multiple mononeuropathies, polyradiculopathies, plexopathies, and radiculoplexus neuropathies are rare peripheral neuropathy localizations that often require extensive diagnostic testing. Atypical neuropathy features, such as acute/subacute onset, asymmetry, and/or motor predominant signs, are frequently present. The most common diffuse, non-length dependent neuropathies are Guillain-Barre syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP), multifocal motor neuropathy (MMN), and amyotrophic lateral sclerosis (ALS). Effective disease modifying therapies exist for many diffuse, non-length dependent neuropathies including GBS, CIDP, MMN, and some paraprotein-associated demyelinating neuropathies. Vasculitic neuropathy (multiple mononeuropathy) also has efficacious treatment options, but definitive evidence of a treatment effect for IgM anti-MAG neuropathy and diabetic amyoptrophy (radiculoplexus neuropathy) is lacking.
Conclusions and Relevance
Recognition of rare localizations of periperhal neuropathy is essential given the implications for diagnostic testing and treatment. Electrodiagnostic studies are an important early step in the diagnostic evaluation and provide information on the localization and pathophysiology of nerve injury.
Introduction
Peripheral neuropathy includes all conditions resulting in injury to the peripheral nervous system and is best categorized by the localization of the nerve injury. Distal symmetric polyneuropathy (DSP), mononeuropathy, and lumbar/cervical radiculopathy are the most common peripheral neuropathies and have been detailed in a separate review. Rare localizations of peripheral neuropathy include diffuse, non-length dependent neuropathies, multiple mononeuropathies, polyradiculopathies, plexopathies, and radiculoplexus neuropathies; these neuropathies are particularly important to recognize because they require different diagnostic evaluations, and potentially different treatments.
Methods
References were identified from PubMed searches with an emphasis on systematic reviews and randomized clinical trials. Articles were also identified through the use of the author's own files. Search terms used included Guillain-Barre syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP), multifocal motor neuropathy (MMN), amyotrophic lateral sclerosis (ALS), vasculitic neuropathy, sensory neuronopathy, motor neuronopathy, polyradiculopathy, plexopathy, and radiculoplexus, epidemiology, pathophysiology, diagnosis, and treatment.
Rare subtypes of peripheral neuropathy
Diffuse, non-length dependent neuropathies present with numbness, tingling, pain, and/or weakness in the arms and legs. Unlike DSP, symptoms can be present in the upper extremities prior to spreading to the level of the knees and can involve proximal extremities early in the disease course. Atypical neuropathy features are commonly observed, including non-length dependent distribution (by definition), acute/subacute onset, asymmetry, and/or motor predominant signs.1 Symptoms and examination features can involve sensory and motor nerves (most common) or can be confined to sensory (sensory neuronopathy) or motor nerves (motor neuronopathy). Sensory neuronopathies typically present with a sensory ataxia, leading to gait imbalance and frequent falls. Proprioception is often quite impaired, such that when patients close their eyes, they move their extremities without their knowledge. Sensory deficits can be asymmetric and pseudoathetosis may be observed. Patients may complain of weakness, but when they visually attend to their muscles, they are able to generate normal force on confrontation testing. A painful form where patients demonstrate mechanical hyperalgesia also exists.2 Motor neuronopathies present with painless weakness, fasciculations, and muscle atrophy without numbness, paresthesias or other sensory symptoms/signs.
Multiple mononeuropathies present with symptoms in the distribution of multiple nerves. For example, patients may present with numbness, paresthesias, and/or pain in the distribution of the right sural, right tibial, left sural, and left peroneal nerves with sparing of the left tibial and right peroneal nerves. Pain is a common presenting symptom. Atypical neuropathy features are frequent, including asymmetry, acute/subacute onset, motor predominant signs, and non-length dependent pattern.
Polyradiculopathies present with symptoms and signs that are in a dermatomal and/or myotomal pattern, although this can be difficult to discern if many nerve roots are involved. Radicular pain is a common presenting symptom and is often accompanied by neck and/or back pain. Plexopathies present with symptoms and signs in the distribution of the brachial or lumbosacral plexus. Clues to this localization include symptoms/signs that involve multiple nerves that pass through the same trunk or cord of the plexus, but do not share the same nerve root origin. Radiculoplexus neuropathies present with abnormalities in the distribution of multiple nerve roots and the brachial or lumbosacral plexus.
Causes of rare subtypes
The differential diagnosis of diffuse, non-length dependent neuropathies depends on the underlying pathophysiology and the types of nerves involved (sensory, motor, both). Common causes are summarized in Table 1.
Table 1.
Common causes of rare subtypes of peripheral neuropathy
| Localization | Condition |
|---|---|
|
| |
| Diffuse, non-length dependent neuropathy | |
| Demyelinating sensory motor | AIDP |
| CIDP | |
| CIDP variants | |
| POEMS | |
| IgM anti-MAG neuropathy | |
| Waldenstrom's acroglobulinemia | |
| MGUS | |
| Diptheria | |
| Toxic exposures (hexane, arsenic, amiodarone) | |
| Demyelinating sensory | Sensory CIDP or AIDP |
| DADS (IgM anti-MAG neuropathy) | |
| Demyelinating motor | MMN |
| Axonal sensory motor | Toxic exposures |
| ASMAN | |
| AIP | |
| Axonal sensory | Paraneoplastic (Hu, CRMP-5, amphyphisin)69 |
| Sjögren's syndrome | |
| Chemotherapy (platinum based, bortezomib) | |
| Vitamin B6 toxicity | |
| Idiopathic | |
| HIV, HTLV | |
| Autoimmune hepatitis | |
| Celiac disease | |
| HSAN | |
| Friedrich's ataxia | |
| CANVAS | |
| SANDO | |
| CANOMAD | |
| Axonal motor | ALS |
| PMA | |
| Post-polio syndrome | |
| HIV, HTLV, WNV, Enterovirus D68 | |
| MMN without conduction block | |
| Radiation | |
| Monomelic amyotrophy | |
| HMN | |
| SMA (including Kennedy's syndrome) | |
| Complicated HSP | |
|
| |
| Multiple mononeuropathies | Systemic vasculitic neuropathy |
| Microscopic polyangiitis | |
| Wegener's granulomatosis | |
| Polyarteritis nodosa | |
| Churg-Strauss syndrome | |
| Cryoglobulinemia | |
