Abstract
Here we document three highly reproducible protocols: 1) A culture system for the derivation of human oigodendrocites (OLs) from human induced pluripotent stem cells (hiPS) and their further maturation; Key features are the concomitant fate restriction, and lineage specification of hiPS towards the neutral and OL phenotypes. The use of very precise amounts of fresh additives and factors also contributes to the shorter periods of time (56days vs. /200days/75days) necessary to obtain hiPS-derived OLs. Our protocol generates viral and integration free OLs that efficiently commit and move forward in the OL lineage recapitulating all the steps known to occur during in vivo development. 2) Protocols for the isolation, propagation and maintenance of neural stem cells (NSCs); and 3) Protocols for the production, isolation, and maintenance of OLs from perinatal rodent and human brain-derived NSCs. Our unique culture systems rely on a series of chemically defined media, specifically designed and carefully characterized for each developmental stage of OL as they advance from OL progenitors to mature, myelinating cells. We are confident that these protocols bring our field a step closer to efficient autologous cell replacement therapies and disease modeling.
Keywords: Human induced pluripotent stem cells, neural stem cells, NSC, oligodendrocyte specification, oligodendrocyte maturation, lineage progression, chemically defined media, oligospheres, neurospheres
Graphical Abstract
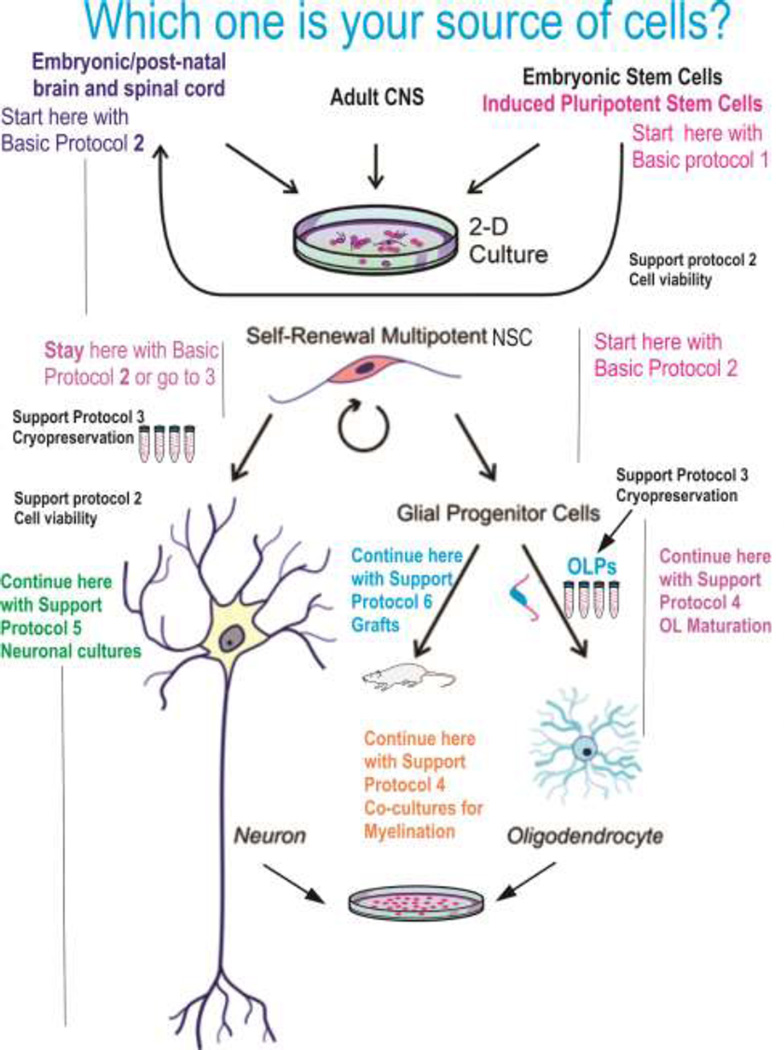
This figure indicates the different sources of neural cells depending of the starting material i.e. embryonic/neonate or adult brain, ES cells, or induced pluripotent stem cells. The starting material would determine where on this chart the user would start the cultures to make oligodendrocytes.
INTRODUCTION
In embryonic life, pluripotent cells proliferate and commit into distinctive cell lineages. During development, in vivo lineage commitment occurs and is maintained by the epigenetic programing of gene expression profiles in which methylation plays a prominent role (Goll and Bestor, 2005). In cell culture, somatic differentiated cells can be made pluripotent with the use of specific pluripotency genes like Oct4 and Nanog. (Takahashi and Yamanaka, 2006). During commitment/differentiation, these genes undergo silencing by de novo DNA methylation in their promoter and enhancer regions maintaining thereafter their hyper-methylated state as differentiated somatic cells (Li et al., 2007). Differences have been found in commitment/differentiation potentials among human pluripotent cell lines and therefore, the culture media can be adjusted, depending on the particular cell line/type being used, to provide the desired results. Our goal was to obtain a “culture system” to implement non-genetic yet stable and irreversible cell commitment. The defined culture medium should contain instructive and selective molecules. There is a lot of interest in deriving OL progenitors (OLPs) from hiPS for cell replacement therapies (Goldman S., 2011) in a shorter period of time than 200 days (Sim et al., 2009). Several protocols have been published aiming at the same goal and they include the use of growth factors and small molecules. More recently it has been reported that OLs can be generated from fibroblasts donated by multiple sclerosis (MS) patients (Douvaras et al., 2014), we appreciate the literature yet it would be inappropriate to include a review of all the literature available in this protocol. We have based our method on three main publications as well as, on the experience we have developed in our laboratory (that expands well over four decades) on the needs of oligodendrocytes as they commit and develop to become functional myelinating cells.
The first publication (Kim et al., 2010) describes a robust enhancement of neural differentiation from human ES and iPS regardless of their innate difference in commitment propensity. The authors used the small molecules ROCK inhibitor, dorsomorphin, and SB431542. In the protocol described herein we shortened their use. Mo and Zecevic (2009) had shown that the numbers of O4-expressing OL progenitors increase when using sonic hedgehog (Shh) in their cultures. Several other authors have used Shh and also retinoic acid (RA) in their medium and in particular, Hu et al., (2009) in their paper described that human OLs derived from ES conserve Shh signaling networks with divergent basic fibroblast growth factor (bFGF) effects. Thus, we incorporated the use of both Shh and RA. The main advantage over any other media described in the literature to generate OLs from human ES or iPS is that with the medium described here OLPs appear much faster. We previously devised a culture system for the production, isolation and maintenance of the OL phenotype from rodent and human neural stem cells (NSC; Espinosa et al., 2009). Here we expand the information and document a protocol for the specification of hiPS to the OL phenotype based on the information we have previously published. Our unique method is reliable because it uses our previously described series of chemically defined media, specifically designed, and carefully characterized for each developmental stage of OLs, as they advance from OLPs to mature myelinating OLs [Figure 1] (Neman and de Vellis, 2008; Espinosa et al., 2009).
Figure 1.
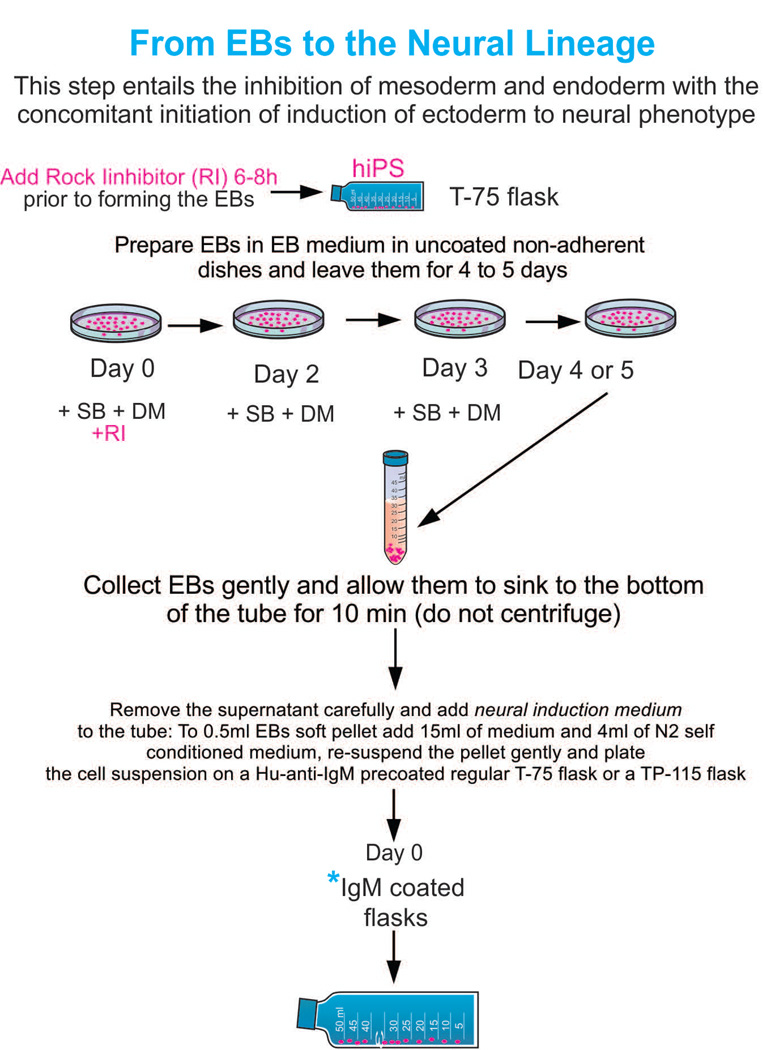
Ectoderm selection can be performed by exposing hiPS to SB431542 (SB) and dorsomorphin (DM), which instruct ectoderm formation preferentially, inhibiting endodermal and mesodermal commitment. All reagents should be freshly prepared as the stock solutions decay fast (between 1 [SB] and 3 [DM] months). Thus, it is suggested to create a stock of hiPS-derived OL while the reagents are fresh. For the procedures described here, the coating of choice was IgM. Nonetheless, if the EBs have been exposed to 4°C while in suspension they would clump together and the vast majority will not adhere to the IgM-coated surface. In this case it is imperative to use Matrigel or poly-D-lysine.
Since our previous chapter describes our “cell culture system” that instructs NSCs to become OLs departing from perinatal brain, in the current chapter we describe the specific nutrients, additives and time windows when the additives should be provided to instruct hiPS to become OLs. We start at the moment when “embryoid bodies (EBs)” are prepared and thereafter to direct and instruct their commitment to the OL phenotype. Once commitment of hiPS cells to OLs has been achieved, the steps of lineage progression is well supported by the culture system we already described (Espinosa et al., 2009). Propagation of hiPS-derived OLs at a desired stage from OLPs to mature premyelinating OLs as well as, lineage progression can be manipulated by controlling the duration of a given developmental stage as needed, in a more “natural” manner, and without using additional gene transfer (Park et al., 2002b; Müller et al., 2006; Ahn et al., 2008), co-cultures, or undefined substrates such as “a different cell line-derived conditioned medium (CM)” or animal serum.
BASIC PROTOCOL 1
Preparation of EBs from hips while starting neural instruction
Preparation of EBs from hiPS while starting neural instruction
Step 1 EBs Preparation
Materials: (See Table 1)
Mouse embryonic fibroblast (MEF) medium
EB medium (Note 1)
hiPS medium (Note 2)
N2 basal medium
Trypsin/EDTA 0.25%
DPBS
60 mm low cell binding culture dishes (NUNC, catalog number 145389)
5mM ROCK inhibitor (1000× stock)
10mM Dorsomorphin (1000× stock)
Matrigel 1/20 (Note 3)
Incubator set to 5% CO2
Table 1.
