Abstract
Somatostatin analogs (SSAs), which were initially used to control hormonal syndromes associated with neuroendocrine neoplasms (NENs), have been successfully proposed as antiproliferative agents, able to control tumor growth in patients affected by gastroenteropancreatic (GEP)-NENs. The development of long-acting formulations of SSAs which require only weekly or monthly injections can improve patient compliance. In particular, lanreotide (LAN) Autogel®, which is a viscous aqueous formulation supplied in ready-to-use prefilled syringes, can be administered every 28–56 days. Since its introduction in the clinical practice, several studies evaluated the clinical utility of LAN Autogel in the medical treatment of GEP-NENs. Although there is no evidence of an overall survival benefit, these studies confirm the efficacy of LAN Autogel in terms of benefit in progression-free survival, and in more than half of cases, a reduction of tumor markers can be observed during treatment with this drug. Moreover, LAN Autogel is widely recognized to be effective in controlling tumor-related symptoms in the majority of patients affected by GEP tumors, especially in patients affected by carcinoid syndrome, improving considerably patients’ quality of life.
Keywords: lanreotide Autogel, gastroenteropancreatic neuroendocrine tumors, gastroentero-pancreatic neuroendocrine neoplasms, somatostatin analogs
Introduction
The discovery of somatotropin-release inhibitory factor, or somatostatin (SST), in hypothalamic extract in the early 1970s, led to important advancements in the comprehension of the regulation of growth hormone (GH) secretion and offered the opportunity to develop drugs mimicking the actions of SST. The synthesis of the first somatostatin analog (SSA), octreotide (OCT), the discovery of 5 SST receptor (SSTR) subtypes, and the development of additional SSTR ligands offered a great opportunity for the medical treatment of acromegaly and neuroendocrine neoplasms (NENs).
With an annual incidence of 5 cases/100,000 in the USA,1 NENs are rare tumors composed of multipotent neuroendocrine cells able to produce, store, and secrete biologically active substances which can cause – if functioning – distinct clinical syndromes. Both functioning and nonfunctioning tumors can also produce symptoms due to mass effects, and even if the majority of these tumors exhibit long periods of relatively small growth, in some cases, they can show massive growth and can be associated with distant metastases. In >50% of cases, tumors originate in the gastrointestinal system or the pancreas (gastroenteropancreatic neuroendocrine neoplasms [GEP-NENs]).
Treatment of NENs requires a multimodal approach, involving both tumor debulking and management of symptoms. Tumor diameter is one of the most important parameters in the decision-making process for nonfunctioning forms. In fact, while surgery represents the treatment of choice for larger nonfunctioning tumors, small lesions can be treated conservatively. Locally advanced and metastatic disease can also be treated with extended resections, considering tumor grading, size, Ki-67 proliferation index, and the presence of extra-abdominal disease. Functioning tumors should be resected regardless of the dimension of the lesion. Other approaches include pharmacological treatment (SSAs, interferon, antiangiogenic agents, tyrosine kinase inhibitors, mammalian-target-of-rapamycin inhibitors, chemotherapy), radionuclide therapy, and chemo- or radioembolization. In particular, SSAs, which were initially used to control hormonal syndromes associated with NENs, have been successfully proposed as antiproliferative agents, able to control tumor growth.2,3 Moreover, long-acting formulations of SSAs have demonstrated their efficacy as antineoplastic agents in the treatment of GEP-NENs.4 Long-acting formulations of SSAs (OCT LAR, slow-release LAN, and LAN Autogel®) assure improved patient compliance with weekly up to monthly injections. In this review, we focus on the clinical utility of LAN Autogel in the treatment of GEP-NENs.
Molecular basis of SSAs action
SST and SSAs
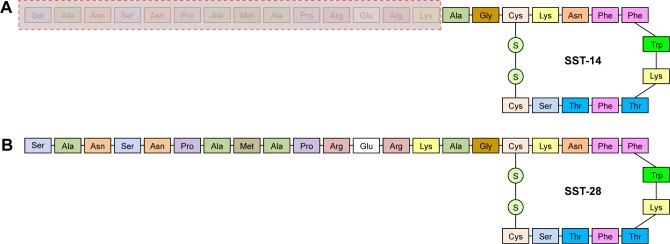
Human SST, isolated in 1973 and soon identified as a hypothalamic inhibitor of GH,5,6 has been subsequently found in several other tissues (central nervous system, endocrine system, and gastrointestinal tract). SST is formed by proteolytic processing of larger precursor molecules: prepro-SST and pro-SST.7 Prepro-SST is a 116-aminoacid precursor which is encoded by a single gene located on chromosome 3q28, and it is processed to pro-SST (96 amino-acids). Pro-SST undergoes tissue-specific enzymatic degradation to produce 2 bioactive proteins: the predominant, but functionally less active 14-aminoacid molecule called SST-14 and the larger and more potent 28-aminoacid form SST-28 (Figure 1).8
Figure 1.
Structure of somatostatin 14 (A) and somatostatin 28 (B). The 2 isoforms differ in the amino acids within the red dashed rectangle, as they are missing in somatostatin 14 but are present in somatostatin 28.
Abbreviation: SST, somatostatin.
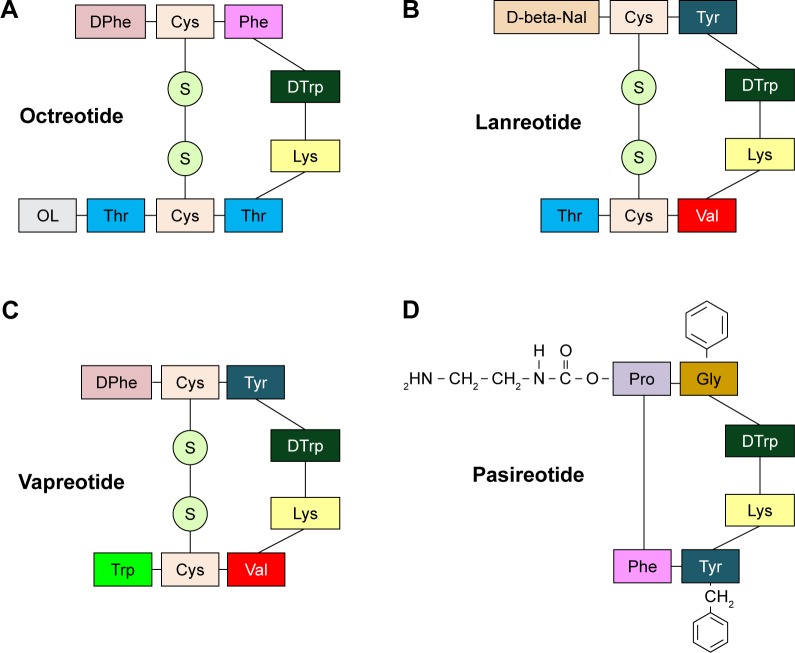
SST isoforms have endocrine, paracrine and autocrine inhibitory effects as well as on exocrine glands. The mechanisms by which SST and SSAs inhibit the neuroendocrine cells are complex and poorly understood.9 Once SST binds to its cell- and tissue-specific receptors, the downstream effects elicited by the ligand–receptor formation are site-specific: in the central nervous system, SST acts as a neurotransmitter, while in the hypothalamic–pituitary region, it acts as a neurohormone.10 SST is also known to inhibit several cellular functions. In particular, in the digestive tract, SST reduces gastrointestinal motility, inhibits gallbladder contraction, decreases portal blood flow, and suppresses the secretion of gastrointestinal hormones (insulin, glucagon, gastrin, cholecystokinin, vasoactive intestinal peptide, and secretin)11 and that of other exocrine gastrointestinal cells (gastric acid, intestinal fluid, and pancreatic enzymes).12 Moreover, it inhibits proliferation of both normal and tumor cells.13 Circulating SST has a short half-life (~2 minutes) because both bioactive isoforms (SST-14 and SST-28) contain multiple enzymatic cleavage sites causing a rapid degradation. This not only makes the analysis of their physiological activity difficult but also represents a serious limitation for their application in clinical practice. Therefore, the development of stable and potent analogs became necessary for therapeutic use. Synthetic SSAs have been developed by reducing the polypeptide chain, leading to an increased affinity for SSTRs and to a longer half-life.9 Structure–activity studies of SST-14 showed that the aminoacid residues Phe, Trp, Lys, and Thr, which comprise a β-turn, are necessary for biological activity. Residues Trp and Lys are essential, while Phe and Thr can be substituted. Compound SMS 201-995 (OCT) exhibits a 3-fold potency in the inhibition of insulin secretion and a 19-fold potency in GH secretion inhibition compared to native SST.14 The introduction of d-Phe at the N-terminal and L-Thr at the C-terminal end, and the substitution of L-Trp by d-Trp in position 8 make the peptide resistant to degradation. Of the many hundreds of SSAs synthesized, 4 are currently used in clinical settings: OCT, LAN, vapreotide (VAP), and pasireotide (PAS; SOM 230) (Figure 2).