| Sjögren's syndrome | |
| Rheumatoid arthritis | |
| SLE | |
| Non-systemic vasculitic neuropathy | |
| Neoplasm (malignant and benign) | |
| HNPP | |
| Sarcoidosis | |
| Amyloidosis | |
| MMN | |
| MADSAM | |
|
| |
| Polyradiculopathy | Compressive |
| Disc herniation/spondylosis | |
| Osteomyelitis | |
| Neoplasm | |
| Non-compressive | |
| Infection (CMV, VZV, Lyme, tuberculosis) | |
| Inflammatory (sarcoidosis) | |
| Neoplastic (leukemia, lymphoma) | |
| Radiation | |
|
| |
| Plexopathy | Compressive |
| Neoplasm | |
| Hemorrhage | |
| Non-compressive | |
| Infection (VZV, HSV, CMV, Lyme) | |
| Inflammatory (sarcoidosis) | |
| Neoplastic (leukemia, lymphoma) | |
| Radiation | |
|
| |
| Radiculoplexus neuropathy | Diabetic lumbar (diabetic amyotrophy) |
| Diabetic cervical | |
| Post-surgical inflammatory | |
| Non-diabetic lumbar or cervical | |
| Infection (VZV, HSV, CMV, Lyme) | |
| Inflammatory (sarcoidosis) | |
| Neoplastic (leukemia, lymphoma) | |
| Radiation | |
AIDP=acute inflammatory demyelinating polyneuropathy, CIDP= chronic inflammatory demyelinating polyneuropathy, POEMS=polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes, MGUS=monoclonal gammopathy of unclear significance, DADS=distal acquired demyelinating syndrome, MMN=multifocal motor neuropathy, ASMAN=acute sensory motor axonal neuropathy, AIP=acute intermittent porphyria, HIV=human immunodeficiency virus, HTLV=human T-lymphotrophic virus, HSAN=hereditary sensory autonomic neuropathy, CANVAS=cerebellar ataxia neuropathy vestibular areflexia syndrome, SANDO=sensory ataxia neuropathy dysarthria ophthalmoplegia, CANOMAD=chronic ataxic neuropathy, ophthalmoplegia, monoclonal IgM protein, cold agglutinins, disialosyl antibodies, ALS=amyotrophic lateral sclerosis, PMA=progressive muscular atropgy, WNV=West Nile virus, HMN=hereditary motor neuropathy, SMA=spinal muscular atrophy, HSP=hereditary spastic paraplegia, SLE=systemic lupus erythrematosus, HNPP=hereditary neuropathy with liability to pressure palsies, MADSAM=multifocal acquired demyelinating sensory and motor neuropathy, CMV=cytomegalovirus, VZV=varicella zoster virus, HSV=herpes simplex virus
In those with demyelinating neuropathy affecting sensory and motor nerves, the most common conditions are GBS and CIDP. GBS typically presents with maximal symptoms before 4 weeks from onset, whereas CIDP symptoms usually nadir after more than 8 weeks.3 In both conditions, electromyogram and nerve conduction studies (EMG/NCS) often reveal acquired demyelinating features (conduction block and/or temporal dispersion) along with other demyelinating features (prolonged latencies, decreased conduction velocities, and prolonged F responses). A lumbar puncture frequently shows elevated protein with a normal WBC count. Mimics include toxic exposures, diphtheria, and CIDP variants such as polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS) syndrome, multifocal acquired demyelinating sensory and motor neuropathy (MADSAM), and IgM anti-MAG neuropathy. Demyelinating motor neuronopathies include MMN, whereas demyelinating sensory neuronopathies include rare sensory-only GBS and CIDP variants. Diffuse, non-length dependent neuropathies that are axonal and involve sensory and motor nerves include toxic exposures, acute sensory motor axonal neuropathy (ASMAN or axonal GBS), and acute intermittent porphyria. Causes of axonal sensory neuronopathies include paraneoplastic syndromes (anti-Hu), Sjögren's syndrome, chemotherapy (cisplatin), vitamin B toxicity, and some remain idiopathic. Slowly progressive forms may be seen in those with Hereditary sensory and autonomic neuropathy (HSAN; autonomic symptoms) and Friedrich's ataxia (cerebellar and upper motor neuron (UMN) signs, and dorsal column dysfunction). Axonal motor neuronopathies include primary muscular atrophy (PMA), ALS (UMN and bulbar signs), post-polio syndrome, human immunodeficiency virus (HIV), human T-lymphotrophic virus (HTLV; thoracic myelopathy), West Nile virus (WNV), enterovirus D68, MMN without conduction block, radiation injury, and monomelic amyotrophy. Slowly progressive forms may be seen in those with hereditary motor neuropathy (HMN), spinal muscular atrophy (SMA; including Kennedy's disease), and complicated hereditary spastic paraplegia (HSP).
The most common cause of multiple mononeuropathies is vasculitic neuropathy (mononeuritis multiplex). Systemic and non-systemic forms exist, emphasizing the need to evaluate for other systemic features. Systemic vasculitic neuropathy often occurs in the setting of longstanding rheumatologic conditions, such as microscopic polyangiitis, Wegener's granulomatosis, polyarteritis nodosa, Churg-Strauss syndrome, cryoglobulinemia, Sjögren's syndrome, rheumatoid arthritis, and systemic lupus erythrematosus (SLE). Patients starting with a non-systemic vasculitic neuropathy rarely evolve systemic manifestations, with most spread restricted to the skin.4 Multiple mononeuropathies can also be seen with neoplasms (including leukemia, lymphoma, lipomas, neurofibromas), hereditary neuropathy with liability to pressure palsies (HNPP), sarcoidosis, amyloidosis, MMN, and MADSAM.
Polyradiculopathy can be caused by compressive lesions such as disc herniation and spondylosis, neoplasm, or osteomyelitis.5 Infectious (cytomegalovirus (CMV), varicella zoster virus (VZV), Lyme, tuberculosis), inflammatory (sarcoid), and neoplastic (infiltrative) causes are also seen, as well as radiation induced injury. The etiology of plexopathy is most commonly trauma/stretch (postpartum, iatrogenic), compression (neoplastic, hemorrhage, iliac/subclavian aneurysm), radiation, ischemic (aortoiliac occlusive disease), infectious (VZV), inflammatory (brachial plexitis, vasculitis), or inherited (HNPP, hereditary neuralgic amyotrophy (HNA)).
Radiculopexus neuropathies are most commonly seen in patients with diabetes. Lumbar involvement (diabetic amyotrophy) is the most common localization and is often seen after weight loss.6 Patients often have severe subacute pain, asymmetric involvement of one leg more than the other, and proximal predominance that improves over the course of several months but often leaves substantial morbidity.7 Cervical involvement is becoming increasingly recognized and patients without diabetes can also develop this subtype of neuropathy.8 Nerve biopsy often shows signs of a microvasculitis.9 Lumbar and cervical radiculoplexus neuropathies can also be seen in patients after surgery (post-surgical inflammatory neuropathy).10 The nerve damage can occur remotely from the surgical site and can develop within 24 hours to a few weeks post-surgery.