Reagents and Materials for Culture Media, Cell Growth and Cryopreservation
| # | Name | Company | Catalog # |
|---|---|---|---|
| 1 | Sodium Selenium | DIFCO | 0608-15 |
| 2 | Insulin | Sigma | 1-5500 |
| 3 | Human Transferrin | Sigma | T-2252 |
| 4 | Putrescine | Sigma | P-7505 |
| 5 | Sodium Bicarbonate | Fisher | S233-500 |
| 6 | Sodium Selenite | Sigma | S5261 |
| 7 | Bovine Serum Albumin | Sigma | A3156 |
| 8 | B-27 Supplement | Invitrogen | 17504-044 |
| 9 | D-MEM/ F12 medium | GIBCO BRL | 12100-046 |
| 10 | D-MEM low glucose medium | GIBCO BRL | 11054-020 |
| 11 | Anti-PSA-NCAM IgM | Iowa DSHB | http://www.uiowa.edu/~dshbwww/ |
| 12 | Recombinant Human EGF | Invitrogen | Cat.132476-051 |
| 13 | Recombinant Human bFGF | Invitrogen | Cat.13256-029 |
| 14 | Tris | Fisher | BP 152-1 |
| 15 | D(+) galactose | Sigma | G0625 |
| 16 | Kanamycin | Sigma | K0254 |
| 17 | 12.5, 75 cm2 T-Flasks | Falcon | 353003 |
| 18 | Petri dishes (Non-TC) | Falcon | 351029 |
| 19 | SYTOX | Invitrogen | S7020 |
| 20 | Creatine | Sigma | C3630-100G |
| 21 | Tissue Culture Test tubes | Fisher | 14-956-6A |
| 22 | 5ml sterile pipettes | Falcon | 357543 |
| 23 | Cell strainers | BD Falcon | REF. 352350 |
| 24 | 12ml Syringes sterile | Tyco Healthcare | 512852 |
| 25 | 20 ml Syringes sterile | Jone Kendall | 520673 |
| 26 | Cryo-Tube Vials | NUNC | 366656 |
| 27 | Cryogenic Freezing container | NALGENE | EW-44400-00 |
| 28 | Neurobasal Medium | GIBCO | 21103-049 |
| 29 | B-27 Supp. without Vitamin A | GIBCO | 12587-010 |
| 30 | GlutaMAX | GIBCO | 35050-061 |
| 31 | Heparin | Sigma | H-3149 |
| 32 | Normocin | InVivoGen | Ant-nr-1 |
| 33 | Leukemia Inhibitory Factor | Millipore | LIF-1010 |
| 34 | Fluorescent Fast Blue | Sigma | F5756 |
| 35 | Progesterone | Sigma | P7556 |
| 36 | Normocin | ||
| 37 | KnockOut DMEM | Gibco-BRL | 10829-018 |
| 38 | KnockOut serum replacement | GIBCO BRL | 10828 |
| 39 | Retinoic acid | Sigma | R2625 |
| 40 | Accutase | Innov. Cell Tech. | AT104 |
| 41 | Tissue Culture Flask, T115 w/ recloseable lid |
TPP | TP90652 |
| 45 | Anti-IgM whole Molecule |
Procedures: (Figure 1)
Culture hiPS on MEFs in six-well plates following standard protocol (Biancotti and Lavon, 2012), until they reach 80–90% confluence.
Prior to preparing the EBs, pre-treat the hiPS cultures with ROCK inhibitor (1µl/ml) in ES medium for 2 h to 6 h.
Remove the old medium from the cells and rinse each well with DPBS.
Aspirate the DPBS and add 0.5 ml of freshly prepared warm trypsin-EDTA (Note 4) to each well of the six-well plate.
Incubate the cultures at room temperature for 1–2 min, until cells start detaching.
Add 0.5 ml of MEF media to each well to inactivate the trypsin, and collect the cells into 15 ml conical tubes, pooling up to 4 wells per tube. Spin them down for 5 min at 400 xg.
Resuspend the cells in 5 ml of EB medium and spin it down to wash the remnant trypsin.
Repeat step 7 one more time.
Resuspend the cells in 3 ml of EB media per well pooled in each tube, supplemented with double amount of ROCK inhibitor (2 µl/mL).
Homogenize the cell suspension by gently pipetting up and down, and distribute 3 ml of the suspension per each low-binding culture dish, so to obtain 1 well/dish ratio.
Add 3 ml of EB media supplemented with double amount of dorsomorphin (2 µl/mL) and SB431542 (2 µl/mL) to each dish to complete 6 ml.
Allow EBs to grow for 2 days without changing the media.
Next day, leave EBs undisturbed.
On the second day, remove 3 ml of media by carefully tilting the dish and aspirating from the surface, and replace same volume of fresh EB media containing double amount of dorsomorphin (2 µl/mL) and SB431542 (2 µl/mL), but not ROCK inhibitor. Gently tilt forward and backward, and from side to side to mix the media and redistribute the EBs.
Next day, EBs should remain undisturbed (Note 5).
NOTE: KOSR performance differs considerably between lots. It is strongly advised to test different lots in parallel to the lot in use, to select the most supportive KOSR.
NOTE: bFGF is very unstable and rapidly degrades at room temperature. Warm up to room temperature only the aliquot of PS medium to be used, keeping always the rest of the medium at +4°C.
NOTE: To prevent gelification, Matrigel has to be thawed on ice. Use cold DPBS to make 1/20 dilution. Cool down pipettes and tips by aspirating cold DPBS solution prior to use with Matrigel, and culture plates or flasks by keeping them on ice. Once diluted, add Matrigel suspension to the culture dish and incubate at room temperature for 2 h. Following aspiration of suspension, dishes are ready to be used. If not used immediately, sealed them with Parafilm and store them in a plastic bag at 4°C.
NOTE: Trypsin auto-degrades when warm. After thawing, keep solution always cold and warm up to 37°C only the aliquot to be used.
NOTE: If the EBs are too small, just a few cells grouped (< 30–40 µm diameter), you may wait one or two more days before moving to next step.
Step 2. Establishing the Neural and OL phenotypes (Figure 2 and 3)
Materials
Matrigel or IgM coated T-75 or T-115 coated flask(s)
N2 medium
GDM culture medium
Incubator set at 4.5% CO2
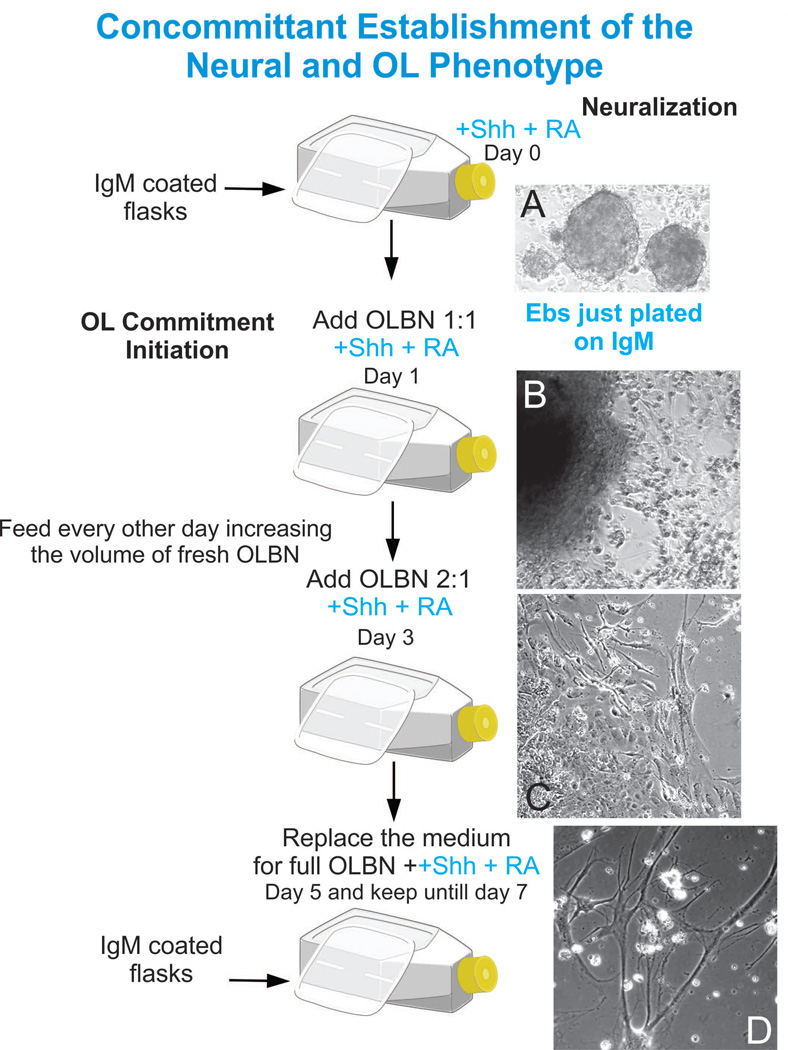
Figure 2.
Once the EBs are transferred to the IgM-coated flask, the culture medium to favor neuralization is the N2 medium, which properties will be enhanced by Shh and RA. A sustained level of these two components leaves virtually no room for other cell types present in the cultures (i.e. flat, fibroblast-like cells, panel C) to prosper. Bi-refringent cells populate both the substratum and flat cells that are often used as anchorage zones (panel D).
Figure 3.
With time in OLBN, flat cells tend to disappear and numerous cells migrate away from the mother clone (panels A and B). These bipolar cells extend their processes to migrate or to connect with other cells while serving as bridges for other cells to migrate on their extensions (panel C). As time in culture increases, cells tend to migrate using other cell processes rather than the substratum.
To be used immediately prior to plating/feeding EB cells
Shh 250 µg/ml (frozen stock aliquoted)
bFGF 100 µg/ml (frozen stock aliquoted)
RA 100 mM in DMSO
N2 supplement
B-27supplement
Preparing OLBN immediately prior to being used
GDM + B-27 +N2 = OLBN
Procedures
On day 4–5, aspirate media and EBs gently, place them in a 15 ml conical tube and let them sink by gravity for 1–2 min (Note 5).
Aspirate the supernatant gently and re-suspend the EBs in 5 ml of N2 medium.
Repeat steps 1 and 2 one more time to eliminate death cells and cell debris.
Plate EBs on matrigel-coated plates, one 60 mm dish into 1 well of a 6 well plate, a T-75, or a T-115 flask in N2 medium supplemented with 20 ng/ml of bFGF, 250 ng/ml of Shh, and 100 nM of RA, at this point discontinue the use of bFGF. This is considered day 0, next day leave cultures undisturbed.
On day 2, very slowly and without shaking the flask, add OLBN with Shh and RA at a volume ratio of 1:1 (for the entire volume of medium in the flask). On day 4, remove 2/3 of the culture medium very slowly and without shaking the flask and replace it with the same volume of GDM.
On day 6 or 7, remove the culture medium and place it in a tube or clean container. Measure the volume and extremely carefully add an equal volume of fresh OLBN to the flask. At this point (day 6 or 7), you should discontinue the Shh and RA.
- From now on, you may maintain these cells in OLBN and feed the cultures every 4th day. OLPs will still proliferate; numerous cells will generate from the mother clone and grow separately. Feed by adding OLBN for the next 4 days.You may start harvesting clones to be re-plated in cell culture containers for your studies, in which case you may re-plate the clone(s) on poly-D-lysine or Matrigel. Alternatively, you may re-plate the clones on anti-human PSA-CAM or anti- human IgM-coated flasks for their propagation. To continue propagating the cells use OLBN.
After 8 days, to continue instructing progenitors toward the OL fate, as well as propagating already specified OLPs, you may remove 1/2 of the volume of conditioned medium and add the same volume of OLBN. If the culture is too rich and the medium becomes orange within 3 days, you may add a larger volume of medium to maintain the medium red rather than orange. Cells will continue to give OLP clones in the original flask at least for 2 months. It is recommended that if the cultures are rich in clones, you pass the clones manually without bringing any flat cells, and re-plate them to either work with them or create a frozen stock (Note 6).
Step 3. Choosing and re-plating single clones
Materials
Poly-D-lysine-coated coverslips, flasks, 24 well plates, 4 well plates, or 16 well glass chambers
OLBN (to continue propagating OLPs) or GDM
IGF-1(88 to 100 µg/ml stock), use 1µl/ml of medium
T3 (20 µg/ml stock solution)
GDM culture medium
IGF-1(88 to 100 µg/ml stock)
T3 (20 µg/ml stock solution)
Procedures
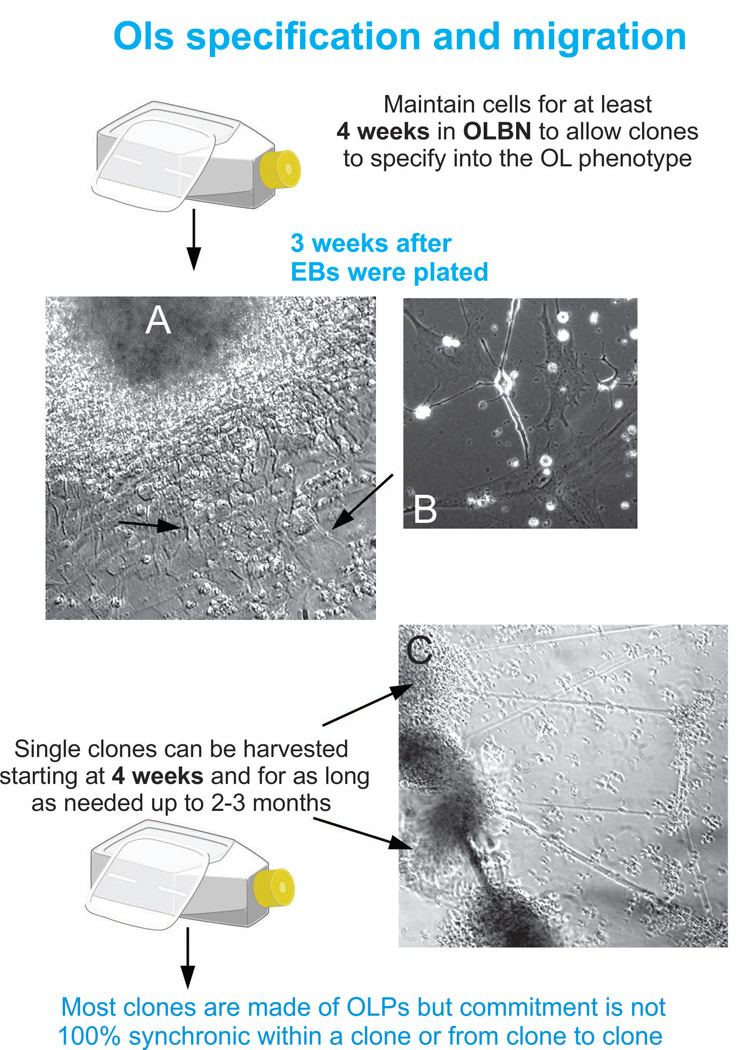
Pick up clones that are clear or partially clear as those shown in Figure 3C with a yellow tip, deposit them on the surface of the new container and let them sit for 5 to 10 min (the tip carries some medium with the clones so they won’t dry out) (Note 6).