Figure 2.
Structure of the most used somatostatin analogs: octreotide (A), lanreotide (B), vapreotide (C), and pasireotide (D). Lanreotide is an octapeptide that self-assembles into nanotubes when placed in an aqueous environment. It has 3 aromatic residues (d-naphthylalanine, tyrosine, and d-tryptophan) that are involved in the formation of these supramolecular structures.
OCT and LAN (“first-generation” SSAs) have been quickly considered first-line medical therapeutic options for the treatment of acromegaly and for the control of hormonal symptoms in patients with NENs.11 OCT and LAN show high-affinity binding to SSTR 2 and 5, with half-lives of 2 hours and <1 hour, respectively. Rebound hypersecretion of hormones does not occur.14 Both drugs have a small volume of distribution and a low clearance that result in a longer duration of exposure and long-lasting biological activity compared with SST. OCT and LAN are administered by multiple subcutaneous injections or by continuous subcutaneous infusion or by the intravenous route (either as a single injection or as a continuous infusion over many hours or days). The OCT LAR formulation has been obtained by combining OCT with microspheres of carboxymethylcellulose which increase its therapeutic action to 24–42 days.15 Two LAR/slow-release formulations have been developed: a slow-release LAN obtained by combining LAN with microspheres of lactide/glycolide copolymers, which allows for administration of every 7–28 days,16 and LAN Autogel which is a viscous aqueous formulation supplied in ready-to-use prefilled syringes administered every 28–56 days.17
PAS (SOM 230), a “second-generation” SSA developed using biodegradable polymers by a method similar to that of OCT LAR,18 is a multireceptor-targeted SST generated by the introduction of 4 synthetic and 2 essential amino acids of SST in a novel cyclohexapeptide structure.
SSTRs and SSA actions
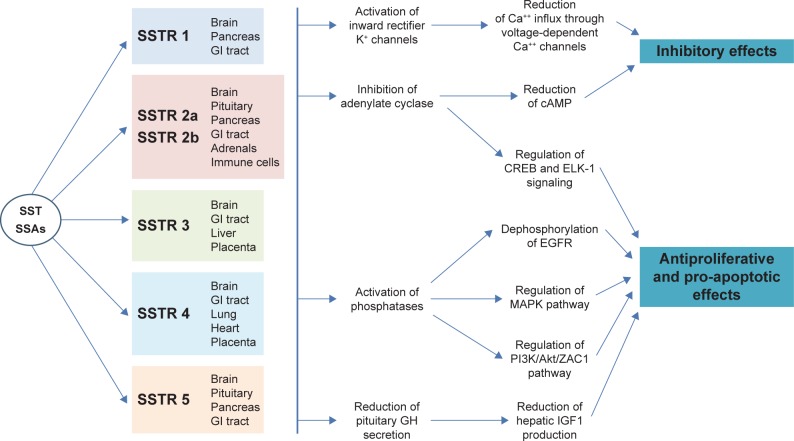
The biological effects of SST are mediated by its interaction with 5 SSTRs (SSTR 1, SSTR 2, SSTR 3, SSTR 4, and SSTR 5) belonging to the family of G-protein-coupled membrane receptors. Each receptor, encoded by genes localized on different chromosomes,19 consists of a single polypeptide chain with 7 transmembrane-spanning domains: the extracellular domain exhibits the ligand-binding sites, while the intracellular domain provides linkage to second messenger activation.20 All 5 SSTRs have been identified in the central nervous system, gastrointestinal tract, endocrine glands, exocrine glands, and inflammatory and immune cells21 (Figure 3).
Figure 3.
Mechanisms explaining the inhibitory and antiproliferative effects of SST and SSAs. There are 5 types of SSTRs, which show different tissue distribution. SSTR 2 has 2 different isoforms (2a and 2b) that derive from alternative splicing at the C-terminus of the receptor – between the 2, the SSTR 2a isoform is far more common in human tissues. SST and SSAs bind to SSTRs with different affinities. All SSTRs are G protein-coupled receptors with 7 transmembrane-spanning domains.
Abbreviations: cAMP, cyclic adenosine monophosphate; EGFR, epidermal growth factor receptor; GH, growth hormone; GI, gastrointestinal; SSAs, somatostatin analogs; SST, somatostatin; SSTR, somatostatin receptor.
SST-14 and SST-28 have approximately equivalent affinity for all the receptor subtypes, except for SSTR 5 which has a 10-fold higher affinity for SST-28 suggesting a potentially different role for this receptor.22 The interaction between SST and its receptor subtypes activates a number of intracellular cascades, including the inhibition of adenylate cyclase activity and the activity of calcium channels, as well as stimulation of phosphotyrosine phosphatase or MAPK activity.19 While all these pathways suppress secretion processes, the activation of phosphotyrosine phosphatase or MAPK by SST may play a role in the regulation of cell proliferation.23,24 In addition, SSTRs may affect the activity of phospholipase C, cyclic guanosine monophosphate, and phospholipase A2 – all involved in signal transduction.25 However, the current understanding of SST/SSTR intracellular signaling is based on in vitro models, and its in vivo relevance remains to be elucidated.