Epidemiology
The most common diffuse, non-length dependent neuropathies are GBS, CIDP, MMN, and ALS. The adjusted incidence rate of GBS in the United States was estimated to be between 1.7 and 1.8 per 100,000 person years from 2000-2004.11 This is similar to the reported average incidence in Canada and Western Europe, described in eTable 1. Electrophysiologically and pathologically, GBS can demonstrate either demyelinating or axonal features. In the United States and Western Europe, most patients have acute inflammatory demyelinating polyradiculoneuropathy (AIDP), as demonstrated by a multi-center study of electrophysiologic features in 369 GBS patients in which 69% were demyelinating, only 3% axonal, and 23% equivocal.12 In contrast, in northern China 65% of GBS patients have the motor predominant axonal form (acute motor axonal neuropathy (AMAN)) and only 24% have the demyelinating form.13 The in-hospital mortality rate in the United State was 2.58% (128/4,954) from 2000-2004.11 Beyond the typical ascending paralysis of classic GBS, there are multiple rare variants of GBS, including the Miller–Fisher syndrome, cranial polyneuritis, and pure sensory loss with areflexia, with incidence rates per 100,000 in Northern Italy of 0.05, 0.03, and 0.01, respectively.14 GBS is preceded by a clinical infection, usually diarrhea or upper respiratory symptoms, in about 2/3 of cases.15 Patients with GBS are more likely than controls with other neuromuscular diseases to have positive acute serologies for campylobacter jejuni (32% vs 12%), CMV (13% vs 2%), and Epstein-Barr virus (10% vs 1%).15 About 2/3 of the GBS patients without a recent clinical illness still had positive serologies, suggesting subclinical infection.
The prevalence of CIDP has been reported to vary from 1.2–8.9 patients per 100,000 (eTable 1). The reasons for this wide range include differences in the electrodiagnostic criteria used, inclusion or exclusion of CIDP variants, and the environment and genetic background of the populations studied. MMN has been less well studied, but a recent study in England estimated a prevalence of 0.5 per 100,000 for MMN, compared with 2.8 for CIDP and 1.0 for paraprotein-associated demyelinating polyneuropathies.16 In Japan, a similar MMN prevalence of 0.3 per 100,000 was reported.17 The incidence and prevalence of ALS is presented in eTable 1.
The most common multiple mononeuropathies are the systemic and non-systemic vasculitic neuropathies. The incidence of systemic vasculitides is approximately 14 cases per 100,000 persons years.18 Peripheral nerve involvement is a common complication of systemic vasculitis, occurring in up to 30% of cases in different case series.19 The incidence of non-systemic vasculitic neuropathy is unknown. The most commonly encountered vasculitic neuropathies are nonsystemic vasculitic neuropathy (26%), microscopic polyangiitis/polyarteritis nodosa (25%), rheumatoid vasculitis (12%), and Churg-Straus (10%).18
The incidence and prevalence of polyradiculopathy, plexopathy, and radiculoplexus neuropathy is unclear.
Pathophysiology
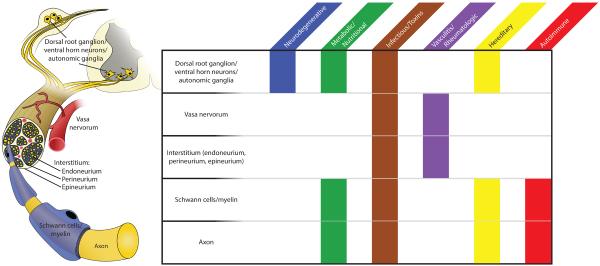
The catalog of pathophysiologic processes that can adversely affect peripheral nerves is quite extensive. Immunologic, metabolic, genetic, infectious, toxic, and even traumatic processes can damage the peripheral nerves at multiple levels via many molecular pathways. To achieve some degree of organization, it can be worthwhile to understand peripheral nerve pathophysiology in the context of the microscopic anatomy and the sites where disease processes occur (Figure 1).
Figure 1.
Pathophysiology of peripheral neuropathy
Nearly every category of disease can manifest within the peripheral nervous system. It is often useful to subdivide the peripheral nervous system into its microanatomical components: neuronal cell bodies within the dorsal root ganglion (DRG) and spinal cord, interstitial tissues (epineurium, perineurium and endoneurium), associated vasa nervorum, Schwann cells/myelin sheaths, and axons. Different diseases tend to affect different subsystems of the peripheral nerve, and careful pathologic study along with clinical history may help narrow the differential diagnosis in complex cases.
The origin of the peripheral nerve lies in the neuronal cell bodies located in the dorsal root ganglion (DRG) for sensory nerves, and in the ventral horn of the spinal cord for motor nerves. Naturally, any pathologic process affecting the cell body will result in downstream degeneration of the cell's axon. Primary motor neuron diseases, such as ALS or SMA, demonstrate axonal pathology peripherally when central neurons degenerate.20 Similarly, metabolic conditions, such as diabetes, the metabolic syndrome, nutritional deficiencies, or chronic renal failure, affect DRG cell bodies by mechanisms involving insulin resistance, oxidative stress, and apoptosis.1
Pathologic damage may also be considered to take place directly at the axon, independent of the cell body. For example, processes that affect the cytoskeletal components of axons may give rise to neuropathy. Thus, chemotherapeutics, particularly those that affect microtubules, have a propensity to produce neuropathic side effects by disrupting axonal structure.21 Other toxic exposures may disrupt metabolic homeostasis, myelin composition, and mitochondrial function, producing demyelinating and axonopathic disease to varying degrees.22
Schwann cells and the myelin sheath are often selectively targeted in immune-mediated processes such as GBS, CIDP, paraproteinemias, and their variants.23,24 It is theorized that a phenomenon of molecular mimicry occurs in these diseases, wherein glycoprotein epitopes found in myelin bear structural similarity to those found in other infectious agents (Campylobacter jejuni, CMV, Epstein-Barr virus, etc.). Immune recognition of these pathogens then spreads to include normal epitopes on the myelin sheath. Pathologic studies reveal both humoral and cellular immune activation and lymphocytic infiltration with patchy demyelination and remyelination (the classic “onion bulb” formation).25
Interestingly, a number of GBS variants which are more prevalent in Asia and Central/South America also damage axons along with myelin, and are commonly associated with Campylobacter jejuni infection.26 Anti-MAG negative IgM gammopathies, as well as less common gammopathies, can also present with a mixed demyelinating/axonopathic picture.27 Hereditary neuropathies can also affect both axons and/or their myelin sheaths. The most common type, hereditary motor sensory neuropathy (HMSN or Charcot-Marie-Tooth disease), is classified into many clinical subtypes. Type 1 encompasses demyelinating processes and results from mutations in proteins integral to myelin formation. Type 2 chiefly results in axonal pathologies and involves mutations that affect cellular structure or metabolism.28 A characteristic of inherited neuropathies is that the entire length of the nerve is affected more or less uniformly given the genetic underpinnings of the disease. Many other genetic syndromes also produce varying disruption of Schwann cell, axon and/or neuronal function.29
Both the Schwann cells and axons of the peripheral nerve depend upon delicate vasa nervorum for perfusion and metabolic support. Many metabolic and inflammatory processes, while directly affecting peripheral nerves and neuronal cell bodies, can also result in damage to nerve vasculature and indirectly produce ischemic damage, particularly to axons.30 Primary vasculitides as well as other rheumatologic disorders (systemic lupus erythematosus, Sjögren's syndrome, nonsystemic vasculitis of the peripheral nerves, etc.) may compromise vascular supply to nerves.31,32 Thus, ischemic changes represent a common neuropathologic mechanism by which peripheral nerves may be damaged.