As long as the mother flask is exposed to fresh OLBN, these committed progenitors will continue proliferating and new clones will grow as shown in Figure 4A. These cultures can be re-plated several times (Figures 4C, D and E).
- For these OLPs to move forward on the lineage, slowly add the culture medium GDM + IGF-1 (final concentration of 88 –100 ng/ml, freshly prepared) and let the culture sit for 7 to 10 min in the clean bench prior to transfer it to the incubator.In order to continue expanding the OL population at this stage, you will need to supplement the GDM with IGF-1 every third day at the same concentration, until the flask or well is confluent. Do this by removing 1/2 of the culture medium and add the same volume of fresh medium containing the full amount of IGF-1 to replenish the entire volume of culture medium. Alternatively, you may feed GDM + IGF-1 only once, and after 4 days you may add GDM with T3 at a final concentration 40 ng/ml triiodothyronine (Sigma-Aldrich, St. Louis, MO). A chart illustrating the steps and culture media that is instructive for pluripotent cells to neural and OL commitment is shown in Figure 5.
Clones should not be disrupted neither mechanically nor enzymatically as they cannot be dissociated without damaging many cells. Moreover, they would form rosary-like floating threads and therefore, they will not anchor properly on the substrate, and as a result they will not mature. Instead, fish the clone and seed it on the new cell culture container. The cells will slowly migrate out and still proliferate while being fed with fresh OLBN.
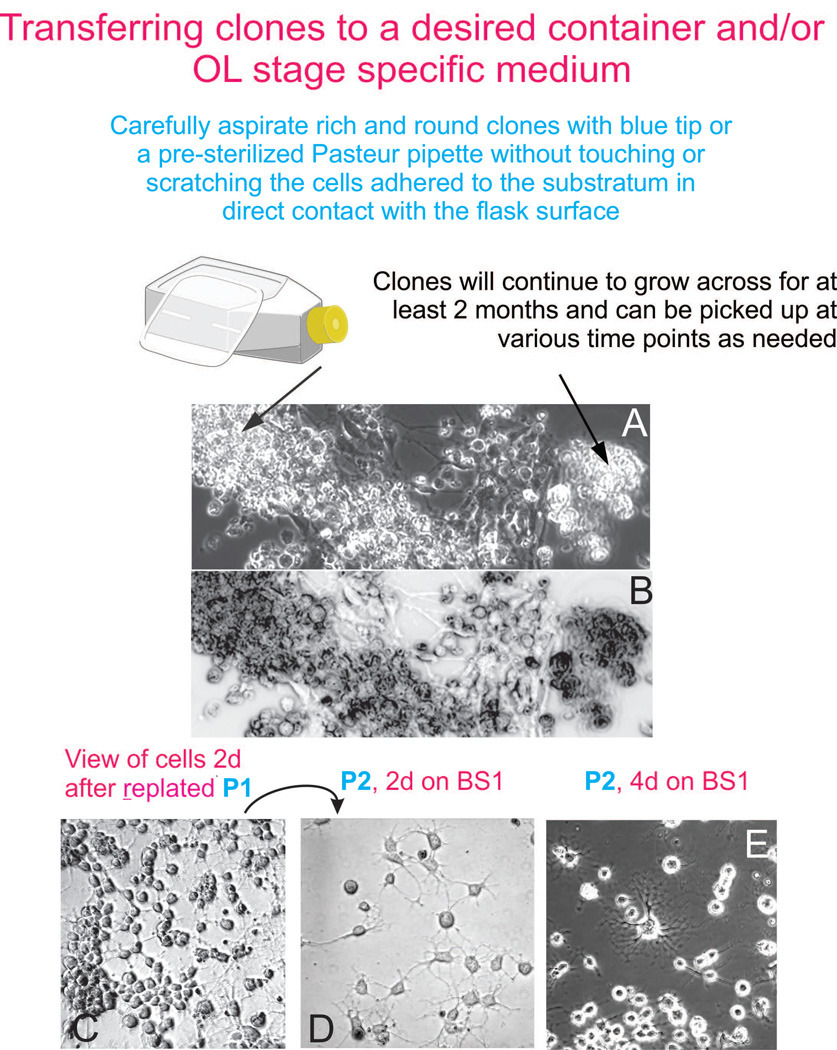
Figure 4.
Transferring Clones successfully. The main aspect to consider is not to scratch the substratum with the yellow tip, aspirating a clone carefully will allow flat cells to remain in the mother flask while bringing the entire clone of round, bright cells to the new cell culture container (panel A). OL will proliferate and arrange as small clones, as well as single cells in a very homogeneous looking culture (panel C). With this cell culture medium-based selection method, there is no need to use FACS to enrich the cultures in OLP cells, and therefore, the cells are healthy and yield large numbers. Cells can be re-plated (transferred to new culture containers) using the same stage specific medium, or the next stage specific medium such as BS1 (panel D). With time, cells tend to become multipolar (panel E).
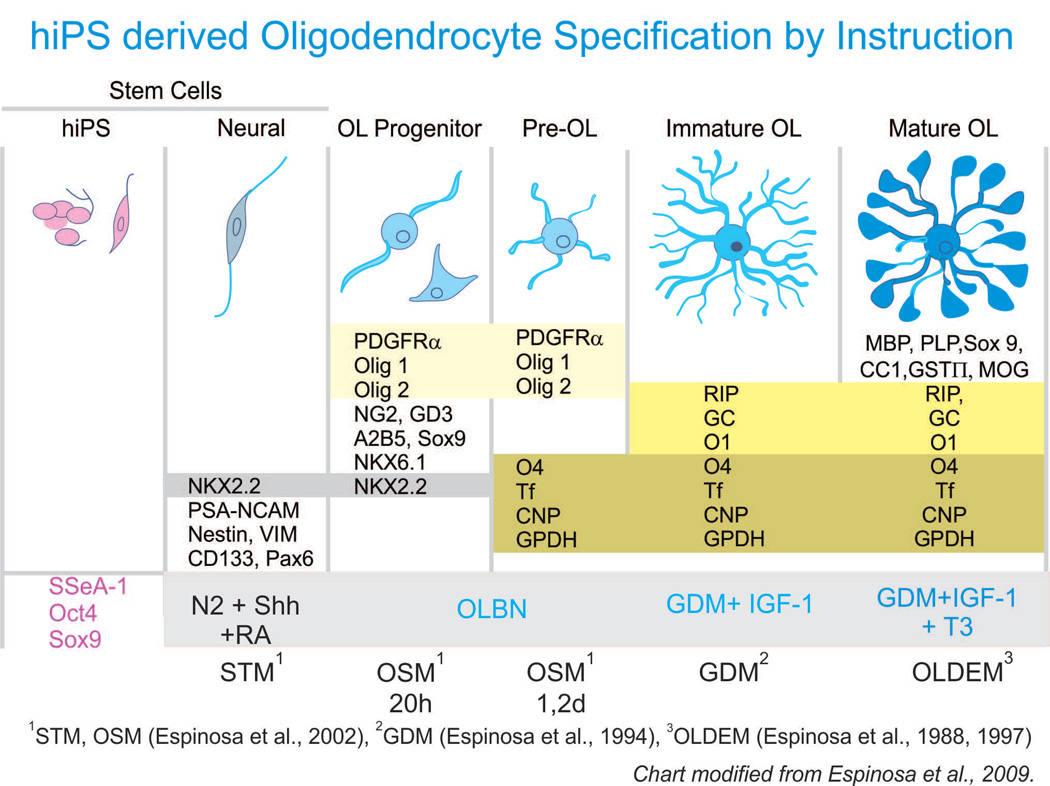
Figure 5. hiPS-derived OL specification by instruction through the specific culture medium.
Human pluripotent stem cells can be obtained from skin fibroblasts, by introducing the 4 transcription factors described by Takahashi and Yamanaka (2006). Then, the components of the culture medium direct and determine the fate of hiPS. Our culture medium previously designed to favor the OL phenotype when starting from neural stem cells (Espinosa et al., 2002), needed to be enriched with the specific molecules present during the development of the CNS to successfully direct human induced pluripotent stem cells to the neural and OL phenotype (see pink highlighted area). Once this transition has been accomplished, OL undergo sequential morphological changes from OLP and acquire characteristics inherent to a functional OL. A partial list of OL markers below each developmental stage is not exhaustive but represents frequently used markers to identify OLs and their specific developmental stage. The main difference between the culture medium for lineage progression and maintenance of hiPS-derived OL is that they need N2 while in the progenitor stage, and IGF-1 + T3 when a mature phenotype is desired. When starting cultures from brain-derived neural stem cells, we have previously described the neural stem cell medium (STM; Espinosa-Jeffrey et al., 2002); OL specification medium (OSM; Espinosa-Jeffrey et al., 2002); glia defined medium (GDM; Espinosa de los Monteros and de Vellis, 1996); OLDEM (Espinosa de los Monteros et al,1988, 1997). Chart Modified from (Espinosa-Jeffrey et al., 2009).
NOTE: Clones should not be disrupted neither mechanically nor enzymatically as they cannot be dissociated without damaging many cells. Moreover, they would form rosary-like floating threads and therefore, they will not anchor properly on the substrate, and as a result they will not mature. Instead, fish the clone and seed it on the new cell culture container. The cells will slowly migrate out and still proliferate while being fed with fresh OLBN.
Support Protocol 1
Immunopanning (alternative to Matrigel)
We developed the following method based on published work (Wysocki and Sato, 1978, and Williams and Gard, 1997). To isolate the rodent and human NSC population from other cell populations of the brain, we used anti-human PSA-NCAM IgM coated dishes during the initial plating. During primary derivation of human NSCs, this method has proven useful (Wakeman et al., 2009a). Here we propose to use IgM coating for the isolation and derivation of OL from hiPS embryoid bodies as an alternative approach to Matrigel.
Materials
Anti-human PSA-NCAM IgM
Bovine Serum Albumin (BSA)
Flasks (T-12.5 cm2, T-75 cm2), or Tissue Culture Flask T-115 (TPP) with recloseable lid
PBS
Procedures
Prepare the immunopanning cocktail by mixing: Tris (pH 9.5) + 1% BSA + 50g/ml anti-human PSA-NCAM.
Coat the bottom surface of the flask with anti-human PSA-NCAM mixture (4 to 5 ml per dish).
Incubate for 30 min at 37°C.
Wash flasks 3 times with PBS and once with PBS + 1% BSA just before using. Do not allow the flasks to dry.
Extra flasks may be covered with foil and stored at 4°C for up to 10 days.
Support Protocol 2
Cell Viability Assay
Cell viability can be determined with the SYTOX blue nucleic acid stain (Molecular Probes, Eugene, OR). Cells with compromised plasma membranes are labeled by SYTOX binding to nucleic acids and detected by fluorometry.
Materials
PBS
SYTOX blue nucleic acid stain
Tris-Buffered Saline (TBS)
Procedures
Harvest and wash cells with 1× TBS.
Incubate for 12 min in 1M SYTOX in PBS.
Remove the solution and wash the cells with 1× TBS, five times.
Determine the number of positive cells per random field and record as a percentage of the total number of cells.
Support Protocol 3
We recommend collecting cells for frozen stocks at low passage number. Human NSCs are cryopreserved using modified methods found elsewhere (Wakeman et al., 2009a). In addition, the method formerly described for rat and mouse NSCs (Espinosa et al., 2002) can also be used to stock human NSCs.
A) Freezing NSCs
Materials
Cryogenic slow-freezing chamber
Cryogenic freezing vials
HBSS
STM media
14-gauze needle
Procedures
Allow NSCs to grow to 70–90% confluency. Remove all of the cell culture medium, add 5 ml of HBSS to each Petri dish or 10 ml to T-75 flasks, and detach cells by gently scraping the culturing surface.
Centrifuge the cells at 45 xg for 8 min and resuspend in 3 ml of STM media.
Gently dissociate cells using a 14-gauge needle, pellet at 45 xg for 8 min in a centrifuge, and discard the supernatant.
Gently resuspend the pellet from one 100 mm Petri dish or T-75 flask in 1 ml of serum-free freezing medium.
Transfer the content to a 1.2 ml cryovial, and place the vial(s) in a cryogenic freezer container overnight for slow freezing.
Next day, place the vials in liquid nitrogen for long-term storage.
B) Thawing NSCs
Materials
Anti-human PSA-NCAM, anti-human whole IgM molecule or poly-D-lysine coated cell culture containers
CM and STM media
NSC Cryovial
Procedures
To “reanimate” NSCs, defrost cryovials quickly in a 37°C water bath, and transfer the content of the vial to a 2 ml tube containing 1 ml of STMc + CM at 37°C.
Centrifuge gently at 35 xg for 5–7 min.
Remove the supernatant, add fresh plating medium (STMc + CM) and remove a small aliquot to test the initial cell viability (as described above).