The antiproliferative mechanisms of SST are different and dependent on the SSTR subtype and target cell type. They involve hyperphosphorylation of the retinoblastoma gene product and G1 cell cycle arrest, but can also be mediated by SSTR 3-induced apoptosis.26 Moreover, SST may exert an indirect antiproliferative effect by inhibiting the release of growth factors and trophic hormones (GH, IGF1, insulin, gastrin, epidermal growth factor) both from neoplastic cells and from the surrounding tumor matrix.9 The latter mechanism also involves the antiproliferative effects on tumor angiogenesis which plays a critical role in tumor progression. SST shows antiangiogenic properties by inhibiting the production and release of pro-angiogenic factors as well as expression of their receptors. In particular, in the pancreatic cancer cell line PC-3, SSTR 2 expression correlates with expression of VEGF and matrix metalloproteinase-2.27
As well as the normal cell lines in SST-target tissues, most tumors originating from these tissues express a high density of SSTR. This is the case of NENs deriving from the neuroendocrine cells of the gastrointestinal and bronchopulmonary systems, as well as of pituitary tumors, medulloblastomas, medullary thyroid carcinomas, and adenocarcinomas of the breast, ovary, and colon.7,28 On the other hand, poorly differentiated or undifferentiated tumors express SSTR at a lower density than their corresponding well-differentiated neoplasias.28
The synthetic SSAs OCT, LAN, and VAP bind preferentially to SSTR 2 and SSTR 5, and with moderate affinity to SSTR 3 and low affinity to SSTR 1 and SSTR 4;29 in contrast, the multireceptor ligand PAS (SOM 230) binds with high affinity to subtypes SSTR 1, SSTR 2, SSTR 3, and SSTR 5.30 Compared with OCT, PAS displays a 40-, 30-, and 5-fold higher binding affinity for SSTR 5, SSTR 1, and SSTR 3, respectively, and a 2.5-time lower binding affinity for SSTR 2. Moreover, PAS has a 106-fold higher affinity for SSTR 5 in comparison with LAN.29
Both OCT and LAN have potent activity against GEP-NENs and inhibit cell proliferation by several mechanisms. A direct antitumor effect may result from the activation of SSTRs on tumor cells leading to modulation of intracellular signaling pathways. Immunohistochemistry and autoradiography reveal that SSTR proteins are highly expressed in gastrinomas, insulinomas, and carcinoid tumors, and their metastases. The majority of the tumors express SSTR 2, followed by SSTR 1, SSTR 5, and SSTR 3, while SSTR 4 is expressed in a minority of cases.31 The frequency and pattern of expression of each subtype can, however, vary in different tumor types and in each patient.32 Indeed, SSTRs are expressed in lower density in undifferentiated GEP-NENs than well-differentiated tumors. The largest expression of SSTR 2 on pancreatic endocrine or carcinoid tumors is the reason for the successful clinical application of OCT and LAN in controlling symptoms related to hormonal hypersecretion.33 Furthermore, SSAs may also produce an indirect antitumor effect by inhibiting mitogenic growth factors (such as IGF) and by inhibiting tumor angiogenesis through interaction with SSTRs on endothelial cells and monocytes. In immortalized human dermal microvascular endothelial cells, expression of VEGF and VEGF receptor-2 and VEGF release are inhibited by SSTR 1 agonists.34
Clinical features of GEP-NENs
Literature regarding GEP-NENs has considerably increased during the past 20–30 years, with changes in classifications, grading systems, and proposed treatments. Over the years, classification systems have aimed at classifying NENs on the basis of their differentiation and grade. The grading system currently used for the classification of all GEP-NENs is based on the Ki-67 proliferation index or mitotic count.35 Grading includes site-specific tumor–node–metastasis staging, referring to the extent of tumor spread.36 Current and previous NEN classifications are reported in Table 1.37,38
Table 1.
Classifications of GEP-NENs
| NEN classification according to embryological origin37 | |
|---|---|
| Foregut | NEN originating from the thymus, respiratory tract, esophagus, stomach, pancreas, duodenum, and ovaries |
| Midgut | NEN originating from the jejunum, ileum, appendix, cecum, ascending colon, and Meckel’s diverticulum |
| Hindgut | NEN originating from the transverse, descending, and sigmoid colon |
| GEP-NEN classification according to grade of differentiation38,a | |
| Well-differentiated tumors (“carcinoid”) | Mild or no atypia, restricted to the mucosa or submucosa, absence of angioinvasion, tumor size <1 cm, and proliferation rate <2 mitoses/10 HPFs or Ki-67 <2% |
| Well-differentiated carcinomas (“malignant carcinoid”) | Malignant endocrine tumor cells, moderate atypia, deep invasion of the gut wall, often metastases to regional lymph nodes or liver, tumor size >1 cm, and proliferation rates >2 mitoses/10 HPFs or Ki-67 index >2% |
| Poorly differentiated carcinomas (“small cell carcinoma”) | Highly atypical, small- to intermediate-sized tumor cells, deep invasion or destruction of the gut wall, often necrosis and angio- and perineural invasion, local and distant metastases, size >1 cm, and proliferation rates of >10 mitoses/10 HPFs or Ki-67 index of >15% |
| Mixed exocrine–endocrine tumors | Unusual bimorphous tumors. Prominent exocrine cells (acinar or ductal) admixed with at least 30% endocrine component. Biological behavior of the exocrine component |
| Tumor-like lesion | |
| WHO 2010 classification35
| |||
|---|---|---|---|
| Neuroendocrine neoplasm typeb | Grade | Ki-67 indexc | Mitotic count (per 10 HPFs)d |
| Neuroendocrine tumor | G1 | ≤2% | <2 |
| Neuroendocrine tumor | G2 | 3%–20% | 2–20 |
| Neuroendocrine carcinoma | G3 | >20% | >20 |
| Mixed adenoneuroendocrine carcinoma | G1–G3 (mostly G3 component) | All ranges | All ranges |
Notes:
In the WHO 2000 classification of GEP-NENs, large cell carcinomas were mentioned under the stomach, colon, and rectum sections, but no other classification criteria were described.
The WHO recommends the use of the term “neuroendocrine neoplasm” to indicate low- to high-grade lesions. However, the term “neuroendocrine tumor” is still widely used. The term “neuroendocrine carcinoma” indicates high-grade lesions.
Ki-67 index: % of 500–2,000 cells in “hot spot areas” stained positive for MIB-1 antibody.
10 HPFs =2 mm2, based on measurement in at least 50 HPFs in hot spot areas.
Abbreviations: GEP, gastroenteropancreatic; HPFs, high-power fields; NENs, neuroendocrine neoplasms; WHO, World Health Organization.