The interstitial spaces between nerve fibers or between fascicles can represent the principal site of pathology.33 Infiltrative disorders such as sarcoidosis or amyloidosis demonstrate accumulation of material in the endoneurium, perineurium and epineurium. In lepromatous leprosy, proliferation of the Mycobacterium leprae organism in the interstitial spaces may also contribute to axonal damage.34 These conditions may invoke pathologic mechanisms of ischemia, or even damage by direct compression of axons.33
As is obvious from the above overview, pathologies of the peripheral nervous system can be quite diverse. Often, results from nerve biopsy are a helpful contribution to the overall corpus of evidence when diagnosing atypical neuropathies.35
Diagnostic evaluation
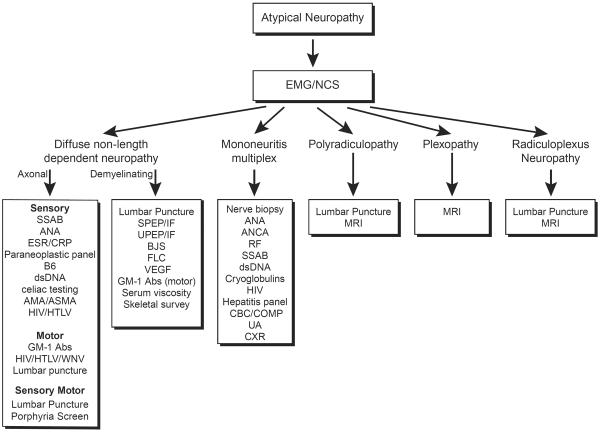
The evaluation of a diffuse, non-length dependent neuropathy, summarized in Figure 2, begins with a comprehensive history and examination. Particular attention should be paid to identifying atypical neuropathy features such as acute/subacute onset, asymmetry, and/or motor predominant signs.1 The past medical history is also important to identify conditions known to be associated with atypical neuropathies. The next step is to perform an EMG/NCS to further define the pattern of nerve injury and underlying pathophysiology. EMG/NCS can determine if the patient has a sensory motor neuropathy, sensory neuronopathy, or motor neuronopathy. Furthermore, occasionally polyradiculopathies and multiple mononeuropathies can be hard to distinguish clinically from diffuse, non-length dependent neuropathies, and EMG/NCS can identify these patterns. Importantly, EMG/NCS can also identify if the underlying pathophysiology is axonal or demyelinating, which has important implications for the differential diagnosis. For demyelinating neuropathies, a lumbar puncture is often helpful to evaluate for elevated protein and/or white blood cells.
Figure 2.
Diagnostic algorithm for the evaluation of atypical neuropathy
Atypical neuropathy features include non-length dependent distribution, acute/subacute onset, asymmetry, and/or motor predominant signs. Electrodiagnostic testing is the first step in further categorizing the peripheral neuropathy subtype and determines further diagnostic evaluation.
Patients with a sensory neuronopathy typically require evaluation with Sjögren's antibodies, antinuclear antibodies (ANA), erythrocyte sedimentation rate and C-reactive protein (ESR/CRP), paraneoplastic panel, and B6 levels.36–39 Additional testing to consider includes dsDNA, celiac testing, and anti-smooth muscle (ASMA) and anti-mitochondrial antibodies (AMA).39–41 History should focus on identifying a past history of chemotherapy (platinum based, bortezomib) or rheumatologic symptoms (dry eyes/mouth). For those with a chronic sensory neuronopathy, a focus on family history including questions regarding autonomic symptoms is important. If a motor neuronopathy is identified on EMG/NCS, then patients need evaluation for UMN signs since ALS is a common cause of this localization. The EMG/NCS should thoroughly investigate for conduction block, which is the hallmark of MMN, although conduction block is not always identified in this condition.42 Physicians should inquire about a history of poliomyelitis, fever, and family history.
The evaluation of multiple mononeuropathies includes detailed questioning regarding rheumatologic symptoms/signs as well as symptoms of systemic vasculitis. The diagnostic evaluation should begin with an EMG/NCS to confirm the pattern of nerve involvement and axonal pathophysiology as well as evaluate for other potential mimics such as MMN (motor nerve conduction block), MADSAM (motor and sensory nerve conduction block), and HNPP (diffuse distal slowing of motor and sensory latencies with slowing across common sites of entrapment), amongst other possibilities. A detailed rheumatologic work up including ANA, Anti-neutrophil cytoplasmic antibodies (ANCA), rheumatoid factor, Sjögren's antibodies, dsDNA, and cryoglobulins should be performed, as well as HIV and hepatitis panel testing. A sural or radial nerve biopsy is needed if concern for vasculitis neuropathy is high. A comprehensive metabolic panel, complete blood count, urinalysis, and chest x-ray are needed to evaluate for systemic manifestations of vasculitis. A detailed family history is important, especially in those with painless multiple mononeuropathies that occur with compression, as is often seen in HNPP.
Those suspected of a polyradiculopathy, plexopathy, or radiculoplexus neuropathies also need a comprehensive history and examination to evaluate for systemic conditions such as infectious, inflammatory, and neoplastic causes as well as previous radiation exposure. EMG/NCS is an invaluable tool to confirm the pattern of injury and distinguish between these three entities. Magnetic resonance imaging (MRI) of the nerve roots and/or plexus is often needed to evaluate for a compressive versus non-compressive etiology, as well as to evaluate for infiltrative conditions. A lumbar puncture is particularly helpful in the evaluation of a polyradiculopathy or radiculoplexus neuropathy.
Disease modifying therapy
The importance of identifying these rare subtypes of peripheral neuropathy, such as GBS, CIDP, POEMS, MMN, and vasculitic neuropathy, is that their course can be modified with particular treatment regimens. The common effective treatments include corticosteroids, intravenous immunoglobulin (IVIG), plasma exchange, and other immunosuppressive medications. Precise categorization of these rare subtypes is essential, as each condition responds to different treatment regimens.