Count the number of viable cells in the tube (approx. 1 × 106 cells expected).
Plate cells onto anti PSA-NCAM-coated surface (Petri dishes or T-75 cm2 flasks, plate the equivalent of 1 vial/T-75 cm2 flask). If the yield is low, utilize T-25 cm2 flasks to increase the cell density necessary for healthy growth. Seeding low-density cultures in large containers decreases the proliferation rate and might be detrimental to the culture.
To propagate NSCs after replating, proceed as described in step 2 for “propagation and maintenance of NSCs”.
NOTE: If cells seem not to grow but look healthy, or if the culture medium is not red but rather purple, you will need to remove 1/2 of the plating medium and complete to 10 ml volume of the mixture (1/3 self-conditioned-STM + 2/3 of fresh STMc freshly prepared). If the opposite is true and the culture medium turns orange overnight, the cells have proliferated heavily, and you will need to replace the entire culture medium and seed more T-75 cm2 flasks (per three T-12.5 cm2).
NOTE: When propagating cells to create frozen stocks, we strongly recommend maintaining a “mother flask/dish” by scraping most, but not all of the cells attached to the flask. After removing the detached cells, feed the mother flask with fresh medium and CM (1:1) to ensure continuity of these cultures (in case re-plated cells do not look healthy, grow slowly or die).
BASIC PROTOCOL 2
Propagation of Rodent and Human Neural Stem Cells (NSCs) and derived OLs (Figure 6)
For details on the the isolation of NSCs from rodent and human brain please refer to Espinosa et al., 2009
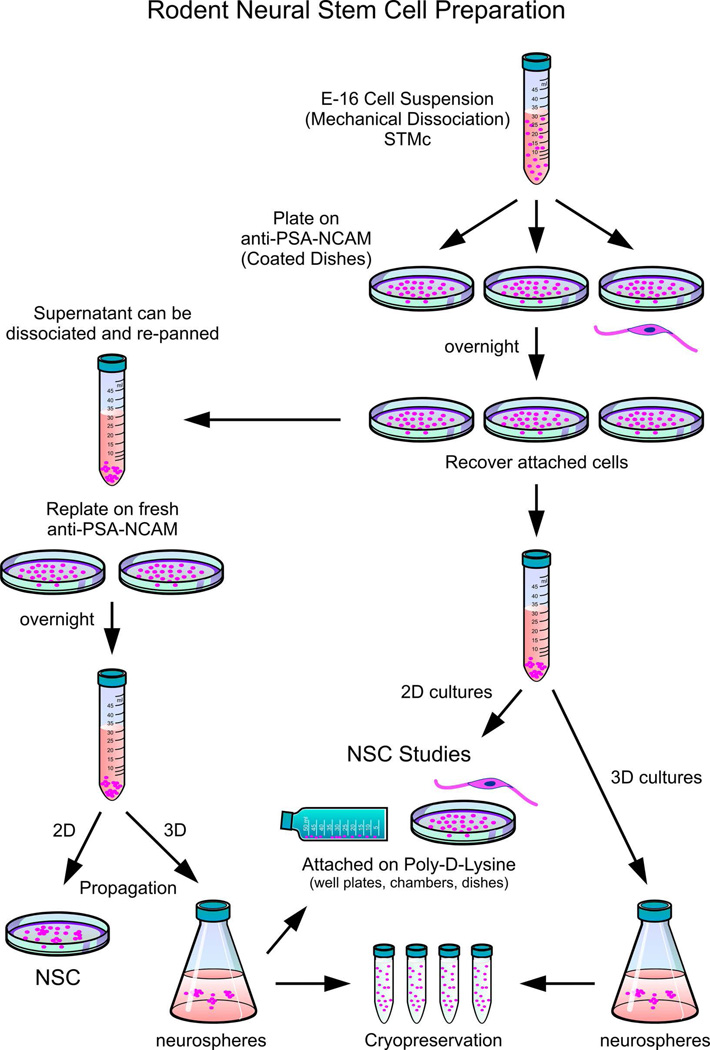
Figure 6. Rodent neural stem cell preparation.
Following dissection, the cell suspension is plated on anti-PSA-NCAM antibody–coated dishes and allowed to adhere. The process can be performed repeatedly to increase the numbers of neural stem cells, as two-dimensional cultures or three-dimensional “sphere” cultures (shown on the left side of the diagram). Every time cells are propagated, use anti-PSA-NCAM-coated dishes. Alternatively, cells can be propagated and immediately used for cell culture experiments (as shown on the right side of the diagram). While we prefer to use committed OL progenitors for cell transplants, other investigators also use uncommitted progenitors for grafting.
OL specification in 2D cultures (Figure 7)
During development, the nutritional and environmental needs of cells change as they lose multipotency and become lineage restricted. The present system is based on the modification of nutrients contained in the cell culture medium and the percentage of CO2 needed to optimize and direct lineage restriction towards the OL phenotype. Like NSCs, OLPs can be propagated in 2D and 3D cultures. When attached (2D cultures), OLPs grow faster, and thus ideal to create an OLP cell stock quickly before starting specific in vitro cell culture or in vivo transplantation studies. A diagram of the following steps can be found in Figure 7.
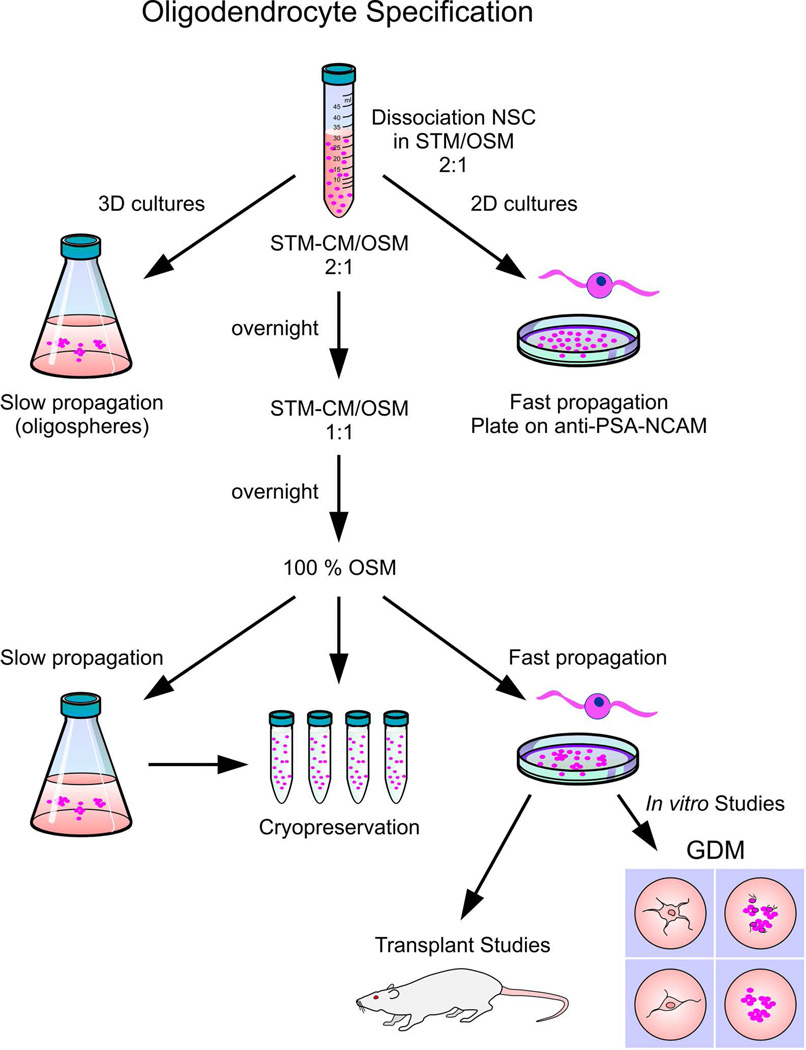
Figure 7.
OL specification by instruction. The transition from NSC to committed OL lineage is brief, but sequential rather than abrupt. In order for cells to survive, they must acclimate to their new environment. OLP can be propagated to create frozen stocks as three-dimensional “oligosphere” cultures (shown on the left of the diagram), or frozen without propagation (as shown in the sequence in the center of the diagram). OLP can also be propagated in two-dimensional cultures for cryopreservation, for specific cell culture experiments, or for cell replacement therapies (as shown on the right side of the diagram).
Materials
If cells seem not to grow but look healthy, or if the culture medium is not red but rather purple, you will need to remove 1/2 of the plating medium and complete to 10 ml volume of the mixture (1/3 self-conditioned-STM + 2/3 of fresh STMc freshly prepared). If the opposite is true and the culture medium turns orange overnight, the cells have proliferated heavily, and you will need to replace the entire culture medium and seed more T-75 cm2 flasks (per three T-12.5 cm2).
When propagating cells to create frozen stocks, we strongly recommend maintaining a “mother flask/dish” by scraping most, but not all of the cells attached to the flask. After removing the detached cells, feed the mother flask with fresh medium and CM (1:1) to ensure continuity of these cultures (in case re-plated cells do not look healthy, grow slowly or die).
BASIC PROTOCOL 3
Propagation of Rodent and Human Neural Stem Cells (NSCs) and derived OLs (Figure 6)
For details on the the isolation of NSCs from rodent and human brain please refer to (Espinosa et al., 2009)
OL specification in 2D cultures (Figure 7)
During development, the nutritional and environmental needs of cells change as they lose multipotency and become lineage restricted. The present system is based on the modification of nutrients contained in the cell culture medium and the percentage of CO2 needed to optimize and direct lineage restriction towards the OL phenotype. Like NSCs, OLPs can be propagated in 2D and 3D cultures. When attached (2D cultures), OLPs grow faster, and thus ideal to create an OLP cell stock quickly before starting specific in vitro cell culture or in vivo transplantation studies. A diagram of the following steps can be found in (Figure 7).
Materials
Media: OSM, GDM, OLDEM
Anti-human PSA-NCAM IgM coated plates
bFGF
Erlenmeyer (EM) flask
HBSS without Ca2+ or Mg2+
Petri dishes
14-gauge needle
15 µm sieve
24-well plate
4.5% CO2 incubator
Procedures
When NSCs reach confluency, remove the supernatant (CM), and add 5 ml of Hank's Buffered Salt Solution (HBSS) without Ca2+ or Mg2+.
Detach the cells with a cell scraper, transfer into a 15 ml tube (if you have 1 to 3 Petri dishes), rinse once with 2 ml of HBSS, and centrifuge at 45 xg for 8 min.
Resuspend the cell pellet in 3 ml OSM media and gently dissociate (3×) using a 14-gauge needle. Centrifuge at 45 xg to pellet the cells.
Resuspend the cells in fresh OSM + STM-CM and seed cells on anti-IgM coated dishes or flasks (use the same procedure as for anti-PSA-NCAM), and maintain the cells as described in Basic Protocol 1 but using OSM/self-CM (2:1) respectively. From this point on, the CO2 concentration in the incubator should remain at 4.5% (Note 9).
Feed the cells with 1/3 self-conditioned-OSM + 2/3 fresh OSM every other day until they reach 80–90% confluency. This process can be repeated several times to attain a large number of cells for freezing (if desired).
Alternatively, to grow OL spheres to create/enrich a frozen stock of OLP, place the equivalent of 2 mm2 (pellet size after dissociated and in suspension) in a 25 ml Erlenmeyer flask with 15 ml of OSM + self-CM (2:1). If the pellet is 4 mm2, use a 50 ml Erlenmeyer flask. Prepare the cell suspension and place in a total volume of 25 ml of OSM + self-CM (2:1). Feed OL-spheres with fresh OSM every other day by adding 3 ml of freshly prepared OSM (no CM). When spheres start to become larger than 2 mm, gently dissociate 1–2 times in the same flask with the 14-gauge needle using a 12 ml syringe (sterile).
When the culture medium starts to turn orange, recover and centrifuge the spheres, and split into more Erlenmeyer flasks. These may be used for experiments or cryopreserved as previously described (cryopreservation section).
We recommended pre-calibrating the percentage of CO2 one day before plating the cells. If the incubator is shared with other people or needed at 5% for NSC propagation and maintenance, we recommend using T-flasks for 2D cultures instead of Petri dishes. Close the cap completely and then open it 1/4 of a turn before placing in the incubator at 5% CO2. For propagation and maintenance of OL-spheres, the Erlenmeyer flask should also be kept open just enough to ensure O2 / CO2 exchange. When using 4.5% CO2, loosen the caps of the flasks until half-way open.