Clinical manifestations of GEP-NENs are very heterogeneous and cover a wide spectrum: from remaining asymptomatic for several years to causing obstructive symptoms (such as abdominal pain, nausea, vomiting, and cholestasis), and from presence of metastases at the time of diagnosis to presenting signs and symptoms due to hormonal hypersecretion. In most cases, because of vagueness and nonspecificity of symptoms, the diagnosis is delayed (3–10 years on average), with an increased risk of developing metastases.39 In cases of functioning GEP-NENs, a specific syndrome develops due to hormonal hypersecretion. Moreover, GEP-NEN cells, which have neuroendocrine differentiation, express both specific neuroendocrine markers such as chromogranin A (CgA)40 and synaptophysin, and less specific markers including CD56 and neuron-specific enolase (NSE).41 Although these biomarkers are not associated with specific syndromes, they can be monitored in both functional and nonfunctional NENs in relation to disease progression and response to treatment. In particular, the use of CgA is recommended in clinical practice, while the utility of serum NSE as a marker of tumor aggressiveness needs to be evaluated by further studies before it can be recommended for routine monitoring. The 5-hydroxyindoleacetic acid (5-HIAA) is the main urinary metabolite of human serotonin, and its determination in 24-hour urine collection has a sensitivity of over 90% and a specificity of 90% for advanced carcinoid syndrome (CS).42 Various blood serotonin assays have been proposed, but their actual accuracy has not been established and serotonin determination is not recommended in clinical practice. Serum gastrin determination is crucial in the diagnosis of gastrinoma and related syndrome (“Zollinger–Ellison syndrome”). Simultaneous measurement of gastric pH is needed to rule out secondary hypergastrinemia due to other causes. For example, in achlorhydria, pernicious anemia, or atrophic gastritis, high gastrin levels are usually associated with high pH values, while elevated serum gastrin levels combined with gastric pH <2 are virtually diagnostic of Zollinger–Ellison syndrome. The occurrence of symptomatic hypoglycemia with non-suppressed endogenous insulin levels is suspicious for insulinoma. Other specific markers include serum glucagon concentrations for the diagnosis of glucagonoma, associated with a characteristic clinical syndrome (diabetes mellitus and cutaneous manifestations, such as migratory necrolytic erythema, nail dystrophies, and stomatitis), and vasointestinal peptide (VIP) for VIP-secreting tumors which cause the Verner–Morrison syndrome, characterized by watery diarrhea, hypokalemia, achlorhydria, weight loss, metabolic acidosis, hypercalcemia, glucose intolerance, and flushing.
Different from the hypersecreting NENs, which can present with specific endocrine syndromes, tumors which do not secrete biologically active substances may be present for years without ever displaying signs or symptoms, except for vague abdominal pain. The most important clinical manifestations of NENs are related to mechanical complications (pain, obstruction, and bleeding) or to the bioactive factors secreted. The CS is characterized by signs and symptoms associated with hypersecretion of vasoactive substances by NENs (serotonin, histamine, tachykinins, and prostaglandins). The symptoms of CS include cutaneous flushing (which occurs in 84% of patients), gastrointestinal hypermotility and diarrhea, heart disease, bronchial constriction, myopathy, and an abnormal increase in skin pigmentation.39
Lanreotide Autogel
LAN Autogel is available at doses of 60 mg, 90 mg, or 120 mg.17 The recommended dose for the treatment of GEP-NENs is 120 mg administered every 4 weeks by deep subcutaneous injection allowing to reach steady-state concentrations after 4–5 injections. It is widely metabolized in the gastrointestinal tract and excreted through the biliary tract. Its half-life is ~30 days.
Even if the use in the geriatric population is associated with differences in pharmacokinetics, no dose adjustment is required because of the wide therapeutic window of LAN. On the other hand, there is no experience with LAN in the pediatric population, in which its use should be avoided. Since in preclinical studies LAN has shown embryocidal effects, in the absence of adequate and well-controlled reproductive studies in humans, its use in pregnancy should be considered with particular care for the potential risk to the fetus. Since LAN may cause a reduction in heart rate, patients affected by underlying cardiac conditions should have their heart rate monitored prior to starting LAN. In a group of 81 patients affected by GEP-NENs treated with LAN Autogel, the incidence of heart rate <60 bpm was 23% (vs 16% in placebo group), while the incidence of episodes of heart rate <50 bpm as well as adverse event of bradycardia was 1% in each group. This finding can be explained by the activity of the bulbospinal neurons in the rostral ventrolateral medulla (RVLM), which are known to be critical for the maintenance of sympathetic vasomotor tone and normal cardiovascular reflex function. In particular, RVLM presympathetic neurons that express SSTR 2A are essential for maintaining and potentially generating sympathetic vasomotor tone.43 In rats, microinjection of either SST or LAN into the RVLM causes a dose-dependent sympathoinhibition, hypotension, and bradycardia that is blocked by the SSTR 2 antagonist.43
Preclinical and clinical pharmacological studies show that LAN, such as SST and other SSAs, inhibits the secretion of insulin and glucagon. Hence, patients treated with LAN may experience hypoglycemia or hyperglycemia. For this reason, glucose levels should be monitored during treatment with LAN, especially in diabetic patients who may require adjustments in their antihyperglycemic therapy.17
In the gastrointestinal system, LAN significantly reduces the levels of pancreatic polypeptide, motilin, and gastric-inhibitory peptide, as well as postprandial gastrin secretion, without affecting secretin. Moreover, it inhibits bile secretion and pancreatic secretion of bicarbonate and enzymes. Moreover, LAN may reduce the intestinal absorption of drugs and may reduce gallbladder motility leading to gall stone formation.17 LAN clearance is reduced by 30% in patients with moderate-to-severe hepatic impairment, but the effects of LAN in patients with hepatic failure have not been studied. At the dose of 120 mg, the clearance of LAN is not modified in patients with mild-to-moderate renal impairment, while patients with severe renal impairment have not been studied. Finally LAN, as other SSAs, may decrease the catalytic pathway of cytochrome P450.17
Use of LAN Autogel in GEP-NENs
LAN Autogel has been approved for the long-term treatment of patients with acromegaly and for the treatment of GEP-NENs in order to delay disease progression in patients with G1 or a subset of G2 (equivalent to Ki-67 <10%), unresectable, locally advanced or metastatic disease.17 The efficacy of LAN Autogel has been shown by studies demonstrating a benefit in progression-free survival (PFS), even if there is no evidence of an overall survival benefit (Table 2). Moreover, LAN Autogel is widely recognized as effective in controlling tumor-related symptoms in the majority of patients affected by GEP-NENs (Table 2).
Table 2.