GBS
Many studies have investigated the effects of steroids, IVIG, and plasma exchange on the rate and degree of recovery in GBS. Each therapy has been the topic of a Cochrane systematic review. The conclusion of the Cochrane systematic review on corticosteroids in 587 patients was that they do not significantly hasten recovery from GBS or affect the long term outcome.43 A detailed description of the larger randomized controlled studies that led to this conclusion and other treatment recommendations in inflammatory demyelinating polyneuropathies are provided in Table 2. In contrast to corticosteroids, treatment of GBS patients with plasma exchange has been shown to increase the rate of recovery and the likelihood of full recovery (five trials with 404 participants, RR 1.24 (95% CI 1.07 to 1.45)) and decrease the likelihood of residual severe weakness compared to supportive care. (six trials with 649 patients, RR 0.65 (95% CI 0.44 to 0.96)).44 Additionally, there have been two large studies evaluating the most efficacious number of plasma exchanges in GBS. Compared to two sessions, four sessions of plasma exchange was shown to reduce the median time to walk with assistance (24 days versus 20 days, P = 0.04) and the median time to hospital discharge (26 days versus 21 days, P = 0.04, respectively), as well as increase the proportion of patients with full recovery of strength at one year (64% versus 48%, P = 0.006).45 In 161 patients, there was no clear difference between four and six plasma exchanges.45 Since plasma exchange was established as an effective treatment of GBS, studies of IVIG in GBS were compared to plasma exchange and not placebo. IVIG appears to have similar improvement in disability at one month as plasma exchange.46 Of note, 5 of the 6 randomized controlled trials included in Cochrane systematic review were not blinded.
Table 2.
Large randomized controlled trials for inflammatory demyelinating polyneuropathies
| GBS | # of Patients | Treatment | Control | Duration | Disability Improvement at 1 month | Common Side Effects |
|---|---|---|---|---|---|---|
| GBS Steroid70 | 242 | 500 mg IV MP | Saline placebo | 48 weeks | 0.8 vs. 0.73 (p=0.06) | Hypertension in placebo |
| Van Koningsveld71 | 225 | 500 mg IV MP and IVIG 0.4 gm/kg for 5 days | IVIG 0.4 gm/kg for 5 days | 1 year | ≥1 point in 68% vs. 56% of (p=0.06) | Hypertension and UTI in placebo, elevated blood glucose in Rx group |
| McKhann72 | 245 | 3–5 PE in 7 to 14 days | Supportive Care | 6 months | ≥1 point in 59% vs. 39% (p=0.01). | No significant difference |
| Raphael73 | 220 | 4 PE in 8 days | Supportive Care | 6 months | ≥1 point in 61.5% vs. 37% (P < 0.001). | Sepsis in Rx group, blood pressure instability and pneumonia in control group |
| PSGBS Study Group74 | 383 | IVIG 0.4 gm/kg for 5 days | 5–6 PE in 8 to 13 days or PE followed by IVIG | 48 weeks | 0.8 IVIG, 0.9 PE, 1.1 PE followed by IVIG (No statistically significant difference between any of these three groups) |
No significant difference |
| CIDP | # of Patients | Treatment | Control | Duration | Disability Improvement (INCAT) | Common Side Effects |
| Hughes47 (Ice trial) | 117 | IVIG 2 g/kg over 2–4 days and then 1 g/kg over 1–2 days every 3 weeks | Albumin | 24 weeks | ≥1 point in 54% vs. 21% (p=0·0002) | Headache and fever were more common in IVIG group |
GBS=Guillain-Barre syndrome, MP=methylprednisolone, IVIG=intravenous immunoglobulin, UTI=urinary tract infection, PE=plasma exchange, CIDP=chronic inflammatory demyelinating polyneuropathy, INCAT=inflammatory neuropathy cause and treatment scale
CIDP
Unlike GBS, patients with CIDP can be treated with corticosteroids, IVIG, or plasma exchange. In CIDP, the largest studies demonstrating efficacy of treatment over placebo are with IVIG. There have been a total of 269 patients in five blinded randomized control trials of IVIG versus placebo with the largest being the ICE trial in 2008 with 117 patients.47 When pooling this data in a Cochrane systematic meta-analysis, IVIG reduces disability in patients with CIDP after one month of treatment as compared with placebo, with a number needed to treat for an additional beneficial outcome of 3.03 (95% CI 2.33 to 4.55).48 There are two small trials of about 30 patients each comparing plasma exchange to sham exchange in patients with CIDP. Both studies demonstrated that plasma exchange provides significant short-term improvement in disability over sham exchange at 4 weeks.49 A single small trial of 32 patients compared IVIG and plasma exchange in patients with CIDP and found no significant difference in the neuropathy disability score at 6 weeks.50 Similarly, there have been two studies comparing either IVIG and oral prednisolone or IVIG and monthly intravenous methylprednisolone that did not show a statistically difference in disability improvement at 6 weeks. More patients discontinued methylprednisolone than IVIG at 6 months for perceived lack of efficacy. Of note, there have not been any blinded randomized control studies demonstrating efficacy of corticosteroids over placebo in CIDP.51 Alternative immunosuppressive medications for the treatment of CIDP currently lack high quality randomized control trials demonstrating efficacy.52 Furthermore, the comparative effectiveness of different treatment regimens for the long term treatment of CIDP is unclear.
Paraprotein-associated demyelinating neuropathy
IgG and IgA associated demyelinating neuropathies usually respond to the same immunotherapies as CIDP. In contrast, IgM anti-MAG neuropathy has not been shown to respond to immunosuppressive therapy. In a blinded randomized control crossover trial of IVIG versus placebo, there was no significant difference in strength or functional outcomes in the 9 patients studied.53 Similarly in two randomized controlled trials of Rituximab versus placebo of 26 patients each, there was no significant difference in the primary outcome of sensory improvement.54,55 POEMS syndrome causes a demyelinating neuropathy in the context of osteosclerotic bone lesions. Radiation of these lesions is one potential therapy for localized disease. For pateints with clonal plasms cells on bone marrow biopsy, high dose melphalan followed by autologous stem cell transplantation results in a 75% 5-year progression free survival.56
MMN
The first line treatment for MMN is IVIG. A Cochrane systematic review in 2005 found four randomized controlled trials with a total of 34 patients that demonstrated improved strength in 78% of patients treated with IVIG compared to only 4% of patients treated with placebo57. Since then, Hahn et al published the largest randomized control trial of IVIG in MMN with 44 patients and showed that strength deteriorated in 35.7% of patients on placebo compared to 11.9% on IVIG (p=.021), and that disability increased in 31% of patients on placebo compared to 7.1% on IVIG (p=.011).58 There have been no randomized-controlled trials of corticosteroids in MMN. Corticosteroids are not used based on case series and reports. In 1991, Feldman et al reported 13 patients that were treated with oral prednisone regimens of 30–100 mg for 6 weeks to 3 years without clinical improvement or reduction of GM1 antibody titer.59 Of note, four of these patients were also treated with plasma exchange without improvement. Four patients reported by Donaghy et al showed marked motor deterioration within four weeks of starting treatment with prednisolone 60 mg/daily.60 Similarly in 2 patients reported by Van den Berg et al treated with dexamethasone every 28 days for 6 months, there was marked deterioration of strength.61 Moreover, there have been no randomized-controlled trials of plasma exchange in MMN and case series and reports have not suggested efficacy.62,63 Little data exists to inform long term treatment of MMN or when refractory to IVIG.