Support Protocol 4
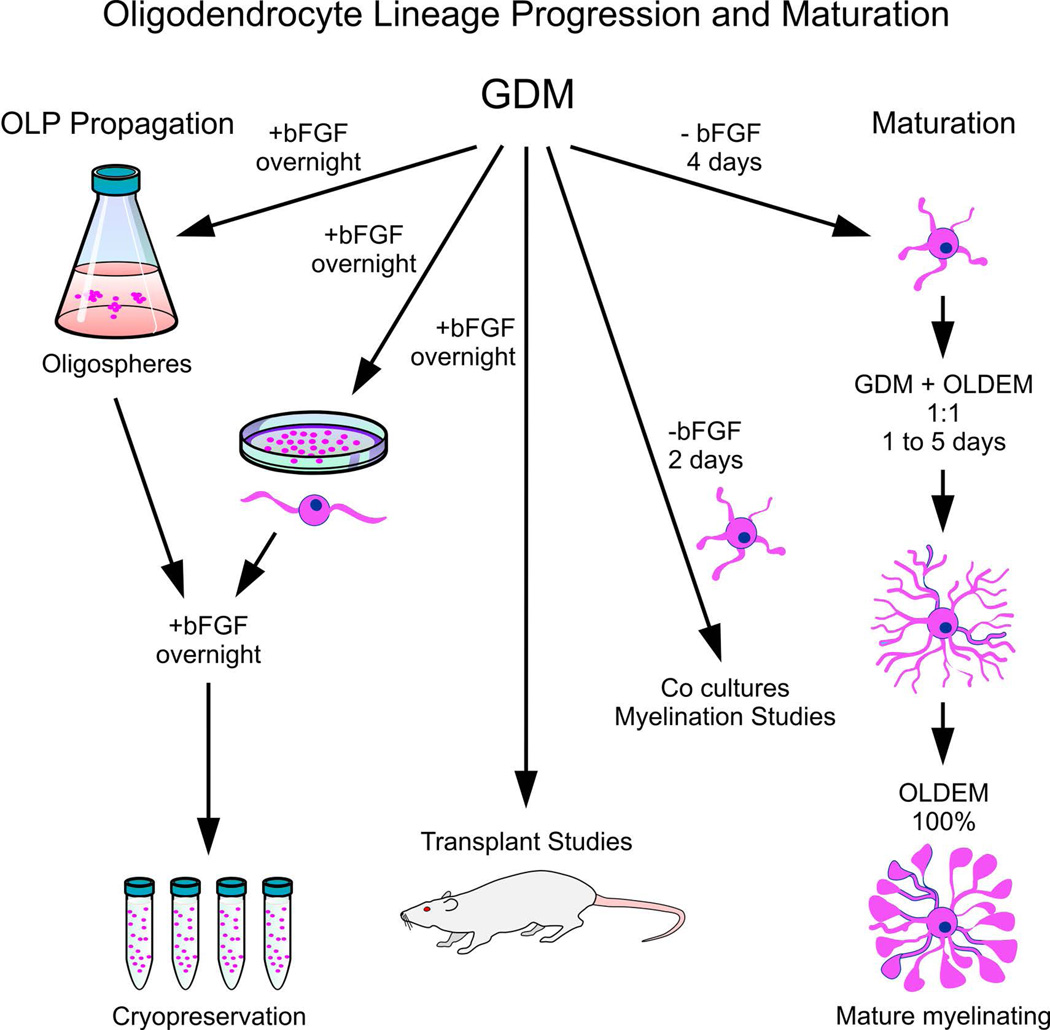
Oligodendrocyte lineage progression and maturation (Figure 8)
The nutritional needs for a committed cell within the OL lineage differ considerably as they progress and mature to the next developmental stage. These cells need to start synthesizing enzymes and proteins related to myelination, therefore, the energy demand is enormous compared to their earlier stage where migration and proliferation are the basic functions. The culture medium “GDM” (glial defined medium) was first designed to maintain 04+ GC+/−, CNP+/− cells (for details see Pre-OL in [Fig.1]). Later, we realized that GDM also induced the transition of OLP to pre-OL (Espinosa et al., 1997).
Figure 8.
OL lineage progression and maturation. After commitment of NSC to the OL lineage, cells are propagated at the OLP stage to create a frozen stock (steps indicated on the left portion of the flow chart) or processed further for transplantation studies (as shown by the middle arrow on the diagram). To allow OLP to further mature along the OL lineage and become myelinating cells, OLP cell cultures are transitioned into OLDEM for at least 48 h. Once OL have reached this stage of maturation, they are excellent for cell culture studies but are not recommended for cell grafting. Detachment from the substrate can damage the numerous delicate cell processes, therefore these cultures are no longer a quality source for cell transplants.
Materials
Media: OSM, GDM, OLDEM
bFGF
Petri dishes with cells
Poly-D-lysine coated wells/plates
24-well plate
Procedures
In order to obtain “pre-OL” (along the OL lineage), plate OLPs using OSM (as in Basic Protocol 2).
Next day, remove 1/2 of the plating medium (OSM) volume and add the same volume of GDM. As in previous steps, they may be propagated as OL-spheres (3D) or as 2D cultures on anti-IgM coated flasks, Petri dishes, or directly on cell culture grade plastic.
To obtain more OLP/pre-OL, cells are grown as 2D or 3D cultures in the presence of bFGF (Fig. 4). Cells will remain at the same stage as the parent cells by adding fresh GDM + [20 ng/ml] bFGF. For cell replacement therapies, we suggest using cells at this stage (1–2 days after plating without bFGF) as cells are still highly motile and readily migrate within the host post-natal and/or adult rodent brain and/or spinal cord.
To enhance maturation of cells into the next developmental stage, OL are cultured as 2D cultures in GDM for at least 2 days (if plated in GDM without bFGF), or 4 days (if plated in GDM + bFGF) without further bFGF supplementation (Figue 5).
After exposure to GDM, cells express myelin enzymes and proteins, and they display multipolar, branched cell processes, but not a myelin-like membrane. In addition, OL maintained in GDM for at least 4 days (without bFGF or any other factors) can be further induced to a fully mature myelinating stage.
To fully mature OL, plate as 2D cultures onto poly-D-lysine coated wells/plates or Petri dishes in GDM/OLDEM (OL maturation medium) 1:1 for 1–5 days, followed by 100% OLDEM thereafter (Figure 4).
Every 4 days, feed the cells by replacing all of the culture medium with fresh OLDEM (Note 10). The medium should look red, not orange. If it turns orange, add more medium while feeding the cells.
NOTE: These cells will express myelin enzyme levels comparable to those found in pure myelin within 5 days after having been introduced to 100% OLDEM. As they mature, cells will synthesize myelin-like membranes in vitro even in the absence of neurons. They can be maintained for various weeks if they are sub-confluent, however, if the culture becomes overcrowded, cells will deteriorate and die.
Preparation for Neurons and OLs Co-Cultures (Figure 9)
To perform myelination studies in vitro, it is recommended to start with OLs plated on plastic alone (rather than poly-D-lysine) and maintained in GDM for 2 days.
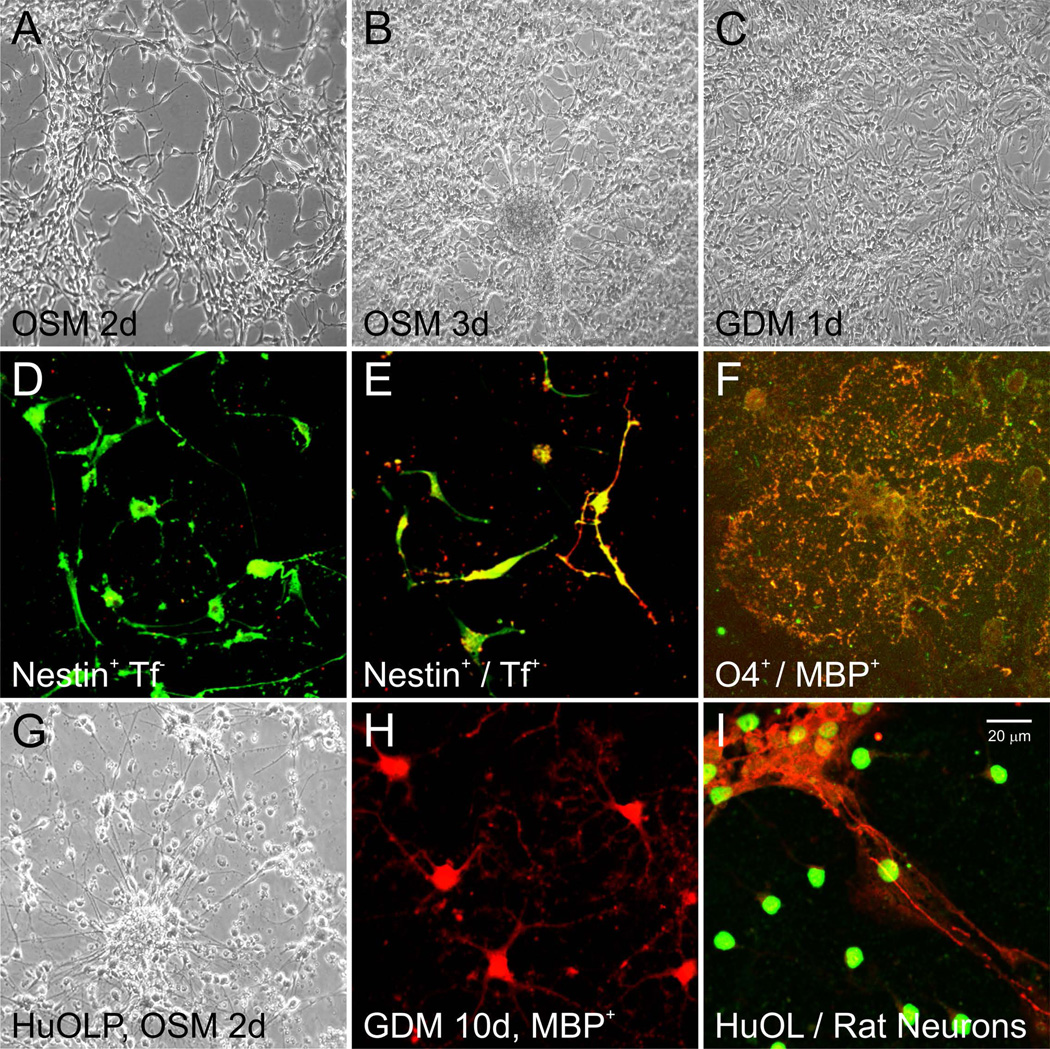
Figure 9.
Phase contrast view of neural stem cells derived from embryonic day 16 rat brain at passage number 2 (P2). NSC were plated and maintained in OSM for 2 days (A), 3 days (B), or 3 days in OSM then switched to GDM for 1 day (C). Cells still proliferate while in OSM. When cells from (A or B) are plated and maintained in OSM on poly-D-lysine-coated coverslips for 1 day, they start to display a bipolar or multipolar morphology (D), and most express the immature precursor marker, nestin (green) but not Tf (red), an early marker for OL. After 2 days in OSM, bipolar nestin+ cells co-express transferrin (Tf) (E). After 4 days in OSM, cells were switched to GDM for 1 day; they developed numerous cell processes and co-expressed sulfatides (recognized by the anti-O4 antibody, green) and myelin basic protein (MBP; red) (F). Panels G to I are human cells. (G) Phase-contrast view of human NSCs (HFB-2050) acclimated and expanded in STM, then replated and maintained in OSM for 2 days. (H) OL derived from human NSCs (HFB-2050) were specified to the OL lineage with OSM and maintained in GDM for 10 days. OL matured and started to express MBP (red). (I) Rat cortical neurons (NFM-200-red) were cultured for 10 days, then human OLP derived from NSC (HFB-2050) were added in co-culture for 24 hr. These cells were labeled with human nuclei marker (HuNu, green).
Materials
Media: GDM and OLDEM,
Cell Scraper
Neuronal Co-culture (See below)
25 µm sieve
24-well plates
Procedures
Detach cells with cell scraper and centrifuge at 45 xg for 8 minutes in the same culture medium.
Remove the supernatant and resuspend the cells in GDM-CM + fresh OLDEM (1:2).
A single cell suspension preparation is necessary for this step. Remove any cell clusters with a 25 µm sieve (as described previously).
Count the cells and adjust the cell suspension to approximately (200,000 cells/ml) of OLDEM medium.
Remove half the volume of culture medium from the neuronal cultures without disturbing the cells (cortical neurons or dorsal root ganglion cells).
Slowly add the OL suspension to one 24-well plate containing the neuronal cultures to complete the original total volume in each well.
Follow the co-cultures for at least 10 days. To feed, replace 1/2 CM with fresh OLDEM. If the cultures are not overcrowded, they can be kept for at least 4 weeks.
Support Protocol 5
Preparation of Cortical Neurons
Materials
Instruments, animals and materials are the same as described in Basic Protocol 1.
Animals: Time-pregnant (ED14–16) Sprague-Dawley rats (Charles-River).
Bovine Serum Albumin (BSA)
Conical tubes
Culture tubes with cap, Sterile. Fisher 17 × 100 mm
Hank’s Balanced Salt Solution (HBSS)
Hemacytometer
Isoflurane
Needle
Poly-D-lysine
Scissors
Sterile Surgical Gauze
230 µm and 140 µm sieves
24 or 12 well plates
Procedures
Prepare the work area and sterile tools in a biosafety hood (Diagram 1).
Euthanize the rats by isofluorane inhalation.
Extract the placenta containing embryos and place into STM Basal + 1% BSA.
Remove the cerebellum.
Dissect the brain of each embryo and place into Neurobasal-N complete (see Media Preparation).
Separate the cortex from the brain and remove the meninges with forceps.
Combine the cortical tissue of all the brains without meninges. Mechanically dissociate with the needle by gently aspirating the brain pieces and releasing the suspension slowly against the wall of the tube ten times (try to minimize foaming).