Relevant clinical studies of LAN Autogel® in GEP-NENs
| Study | Type of the study | Patients | Aim of the study | Results |
|---|---|---|---|---|
| Ruszniewski et al53 | 6-month, open, non-controlled, multicenter, dose-titration study | 71 patients affected by CS treated with LAN. The dose for the first 2 injections was 90 mg. Subsequent doses could be titrated (60 mg, 90 mg, 120 mg) according to symptom response | Efficacy and safety of 28-day PR-LAN in the treatment of CS | Symptom frequency decreased further after the second and third injections. In 6 months, flushing and diarrhea had significantly decreased from baseline. Median urinary 5-HIAA and CgA levels decreased by 24% and 38%, respectively |
| Bajetta et al44 | Phase III, multicenter, randomized trial | 60 patients randomized; 46 completed the study. Comparison between LAN Autogel 120 mg/6 weeks vs LAN microparticles 60 mg/3 weeks | Efficacy of LAN Autogel vs LAN microparticles in sporadic, well-differentiated NENs with a low grade of malignancy | LAN Autogel was not inferior to LAN microparticles for tumor markers (55% and 59%, respectively) and tumor size reduction (68% and 66%, respectively) |
| Khan et al58 | Retrospective study (9 years) | 69 patients treated (initial dose) with LAN 60 mg/28 days (23 patients), 90 mg/28 days in 36 patients and 120 mg in 7 patients | Clinical response and tolerance of LAN in metastatic midgut NENs and CS | 94% of patients achieved symptomatic response at first follow-up visit. 46% had loss of symptomatic response, but 44% of these achieved control increasing LAN dose. Overall, symptoms were controlled with LAN in 74% of patients. 26% required additional treatment despite good initial response; 30% showed radiological progression |
| Bianchi et al45 | Retrospective study (4 years) | 23 patients receiving LAN Autogel (120 mg) every 28 days | Relief of disease symptoms, behavior of tumor markers response rate in terms of time to tumor progression, and safety and tolerability of LAN in metastatic well-differentiated NENs | Improvement of flushing and diarrhea in 85.7% and 55.6% of patients, respectively. Complete, partial, or no-changed response in the tumor markers behavior in 42.9%, 22.9%, and 17.1% of cases, respectively. Tumor partial regression: 8.7%; stable disease: 65.3%; tumor progression: 26.0%. No severe adverse reaction |
| Martín-Richard et al46 | Multicenter, open-label, Phase II trial | 30 patients receiving LAN Autogel (120 mg) every 28 days | PFS, response rate, tumor biomarkers, symptom control, QoL, and safety in well-differentiated NENs | PFS time: 12.9 months; disease stabilization: 89% of patients; partial response: 4% of cases. No deterioration in QoL. Treatment-related adverse events (most frequently diarrhea and asthenia): 63% |
| Palazzo et al50 | Retrospective study | 68 patients with well-differentiated NENs treated with LAN | To identify factors associated with tumor control in a group of patients with well-differentiated, malignant GEP-NENs treated with LAN | Tumor progression in 57.4%. Median PFS was 29 months. Ki-67 index of up to 5%, pretreatment stability, and hepatic tumor load of up to 25% were significantly associated with disease stability under LAN therapy |
| Vinik et al54 | 16-week, randomized, double-blind, Phase III trial | LAN 120 mg/28 days, n=59; placebo, n=56 | Efficacy of LAN 120 mg/4 weeks in treatment of CS | The percentage days with rescue OCT use is significantly lower in the LAN vs placebo (33.7% vs 48.5%) |
| Caplin et al47 | 96-week, randomized, double-blind, placebo-controlled, multinational study (CLARINET) | LAN Autogel 120 mg/28 days, n=101; placebo, n=103 | PFS, overall survival, QoL, and safety in SSAs-positive, G1 or G2 NENs and documented disease-progression status | PFS at 24 months was 65.1% for LAN and 33.0% for placebo group. No significant differences in QoL or overall survival. The most common treatment-related adverse event was diarrhea (in 26% of the patients in the LAN group and 9% of those in the placebo group) |
| Orlewska et al59 | National, multicenter, non-interventional, observational study (LANRONET) | 52 patients receiving LAN 120 mg/28 days | To examine characteristics and treatment patterns of symptomatic NEN patients treated with LAN Autogel 120 mg for at least 3 months before inclusion, administered as part of routine clinical practice | Primary tumors were GEP-NENs (51.2%); all tumors were metastatic. Most commonly reported symptoms were flushing and diarrhea (55.8%). During the 12-month observation, 28% received LAN Autogel |
| Ruszniewski et al55 | International, open-label, observational study (SYMNET) | 273 patients | Satisfaction with diarrhea control; severity, change in symptoms, and impact on daily life of diarrhea; and satisfaction with flushing control | 76% were “completely” or “rather” satisfied with diarrhea control; 79% had improvement in diarrhea with LAN. 75% were unconcerned about the impact of diarrhea on daily life. Satisfaction with flushing control was 73% |
| Caplin et al49 | Open-label extension of CLARINET study | 88 patients. LAN, n=41; placebo, n=47 | Long-term safety and additional efficacy | Patients continuing LAN reported fewer adverse events than core study. Placebo-to-LAN switch patients reported similar adverse effects |
| Faggiano et al51 | Observational multicenter study | 106 patients with a histologically confirmed GEP or thoracic NENs or unknown primary NENs, initially treated with LAN Autogel (120 mg/28 days) or OCT LAR (30 mg/28 days). The initial SSA dose has been subsequently changed in 14 patients. OCT was switched to LAN in 6 cases | Evaluate the efficacy of long-acting SSAs in NENs according to Ki-67 index | Tumor response in 11%, stability in 58%, and progression in 31%. No differences between G1 and G2 NENs. PFS was longer but not significantly different in G1 than G2 NENs. The median PFS was significantly longer in NENs showing Ki-67 <5% than in those showing Ki-67 ≥5% |
Abbreviations: 5-HIAA, 5-hydroxyindoleacetic acid; CgA, chromogranin A; CS, carcinoid syndrome; GEP, gastroenteropancreatic; LAN, lanreotide; NENs, neuroendocrine neoplasms; OCT, octreotide; PFS, progression-free survival; PR, prolonged release; QoL, quality of life; SSAs, somatostatin analogs; LAR, long acting repeatable.
The rationale of using SSAs as medical therapy in patients with NENs is based on the expression of SSTRs on the cell surfaces of the majority of these tumors. LAN and OCT, the most used SSAs in this context, bind with high affinity to receptor subtypes 2 and 5, inhibiting the signal-transmission pathways, causing a reduction in secretion of hormone and amine which can ameliorate tumor-related syndromes and stabilize tumor growth. Moreover, compared with the earlier formulation of SSAs, newer long-acting formulations reduce the number of injections required, increasing patients’ compliance. Since the introduction of LAN Autogel in the clinical practice, several studies have compared the therapeutic equivalence between LAN Autogel formulation (injected every 4 weeks at a dose of 60 mg, 90 mg, or 120 mg) and LAN microparticles (injected every 7–14 days at the dose of 30 mg, or every 14–28 days at the dose of 60 mg) in GEP-NENs. In a Phase III randomized clinical trial, involving 46 patients who completed the study, LAN Autogel (120 mg/6 weeks) demonstrated the same efficacy of LAN microparticles (30 mg/3 weeks) in terms of reduction of tumor markers and tumor size, offering the possibility to use a more delayed formulation of SSA.44
The efficacy, safety, and tolerability of LAN Autogel have been evaluated in metastatic, well-differentiated NENs in an Italian retrospective evaluation performed by Bianchi et al.45 The study included 23 patients affected by metastatic NENs, and in ~65% of cases, tumor was localized in the gastrointestinal system. Functional tumors were 43.5%. In this clinical study, LAN Autogel 120 mg, given once a month by deep subcutaneous injection for at least 24 months, was well tolerated and induced long-lasting responses in terms of clinical symptoms. A partial response in terms of tumor reduction was observed only in 2 out of 5 lung tumors, but not in GEP-NENs, which remained either stable or showed progression (Table 2).
Prospective evaluations investigating the antiproliferative effects of LAN have showed an effective tumor stabilization and a PFS >12 months in patients with progressive NENs ineligible for surgery or chemotherapy (Table 2).46
The CLARINET study represents a fundamental contribution to the evaluation of efficacy of LAN Autogel in GEP-NENs (Table 2). This randomized, double-blind, placebo-controlled, 96-week study assessed the effects of LAN Autogel 120 mg/monthly in patients with advanced, well-differentiated, nonfunctioning, SSTR-positive, G1 or G2 GEP-NENs and documented disease-progression status. Tumors originated in the pancreas, midgut, hindgut, or unknown primary location. Compared to placebo, LAN Autogel at a dose of 120 mg/monthly was associated with significant increase in PFS and a 53% reduction in the risk of tumor progression.47 Quality of life did not differ significantly between treatment groups. Moreover, in patients with baseline levels of CgA above the upper limit of the normal range, LAN Autogel induced a significantly greater reduction compared to placebo. The overall incidence of adverse events was similar between LAN Autogel and placebo (~90%), but half the patients treated with LAN Autogel experienced drug-related adverse events (diarrhea, hyperglycemia, or cholelithiasis).