Vasculitic neuropathy
Non-viral associated systemic vasculitis is typically treated with corticosteroids and cyclophosphamide to induce remission.7 Rituximab is an alternative therapy, with evidence supporting that it is non-inferior to cyclophophasmide in ANCA-associated vasculitis.64,65 Maintanence therapy with azathioprine, methotrexate, or rituximab is often given for 24 months after remission has been achieved.7 Recent data in a population with ANCA-associated vasculitis revealed that maintenance with rituximab was superior to azathioprine.66 Randomized controlled trials in other forms of systemic vasculitis and in patients presenting with vasculitic neuropathy and/or reporting on neuropthy outcomes have not been performed. Similarly, a 2007 Cochrane review revealed no randomized controlled clinical trials have been performed on which to base immunosuppressive treatment for non-systemic vasculitic neuropathy.67 In the largest observational study in this population (48 patients followed for at least 6 months), 61% of patients treated with corticosteroids monotherapy had a long term response whereas 95% of patients treated with corticosteroids in combination with another immunosuppressive agent, usually cyclophosphamide, had a long term response.4 Further high quality treatment trials are needed to determine the appropriate initial and long term therapeutic strategy in patients with systemic and non-sytemic vasculitic neuropathy.
Diabetic amyotrophy
Only one randomized trial has been performed in patients with DLRPN.68 Seventy five patients were randomized to IV methylprednisolone versus placebo. The study failed to show a statistically significant difference in the primary endpoint, but neuropathic pain symptoms were reduced. Overall, this study provides evidence that IV methylprednisolone is effective at neuropathic pain relief, but not in reducing neurologic deficits.
Conclusion
The presence of key warning signs, such as a non-length dependent distribution, acute/subacute onset, asymmetry, and/or motor predominant signs, increases the likelihood of a rare localization of peripheral neuropathy. Importantly, identification of these rare periperhal neuropathy localizations has important diagnostic testing and treatment implications. Electrodiagnostic studies are an important early piece of the diagnostic evaluation by providing information on the localization and pathophysiology of nerve injury.
Supplementary Material
Acknowledgements
Dr. Callaghan receives research support from Impeto Medical Inc. and honorarium from the British Medical Journal; he also certifies ALS centers for the ALS Association, performs medical consultations for Advance Medical, and consults for a PCORI grant. Dr. Price has received honorarium from the Critical Thinking Company for teaching the diagnostic criteria for CIDP and from Accenture for the treatment of multiple sclerosis. Dr. Feldman has nothing to disclose.
Funding support during the preparation of this article was provided by the National Institutes of Health (DP3 DK094292 and R24 DK082841 to E.L.F.; K23 NS079417-01 to B.C.C.; and University of Michigan Clinician Scientist Training Program NINDS R25NS089450R25 to K.S.C.), the American Diabetes Association (to E.L.F.), and the A. Alfred Taubman Medical Research Institute. The funders had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review or approval of the manusctipt; and decision to submit the manuscript for publication.
Footnotes
All authors contributed equally to the preparation of content, writing, and revision of the manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis.
References
- 1.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012 Jun;11(6):521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oki Y, Koike H, Iijima M, et al. Ataxic vs painful form of paraneoplastic neuropathy. Neurology. 2007 Aug 7;69(6):564–572. doi: 10.1212/01.wnl.0000266668.03638.94. [DOI] [PubMed] [Google Scholar]
- 3.Hughes R, Sanders E, Hall S, Atkinson P, Colchester A, Payan P. Subacute idiopathic demyelinating polyradiculoneuropathy. Archives of Neurology. 1992 Jun;49(6):612–616. doi: 10.1001/archneur.1992.00530300044009. [DOI] [PubMed] [Google Scholar]
- 4.Collins MP, Periquet MI, Mendell JR, Sahenk Z, Nagaraja HN, Kissel JT. Nonsystemic vasculitic neuropathy: insights from a clinical cohort. Neurology. 2003 Sep 9;61(5):623–630. doi: 10.1212/01.wnl.0000082715.48844.3e. [DOI] [PubMed] [Google Scholar]
- 5.McGonagle TK, Levine SR, Donofrio PD, Albers JW. Spectrum of patients with EMG features of polyradiculopathy without neuropathy. Muscle Nerve. 1990 Jan;13(1):63–69. doi: 10.1002/mus.880130112. [DOI] [PubMed] [Google Scholar]
- 6.Bastron JA, Thomas JE. Diabetic polyradiculopathy: clinical and electromyographic findings in 105 patients. Mayo Clinic Proceedings. 1981 Dec;56(12):725–732. [PubMed] [Google Scholar]
- 7.Gwathmey KG, Burns TM, Collins MP, Dyck PJ. Vasculitic neuropathies. Lancet Neurol. 2014 Jan;13(1):67–82. doi: 10.1016/S1474-4422(13)70236-9. [DOI] [PubMed] [Google Scholar]
- 8.Dyck PJ, Norell JE, Dyck PJ. Non-diabetic lumbosacral radiculoplexus neuropathy: natural history, outcome and comparison with the diabetic variety. Brain. 2001 Jun;124(Pt 6):1197–1207. doi: 10.1093/brain/124.6.1197. [DOI] [PubMed] [Google Scholar]
- 9.Dyck PJ, Norell JE, Dyck PJ. Microvasculitis and ischemia in diabetic lumbosacral radiculoplexus neuropathy. Neurology. 1999 Dec 10;53(9):2113–2121. doi: 10.1212/wnl.53.9.2113. [DOI] [PubMed] [Google Scholar]
- 10.Staff NP, Engelstad J, Klein CJ, et al. Post-surgical inflammatory neuropathy. Brain. 2010 Oct;133(10):2866–2880. doi: 10.1093/brain/awq252. [DOI] [PubMed] [Google Scholar]
- 11.Alshekhlee A, Hussain Z, Sultan B, Katirji B. Guillain-Barre syndrome: incidence and mortality rates in US hospitals. Neurology. 2008 Apr 29;70(18):1608–1613. doi: 10.1212/01.wnl.0000310983.38724.d4. [DOI] [PubMed] [Google Scholar]
- 12.Hadden RD, Cornblath DR, Hughes RA, et al. Electrophysiological classification of Guillain-Barre syndrome: clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barre Syndrome Trial Group. Ann Neurol. 1998 Nov;44(5):780–788. doi: 10.1002/ana.410440512. [DOI] [PubMed] [Google Scholar]
- 13.Ho TW, Mishu B, Li CY, et al. Guillain-Barre syndrome in northern China. Relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain. 1995 Jun;118(Pt 3):597–605. doi: 10.1093/brain/118.3.597. [DOI] [PubMed] [Google Scholar]