Recover the supernatant with the cells in suspension and transfer to a 15 ml tube.
Add 2 to 4 ml of Neurobasal-N medium to the chunks left over in the dissociation tube and dissociate again 5 to 8 times.
Filter the suspension of dissociated cells through 230 µm and 140 µm sieves to remove cell clusters.
Rinse the sieves sequentially with basal Neurobasal-N + 1% BSA at room temperature and add this medium to the tubes containing the cells.
Collect the cells by centrifugation in the culture tubes at 40 xg for 8 min.
Discard the supernatant very gently as the pellet is very loose.
Resuspend the pellet in 4 ml of fresh Neurobasal-N medium with a 5 ml pipette by gently triturating 2 or 3 times. Complete the volume to 12 ml (or the equivalent of 1 embryo/ml) with 2 parts of fresh medium and 1 part of conditioned medium.
Assess *cell viability, count cells using a hemacytometer, and plate onto poly-D-lysine coated well plates (Note 12).
Incubate plated cells at 37°C in 4.5% CO2/95% humidity and monitor with a Combustion Test Kit (Bacharach # 10–500) (most electronic panels do not give an accurate reading).
NOTE: Cortical neurons maintained in Neurobasal-N do not need to be fed as frequently as other cell types. Simply add 500 µl of Neurobasal-Nc every third day. Six days after plating, remove 1/4 of the culture medium and add the same volume of fresh Neurobasal-Nc.
NOTE: Cell viability can be determined with SYTOX as described in Cell Viability Assay in Basic Protocol 1.
Support Protocol 6
Transplantation of OL Progenitors into Neonatal Rats
Neural progenitor cells and their differentiated OL counterparts can be stereotaxically transplanted into the newborn developing rat brain relatively non-invasively as previously described (Snyder et al., 1997; Flax et al., 1998; Espinosa et al., 2002). Similar results can be obtained with variations on the transplant method that are more suitable depending on the needs of the host brain and the type of study (Yandava et al., 1999; Ourednik et al., 2001, 2002; Park et al., 2002a; Teng et al., 2002; Wakeman et al., 2006; Redmond et al., 2007). A selection of detailed protocols for neonatal and adult mouse transplantation are described elsewhere (Espinosa et al., 1992; 1993a,b; Lee, 2008; Wakeman et al., 2009a,b; Yan et al., 2004). Upon implantation in the lateral ventricles, donor cells engraft and migrate from the subventricular zone into the host RMS in much the same manner as host NSC.
Materials: (may vary depending on the grafting method of choice)
Aspirator tube assembly (Sigma, A5177–5EA)
Borosilicate glass tube (Sutter Instrument, B100–75-15)
DPBS
Transillumination light source
Microfuge tube with cell sample
Micropipette puller (Sutter Instrument Co., Model P-87)
Neonatal rat (P0–P5)
Warming pad
Warm Water Glove Balloon
Wet Ice
Procedures
Anesthetize the rat pup until the animal no longer retains locomotion or responds to gentle toe and tail pinch. Carefully monitor the pup and proceed to transplantation.
Insert a calibrated, drawn borosilicate glass micropipette into the aspirator tube assembly and rinse the micropipette by drawing and expelling 70% Et-OH five times followed by sterile DPBS ten times to clean the needle.
Gently flick sample in microcentrifuge tube prior to filling the needle, wipe the tube with 70% Et-OH, and uncap the tube.
Slowly draw 4–5 µl cell suspension into the micropipette.
Loosely secure the head of the anesthetized pup and place directly over the light source to visualize the eyes and bregma.
Carefully insert the glass needle into the head at the midline between eye and bregma and slowly inject 2–5 µl cell suspension at 5×104 cells/µl into both lateral ventricles. Slowly remove the needle and check for leakage through needle tract. Repeat step 6 into the contralateral hemisphere.
After the injection, warm the pup by placing on a warm water balloon glove or heating pad to increase the body temperature before returning to the mother.
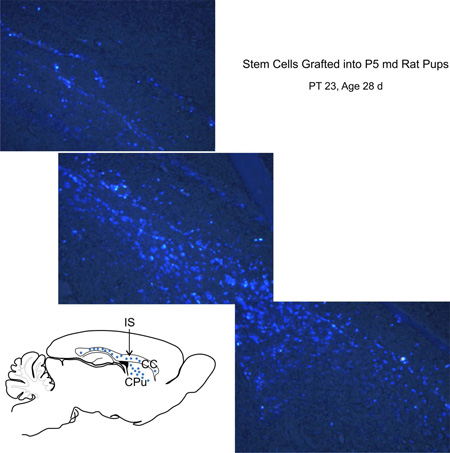
NOTE: In addition to the lateral ventricles, NSC can also be transplanted into the striatum (SN) (Bjugstad et al., 2005, Redmond et al., 2007, and Bjugstad et al., 2008) and corpus callosum (CC). Upon implantation into the CC of the host, HFB-2050 donor cells recognized by the fluorescent Fast Blue (FB) label migrated along the CC and into the CPu (Figure 10). Pre-committed-OL can also be placed locally within focal sites of injury to decrease the need for extensive migration.
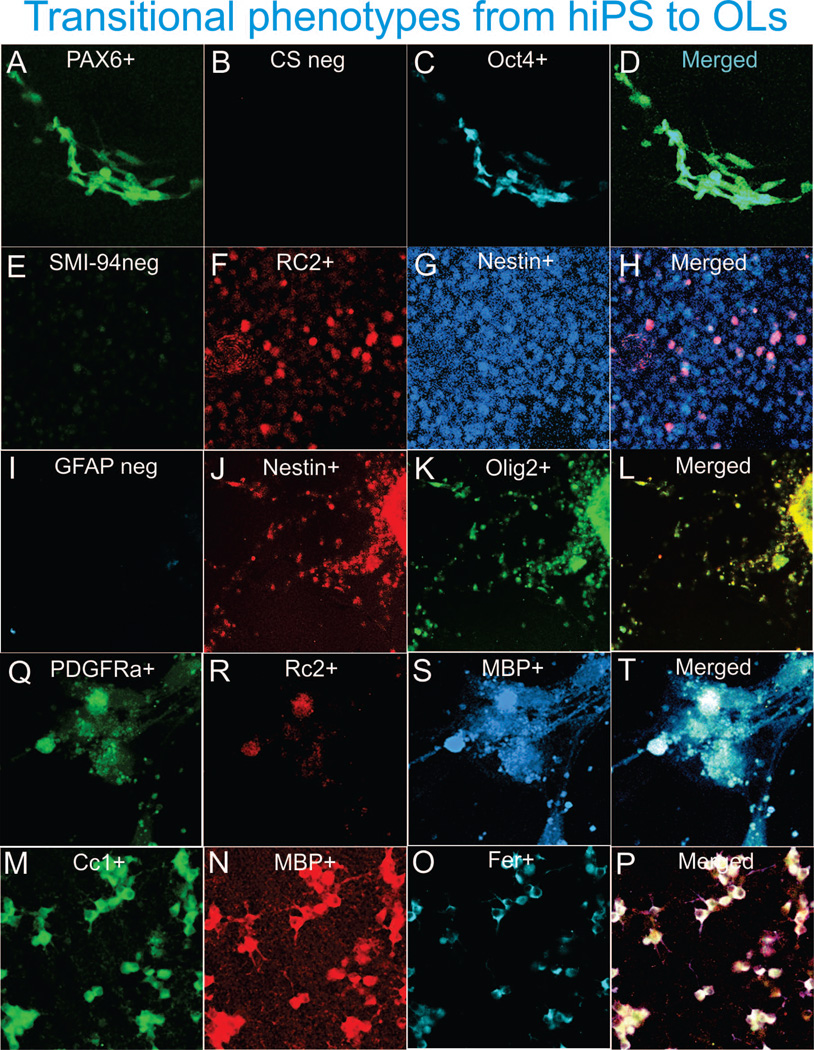
Figure 10. hiPS derived OL express neural progenitor and OL cell lineage and stage specific markers recapitulating their development in vivo.
Two days after EB’s were plated cells express  and
and
 (panels A–D;
developmental stage corresponding to
Figure 2
panel B). By day 6 (panels F–H) expression
of
(panels A–D;
developmental stage corresponding to
Figure 2
panel B). By day 6 (panels F–H) expression
of  a marker for radial glia (F) at different
intensity could be observed with a concomitant expression of
a marker for radial glia (F) at different
intensity could be observed with a concomitant expression of  (H) by virtually all cells (panel G), and not all nestin +
cells bared the RC2 staining (stage corresponding to Figure 2 panel C). The co-expression of
(H) by virtually all cells (panel G), and not all nestin +
cells bared the RC2 staining (stage corresponding to Figure 2 panel C). The co-expression of  and
and
 can be observed within the next three weeks
(panels J–L) most nestin expressing cells co-express
Olig2 (panel L; developmental stage corresponding to
Figure 2
panel C). When clones are harvested 4 weeks after starting
their fate specification and re-plated on poly-D-Lysine in BS1 and allowed to
become mature i.e. 6 days after seeding (panels M–P) cells
expressing faintly
can be observed within the next three weeks
(panels J–L) most nestin expressing cells co-express
Olig2 (panel L; developmental stage corresponding to
Figure 2
panel C). When clones are harvested 4 weeks after starting
their fate specification and re-plated on poly-D-Lysine in BS1 and allowed to
become mature i.e. 6 days after seeding (panels M–P) cells
expressing faintly  (M) in their extended membrane and a few cells
still bearing it in their cell body, they appear to be losing the RC2 staining
(N) while intensely expressing
(M) in their extended membrane and a few cells
still bearing it in their cell body, they appear to be losing the RC2 staining
(N) while intensely expressing  in
the cell body as well as, in their extended membrane sheath (O) the
colocalization of markers is shown in panel (P). OLs in these cultures also
express intensely in their cell body and immediate processes the mature markers
in
the cell body as well as, in their extended membrane sheath (O) the
colocalization of markers is shown in panel (P). OLs in these cultures also
express intensely in their cell body and immediate processes the mature markers
 and
and  as
well as
as
well as  (panels Q–R). Panel B shows control
serum for Alexa-fluor 408,540 and 633. Panel I shows immunoreactivity for
(panels Q–R). Panel B shows control
serum for Alexa-fluor 408,540 and 633. Panel I shows immunoreactivity for
 is absent from these cultures.
is absent from these cultures.
Media Preparation
Mouse Embryonic Fibroblast Medium (MEF medium)
Add to 450 ml of DMEM (high glucose with L-glutamine; Sigma cat # 51441C) 50 ml of fetal bovine serum (FBS) and 2.5 ml of penicillin (stock 10,000 U/ml)/streptomycin (10 mg/ml) (Pen/Strep). The medium can be stored at +4°C for up to 2 weeks.
Embryoid Body Medium (EB medium)
Prepare 400 ml of Knock Out-DMEM (KO-DMEM, Life Technologies, cat # 10828-018) + 70 ml of Knock Out Serum Replacement (KOSR, Life Technologies, cat # 10828-028) + 5 ml of 200 mM glutamine + 5 ml of 100× Non-Essential Aminoacids (NEAA) + 2 ml of Insulin-Transferrin-Selenite (ITS) + 40 µl of 1M β-mercaptoethanol (βME) + 2.5 ml of penicillin (stock 10,000 U/ml)/streptomycin (10 mg/ml) (Pen/Strep).
Store EB media at +4°C for up to 2 weeks.
Pluripotent Stem Cell Medium (PS medium)
Add 1.2 ml of 2µg/ml stock of bFGF to 480 ml of EB medium (5 ng bFGF/ml).
Store PS medium at +4°C for up to 1 week.
N2 Basal Medium (N2 medium)
Add to 500 ml of DMEM/F12, 5 ml of N2 supplement (stock 100×), 5 ml of 200 mM L-glutamine, and 5 ml of Pen/Strep.
Store N2 media at +4°C for up to 2 weeks.
Basal Stem Cell Medium (*STM-II; for rat and human NSC)
One liter of D-MEM, SKU # 11054-020 (low glucose, without glutamine, without sodium pyruvate) + 1 liter of Neurobasal medium without normocin, heparin, Vit A or LIF + the following additives:
| Insulin | 5 mg |
| Transferrin | 50mg |
| Putrescine | 16.1mg |
| Progesterone | 20nM |
| Sodium Selenium | 4µg 0.4mg/ml (4ml/litter of medium) |
| Sodium Bicarbonate | 2.2g |
| Penicillin-Streptomycin | 1 ml |
| Kanamycin | 1 ml |
Complete STM (STMc) medium for plating, maintenance and propagation of NSCs
Just before feeding the cells, prepare the desired volume of STM complete by mixing Basal STM and the following supplements to reach the final concentrations as indicated: B-27 (1:50), recombinant Human EGF [20 ng/ml], recombinant human bFGF [20 ng/ml], and Creatine [3.8mg/L].
NOTE: STM-II is a variation of the original STM medium we previously described (Espinosa et al., 2002); UCLA case # 2002-475, which formula is available upon MTA. STM-II yields results comparable to those obtained with STM.
“OSM-II” OL specification medium
Formerly named OTM; (Espinosa et al., 2002); OSM-II derives from STM-II. Add STM-II complete freshly prepared to GDM 1:1 v/v.