The decrease of CgA levels during treatment with LAN Autogel from baseline was associated with a significant reduction of the hazard of disease progression, confirming the utility of this marker in the clinical follow-up of patients with NENs.48 Recent data from the open-label extension of the CLARINET study confirm that LAN Autogel maintains favorable risk/benefit profile, providing new evidence of the LAN antitumor benefits in indolent and progressive GEP-NENs (Table 2).49
The Ki-67 proliferation index represents a good marker in predicting tumor response to SSAs treatment (Table 2). In patients with well-differentiated GEP-NENs, a Ki-67 proliferation index of up to 5%, stable disease prior to treatment, and low-to-moderate hepatic tumor involvement (≤25%) have been associated with tumor control during LAN Autogel treatment.50 The antiproliferative effects of long-acting SSAs according to Ki-67 index have been recently evaluated by an Italian multicenter observational study using both OCT LAR 30 mg/28 days and LAN 120 mg/28 days.51 Objective response and tumor stability were not significantly different between G1 and G2 NENs, and between locoregional disease and distant metastases. Interestingly, the clinical benefit (improvement of symptoms) was significantly greater in patients with locoregional disease than in those with distant metastases as well as in patients with GEP-NENs than those with other primary tumors. Although PFS was longer in G1 than G2 NENs, the difference was not significant. However, in the subgroup of NENs with Ki-67 <5%, the PFS was significantly longer compared to NENs with Ki-67 ≥5%, consistent with previous data.46 Furthermore, while PFS was not different between GEP and thoracic NENs, it was longer in GEP and thoracic NENs compared to those with unknown primary tumors.51
Some functional NENs release peptides and amines which produce a characteristic set of symptoms of the CS. This syndrome occurs in ~10% of patients with metastatic NENs, and it is most prevalent in those with NENs of the small intestine (~20%). It is caused by the release of serotonin, which is no longer metabolized in the liver, and other substances, such as tachykinins, prostaglandins, and bradykinins.52 The predominant signs and symptoms of CS are flushing (90%), diarrhea (70%), and abdominal pain (40%); less frequent events are lacrimation, profuse sweating, telangiectasias, cardiac fibrosis, and cutaneous pellagra-like manifestations due to lack of niacin.39 These can be very distressing for patients and have a negative impact on their quality of life. SSAs are currently considered the “standard of care” for symptom control in CS, demonstrating a significant reduction of flushing and diarrhea since the first day of treatment. Moreover, a reduction of CgA and 5-HIAA can be detected (Table 2).53 A recent 16-week, randomized, double-blind, Phase III trial involving 115 patients evaluated the response of CS symptoms to treatment with LAN Autogel 120 mg/4 weeks. In this study, patients had access to short-acting OCT as rescue medication. Results showed that the proportion of patients requiring OCT rescue was significantly lower in the LAN-treated group than in the placebo (Table 2).54 Satisfaction with diarrhea control and flushing control and a good impact on the quality of life have also been reported in an open-label observational study (Table 2).55 Moreover, the once-monthly dosing regimen of LAN is associated with improved patient adherence and patient perception of LAN injection.56,57
Although in the majority of patients with metastatic carcinoids and pancreatic endocrine tumors treatment with SSAs induces a rapid improvement of clinical symptomatology related to hormonal hypersecretion, most patients can develop desensitization within weeks to months.58,59 The potential mechanisms responsible for this phenomenon, as well as for the considerable variability in the duration of the responses to medical therapy, are not known at present. Potential mechanisms of resistance to SSAs therapy in patients with SST-positive tumors are the possibility of receptor downregulation as well as reduction in the number and/or affinity of SSTRs. Moreover, a decrease in responsiveness due to receptor uncoupling from second messenger activation can determine desensitization. Other potential causes could be a non-homogeneous expression of SSTRs in tumors, outgrowth of SST-negative cell clones, absence of SSTR subtypes with high affinity for SSAs, tachyphylaxis of the inhibitory effect of SSAs on indirect tumor growth-promoting mechanisms, and mutations in SSTR genes leading to the absence of functional receptor proteins.7
Conclusion
To date, SSAs represent the main symptomatic therapeutic approach for the management of NENs. In this context, LAN Autogel is one of the most used SSAs. There are no differences between LAN microparticulate and LAN Autogel in their ability to control tumor growth and tumor hypersecretion. Their long-acting formulation, which allows up to once-monthly administration regimens, increases the compliance of patients with NENs. Although several studies have been conducted since the approval of LAN Autogel, their conclusions are often difficult to compare due to differences in study design, eligibility criteria, and study end points. Nonetheless, the majority of the studies indicate that the effects of LAN Autogel are mainly on improving symptoms and stabilization of the disease progress, and even if a benefit in PFS has been demonstrated, there is no evidence of an overall survival benefit. This may partly be explained by the fact that NENs are frequently tumors showing slow progression and an indolent behavior. Therefore, more data must be derived by the extension of studies that have already been published. In addition, the molecular bases of tumor response to SSAs are still unclear, although the application of proteomics to this field has led to promising results.60 Finally, the ongoing drug research has shown that LAN – above a critical assembly concentration of 20 mM – spontaneously forms hollow nanotubes when solubilized in pure water.61,62 Moreover, a recent study evaluated the utility of a self nano-emulsifying delivery system for model peptide LAN which provided a protective effect toward thiol–disulfide exchange reactions and can be useful to overcome sulfhydryl barrier of the gastrointestinal tract.63 Therefore, LAN is an interesting self-assembly model that can provide insights on how these mechanisms can be controlled, and applied to nanotechnology and drug delivery.64
Footnotes