- 14.Bogliun G, Beghi E. Incidence and clinical features of acute inflammatory polyradiculoneuropathy in Lombardy, Italy, 1996. Acta Neurol Scand. 2004 Aug;110(2):100–106. doi: 10.1111/j.1600-0404.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs BC, Rothbarth PH, van der Meche FG, et al. The spectrum of antecedent infections in Guillain-Barre syndrome: a case-control study. Neurology. 1998 Oct;51(4):1110–1115. doi: 10.1212/wnl.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 16.Mahdi-Rogers M, Hughes RA. Epidemiology of chronic inflammatory neuropathies in southeast England. Eur J Neurol. 2014;21(1):28–33. doi: 10.1111/ene.12190. [DOI] [PubMed] [Google Scholar]
- 17.Miyashiro A, Matsui N, Shimatani Y, et al. Are multifocal motor neuropathy patients underdiagnosed? An epidemiological survey in Japan. Muscle Nerve. 2014 Mar;49(3):357–361. doi: 10.1002/mus.23930. [DOI] [PubMed] [Google Scholar]
- 18.Collins MP. The vasculitic neuropathies: an update. Curr Opin Neurol. 2012 Oct;25(5):573–585. doi: 10.1097/WCO.0b013e3283580432. [DOI] [PubMed] [Google Scholar]
- 19.Callaghan B, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer-Hayes LR, Brotherton T, Glass JD. Axonal degeneration in the peripheral nervous system: implications for the pathogenesis of amyotrophic lateral sclerosis. Exp Neurol. 2013 Aug;246:6–13. doi: 10.1016/j.expneurol.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014 Aug;40(7):872–882. doi: 10.1016/j.ctrv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Diezi M, Buclin T, Kuntzer T. Toxic and drug-induced peripheral neuropathies: updates on causes, mechanisms and management. Curr Opin Neurol. 2013 Oct;26(5):481–488. doi: 10.1097/WCO.0b013e328364eb07. [DOI] [PubMed] [Google Scholar]
- 23.Joint Task Force of the E, the PNS European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of paraproteinemic demyelinating neuropathies. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society--first revision. J Peripher Nerv Syst. 2010 Sep;15(3):185–195. doi: 10.1111/j.1529-8027.2010.00278.x. [DOI] [PubMed] [Google Scholar]
- 24.Dimachkie MM, Barohn RJ, Katz J. Multifocal motor neuropathy, multifocal acquired demyelinating sensory and motor neuropathy, and other chronic acquired demyelinating polyneuropathy variants. Neurologic Clinics. 2013 May;31(2):533–555. doi: 10.1016/j.ncl.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorson KC, Katz J. Chronic inflammatory demyelinating polyneuropathy. Neurologic Clinics. 2013 May;31(2):511–532. doi: 10.1016/j.ncl.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Hughes RA, Cornblath DR. Guillain-Barre syndrome. Lancet. 2005 Nov 5;366(9497):1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 27.Joint Task Force of the E, the PNS European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society--First Revision. J Peripher Nerv Syst. 2010 Mar;15(1):1–9. doi: 10.1111/j.1529-8027.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- 28.Saporta MA, Shy ME. Inherited peripheral neuropathies. Neurologic Clinics. 2013 May;31(2):597–619. doi: 10.1016/j.ncl.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassam BA. Charcot-Marie-Tooth disease variants-classification, clinical, and genetic features and rational diagnostic evaluation. J Clin Neuromuscul Dis. 2014 Mar;15(3):117–128. doi: 10.1097/CND.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 30.Nukada H, Dyck PJ. Acute ischemia causes axonal stasis, swelling, attenuation, and secondary demyelination. Ann Neurol. 1987 Sep;22(3):311–318. doi: 10.1002/ana.410220306. [DOI] [PubMed] [Google Scholar]
- 31.Collins MP, Arnold WD, Kissel JT. The neuropathies of vasculitis. Neurologic Clinics. 2013 May;31(2):557–595. doi: 10.1016/j.ncl.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis and Rheumatism. 2013 Jan;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 33.Dyck PJ, Dyck JB, Engelstad J. Pathologic Alterations of Nerves. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. 4th ed Saunders; Philadelphia: 2005. pp. 733–829. [Google Scholar]
- 34.Said G. Infectious neuropathies. Neurologic Clinics. 2007 Feb;25(1):115–137. doi: 10.1016/j.ncl.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Mellgren SI, Lindal S. Nerve biopsy--some comments on procedures and indications. Acta Neurologica Scandinavica. Supplementum. 2011;(191):64–70. doi: 10.1111/j.1600-0404.2011.01546.x. [DOI] [PubMed] [Google Scholar]
- 36.Graus F, Cordon-Cardo C, Posner JB. Neuronal antinuclear antibody in sensory neuronopathy from lung cancer. Neurology. 1985 Apr;35(4):538–543. doi: 10.1212/wnl.35.4.538. [DOI] [PubMed] [Google Scholar]
- 37.Malinow K, Yannakakis GD, Glusman SM, et al. Subacute sensory neuronopathy secondary to dorsal root ganglionitis in primary Sjogren's syndrome. Ann Neurol. 1986 Oct;20(4):535–537. doi: 10.1002/ana.410200416. [DOI] [PubMed] [Google Scholar]
- 38.Schaumburg H, Kaplan J, Windebank A, et al. Sensory neuropathy from pyridoxine abuse. A new megavitamin syndrome. N Engl J Med. 1983 Aug 25;309(8):445–448. doi: 10.1056/NEJM198308253090801. [DOI] [PubMed] [Google Scholar]
- 39.Wang JC, Lin YC, Yang TF, Lin HY. Ataxic sensory neuronopathy in a patient with systemic lupus erythematosus. Lupus. 2012 Jul;21(8):905–909. doi: 10.1177/0961203311434105. [DOI] [PubMed] [Google Scholar]
- 40.Brannagan TH, 3rd, Hays AP, Chin SS, et al. Small-fiber neuropathy/neuronopathy associated with celiac disease: skin biopsy findings. Archives of Neurology. 2005 Oct;62(10):1574–1578. doi: 10.1001/archneur.62.10.1574. [DOI] [PubMed] [Google Scholar]
- 41.Merchut MP, Adams EM, Morrissey M. Sensory neuronopathy in autoimmune chronic active hepatitis. Neurology. 1993 Nov;43(11):2410–2411. doi: 10.1212/wnl.43.11.2410. [DOI] [PubMed] [Google Scholar]
- 42.Delmont E, Azulay JP, Giorgi R, et al. Multifocal motor neuropathy with and without conduction block: a single entity? Neurology. 2006 Aug 22;67(4):592–596. doi: 10.1212/01.wnl.0000234063.51897.20. [DOI] [PubMed] [Google Scholar]
- 43.Hughes RA, van Doorn PA. Corticosteroids for Guillain-Barre syndrome. Cochrane Database Syst Rev. 2012;8:CD001446. doi: 10.1002/14651858.CD001446.pub4. [DOI] [PubMed] [Google Scholar]