“GDM”(Glia defined medium) (Espinosa et al., 1988; 1997)
*One liter of double distilled water + one package D-MEM/F12 medium (High glucose) + the following additives:
| Insulin | 5 mg | |
| Transferrin | 50 mg | |
| Putrescine | 16.1 mg | |
| Sodium Bicarbonate | 2.2 g | |
| D(+) galactose | 4.6 g | |
| **Sodium Selenite | 8µg Kanamycin | 1 ml |
| *Adjust the pH to 7.4 (after filtering) | ||
| ** Prepare a “stock solution” 0.8mg/ml in PBS. Add 8 µl of the stock solution for 1 liter. | ||
Neurobasal-B27 “NB-B27” (Human Neural Stem Cell Proliferation Medium)
| Neurobasal medium | 97% |
| B-27 w/o Vitamin A | 2% |
| GlutaMAX | 1% |
| Normocin (optional) | 0.2% |
| Heparin | [8 µg/mL] |
| bFGF | [20 ng/mL] |
| LIF | [10 ng/mL] |
“Neurobasal-N” medium for cortical neurons
| Neurobasal medium | 1 L |
| Insulin | 5 mg/L |
| Transferrin | 50mg/L |
| Progesterone | 20nM * |
| Sodium Selenite | 8µl ** |
| Sodium Bicarbonate | 2.2g |
| Kanamycin | 1 ml |
---These additives should be added just before using Neurobasal-N to make complete “Neurobasal-Nc”
| B-27 with vit. A | 1:50 |
| Recombinant Human bFGF | [20ng/ml] |
| Creatine | 4mg/ml |
COMMENTARY
Background
The culturing system allows for the production of relatively homogeneous primary OL cultures in adequate numbers for cryopreservation. These cell stocks can be used for basic research in further in vitro studies. Moreover, these cells are never exposed to animal or human sera, and therefore remain as suitable candidates for cell replacement therapies in developmental disorders of the central nervous system (CNS) as well as neurodegenerative diseases.
Numerous methods and culture media described in the literature (even before, the times of NSC), were the basis for the optimization of the culture media formulations described here (some examples are, Botenstein and Sato, 1979; Saneto and de Vellis, 1985; Espinosa et al., 1988; Yang et al., 2005, Larsen et al., 2008. etc). Undoubtedly, all previous reports on how to obtain and culture OL derived from NSC have also been instrumental in designing the present protocol. For example, the group of Lachapelle, and Baron-Van Evercooren described floating oligospheres derived from newborn rat brain (1996). This concept has been applied to NSC to generate OLs by Zhang et al, 1998, Espinosa et al 2002, and in the present protocol. Zhang and coworkers (1998) described the use of B104 neuroblastoma cell-conditioned medium (B104CM) to induce the oligodendrocyte phenotype on neurospheres and induce proliferation. This approach provides OL for many kinds of studies, but they are unsuitable as donor cells for cell replacement therapies to be used in translational studies, having used uncharacterized conditioned medium from B104 cells that have been grown in the presence of fetal bovine serum (as originally described by Louis et al., 1992). An example on the use of the protocols described can be found in Chattopadhyay et al., 2008.
Critical Parameters
We want to emphasize that fate restriction towards commitment from NSC to OLP (as defined in “Basic Protocol 2”) becomes irreversible after NSCs have been in OSM for at least 20 hours (2D and 3D cultures). Therefore, the progeny of these cells will define a homogeneous OLP population, ideal for biochemical, toxicological and pharmacological studies, as well as an appropriate and reproducible source of committed cells to be used in cell therapy studies. Phenotype reversal of induced OLPs may be possible with genetic manipulation but we have not attempted such studies to date.
HuNSC-derived OLs and hiPS-derived OLs are more sensitive to CO2 variations than rodent and primate non-human OLs. Thus monitoring of your incubator with Fyrite is essential for the yield of homogeneous populations of OLs and disfavor of astrocytes and fibroblasts.
Always monitor the concentration of CO2 with a Combustion Test Kit (Bacharach # 10–500), as most electronic panels do not provide an accurate reading. The proper lineage progression relies on precise control of CO2 to maintain a pH that should remain accurate and controlled.
Troubleshooting
Human NSC are more fragile than their rodent counterparts, therefore, we recommend dissociation protocols that favor as little mechanical stress as possible. In our hands, enzymatic dissociation with 2–4 ml Accutase (Millipore) at 37°C for 3–5 minutes or light mechanical trituration through a 14-gauze needle (3–5 times) is sufficient to dissociate hNSC into single cells and small 2–6 cell clusters. Detailed methodology can be found elsewhere (Wakeman et al., 2009a,b).
If the EBs are exposed to 4°C while in suspension, IgM will make them clump in a virtually irreversible manner. In this case place the EBs in a 60 mm Petri dish and use curved scissors for eye surgery to cut the clumps in smaller pieces. Then resuspend EBs without dissociation and plate them as indicated in Figure 1 on matrigel rather than poly-D-lysine.
Anticipated Results
OLPs obtained by the isolation of NSC and their subsequent commitment to OLs utilizing this system are plated on anti-PSA-NCAM plates and will attain a bipolar morphology if maintained in freshly supplemented OSM. Cells can also be plated directly onto plastic (tissue culture grade). The morphology may look more flattened or “fibroblast-like”, but if maintained in fresh OSM, the early markers such as Olig2, Tf, PDGF-R and NG2 will be expressed. At this stage, cells are still highly motile but will migrate less if plated onto poly-D-lysine. During this time, cells attain a more mature phenotype that truly represents their in vivo counterparts.
Each of our culture media formulations as previously described (Espinosa et al., 2002; 2009), have proven to be effective for the isolation, commitment and maintenance of NSCs and OLPs because they include the minimum and sufficient nutrients to support a given developmental stage in this lineage. Cultures at a given developmental stage could virtually be kept indefinitely. Nonetheless this is not possible only because the substrata used to coat the containers to enhance cell adhesion and well being for the cells, decays with time and therefore, cells cannot be kept indefinitely in these conditions. Because the substratum dictates the organization of the molecules on the cell membrane and the subsequent signal transduction chain of events, the substratum of choice to isolate or to study the cells may yield slightly different results in terms of yield (i.e. OL numbers) (rev. Linnemann and Bock, 1989; Mauro et al., 1994).
Lastly, the needs and dynamics of a totipotent stem cell such as hiPS are much more numerous than a lineage restricted cell such as NSCs. The pace at which they develop is also much faster and therefore, lineage restriction needs to be aggressively, yet methodically performed to keep that pace at which these cells wish to develop. Keeping the finesse in the stoichiometry between reactants and products involved in lineage restriction is of the essence hence, the modifications performed to our chemically defined cell culture media to meet the requirements of hiPS to commit to the neural and OL phenotype. We believe that the levels of nutrients used in the current protocol have met the pace at which these cells are expected to develop.
Time Considerations
The initial dissection and preparation of the primary cell suspension takes approximately 2 h. From the moment cells are plated on anti-PSA-NCAM (if fed regularly with fresh humoral factors), 100 mm dishes can be confluent within 3–4 days. Thus, generating 20 vials of rat NSCs for cryostorage would take approximately 16 days. The generation of OLP from rNSC takes approximately 24 h; yet, generating OLP in high numbers (15 vials) for storage would take 4–6 weeks. Lineage progression of rat OL towards more mature phenotypes takes approximately 48 h in the specific culture medium (GDM or OLDEM). In addition, OL will still proliferate in GDM but at a much slower rate. Both, GDM and OLDEM media are favorable to protein synthesis but less favorable for cell proliferation.
Previously isolated ES cells and their NSC derivatives will need a longer period of time (approx. five times longer) to provide high numbers of NSC for frozen stocks. This time will vary depending on the origin of the sample. We have had similar success directing NSC from several species, utilizing the same chemically defined media; however, incubation times may need to be increased for full maturation in higher order mammals, such as primates. Induced cells loose NSC characteristics and acquire OLP features within 72 h, yet their cell cycle is much slower and therefore, it would be necessary to propagate these cells 8 to 10 weeks to be able to create a healthy stock (6 to 8 vials) of human (Hu) OLP. Previously established NSC lines (Snyder et al., 1992) can also be propagated and specified into the OL phenotype using the system described here.
As for the time needed to obtain adequate numbers of human OLs from hiPS now that we have standardized and optimized the culture system described here, it takes us around 8 weeks to obtain enough OLs derived from human embryonic brain using the method described in Figure 6 and 10. This is because cells need either to be isolated, propagated and then committed to OLs, or isolated and specified to the OL lineage and then propagated to obtain the desired yield. Both methods offer advantages in terms of the type of studies to be performed, i.e. disease models, or cell replacement therapies where the method of choice might be using hiPS as the starting material for transplantation. These cells may also be used in global gene and cell replacement strategies (Rev: Park 2002b)
Figure 11.
Human OL derived from (HFB-2050) human fetal NSC were labeled with fluorescent fast blue (FB; Sigma, cat. no. F-5756). A total of 60,000 cells were grafted into the corpus callosum (CC) of P(5) rat pups born to a myelin-deficient (md) carrier mother. At 23 days after grafting, samples were harvested and examined. Grafted NSC survived and migrated extensively within the host brain parenchyma extending along the corpus callosum (CC) and caudate putamen (CPu). In the sketch, dots represent the location where FB+ cells were found. The sketch represents a sagittal view of the transplanted rat brain at 28 days of age, IS indicates where cells were implanted.
Acknowledgments
We thank Dr. W. Lowry for hiPS21 and 23. This work was supported in part by NIH HD06576 and by a Pilot Grant from the National Multiple Sclerosis Society PP1498 (de Vellis/Espinosa-Jeffrey).