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Saltz L, Trochanowski B, Buckley M, et al. Octreotide as an antineoplastic agent in the treatment of functional and nonfunctional neuroendocrine tumors. Cancer. 1993;72(1):244–248. doi: 10.1002/1097-0142(19930701)72:1<244::aid-cncr2820720143>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.di Bartolomeo M, Bajetta E, Buzzoni R, et al. Clinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. A study by the Italian Trials in Medical Oncology Group. Cancer. 1996;77(2):402–408. doi: 10.1002/(SICI)1097-0142(19960115)77:2<402::AID-CNCR25>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Strosberg J, Kvols L. Antiproliferative effect of somatostatin analogs in gastroenteropancreatic neuroendocrine tumors. World J Gastroenterol. 2010;16(24):2963–2970. doi: 10.3748/wjg.v16.i24.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brazeau P, Vale W, Burgus R, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 6.Brazeau P, Gullemin R. Editorial: Somatostatin: newcomer from the hypothalamus. N Engl J Med. 1974;290(17):963–964. doi: 10.1056/NEJM197404252901711. [DOI] [PubMed] [Google Scholar]
- 7.de Herder WW, Hofland LJ, van der Lely AJ, Lamberts SW. Somatostatin receptors in gastroentero-pancreatic neuroendocrine tumours. Endocr Relat Cancer. 2003;10(4):451–458. doi: 10.1677/erc.0.0100451. [DOI] [PubMed] [Google Scholar]
- 8.Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocr Rev. 1995;16(4):427–442. doi: 10.1210/edrv-16-4-427. [DOI] [PubMed] [Google Scholar]
- 9.Susini C, Buscail L. Rationale for the use of somatostatin analogs as antitumor agents. Ann Oncol. 2006;17(12):1733–1742. doi: 10.1093/annonc/mdl105. [DOI] [PubMed] [Google Scholar]
- 10.Grozinsky-Glasberg S, Shimon I, Korbonits M, Grossman AB. Somatostatin analogues in the control of neuroendocrine tumours: efficacy and mechanisms. Endocr Relat Cancer. 2008;15(3):701–720. doi: 10.1677/ERC-07-0288. [DOI] [PubMed] [Google Scholar]
- 11.Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. N Engl J Med. 1996;334(4):246–254. doi: 10.1056/NEJM199601253340408. [DOI] [PubMed] [Google Scholar]
- 12.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20(3):157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 13.Bousquet C, Puente E, Buscail L, Vaysse N, Susini C. Antiproliferative effect of somatostatin and analogs. Chemotherapy. 2001;47(Suppl 2):30–39. doi: 10.1159/000049159. [DOI] [PubMed] [Google Scholar]
- 14.Bauer W, Briner U, Doepfner W, et al. SMS 201–995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982;31(11):1133–1140. doi: 10.1016/0024-3205(82)90087-x. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Shlomo A, Melmed S. Somatostatin agonists for treatment of acromegaly. Mol Cell Endocrinol. 2008;286(1–2):192–198. doi: 10.1016/j.mce.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melmed S. Medical progress: acromegaly. N Engl J Med. 2006;355(24):2558–2573. doi: 10.1056/NEJMra062453. [DOI] [PubMed] [Google Scholar]
- 17.Somatuline® Autogel® lanreotide injection [product monograph] Ipsen Biopharm Limited; UK: 2015. [Google Scholar]
- 18.Fleseriu M. Advances in the pharmacotherapy of patients with acromegaly. Discov Med. 2014;17(96):329–338. [PubMed] [Google Scholar]
- 19.Patel YC. Molecular pharmacology of somatostatin receptor subtypes. J Endocrinol Invest. 1997;20(6):348–367. doi: 10.1007/BF03350317. [DOI] [PubMed] [Google Scholar]
- 20.Maurer R, Reubi JC. Somatostatin receptors. JAMA. 1985;253(18):2741. [PubMed] [Google Scholar]
- 21.Krantic S, Goddard I, Saveanu A, et al. Novel modalities of somatostatin actions. Eur J Endocrinol. 2004;151(6):643–655. doi: 10.1530/eje.0.1510643. [DOI] [PubMed] [Google Scholar]
- 22.Guillermet-Guibert J, Lahlou H, Cordelier P, Bousquet C, Pyronnet S, Susini C. Physiology of somatostatin receptors. J Endocrinol Invest. 2005;28(11 Suppl International):5–9. [PubMed] [Google Scholar]
- 23.Schally AV. Oncological applications of somatostatin analogues. Cancer Res. 1988;48(24 Pt 1):6977–6985. [PubMed] [Google Scholar]; Erratum. Cancer Res. 1989;49(6):1618. [Google Scholar]
- 24.Lamberts SW, Krenning EP, Reubi JC. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev. 1991;12(4):450–482. doi: 10.1210/edrv-12-4-450. [DOI] [PubMed] [Google Scholar]
- 25.Cervia D, Bagnoli P. An update on somatostatin receptor signaling in native systems and new insights on their pathophysiology. Pharmacol Ther. 2007;116(2):322–341. doi: 10.1016/j.pharmthera.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Patel YC, Greenwood MT, Panetta R, Demchyshyn L, Niznik H, Srikant CB. The somatostatin receptor family. Life Sci. 1995;57(13):1249–1265. doi: 10.1016/0024-3205(95)02082-t. [DOI] [PubMed] [Google Scholar]
- 27.Kumar M, Liu ZR, Thapa L, Chang Q, Wang DY, Qin RY. Antiangiogenic effect of somatostatin receptor subtype 2 on pancreatic cancer cell line: inhibition of vascular endothelial growth factor and matrix metalloproteinase-2 expression in vitro. World J Gastroenterol. 2004;10(3):393–399. doi: 10.3748/wjg.v10.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reubi JC. Somatostatin and other peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology. 2004;80(Suppl 1):51–56. doi: 10.1159/000080742. [DOI] [PubMed] [Google Scholar]
- 29.Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146(5):707–176. doi: 10.1530/eje.0.1460707. [DOI] [PubMed] [Google Scholar]
- 30.Lesche S, Lehmann D, Nagel F, Schmid HA, Schulz S. Differential effects of octreotide and pasireotide on somatostatin receptor internalization and trafficking in vitro. J Clin Endocrinol Metab. 2009;94(2):654–661. doi: 10.1210/jc.2008-1919. [DOI] [PubMed] [Google Scholar]
- 31.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30(5):781–793. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 32.Papotti M, Bongiovanni M, Volante M, et al. Expression of somatostatin receptor types 1–5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002;440(5):461–475. doi: 10.1007/s00428-002-0609-x. [DOI] [PubMed] [Google Scholar]
- 33.Lamberts SW, van der Lely AJ, Hofland LJ. New somatostatin analogs: will they fulfil old promises? Eur J Endocrinol. 2002;146(5):701–705. doi: 10.1530/eje.0.1460701. [DOI] [PubMed] [Google Scholar]
- 34.Bocci G, Culler MD, Fioravanti A, et al. In vitro antiangiogenic activity of selective somatostatin subtype-1 receptor agonists. Eur J Clin Invest. 2007;37(9):700–708. doi: 10.1111/j.1365-2362.2007.01848.x. [DOI] [PubMed] [Google Scholar]
- 35.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer (IARC); 2010. [Google Scholar]
- 36.Capelli P, Fassan M, Scarpa A. Pathology – grading and staging of GEP-NETs. Best Pract Res Clin Gastroenterol. 2012;26(6):705–717. doi: 10.1016/j.bpg.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Williams ED, Sandler M. The classification of carcinoid tumors. Lancet. 1963;1(7275):238–239. doi: 10.1016/s0140-6736(63)90951-6. [DOI] [PubMed] [Google Scholar]
- 38.Solcia E, Kloeppel G, Sobin LH. Histological Typing of Endocrine Tumours. New York, NY: Springer; 2000. [Google Scholar]