- 44.Raphael JC, Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain-Barre syndrome. Cochrane Database Syst Rev. 2012;7:CD001798. doi: 10.1002/14651858.CD001798.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Appropriate number of plasma exchanges in Guillain-Barre syndrome. The French Cooperative Group on Plasma Exchange in Guillain-Barre Syndrome. Ann Neurol. 1997 Mar;41(3):298–306. doi: 10.1002/ana.410410304. [DOI] [PubMed] [Google Scholar]
- 46.Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barre syndrome. Cochrane Database Syst Rev. 2014;9:CD002063. doi: 10.1002/14651858.CD002063.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes RA, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 2008 Feb;7(2):136–144. doi: 10.1016/S1474-4422(07)70329-0. [DOI] [PubMed] [Google Scholar]
- 48.Eftimov F, Winer JB, Vermeulen M, de Haan R, van Schaik IN. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2013;12:CD001797. doi: 10.1002/14651858.CD001797.pub3. [DOI] [PubMed] [Google Scholar]
- 49.Mehndiratta MM, Hughes RA. Plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2012;9:CD003906. doi: 10.1002/14651858.CD003906.pub3. [DOI] [PubMed] [Google Scholar]
- 50.Dyck PJ, Litchy WJ, Kratz KM, et al. A plasma exchange versus immune globulin infusion trial in chronic inflammatory demyelinating polyradiculoneuropathy. Ann Neurol. 1994 Dec;36(6):838–845. doi: 10.1002/ana.410360607. [DOI] [PubMed] [Google Scholar]
- 51.Hughes RA, Mehndiratta MM. Corticosteroids for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2015 Jan 5;1:CD002062. doi: 10.1002/14651858.CD002062.pub3. [DOI] [PubMed] [Google Scholar]
- 52.Mahdi-Rogers M, van Doorn PA, Hughes RA. Immunomodulatory treatment other than corticosteroids, immunoglobulin and plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. 2013;6:CD003280. doi: 10.1002/14651858.CD003280.pub4. [DOI] [PubMed] [Google Scholar]
- 53.Dalakas MC, Quarles RH, Farrer RG, et al. A controlled study of intravenous immunoglobulin in demyelinating neuropathy with IgM gammopathy. Ann Neurol. 1996 Nov;40(5):792–795. doi: 10.1002/ana.410400516. [DOI] [PubMed] [Google Scholar]
- 54.Dalakas MC, Rakocevic G, Salajegheh M, et al. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein antibody demyelinating neuropathy. Ann Neurol. 2009 Mar;65(3):286–293. doi: 10.1002/ana.21577. [DOI] [PubMed] [Google Scholar]
- 55.Leger JM, Viala K, Nicolas G, et al. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein neuropathy. Neurology. 2013 Jun 11;80(24):2217–2225. doi: 10.1212/WNL.0b013e318296e92b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D'Souza A, Lacy M, Gertz M, et al. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood. 2012 Jul 5;120(1):56–62. doi: 10.1182/blood-2012-04-423178. [DOI] [PubMed] [Google Scholar]
- 57.van Schaik IN, van den Berg LH, de Haan R, Vermeulen M. Intravenous immunoglobulin for multifocal motor neuropathy. Cochrane Database Syst Rev. 2005;(2):CD004429. doi: 10.1002/14651858.CD004429.pub2. [DOI] [PubMed] [Google Scholar]
- 58.Hahn AF, Beydoun SR, Lawson V, et al. A controlled trial of intravenous immunoglobulin in multifocal motor neuropathy. J Peripher Nerv Syst. 2013 Dec;18(4):321–330. doi: 10.1111/jns5.12046. [DOI] [PubMed] [Google Scholar]
- 59.Feldman EL, Bromberg MB, Albers JW, Pestronk A. Immunosuppressive treatment in multifocal motor neuropathy. Ann Neurol. 1991 Sep;30(3):397–401. doi: 10.1002/ana.410300312. [DOI] [PubMed] [Google Scholar]
- 60.Donaghy M, Mills KR, Boniface SJ, et al. Pure motor demyelinating neuropathy: deterioration after steroid treatment and improvement with intravenous immunoglobulin. J Neurol Neurosurg Psychiatry. 1994 Jul;57(7):778–783. doi: 10.1136/jnnp.57.7.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van den Berg LH, Lokhorst H, Wokke JH. Pulsed high-dose dexamethasone is not effective in patients with multifocal motor neuropathy. Neurology. 1997 Apr;48(4):1135. doi: 10.1212/wnl.48.4.1135. [DOI] [PubMed] [Google Scholar]
- 62.Lehmann HC, Hoffmann FR, Fusshoeller A, et al. The clinical value of therapeutic plasma exchange in multifocal motor neuropathy. J Neurol Sci. 2008 Aug 15;271(1–2):34–39. doi: 10.1016/j.jns.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 63.Carpo M, Cappellari A, Mora G, et al. Deterioration of multifocal motor neuropathy after plasma exchange. Neurology. 1998 May;50(5):1480–1482. doi: 10.1212/wnl.50.5.1480. [DOI] [PubMed] [Google Scholar]
- 64.Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013 Aug 1;369(5):417–427. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010 Jul 15;363(3):221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014 Nov 6;371(19):1771–1780. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 67.Vrancken AF, Hughes RA, Said G, Wokke JH, Notermans NC. Immunosuppressive treatment for non-systemic vasculitic neuropathy. Cochrane Database Syst Rev. 2007;(1):CD006050. doi: 10.1002/14651858.CD006050.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dyck PJBOBP, Bosch P. The multi-center double-blind controlled trial of IV methylprednisolone in diabetic lumbosacral radiculoplexus neuropathy. Neurology. 2006;(suppl 2):A191. [Google Scholar]
- 69.Graus F, Dalmau J. Paraneoplastic neuropathies. Curr Opin Neurol. 2013 Oct;26(5):489–495. doi: 10.1097/WCO.0b013e328364c020. [DOI] [PubMed] [Google Scholar]
- 70.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993 Sep 30;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 71.van Koningsveld R, Schmitz PI, Meche FG, Visser LH, Meulstee J, van Doorn PA. Effect of methylprednisolone when added to standard treatment with intravenous immunoglobulin for Guillain-Barre syndrome: randomised trial. Lancet. 2004 Jan 17;363(9404):192–196. doi: 10.1016/s0140-6736(03)15324-x. [DOI] [PubMed] [Google Scholar]
- 72.Plasmapheresis and acute Guillain-Barre syndrome. The Guillain-Barre syndrome Study Group. Neurology. 1985 Aug;35(8):1096–1104. [PubMed] [Google Scholar]
- 73.Efficiency of plasma exchange in Guillain-Barre syndrome: role of replacement fluids. French Cooperative Group on Plasma Exchange in Guillain-Barre syndrome. Ann Neurol. 1987 Dec;22(6):753–761. doi: 10.1002/ana.410220612. [DOI] [PubMed] [Google Scholar]
- 74.Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barre syndrome. Plasma Exchange/Sandoglobulin Guillain-Barre Syndrome Trial Group. Lancet. 1997 Jan 25;349(9047):225–230. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.