Abbreviations
- Oct4
octamer binding transcription factor 4
- DNA
deoxyribonucleic acid
- MS
Multiple Sclerosis
- ROCK
Rho-associated protein kinase
- SB431542
TGFbeta/Smad Inhibitor
- O4
anti-sulfatides monoclonal antibody
- Shh
sonic hedgehog
- RA
retinoic acid
- bFGF
basic fibroblast growth factor
- EBs
embryoid bodies
- MEF medium
Mouse embryonic fibroblast expansion and freezing medium
- PS medium
- N2
A suplement containing: Recombinant human insulin Human transferrin (iron-saturated) Sodium selenite, Putrescine, Progesterone
- EDTA
Ethylenediaminetetraacetic acid
- KOSR
Knock Out Serum Replacement
- KO-DMEM
DMEM supplemented with KO
- IgM
Immunoglobulin M
- GDM
glial defined culture medium
- DMSO
Dimethyl Sulfoxide
- OLSBN
OLS medium +B27 +N2
- nM
nano mOLsar
- pOLsy-D-lysine
Multi-pOLsymer of lysine
- PSA-NCAM
pOLsysialic acid neural cell adhesion mOLsecule
- PSA-NCAM IgM
ant-PSA-NCAM IgM
- IGF-1
insulin growth factor 1
- T3
Triiodothyronine
- SYTOX
- STM
stem cell medium
- STMc
stem cell medium supplemented with additives
- Basal STM
stem medium without supplements
- MTA
material transfer agreement
- STM-II
stem culture medium alternative formula
- OLSDEM
OLsigodendrocyte defined medium
- STM-CM
stem conditioned medium
- self-CM
self-conditioned medium
- 2-D cultures
bidimensional cultures
- GC+/−
galactocerebrosides positive or negative
- CNP+/−
2',3'-Cyclic-nucleotide 3'-phosphodiesterase positive or negative
- ED14–16
embryonic day 14 to 15
- CM
conditioned medium
- RCF
relative centrifugal force
- RMS
rostral migratory stream
- P0–P5
Postnatal day 0 to 5
- dPBS
distilled phosphate buffer
- Et-OH
ethylic alcohOLs
- SN
supernatant
- FBS
Fetal Bovine Serum
- EB medium
Embryoid Body Medium
- NEAA
Non-Essential Amionacids
- ITS
Insulin-Transferrin-Selenite
- D-MEM
Dulbecco minimal essential medium
- CNS
Central Nervous System
- B104CM
B104 neuroblastoma cell-conditioned medium
- hNSC
- Tf
transferrin
- PDGF-R
Platelet-derived growth factor
- NG2
neural/glial antigen 2
- rNSC
rodent neural stem cell
- Hu OLSP
uman OLsigodendrocytes
- FACS
fluorescent activated flow sorting
- MBP
myelin basic protein
- NF-200
- HuNu
Human Nuclei marker
- PAX6
Paired box protein Pax-6
- RC2
anti-radial glia antibody
- CC1
also known as APC anti-adenomatous pOLsyposis cOLsi protein
- GFAP
glial fibrillary acidic protein
- md
myelin-deficient
- FB+
fluorescent fast blue positive
- IS
implant site
Footnotes
Conflict of Interest Statement:
The authors acknowledge no conflict of interest
Literature Cited
- Ahn SM, Byun K, Kim D, Lee K, Yoo JS, Kim SU, Jho EH, Simpson RJ, Lee B. Olig2-induced neural stem cell differentiation involves downregulation of Wnt signaling and induction of Dickkopf-1 expression. PLoS ONE. 2008;3(12):e3917. doi: 10.1371/journal.pone.0003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancotti JC, Lavon N. Derivation, expansion, and characterization of human embryonic stem cell lines from aneuploid embryos. Methods in Molecular Biology (Clifton, N.J.) 2012;873:163–178. doi: 10.1007/978-1-61779-794-1_10. [DOI] [PubMed] [Google Scholar]
- Bjugstad KB, Redmond DE, Jr, Teng YD, Elsworth JD, Roth RH, Blanchard BC, Snyder EY, Sladek JR., Jr Neural stem cells implanted into MPTP-treated monkeys increase the size of endogenous tyrosine hydroxylase-positive cells found in the striatum: a return to control measures. Cell Transplant. 2005;14(4):183–192. doi: 10.3727/000000005783983098. [DOI] [PubMed] [Google Scholar]
- Bjugstad KB, Teng YD, Redmond DE, Jr, Elsworth JD, Roth RH, Cornelius SK, Snyder EY, Sladek JR., Jr Human neural stem cells migrate along the nigrostriatal pathway in a primate model of Parkinson's disease. Exp. Neurol. 2008;211(2):362–369. doi: 10.1016/j.expneurol.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc. Natl. Acad Sci. U.S.A. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay N, Espinosa-Jeffrey A, Tfelt-Hansen J, Yano S, Bandyopadhyay S, Brown EM, de Vellis J. Calcium receptor expression and function in oligodendrocyte commitment and lineage progression: potential impact on reduced myelin basic protein in CaR-null mice. J. Neurosci. Res. 2008;86(10):2159–2167. doi: 10.1002/jnr.21662. [DOI] [PubMed] [Google Scholar]
- De Filippis L, Lamorte G, Snyder EY, Malgaroli A, Vescovi AL. A novel, immortal, and multipotent human neural stem cell line generating functional neurons and oligodendrocytes. Stem Cells. 2007;25(9):2312–2321. doi: 10.1634/stemcells.2007-0040. [DOI] [PubMed] [Google Scholar]
- Douvaras P, JingWang MZ, Hanchuk S, O’Bara MA, Sadiq S, Sim FJ, Goldman J, Fossati V. Efficient Generation of Myelinating Oligodendrocytes from Primary Progressive Multiple Sclerosis Patients by Induced Pluripotent Stem Cells. Stem Cell Reports. 2014;3:250–259. doi: 10.1016/j.stemcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa de los Monteros A, Roussel G, Neskovic NM, Nussbaum JL. A chemically defined medium for the culture of mature oligodendrocytes. J. Neurosci. Res. 1988;19(2):202–211. doi: 10.1002/jnr.490190205. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros A, Zhang M, Gordon M, Aymie M, de Vellis J. Transplantation of Cultured Premyelinating Oligodendrocytes into Normal and Myelin-Deficient Rat Brain. Dev. Neurosci. 1992;14(2):98–104. doi: 10.1159/000111653. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros A, Zhang M-S, de Vellis J. O2A Progenitor Cells Transplanted into the Neonatal Rat Brain Develop Into Oligodendrocytes but not Astrocytes. Proc. Natl. Acad. Sci. 1993a;90(1):50–54. doi: 10.1073/pnas.90.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa de los Monteros A, Bernard R, Tiller B, Rouget P, de Vellis J. Grafting of fast blue labeled glial cells into neonatal rat brain: differential survival and migration among cell types. Int. J. of Dev. Neurosci. 1993b;11(5):625–639. doi: 10.1016/0736-5748(93)90051-e. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros A, Yuan J, McCartney D, Madrid BR, Cole R, Kanfer JN, de Vellis J. Acceleration of the maturation of oligodendroblasts into oligodendrocytes and enhancement of their myelinogenic properties by a chemically defined medium. Dev. Neurosci. 1997;19(4):297–311. doi: 10.1159/000111226. [DOI] [PubMed] [Google Scholar]
- Espinosa-Jeffrey A, Becker-Catania S, Zhao PM, Cole R, de Vellis J. Phenotype Specification and Development of Oligodendrocytes and Neurons from Rat Stem Cell Cultures Using two Chemically Defined Media. Special Issue on Stem cells. J. Neurosci. Res. 2002;69:810–825. doi: 10.1002/jnr.10344. [DOI] [PubMed] [Google Scholar]
- Espinosa-Jeffrey A, Wakeman DR, Kim SU, Snyder EY, de Vellis J. Culture System for Rodent and Human Oligodendrocyte Specification, Lineage Progression and Maturation. Current Protocols in Stem Cell Biology. 2009:2D.4.1–2D.4.26. doi: 10.1002/9780470151808.sc02d04s10. Published: Wiley Interscience. [DOI] [PubMed] [Google Scholar]
- Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU, Snyder EY. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat. Biotechnol. 1998;16(11):1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- Goldman SA. Progenitor cell–based treatment of the pediatric myelin disorders. Arch Neurol. 2011;68:848–856. doi: 10.1001/archneurol.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll MG, Bestor TM. Eukaryotic Cytosine Methyl-transferases. Annu. Rev. Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. (First published online as a Review in Advance on March 11, 2005) [DOI] [PubMed] [Google Scholar]
- Hu BY, Du ZW, Li XJ, Ayala M, Zhang SC. Human oligodendrocytes from embryonic stem cells: Conserved Shh signaling networks and divergent FGF effects. Development. 2009;136:1443–1452. doi: 10.1242/dev.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Lee JS, Leem JW, Huh YJ, Kim JY, Kim HS, Park IH, Daley GQ, Hwang DY, Kim DW. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev. 2010;6(2):270–81. doi: 10.1007/s12015-010-9138-1. [DOI] [PubMed] [Google Scholar]
- Larsen EC, Kondo Y, Fahrenholtz CD, Duncan ID. Generation of cultured oligodendrocyte progenitor cells from rat neonatal brains. Curr. Protoc. Stem Cell Biol. 2008;Chapter 2(Unit 2D.1.1–2D.1.13) doi: 10.1002/9780470151808.sc02d01s6. [DOI] [PubMed] [Google Scholar]
- Lee JP, McKercher S, Muller FJ, Snyder EY. Neural stem cell transplantation in mouse brain. Curr. Protoc. Neurosci. 2008;3( 3.10) doi: 10.1002/0471142301.ns0310s42. [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman J. Cell. Vol. 128. Elsevier Inc.; 2007. The Role of Chromatin during Transcription; pp. 707–719. [DOI] [PubMed] [Google Scholar]
- Linnemann D, Bock E. Cell Adhesion Molecules in Neural Development. Dev. Neurosci. 1989;11(3):149–173. doi: 10.1159/000111896. [DOI] [PubMed] [Google Scholar]
- Louis JC, Magal E, Muir D, Manthorpe M, Varon S. CG4, a new bipotential glial cell line from rat brain, is capable of differentiating in vitro either mature oligodendrocytes or type-2 astrocytes. J. Neurosci. Res. 1992;31(1):193–204. doi: 10.1002/jnr.490310125. [DOI] [PubMed] [Google Scholar]
- Mauro VP, Wood IC, Krushel C, Crossin KL, Edelman GM. Cell adhesion alters gene transcription in chicken embryo brain cells and mouse embryonal carcinoma cells. Proc. Natl. Acad. Sci. 1994;.91(7):2868–2872. doi: 10.1073/pnas.91.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z, Zecevic N. Human fetal radial glia cells generate oligodendrocytes in vitro Glia. [1 April, 2009];2009 57(5):490–498. doi: 10.1002/glia.20775. April, 2009. 10.1002/glia.20775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat. Rev. Neurosci. 2006;7(1):75–84. doi: 10.1038/nrn1829. Review. [DOI] [PubMed] [Google Scholar]
- Neman J, de Vellis J, editors. Handbook of Neurochemistry and Molecular Neurobiology: Myelinating Cells in the Central Nervous System- Development, Aging, and Disease. US: Springer; 2008. [Google Scholar]
- Ourednik V, Ourednik J, Flax JD, Zawada WM, Hutt C, Yang C, Park KI, Kim SU, Sidman RL, Freed CR, Snyder EY. Segregation of human neural stem cells in the developing primate forebrain. Science. 2001;293(5536):1820–1824. doi: 10.1126/science.1060580. [DOI] [PubMed] [Google Scholar]
- Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat. Biotechnol. 2002;20(11):1103–1110. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- Park KI, Teng YD, Snyder EY. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat. Biotechnol. 2002a;20(11):1111–1117. doi: 10.1038/nbt751. [DOI] [PubMed] [Google Scholar]
- Park KI, Ourednik J, Ourednik V, Taylor RM, Aboody KS, Auguste KI, Lachyankar MB, Redmond DE, Snyder EY. Global gene and cell replacement strategies via stem cells. Gene Ther. 2002b;9(10):613–24. doi: 10.1038/sj.gt.3301721. Review. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Jr, Bjugstad KB, Teng YD, Ourednik V, Ourednik J, Wakeman DR, Parsons XH, Gonzalez R, Blanchard BC, Kim SU, Gu Z, Lipton SA, Markakis EA, Roth RH, Elsworth JD, Sladek JR, Jr, Sidman RL, Snyder EY. Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proc. Natl. Acad. Sci. 2007;104(29):12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneto RP, de Vellis J. Characterization of cultured rat oligodendrocytes proliferating in a serum-free chemically defined medium. Proc. Natl. Acad. Sci. 1985;82(10):3509–3513. doi: 10.1073/pnas.82.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim FJ, Windrem MS, Goldman SA. Fate determination of adult human glial progenitor cells. Neuron Glia Biol. 2009 Nov;5(3–4):45–55. doi: 10.1017/S1740925X09990317. [DOI] [PubMed] [Google Scholar]
- Snyder EY, Deitcher DL, Walsh C, Arnold-Aldea S, Hartwieg EA, Cepko CL. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68(1):33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- Snyder EY, Yoon C, Flax JD, Macklis JD. Multipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc. Natl. Acad. Sci. 1997;94(21):11663–11668. doi: 10.1073/pnas.94.21.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. [25 August, 2006];2006 126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. 2002;99(5):3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen CN, Caldwell MA, Ostenfeld T. Human neural stem cells: isolation, expansion and transplantation. Brain Pathol. 1999;9(3):499–513. doi: 10.1111/j.1750-3639.1999.tb00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A, Snyder EY, Vescovi A, Martínez-Serrano A. Establishment and properties of a growth factor-dependent, perpetual neural stem cell line from the human CNS. Exp Neurol. 2000;161(1):67–84. doi: 10.1006/exnr.1999.7237. [DOI] [PubMed] [Google Scholar]
- Wakeman DR, Crain AC, Snyder EY. Large animal models are critical for rationally advancing regenerative therapies. Regenerative Med. 2006;1(4):405–413. doi: 10.2217/17460751.1.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeman DR, Hofmann MR, Teng YD, Snyder EY. Derivation, Expansion, and Characterization of Human Fetal Forebrain Neural Stem Cells. In: Masters JR, Palsson BO, editors. Human Cell Culture: Adult Stem Cells. Vol. 7. Dordrecht: Springer; 2009a. [Google Scholar]
- Wakeman DR, Hofmann MR, Redmond DE, Jr, Teng YD, Snyder EY. Long-term multilayer adherent network (MAN) expansion, maintenance, and characterization, chemical and genetic manipulation, and transplantation of human fetal forebrain neural stem cells. Curr Protoc Stem Cell Biol. 2009b;Chapter 2(Unit2D.3) doi: 10.1002/9780470151808.sc02d03s9. [DOI] [PubMed] [Google Scholar]
- Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Gee L, Maherali N, Hockendlinger K, Studer L, Windrem M, Goldman S. High efficiency myelination of the hypomyelinatedshiverer mouse brain using human iPS cell-derived glial progenitor cells. ISSCR 9th Annual Meeting; Toronto, poster No. 2002. 2011. [Google Scholar]
- Williams WC, 2nd, Gard AL. In vitro death of jimpy oligodendrocytes: correlation with onset of DM-20/PLP expression and resistance to oligodendrogliotrophic factors. J. Neurosci. Res. 1997;50(2):177–189. doi: 10.1002/(SICI)1097-4547(19971015)50:2<177::AID-JNR7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wysocki LJ, Sato VL. "Panning" for lymphocytes: a method for cell selection. Proc. Natl. Acad. Sci. 1978;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Welsh AM, Bora SH, Snyder EY, Koliatsos VE. Differentiation and tropic/trophic effects of exogenous neural precursors in the adult spinal cord. J. Comp. Neurol. 2004;480(1):101–114. doi: 10.1002/cne.20344. [DOI] [PubMed] [Google Scholar]
- Yandava BD, Billinghurst LL, Snyder EY. "Global" cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci. 1999;96(12):7029–7034. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Watanabe M, Nishiyama A. Optimization of Oligodendrocyte progenitor cell culture method for enhanced survival. J. Neurosci. Methods. 2005;149(1):50–56. doi: 10.1016/j.jneumeth.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Zhang SCh, Lundberg C, Lipitz D, O’connor LT, Duncan ID. Generation of oligodendroglial progenitors from neural stem cells. Journal of Neurocytol. 1998;27(7):475–489. doi: 10.1023/a:1006953023845. [DOI] [PubMed] [Google Scholar]