- 39.Massironi S, Sciola V, Peracchi M, Ciafardini C, Spampatti MP, Conte D. Neuroendocrine tumors of the gastro-entero-pancreatic system. World J Gastroenterol. 2008;14(35):5377–5384. doi: 10.3748/wjg.14.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peracchi M, Conte D, Gebbia C, et al. Plasma chromogranin A in patients with sporadic gastro-entero-pancreatic neuroendocrine tumors or multiple endocrine neoplasia type 1. Eur J Endocrinol. 2003;148(1):39–43. doi: 10.1530/eje.0.1480039. [DOI] [PubMed] [Google Scholar]
- 41.Baudin E, Gigliotti A, Ducreux M, et al. Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumours. Br J Cancer. 1998;78(8):1102–1107. doi: 10.1038/bjc.1998.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimaldi F, Fazio N, Attanasio R, et al. Italian Association of Clinical Endocrinologists (AME) position statement: a stepwise clinical approach to the diagnosis of gastroenteropancreatic neuroendocrine neoplasms. J Endocrinol Invest. 2014;37(9):875–909. doi: 10.1007/s40618-014-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burke PG, Li Q, Costin ML, McMullan S, Pilowsky PM, Goodchild AK. Somatostatin 2A receptor-expressing presympathetic neurons in the rostral ventrolateral medulla maintain blood pressure. Hypertension. 2008;52(6):1127–1233. doi: 10.1161/HYPERTENSIONAHA.108.118224. [DOI] [PubMed] [Google Scholar]
- 44.Bajetta E, Procopio G, Catena L, et al. Lanreotide autogel every 6 weeks compared with lanreotide microparticles every 3 weeks in patients with well differentiated neuroendocrine tumors: a Phase III study. Cancer. 2006;107(10):2474–2481. doi: 10.1002/cncr.22272. [DOI] [PubMed] [Google Scholar]
- 45.Bianchi A, De Marinis L, Fusco A, et al. The treatment of neuroendocrine tumors with long-acting somatostatin analogs: a single center experience with lanreotide autogel. J Endocrinol Invest. 2011;34(9):692–697. doi: 10.3275/8058. [DOI] [PubMed] [Google Scholar]
- 46.Martín-Richard M, Massutí B, Pineda E, et al. TTD (Tumores del Tracto Digestivo) Study Group Antiproliferative effects of lanreotide autogel in patients with progressive, well-differentiated neuroendocrine tumours: a Spanish, multicentre, open-label, single arm phase II study. BMC Cancer. 2013;13:427. doi: 10.1186/1471-2407-13-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caplin ME, Pavel M, Ćwikła JB, et al. CLARINET Investigators Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 48.Buil-Bruna N, Dehez M, Manon A, Nguyen TX, Trocóniz IF. Establishing the quantitative relationship between lanreotide Autogel®, chromogranin A, and progression-free survival in patients with nonfunctioning gastroenteropancreatic neuroendocrine tumors. AAPS J. 2016;18(3):703–712. doi: 10.1208/s12248-016-9884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caplin ME, Pavel M, Ćwikła JB, et al. CLARINET Investigators Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr Relat Cancer. 2016;23(3):191–199. doi: 10.1530/ERC-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palazzo M, Lombard-Bohas C, Cadiot G, et al. Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumors. Eur J Gastroenterol Hepatol. 2013;25(2):232–238. doi: 10.1097/MEG.0b013e328359d1a6. [DOI] [PubMed] [Google Scholar]
- 51.Faggiano A, Carratù AC, Guadagno E, et al. Somatostatin analogues according to Ki67 index in neuroendocrine tumours: an observational retrospective-prospective analysis from real life. Oncotarget. 2016;7(5):5538–5547. doi: 10.18632/oncotarget.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128(6):1717–1751. doi: 10.1053/j.gastro.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 53.Ruszniewski P, Ish-Shalom S, Wymenga M, et al. Rapid and sustained relief from the symptoms of carcinoid syndrome: results from an open 6-month study of the 28-day prolonged-release formulation of lanreotide. Neuroendocrinology. 2004;80(4):244–251. doi: 10.1159/000082875. [DOI] [PubMed] [Google Scholar]
- 54.Vinik AI, Wolin EM, Liyanage N, Gomez-Panzani E, Fisher GA, ELECT Study Group Evaluation of Lanreotide Depot/Autogel efficacy and safety as a carcinoid syndrome treatment (ELECT): a randomized, double-bind, placebo-controlled trial. Endocr Pract. 2016;22(9):1068–1080. doi: 10.4158/EP151172.OR. [DOI] [PubMed] [Google Scholar]
- 55.Ruszniewski P, Valle JW, Lombard-Bohas C, et al. SYMNET Study Group Patient-reported outcomes with lanreotide Autogel/Depot for carcinoid syndrome: an international observational study. Dig Liver Dis. 2016;48(5):552–558. doi: 10.1016/j.dld.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Ricci S, Antonuzzo A, Galli L, et al. Long-acting depot lanreotide in the treatment of patients with advanced neuroendocrine tumors. Am J Clin Oncol. 2000;23(4):412–415. doi: 10.1097/00000421-200008000-00020. [DOI] [PubMed] [Google Scholar]
- 57.Johanson V, Wilson B, Abrahamsson A, et al. Randomized crossover study in patients with neuroendocrine tumors to assess patient preference for lanreotide Autogel(®) given by either self/partner or a health care professional. Patient Prefer Adherence. 2012;6:703–1017. doi: 10.2147/PPA.S34337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khan MS, El-Khouly F, Davies P, Toumpanakis C, Caplin ME. Long-term results of treatment of malignant carcinoid syndrome with prolonged release Lanreotide (Somatuline Autogel) Aliment Pharmacol Ther. 2011;34(2):235–242. doi: 10.1111/j.1365-2036.2011.04693.x. [DOI] [PubMed] [Google Scholar]
- 59.Orlewska E, Bednarczuk T, Kaminski G, Kos-Kudla B, LanroNET Study Group LanroNET, a non-interventional, prospective study to assess the resource utilization and cost of lanreotide autogel 120 mg in Polish patients with neuroendocrine tumors – results of interim analysis. Contemp Oncol (Pozn) 2014;18(6):442–447. doi: 10.5114/wo.2014.47908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fotouhi O, Kjellin H, Larsson C, et al. Proteomics suggests a role for APC-survivin in response to somatostatin analog treatment of neuroendocrine tumors. J Clin Endocrinol Metab. 2016 Jul 26; doi: 10.1210/jc.2016-2028. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gobeaux F, Fay N, Tarabout C, et al. Structural role of counterions adsorbed on self-assembled peptide nanotubes. J Am Chem Soc. 2012;134(1):723–733. doi: 10.1021/ja210299g. [DOI] [PubMed] [Google Scholar]
- 62.Valéry C, Pouget E, Pandit A, et al. Molecular origin of the self-assembly of lanreotide into nanotubes: a mutational approach. Biophys J. 2008;94(5):1782–1795. doi: 10.1529/biophysj.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ijaz M, Bonengel S, Zupančič O, et al. Development of oral self nano-emulsifying delivery system(s) of lanreotide with improved stability against presystemic thiol-disulfide exchange reactions. Expert Opin Drug Deliv. 2016;13(7):923–929. doi: 10.1517/17425247.2016.1167034. [DOI] [PubMed] [Google Scholar]
- 64.Leite DM, Barbu E, Pilkington GJ, Lalatsa A. Peptide self-assemblies for drug delivery. Curr Top Med Chem. 2015;15(22):2277–2289. doi: 10.2174/1568026615666150605120456. [DOI] [PubMed] [Google Scholar]